Summary
Because of their capacity to give rise to various types of cells in vitro, embryonic stem and embryonal carcinoma (EC) cells have been used as convenient models to study the mechanisms of cell differentiation in mammalian embryos. In this study, we explored the mouse P19 EC cell line as an effective tool to investigate the factors that may play essential roles in mesoderm formation and axial elongation morphogenesis. We first demonstrated that aggregated P19 cells not only exhibited gene expression patterns characteristic of mesoderm formation but also displayed elongation morphogenesis with a distinct anterior–posterior body axis as in the embryo. We then showed by RNA interference that these processes were controlled by various regulators of Wnt signaling pathways, namely β-catenin, Wnt3, Wnt3a, and Wnt5a, in a manner similar to normal embryo development. We further showed by inhibitor treatments that the axial elongation morphogenesis was dependent on the activity of Rho-associated kinase. Because of the convenience of these experimental manipulations, we propose that P19 cells can be used as a simple and efficient screening tool to assess the potential functions of specific molecules in mesoderm formation and axial elongation morphogenesis.
Keywords: Wnt3, Wnt3a, Wnt5a, β-catenin, epiblast, convergent extension, ROCK
INTRODUCTION
The basic body plan is conserved among all vertebrate embryos. However, the cellular and molecular mechanisms of body plan formation is less understood for mammalian embryos compared to other vertebrates, particularly with respect to germ layer specification and axis formation (Takaoka et al., 2007; Tam and Loebel, 2007). The difficulty in studying the mechanisms of mammalian development is partly due to its viviparous nature: the development of the basic body plan takes place within the maternal reproductive tract. Normally, oocytes are fertilized in the oviduct and undergo a series of cell divisions to form the blastocyst, which is composed of the outer layer of trophectoderm (TE) and the internal clump of inner cell mass (ICM). TE of the blastocyst interacts with the endometrial wall of the uterus for implantation, and develops into trophoblast and extra-embryonic ectoderm. On the other hand, ICM gives rise to the epiblast and primitive endoderm around the time of implantation. The future posterior side of the epiblast forms the primitive streak, from which cells emigrate to generate mesoderm and definitive endoderm. Mesoderm is continuously generated through the primitive streak at the posterior end of the embryo, whereas the embryonic body elongates along the anterior–posterior axis.
Because of the viviparous nature of the mammalian development, various experimental procedures, such as micromanipulations, over-and under-expression of specific gene products, and treatment with pharmacological reagents, are not easily implemented when compared with lower vertebrate embryos, like frogs and fish. In addition, maternal and extraembryonic tissues are connected to the mammalian embryo during most of development, so that the analysis of developmental mechanisms is more complicated than the lower vertebrate embryos. Mouse whole embryos can be genetically manipulated by transgenic and gene-targeting techniques, and can be cultured outside of the uterus for certain durations during experimentations (Nagy et al., 2003). Nonetheless, these procedures are still laborious, time-consuming, and expensive. To compensate for these difficulties, many researchers have used in vitro model systems, namely cultured cell lines, which can recapitulate certain aspects of the developmental processes. For example, in vitro differentiation of embryonic stem (ES) cells and embryonal carcinoma (EC) cells has been used to study the mechanisms of germ layer formation because various experimental manipulations are easily implemented in these cell lines (Keller, 2005; Solter, 2006).
In the present study, we explored the mouse P19 EC cell line as an in vitro model to investigate the molecular mechanisms of germ layer formation and axial elongation morphogenesis. P19 cells, which were initially isolated from teratocarcinoma of normal embryo origin, possess properties similar to the epiblast, and can differentiate in vitro into various cell types, such as muscles and neurons (McBurney, 1993; van der Heyden and Defize, 2003). Here, we demonstrated that aggregated P19 cells exhibited gene expression patterns and axial elongation morphogenesis characteristic of posterior mesoderm, and that these processes were dependent on the same molecular components as the corresponding processes during normal development. Because various experimental manipulations can be easily and effectively applied, we propose that P19 cells can serve as a simple and convenient in vitro tool to assess the actions of specific molecules in mesoderm formation and axial elongation.
RESULTS
Aggregated P19 Cells Exhibit Gene Expression Patterns Characteristic of Posterior Mesoderm Development
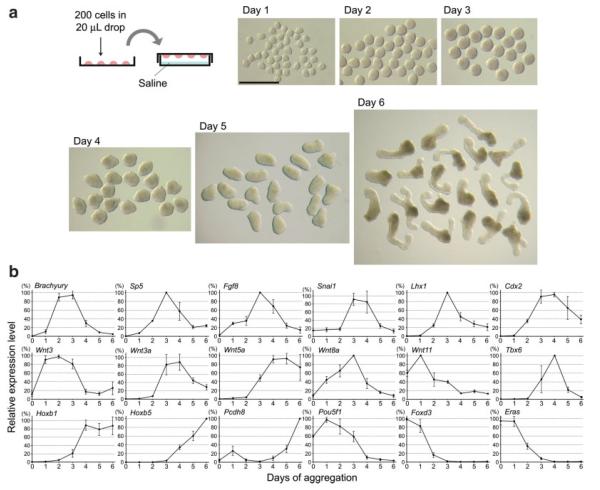
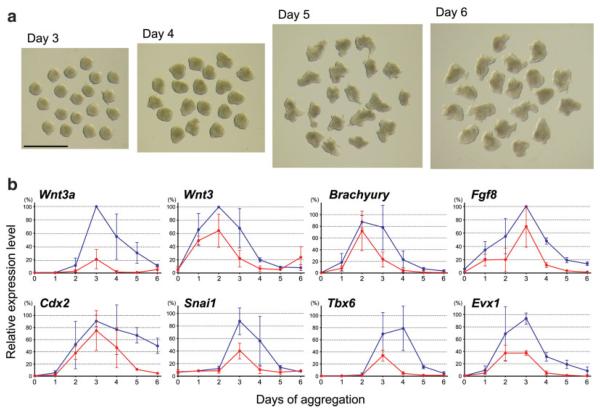
Mesodermal derivatives, including cardiac and skeletal muscle, can be derived from P19 cells that are aggregated in culture medium containing 1% dimethyl sulfoxide (DMSO), indicating that mesoderm development occurs under this condition (McBurney, 1993; van der Heyden and Defize, 2003). Here, we examined by qRT-PCR the expressions of various genes during aggregation culture of P19 cells, particularly those genes that are normally expressed in the primitive streak and posterior mesoderm. P19 cells were aggregated in hanging drops of culture medium containing 1% DMSO (Fig. 1a). Each hanging drop was 20 μL in volume, and initially contained 200 dissociated cells. After 1 day of culture (designated as Day 1), almost all cells in a drop gathered at the bottom, adhered to each other, and formed a single aggregate (Fig. 1a). Cells were harvested for RNA extraction everyday from Day 1 to Day 6 of hanging drop culture.
FIG. 1.
