Abstract
Volicitin (17-hydroxylinolenoyl-l-Gln) present in the regurgitant of Spodoptera exigua (beet armyworm caterpillars) activates the emission of volatile organic compounds (VOCs) when in contact with damaged Zea mays cv Delprim (maize) leaves. VOC emissions in turn serve as a signaling defense for the plant by attracting female parasitic wasps that prey on herbivore larvae. A tritiated form of volicitin was synthesized and shown to induce volatiles in the same fashion as the biological form. [3H]-l-volicitin rapidly, reversibly, and saturably bound to enriched plasma membrane fractions isolated from Z. mays leaves with an apparent Kd of 1.3 nM and a Hill coefficient of 1.07. Analog studies showed that the l-Gln and hydroxy moieties of volicitin play an important role in binding. Treatment of plants with methyl jasmonate (MeJA) increased the total binding of [3H]-l-volicitin to the enriched plasma membrane more than threefold, suggesting that MeJA activates transcription of the gene encoding the binding protein. S. exigua feeding also increased total binding fourfold. Cycloheximide pretreatment of plants significantly decreased binding of radiolabeled volicitin to the enriched plasma membrane. These data provide the first experimental evidence that initiation of plant defenses in response to herbivore damage can be mediated by a binding protein–ligand interaction.
INTRODUCTION
The coevolution of plants and insects has resulted in a wide array of chemical plant defenses that effectively reduce damage caused by feeding herbivores. Certain of these defenses, such as toxic alkaloids, terpenoids, and phenolics, are expressed constitutively (Wittstock and Gershenzon, 2002), whereas others such as proteinase inhibitors (Tamayo et al., 2000) and polyphenol oxidases (Constabel et al., 1995) are not normally triggered unless the plant is damaged. Although these latter defense responses are known to be wound inducible, there are numerous studies showing that injury by insects can result in augmented or attenuated physiological and biochemical plant responses compared with mechanical damage alone. These conclusions are based on measurements of a number of parameters such as plant regrowth (Dyer et al., 1995), timing of transcript accumulation (Korth and Dixon, 1997), and volatile organic compound (VOC) emissions (Paré and Tumlinson, 1999). Most recently, microarray studies that have compared mRNA levels in mechanically wounded and caterpillar-wounded plants have revealed very different transcript profiles. In Arabidopsis thaliana, insect wounding resulted in a downregulation of water stress–induced genes compared with wounding alone (Reymond et al., 2000), whereas in Nicotiana tabacum (tobacco), wound-induced transcripts are modified by insect regurgitant either by suppressing wound-induced transcripts systemically or by amplifying the wound response in the attacked leaves (Hermsmeier et al., 2001).
Herbivore-specific elicitors have been isolated from two insect fluids that regularly are exposed to plant wounds. Oviposition fluid of Callosobruchus maculatus (cowpea weevils) contains long-chain diols that are monoesterified and diesterified with 3-hydroxypropanoic acid, referred to as bruchins (Doss et al., 2000). These long-chain hydrocarbons elicit neoplastic growth of Pisum sativum (pea) pods that results in C. maculatus eggs being lifted out of the oviposition site and impedes larval entry into the pod. From larval oral secretions, two classes of elicitors have been isolated that trigger defense responses in plants. The lytic enzyme group includes β-glucosidase (Mattiacci et al., 1995), glucose oxidase (Felton and Eichenseer, 1999), and alkaline phosphatase (Funk, 2001), whereas the fatty acid–amino acid conjugate group includes a series of saturated and unsaturated fatty acids that are 16 or 18 carbons in length and coupled to α-Glu or α-Gln through a peptide bond (Alborn et al., 1997; Pohnert et al., 1999; Halitschke et al., 2001).
Plant VOCs released by herbivore attack have been shown to be attractive to arthropod predators and parasitoids in the laboratory (Dicke and van Loon, 2000) as well as outdoors in natural field conditions (Kessler and Baldwin, 2001). The VOC response can be highly specific, and this specificity can be used by certain parasitic wasps to locate particular herbivore species (De Moraes et al., 1998). In addition to attracting natural enemies of herbivores, VOC emissions can function as a direct defense by repelling the ovipositing herbivore (De Moraes et al., 2001). Such plant VOCs usually originate from at least three distinct biosynthetic pathways. The most consistently released set of VOCs are the terpenes (Paré and Tumlinson, 1997), a group of compounds derived from the mevalonate or nonmevalonate isoprenoid pathway. Many of these terpenes are emitted transiently and systemically with insect damage, suggesting that this VOC release may be under transcriptional regulation (Paré and Tumlinson, 1997). C6 alcohols and C6 aldehydes are produced from α-linolenic acid and linoleic acid via their respective hydroperoxides (Blee, 2002); the electrophilic nature of the α,β-unsaturated carbonyl motif, common to many members of this class, has been suggested to be responsible for their biological activity (Farmer, 2001). The shikimic acid pathway can also generate VOCs (e.g., methyl salicylate and indole). In fact, indole-3-glycerol phosphate lyase, a branch point enzyme in the catalysis of free indole, was the first identified enzymatic step selectively activated at the transcription level by the herbivore elicitor N-(17-hydroxylinolenoyl)-l-Gln (l-volicitin) (Frey et al., 2000).
The linolenic acid derivative volicitin induces Zea mays seedlings to release a blend of volatile terpenoids and indole that are qualitatively and quantitatively similar to those released from plants damaged by caterpillar feeding (Alborn et al., 1997). Interestingly, when a series of analogs were tested for biological activity in triggering VOC emissions, the substitution of linoleic acid for linolenic acid or the removal of the Gln or hydroxyl substituents resulted in loss-of-elicitor activity. This suggests a rigid set of chemical requirements to confer elicitor activity. In fact, only when the natural l form of Gln was coupled to the hydroxylated linolenic acid was volicitin active as an elicitor of VOC emissions. This stereoisomer specificity reduces the likelihood that nonspecific pH or polarity effects are predominantly responsible for the induction of VOC emissions and indeed points to a ligand–receptor interaction.
In this study, we have identified a plasma membrane binding protein for volicitin in Z. mays. The herbivore elicitor was synthesized in a radiolabeled form with tritiated Gln and tested for biological activity using an in vitro Z. mays bioassay system (Alborn et al., 1997). Binding saturability, reversibility, and affinity were determined through saturation experiments and competition assays with structural analogs. The induction of volicitin binding sites was stimulated by plant exposure to methyl jasmonate (MeJA) or herbivore damage.
