Abstract
Male-male conflict is common among animals, but questions remain as to when, how and by whom aggression should be initiated. Factors that affect agonistic strategies include residency, the value of the contested resource and the fighting ability of the two contestants. We quantified initiation of aggression in a fish, the desert goby, Chlamydogobius eremius, by exposing nest-holding males to a male intruder. The perceived value of the resource (the nest) was manipulated by exposing half of the residents to sexually receptive females for two days before the trial. Resident male aggression, however, was unaffected by perceived mating opportunities. It was also unaffected by the absolute and relative size of the intruder. Instead resident aggression was negatively related to resident male size. In particular, smaller residents attacked sooner and with greater intensity compared to larger residents. These results suggest that resident desert goby males used set, rather than conditional, strategies for initiating aggression. If intruders are more likely to flee than retaliate, small males may benefit from attacking intruders before these have had an opportunity to assess the resident and/or the resource.
Introduction
Male aggression is widespread among animals and can have a direct bearing on male reproductive success. At the same time, aggression can be very taxing, and animals have evolved various strategies to avoid physical combat and its associated costs. Considerable research has been focused on understanding male strategies for the initiation and escalation of aggressive behaviours. A number of important factors have been identified that influence whether or not a particular individual should initiate aggression [1], [2]. These typically relate to asymmetries between the two contestants, such as asymmetry in resource holding potential (RHP), often determined by body size asymmetry in information about the 46 contested resource or opponent, and asymmetry in the value placed on the resource. For example, both previous fighting experience [3], information about the opponent [4], and value placed on the resource [5] can affect the probability of an individual initiating aggression.
The classic game-theoretical framework of Maynard Smith and Parker [1] suggests that conflicts should mainly be resolved through an ordered exchange of agonistic displays. Such displays are thought to facilitate mutual assessment of resource holding potential (RHP), ensuring that more costly types of aggression are avoided, unless the contestants are evenly matched. In practice, however, animals often need to decide on a course of action while still having imperfect information about their opponent or of the contested resource. Furthermore, it may not always be in the interest of a contestant to partake in mutual assessment. In particular, individuals with low RHP may want to avoid drawn-out rituals that can reveal their weakness to an opponent [6]. Similarly, when there is an asymmetry in residency (that is, when an resident confronts an intruder), it may be advantageous for the resident to attack before the intruder has had time to assess the resource, especially if the resident has low RHP. Confronted with an aggressive resident, the intruder may flee rather than stay and fight. For example, in the mangrove killifish, Kryptolebias marmoratus, a large proportion of attacked individuals will retreat immediately from the aggressor [3]. Likewise, territory-holding northern harriers, Circus cyaneus, are able to successfully drive off competing raptor species much larger than themselves [7].
Resource value is often seen as a “key component” of strategic models of aggression [8]. Defenders have been shown to increase their efforts as the value of the resource increases [9], [10]. Increasing the value of the resource does not simply increase aggression, but may also modify the strategy for aggression toward intruders [11]. Males defending a breeding resource are expected to be more aggressive if they have direct mating opportunities. For example, in butterflies, males that had recently encountered females were more motivated to defend their territory [12]. In autumn spiders, Metellina segmentata, residents fought longer over more fecund females, indicating that the perceived resource value affected fighting effort [13]. Similarly, in dung flies, Scathophaga stercoraria, intruders are more aggressive if the contested female is larger [9], and in skinks, Eumeces laticeps, males aggressively exclude other males from the vicinity of females [14].
Males of many species defend territories or breeding resources, such as nests. For example among substrate brooding fishes, where males also often provide exclusive care of the eggs, nesting substrates are often aggressively defended against other males. Such species are excellent models for testing predictions of male-male aggression when there are asymmetries associated with residency. The aggression strategy of a resident (nest holding) male toward an intruder can be affected by the availability of nest sites, body size asymmetry, and resource value. In addition, longer ownership increases aggression in nest defence [15]. Understanding this aggression is important, because fighting over nests can be very costly, and can significantly shorten the resident’s life span [16].