Aggregated P19 cells exhibit distinct morphological changes and gene expression patterns. (a) A schematic diagram of hanging drop culture (top left), and morphology of aggregates from Day 1 to Day 6 of aggregation culture. Aggregates were removed from hanging drops and placed together for photography. Scale bar = 1 mm. (b) qRT-PCR analyses showing the expression levels of various genes in P19 cell aggregates from Day 0 (immediately before aggregation) to Day 6. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.comy.]
Many of the genes that are normally expressed in the primitive streak at E6.5 to E7.5, such as Brachyury (Rashbass et al., 1991), Sp5 (Harrison et al., 2000), Fgf8 (Crossley and Martin, 1995), Snai1 (Smith et al., 1992), Lhx1 (also known as Lim1; Barnes et al., 1994), Tbx6 (Chapman et al., 1996), and Hoxb1 (Forlani et al., 2003), were up-regulated during the first 4 days of hanging drop culture (Fig. 1b). Several members of the Wnt gene family, specifically Wnt3 (Liu et al., 1999), Wnt3a (Yoshikawa et al., 1997), Wnt5a (Yamaguchi et al., 1999a), and Wnt8a (Bouillet et al., 1996), are normally expressed in the primitive streak and posterior mesoderm at E6.5 to E8.5, and they were also up-regulated in aggregated P19 cells (Fig. 1b). However, Wnt11, which is normally expressed in the node and extraembryonic mesoderm at E6.5 to E7.5 (Kispert et al., 1996), was not up-regulated in aggregated P19 cells. Cdx2, which is normally expressed in the definitive endoderm, the posterior mesoderm and the posterior neural tube at E8.5 (Beck et al., 1995), was also up-regulated during aggregation culture. By contrast, the genes that are normally associated with the maintenance of pluripotency in ES cells, such as Pou5f1 (also known as Oct4; Nichols et al., 1998), Foxd3 (Hanna et al., 2002), and Eras (Takahashi et al., 2003), were gradually down-regulated during aggregation culture (Fig. 1b).
The temporal order of expression of these genes during aggregation culture was also similar to that in normal development. Specifically, in aggregated P19 cells, Wnt3 was markedly up-regulated by Day 1, followed by the upregulation of Brachyury by Day 2 (Fig. 1b), which is consistent with the temporal expression patterns of these genes in normal embryos (Rivera-Perez and Magnuson, 2005). Fgf8, Snai1, Lhx1, Wnt3a, Wnt5a Wnt8a, Tbx6, and Hoxb1 reached the highest expression level later than Wnt3 and Brachyury (Fig. 1b), which is also similar to the temporal expression patterns during normal embryo development (Pfister et al., 2007). In addition, the up-regulation of Hoxb5 started later than that of Hoxb1, and continued until Day 6 (Fig. 1b), which is consistent with the temporal expression patterns of the Hox genes in normal embryos.
Elongation Morphogenesis of P19 Cell Aggregates
P19 cell aggregates exhibited a distinct morphological change (Fig. 1a) that is reminiscent of convergent extension of axial and paraxial mesoderm that normally takes place at the posterior end of normal embryos. Most P19 cell aggregates were nearly spherical until Day 3. However, aggregates were slightly irregular in shape at Day 4, and were clearly elongated by Day 5 and Day 6. This elongation morphogenesis was observed in almost all aggregates examined (98.5%; n = 199), indicating that the shape change of aggregates in hanging drops occurs in a consistent and synchronous manner. Many of the aggregates (66.4%) had a single axis of elongation, whereas some aggregates had two axes (25.6%) or more than two axes (6.5%) of elongation (Fig. 1a). The elongated aggregates with a single axis exhibited a distinct morphological polarity: one end was narrower and more transparent, whereas the other end was wider and more opaque. From Day 4 to Day 6 of culture, aggregates became not only elongated in length but also constricted in width (Fig. 1a). Thus, this morphological change appears to be caused by convergent extension, which has been extensively studied in frogs and fish embryos as a unique behavior of axial and paraxial mesoderm to drive the elongation of embryo along the anterior–posterior axis (Jenny and Mlodzik, 2006; Keller et al., 1985; Wallingford et al., 2002). After Day 6, aggregates did not appear to exhibit further morphological changes, although some of them started to degenerate, as judged by the emergence of loose cells that were dissociated from the aggregates. Thus, we did not analyze aggregates beyond Day 6 in this study.
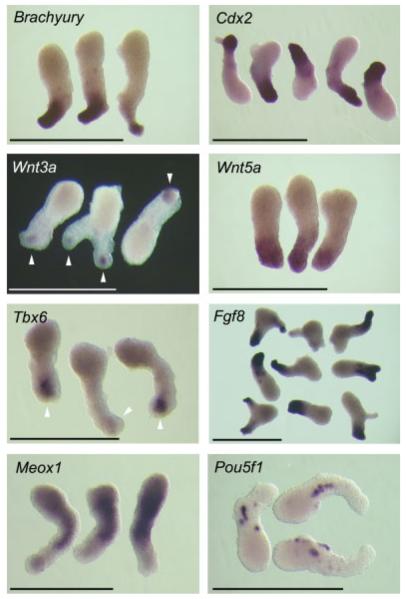
To gain insight into the relationship of aggregate elongation with respect to normal embryological events, we examined the spatial expression patterns of several genes in Day 6 aggregates by whole-mount in situ hybridization, including those that are normally expressed in the posterior mesoderm (see Fig. 2). Brachyury, Cdx2, Wnt5a, and Fgf8 were strongly expressed in the narrower side of elongated aggregates. Wnt3a and Tbx6 were also expressed in the narrower side of aggregates, although their expression domains were more localized to the distal end. On the other hand, Meox1, which is normally expressed in the somites and anterior presomitic mesoderm but not in the posterior mesoderm at E8.5 (Conlon et al., 1995), was broadly expressed in Day 6 aggregates except in the narrower end. These results suggest that the wider-narrower axis of elongated aggregates corresponds to the anterior–posterior axis of the normal embryo. Pou5f1, which is normally expressed only in the primordial germ cells (PGCs) at E8.5 (Scholer et al., 1990), was expressed at Day 6 in a punctate manner around the middle part of aggregates (see Fig. 2). Whether these Pou5f1-positive cells are similar to PGCs requires further investigations.
FIG. 2.
