Abstract
Objective
Obesity, represented by high body mass index (BMI), is a major complication after treatment for childhood cancer. However, it has been shown that high total fat percentage and low lean body mass are more reliable predictors of cardiovascular morbidity. In this study longitudinal changes of BMI and body composition, as well as the value of BMI and waist-hip ratio representing obesity, were evaluated in adult childhood cancer survivors.
Methods
Data from 410 survivors who had visited the late effects clinic twice were analyzed. Median follow-up time was 16 years (interquartile range 11–21) and time between visits was 3.2 years (2.9–3.6). BMI was measured and body composition was assessed by dual X-ray absorptiometry (DXA, Lunar Prodigy; available twice in 182 survivors). Data were compared with healthy Dutch references and calculated as standard deviation scores (SDS). BMI, waist-hip ratio and total fat percentage were evaluated cross-sectionally in 422 survivors, in who at least one DXA scan was assessed.
Results
BMI was significantly higher in women, without significant change over time. In men BMI changed significantly with time (ΔSDS = 0.19, P<0.001). Percentage fat was significantly higher than references in all survivors, with the highest SDS after cranial radiotherapy (CRT) (mean SDS 1.73 in men, 1.48 in women, P<0.001). Only in men, increase in total fat percentage was significantly higher than references (ΔSDS = 0.22, P<0.001). Using total fat percentage as the gold standard, 65% of female and 42% of male survivors were misclassified as non-obese using BMI. Misclassification of obesity using waist-hip ratio was 40% in women and 24% in men.
Conclusions
Sixteen years after treatment for childhood cancer, the increase in BMI and total fat percentage was significantly greater than expected, especially after CRT. This is important as we could show that obesity was grossly underestimated using BMI and waist-hip ratio.
Introduction
Childhood cancer survival rates have increased enormously over the last few decades [1], [2]. As a consequence, the incidence of treatment related complications is increasing. One of the major sequelae is obesity, with a prevalence of 9 to 30% depending on former treatment modalities [3], [4], [5]. Body mass index (BMI) is the most widely used measure for obesity. However, it has been shown that a high amount of total body fat, high intra-abdominal fat percentage and low lean body mass are more reliable determinants than high BMI in predicting the development of cardiovascular disease or diabetes mellitus [6], [7], [8], [9], [10]. In a recent study, Shah and Braverman showed that BMI misclassified 48% of the female and 25% of the male population using total fat percentage measured by dual X-ray absorptiometry (DXA) as the gold standard. This lead to an underestimation of the prevalence of obesity [10]. Waist circumference, which approximates the amount of intra-abdominal fat, is a more accurate marker than BMI and is one of the criteria used to define metabolic syndrome [11], [12].
In childhood cancer survivors, abnormal body composition has been thoroughly described and is caused by several factors including damage to the hypothalamus and/or pituitary due to cranial radiotherapy and use of corticosteroids [5], [11], [12], [13], [14], [15]. Most studies that investigated changes in body composition, in addition to BMI, were cross-sectional and included small cohorts and selected subgroups, such as acute lymphoblastic leukemia (ALL) survivors [11], [16], [17], [18]. Currently, long-term survivor studies measuring body composition longitudinally are not available. The Childhood Cancer Survivor Study has reported changes in BMI with a very long-term follow-up of 25 years [19]. The aim of our study was to investigate longitudinal changes in BMI and body composition, and to evaluate the value of BMI and waist-hip ratio as compared with total fat percentage measured by DXA in long-term adult childhood cancer survivors in a single center in the Netherlands.
Subjects and Methods
Ethics Statement
The data described in the current retrospective study were obtained during regular visits at the late effects clinic, and clinical investigations were assessed using the standard guidelines for screening late effects after childhood cancer following Good Clinical Practice (GCP). An official written informed consent from every patient that visited the outpatient clinic was obtained according to standards of the Institutional Review Board (IRB).