The Australian desert goby, Chlamydogobius eremius, is a freshwater fish endemic to the arid region of Central Australia [17]. As with most other gobies [18], desert gobies spend most of the day in shelters, such as crevices and under rocks. Males aggressively defend these daytime shelters, especially when they are also suitable as nest substrates. Indeed, nest holding males will typically attack other males, regardless of whether eggs are present in the nest. In the current study, we used the desert goby as a model to redress gaps in empirical knowledge on initiation of aggressive behaviour. Specifically, we investigated the strategies of nest holding males to initiate aggression when confronted with an intruding male, and tested whether an increase in perceived mating opportunities affects the level of aggression. We predicted that the presence of females (i.e. an increase in perceived mating opportunities) would increase the value of the nest to the resident and would cause an increased level of aggression. We also predicted that the size of the intruder (either absolute size or relative to the resident) would influence the aggression strategy of the resident.
Materials and Methods
Model System
The desert goby is a small (<8 cm), colourful species with paternal egg-care. It can be locally abundant in both permanent and temporary bodies of water, from spring-fed pools to ephemeral desert streams (Allen et al. 2002). Nest-holding males perform elaborate courtship displays to attract females to spawn [19], [20], [21], whereas rivals are expelled aggressively. Aggression typically occurs in distinctive “bouts”, which consist of the resident male darting out from the nest, repeatedly attacking the intruding male and then returning back to the nest. If males are allowed to interact physically, aggression can escalate to combat with males locking jaws and grappling with their opponent. In such fights, the winner will typically be the larger male (authors’ personal observations).
Fish Origin and Housing
Desert gobies, originating from waterholes and springs west of Lake Eyre in South Australia, were housed in single-sex holding tanks. All aquaria were kept at a temperature of 24–26°C on a 12 hour light: dark cycle and a salinity of 5‰ to mimic field conditions. Fish were fed daily on a diet of commercially prepared pellets and frozen Artemia. In the male holding tanks, rocks, plants and shelters (halved flower pots) were provided. The experiment was carried out in June and July of 2009.
Experimental Design
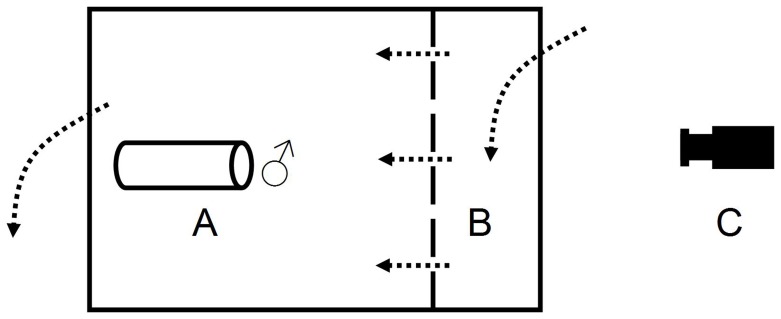
Thirty males in breeding coloration were removed from holding tanks and placed in individual aquaria (length × width) 30×20 cm. These were filled to a depth of 13 cm with water and contained a nest in the form of a 9 cm long PVC pipe (3 cm diameter), secured by a ceramic tile that was buried into the gravel substrate [19], [20]. These tanks shared a re-circulating water supply with a central filter. The males were allowed to acclimatize to the new tanks for one week. A transparent divider was affixed 7 cm from the far end of the tank, dividing each tank into two compartments (Figure 1). In addition, an opaque plastic sheet was placed to cover the transparent divider. The flow of water went from the smaller compartment to an outlet behind the resident male’s nest. The transparent divider had holes to allow water flow and transmission of olfactory cues. The males (hereafter referred to as “resident males”) were haphazardly assigned to one of two treatments (n = 15 per treatment).
Figure 1. Top-view of the experimental setup.
A) the resident male and the nest substrate. B) the compartment to which females and intruder males were introduced. C) the position of the video camera. The dashed line denotes the transparent divider and the dotted arrows demonstrate the direction of water flow through the tank.