RESULTS
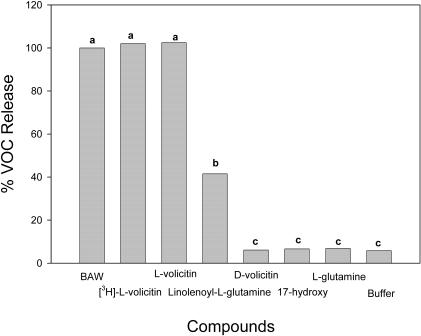
Bioactivity and Characterization of 17-Hydroxylinolenoyl [3,4-3H(N)]–l-Gln
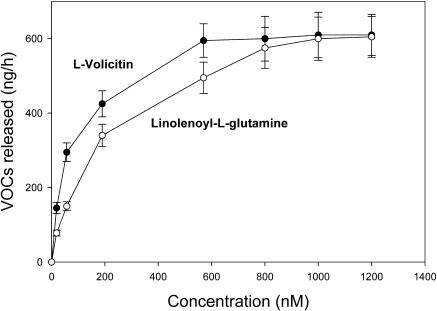
A biologically active tritiated form of volicitin ([3H]-l-volicitin) and a series of nonradioactive analogs were synthesized. Synthetic components were purified by HPLC and assayed for their ability to induce VOC emission in Z. mays bioassays (Figure 1). After the procedure reported by Alborn et al. (1997), the sum of emissions from caryophyllene, α-trans-bergamotene, (E)-β-farnesene, (E)-nerolidol, and (3E,7E)-4,8,12-trimethyl-1,3,7,11-tridecatetraene were used to measure plant induction responses by different elicitors. The results obtained using radiolabeled volicitin were not significantly different from those results obtained in experiments using nonradiolabeled synthetic volicitin or Spodoptera exigua (beet armyworm caterpillars, or BAW) oral secretions containing natural volicitin. The synthetic analog of volicitin, linolenoyl-l-Gln, was significantly less active in triggering VOC emissions, with an approximate 40% relative release rate compared with BAW regurgitant or synthetic volicitin. The other volicitin analogs tested, including 17-hydroxylinolenoyl-d-Gln (D-volicitin), 17-hydroxylinolenic acid, and l-Gln (Figure 2), did not trigger Z. mays volatile emissions at a level greater than emissions observed for the buffer control treatment.
Figure 1.
Verification of Synthetic Volicitin Activity Using a Z. mays Bioassay.
Release of volatiles collected for 2 h from six Z. mays seedlings that had been treated with 300 pmol per plant of l-volicitin, d-volicitin, 17-hydroxylinolenic acid, l-Gln, and linolenoyl-l-Gln, with concentrations ranging from 50 μg/μL to 100 μg/μL plus buffer for a final volume of 500 μL. The combined amount in nanograms of caryophyllene, α-trans-bergamontene, (E)-β-farnesene, (E)-nerolidol, and (3E,7E)-4,8,12-trimethyl-1,3,7,11-tridecatetraene was used to calculate the relative release to that of seedlings treated with 15 μL of BAW oral secretions plus 485 μL buffer, which is equivalent to ∼300 pmol natural volicitin. Data labeled with the same letter do not differ significantly (ANOVA, P < 0.05).
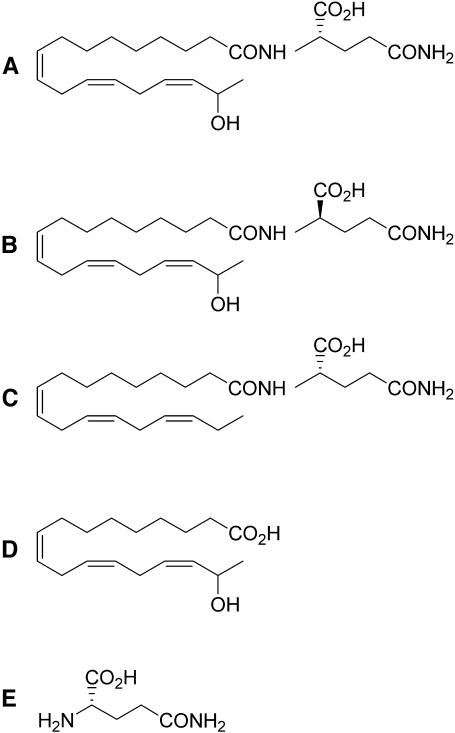
Figure 2.
Chemical Structure of Volicitin and Analogs Assayed.
(A) l-volicitin.
(B) d-volicitin.
(C) Linolenoyl-l-Gln.
(D) 17-hydroxylinolenic acid.
(E) l-Gln.
Plasma Membrane Isolation
To identify different membrane fractions and the degree of enrichment, marker enzymes ATPase (plasma membrane), cytochrome c oxidase (mitochondrial membrane), and NADPH–cytochrome c reductase (endoplasmic reticulum [ER]) were assayed. The upper plasma membrane enriched fraction (U3) contained 9.5% of the total protein and 94% of the plasma membrane marker, whereas the lower fraction (L1) contained 61% of the protein and 4% of the plasma membrane marker (Table 1). This signified a 17-fold enrichment of the plasma membrane marker in U3 versus L1. Conversely, the marker enzymes for mitochondrial membranes and ER membranes were present predominately in L1.
Table 1.
Analysis of Marker Enzymes in Membrane Fractions Isolated by Aqueous Two-Phase Partioning Procedure with the Total Protein and Marker Enzyme Activities in the Microsomal, Plasma Membrane, and Intracellular Membrane Fractions
| Fraction | Protein mg (%) | Plasma membrane ATPase nmol s−1 (%) | Mitochondria membrane Cyt c oxid. nmol s−1 (%) | ER membrane Cyt c red. nmol s−1 (%) |
|---|---|---|---|---|
| MF | 60 | 380 | 21 | 200 |
| U3 | 5.7 (9.5) | 357 (94) | 0.42 (2) | 62 (31) |
| L1 | 36.6 (61) | 15.2 (4) | 13.4 (62) | 108 (54) |
ATPase activity for plasma membrane was determined in the presence of 0.025% (v/v) Triton X-100 and 50 μM vanadate. The enzymes assayed for the mitochondria and ER were NADPH–cytochrome c oxidase and NADPH–cytochrome c reductase, respectively. Cyt c oxid., NADPH–cytochrome c oxidase; cyt c red., NADPH–cytochrome c reductase; L1, intracellular membrane fractions; MF, microsomal fractions; U3, plasma membrane fractions.
Binding Kinetics
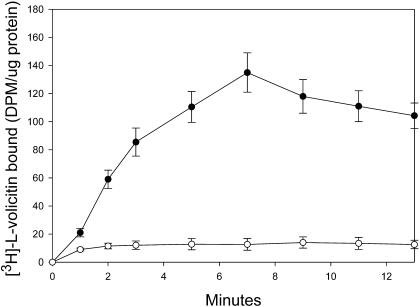
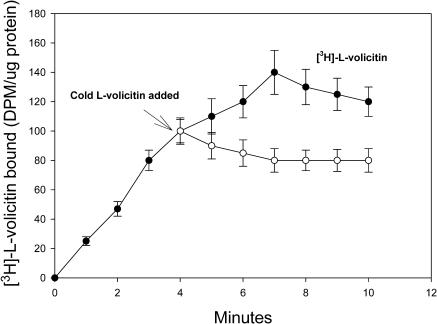
The kinetics of radiolabeled volicitin binding in an enriched plasma membrane preparation are illustrated in Figure 3. Binding was observed as early as 1 min, and the extent of binding increased for 7 min followed by a slight decrease through 13 min (Figure 3). Nonspecific binding remained constant throughout the experiment and represented only 7 to 10% of the total binding between the 5- to 13-min period. All subsequent binding studies were performed at 7 min after the addition of volicitin and volicitin analogs.
Figure 3.
Time Course of [3H]-l-Volicitin Binding to Z. mays Enriched Plasma Membranes.
