Abstract
Purpose
Proliferative vitreoretinopathy (PVR) is the primary cause of failure of retinal reattachment surgery. Growth factors such as platelet-derived growth factor (PDGF) are strongly associated with PVR. Of the five PDGF family members, PDGF-C predominates in the vitreous of experimental and clinical PVR. PDGF-C is secreted as a latent protein that requires proteolytic processing for activation. Although tissue plasminogen activator (tPA) is primarily responsible for processing PDGF-C in cultured cells, it constitutes a minority of the processing activity in the vitreous of experimental animals and in patients with PVR. Identifying the major PDGF-C processing protease was the purpose of this study.
Methods
The presence of serum proteins in the vitreous was detected by Coomassie blue staining and Western blotting. PDGF-C processing activity was detected in an in vitro processing assay using either native or recombinant PDGF-C as the substrate. Plasmin activity was blocked using α2-plasmin inhibitor. Phosphorylation of the PDGF receptor (PDGFR) was monitored by antiphosphotyrosine Western blotting. Vitreous specimens were collected from experimental rabbits or from patients undergoing vitrectomy to repair retinal detachment or for other reasons.
Results
A number of prominent serum proteins (albumin and IgG) were detected in the vitreous of all patients undergoing retinal surgery. The level of these proteins markedly increased in the vitreous of rabbits as they developed PVR. These observations suggested that serum-borne proteases are also likely to be present in the vitreous. Indeed, plasmin (a protease capable of processing PDGF-C) was present in the vitreous from PVR rabbits and retinal surgery patients. Plasmin was dramatically more effective than tPA in processing PDGF-C in an in vitro assay. Blocking plasmin activity eliminated most of the processing activity in the vitreous of patients and rabbits with PVR.
Conclusions
Plasmin was the major PDGF-C processing protease in the vitreous of PVR rabbits and patients undergoing retinal surgery. Blocking plasmin prevented the generation of active PDGF-C, which is the major PDGF isoform relevant for PVR. These observations are the first report of an in vivo protease responsible for processing PDGF-C. In addition, plasmin was identified as a novel therapeutic target for patients with PVR.
Proliferative vitreoretinopathy (PVR) is a problematic complication of retinal detachment and the major cause of failure of retinal reattachment surgery.1–3 Disease progression is thought to involve a series of events.4–6 Once the neural retinal barrier is breached, retinal pigment epithelial (RPE) cells migrate to the vitreous, where they proliferate and synthesize extracellular matrix proteins. This leads to the development of an epiretinal membrane; contraction of this membrane results in retinal detachment.
There are several reasons to suspect that growth factors are prominent contributors to PVR. First, they are capable of driving all the cellular events that contribute to the manifestation of PVR. Second, events intrinsic to retinal detachment increase the level of growth factors. For instance, retinal damage stimulates the production of PDGF by RPE cells.7 Furthermore, vascular hemorrhage results in spillage of blood component such as platelets, which contain high levels of numerous growth factors including PDGF.
The PDGF family consists of four gene products that assemble into five dimeric isoforms: PDGF-A, -AB, -B, -C, and -D.8,9 Although all PDGF family members exert their biological effects by activating PDGFRs, individual family members differ in their ability to assemble and activate PDGFRs. There are two PDGFR subunits (α and β), and PDGF drives their homodimerization and heterodimerization. For instance, PDGF-B is the universal ligand, and it assembles all possible dimeric receptor combinations. PDGF-A and -D are the most selective; they activate homodimers of α or β PDGFRs, respectively. PDGF-C and -AB assemble αα homodimers (PDGFRα) and αβ heterodimers (PDGFRαβ). Given that PDGF-C is the predominant PDGF isoform in both clinical and experimental PVR, this family member appears to be the most relevant for PVR.10
Although certain PDGF family members (PDGF-A, -B and -AB) are secreted fully capable of activating PDGFRs, the secreted form of PDGF-C (and -D) is inactive.8,9 The mature, secreted from of PDGF-C consists of an N-terminal CUB domain followed by a linker, which must be proteolytically removed to permit the C-terminal growth factor domain to bind and activate PDG-FRs.11–13 There has been much interest in identifying the proteases capable of processing PDGF-C. Testing a panel of five purified proteases quickly identified plasmin as capable of activating PDGF-C in vitro.12 A more elegant approach was necessary to identify the proteases that process PDGF-C in living cells, and these studies revealed the tissue plasminogen activator (tPA) was necessary and sufficient for processing PDGF-C in a tissue culture setting.13 Although good evidence exists that PDGF-C is processed in vivo,12 the responsible proteases have not been identified in either normal or pathologic contexts.
We previously reported that tPA was not the primary PDGF-C processing protease in the vitreous of patients with PVR.10 Although tPA was the major PDGF-C processing protease in the vitreous of normal rabbits, another protease became the dominant vitreal protease in rabbits that had developed PVR.10 We hypothesized that the PVR-related protease was plasmin for the following reasons. Plasmin was capable of processing PDGF-C.12 Furthermore, plasmin was present in serum, which was likely to contaminate the vitreous of patients who experienced retinal trauma.14 Finally, vascular hemorrhage is a risk factor associated with PVR.15,16 The results presented herein strongly support the idea that plasmin is the major protease that processes PDGF-C in the vitreous of PVR patients and thereby identifies an in vivo PDGF-C processing protease.