In the “experimental treatment”, we manipulated perceived mating opportunity by allowing nest holding males to interact with two gravid females prior to the trials. Specifically, two visibly round females (mean total length, TL±SE: 52.5±0.67 mm) were introduced in the smaller of the two compartments (Figure 1). In the “control treatment”, this compartment was left empty. There was no difference in resident male size between the two treatments (TL: 64.5±1.07 mm, two-sample t-test, t 28 = 0.04, P = 0.97). After 10 min acclimation, the opaque sheets were lifted, exposing the females (or empty compartment) to the resident but precluding contact, and the tanks were left for 48 h. Presenting females in this way quickly entices resident males to commence courtship [19], [20], and observations confirmed that resident males were indeed courting the females. After 48 h, the opaque sheets were put back, and all females were removed. One male in breeding coloration (hereafter referred to as “intruder males”) was introduced to the front compartment of each tank. Each resident male was given a different intruder male. There was no difference in intruder male size between the two treatments (TL: 59.0±0.99 mm, two-sample t-test, t 28 = 1.27, P = 0.28). After 10 min acclimation, the opaque sheets were lifted again. The behaviour of the resident males was recorded for 2 hours using a video surveillance system (Signet digital video recorder and cameras, Jaycar, Australia). At the end of the 2 hours, the experiment was terminated and all males were photographed on a 1 cm grid for measurement of total length.
Video Analysis of Behaviours
The video clips were analyzed (blind with respect to treatment) on a PC which allowed accurate measurements of the different behaviours. The following response variables were quantified: (1) the time from the start of the trial until the first attack (maximum time: 2 h), (2) the intensity of the first aggressive bout (attacks ⋅ min−1) within the first 30 min and (3) the total number of attacks within the first 30 min. An “attack” was defined as the resident male darting toward the transparent divider, with fins fully raised and the body unambiguously oriented toward the intruder. “Aggressive bouts” were defined as a series of rapidly repeated attacks ending either with the resident male returning to its nest or being inactive for more than 20 s. Note that we used the intruder males merely as stimuli to elicit an aggressive response in the resident males, and that the main focus was on the initial attack of the resident. This was done to avoid the issues of stimulus habituation, physical exhaustion and the lack of an option to flee that arise when males are in a confined area [11].
Statistics
All statistical analyses were conducted using the software R 2.10.1 [22]. The time until the first attack was analyzed as a time-to-event analysis, using a Cox proportional hazards model in the “survival” package, version 2.35–8 [23]. This was done to accommodate censoring in the data because some residents did not attack within the 2 h video recording. When the data included influential outliers, we present both analyses with and without these data points. The intensity of the first attack and the number of attacks were analyzed with linear models, and response variables were square-root transformed if this improved normality of model residuals. In all statistical models, the predictor variables were: resident male size (RMS), intruder male size (IMS) and treatment (T, a factor with two levels: either exposed or not exposed to females prior to the trial). We chose to use RMS and IMS as separate predictors rather than some composite measure of “size difference” (e.g. RMS-IMS or RMS/IMS), because the latter may lead to spurious results [24]. Fully factorial models (with all three factors and all four interactions) were simplified by hierarchical deletion of non-significant terms until a minimal adequate model was obtained, using a deletion criterion of P>0.05 [25]. We also performed Bayesian model averaging on the full models, using the BMA package, version 3.13 [26]. This method circumvents the problems inherent in traditional best-model procedures by instead averaging over several models according to posterior model probability. We used the posterior probability of a variable having a non-zero coefficient in the model (Princ) as a measure of the influence of that variable on the response. That is, Princ is the probability that a variable is an contributing factor. As rules of thumb, values of Princ <0.50 show no evidence, values between 0.50 and 0.75 show weak evidence and values >0.75 show positive evidence that the predictor is a contributing factor [27].
Ethics Statement
This study complies with all the relevant Federal and State laws of Australia and was carried out under ethics permit no. BSCI/2007/12 from the Biological Sciences Animal Ethics Committee of Monash University. The Monash University Animal Ethics Committee specifically approved this study.