Binding of [3H]-l-volicitin (10 nM) in the absence (closed circle, total binding) or presence (open circle, nonspecific binding) of a 100-fold excess of unlabeled l-volicitin over time. At the indicated times, the membranes were collected, washed, and analyzed for radioactivity. Error bars indicate standard error (n = 6).
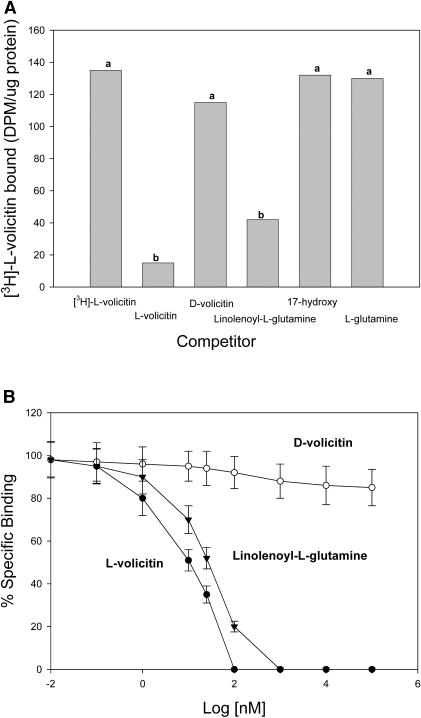
The competition by volicitin and volicitin analogs was assessed in experiments in which nonradioactive volicitin and volicitin analogs were added to the enriched plasma membrane binding assay mixture. The competition assays were performed by the addition of a 100-fold molar excess of analog to the reaction mixture before the addition of 10 nM [3H]-l-volicitin (Figure 4A). The binding of [3H]-l-volicitin to the enriched plasma membrane preparation decreased to background levels in the presence of unlabeled l-volicitin. d-volicitin containing the same charge and hydrophobicity produced only a 15% decrease for [3H]-l-volicitin bound by the enriched plasma membrane preparation. Unlabeled l-volicitin and linolenoyl-l-Gln competed for the binding sites with half-maximal inhibitory concentrations of 9 and 22 nM, respectively (Figure 4B). d-volicitin and the fatty acid and amino acids of volicitin did not produce greater than a 15% decrease in [3H]-l-volicitin binding at concentrations up to 1 mM. To relate our binding data with the volatile assays, half-maximal effective concentration (EC50) of l-volicitin and linolenoyl-l-Gln was determined by measuring volatile release from Z. mays seedlings with increasing concentrations of elicitor (Figure 5). EC50 values for l-volicitin and linolenoyl-l-Gln were measured to be 58 and 165 nM, respectively.
Figure 4.
Competition of Unlabeled Volicitin and Volicitin Analogs with [3H]-l-Volicitin for Binding Sites on the Z. mays Enriched Plasma Membranes.
(A) Competition of [3H]-l-volicitin binding to enriched plasma membranes with unlabeled l-volicitin, d-volicitin, linolenoyl-l-Gln, 17-hydroxylinolenic acid (17-hydroxy), and l-Gln. The competing analogs were added at a 100-fold molar excess (1 μM) over [3H]-l-volicitin (10 nM). Data labeled with the same letter do not differ significantly (ANOVA, P < 0.05).
(B) Competition analysis of [3H]-l-volicitin binding by l-volicitin, d-volicitin, and linolenoyl-l-Gln were determined by treating the enriched plasma membrane preparations with the indicated concentrations of unlabeled analog and by calculating the percentage of specific binding as a ratio of specific binding at the indicated concentration to maximal specific binding found in the presence of 100-fold excess of unlabeled volicitin. Closed circles, L-volicitin; open circles, D-volicitin; triangles, linolenoyl-L-Gln. Error bars indicate standard error (n = 6).
Figure 5.
Saturation Analysis of l-Volicitin and Linolenoyl-l-Gln Induction of VOCs in Z. mays Seedlings.
Maximum release of volatiles collected for 2 h from six Z. mays seedlings that had been treated with the indicated concentration of either l-volicitin (closed circles) or linolenoyl-l-Gln (open circles). The combined amount in nanograms of caryophyllene, α-trans-bergamontene, (E)-β-farnesene, (E)-nerolidol, and (3E,7E)-4,8,12-trimethyl-1,3,7,11-tridecatetraene was used to calculate the release of seedlings treated with 15 μL of test solution. Error bars indicate standard error (n = 6).
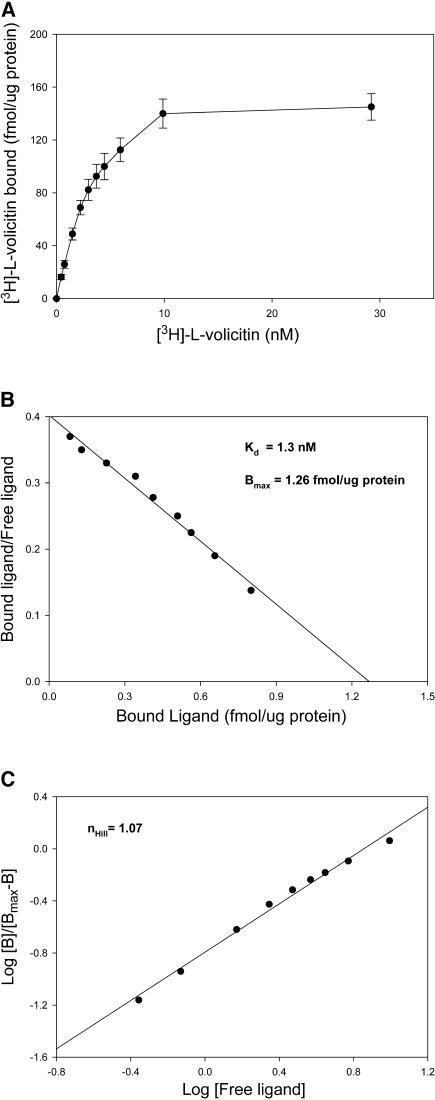
To further characterize the binding protein–ligand interaction, enriched plasma membrane fractions were incubated with increasing concentrations of ligand. The experiments were performed by incubating [3H]-l-volicitin in the presence or absence of unlabeled volicitin. Nonspecific binding was subtracted from the total binding to determine specific binding of [3H]-l-volicitin. A plot of specific binding as a function of increasing [3H]-l-volicitin shows that the binding was saturated at a level of 10 nM volicitin (Figure 6A). The saturation data was used to calculate the apparent Kd and maximal binding (Bmax) by Scatchard analysis (Figure 6B). The Kd for binding of [3H]-l-volicitin to the enriched plasma membrane was determined by taking the negative inverse of the slope in Figure 6B and calculating a Kd of 1.3 nM. From the same plot, the Bmax was determined by the x-intercept and indicated a Bmax of 1.26 fmol·μg protein−1. To determine the cooperativity of binding, the slope generated from a Hill plot was used (Figure 6C). The Hill coefficient was calculated to be 1.07. Assuming one ligand per receptor, the number of volicitin binding sites per cell was estimated at 3000.
Figure 6.
Saturation Analysis of [3H]-l-Volicitin Binding to Z. mays Enriched Plasma Membranes.