Whole-mount in situ hybridization analyses of various genes in P19 cell aggregates at Day 6. White arrowheads indicate the locations of Wnt3a and Tbx6 expression. Scale bars = 1 mm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
The Activation of Wnt/β-catenin Signaling Is Necessary to Up-regulate the Expression of Mesoderm Genes in P19 Cells
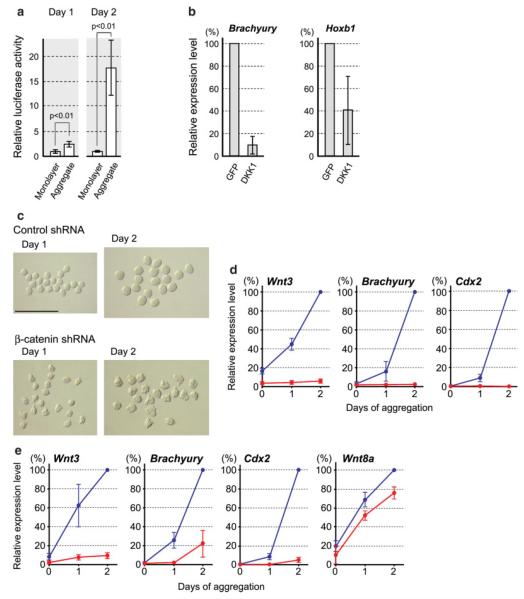
In normal embryos, the activation of Wnt/β-catenin signaling is critical for the generation of mesoderm through the primitive streak (Marikawa, 2006). Thus, we investigated whether the activation of Wnt/β-catenin signaling also plays a critical role in the up-regulation of mesoderm genes in P19 cell aggregates. First, we measured the level of endogenous Wnt/β-catenin signaling during cell aggregation, using the TOPFLASH reporter plasmid. The TOPFLASH plasmid contains a luciferase reporter gene under the transcriptional control of Lef/Tcf-response elements, and is turned on in response to active Wnt/β-catenin signaling (Korinek et al., 1997). P19 cells were transfected with TOPFLASH, and then cultured either as a monolayer or as aggregates. The luciferase activity was higher by about 2.5-and 17.5-fold in aggregates than in a monolayer after 1 day and 2 days of culture, respectively (Fig. 3a). By contrast, the control reporter plasmid FOPFLASH, in which the Lef/Tcf-response elements are mutated, did not exhibit a significant difference between aggregate and monolayer cultures (data not shown). These results indicate that aggregation leads to the activation of endogenous Wnt/β-catenin signaling in P19 cells.
FIG. 3.
The activation of Wnt/β-catenin signaling is necessary to up-regulate the expression of mesoderm genes in P19 cells. (a) The endogenous Wnt/β-catenin signaling is elevated in response to cell aggregation. The activity of TOPFLASH is normalized by that of the cotransfected pRL-TK control plasmid. The data is presented as mean ± standard deviation of three independent experiments. Statistical significance was examined by Student’s t-test. (b) Ectopic expression of Dkk1 impairs the up-regulation of Brachyury and Hoxb1 in aggregates at Day 2. (c) Morphology of Day 1 and Day 2 aggregates that are expressing control shRNA or β-catenin-specific shRNA. Aggregates expressing β-catenin shRNA have a rougher surface. Scale bar = 1 mm. (d) The up-regulation of Wnt3, Brachyury, and Cdx2 is essentially absent in cell aggregates expressing β-catenin-specific shRNA (red lines), as compared to those expressing the control shRNA (blue lines). (e) The up-regulation of Brachyury and Cdx2 is diminished in cell aggregates expressing Wnt3-specific shRNA (red lines), as compared to those expressing the control shRNA (blue lines). Wnt8a up-regulation is unaffected by Wnt3a knockdown. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
We then examined whether the activation of Wnt/β-catenin signaling is necessary for the up-regulation of mesoderm genes in P19 cell aggregates. First, P19 cells were transiently transfected with the expression plasmid encoding Dkk1, a secreted inhibitor of Wnt co-receptor Lrp5/6 (Glinka et al., 1998; Mao et al., 2001; Semenov et al., 2001). As a control, P19 cells were transfected with the expression plasmid encoding green fluorescent protein (GFP). The expression levels of Brachyury and Hoxb1 at Day 2 of hanging drop culture were lower in Dkk1-expressing aggregates than in GFP-expressing aggregates (Fig. 3b). This suggests that the inhibition of Wnt/β-catenin signaling impairs the up-regulation of mesoderm genes in P19 cell aggregates.
As an alternative way to inhibit Wnt/β-catenin signaling, we suppressed the expression of β-catenin in P19 cells by stably transfecting with the plasmid encoding a β-catenin-specific short hairpin RNA (shRNA). As a control, P19 cells were stably transfected with the plasmid encoding nontarget shRNA sequence. The stably transfected P19 cells were then aggregated in hanging drops and cultured for 2 days. In addition to its role as a mediator of Wnt/β-catenin signaling, β-catenin protein is known to regulate cadherin-mediated cell–cell adhesion (Gottardi and Gumbiner, 2001). Nonetheless, P19 cells expressing β-catenin-specific shRNA adhered to each other in hanging drops and formed cohesive aggregates, although their surface appeared rougher than that of control aggregates (Fig. 3c). In spite of their consistent aggregation, Wnt3, Brachyury, and Cdx2 were not up-regulated in P19 cells expressing β-catenin-specific shRNA (Fig. 3d). This result further supports that the activation of Wnt/β-catenin signaling is essential for the up-regulation of mesoderm genes in P19 cell aggregates. Notably, the up-regulation of Wnt3 was totally eliminated by the suppression of β-catenin. This suggests that the expression of Wnt3 is regulated by the activation of Wnt/β-catenin signaling through a positive feedback mechanism.
In normal embryos, Wnt3 expression in the epiblast is essential for the formation of the primitive streak (Barrow et al., 2007; Liu et al., 1999). Thus, we examined the role of Wnt3 in the up-regulation of mesoderm genes in P19 cell aggregates. P19 cells were stably transfected with the plasmid encoding a Wnt3-specific shRNA, and then cultured as aggregates for 2 days. Wnt3-specific shRNA effectively reduced the expression of Wnt3 down to about 10% of that in the control aggregates (Fig. 3e). The up-regulation of Brachyury and Cdx2 was diminished in the aggregates expressing Wnt3-specific shRNA, when compared with those expressing the control shRNA. However, a small but distinct up-regulation of Brachyury and Cdx2 was observed at Day 2. This up-regulation may be driven by Wnt8a, whose expression was essentially unaffected by the suppression of Wnt3. Nonetheless, most of the Brachyury and Cdx2 expressions (more than 75% and 90%, respectively) were abolished by Wnt3-specific shRNA. This suggests that the up-regulation of mesoderm genes in P19 cell aggregates is mainly dependent on Wnt3, consistent with its role in normal embryos.