Subjects
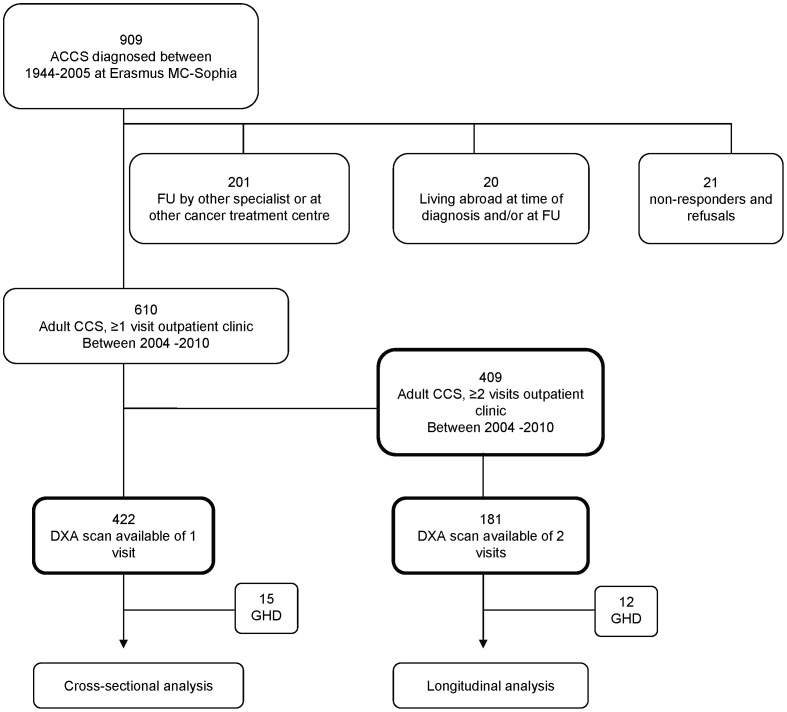
We performed a retrospective single center study. Follow-up of subjects at our late effects outpatient clinic for long-term childhood cancer survivors starts 5 years after cessation of therapy and is individualized based on cancer diagnosis and treatment protocol. Out of 909 adult childhood cancer survivors diagnosed and treated between 1964 and 2005, 610 had visited the outpatient clinic and 410 had visited the outpatient clinic twice (figure 1). Longitudinal changes in BMI were evaluated in survivors with 2 visits. Cross-sectional evaluation of BMI, waist-hip ratio and total fat percentage was assessed in 423 survivors. One survivor was diagnosed with Down syndrome and was therefore excluded.
Figure 1. Flowchart of included survivors.
FU follow-up; CCS childhood cancer survivors; GHD Growth hormone deficiency.
Methods
Treatment details, as well as disease and patient characteristics, were retrieved from our local database (table 1). Information regarding pituitary dysfunction and hormone supplementation were evaluated. Body mass index (BMI) was calculated from height and weight [20]. Waist-hip ratio was calculated from waist and hip circumference [21]. Lean body mass and total fat percentages were measured by DXA using a single machine (DXA, Lunar Prodigy, GE Healthcare, Madison, WI, USA). Data from sequential DXA scans was available in 182 survivors. Obesity was defined as BMI ≥30 [22], total fat percentage ≥25% (M) and ≥30% (W) [23] and waist-hip ratio ≥0.85 (W) and ≥0.90 (M) [21].
Table 1. Baseline characteristics of survivors included in this study.
| Survivors with DXA scan (cross-sectional data) | Survivors with two visits (longitudinal data) | |||
| Male N = 243 | Female N = 179 | Male N = 229 | Female N = 180 | |
| Age at diagnosis (yrs) | 6.3 (3.4–11.8) | 6.5 (3.2–11.7) | 6.7 (3.3–11.8) | 6.2 (2.9–11.7) |
| Age at follow-up (yrs) | 25.8 (21.9–31.4) | 26.6 (22.3–32.7) | 23.8 (20.2–28.0) | 25.4 (21.0–30.1) |
| Time between DXA’s (yrs) | n.a. | n.a. | 3.2 (2.9–3.6) | 3.1 (2.9–3.4) |
| Follow-up time (yrs) | 17.5 (12.1–23.0) | 17.9 (13.2–23.7) | 15.9 (11.2–20.3) | 17.0 (11.9–22.4) |
| Diagnosis | ||||
| ALL, T-NHL | 107 (44) | 82 (46) | 79 (35) | 58 (33) |
| AML | 10 (4) | 7 (4) | 8 (4) | 8 (4) |
| B-NHL | 28 (12) | 14 (8) | 24 (11) | 14 (8) |
| Hodgkin lymphoma | 32 (13) | 14 (8) | 25 (11) | 16 (9) |
| Bone tumor | 4 (2) | 6 (3) | 11 (5) | 8 (4) |
| Wilms tumor | 26 (11) | 21 (12) | 26 (11) | 24 (13) |
| Neuroblastoma | 5 (2) | 10 (6) | 11 (5) | 18 (10) |
| Germ cell tumor | 1 (1) | 1 (1) | 4 (2) | 5 (3) |
| MMT | 6 (3) | 5 (3) | 17 (7) | 13 (7) |
| Brain tumor | 15 (6) | 9 (5) | 15 (7) | 7 (4) |
| Other | 17 (7) | 16 (9) | 9 (4) | 9 (5) |
| Therapy | ||||
| CRT | 43 (18) | 28 (16) | 26 (11) | 19 (11) |
| BRT | 9 (4) | 3 (2) | 31 (14) | 8 (4) |
| Corticosteroids | 124 (51) | 88 (49) | 75 (33) | 68 (38) |
| No CRT/corticosteroids | 54 (22) | 50 (28) | 91 (40) | 79 (44) |
| TBI | 13 (5) | 10 (6) | 6 (3) | 6 (3) |
| Abdominal RT | 15 (6) | 14 (8) | n.a. | n.a. |
Data are expressed as median (interquartile range) or as frequencies (N %).
ALL Acute lymphoblastic leukemia; AML Acute myeloid leukemia; B-NHL B-cell non hodgkin lymphoma; T-NHL T-cell non hodgkin lymphoma; MMT Malignant mesenchymal tumor; CRT Cranial radiotherapy; BRT Local radiotherapy on cranium; TBI Total body irradiation.