Materials and Methods
Reagents
Recombinant human PDGF-C core domain and recombinant glutathione S-transferase (GST) full-length PDGF-C were prepared as previously described.10,11 The rabbit anti–PDGF-C core domain antibody was produced by immunizing New Zealand White rabbits with the peptides 3-3 (residues 299–326) and was purified using a peptide affinity column.11 Goat anti–PDGF-C antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). The two anti–PDGFRα antibodies (27P and 80.8) were produced and characterized as previously described.17 The two antiphosphotyrosine antibodies, 4G10 and PY20, were purchased from Upstate Biotechnology (Lake Placid, NY) and BD Transduction Laboratories (Madison, WI), respectively. Antihuman albumin and human plasmin antibodies were purchased from Sigma (Rocky Hill, NJ), and human IgG horseradish peroxidase (HRP)-conjugated antibody was from Bethyl (Montgomery, TX). tPA was purchased from Sigma (St. Louis, MO). Human urokinase, human plasmin, and humanα2-plasmin inhibitor were purchased from Hematologic Technologies Inc. (Essex Junction, VT). HRP-conjugated anti–rabbit albumin antibody was from Bethyl (Montgomery, TX). Chicken anti–rabbit plasminogen antibody and HRP-conjugated goat anti–chicken IgG h+1 antibodies were from Immunology Consultants Laboratory Inc. (Newberg, OR).
Cell Culture
The human retinal pigment epithelial cell line ARPE-19 (RPE) was purchased from American Type Culture Collection (ATCC; Manassas, VA). Primary fetal RPE cells (passage 3) were provided by Jing Cui and Joanne Matsubara (University of British Columbia, Vancouver, BC, Canada). The primary cells and the established RPE cell line were cultured in a 1:1 mixture of Dulbecco modified Eagle medium (DMEM; high glucose; Gibco-BRL, Grand Island, NY) and Ham F12 medium (Gibco-BRL) supplemented with 10% fetal bovine serum (FBS; Gemini Bio Products, Calabasas, CA), 500 U/mL penicillin, and 500 μg/mL streptomycin. RPE cells expressing PDGFRα (RPEα) were established by infecting RPE cells with a replication-incompetent retrovirus harboring pLHDCX2-PDGFRα plasmid. Infected cells were grown in DMEM supplemented with histidinol (2 mM) to select the successfully infected cells.18,19 The cells were cultured at 37°C in a humidified 5% CO2 atmosphere.
The following protocol was used to produce conditioned medium. After cells grew to approximately 90% confluence, they were rinsed once with phosphate-buffered saline (PBS), and the medium was replaced with a 1:1 mixture of DMEM and Ham F12. The general viability of the cells was monitored by observation under the light microscope, and medium was collected after 2 weeks, when the cells appeared healthy. The harvested medium was centrifuged for 10 minutes at 2000g and then frozen until analysis.
Rabbit Model for PVR and Preparation of Rabbit Vitreous
Rabbits (Dutch belted) were purchased from Covance (Denver, PA). PVR was induced in the right eye of each rabbit, as previously described.20,21 We used two types of controls for these experiments. The first was the uninjected left eye, and the data from these samples in shown in Figure 1. The second control consisted of rabbits injected with platelet-rich plasma and 0.1 mL DMEM (instead of cells); these rabbits never developed PVR (stage 0 at all examinations). At the end of the experiment, the level of serum proteins in the vitreous of these animals was equivalent to the level in uninjected eyes (data not shown). This type of control (PRP-injected) was used in one experiment (see Fig. 5). On day 28, the animals were killed, and the eyes were enucleated and frozen at −80°C. All surgeries were performed under aseptic conditions and in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The protocol for the use of animals was approved by the Schepens Animal Care and Use Committee.
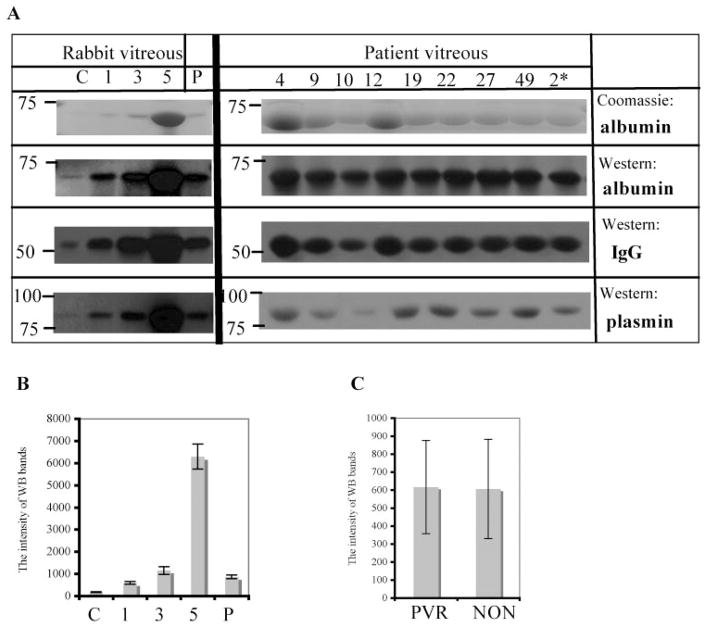
Figure 1.