Ectopic Activation of Wnt/β-catenin Signaling Is Sufficient to Up-regulate Mesoderm Genes in P19 Cells Without Cell Aggregation
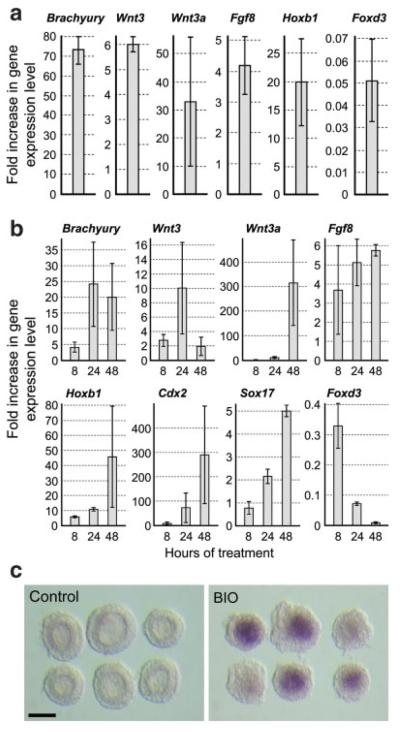
We examined whether a forced activation of Wnt/β-catenin signaling is sufficient to up-regulate mesoderm genes in P19 cells without cell aggregation. When monolayer-cultured P19 cells were treated with Wnt3a protein for 1 day, many genes that are normally expressed in the primitive streak and posterior mesoderm were up-regulated, including Brachyury, Wnt3, Wnt3a, Fgf8, and Hoxb1 (Fig. 4a). By contrast, Foxd3 was down-regulated by the Wnt3a treatment (Fig. 4a). Likewise, the activation of Wnt/β-catenin signaling with [2′Z,3′E]-6-bromoindirubin-3′-oxime (BIO), a specific inhibitor of GSK3 (Sato et al., 2004), also induced the up-regulation of various mesoderm genes and the down-regulation of Foxd3 in monolayer-cultured P19 cells (Fig. 4b). The expressions of Wnt3 and Brachyury reached the highest levels in 24 h, whereas those of Wnt3a, Hoxb1, Cdx2, and Sox17 did so only after 48 h of BIO treatment. This temporal order of mesoderm gene expression was similar to those in aggregation culture (Fig. 1b). These results suggest that the activation of Wnt/β-catenin signaling is sufficient to up-regulate mesoderm genes in a distinct temporal order. Notably, the expression of Wnt3 was upregulated by the activation of Wnt/β-catenin signaling (Fig. 4a,b), which further supports that the expression of Wnt3 is regulated by a positive feedback mechanism.
FIG. 4.
The activation of Wnt/β-catenin signaling is sufficient to induce the up-regulation of mesoderm genes in P19 cells without cell aggregation. Various mesoderm genes are up-regulated when monolayer-cultured P19 cells are treated with 100 ng/mL of Wnt3a protein (a) or with 2 μM BIO (b). The vertical axes of the graphs represent fold increase of gene expressions in the treated cells relative to nontreated control cells. (c) Brachyury expression is activated in the distal explants of mouse E5.5 embryos by the treatment with 2 μM BIO, as assessed by in situ hybridization. Scale bar = 100 μm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
To compare the characteristics of P19 cells with those of the epiblast, we tested whether the activation of Wnt/β-catenin signaling with BIO is sufficient to up-regulate Brachyury in cultured embryo explants. The distal portion of E5.5 embryos were explanted and cultured for 24 h in the presence of BIO. Control explants that were cultured in the absence of BIO maintained a proamniotic cavity surrounded by the epithelial epiblast layer (Fig. 4c). None of the control explants (0 out of 17) displayed expression of Brachyury, as examined by in situ hybridization. By contrast, Brachyury was distinctly expressed in many of the BIO-treated explants (21 out of 24). The Brachyury expression was found in the inner portion (corresponding to the epiblast) but not in the outer layer (corresponding to the primitive endoderm) of the explants. These results suggest that only the epiblast, but not the primitive endoderm, possess the competence to express Brachyury in response to the activation of Wnt/β-catenin signaling. Furthermore, the proamniotic cavity was indistinct in the BIO-treated explants (Fig. 4c), suggesting that the epithelial feature of the epiblast was diminished by the activation of Wnt/β-catenin signaling. These results are consistent with the previous study that the constitutive activation of β-catenin in early embryos results in an ectopic expression of Brachyury and precocious epithelial-to-mesenchymal transformation of the epiblast (Kemler et al., 2004). Thus, the activation of Wnt/β-catenin signaling is sufficient to induce Brachyury expression in both the epiblast and P19 cells, demonstrating a similarity between these two types of cells.
Wnt3a Is Essential for Mesoderm Formation and Axial Elongation in P19 Cell Aggregates
In addition to Wnt3, Wnt3a also plays a critical role in mesoderm development and axial morphogenesis in mouse embryos. In contrast to Wnt3, initial activation of Brachyury and Tbx6 in the primitive streak is independent of Wnt3a, as these genes are expressed normally in Wnt3a-null mutant embryos at E7.5. However, Wnt3a is essential to maintain the expression of Brachyury and Tbx6 in the posterior mesoderm, as they are markedly diminished in the mutant embryos at E8.5 (Yamaguchi et al., 1999b). As a result, Wnt3a-null mutant embryos lack posterior somites and a tailbud at E9.5 (Takada et al., 1994).
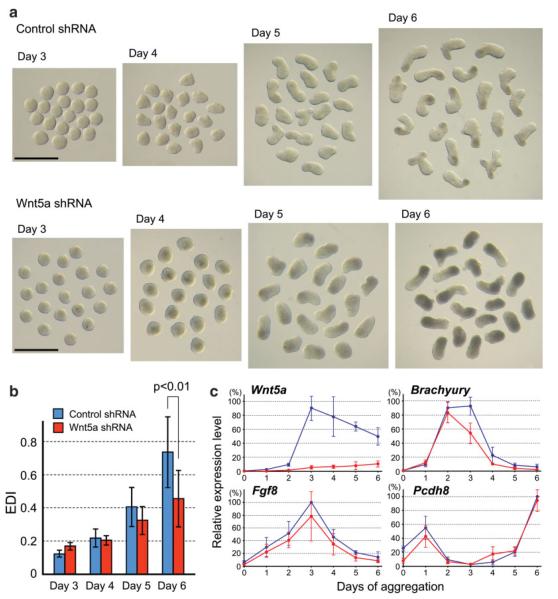
To assess the role of Wnt3a in mesoderm development and axial elongation in P19 cell aggregates, we suppressed the expression of Wnt3a by stably transfecting the plasmid encoding a Wnt3a-specific shRNA. Morphologically, aggregates expressing the Wnt3a-specific shRNA were indistinguishable from the control aggregates until Day 4 (Fig. 5a; compare with Fig. 1a). However, aggregates expressing the Wnt3a-specific shRNA neither elongated nor exhibited a morphological polarity by Day 6. The expression level of Wnt3a was effectively suppressed by shRNA, as it was reduced down to about 20% of that in the control aggregates at Day 3 (Fig. 5b). The levels of Brachyury were comparable at Day 2 between the aggregates expressing Wnt3a-specific shRNA and those expressing a control shRNA (Fig. 5b). However, the Brachyury level was distinctly lower in the aggregates expressing Wnt3a-specific shRNA after Day 2, suggesting that the maintenance of Brachyury expression is dependent on Wnt3a. Likewise, the initial up-regulation of Cdx2 appeared unaffected until Day 3, but its expression at later stages was diminished by the suppression of Wnt3a (Fig. 5b). Furthermore, although the expression of early mesoderm genes, namely Wnt3 and Fgf8, were not severely affected, the up-regulations of Snai1, Tbx6, and Evx1, which peaked at Day 3 to Day 4 in the control aggregates, were diminished by more than 50% in those expressing Wnt3a-specific shRNA at Day 3 (Fig. 5b). These results suggest that Wnt3a is essential for the up-regulation and maintenance of various mesoderm genes.
FIG. 5.