Final height data were compared with Dutch adult references described by Fredriks et al. [24]. BMI data were compared with data from self-reported questionnaires, which are sent out yearly to 10 000 subjects by the central office for statistics (CBS) in the Netherlands [25]. Lean body mass and total fat percentage data were compared with healthy Dutch references [26], [27]. To adjust for age and gender, standard deviation scores were calculated for final height, BMI and body composition measures.
Growth hormone deficiency (GHD) was defined by an insufficient growth hormone stimulation test (growth hormone peak <3 µg/L during an insulin tolerance test or <9 µg/L during a GHRH-Arginine test [28]). Hypothyroidism was defined as an fT4 level <11 pmol/L in combination with normal or high TSH levels (>4.3 mU/L; primary hypothyroidism) or a TSH level <0.4 mU/L (secondary hypothyroidism). Hypogonadism was defined as a testosterone level <9 nmol/L in men or oligo/amenorrhea in women.
Laboratory Measurements
Serum insulin-like growth factor-I (IGF-I; nmol/L) was measured using a chemi-luminescence-based immunoassay (Immulite 2000, Siemens DPC, Los Angeles CA, USA). Intra- and interassay coefficients of variation (CV) were <5 and <7%. IGF-I levels were compared with reference values by using standard deviation scores (SDS) [29]. In cases where GHD was suspected (i.e. IGF-I below −2 SDS) a growth hormone stimulation test was assessed. Testosterone (nmol/l) was measured by coated tube radioimmuno-assay (Siemens DPC). Intra- and interassay CVs were <6 and <9% respectively. Thyroid stimulating hormone (TSH) (U/L) and free thyroxine (fT4) (pmol/l) were measured using chemoluminescence assays (Vitros ECi Immunodiagnostic System; Ortho Clinical Diagnostics, Rochester, NY). Interassay CV was 4.7–5.4% for fT4 and 2.5–4.1% for TSH.
Statistics
To evaluate longitudinal changes in BMI and body composition, treatment modalities were categorized into five groups: cranial radiotherapy as defined by central nervous system irradiation in leukemia/lymphoma survivors (CRT), brain tumor irradiation directed to the indicated tumor field (BRT), total body irradiation (TBI), corticosteroids (without irradiation), and other treatment (neither cranial irradiation nor corticosteroids). Standard deviation scores were calculated for BMI, total fat percentage and lean body mass and were compared with healthy references using the one sample t-test. When analyzing the rough data, differences between 1st and 2nd assessments were tested using the paired t-test (figure 2). Growth hormone deficient subjects were excluded from the analysis because growth hormone treatment causes a significant decrease of total body fat [30]. Multivariate regression analysis was performed to determine the influence of age at diagnosis, hypothyroidism, hypogonadism and use of oral contraceptives on BMI and measures of body composition.
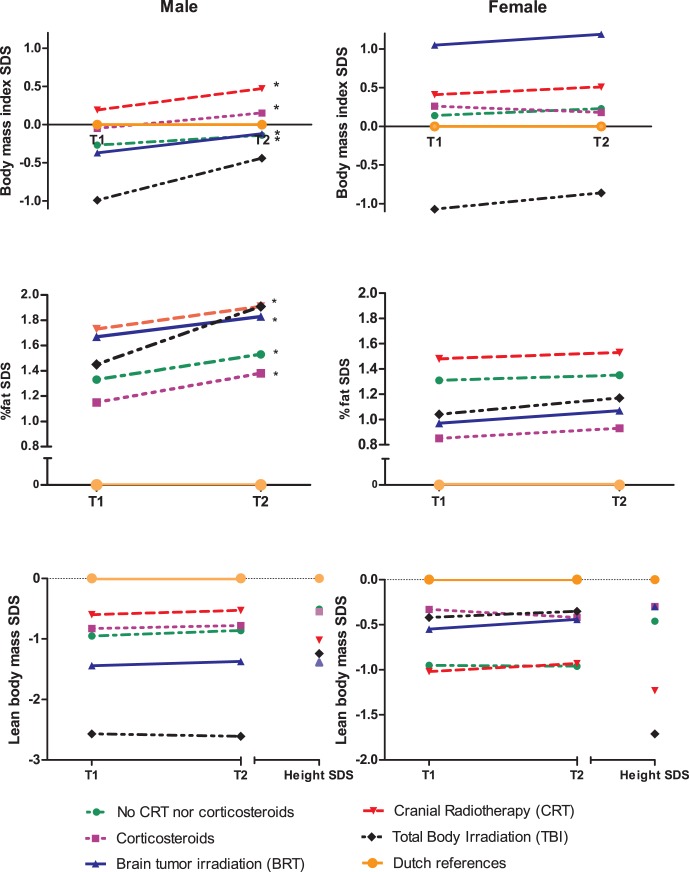
Figure 2. Standard deviation scores (SDS) of body mass index, total fat percentage, lean body mass and height in different therapy groups expressed as mean at T1 and T2.