(A) Enriched plasma membrane preparations were treated with increasing concentrations of [3H]-l-volicitin. The specific binding (total binding minus nonspecific binding) is shown. The specific activity of [3H]-l-volicitin was ∼1.2 Ci/mmol (140 dpm/fmol). Error bars indicate standard error (n = 6).
(B) Scatchard analysis of [3H]-l-volicitin binding to enriched plasma membranes from data shown in (A). The Kd was calculated from the negative inverse of the slope and Bmax from the x-intercept.
(C) Hill analysis of [3H]-l-volicitin binding to enriched plasma membranes derived from the data shown in (A). The slope of the plot is the Hill coefficient (nHill).
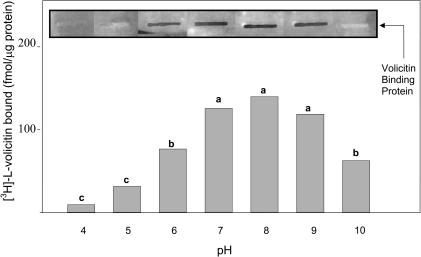
The reversibility of volicitin binding was studied by the addition of unlabeled l-volicitin to enriched plasma membrane fractions 4 min after the addition of [3H]-l-volicitin (Figure 7). The addition of unlabeled l-volicitin caused a time-dependent decrease of the radioactive retention on the filters that equilibrated within 3 min to ∼60% of the total bound. The pH protein–ligand binding optimum measured by filter and slot-blot binding assays was between 7 and 9, with ligand binding rapidly decreasing above and below this pH range (Figure 8). Based on these results, subsequent binding assays were maintained at pH 7.2.
Figure 7.
Reversibility of [3H]-l-Volicitin Binding to Z. mays Enriched Plasma Membranes.
Two sets of cells were treated with saturating levels of [3H]-l-volicitin (10 nM), and the total radioactivity associated with the enriched plasma membrane was determined at the indicated time. A 100-fold excess (1 μM) of unlabeled volicitin was added to one set of enriched plasma membrane preparations 4 min after the addition of [3H]-l-volicitin. The total radioactivity associated with both sets of enriched plasma membranes was determined at the indicated times. Error bars indicate standard error (n = 6).
Figure 8.
Assay pH Influences [3H]-l-Volicitin–Plasma Membrane Binding.
[3H]-l-Volicitin (10 nM) filter binding and slot-blot assays for plasma membrane enriched fractions adjusted to the selected pH. Slot-blot insets display [3H]-l-volicitin binding to enriched plasma membranes bound to nitrocellulose and analyzed by autoradiography. Data labeled with the same letter do not differ significantly (ANOVA, P < 0.05).
Induction of Binding Sites
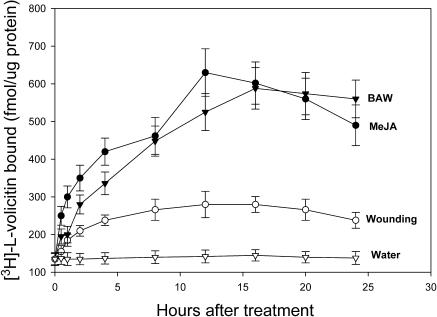
Jasmonic acid (JA) and mechanical damage are both inducers of VOC emissions in Z. mays seedlings (Schmelz et al., 2001, 2003); their effect on [3H]-l-volicitin binding to enriched plasma membrane was assayed over time (Figure 9). The application of 50 mL of 1 μM MeJA produced a 2× increase in [3H]-l-volicitin binding 2 h after treatment and a 4× increase in [3H]-l-volicitin binding by 12 h after treatment. Beyond the 12-h period, [3H]-l-volicitin binding decreased. The initial response to BAW wounding showed an approximate twofold increase within the first 2 h, a steady increase to an approximate fourfold peak at 16 h, followed by a leveling of induction over the remaining 24-h time course. By contrast, mechanical damage had only a modest 2× effect on the binding of [3H]-l-volicitin to the enriched plasma membrane preparation.
Figure 9.
Induction by MeJA, BAW, and Mechanical Wounding on [3H]-l-Volicitin–Plasma Membrane Binding.
Saturating levels of [3H]-l-volicitin (10 nM) were used to determine the total level of binding at the indicated times after treatment with MeJA, BAW, or razor blade. The total binding is shown in fmol·μg protein−1. Error bars indicate standard error (n = 6).
To examine whether the increase in [3H]-l-volicitin binding in response to mechanical damage, MeJA, and BAW required de novo protein synthesis, plants were cotreated with 2 μM cycloheximide and compared with plants not treated with the protein translation inhibitor. Binding studies with radiolabeled volicitin were performed with enriched plasma membrane isolated 16 h after treatment with mechanical damage, MeJA, and BAW, with or without cycloheximide. Volicitin binding for plants treated with cycloheximide versus those without cycloheximide treatment showed a decreased binding in all cases tested (Table 2): water (fourfold), mechanical damage (eightfold), BAW (16-fold), and MeJA (17-fold).
Table 2.
Induction of [3H]-l-volicitin–Plasma Membrane Binding with Mechanical Damage, BAW, MeJA, or Water Treatment in the Presence or Absence of Cycloheximide
| Specific Binding (dpm/μg protein)
|
||
|---|---|---|
| Plant Treatment | Without Cycloheximide | With Cycloheximide |
| Mechanical Damage1 | 260 ± 34 | 30 ± 6.3 |
| BAW1 | 510 ± 62 | 32 ± 7.2 |
| MeJA1 | 455 ± 57 | 27 ± 6.9 |
| Water1 | 130 ± 15 | 28 ± 5.5 |
| Water2 | 128 ± 18 | 121 ± 20 |
Binding of volicitin was performed with enriched plasma membrane isolated 16 h after treatment, except where noted.
A 16 h delay between treatment and harvest.
No delay between treatment and harvest.
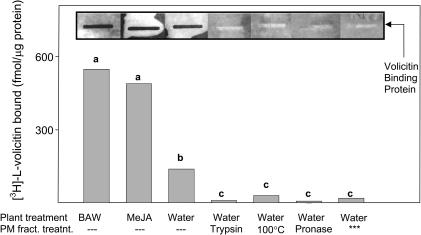
To validate whether the specific binding of [3H]-l-volicitin involved a proteinacous receptor, enriched Z.mays plasma membrane fractions were treated with the proteases trypsin and pronase. Protease treatment reduced [3H]-l-volicitin–specific binding by ∼90% in filter binding assays and to background levels in slot-blot binding assays (Figure 10). Enriched plasma membrane heat treatment also significantly reduced protein–ligand binding (≥80%), whereas heat-inactivated proteases had no effect on ligand binding (data not shown). Plant exposure for 16 h to BAW leaf damage or MeJA before plasma membrane isolation resulted in induction of protein–ligand binding in both the filter and slot-blot binding assays.
Figure 10.
Protein Requirement for [3H]-l-Volicitin–Plasma Membrane Binding.
[3H]-l-volicitin (10 nM) filter binding and slot-blot assays with indicated plant and plasma membrane fraction treatments. Slot-blot insets display [3H]-l-volicitin (1.6 × 106 cpm·mL−1) binding to enriched plasma membranes bound to nitrocellulose and analyzed by autoradiography. Asterisks indicate the lower fraction (L1) containing a non-plasma membrane enriched fraction. Data labeled with the same letter do not differ significantly (ANOVA, P < 0.05).