Results
Timing of First Attack
The time until the first attack was not significantly affected by treatment (mating opportunity), intruder male size or any of the interactions (Table 1). However, there was a significant positive relationship between resident male size (RMS) and the time until the first attack (Cox proportional hazards regression model, N = 30, z = 2.64, P = 0.008). That is, smaller residents attacked earlier than larger residents (Figure 2). Similarly, Bayesian model averaging suggested that RMS was the only factor of importance (Princ = 0.82; Table 2). There were seven censored data points, that is, seven of 30 males did not attack within the 2 hr video recording. This frequency was not affected by treatment (control treatment: 3, experimental treatment: 4, Fisher exact test P = 1).
Table 1. Deleted terms and order or deletion from hierarchical simplification of three statistical models of male-male aggression.
| Response | Time to first attack | Intensity of first attack bout | Number of attacksa | |||||||||
| Model type | Cox proportional hazardsregression model | Linear model | Linear model | |||||||||
| Variable | order | χ2 | df | P | order | F | df | P | order | F | df | P |
| T:RMS:IMS | 1 | 0.30 | 1 | 0.58 | 1 | 0.32 | 12,13 | 0.58 | 1 | 0.09 | 22,23 | 0.77 |
| T:RMS | 2 | <0.01 | 1 | 0.96 | 4 | <0.01 | 13,14 | 0.99 | 2 | 0.13 | 23,24 | 0.72 |
| T:IMS | 3 | 0.45 | 1 | 0.50 | 3 | <0.01 | 14,15 | 0.96 | 3 | 1.23 | 24,25 | 0.28 |
| RMS:IMS | 4 | 0.60 | 1 | 0.44 | 2 | 0.18 | 15,16 | 0.68 | 4 | 0.93 | 25,26 | 0.34 |
| IMS | 5 | 0.03 | 1 | 0.85 | 5 | 0.40 | 16,17 | 0.53 | 5 | 0.14 | 26,27 | 0.71 |
| T | 6 | 0.56 | 1 | 0.46 | 6 | 2.11 | 17,18 | 0.16 | 6 | 1.20 | 27,28 | 0.28 |
The predictor variables were treatment (T, presence or absence of females prior to trial), resident male size (RMS), intruding male size (IMS) and all their interactions.
The response variable was square-root transformed.
Figure 2. Kaplan-Meier survival curves for time to first attack after dividing resident males into large and small individuals.
Solid line: resident males that were larger than the median length (TL = 62.2 mm). Dashed line: resident males smaller than median length. Crosses (+) indicate censored data.
Table 2. Probability of a non-zero coefficient of predictors (Princ) determined by Bayesian model averaging in three statistical models of male-male aggression.
| Response | Time to first attack | Intensity of first attack bout | Number of attacks | |
| Predictor | all data two outliers removed | |||
| T | 0.15 | 0.21 | 0.16 | 0.14 |
| RMS | 0.82 | 0.46 | 0. 71 | 0.82 |
| IMS | 0.25 | 0.19 | 0.25 | 0.27 |
| T:RMS | 0.15 | 0.22 | 0.17 | 0.14 |
| T:IMS | 0.16 | 0.22 | 0.16 | 0.16 |
| RMS:IMS | 0.29 | 0.33 | 0.33 | 0.32 |
| T:RMS:IMS | 0.16 | 0.22 | 0.16 | 0.16 |
The predictor variables were treatment (T, presence or absence of females prior to trial), resident male size (RMS), intruding male size (IMS) and all their interactions. Bold values indicate important predictors (Princ >0.75).
Attack Intensity
There were no significant effects of treatment, intruder male size or any of the interactions on the intensity of the first bout of aggression (Table 1). However, there was a significant negative relationship between resident male size (RMS) and attack intensity (linear model, R2 = 0.20, F 1,18 = 4.52, P = 0.048; Figure 3). That is, smaller residents attacked the intruder with higher intensity. There were two influential outliers in this data (Cook’s D>0.5), and the analysis was performed also without these values. However, removing the outliers only strengthened the result (linear model, R2 = 0.38, F 1,16 = 9.65, P = 0.007; Figure 3). Similarly, Bayesian model averaging attributed resident male size the highest probability of inclusion of all the predictor variables (Table 2). However, the value was low (Princ = 0.46) and this is not regarded as positive evidence. Removing the two outliers increased the probability of inclusion of RMS (Princ = 0.71; Table 2) which can be considered weak evidence for resident male size being an important factor for attack intensity.