* Significantly higher increase as compared to references (one-paired T-test, p<0.05).
To evaluate the value of BMI and waist-hip ratio, total fat percentage, retrieved from DXA scans, was used as the gold standard. Total fat percentage was compared with BMI and waist-hip ratio, to determine percent agreement and disagreement, separately in men and women. A Receiver Operating Curve (ROC) analysis stratified by sex was used to evaluate sensitivity and specificity for BMI and waist-hip ratio and to determine more accurate cut-off points relative to total fat percentage. Survivors who had been treated with abdominal radiotherapy were excluded from the waist-hip ratio analysis.
P-values were considered statistically significant if p<0.05. Statistical analysis was performed using SPSS 17.0 software (SPSS, Chicago, IL).
Results
Survivors
Baseline and treatment characteristics are shown in Table 1. Median follow-up time between cessation of therapy and the first visit at the outpatient late effects clinic was 16 years (interquartile range (IQR) 11–21). Median time between the 1st assessment (defined as T1) and 2nd assessment (defined as T2) was 3.2 years (IQR 2.9–3.6). Forty-five, mainly leukemia survivors, had been treated with CRT (25 Gy (24–25)), including 3 survivors that had received craniospinal irradiation; thirty-nine, mainly brain tumor survivors, had been treated with BRT (40 Gy (35–44)). Two leukemia survivors had been treated with CRT and TBI and were analyzed in the CRT group. One brain tumor survivor had received BRT and TBI and was analyzed in the BRT group. One hundred and forty-three survivors had been treated with corticosteroids but not cranial irradiation, and 170 had only been treated with chemotherapy, not with corticosteroids or cranial irradiation.
Fifteen survivors had been diagnosed with GHD earlier and were treated with growth hormone (GH) therapy at time of follow-up, and were excluded from further analysis. Hypothyroidism was present in 14 survivors at T1 and 18 survivors at T2. Hypogonadism was present in 21 survivors at T1 and 25 survivors at T2. There were no subjects with untreated hypogonadism and hypothyroidism. Oral contraceptives were used by 94 women at T1 and 86 at T2. Multivariate analysis showed that total fat percentage was not associated with hypogonadism or hypothyroidism during adequate replacement therapy, nor with oral contraceptive use. After correction for height SDS, lean body mass was significantly associated with hypothyroidism in female survivors, which were therefore excluded from the analysis (β −1.20, p = 0.039). BMI was significantly associated with hypogonadism in male survivors, which were therefore excluded from the analysis (β 0.92, p = 0.015). Age at diagnosis was not significantly associated with BMI and body composition.
Body Mass Index
Male survivors had a significantly lower BMI at T1 (SDS = −0.17, P = 0.022) and showed a significant change over time (ΔSDS = 0.19, P<0.001). When analyzing treatment groups separately BMI at T1 was not significantly different from Dutch references, but changed significantly over time in all groups except for TBI (figure 2). In female survivors BMI was significantly higher compared with references, without significant change over time (SDS T1 = 0.22, P = 0.024, T2 = 0.25, P = 0.006). When analyzing treatment groups separately only cranial irradiated female leukemia/lymphoma survivors had significantly higher BMI without significant change over time (SDS T1 = 0.41, P = 0.021, T2 0.51, P = 0.031). Both male and female TBI survivors had a significantly lower BMI compared with references (figure 2).
Total Fat Percentage
Fat percentage was significantly higher compared with Dutch references in both men (SDS T1 1.37, P<0.001) and women (SDS T1 1.05, P<0.001). The highest fat percentage was found after CRT (mean SDS T1 1.73 in men, 1.48 in women, P<0.001) (figure 2). In men, fat percentage increased significantly (ΔSDS = 0.22, P<0.001). Evaluating treatment groups separately, fat percentage increased significantly in all groups except for the TBI group (figure 2). In female survivors no significant increase of fat percentage was found (ΔSDS = 0.07, P = 0.22).
Height and Lean Body Mass
Lean body mass, as well as height, were significantly lower in all treatment groups, compared with Dutch references, except for female BRT survivors (figure 2). Adjusted for height SDS, lean body mass SDS was significantly lower in male TBI survivors only (T1, P = 0.01; T2, P = 0.004). Lean body mass did not change over time in either men or women in any of the treatment groups (figure 2).