The possibility that volicitin intercalated into membranes based on the hydrophobic nature of the ligand combined with the Gln moiety nonselectively interacting with integral membrane proteins was ruled out by assaying membrane fractions not enriched with plasma membrane proteins. The absence of volicitin binding to the lower fraction from the phase partitioning procedure (Figure 10, last lane), which was enriched in non-plasma membrane bound proteins (Table 1, fraction L1), indicates that a protein-rich lipid bilayer is not sufficient to cause volicitin binding. Indeed, the volicitin binding protein appears to be located in or associated with the plasma membrane.
DISCUSSION
These data provide biochemical evidence for the existence of a plant binding protein that can selectively bind an herbivore ligand (elicitor) that triggers the emissions of plant VOCs. We demonstrate that radiolabeled volicitin rapidly, reversibly, and saturably binds to enriched plasma membrane fractions. The Kd observed by Scatchard plot analysis was 1.3 nM, indicating a high affinity association; this binding constant reflects the ability of low volicitin concentrations to serve as elicitors of VOC emissions in plants. Bmax was calculated at 1.26 fmol·μg protein−1 or ∼3000 sites per cell. Density estimates of other defense signal receptors on the cell's surface include 3000 sites per cell for systemin binding to Lycopersicon esculentum (tomato) cell suspensions (Scheer and Ryan, 1999) and 2900 sites per cell for the peptide elicitor Pep-13 binding to Petroselinum crispum (parsley) (Nurnberger et al., 1994). The binding of volicitin did not facilitate additional volicitin binding (i.e., noncooperative binding) based on the Hill plot coefficient (n = 1.07).
Correlations between ligand binding and biological activity were used as the criterion for assessing authentic protein binding. Several synthesized volicitin analogs were tested to correlate binding and biological activity. Analogs tested for their ability to competitively displace [3H]-l-volicitin bound to plasma membrane enriched fractions (half-maximal inhibitory concentration values) closely overlapped biological activity with D-volicitin, 17-hydroxylinolenic acid, and l-Gln inactive in triggering VOCs and ineffective in serving as an antagonist of [3H]-l-volicitin binding to the enriched plasma membrane, whereas the unlabeled elicitor molecules volicitin and linolenoyl-l-Gln were competent in both triggering VOCs and competing for protein binding sites occupied by radiolabeled volicitin.
The binding of radiolabeled volicitin was reversible, as determined by the decrease in binding within 3 min of a 100-fold molar excess addition of unlabeled volicitin. Approximately 40% reduction of total binding was observed, suggesting that volicitin–protein binding is quite strong. Other studies of elicitor binding have shown similar levels of reversibility binding; for example, with a 200-fold molar addition of cold systemin, a 50% reduction of total binding was observed in L. esculentum cell suspensions (Scheer and Ryan, 1999), and with a 10,000-fold molar addition of cold Pep-13, a 60% reduction of total binding was observed in P. crispum (Nurnberger et al., 1994).
To further characterize this volicitin binding protein, either enriched plasma membrane preparations or whole plants were exposed to previously identified chemical regulators. Loss of ligand binding was observed when enriched plasma membrane preparations were pretreated with proteases, indicating that ligand binding is mediated at least in part through a protein-containing entity. Treatment of plants with cycloheximide 16 h before harvesting plant material caused a marked decrease in binding of radiolabeled volicitin to the enriched plasma membrane preparation. This decrease in ligand–protein binding suggests that the volicitin binding protein is in constant turnover and that loss of synthesis quickly translates in an absence of the binding protein. For example, cycloheximide treatment, which results in an inhibition of protein synthesis, compared with water treatment resulted in a decrease of volicitin-specific binding from 130 to 28 dpm (Table 2), suggesting that the binding protein proceeds through two half-lives during the 16 h treatment, or t1/2 = 8 h. This rapid turnover for a volicitin binding protein was unexpected, although precedent for such turnover rates does exist in the case of the defense signaling receptor systemin isolated from L. esculentum, which exhibited a half-life of 7.5 h (Scheer and Ryan, 1999). Plant exposure to insect damage or MeJA treatment triggered an induction of binding sites on the enriched plasma membrane, suggesting that the volicitin binding protein may be transcriptionally upregulated to enhance the mechanism for perception of elicitor signals with herbivore feeding. Insect wounding also induces the emission of volatile terpenes in a wide range of plant taxa (Paré and Tumlinson, 1999), including Z. mays, and studies have implicated a role for JA in triggering plant VOC emissions. Indeed the exogenous application of JA to intact (Schmelz et al., 2001) or excised plants (Dicke et al., 1999) triggers elevated VOC emissions. In some cases, endogenous increases of JA with insect damage either parallel or precede VOC emissions. In fact, Schmelz et al. (2003) have observed a direct positive relationship between endogenous JA levels and VOC emissions in Z. mays seedlings with insect damage.
Early steps in the herbivore elicitation process have yet to be elucidated (Kessler and Baldwin, 2002). Although there are no previous reports of herbivore-specific elicitor plant receptors, plasma membrane binding proteins for pathogen elicitors have been identified and characterized. Using radiolabeled β-1,3-glucan and hepta-β-glucoside elicitors characterized from fungal cell walls of Phytophthora megasperma f sp glycinea (Schmidt and Ebel, 1987), high affinity binding proteins have been identified in isolated legume plasma membrane fractions (Cheong and Hahn, 1991; Cote et al., 2000). Elicitor binding receptors for the N-acetylchitooligossaccharide elicitor have also been detected in plasma membrane fractions from several plant species including Oryza sativa (rice), Triticum aestivum (wheat), Hordeum vulgare (barley), and Daucus carota (carrot) (Okada et al., 2001, 2002). Each of these pathogen elicitor–plant receptor interactions is characterized by nanomolar EC50 concentrations as well as Kd values in the low nanomolar range. A comparable EC50 for VOC emissions in Z. mays with volicitin exposure suggested that this elicitor, like plant pathogen elicitors, is perceived by a cell-specific receptor.
The biogenetic origin of volicitin has been established by a series of stable isotope–labeling studies that demonstrated that BAW acquires linolenic acid (an essential fatty acid in the diet of Lepidoptera) from plants and that the insect subsequently hydroxylates and conjugates the fatty acid with Gln (Paré et al., 1998). Mechanical damage causes the release of membrane-bound linolenic acid and initiates the octadecanoid signaling pathway. Modification of linolenic acid by the feeding insect appears to provide a distinct chemical cue that can selectively bind to a specific volicitin binding protein, upregulate the binding of volicitin to the enriched plasma membrane, and ultimately allow the plant to differentiate between herbivore damage and nonspecific leaf damage that triggers the lipoxygenase pathway. In subsequent studies, how a volicitin binding protein might function in the cell either as a membrane transporter shuttling volicitin across the membrane and serving as the primary messenger of VOC synthesis or as an activator of second messenger(s) cascade will be examined. Because the volicitin binding protein is detectable by slot-blot binding assays, screening of a Z. mays cDNA phage–display library with radiolabeled volicitin probe may identify volicitin binding phage proteins(s) that can be subsequently cloned and analyzed.