Wnt3a is essential for mesoderm formation and axial elongation in P19 cell aggregates. (a) Morphology of aggregates expressing Wnt3a-specific shRNA. Scale bar = 1 mm. (b) Comparison of temporal gene expression patterns between aggregates expressing Wnt3a-specific shRNA (red lines) and those expressing the control nontarget shRNA sequence (blue lines). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Wnt5a Is Essential for Axial Elongation in P19 Cell Aggregates
Wnt5a also plays essential roles in normal axial morphogenesis in mouse embryos. Wnt5a-null mutant embryos exhibit a diminished caudal extension by E8.5 (Yamaguchi et al., 1999a). However, Brachyury, Fgf8, and Tbx6 are normally expressed in the caudal end of the mutant embryos, suggesting that Wnt5a is not essential for the initial specification of posterior mesoderm but is involved in the morphogenetic regulation of posterior mesoderm (Yamaguchi et al., 1999a).
To determine whether Wnt5a regulates axial elongation in P19 cell aggregates, the expression of Wnt5a was suppressed by stably transfecting the plasmid encoding a Wnt5a-specific shRNA. The aggregates expressing a control shRNA as well as those expressing the Wnt5a-specific shRNA exhibited axial elongation by Day 6 (Fig. 6a,b). However, it appeared that axial elongation was less pronounced in the aggregates expressing Wnt5a-specific shRNA. This notion was confirmed by the measurement of the elongation distortion index (EDI; see Materials and Methods). The average EDI at Day 6 was significantly lower in the aggregates expressing Wnt5a-specific shRNA when compared with the control aggregates (Fig. 6b).
FIG. 6.
Wnt5a is essential for axial elongation in P19 cell aggregates. (a) Morphology of aggregates expressing the control shRNA or Wnt5a-specific shRNA. Scale bar = 1 mm. (b) EDI measurement reveals that the elongation of aggregates is diminished by the suppression of Wnt5a. Statistical significance was examined by Student’s t-test. (c) Comparison of temporal gene expression patterns between aggregates expressing Wnt5a-specific shRNA (red lines) and those expressing the control shRNA (blue lines). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
We further examined gene expressions in the aggregates expressing Wnt5a-specific shRNA. The expression level of Wnt5a was substantially reduced by shRNA down to about 10% of that in the control aggregates at Day 3 (Fig. 6c). However, the expression of Fgf8 was apparently unaffected by the suppression of Wnt5a throughout the six days of aggregation culture. Also, the up-regulation of Brachyury was unaffected until Day 2, although its expression was diminished by about 40% by Day 3 (Fig. 6c). These results suggest that Wnt5a regulates axial elongation of P19 cell aggregates without substantially affecting the initial specification of mesoderm.
Interestingly, the expression of Pcdh8 in P19 cell aggregates was apparently unaffected by the suppression of Wnt5a (Figs. 1b and 6c). In Xenopus embryos, Wnt5a regulates convergent extension along the anterior–posterior body axis by activating the expression of the Pcdh8 homolog XPAPC, and the depletion of Wnt5a by antisense morpholino oligonucleotides results in significant down-regulation of the XPAPC expression (Schambony and Wedlich, 2007). Thus, unlike in Xenopus, Wnt5a may not regulate axial elongation by activating the expression of Pcdh8 in mouse embryos, although the expression pattern of Pcdh8 in Wnt5a-null mutant mouse embryos is yet to be determined.
The Activity of Rho-Associated Kinase Is Essential for Axial Elongation in P19 Cell Aggregates
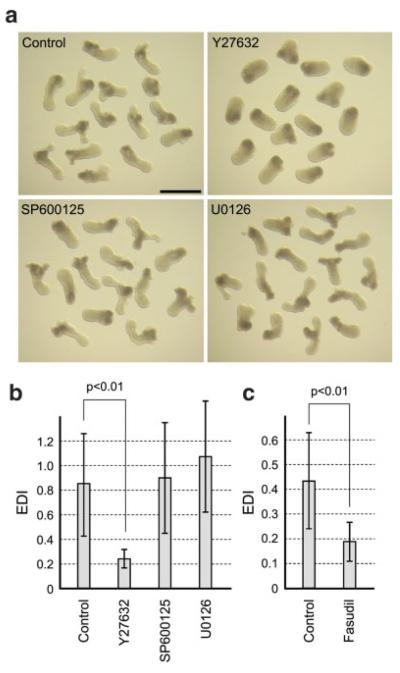
Several studies have shown that Wnt5a protein regulates convergent extension and posterior elongation of Xenopus and zebrafish embryos through the Wnt/planar cell polarity (PCP) signaling pathway, which is distinct from the Wnt/β-catenin signaling pathway (Barrow, 2006; Karner et al., 2006). Various components of the Wnt/PCP pathway have been identified that regulate convergent extension, including Rho-associated kinase (ROCK) and Jun N-terminal kinase (JNK) (Kim and Han, 2005; Marlow et al., 2002; Yamanaka et al., 2002). We investigated whether these PCP components are involved in the axial elongation of P19 cell aggregates. Aggregates were cultured in hanging drops of normal culture medium until Day 4, and then transferred into hanging drops containing specific inhibitors: Y27632 (ROCK inhibitor; 10 μM), SP600125 (JNK inhibitor; 10 μM), and U0126 (MEK inhibitor; 10 μM). As a control, Day 4 aggregates were transferred into new hanging drops with no inhibitor. After two more days of culture (i.e., Day 6), the morphology of aggregates were assessed by measuring EDI. Neither SP600125 nor U0126 significantly inhibited the axial elongation, when compared with the control aggregates (Fig. 7a,b). SP600125 did not block axial elongation even at a near-toxic concentration (50 μM; data not shown), which is far in excess of that typically used in cell culture. By contrast, Y27632 significantly interfered with the elongation of aggregates (Fig. 7a,b). The elongation of aggregates was also significantly inhibited by another ROCK inhibitor Fasudil (50 μM; Fig. 7c). These results suggest that ROCK, but not JNK or MEK, is involved in the regulation of axial elongation in P19 cell aggregates.
FIG. 7.
The activity of Rho-associated kinase is essential for axial elongation in P19 cell aggregates. (a) Morphology of aggregates that are treated with a specific inhibitor from Day 4 to Day 6. Scale bar = 1 mm. (b, c) EDI measurement reveals that the elongation of aggregates is dependent on the activity of ROCK. Statistical significance was examined by Student’s t-test. [Color figure can be viewed in the online issue, which is available at www.interscience. wiley.com.]
DISCUSSION
In the present study, we showed that aggregated P19 cells recapitulated several critical aspects of mesoderm development in vitro. The genes that are normally expressed in the primitive streak and posterior mesoderm were up-regulated upon cell aggregation. The initial up-regulation of mesoderm genes in P19 cell aggregates was induced by the activation of Wnt/β-catenin signaling, which depended on Wnt3 and β-catenin, as in normal embryos. Later on, aggregates elongated by convergent extension, which is normally driven by axial and paraxial mesoderm. Elongated aggregates displayed a morphological polarity with distinct spatial gene expression patterns, which appeared to correspond to the anterior–posterior axis of the normal embryo. Elongation morphogenesis of P19 cell aggregates was dependent on the distinct actions of Wnt3a and Wnt5a, in a manner similar to the posterior development of normal embryos. Here, we propose that P19 cells can serve as a simple and convenient in vitro tool to investigate the molecular mechanisms of mesoderm formation and axial elongation morphogenesis.