BMI and Waist-hip Ratio as Compared with Total Fat Percentage
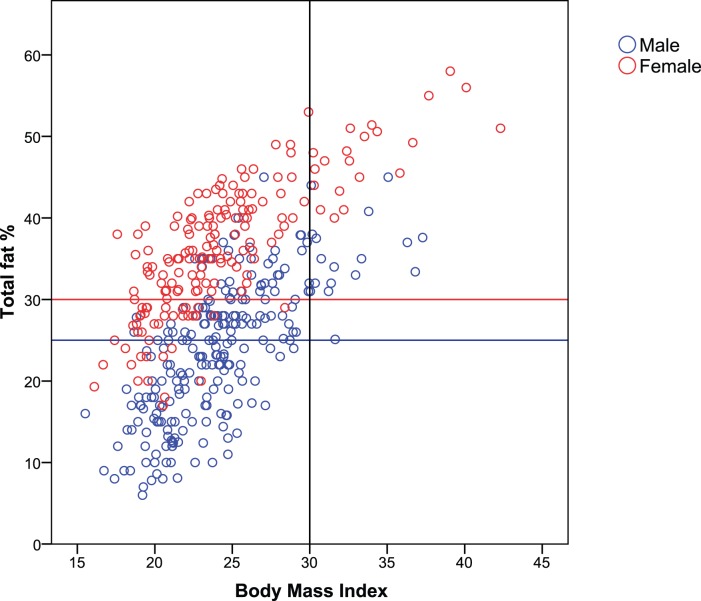
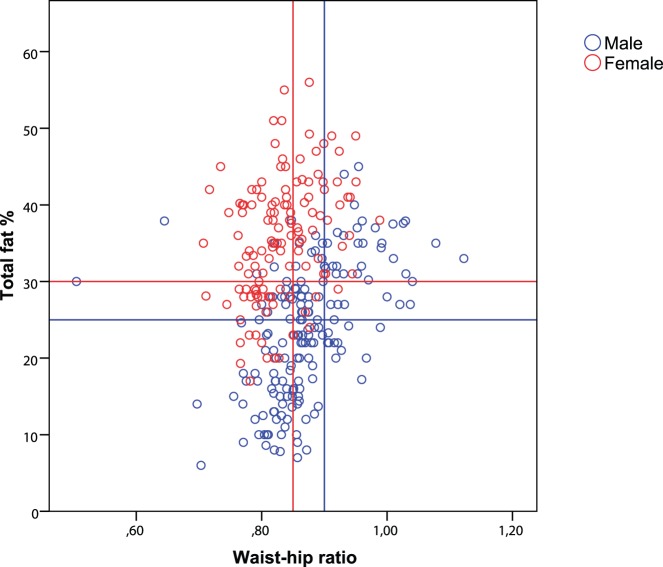
Out of 407 survivors 210 (52%) were misclassified as non-obese based on BMI, while meeting the obesity criteria based on total fat percentage. In female survivors this percentage of misclassification was even higher (65%) than in men (42%) (Table 2, figure 3). Waist-hip ratio was classified as non-obese and total fat percentage as obese in 92 out of 299 survivors (31%). This percentage of misclassification was 40% in female and 24% in male survivors (figure 4). We attempted to define new cut-off points for BMI and waist-hip ratio to better identify childhood cancer survivors as obese, using total fat percentage as the gold standard (table 3). Sensitivity improved by 58% (from 14% to 72%) in males, with a loss in specificity of only 24% (100% to 76%) using a BMI cut-off of 24 instead of 30. In females a cut-off of 22 would give an increase in sensitivity of 64% (16 to 80%) with a loss in specificity of only 18% (100% to 82%). Cut-off points for waist-hip ratio, as defined by the WHO, were more accurate than BMI in childhood cancer survivors (table 3), although a cut-off limit of 0.82 instead of 0.85 in women would give an increase in sensitivity of 26% (45% to 71%), with a loss in specificity of 14% (85% to 71%).
Table 2. Total fat percentage, BMI and waist-hip ratio for adult survivors of childhood cancer.
| Body Mass Index | Waist-hip Ratio* | |||||
| Men N = 235 | Women N = 172 | Total N = 407 | Men N = 174 | Women N = 125 | Total N = 299 | |
| Concordant | ||||||
| BMI/WHR non obese, total fat % non-obese | 120 (51) | 39 (23) | 159 (39) | 80 (46) | 29 (23) | 109 (37) |
| BMI/WHR obese, total fat % obese | 17 (7) | 21 (12) | 38 (9) | 40 (23) | 41 (33) | 81 (27) |
| Discordant | ||||||
| BMI/WHR non-obese, total fat % obese | 98 (42) | 112 (65) | 210 (52) | 42 (24) | 50 (40) | 92 (31) |
| BMI/WHR obese, total fat % non-obese | 0 | 0 | 0 | 12 (7) | 5 (4) | 17 (6) |
Survivors treated with abdominal radiotherapy were excluded.
Figure 3. BMI versus total fat percentage.
Female and male survivors in the upper quadrant corner, marked by the red respectively the blue horizontal line and black vertical line, are classified as non-obese by BMI, but as obese by total fat percentage.
Figure 4. Waist-hip ratio versus total fat percentage.
Female and male survivors in the upper quadrant corner, marked by the red respectively the blue lines, are classified as non-obese by waist-hip ratio, but as obese by total fat percentage.