Figure 3. Relationship between the intensity of the first aggressive bout and resident male size in male Australian desert gobies (Chlamydogobius eremius).
The circled symbols indicate two influential outliers.
Number of Attacks
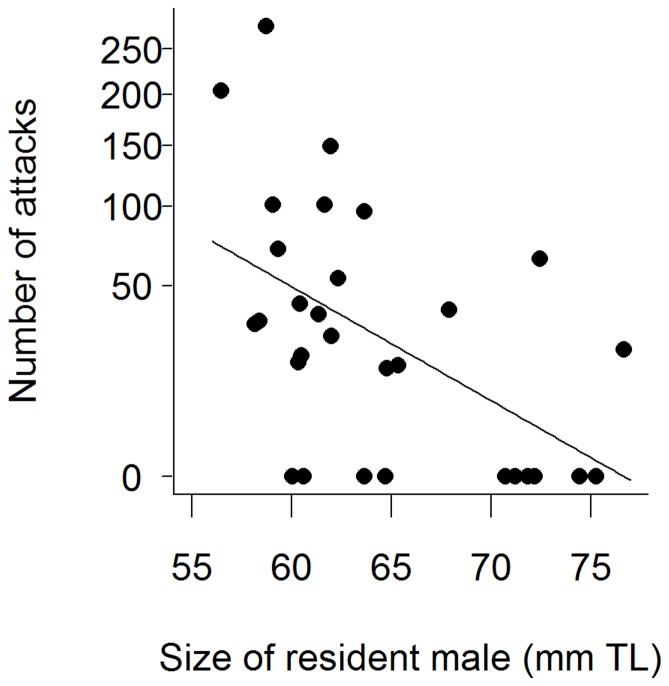
There were no significant effects of treatment, intruder male size or any of the interactions on the total number of attacks resident males performed during the observation period (Table 1). However, there was a significant negative relationship between resident male size (RMS) and the total number of attacks (linear model, R2 = 0.28, F 1,28 = 10.76, P = 0.003; Figure 4). That is, smaller males performed a greater number of attacks. Similarly, Bayesian model averaging suggested that RMS was the only factor of importance (Princ = 0.82; Table 2).
Figure 4. Relationship between the number of attacks (in 30 min) and the size of resident male desert gobies, Chlamydogobius eremius.
Note square root scale on y-axis.
Discussion
Nest-holding desert goby males displayed clear aggression toward intruders. However, timing and intensity of aggression was not affected by our attempt to manipulate their perceived mating opportunities. That is, presenting two gravid females for two days prior to the trial did not influence the response of the residents to intruders, even though resident males had actively courted the females presented to them. In comparison, autumn spiders increase territory and nest defence behaviours when given improved access to mates, probably because this increases the perceived value of the resource [13]. However, other studies have, like ours, failed to find effects of mating opportunities on aggression. For example, in cottonwood borers, Plectrodera scalator, prior possession of a female did not affect male aggression [28].
The lack of effect from the females in our study could potentially be explained if the overriding importance of a nest is for a purpose other than breeding. In many substrate brooders, nests also function as shelters for the resident male. In gobies, nests work both as breeding substrates and as refuges [29], [30]. This dual purpose of nests can potentially impact on male defence strategies. For instance, if nest sites are rare and very important as shelters, defence strategies may be less sensitive to perceived mating opportunities. Future studies should investigate if manipulating the quality of the nest/shelter will affect resident male aggression.