As demonstrated in this study, P19 cell aggregates can be effectively manipulated to examine the roles of specific genes in mesoderm development and axial elongation. Particularly, the knockdown of a specific gene expression can be efficiently achieved by stable transfection of a plasmid construct encoding a specific shRNA, as we have effectively down-regulated the expression of β-catenin, Wnt3, Wnt3a, and Wnt5a. Typically, by puromycin selection, it takes less than two weeks to obtain sufficient numbers of P19 cells that are stably transfected with shRNA plasmids to use for cell aggregation. Because libraries of predesigned shRNA plasmids are commercially available to target most genes in the mouse genome, P19 cells can be used as a convenient tool for the rapid screenings of candidate genes that are involved in mesoderm formation and axial elongation morphogenesis.
P19 cells can also serve as a tool to identify new genes that may be important for the initial steps of mesoderm formation. We have shown that not only cell aggregation but also ectopic activation of Wnt/β-catenin signaling without aggregation is sufficient to up-regulate a series of mesoderm genes that are normally expressed in the primitive streak (see Fig. 4). Microarray analyses of P19 cells that are aggregated or treated with an activator of Wnt/β-catenin signaling should yield a list of candidate genes that may play roles in the initial stages of mesoderm development. Furthermore, because a relatively large number of P19 cells can be easily treated with an activator of Wnt/β-catenin signaling, proteomics and other biochemical analyses can be implemented to identify protein modifications that may occur during mesoderm formation.
The experiment using specific inhibitors showed that the activity of ROCK between Day 4 and Day 6 is essential for the elongation of P19 cell aggregates (see Fig. 7). Elucidation of how the ROCK activity is temporally and spatially regulated during elongation morphogenesis needs further investigations, particularly with respect to the roles of Wnt/PCP pathway components, including Wnt5a. Our finding is consistent with the recent study that convergent extension and neural tube closure of cultured mouse embryo is diminished by the treatment with Y27632 but not with SP600125, indicating that ROCK, but not JNK, is essential for these morphogenetic processes (Ybot-Gonzales et al., 2007). Thus, P19 cell aggregates can be used for simple drug treatment experiments to examine which signaling events are essential for axial elongation. Importantly, the extent of axial elongation can be quantitatively evaluated by the measurement of EDI (Fig. 7b,c).
We showed that an activator of Wnt/β-catenin signaling is sufficient to induce the expression of various mesoderm genes in P19 cells. In this respect, the property of P19 cells is similar to that of the epiblast in normal embryos. The activation of Wnt/β-catenin signaling in early embryos by a constitutive activation of β-catenin (Kemler et al., 2004) or by a loss-of-function mutation of negative regulator Apc (Chazaud and Rossant, 2006) results in precocious and excessive expression of mesoderm genes in the epiblast. Consistently, the treatment of E5.5 distal explants with an activator of Wnt/β-catenin signaling caused robust expression of Brachyury in the epiblast, as shown in the present study. By contrast, in ES cells, the activation of Wnt/β-catenin signaling promotes the maintenance of the undifferentiated state (Hao et al., 2006; Ogawa et al., 2006; Sato et al., 2004; Singla et al., 2006), suggesting that the response of ES cells to active Wnt/β-catenin signaling is fundamentally different from those of the epiblast and P19 cells. Similarity between the epiblast and P19 cells is also exemplified by the regulatory mechanism of the Pou5f1 gene expression. The Pou5f1 expression in the epiblast and P19 cells are both driven by the proximal enhancer, whereas expression in ES cells is controlled by the distal enhancer, which is also responsible for the expression in ICM and PGC (Yeom et al., 1996). Although ES cells have been widely used by many researchers to study the mechanisms of mesoderm formation, the use of P19 cells should also complement the efforts to elucidate the molecular mechanisms of mesoderm formation and axial elongation.
In the past, many researchers have routinely generated mesoderm from P19 cells by aggregation culture in the presence of DMSO (van der Heyden and Defize, 2003). Nonetheless, to our knowledge, the elongation morphogenesis that we reported in the present study had not been documented. We speculate two possible reasons why such a morphogenetic event might have been overlooked. One possibility is that the duration of aggregation culture was not long enough to observe elongation morphogenesis. In many studies (e.g., Habara-Ohkubo, 1996; Ridgeway et al., 2000; van der Heyden et al., 2003; Vidricaire et al., 1994), P19 cells were allowed to aggregate for 3 to 4 days, and then transferred to a culture dish with an adhesive substratum on which cells spread out and differentiate. By contrast, the elongation morphogenesis was most dramatic after 5 days of aggregation culture, as shown in the present study (see Fig. 1). The other possibility is that the initial cell number of each aggregate may be critical to yield elongation morphogenesis. In the present study, two hundred cells were placed in each hanging drop at the beginning of aggregation (see Fig. 1). We used this number with an intent to generate an aggregate that carries the number of cells that is similar to the epiblast of a normal embryo before primitive streak formation (Kaufman, 1995). By contrast, in several studies (e.g., Anisimov et al., 2002; Smith et al., 1987; Zhang et al., 2002), many more cells, up to 5,000 cells, were placed in each hanging drop. Differences in aggregate size influence the type as well as the amount of mesoderm cells generated (Smith et al., 1987), which may affect the pattern of morphogenetic processes.
Whether aggregates of other EC cell lines or ES cells can also exhibit the elongation morphogenesis with an anterior–posterior axis is currently unclear. F9 is one of the most commonly used EC cell lines (Lehtonen et al., 1989). Although F9 cells share several features similar to ICM and ES cells, they are considered to be nullipotent and capable of differentiating only into primitive endoderm but not embryonic mesoderm (Sennerstam and Stromberg et al., 1984; Zakany et al., 1984). Therefore, it is unlikely that aggregates of F9 cells would exhibit convergent extension morphogenesis, which requires the activity of posterior mesoderm. By contrast, ES cells are pluripotent and capable of giving rise to any type of embryonic cells. Thus, it may be possible for ES cell aggregates to exhibit elongation morphogenesis, although it is likely to depend on a specific culture condition to effectively induce the formation of posterior mesoderm. Recently, pluripotent cell lines, called EpiSC, have been generated from the epiblast (Brons et al., 2007; Tesar et al., 2007). Because EpiSC cells, like P19 cells, retain various characteristics of the epiblast, it is of particular interest to examine whether EpiSC aggregates exhibit the elongation morphogenesis.