Table 3. Sensitivity and specificity for different cut-off points of BMI and waist-hip ratio as defined by total fat percentage of ≥25% for men and ≥30% for women.
| Male | Female | |||
| Sensitivity | Specificity | Sensitivity | Specificity | |
| BMI cut-off point | ||||
| 18 | 100 | 4 | 99 | 10 |
| 20 | 98 | 25 | 92 | 54 |
| 22 | 91 | 58 | 80 | 82 |
| 24 | 72 | 76 | 53 | 97 |
| 26 | 47 | 95 | 32 | 97 |
| 28 | 28 | 98 | 23 | 97 |
| 30 * | 14 | 100 | 16 | 100 |
| 32 | 6 | 100 | 11 | 100 |
| WHR cut-off point | ||||
| 0.77 | 98 | 3 | 93 | 9 |
| 0.78 | 98 | 8 | 89 | 21 |
| 0.79 | 98 | 9 | 84 | 32 |
| 0.80 | 96 | 12 | 80 | 50 |
| 0.81 | 94 | 15 | 75 | 59 |
| 0.82 | 91 | 22 | 71 | 71 |
| 0.83 | 88 | 34 | 60 | 76 |
| 0.84 | 86 | 42 | 54 | 82 |
| 0.85 * | 81 | 48 | 45 | 85 |
| 0.86 | 73 | 56 | 42 | 88 |
| 0.87 | 63 | 70 | 33 | 88 |
| 0.88 | 59 | 76 | 31 | 91 |
| 0.89 | 54 | 85 | 24 | 94 |
| 0.90 * | 49 | 87 | 20 | 97 |
| 0.91 | 42 | 88 | 13 | 97 |
| 0.92 | 40 | 91 | 12 | 97 |
| 0.93 | 33 | 95 | 9 | 100 |
| 0.94 | 28 | 96 | n.a. | n.a. |
| 0.95 | 28 | 97 | n.a. | n.a. |
| 0.96 | 22 | 97 | n.a. | n.a. |
| 0.97 | 19 | 98 | n.a. | n.a. |
| 0.98 | 17 | 99 | n.a. | n.a. |
Cut-off limits of BMI respectively waist-hip ratio generally used for the classification of obesity.
Discussion
Male survivors showed an increase of BMI and total fat percentage during a median follow-up time of 3.2 years, more than 15 years after treatment for childhood cancer. Female survivors, especially the cranial irradiated leukemia/lymphoma subgroup, showed higher BMI and total fat percentage at both time points but without a significant change over time. To our knowledge, the childhood cancer survivor study (CCSS) is the only study to date that describes longitudinal changes in childhood cancer survivors at very long-term follow-up [19]. In this study, self-reported BMI was evaluated in ALL survivors 25 years after diagnosis, and CRT was defined as a risk factor for BMI change, particularly in women [19]. Brouwer et al. showed that BMI increased in Dutch childhood cancer survivors, from the end of treatment until attainment of final height and observed that high dose CRT was the most important risk factor for this BMI increase [31]. Long after attainment of final height, all of our male survivors showed a higher BMI increase compared with the reference population, suggesting that cancer treatment effects persist over time. This BMI increase did not result in a significantly higher BMI 3 years later. However, taking into account the short time-interval, this significant increase in BMI at a relatively young age might indicate an emerging problem in the future. In female survivors BMI was significantly higher after CRT treatment; however, there was no significant change over time, suggesting that changes in BMI had already occurred at a younger age. Furthermore, the interval between measurements was only 3 years and survivors had a younger age at the 1st assessment (median 24 years) as compared with the CCSS (median 32 years) which might explain the different findings. Additionally, since annual reference data from the CBS are self-reported, BMI could be underestimated, possibly affecting the comparison between BMI in survivors and controls in this study.
Previous studies have been mainly based on self-reported or measured BMI values. Recently it was shown that BMI is an inaccurate marker for total body fat. Obesity was underestimated in 39% of subjects, using BMI [10]. Therefore, we used DXA to assess total fat percentage and lean body mass, which are considered to be more relevant predictors for cardiovascular disease or diabetes mellitus [6], [7], [8], [9]. In the current study, total fat percentage was persistently and significantly higher than the reference population and was more pronounced in CRT survivors. Furthermore, while mean BMI standard deviation scores varied around zero, increases in total fat percentage SDS and decreases in lean body mass SDS were more pronounced. This implies that although BMI may seem normal, the distribution of total fat and lean body mass might be dramatically changed. Therefore, in order to test the hypothesis that BMI underestimates the adiposity risk in childhood cancer survivors, BMI data were compared with total fat percentage of all the survivors in whom one DXA scan was performed. This revealed that indeed 42% of male survivors and 65% of female survivors were classified as non-obese by BMI, but as obese by total fat percentage. These percentages of misclassification are even greater than compared with earlier findings in the general population by Shah and Braverman, although our subjects were much younger [10]. This finding emphasizes the need for more precise measurements of total fat percentage in order to define obesity. Since DXA is costly and not easily accessible for less developed countries, we attempted to define more accurate cut-off limits for BMI in childhood cancer survivors. It was shown that a cut-off limit of 24 for males and 22 for females resulted in greater sensitivity, with only small reductions in specificity. Although the group was too small to draw definite conclusions, these findings indicate that the current cut-off of 30 is not useful in defining obesity in childhood cancer survivors. A more reliable determinant than BMI that predicts the development of cardiovascular disease is the amount of intra-abdominal fat, which is approximated by the waist-hip ratio [11], [12]. Above all, waist-hip ratio is an easy to use clinical tool. However, when we compared waist-hip ratio with total fat percentage in childhood cancer survivors, percentage misclassification was lower than with BMI, but still 31%.