The size of the intruding male - either absolute size or size relative to the resident - did not affect resident male aggression. This was contrary to the common assumption that animals should assess their opponents before attacking [1]. Taken together, the lack of effects from the treatment (presence of females) and from intruder size, suggest that nest holding desert gobies have set strategies for initiating aggression. The earliest game-theoretical models often assumed fixed strategies for aggressive behaviours [31]. However, these models have been viewed as too simplistic [1], and have largely been replaced by models that allow for conditional strategies, that is, where the participants change their behaviour depending on the opponents’ appearance and/or behaviour [32]. It is interesting that our data suggest that resident male aggression level was decided before the encounter, in accordance with the simpler evolutionary game models.
We found that smaller residents attacked an intruder sooner and more vigorously than larger residents. This is in contrast with, for example, three-spined sticklebacks, Gasterosteus aculeatus, where smaller males were more reluctant to attack intruders [33]. However, other studies have found, like us, that smaller males can be more aggressive. For instance, in jumping spiders, Plexippus paykulli, smaller individuals are more likely to escalate fights [24]. Likewise, in swordtails, Xiphophorus spp., smaller males are more prone to initiate and escalate aggression [6], [34], [35]. Why should small males behave in this way? It is well established that larger body size is an important predictor for winning escalated fights [36], [37], [38]. Indeed, in situations where two opponents are forced to “fight it out”, the smaller, more aggressive, male will typically end up losing [24], [39]. Hence, prima facie, it may seem paradoxical for smaller males to have a high aggression strategy. Several evolutionary game models have attempted to explain this apparent paradox [the so called ‘Napoleon complex’, 40,41,42,43]. These models suggest that a high aggression strategy in small males may be adaptive (i.e. evolutionarily stable) if one assumes either assessment error on the part of the smaller male [40], [42], or that there is a some chance of the smaller male actually winning an escalated combat [41]. These models attempt to explain observations that small males initiate fights they are likely to lose. However, because escalation of fights is typically rare in the wild, it may be misleading to use the losses of smaller males in confined laboratory conditions as the premise of whether a high aggression strategy is beneficial or not [11]. We suggest that if intruders are more likely to flee than retaliate in the wild, small residents may in fact benefit from attacking early, before the intruder can assess him or his resource.
Resident males in our experimental set up had access to both visual and olfactory cues. However, other sensory cues may also be important. The lateral line system of gobies, for example, contain superficial and canal neuromasts that are distributed in patterns different from those in other fish families [44]. The potential role for neuromasts in mechanosensory intruder detection [45], especially in absence of visual cues in turbid desert waters, would be an interesting topic for further research.
In conclusion, we found nest-holding male aggression to be independent of perceived mating opportunities and of the size of the intruding male. Instead, residents appeared to have set strategies for initiating aggression related to their own body size. In order to examine the adaptive value of the commonly observed high aggression strategy in small males, future studies should be conducted under field-like conditions that allow the full range of behavioural options for both residents and intruders.
Acknowledgments
We thank Sheila Hamilton-Brown and Ricardo San Martin for logistical support.