Albumin, IgG, and plasmin were present in PVR vitreous. (A) Vitreous from rabbits and patient was tested for albumin, IgG, and plasmin. Left: C, vitreous from control (uninjected) eyes; 1, 3, and 5 indicate the stage of PVR; P, 10% platelet-rich plasma. For the patient vitreous (right), samples 4, 9, 10, 12, 19, 22, 27, and 49 were from PVR patients, and sample 2 was from a non-PVR patient. Samples (1 μL rabbit vitreous or 10 μL patient vitreous) were separated by 10% SDS-PAGE, and proteins in the resultant gel were visualized by Coomassie brilliant blue staining (top row). A parallel set of samples was subjected to Western blot analysis using antibodies against albumin (second row from the top), IgG (third row from the top), or plasmin (bottom row). The data shown are representative of the entire data set, which included vitreous from 8 control rabbit eyes, 22 PVR rabbit eyes (3, 5, and 14 [stages 1, 3, and 5, respectively]), 10 patients with PVR, and 16 non-PVR patients. (B, C) The intensity of Western blot bands for plasmin of all rabbit and patient samples was quantitated, and the resultant data are presented as mean ± SD. Comparison of the plasmin band intensity with known amounts of purified plasmin standards permitted calculation of the amount of plasmin in each of the samples. (B) 0.31 ± 0.05, 1.06 ± 0.15, 2.07 ± 0.26, 11.3 ± 1.89, and 1.55 ± 0.15 nM plasmin for C, 1, 3, 5, and P, respectively. Similar analysis indicated 29.2 ± 9.8 nM plasmin in the vitreous of patients with PVR.
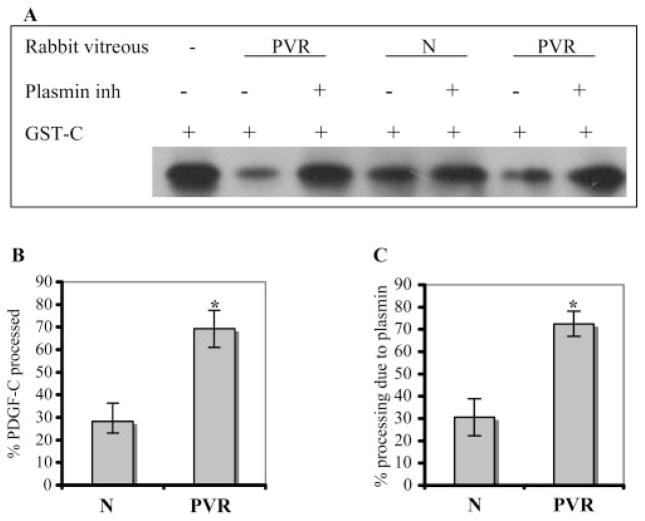
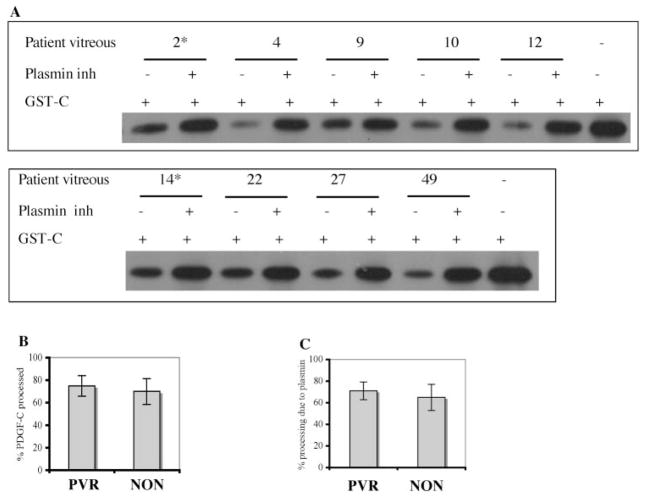
Figure 5.
Plasmin was the major protease responsible for processing PDGF-C in the rabbit vitreous. (A) Rabbit vitreous (1 μL) was preincubated with or without α2-plasmin inhibitor (2 μg) for 1 hour at room temperature and then incubated with GST-PDGF-C (GST-C, 100 ng) in plasmin buffer for 2 hours. The samples were subjected to PDGF-C Western blot analysis. (B) Results from three independent experiments were quantified, and the resultant data are presented as a bar graph. Vitreous from normal (PRP-injected) and PVR rabbits processed 28% ± 5% and 69% ± 8%, respectively. (C) Processing activity was blocked by the plasmin inhibitor (30% ± 3% and 72% ± 5% in the normal and PVR rabbit vitreous, respectively). The extent of inhibition was calculated as the ratio of the actual inhibition/maximum possible inhibition × 100%, as previously described.10 *P < 0.05.
To prepare the rabbit vitreous, the vitreous was dissected from the eyeball while it was still frozen, permitted to thaw, and then centrifuged at 4°C for 5 minutes at 10,000g. The resultant supernatant was used for all analyses.10 The pellet did not contain detectable levels of PDGF-C or plasmin (data not shown).