Mechanisms that contribute to abnormal body composition in childhood cancer survivors include decrease in physical activity, hypothalamic-, pituitary and/or gonadal damage, due to cranial and abdominal radiotherapy and leading to endocrinopathies and use of corticosteroids, followed by metabolic changes [5], [11], [12], [13], [14], [15].
Limitations
In the current retrospective study no data concerning socio-economic status, smoking, diet and physical activity for the total cohort of survivors, which could be important predictors for BMI change, were available. Reduced energy expenditure and excess energy intake may play a role in the development of overweight in childhood cancer survivors, and has been described during and shortly after treatment [32], [33]. Furthermore, since obesity is not an independent risk factor for cardiovascular morbidity, prospective studies are needed, investigating multiple risk factors such as hypertension, dyslipidemia, insulin resistance and life style changes in very long-term survivors of childhood cancer.
Conclusion
In this large and unique longitudinal study among childhood cancer survivors, a greater increase in BMI and total fat percentage compared with the reference population was found, especially in adult male survivors,. Furthermore, we found that BMI underestimated obesity by 52% of adult survivors, while waist-hip ratio underestimated 31% of survivors when compared with measurements of total fat percentage. Therefore, we have suggested new cut-off limits, which need to be confirmed in future prospective studies, in order to define obesity more precisely in childhood cancer survivors.
Funding Statement
The authors have no support or funding to report.
References
- 1.Hewitt M WS, Simone JV. (2003) Childhood cancer survivorship: improving care and quality of life. Washington, DC: National Academies Press. [PubMed]
- 2.Ries LAG EM, Kosary CL. (2005) SEER cancer statistics review, 1975–2002. National Cancer Institute.
- 3. Oeffinger KC, Mertens AC, Sklar CA, Yasui Y, Fears T, et al. (2003) Obesity in adult survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. J Clin Oncol 21: 1359–1365. [DOI] [PubMed] [Google Scholar]
- 4. Pagano L, De Rosa G, Voso MT, Marra R, Testa A, et al. (1994) Prevalence of obesity in young adults with acute lymphoblastic leukemia. Int J Clin Lab Res 24: 117–119. [DOI] [PubMed] [Google Scholar]
- 5. Van Dongen-Melman JE, Hokken-Koelega AC, Hahlen K, De Groot A, Tromp CG, et al. (1995) Obesity after successful treatment of acute lymphoblastic leukemia in childhood. Pediatr Res 38: 86–90. [DOI] [PubMed] [Google Scholar]
- 6. Sardinha LB, Teixeira PJ, Guedes DP, Going SB, Lohman TG (2000) Subcutaneous central fat is associated with cardiovascular risk factors in men independently of total fatness and fitness. Metabolism 49: 1379–1385. [DOI] [PubMed] [Google Scholar]
- 7. Larsson B, Svardsudd K, Welin L, Wilhelmsen L, Bjorntorp P, et al. (1984) Abdominal adipose tissue distribution, obesity, and risk of cardiovascular disease and death: 13 year follow up of participants in the study of men born in 1913. Br Med J (Clin Res Ed) 288: 1401–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Paradisi G, Smith L, Burtner C, Leaming R, Garvey WT, et al. (1999) Dual energy X-ray absorptiometry assessment of fat mass distribution and its association with the insulin resistance syndrome. Diabetes Care 22: 1310–1317. [DOI] [PubMed] [Google Scholar]
- 9. Bjorntorp P (1997) Body fat distribution, insulin resistance, and metabolic diseases. Nutrition 13: 795–803. [DOI] [PubMed] [Google Scholar]
- 10. Shah NR, Braverman ER (2012) Measuring Adiposity in Patients: The Utility of Body Mass Index (BMI), Percent Body Fat, and Leptin. PLoS One 7: e33308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jarfelt M, Lannering B, Bosaeus I, Johannsson G, Bjarnason R (2005) Body composition in young adult survivors of childhood acute lymphoblastic leukaemia. Eur J Endocrinol 153: 81–89. [DOI] [PubMed] [Google Scholar]
- 12. van Waas M, Neggers SJ, Pieters R, van den Heuvel-Eibrink MM (2010) Components of the metabolic syndrome in 500 adult long-term survivors of childhood cancer. Ann Oncol 21: 1121–1126. [DOI] [PubMed] [Google Scholar]
- 13. van Waas M, Neggers SJ, van der Lelij AJ, Pieters R, van den Heuvel-Eibrink MM (2010) The metabolic syndrome in adult survivors of childhood cancer, a review. J Pediatr Hematol Oncol 32: 171–179. [DOI] [PubMed] [Google Scholar]