Funding Statement
Funding was provided by the Australian Research Council Discovery Project, grant number DP0771070 (to BBMW), an Alfred Deakin Postdoctoral Research Fellowship from Deakin University (to PAS), and the Academy of Finland and Department of Biology, University of Turku (to TKL). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Maynard Smith JM, Parker GA (1976) Logic of asymmetric contests. Animal Behaviour 24: 159–175. [Google Scholar]
- 2. Parker GA (1974) Assessment strategy and evolution of fighting behavior Journal of Theoretical Biology. 47: 223–243. [DOI] [PubMed] [Google Scholar]
- 3. Hsu YY, Wolf LL (2001) The winner and loser effect: What fighting behaviours are influenced? Animal Behaviour 61: 777–786. [Google Scholar]
- 4. Magnhagen C (2006) Information about an opponent can decrease aggression in male common gobies competing for nest sites. Animal Behaviour 71: 879–884. [Google Scholar]
- 5. Johnsson JI, Forser A (2002) Residence duration influences the outcome of territorial conflicts in brown trout (Salmo trutta). Behavioral Ecology and Sociobiology 51: 282–286. [Google Scholar]
- 6. Franck D, Ribowski A (1989) Escalating fights for rank-order position between male swordtails (Xiphophorus helleri) - effects of prior rank-order experience and information-transfer. Behavioral Ecology and Sociobiology 24: 133–143. [Google Scholar]
- 7. Temeles EJ (1990) Interspecific territoriality of northern harriers - the role of kleptoparasitism. Animal Behaviour 40: 361–366. [Google Scholar]
- 8. Brown WD, Chimenti AJ, Siebert JR (2007) The payoff of fighting in house crickets: Motivational asymmetry increases male aggression and mating success. Ethology 113: 457–465. [Google Scholar]
- 9. Sigurjonsdottir H, Parker GA (1981) Dung fly struggles - evidence for assessment strategy. Behavioral Ecology and Sociobiology 8: 219–230. [Google Scholar]
- 10. Nijman V, Heuts BA (2011) Aggression and dominance in cichlids in resident-intruder tests: the role of environmental enrichment. Neotropical Ichthyology 9: 543–545. [Google Scholar]
- 11. Dugatkin LA, Ohlsen SR (1990) Contrasting asymmetries in value expectation and resource holding power: effects on attack behavior and dominance in the pumpkinseed sunfish, Lepomis gibbosus. . Animal Behaviour 39: 802–804. [Google Scholar]
- 12. Bergman M, Olofsson M, Wiklund C (2010) Contest outcome in a territorial butterfly: the role of motivation. Proceedings of the Royal Society B-Biological Sciences 277: 3027–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hack MA, Thompson DJ, Fernandes DM (1997) Fighting in males of the autumn spider, Metellina segmentata: Effects of relative body size, prior residency and female value on contest outcome and duration. Ethology 103: 488–498. [Google Scholar]
- 14. Cooper WE Jr, Vitt LJ (1997) Maximizing male reproductive success in the broad-headed skink (Eumeces laticeps): Preliminary evidence for mate guarding, size-assortative pairing, and opportunistic extra-pair mating. Amphibia-Reptilia 18: 59–73. [Google Scholar]
- 15. McPhee MV, Quinn TP (1998) Factors affecting the duration of nest defense and reproductive lifespan of female sockeye salmon, Oncorhynchus nerka. Environmental Biology of Fishes 51: 369–375. [Google Scholar]
- 16. Lindström K (2001) Effects of resource distribution on sexual selection and the cost of reproduction in sandgobies. American Naturalist 158: 64–74. [DOI] [PubMed] [Google Scholar]
- 17.Glover CJM (1971) The taxonomy and biology of Chlamydogobius eremius (Zietz, 1896): Masters Thesis, Department of Zoology, University of Adelaide.
- 18. Dubs DOL, Corkum LD (1996) Behavioral interactions between round gobies (Neogobius melanostomus) and mottled sculpins (Cottus bairdi). Journal of Great Lakes Research 22: 838–844. [Google Scholar]
- 19. Wong BBM, Svensson PA (2009) Strategic male signalling effort in a desert-dwelling fish. Behavioral Ecology and Sociobiology 63: 543–549. [Google Scholar]
- 20. Svensson PA, Lehtonen TK, Wong BBM (2010) The interval between sexual encounters affects male courtship tactics in a desert-dwelling fish. Behavioral Ecology and Sociobiology 64: 1967–1970. [Google Scholar]
- 21. Lehtonen TK, Svensson PA, Wong BBM (2011) Both male and female identity influence variation in male signalling effort. BMC Evolutionary Biology 11: 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.R-Development-Core-Team (2009) R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
- 23.Therneau T, Lumley T (2009) Survival: survival analysis, including penalized likelihood. R Package Version 2.35–8.
- 24. Taylor PW, Hasson O, Clark DL (2001) Initiation and resolution of jumping spider contests: roles for size, proximity, and early detection of rivals. Behavioral Ecology and Sociobiology 50: 403–413. [Google Scholar]
- 25.Crawley MJ (2007) The R book. Chichester: John Wiley & Sons Ltd.