Patient Vitreous
All human vitreous specimens were obtained from patients at Schepens Retina Associates who were undergoing vitrectomy at Massachusetts Eye and Ear Infirmary (Boston, MA). Institutional review board approval to perform these studies was obtained (protocol 05-03-019X, Assay of Human Vitreous for Activity that Processes PDGF-C) before any experiments were conducted. The reasons for surgery were varied (e.g., PVR, vitreous hemorrhage, retained lens fragments, retinal detachment, retinoschisis, macular hole), and patients were accepted for specimen donation in a consecutive fashion, regardless of preoperative diagnosis. Ten patients had diagnoses of PVR, and 16 had other retinal diagnoses. Undiluted vitreous specimens were obtained with a vitrector through a standard sclerotomy port before the infusion of fluid. In eyes that had previously undergone vitrectomy, an undiluted vitreous sample of 0.2 mL was drawn from the core of the vitreous cavity with a TB syringe and a 30-gauge needle before the infusion cannula was opened. Samples thus obtained represented the undiluted initial material obtained from a core vitrectomy. This method did not significantly alter the normal procedure, nor did the patients assume any additional risk by their donation of the specimen. The research adhered to the tenets of the Declaration of Helsinki.10
PDGF-C Processing Assay
Native and recombinant GST-PDGF-C were used as substrates in the processing assay. The source of native PDGF-C was RPE-conditioned medium (40 μL/sample). To control for factors in the conditioned medium that might influence the processing assay, the same volume of conditioned medium from RPE cells in which PDGF-C expression was suppressed with shRNA oligos was added to 60 ng GST-PDGF-C. PDGF-C expression was suppressed in RPE by PDGF-C shRNA by 93.5% ± 1.5.10 Plasmin (0.2 μg) or plasmin that had been preincubated for 1 hour at 25°C with inhibitor (0.2 μg plasmin + 2 μg α2-plasmin inhibitor) was added to substrate (PDGF-C) and incubated at 37°C for the indicated time, and the samples were subjected to a PDGF-C Western blot using the antibody that recognized the core domain.
To assess the extent to which plasmin contributed to the processing activity, we performed all assays in the presence or absence of the α2-plasmin inhibitor. Experimental samples were preincubated with α2-plasmin inhibitor (2 μg) for 1 hour at 25°C before the standard processing assay was performed. Volumes of samples used in the processing experiments were 1 μL rabbit vitreous and 5 μL patient vitreous. Experimental samples were incubated with 60 ng GST-PDGF-C in plasmin buffer (20 mM Tris-HCl, 1 mM CaCl2, 1 mM MgCl2, 0.01% Tween-20) for 2 hours at 37°C. Total reaction volume was 50 μL. The “input” consisted of an equivalent amount of GST-PDGF-C that was treated identically except that it was not incubated with vitreous. At the conclusion of the incubation period, the proteins were resolved by reducing SDS-PAGE and then were immunoblotted using a goat anti–PDGF-C core domain antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Pilot experiments indicated that a 2-hour incubation period was optimal, so this was the duration of the incubation in all processing assays unless otherwise indicated. tPA-dependent processing was assayed exactly as previously described.10
Immunoprecipitation and Immunoblotting
RPEα cells were grown to 90% confluence and then incubated for 24 hours in DMEM/F12 without serum. The desired agents were added for 5 minutes at 37°C, and the cells were washed twice with H/S (20 mM HEPES, pH 7.4, 150 mM NaCl) and then lysed in extraction buffer (10 mM Tris-HCl, pH 7.4, 5 mM EDTA, 50 mM NaCl, 50 mM NaF, 1% Triton X-100, 20 μg/mL aprotinin, 2 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride). Lysates were centrifuged for 15 minutes at 13,000g, and PDGFRα was immunoprecipitated from 1.5 mg clarified lysate, as previously described,17 except that protein A Sepharose was used to collect the immune complexes instead of Staphylococcus aureus membranes. Immunoprecipitating antibody was a crude rabbit polyclonal (27P). Blotting antibody was the mixture of antiphosphotyrosine antibodies (4G10/PY20). The primary blot was stripped and reprobed with a mixture of two PDGFRα antibodies (27P and 80.8). The extent of phosphorylation was analyzed by densitometry (Quantity One; Bio-Rad, Hercules, CA) and was normalized for the amount of PDGFRα in each sample.
Statistical Analysis
Comparisons were made using unpaired and paired t-tests; a confidence level of P < 0.05 was considered statistically significant.
Results
We previously found that the vitreous of normal rabbits could process PDGF-C and that most (68.5% ± 14.2%) of this activity was attributed to tPA.10 Inducing PVR altered processing activity quantitatively and qualitatively. There was a 2.4-fold increase in processing activity, and only 30.0% ± 2.9% was blocked by the tPA inhibitor.10 Although it was not possible to perform the same kind of comparison in human samples (because of the lack of vitreal specimens from healthy people), tPA accounted for only a minority (34.0% ± 5.8%) of the processing activity in the vitreous of patients with PVR.10 These studies indicate that tPA was not the major PDGF-C processing protease in the vitreous of patients or experimental animals with PVR. This protease is a potential therapeutic target; therefore, we set out to identify it.