- 14. van Beek RD, de Muinck Keizer-Schrama SM, Hakvoort-Cammel FG, van der Sluis IM, Krenning EP, et al. (2006) No difference between prednisolone and dexamethasone treatment in bone mineral density and growth in long term survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer 46: 88–93. [DOI] [PubMed] [Google Scholar]
- 15. Janiszewski PM, Oeffinger KC, Church TS, Dunn AL, Eshelman DA, et al. (2007) Abdominal obesity, liver fat, and muscle composition in survivors of childhood acute lymphoblastic leukemia. J Clin Endocrinol Metab 92: 3816–3821. [DOI] [PubMed] [Google Scholar]
- 16. Nysom K, Holm K, Michaelsen KF, Hertz H, Muller J, et al. (2003) Degree of fatness after treatment of malignant lymphoma in childhood. Med Pediatr Oncol 40: 239–243. [DOI] [PubMed] [Google Scholar]
- 17. Brouwer CA, Gietema JA, Kamps WA, de Vries EG, Postma A (2007) Changes in body composition after childhood cancer treatment: impact on future health status–a review. Crit Rev Oncol Hematol 63: 32–46. [DOI] [PubMed] [Google Scholar]
- 18. van der Sluis IM, van den Heuvel-Eibrink MM, Hahlen K, Krenning EP, de Muinck Keizer-Schrama SM (2000) Bone mineral density, body composition, and height in long-term survivors of acute lymphoblastic leukemia in childhood. Med Pediatr Oncol 35: 415–420. [DOI] [PubMed] [Google Scholar]
- 19. Garmey EG, Liu Q, Sklar CA, Meacham LR, Mertens AC, et al. (2008) Longitudinal changes in obesity and body mass index among adult survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. J Clin Oncol 26: 4639–4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Health BNIo (2000) National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (NHLBI). The practical guide: identification, evaluation, and treatment of overweight and obesity in adults. NIH publication 00–4084.
- 21.World Health Organisation website. Available: http://whqlibdoc.who.int/publications/2011/9789241501491_eng.pdf. Accessed 2012 Apr 20.
- 22.The National Institue of Health Website. Available: http://www.nhlbi.nih.gov/guidelines/obesity/bmi_tbl.htm. Accessed 2012 Apr 20.
- 23.American Society of Bariatric Physicians Website. Available: http://www.asbp.org/siterun_data/about_asbp/position_statements/doc7270523281295654373.html. Accessed 2012 Apr 20.
- 24. Fredriks AM, van Buuren S, Burgmeijer RJ, Meulmeester JF, Beuker RJ, et al. (2000) Continuing positive secular growth change in The Netherlands 1955–1997. Pediatr Res 47: 316–323. [DOI] [PubMed] [Google Scholar]
- 25.Centraal Bureau voor Statistiek website. Available: http://www.cbs.nl/nl-NL/menu/home/default.htm. Accessed 2012 Mar 1.
- 26.van der Sluis IM, de Ridder MA, Boot AM, Krenning EP, de Muinck Keizer-Schrama SM (2002) Reference data for bone density and body composition measured with dual energy x ray absorptiometry in white children and young adults. Arch Dis Child 87: 341–347; discussion 341–347. [DOI] [PMC free article] [PubMed]
- 27. Boot AM, de Ridder MA, van der Sluis IM, van Slobbe I, Krenning EP, et al. (2010) Peak bone mineral density, lean body mass and fractures. Bone 46: 336–341. [DOI] [PubMed] [Google Scholar]
- 28. Consensus guidelines for the diagnosis and treatment of adults with growth hormone deficiency: summary statement of the Growth Hormone Research Society Workshop on Adult Growth Hormone Deficiency. J Clin Endocrinol Metab 83: 379–381. [DOI] [PubMed] [Google Scholar]
- 29. Elmlinger MW, Kuhnel W, Weber MM, Ranke MB (2004) Reference ranges for two automated chemiluminescent assays for serum insulin-like growth factor I (IGF-I) and IGF-binding protein 3 (IGFBP-3). Clin Chem Lab Med 42: 654–664. [DOI] [PubMed] [Google Scholar]
- 30. Hazem A, Elamin MB, Bancos I, Malaga G, Prutsky G, et al. (2012) Body composition and quality of life in adults treated with GH therapy: a systematic review and meta-analysis. Eur J Endocrinol 166: 13–20. [DOI] [PubMed] [Google Scholar]
- 31.Brouwer CA, Gietema JA, Vonk JM, Tissing WJ, Boezen HM, et al.. (2011) Body mass index and annual increase of body mass index in long-term childhood cancer survivors; relationship to treatment. Support Care Cancer. [DOI] [PMC free article] [PubMed]
- 32. Marinovic D, Dorgeret S, Lescoeur B, Alberti C, Noel M, et al. (2005) Improvement in bone mineral density and body composition in survivors of childhood acute lymphoblastic leukemia: a 1-year prospective study. Pediatrics 116: e102–108. [DOI] [PubMed] [Google Scholar]
- 33. Warner JT, Bell W, Webb DK, Gregory JW (1997) Relationship between cardiopulmonary response to exercise and adiposity in survivors of childhood malignancy. Arch Dis Child 76: 298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]