MATERIALS AND METHODS
Cell Culture
P19 mouse embryonal carcinoma cells were obtained from the American Type Culture Collection (Manassas, VA), and cultured in MEM Alpha Medium with 2.5% fetal bovine serum (FBS) and 7.5% calf serum (Invitrogen, Carlsbad, CA). P19 cells were aggregated by hanging drop culture (Fig. 1a). Cells were first dissociated in Trypsin-EDTA and suspended in culture medium containing 1% DMSO at 10 cells/lL density. Twenty drops of cell suspension (20 μL each) were placed on the inner surface of the lid of a 60 mm Petri dish (351007; Becton Dickinson, Franklin Lakes, NJ). The lid with drops was carefully inverted and placed on the bottom part of a Petri dish, which was filled with about 5 mL of phosphatebuffered saline (PBS) to minimize the evaporation of hanging drops. To activate Wnt/β-catenin signaling in monolayer-cultured P19 cells, the culture medium was supplemented with 100 ng/mL of recombinant mouse Wnt3a protein (R&D Systems, Minneapolis, MN) or 2 lM [2′Z,3′E]-6-bromoindirubin-3′-oxime (BIO; EMD Biosciences, San Diego, CA). The other pharmacological inhibitors, namely Y27632, SP600125, U0126, and Fasudil, were obtained commercially (EMD Biosciences).
Quantitative Reverse Transcription and Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted using TRI Reagent (Molecular Research Center, Cincinnati, OH), according to the manufacturer’s instruction. cDNA was synthesized from 1 lg of total RNA, using oligo dT(18) primer and M-MLV Reverse Transcriptase (Promega, Madison, WI) in a 25 lL reaction volume. PCR was performed using iCycler Thermal Cycler with MyiQ Single Color Real-Time PCR Detection System (Bio-Rad, Hercules, CA). 0.5 μL of cDNA was amplified using iQ SYBR Green Supermix (Bio-Rad) in a 20 μL reaction volume with the following condition: the initial denaturation at 94°C (5 min) followed by up to 45 cycles of 94°C (15 s), 60°C (20 s) and 72°C (40 s). The sequences of PCR primers are listed in Supporting Information. The Gapdh gene was used to normalize the expression levels of all other genes. At least three independent series of samples were analyzed for each experiment, and the results are presented as mean ± standard deviation. For those experiments that are presented in Figs. 1b, 3d, 3e, 5b, and 6c, the highest gene expression level in control aggregates was set as 100% in the graphs.
Whole-Mount in situ Hybridization
Digoxygenin-labeled antisense RNA probes were synthesized from the plasmid DNA template, using digoxygenin-11-UTP (Roche, Indianapolis, IN) and RNA polymerases (Promega). The plasmid containing the Brachyury cDNA was a gift from Dr. B. G. Herrmann. The cDNA fragments for Cdx2, Wnt3a, Wnt5a, Tbx6, Fgf8, Meox1, and Pou5f1 were isolated by RT-PCR from mouse embryonic cDNA, using the primers listed in Supporting Information, and were subcloned into pGEM-T Easy vector (Promega). P19 cell aggregates and embryo explants were fixed in 4% paraformaldehyde in PBS. Probe hybridization and detection were performed according to Belo et al., 1997.
Plasmid Transfection and Luciferase Assay
A day before transfection, 2 × 104 of P19 cells were plated per well in a 24-well plate. The plasmids were transfected using Lipofectamine2000 (Invitrogen) according to the manufacturer’s instruction. The full-length cDNA of mouse Dkk1 was obtained by RT-PCR using the primers (forward: 5′-CCA TGG TTG TGT GTG CAG CGG CAG CTG TCC GG-3′ and reverse: 5′-ATT TGC GGC CGC GTG TCT CTG GCA GGT GTG GAG CCT AGA A-3′), and subcloned into the NcoI/NotI sites of pEF/cyto/myc (Invitrogen). The following plasmids were obtained commercially: the GFP expression plasmid (pEF/cyto/myc/GFP; Invitrogen), TOPFLASH (Upstate, Charlottesville, VA), FOPFLASH (Upstate), pRL-TK (Promega), and shRNA-encoding plasmids (TRCN 0000012692 for β-catenin, TRCN0000071499 for Wnt3, TRCN0000089120 for Wnt3a, TRCN0000071831 for Wnt5a, and SHC002 for nontarget control; Sigma-Aldrich, St. Louis, MO). The dual-luciferase assay was conducted using the Dual-Luciferase Reporter Assay System (Promega) with Gene Light 55 Luminometer (Microtech, Chiba, Japan), according to the manufacturer’s instructions. For stable transfection, P19 cells were transfected with the shRNA-encoding plasmid that was linearized with SfiI, and cultured in the presence of 10 lg/mL puromycin for at least 10 days.
Animals and Embryo Explants
F1 (C57BL/6 × DBA/2) mice were obtained from the National Cancer Institute (Frederick, MD). Animals were maintained according to the guidelines of the Laboratory Animal Service at the University of Hawaii and the Guide for the Care and Use of Laboratory Animals of the National Research Council (Committee to Revise the Guide, Institute of Laboratory Animal Resources Council, Commission on Life Sciences, National Research Council, 1996). The protocol of animal handling and treatment was reviewed and approved by the Institutional Animal Care and Use Committee. Pregnant female mice were sacrificed at around noon of the fifth day post coitum to isolate E5.5 embryos. The distal portion of the egg cylinder was dissected using a pair of metal needles, and cultured in hanging drops of MEM Alpha Medium with 2.5% FBS and 7.5% calf serum.
Measurement of the Elongation Distortion Index
Cell aggregates were removed from hanging drops and placed in PBS containing 5% FBS for photography. The ImageJ program (http://rsb.info.nih.gov/ij) was used to measure the circumference and area of individual aggregates in photographs. The elongation distortion index (EDI) was calculated as {(circumference)2/(area) × 4π − 1}. With this formula, when an aggregate is completely spherical, which appears circular in a photograph, the EDI is zero. The more an aggregate is elongated or distorted, the higher the EDI. EDI reflects the shape of an aggregate, and is not affected by the size of an aggregate.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. B. G. Herrmann for the Brachyury plasmid.
Contract grant sponsor: Research Center in Minority Institutions program of the National Center for Research Resources, Contract grant number: G12RR003061, Contract grant sponsor: The National Institute of Child Health and Human Development, Contract grant number: HD040208
Footnotes
Additional Supporting Information may be found in the online version of this article.