Serum Proteins Were Readily Detected in the PVR Vitreous
Because the development of PVR is thought to involve the entry of serum components into the vitreous,5,6 we considered whether plasmin, a blood-borne protease capable of processing PDGF-C,12 was contributing to the processing activity of the PVR vitreous. The first step in testing this hypothesis was to determine whether the PVR vitreous contained serum proteins. The vitreous from control (uninjected) eyes had a low, albeit detectable, level of albumin, IgG, and plasmin (Fig. 1). Levels of these proteins were higher in PVR eyes, and the amounts of the protein correlated with the stage of PVR (Fig. 1). Although platelet-rich plasma contains all these proteins (Fig. 1), the contribution from this source was negligible. Rabbits injected with platelet-rich plasma and medium (instead of cells) did not develop PVR, and the levels of albumin, IgG, and plasmin were not elevated over the level in uninjected eyes (data not shown). This finding suggests that the injected proteins were cleared from the vitreous by the end of the 4-week duration of the experiment. The data in Figure 1B are of the mean ± SD of 8 control and 22 PVR rabbits of which 3, 5, and 14 were stage 1, 3, and 5, respectively.
Similar to the rabbit findings, the vitreous from patients with PVR had readily detectable levels of albumin, IgG, and plasmin (Fig. 1). The presence of these serum proteins was not unique to patient with PVR; sample 2 is from a patient undergoing retinal surgery unrelated to PVR. Serum proteins were detected in all the patient samples (10 PVR and 16 non-PVR patients), and there was no difference in the amount of plasmin between these two patient populations (Fig. 1C). Taken together, these results indicate that one of the vitreal changes that accompany PVR is a dramatic increase in serum proteins, including plasmin.
Plasmin Activated PDGF-C
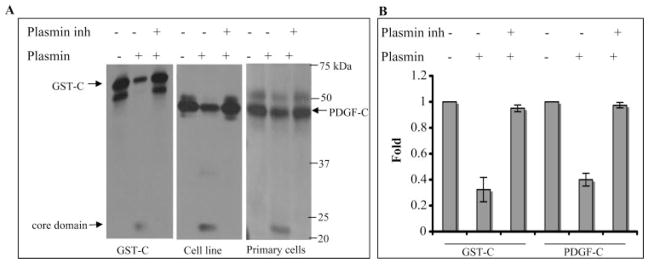
Li et al.12 report that purified plasmin was capable of processing purified PDGF-C in an in vitro setting. To test whether plasmin was able to do so under physiologically relevant conditions, we collected conditioned medium from primary fetal RPE cells and an established RPE cell line (ARPE-19) that secrete PDGF-C. As shown in the middle and right-hand panels of Figure 2A, plasmin processed native PDGF-C present in the conditioned medium. Processing was scored as a reduction in the amount of the full-length protein and the concomitant appearance of the lower molecular mass core domain (also called the growth factor domain). Pharmacologically inhibiting plasmin prevented processing, indicating that the processing was attributed to plasmin instead of contaminating proteases. Plasmin was also able to process a recombinant GST-PDGF-C fusion protein (Fig. 2A, left), and this observation, in addition to our previous substrate characterization studies,10 indicated the suitability of this substrate in the processing assay.
Figure 2.
Plasmin processed PDGF-C. (A) Plasmin (0.2 μg) was preincubated with or without α2-plasmin inhibitor (2 μg) for 1 hour at room temperature and was added to GST-PDGF-C (GST-C, 60 ng, left) or conditioned medium (40 μL) from either an RPE cell line (middle) or primary fetal RPE cells (right). After 10-minute incubation at 37°C, the samples were subjected to PDGF-C Western blot analysis. Top bands: latent PDGF-C is the higher molecular mass in the right-hand panel (GST adds 26 kDa), and the core domain is the lower molecular mass species. (B) Quantification of the Western blot results from three independent experiments (mean ± SD). GST-PDGF-C (68% ± 9%) and latent PDGF-C (60% ± 5%) were processed by plasmin. The plasmin inhibitor blocked the processing of GST-C and native PDGF-C by 95% ± 3% and 97% ± 2%, respectively.
To investigate whether processed PDGF-C was capable of activating PDGFRα, we added processed or unprocessed PDGF-C to RPE cells and monitored activation of the PDGFRα. As shown in Figure 3, both the native and the recombinant PDGF-C induced tyrosine phosphorylation of PDGFRα provided that the PDGF had been processed by plasmin. We concluded that plasmin-dependent processing of PDGF-C can occur in the physiologically relevant setting of RPE-conditioned medium.
Figure 3.
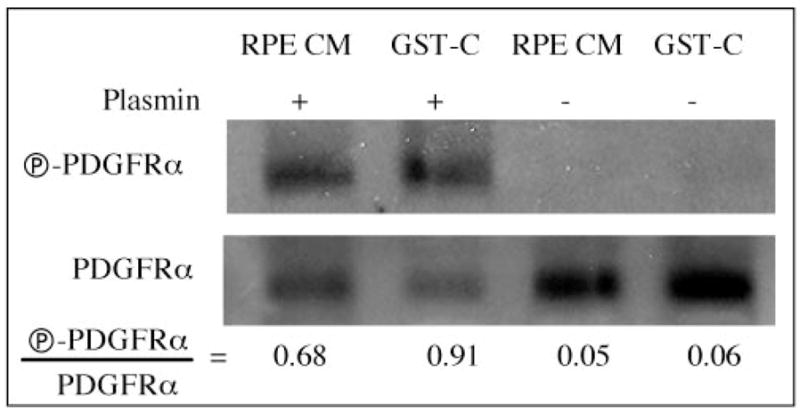
Plasmin-processed PDGF-C–activated PDGFRα. Latent PDGF-C (recombinant [GST-C] or native [RPE CM]) was processed by plasmin, as described in the Figure 2 legend, and was added to RPEα cells for 5 minutes. Controls for this experiment included unprocessed PDGF-C (two right lanes). The cells were lysed, and PDGFRα was immunoprecipitated and subjected to an antiphosphotyrosine Western blot (top), followed by an anti-PDGFRα Western blot (bottom). Signal intensities were quantified, and the resultant ratios are given. Similar results were observed in three independent experiments.