- 26.Raftery AE, Hoeting JA, Volinsky CT, Painter IS, Yeung KY (2010) BMA: Bayesian Model Averaging. R package version 3.13.
- 27. Viallefont V, Raftery AE, Richardson S (2001) Variable selection and Bayesian model averaging in case-control studies. Statistics in Medicine 20: 3215–3230. [DOI] [PubMed] [Google Scholar]
- 28. Goldsmith SK, Stewart Z, Adams S, Trimble A (1996) Body size, male aggression, and male mating success in the cottonwood borer, Plectrodera scalator (Coleoptera: Cerambycidae). Journal of Insect Behavior 9: 719–727. [Google Scholar]
- 29. Balshine S, Verma A, Chant V, Theysmeyer T (2005) Competitive interactions between round gobies and logperch. Journal of Great Lakes Research 31: 68–77. [Google Scholar]
- 30. Grossman GD (1980) Food, fights, and burrows - the adaptive significance of intraspecific aggression in the bay goby (Pisces, Gobiidae). Oecologia 45: 261–266. [DOI] [PubMed] [Google Scholar]
- 31. Maynard Smith J, Price GR (1973) The logic of animal conflict. Nature London 246: 15–18. [Google Scholar]
- 32.Maynard Smith J (1982) Evolution and Theory of Games. Cambridge, Massachusetts: Cambridge University Press.
- 33. Mehlis M, Bakker TCM, Engqvist L, Frommen JG (2010) To eat or not to eat: egg-based assessment of paternity triggers fine-tuned decisions about filial cannibalism. Proceedings of the Royal Society B-Biological Sciences 277: 2627–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ribowski A, Franck D (1993) Subordinate swordtail males escalate faster than dominants - a failure of the social conditioning principle. Aggressive Behavior 19: 223–229. [Google Scholar]
- 35. Morris MR, Gass L, Ryan MJ (1995) Assessment and individual recognition of opponents in the pygmy swordtails Xiphophorus nigrensis and Xiphophorus multilineatus . Behavioral Ecology and Sociobiology 37: 303–310. [Google Scholar]
- 36. Wong BBM, Candolin U (2005) How is female mate choice affected by male competition? Biological Reviews 80: 559–571. [DOI] [PubMed] [Google Scholar]
- 37. Berglund A, Bisazza A, Pilastro A (1996) Armaments and ornaments: An evolutionary explanation of traits of dual utility. Biological Journal of the Linnean Society 58: 385–399. [Google Scholar]
- 38. Thünken T, Baldauf SA, Kullmann H, Schuld J, Hesse S, et al. (2011) Size-related inbreeding preference and competitiveness in male Pelvicachromis taeniatus (Cichlidae). Behavioral Ecology 22: 358–362. [Google Scholar]
- 39. Ribowski A, Franck D (1993) Demonstration of strength and concealment of weakness in escalating fights of male swordtails (Xiphophorus helleri). Ethology 93: 265–274. [Google Scholar]
- 40. Just W, Morris MR, Sun XL (2007) The evolution of aggressive losers. Behavioural Processes 74: 342–350. [DOI] [PubMed] [Google Scholar]
- 41. Morrell LJ, Lindstrom J, Ruxton GD (2005) Why are small males aggressive? Proceedings of the Royal Society B-Biological Sciences 272: 1235–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Just W, Morris MR (2003) The Napoleon Complex: why smaller males pick fights. Evolutionary Ecology 17: 509–522. [Google Scholar]
- 43. Just W, Wu MY, Holt JP (2000) How to evolve a Napoleon complex. Proceedings of the 2000 Congress on Evolutionary Computation, Vols 1 and 2: 851–856. [Google Scholar]
- 44.Van Tassell JL, Tornabene L, Taylor MS (2011) A history of Gobioid morphological systematics. In: Patzner R, Van Tassell JL, Kovacic M, Kapoor BG, editors. The Biology of Gobies: Science Publishers. 3–22.
- 45.Janssen J (1990) Localization of substrate vibrations by the mottled sculpin (Cottus bairdi). Copeia: 349–355.