METHODS
Plants, Insects, and Reagents
Z. mays plants (cv Delprim) grown from seed in Pro-Grow potting soil supplemented with Osmocote fertilizer (Scotts-Sierra, Marysville, OH) were maintained in an insect-free facility in which temperature and relative humidity were monitored and maintained at 29°C ± 4°C and 40% ± 10%, respectively. Metal halide and high-pressure sodium lamps on a 16-h/8-h light/dark photoperiod provided a light intensity of 700 μmol·m−2·s−1. Two-week-old plants were used for experiments described below, except where noted, and BAW eggs obtained from USDA-ARS (Tifton, GA) were incubated at 26°C, relative humidity ∼90%, and 16-h/8-h light/dark cycle. Hatched larvae were reared on an artificial pinto bean diet, following the method of King and Leppla (1984), and used at the late third instar stage. MeJA was obtained from Bedoukian Research (Danbury, CT) and was of ≥99% purity, as determined by capillary gas chromatography–flame ionization detector analysis. MeJA (1 μM) was prepared by adding 10 μL to 1 L of water (50 μM) and taking a 1 mL aliquot with 50 mL of water before application. Other compounds were purchased form Sigma-Aldrich (St. Louis, MO) and were of ≥99% purity, except where noted.
Ligand Synthesis
The synthesis of the l- and d-enantiomers of volicitin, linolenoyl-l-Gln, and 17-hydroxylinolenic acid is described by Wei et al. (2003). All synthetic compounds were analyzed by mass spectrometry using a direct-inserted probe before use. The radiolabeling of volicitin was prepared in the same method with the use of 1 mCi of l-[3,4-3H(N)]Gln with a specific activity of 45 Ci/mmol (Dupont NEN, Boston, MA).
Seedling Bioassays
Volatile analyses were performed on 10-d-old seedlings following the protocol by Alborn et al. (1997), except that 15 μL of test solution was added to 500 μL of 50 mM Na2HPO4 buffer titrated to pH 8.0 with 1 M NaH2PO4. The final assay concentration of volicitin and analogs was 600 nM.
Preparation of Enriched Plasma Membranes
Membrane fractions enriched in plasma membrane material were isolated from Z. mays leaves following the protocol of Robinson et al. (1988), with all subsequent operations conducted at 4°C. Described briefly, leaves were homogenized in 15 mM Tris-HCl, pH 7.8, with 1 mM EDTA, 3 mM DTT, 0.5 M sucrose, and 0.6% polyvinylpyrrolidone in a 1:4 ratio (g/mL) and filtered through four layers of cheesecloth. The filtrate was centrifuged at 10,000g for 10 min, and the decanted supernatant was spun a second time at 50,000g for 30 min. The resulting microsomal pellet (8.4 mg protein/mL) was resuspended in phosphate buffer (5 mM K-phosphate, pH 7.8, and 0.25 M sucrose), and the plasma membrane fraction was isolated using the aqueous two-phase partitioning method described by Larsson et al. (1987). Ten grams of the microsomal fractions was added to 11.52 g dextran (Mr = 500,000) and 5.76 g polyethylene glycol (Mr = 3350) in 5 mM phosphate buffer, pH 7.8, containing 0.3 M sucrose and 3 mM KCl for a final weight of 36 g. The system was equilibrated overnight at 4°C after vigorous shaking for 30 s. The phases were separated by centrifugation at 2000g for 10 min (HB-4 rotor; Sorvall, Newton, CT). The upper fraction was combined with a fresh lower fraction obtained from another phase system prepared as above, except with 10 g of water, whereas the lower phase was combined with fresh upper phase. The two systems were mixed, equilibrated, and centrifuged as above, followed by repetition of same protocol. The final upper phases containing enriched plasma membrane were combined, diluted in Tris buffer (5 mM Tris-MES, pH 7.2, 0.1 mM DTT, and 0.25 M sucrose) and centrifuged at 100,000g. The pellet was resuspended in the same Tris buffer and stored at −70°C. For pH assays, the pellet was resuspended in a modified buffer (5 mM sodium citrate, 5 mM phosphate, pH 7.2, 0.1 mM DTT, and 0.25 M sucrose) with the concentrations of citric acid and phosphate adjusted to achieve pH values between 4 and 8. In addition, 10 mM ethanolamine buffer was used to assay binding between pH 9.0 and 10. Protein concentrations were assayed based on the method of Bradford (1976) using BSA as a standard.
Membrane Purity Assays
Marker enzymes were used to assay plasma membrane, ER, and mitochondria purity following the protocol of Hodges and Leonard (1972), with all subsequent reactions done at 25°C unless otherwise stated. Plasma membrane was assayed for vanadate-inhibited, Mg2+-ATPase activity. The reaction was performed in a 1-mL reaction volume containing 3 mM ATP, 3 mM MgSO4, 30 mM Tris-MES buffer, pH 6.5, 50 mM KCl, and 30 μg membrane protein in the presence or absence of 0.025% (v/v) Titron X-100 and/or 50 μM Na3VO4. After 30 min incubation at 38°C, 2.6 mL of a stopping reagent, 0.42% (w/v) ammonium molybdate in 1 M H2SO4 with one part 10% (w/v) ascorbic acid, was added to each assay. After 20 min incubation at room temperature, the absorbance at 650 nm was measured, and the amount of inorganic phosphate was calculated with 0.01 μmol equal to an absorbance of 0.240 (Ames, 1966). ER was assayed for the NADPH–cytochrome c reductase, a cyanide-insensitive enzyme. A 3-mL reaction volume with 0.1 mL of membrane suspension (30 μg protein), 0.1 mL of 50 mM NaCN, 0.2 mL of 0.45 mM cytochrome c, and 2.5 mL of 50 mM sodium phosphate buffer, pH 7.5, was started by the addition of 0.1 mL of 3 mM NADPH, with the reduction of cytochrome c change measured at 550 nm. Mitochondrial membranes were assayed for cytochrome c oxidase activity using NADPH–cytochrome c reductase. Cytochrome c was reduced by addition of excess of sodium dithionite, followed by aeration of the solution to oxidize excess dithionite. Reactions contained 0.1 mL of membrane suspension (30 μg protein), 0.1 mol of 0.3% Triton X-100, and 2.7 mL of 50 mM sodium phosphate, pH 7.5. Reactions proceeded by addition of 0.1 mL of reduced cytochrome c, and change at 550 nm was measured. The rate of cytochrome c reduction and oxidation was calculated using an extinction coefficient of 18.5 mM−1·cm−1.