Plasmin versus tPA
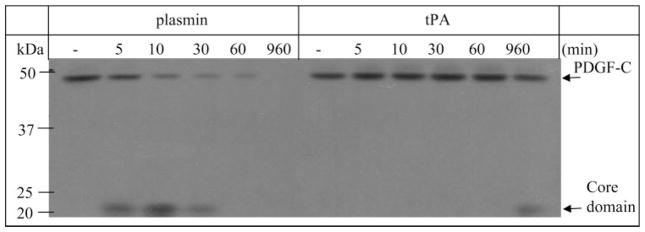
Having identified two proteases capable of processing PDGF-C, we determined which of them was more efficient. As shown in Figure 4, plasmin was vastly more potent than tPA. More specifically, it took 5 minutes for plasmin to process 33.9% ± 3.5% of the PDGF-C, whereas 192 times longer (960 minutes) was required for tPA to process this amount of the substrate. These findings reveal that plasmin was the more potent PDGF-C processing protease and, hence, was more likely than tPA to be responsible for processing PDGF-C when both proteases were present. Importantly, the amount of vitreal plasmin in experimental and clinical PVR (11.3 ± 1.89 [Fig. 1B] and 29.2 ± 9.9 nM [Fig. 1C]) was sufficient to process PDGF-C and constituted a major portion of the processing activity found in the vitreous from rabbits and patients with PVR (Figs. 5, 6).
Figure 4.
Plasmin processed PDGF-C better than tPA. RPE-conditioned medium (38 μL) containing latent native PDGF-C was incubated with plasmin (25 nM) or tPA (25 nM) for the indicated times at 37°C. This is the optimum concentration for tPA10 and was slightly below the optimum concentration for plasmin (30 mM). The reactions were stopped, and the samples were subjected to PDGF-C Western blot analysis. In three independent experiments, we found that 33.9% ± 3.5% PDGF-C was processed in 5 minutes by plasmin, whereas it took 960 minutes for tPA to process this amount of PDGF-C.
Figure 6.
Plasmin was the major protease responsible for processing PDGF-C in the patient PVR vitreous. (A) Vitreous from patients who did or did not have PVR (asterisk) was assayed for PDGF-C processing activity, as described in the Figure 5 legend. (B) Results from three independent experiments were quantified, and the resultant data are presented as a bar graph. Vitreous from a PVR and a non-PVR (NON) patient processed 75% ± 9% and 70% ± 11%, respectively. (C) The extent of inhibition was calculated as described in Figure 5. Processing activity was blocked by the plasmin inhibitor: 71% ± 8% in the PVR and 65% ± 12% in the non-PVR patient vitreous.
Plasmin Was the Major Protease Responsible for Processing PDGF-C in the PVR Rabbit Vitreous
Having identified plasmin as a potent PDGF-C–processing protease, we next addressed the relative contribution of plasmin to the overall processing activity in the vitreous. To this end, PDGF-C substrate was added to normal (PRP-injected) or PVR vitreous that had or had not been supplemented with a plasmin inhibitor. As expected from our previous studies,10 the vitreous from PVR rabbits processed PDGF-C more efficiently than the vitreous from normal rabbits (Figs. 5A, 5B). By comparing the extent of processing in samples that did and did not receive the plasmin inhibitor, we learned that plasmin was responsible for 72.5% ± 5.6% and 30.6% ± 3.5% of processing activity in PVR and normal samples, respectively (Fig. 5C). A representative experiment is shown in Figure 5A; in total, the vitreous from 22 rabbits (8 normal [PRP injected], 14 PVR) was analyzed. These studies demonstrated that PVR increased the amount of processing activity in the vitreous; plasmin accounted for most of this increase.
Plasmin Was the Major PDGF-C Processing Protease in the Vitreous of Patients Undergoing Retinal Surgery
We subjected the vitreous from patients undergoing retinal surgery to the same analysis shown in Figure 5. Figure 6A is a representative experiment; in total, the vitreous from 26 patients (10 PVR, 16 non-PVR) was analyzed. Consistent with our previous findings,10 a similar amount of processing activity was present in all specimens (Fig. 6B). Plasmin accounted for most of this activity (71.6% ± 8.2% in PVR samples and 65.0% ± 12.1% in non-PVR samples) (Fig. 6C). These data indicate that plasmin is the major PDGF-C–processing protease in the vitreous of patients undergoing retinal surgery, and they resonate with the findings that all these samples contain readily detectable levels of serum proteins, including plasmin (Fig. 1).
Discussion
In this article we provide evidence that plasmin was the major PVR-inducible protease that processed PDGF-C in the rabbit model of PVR. Plasmin also predominated in the vitreous of patients with PVR, though patients undergoing retinal surgery for other conditions also had readily detectable levels of plasmin in the vitreous.