Binding Experiments
Radioligand filter binding assays were based on a procedure by Scheer and Ryan (1999). Binding was initiated by adding 50 μL of radiolabeled volicitin (10 nM) to 100 μL of enriched plasma membrane (200 μg of protein) in 1.5 mL Eppendorf tubes at 25°C. Precisely 7 min were given for binding, except when measuring the time course of binding. The reaction mixtures were then filtered through GF/B binder-free glass fiber filters (Fisher Scientific, Loughborough, Leicestershire, UK) housed in a 12-well vacuum filtration manifold (Millipore, Bedford, MA). Filters were prewashed with 1 mL of 4°C buffer (5 mM Tris-MES, pH 7.2, 0.1 mM DTT, and 0.25 M sucrose) and then rinsed with an additional 3 mL buffer once the radioligand was applied; the amount of [3H]-l-volicitin retained by the filter was measured by liquid scintillation (LS5000 TD; Beckman Instruments, Fullerton, CA). In competition assays, unlabeled volicitin or a volicitin analog was added just before the addition of radiolableled volicitin. Specific binding was calculated by subtracting nonspecific binding (binding in the presence of excess nonradioactive volicitin) from total binding. Binding assays were replicated (6×) using saturating levels of volicitin (10 nM) unless otherwise indicated. Volicitin analogs were added at 100-fold molar excess (1 μM). The percentage of specific binding used in displacement experiments was calculated by dividing the specific binding at the indicated concentrations of competing analog by the maximum specific binding determined in the presence of an excess of volicitin. Enriched plasma membrane preparations incubated in filtration buffer for 16 h at 37°C showed no decrease in specific binding, indicating the absence of endogenous protease activity.
For slot-blot binding assays, 10 μL of plasma membrane enriched protein (1 μg/μL) was applied to nitrocellulose filters in a chilled slot-blot manifold (Bio-Dot SF microfiltration apparatus; Bio-Rad, Hercules, CA); temperature was maintained at 4°C throughout the hybridization procedure. Membranes were presoaked for 20 min in 10 mL hybridization buffer (20 mM potassium phosphate, 200 mM KCl, 1 mM EDTA, and 1 mM DTT) adjusted to pH 7.9, and then incubated for 7 min in 10 mL of fresh buffer containing the [3H]-l-volicitin probe (1.6 × 106 cpm/mL buffer). Membranes were rinsed two times for 15 min in 10 mL hybridization buffer and placed in x-ray film intensifying cassettes at −70°C for 30 d before film development.
The receptor number per cell estimation was based on a generic cell with the following features: the plasma membrane contained 40% protein by weight; the plasma membrane volume was 8.1 × 10−12 mL (30 × 30 × 0.009 μm) (Larsson and Moller, 1989); and the average plasma membrane density was 1.15 g·mL−1 (Larsson et al., 1987). Plasma membrane number per microliter of extract was calculated from the binding assay protein concentration, factoring in the enrichment of plasma membrane protein from the marker enzyme. This value was then divided by the receptor number per microliter calculated from Bmax, assuming a 1:1 mole ratio of volicitin binding protein to ligand.
Protease and Heat Treatment
Enriched plasma membrane preparations (2 mg protein/mL) were incubated in filtration buffer for 30 min in a 37°C water bath with the following protease concentrations: 50 μg/mL trypsin and 12 μg/mL Pronase E (Sigma). Treatments were ended using the antiprotease mixture of 50 μg/mL of phenylmethylsulfonyl fluoride to inhibit trypsin and 1 μg/mL of pepstatin for Pronase E. Enriched plasma membrane preparations were then used for [3H]-l-volicitin binding assays. Control assays contained the same antiprotease mixture with the proteases inactivated by heating at 100°C for 15 min before binding.
Volicitin Binding Protein Induction
Binding protein induction was assayed under the following conditions: (1) plants sprayed with 1 μM MeJA; (2) plants mechanically razor-blade damaged covering an area of 2 cm2 on two lower leaves; and (3) plants insect damaged with five BAW larvae covering an area of 2 cm2 on the two lower leaves. All plants were sprayed with 50 mL of water and Tween 20 (0.025%) to account for solvent effects. Plants were maintained under previously described growth conditions until the time of harvest. The specific binding was determined in the water-treated, mechanically damaged, BAW-treated, and MeJA-treated enriched plasma membrane preparations and expressed as a fold increase over water-treated controls. To measure the effect of protein translation on binding, plants were sprayed with 2 μM cycloheximide concomitantly with mechanical damage, BAW feeding, or MeJA treatment, and plants were harvested 16 h after treatment.
Statistical Analyses
Analyses of variance, using SAS statistical software version 8 (SAS Institute, Cary, NC), were performed on all volatile collection data and induction of binding results. Means were separated using Duncan's multiple range test with a P value <0.05. Significant treatment effects were investigated when analysis of variance was significant (P < 0.05).
Acknowledgments
We thank Richard Jasoni for statistical analysis training, Mohamed A. Farag for slot-blot binding assay instructions, and James G. Harmon for constructive comments concerning the initial manuscript. Financial support was provided by USDA Grant 35320-9378, the Herman Frasch Foundation for Chemical Research, and the Robert A. Welch Foundation Grant D-1478.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Paul W. Paré (paul.pare@ttu.edu).
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.017723.
References
- Alborn, H.T., Turlings, T.C.J., Jones, T.H., Stenhagen, G., Loughrin, J.H., and Tumlinson, J.H. (1997). An elicitor of plant volatiles from beet armyworm oral secretion. Science 276, 945–949. [Google Scholar]
- Ames, B.N. (1966). Assay of inorganic phosphate, total phosphate and phosphatase. Meth. Enzymol. 8, 115–118. [Google Scholar]
- Blee, E. (2002). Impact of phyto-oxylipins in plant defense. Trends Plant Sci. 7, 315–321. [DOI] [PubMed] [Google Scholar]
- Bradford, M.M. (1976). A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–255. [DOI] [PubMed] [Google Scholar]
- Cheong, J.J., and Hahn, M.G. (1991). A specific, high-affinity binding site for the hepta-β-glucoside elicitor exists in soybean membranes. Plant Cell 3, 137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constabel, C.P., Bergey, D.R., and Ryan, C.A. (1995). Systemin activates synthesis of wound-inducible tomato leaf polyphenol oxidase via the octadecanoid defense signaling pathway. Proc. Natl. Acad. Sci. USA 92, 407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote, F., Roberts, K.A., and Hahn, M.G. (2000). Identification of high affinity binding sites for the hepta-β-glucoside elicitor in membranes of the model legumes Medicago truncatula and Lotus japonicus. Planta 211, 596–605. [DOI] [PubMed] [Google Scholar]
- De Moraes, C.M., Lewis, W.J., Paré, P.W., Alborn, H.T., and Tumlinson, J.H. (1998). Herbivore-infested plants selectively attract parasitoids. Nature 393, 570–573. [Google Scholar]
- De Moraes, C.M., Mescher, M.C., and Tumlinson, J.H. (2001). Caterpillar-induced nocturnal plant volatiles repel nonspecific females. Nature 410, 577–580. [DOI] [PubMed] [Google Scholar]
- Dicke, M., Gols, R., Ludeking, D., and Posthumus, M.A. (1999). Jasmonic acid and herbivory differentially induce carnivore-attracting plant volatiles in lima bean plants. J. Chem. Ecol. 25, 1907–1922. [Google Scholar]
- Dicke, M., and van Loon, J.A. (2000). Multitrophic effects of herbivore-induced plant volatiles in an evolutionary context. Entomol. Exp. Appl. 97, 237–249. [Google Scholar]
- Doss, R.P., Oliver, J.E., Proebsting, W.M., Potter, S.W., Kuy, S.R., Clement, S.L., Williamson, R.T., Carney, J.R., and DeVilbiss, E.D. (2000). Bruchins: Insect-derived plant regulators that stimulate neoplasm formation. Proc. Natl. Acad. Sci. USA 97, 6218–6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer, M.I., Moon, A.M., Brown, M.R., and Crossley, D.A. (1995). Grasshopper crop and midgut extract effects on plants- an example of reward feedback. Proc. Natl. Acad. Sci. USA 92, 5475–5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer, E.E. (2001). Surface-to-air signals. Nature 411, 854–856. [DOI] [PubMed] [Google Scholar]
- Felton, G.W., and Eichenseer, H. (1999). Herbivore saliva and induction of resistance to herbivores and pathogens. In Induced Plant Defenses Against Pathogens and Herbivores: Biochemistry, Ecology and Agriculture, A. Agurwal, S. Tuzun, and E. Bent, eds (St. Paul, MN: American Phytopathological Society), pp. 19–36.