Vascular hemorrhage is common in patients with PVR, retinal detachment, diabetic retinopathy, and idiopathic vitreous hemorrhage and is, consequently, the likely source of plasmin in the vitreous. In patients with other retinal diagnoses (retained lens fragments, venous occlusions, preretinal proliferation) vascular permeability and breakdown of the blood-ocular barrier may be sufficient to allow for the accumulation of plasmin in the vitreous cavity. Our human vitreous specimens were obtained from patients with significant retinal disease in whom any of these conditions might have contributed to the presence of plasmin. Although vitreal samples from healthy rabbits were included in our study, specimens from patients without retinal disease are not available for comparison.
We know from the literature that the presence of a vitreous hemorrhage in the context of a retinal detachment increases the risk for PVR and that the severity of PVR correlates with the extent of hemorrhage.15,16 Because of the abundance of growth factors in the blood, the underlying mechanism by which hemorrhage exacerbates PVR was thought to be by increasing the level of growth factors. Our observation that blood-borne proteases (tPA and plasmin) promote processing of PDGF-C suggest that activating the existing pool of growth factors is a second mechanism by which hemorrhage may contribute to PVR (Fig. 7).
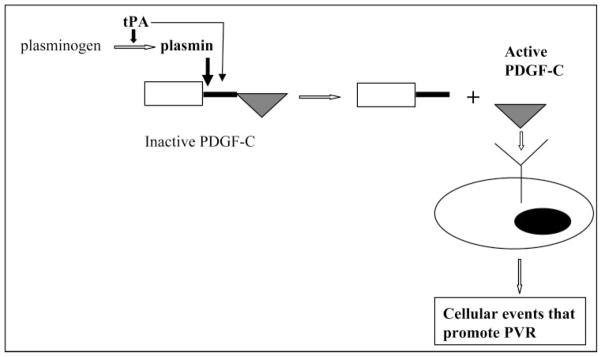
Figure 7.
Model for activation of PDGF-C in the vitreous of PVR patients. Two proteases are present in the vitreous that process PDGF-C: tPA and plasmin. Plasmin processes PDGF-C more efficiently than tPA; hence, it is probably the major processing protease. Although tPA can also process PDGF-C, its ability to generate plasmin from plasminogen suggests an indirect contribution to processing. Once PDGF-C has been processed—i.e., the core domain (triangle) is separated from the CUB domain and linker (rectangle and vertical line)—it activates PDGFRs to drive cellular responses (migration, proliferation, synthesis of extracellular matrix proteins, contraction) that promote PVR.
Given that plasmin processed PDGF-C much more effectively than tPA, it seems likely that PDGF-C is processed primarily by plasmin. However, tPA may still play an important, albeit indirect, role in processing PDGF-C by generating active plasmin from plasminogen (Fig. 7). These insights suggest that blocking the processing of PDGF-C should include inhibition of plasmin and tPA.
The discovery of multiple proteases capable of processing PDGF-C raises the possibility of more such enzymes. However, this does not seem to be the case. In the vitreous of rabbits with PVR, plasmin and tPA accounted for most of the processing activity (72.5% ± 5.6% and 30.0% ± 2.9%, respectively). Similarly, these two proteases appeared to account for all the processing activity in the vitreous of patients with PVR (71.6% ± 8.2% and 34.0% ± 5.8%, respectively). These findings suggest that there are no additional major PDGF-C processing proteases in the vitreous.
Although we focused on proteases in the vitreous in this study, the PVR membrane is capable of producing proteases that process PDGF-C (such as tPA).22 In addition to generating these proteases, the PVR membrane may act as a scaffold for PDGF-C and the proteases to promote or prevent processing. The contribution of membrane-based proteases to the processing of PDGF-C remains an open question.
Identifying PDGF-C and its processing proteases as potential contributors to PVR provides additional insight into why patients differ in their susceptibility to PVR. We speculate that high levels of PDGF-C and active proteases capable of converting it to its active form (plasmin and, to a lesser extent, tPA) predispose patients to PVR. Importantly, blocking the processing of PDGF-C and neutralizing it once it has been activated are exciting new therapeutic opportunities that await evaluation in animal models.
Acknowledgments
Supported by National Institutes of Health Grant EY012509 (AK).
The authors thank Debra Gilbertson (Zymogenetics) for antibodies to PDGF-C and Jing Cui and Joanne Matsubara (University of British Columbia) for the primary fetal RPE cells.