- Frey, M., Stettner, C., Paré, P.W., Schmelz, E.A., Tumlinson, J.H., and Gierl, A. (2000). An herbivore elicitor activates the gene for indole emission in maize. Proc. Natl. Acad. Sci. USA 97, 14801–14806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk, C.J. (2001). Alkaline phosphatase activity in whitefly salivary glands and saliva. Arch. Insect Biochem. Physiol. 46, 165–174. [DOI] [PubMed] [Google Scholar]
- Halitschke, R., Schittko, U., Pohnert, G., Boland, W., and Baldwin, I.T. (2001). Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. III. Fatty acid-amino acid conjugates in herbivore oral secretions are necessary and sufficient for herbivore-specific plant responses. Plant Physiol. 125, 711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermsmeier, D., Schittko, W., and Baldwin, I.T. (2001). Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. I. Large-scale changes in the accumulation of growth- and defense-related plant mRNAs. Plant Physiol. 125, 683–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges, T.K., and Leonard, R.T. (1972). Purification of a plasma membrane-bound adenosine triphosphatase form plant roots. Meth. Enzymol. 32, 392–406. [DOI] [PubMed] [Google Scholar]
- Kessler, A., and Baldwin, I.T. (2001). Defensive function of herbivore-induced plant volatile emissions in nature. Science 291, 2141–2144. [DOI] [PubMed] [Google Scholar]
- Kessler, A., and Baldwin, I.T. (2002). Plant responses to insect herbivory: The emerging molecular analysis. Annu. Rev. Plant Biol. 53, 299–328. [DOI] [PubMed] [Google Scholar]
- King, E.G., and Leppla, N.C. (1984). Advances and Challenges in Insect Rearing. U.S. Government Printing Services, Washington, D.C.
- Korth, K.L., and Dixon, R.A. (1997). Evidence for chewing insect-specific molecular events distinct from a general wound response in leaves. Plant Physiol. 115, 1299–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson, C., and Moller, I.M. (1989). Introduction to the plant plasma membrane and its molecular composition and organization. In The Plant Plasma Membrane: Structure, Function, and Molecular Biology, C. Larsson and I.M. Moller, eds (New York: Springer-Verlag), pp. 1–15.
- Larsson, C., Widell, S., and Kjelbom, P. (1987). Preparation of high-purity plasma membranes. Meth. Enzymol. 148, 558–568. [Google Scholar]
- Mattiacci, L., Dicke, M., and Posthumus, M.A. (1995). β-Glucosidase: An elicitor of herbivore-induced plant odor that attracts host-searching parasitic wasps. Proc. Natl. Acad. Sci. USA 92, 2036–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurnberger, T., Nennstiel, D., Jabs, T., Sacks, W.R., Hahlbrock, K., and Scheel, D. (1994). High affinity binding of a fungal oligopeptide elicitor to parsley plasma membranes triggers multiple defense responses. Cell 78, 449–460. [DOI] [PubMed] [Google Scholar]
- Okada, M., Matsumura, M., and Shibuya, N. (2001). Identification of a high-affinity binding protein for N-acetylchitooligosaccharide elicitor in the plasma membrane from rice leaf and root cells. J. Plant Physiol. 158, 121–124. [Google Scholar]
- Okada, M., Matsumura, M., and Shibuya, N. (2002). High-affinity binding proteins for N-acetylchitooligosaccharide elicitor in the plasma membranes from wheat, barley and carrot cells: Conserved presence and correlation with the responsiveness to the elicitor. Plant Cell Physiol. 43, 505–512. [DOI] [PubMed] [Google Scholar]
- Paré, P.W., Alborn, H.T., and Tumlinson, J.H. (1998). Concerted biosynthesis of an insect elicitor of plant volatiles. Proc. Natl. Acad. Sci. USA 95, 13971–13975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré, P.W., and Tumlinson, J.H. (1997). Induced synthesis of plant volatiles. Nature 385, 30–31. [Google Scholar]
- Paré, P.W., and Tumlinson, J.H. (1999). Plant volatiles as a defense against insect herbivores. Plant Physiol. 121, 325–331. [PMC free article] [PubMed] [Google Scholar]
- Pohnert, G., Jung, V., Haukioja, E., Lempa, K., and Boland, W. (1999). New fatty acid amides from regurgitant of Lepidopteran (Noctuidae, Geometridae) caterpillars. Tetrahedron Lett. 55, 11275–11280. [Google Scholar]
- Reymond, P., Weber, H., Damond, M., and Farmer, E.E. (2000). Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell 12, 707–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, C., Larsson, D., and Buckhout, T.J. (1988). Identification of a calmodulin-stimulated (Ca2+ + Mg2+)-ATPase in a plasma membrane fraction isolated from maize (Zea mays) leaves. Physiol. Plant 72, 177–184. [Google Scholar]
- Scheer, J.M., and Ryan, C.A. (1999). A 160-kD systemin receptor on the surface of Lycopersicon peruvianum suspension-cultured cells. Plant Cell 11, 1525–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz, E.A., Alborn, H.T., Banchio, E., and Tumlinson, J.H. (2003). Quantitative relationships between induced jasmonic acid levels and volatile emission in Zea mays during Spodoptera exigua herbivory. Planta 216, 665–673. [DOI] [PubMed] [Google Scholar]
- Schmelz, E.A., Alborn, H.T., and Tumlinson, J.H. (2001). The influence of intact-plant and excised-leaf bioassay designs on volicitin- and jasmonic acid-induced sesquiterpene volatile release in Zea mays. Planta 214, 171–179. [DOI] [PubMed] [Google Scholar]
- Schmidt, W.E., and Ebel, J. (1987). Specific binding of a fungal glucan phytoalexinelicitor to membrane fractions from soybean Glycine max. Proc. Natl. Acad. Sci. USA 84, 4117–4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamayo, M.C., Rufat, M., Bravo, J.M., and San Segundo, B. (2000). Accumulation of a maize proteinase inhibitor in response to wounding and insect feeding, and characterization of its activity toward digestive proteinases of Spodoptera littoralis larvae. Planta 211, 62–71. [DOI] [PubMed] [Google Scholar]
- Wei, H.X., Truitt, C.L., and Paré, P.W. (2003). Synthesis of hydroxy-substituted unsaturated fatty acids and the amino-acid insect-derivative volicitin. Tetrahedron Lett. 44, 831–833. [Google Scholar]
- Wittstock, U., and Gershenzon, J. (2002). Constitutive plant toxins and their role in defense against herbivores and pathogens. Curr. Opin. Plant Biol. 5, 300–307. [DOI] [PubMed] [Google Scholar]