Footnotes
Disclosure: H. Lei, None; G. Velez, None; P. Hovland, None; T. Hirose, None; A. Kazlauskas, None
References
- 1.Ryan SJ. Traction retinal detachment: XLIX Edward Jackson Memorial Lecture. Am J Ophthalmol. 1993;115:1–20. doi: 10.1016/s0002-9394(14)73518-4. [DOI] [PubMed] [Google Scholar]
- 2.Glaser BM, Cardin A, Biscoe B. Proliferative vitreoretinopathy: the mechanism of development of vitreoretinal traction. Ophthalmology. 1987;94:327–332. doi: 10.1016/s0161-6420(87)33443-8. [DOI] [PubMed] [Google Scholar]
- 3.Campochiaro PA. Mechanisms in ophthalmic disease: pathogenic mechanisms in proliferative vitreoretinopathy. Arch Ophthalmol. 1997;115:237–241. doi: 10.1001/archopht.1997.01100150239014. [DOI] [PubMed] [Google Scholar]
- 4.Ando A, Ueda M, Uyama M, Masu Y, Ito S. Enhancement of dedifferentiation and myoid differentiation of retinal pigment epithelial cells by platelet derived growth factor. Br J Ophthalmol. 2000;84:1306–1311. doi: 10.1136/bjo.84.11.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campochiaro PA, Bryan JA, 3rd, Conway BP, Jaccoma EH. Intravitreal chemotactic and mitogenic activity: implication of blood-retinal barrier breakdown. Arch Ophthalmol. 1986;104:1685–1687. doi: 10.1001/archopht.1986.01050230123046. [DOI] [PubMed] [Google Scholar]
- 6.Cleary PE, Ryan SJ. Experimental posterior penetrating eye injury in the rabbit, I: method of production and natural history. Br J Ophthalmol. 1979;63:306–311. doi: 10.1136/bjo.63.5.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campochiaro PA, Hackett SF, Vinores SA, et al. Platelet-derived growth factor is an autocrine growth stimulator in retinal pigmented epithelial cells. J Cell Sci. 1994;107:2459–2469. doi: 10.1242/jcs.107.9.2459. [DOI] [PubMed] [Google Scholar]
- 8.Fredriksson L, Li H, Eriksson U. The PDGF family: four gene products form five dimeric isoforms. Cytokine Growth Factor Rev. 2004;15:197–204. doi: 10.1016/j.cytogfr.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Reigstad LJ, Varhaug JE, Lillehaug JR. Structural and functional specificities of PDGF-C and PDGF-D, the novel members of the platelet-derived growth factors family. FEBS J. 2005;272:5723–5741. doi: 10.1111/j.1742-4658.2005.04989.x. [DOI] [PubMed] [Google Scholar]
- 10.Lei H, Hovland P, Velez G, et al. A potential role for PDGF-C in experimental and clinical proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 2007;48:2335–2342. doi: 10.1167/iovs.06-0965. [DOI] [PubMed] [Google Scholar]
- 11.Gilbertson DG, Duff ME, West JW, et al. Platelet-derived growth factor C (PDGF-C), a novel growth factor that binds to PDGF alpha and beta receptor. J Biol Chem. 2001;276:27406–27414. doi: 10.1074/jbc.M101056200. [DOI] [PubMed] [Google Scholar]
- 12.Li X, Pontén A, Aase K, et al. PDGF-C is a new protease-activated ligand for the PDGF alpha-receptor [see comments] Nat Cell Biol. 2000;2:302–309. doi: 10.1038/35010579. [DOI] [PubMed] [Google Scholar]
- 13.Fredriksson L, Li H, Fieber C, Li X, Eriksson U. Tissue plasminogen activator is a potent activator of PDGF-CC. EMBO J. 2004;23:3793–3802. doi: 10.1038/sj.emboj.7600397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tripathi RC, Tripathi BJ. Tissue plasminogen activator therapy for the eye. Br J Ophthalmol. 2005;89:1390–1391. doi: 10.1136/bjo.2005.074401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonnet M. The development of severe proliferative vitreoretinopathy after retinal detachment surgery: grade B: a determining risk factor. Graefes Arch Clin Exp Ophthalmol. 1988;226:201–205. doi: 10.1007/BF02181181. [DOI] [PubMed] [Google Scholar]
- 16.Tseng W, Cortez RT, Ramirez G, Stinnett S, Jaffe GJ. Prevalence and risk factors for proliferative vitreoretinopathy in eyes with rhegmatogenous retinal detachment but no previous vitreoretinal surgery. Am J Ophthalmol. 2004;137:1105–1115. doi: 10.1016/j.ajo.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Gelderloos JA, Rosenkranz S, Bazenet C, Kazlauskas A. A role for Src in signal relay by the platelet-derived growth factor alpha receptor. J Biol Chem. 1998;273:5908–5915. doi: 10.1074/jbc.273.10.5908. [DOI] [PubMed] [Google Scholar]
- 18.Lei H, Venkatakrishnan A, Yu S, Kazlauskas A. Protein kinase A-dependent Hsp90α translocation impairs endothelial nitric-oxide synthase activity in high glucose and diabetes. J Biol Chem. 2007;282:9364–9371. doi: 10.1074/jbc.M608985200. [DOI] [PubMed] [Google Scholar]
- 19.Lei H, Romeo G, Kazlauskas A. Heat shock protein 90alpha-dependent translocation of annexin II to the surface of endothelial cells modulates plasmin activity in the diabetic rat aorta. Circ Res. 2004;94:902–909. doi: 10.1161/01.RES.0000124979.46214.E3. [DOI] [PubMed] [Google Scholar]
- 20.Nakagawa M, Refojo MF, Marin JF, Doi M, Tolentino FI. Retinoic acid in silicone and silicone-fluorosilicone copolymer oils in a rabbit model of proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 1995;36:2388–2395. [PubMed] [Google Scholar]
- 21.Andrews A, Balciunate E, Leong L, et al. Platelet-derived growth factor plays a key role in proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 1999;40:2683–2689. [PubMed] [Google Scholar]
- 22.Siren V, Immonen I. uPA, tPA and PAI-1 mRNA expression in periretinal membranes. Curr Eye Res. 2003;27:261–267. doi: 10.1076/ceyr.27.5.261.17223. [DOI] [PubMed] [Google Scholar]