LITERATURE CITED
- Anisimov SV, Tarasov KV, Riordon D, Wobus AM, Boheler KR. SAGE identification of differentiation responsive genes in P19 embryonic cells induced to form cardiomyocytes in vitro. Mech Dev. 2002;117:25–74. doi: 10.1016/s0925-4773(02)00177-6. [DOI] [PubMed] [Google Scholar]
- Barnes JD, Crosby JL, Jones CM, Wright CV, Hogan BL. Embryonic expression of Lim-1, the mouse homolog of Xenopus Xlim-1, suggests a role in lateral mesoderm differentiation and neurogenesis. Dev Biol. 1994;161:168–178. doi: 10.1006/dbio.1994.1018. [DOI] [PubMed] [Google Scholar]
- Barrow JR. Wnt/PCP signaling: A veritable polar star in establishing patterns of polarity in embryonic tissues. Semin Cell Dev Biol. 2006;17:185–193. doi: 10.1016/j.semcdb.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Barrow JR, Howell WD, Rule M, Hayashi S, Thomas KR, Capecchi MR, McMahon AP. Wnt3 signaling in the epiblast is required for proper orientation of the anteroposterior axis. Dev Biol. 2007;312:312–320. doi: 10.1016/j.ydbio.2007.09.030. [DOI] [PubMed] [Google Scholar]
- Beck F, Erler T, Russell A, James R. Expression of Cdx-2 in the mouse embryo and placenta: Possible role in patterning of the extra-embryonic membranes. Dev Dyn. 1995;204:219–227. doi: 10.1002/aja.1002040302. [DOI] [PubMed] [Google Scholar]
- Belo JA, Bouwmeester T, Leyns L, Kertesz N, Gallo M, Follettie M, De Robertis EM. Cerberus-like is a secreted factor with neuralizing activity expressed in the anterior primitive endoderm of the mouse gastrula. Mech Dev. 1997;68:45–57. doi: 10.1016/s0925-4773(97)00125-1. [DOI] [PubMed] [Google Scholar]
- Bouillet P, Oulad-Abdelghani M, Ward SJ, Bronner S, Chambon P, Dolle P. A new mouse member of the Wnt gene family, mWnt-8, is expressed during early embryogenesis and is ectopically induced by retinoic acid. Mech Dev. 1996;58:141–152. doi: 10.1016/s0925-4773(96)00569-2. [DOI] [PubMed] [Google Scholar]
- Brons IG, Smithers LE, Trotter MW, Rugg-Gunn P, Sun B, Chuva de Sousa Lopes SM, Howlett SK, Clarkson A, Ahrlund-Richter L, Pedersen RA, Vallier L. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–195. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- Chapman DL, Agulnik I, Hancock S, Silver LM, Papaioannou VE. Tbx6, a mouse T-Box gene implicated in paraxial mesoderm formation at gastrulation. Dev Biol. 1996;180:534–542. doi: 10.1006/dbio.1996.0326. [DOI] [PubMed] [Google Scholar]
- Chazaud C, Rossant J. Disruption of early proximodistal patterning and AVE formation in Apc mutants. Development. 2006;133:3379–3387. doi: 10.1242/dev.02523. [DOI] [PubMed] [Google Scholar]
- Committee to Revise the Guide, Institute of Laboratory Animal Resources Council. Commission on Life Sciences, National Research Council . Guide for the Care and Use of Laboratory Animals. 7th edn National Academy Press; Washington, DC: 1996. [Google Scholar]
- Conlon RA, Reaume AG, Rossant J. Notch1 is required for the coordinate segmentation of somites. Development. 1995;121:1533–1545. doi: 10.1242/dev.121.5.1533. [DOI] [PubMed] [Google Scholar]
- Crossley PH, Martin GR. The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development. 1995;121:439–451. doi: 10.1242/dev.121.2.439. [DOI] [PubMed] [Google Scholar]
- Forlani S, Lawson KA, Deschamps J. Acquisition of Hox codes during gastrulation and axial elongation in the mouse embryo. Development. 2003;130:3807–3819. doi: 10.1242/dev.00573. [DOI] [PubMed] [Google Scholar]
- Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- Gottardi CJ, Gumbiner BM. Adhesion signaling: How beta-catenin interacts with its partners. Curr Biol. 2001;11:R792–R794. doi: 10.1016/s0960-9822(01)00473-0. [DOI] [PubMed] [Google Scholar]
- Habara-Ohkubo A. Differentiation of beating cardiac muscle cells from a derivative of P19 embryonal carcinoma cells. Cell Struct Funct. 1996;21:101–110. doi: 10.1247/csf.21.101. [DOI] [PubMed] [Google Scholar]
- Hanna LA, Foreman RK, Tarasenko IA, Kessler DS, Labosky PA. Requirement for Foxd3 in maintaining pluripotent cells of the early mouse embryo. Genes Dev. 2002;16:2650–2661. doi: 10.1101/gad.1020502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Li TG, Qi X, Zhao DF, Zhao GQ. Wnt/βeta-catenin path-way up-regulates Stat3 and converges on LIF to prevent differentiation of mouse embryonic stem cells. Dev Biol. 2006;290:81–91. doi: 10.1016/j.ydbio.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Harrison SM, Houzelstein D, Dunwoodie SL, Beddington RS. Sp5, a new member of the Sp1 family, is dynamically expressed during development and genetically interacts with Brachyury. Dev Biol. 2000;227:358–372. doi: 10.1006/dbio.2000.9878. [DOI] [PubMed] [Google Scholar]
- Jenny A, Mlodzik M. Planar cell polarity signaling: A common mechanism for cellular polarization. Mt Sinai J Med. 2006;73:738–750. [PubMed] [Google Scholar]
- Karner C, Wharton KA, Jr, Carroll TJ. Planar cell polarity and vertebrate organogenesis. Semin Cell Dev Biol. 2006;17:194–203. doi: 10.1016/j.semcdb.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Kaufman MH. The atlas of mouse development (revised edition) Academic Press; San Diego: 1995. [Google Scholar]
- Keller G. Embryonic stem cell differentiation: Emergence of a new era in biology and medicine. Genes Dev. 2005;19:1129–1155. doi: 10.1101/gad.1303605. [DOI] [PubMed] [Google Scholar]
- Keller RE, Danilchik M, Gimlich R, Shih J. The function and mechanism of convergent extension during gastrulation of Xenopus laevis. J Embryol Exp Morphol. 1985;89:185–209. [PubMed] [Google Scholar]
- Kemler R, Hierholzer A, Kanzler B, Kuppig S, Hansen K, Taketo MM, de Vries WN, Knowles BB, Solter D. Stabilization of betacatenin in the mouse zygote leads to premature epithelial-mesenchymal transition in the epiblast. Development. 2004;131:5817–5824. doi: 10.1242/dev.01458. [DOI] [PubMed] [Google Scholar]
- Kim GH, Han JK. JNK and ROKalpha function in the noncanonical Wnt/RhoA signaling pathway to regulate Xenopus convergent extension movements. Dev Dyn. 2005;232:958–968. doi: 10.1002/dvdy.20262. [DOI] [PubMed] [Google Scholar]
- Kispert A, Vainio S, Shen L, Rowitch DH, McMahon AP. Proteoglycans are required for maintenance of Wnt-11 expression in the ureter tips. Development. 1996;122:3627–3637. doi: 10.1242/dev.122.11.3627. [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/-colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- Lehtonen E, Laasonen A, Tienari J. Teratocarcinoma stem cells as a model for differentiation in the mouse embryo. Int J Dev Biol. 1989;33:105–115. [PubMed] [Google Scholar]
- Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A. Requirement for Wnt3 in vertebrate axis formation. Nat Genet. 1999;22:361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, Niehrs C. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- Marikawa Y. Wnt/βeta-catenin signaling and body plan formation in mouse embryos. Semin Cell Dev Biol. 2006;17:175–184. doi: 10.1016/j.semcdb.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Marlow F, Topczewski J, Sepich D, Solnica-Krezel L. Zebrafish Rho kinase 2 acts downstream of Wnt11 to mediate cell polarity and effective convergence and extension movements. Curr Biol. 2002;12:876–884. doi: 10.1016/s0960-9822(02)00864-3. [DOI] [PubMed] [Google Scholar]
- McBurney MW. P19 embryonal carcinoma cells. Int J Dev Biol. 1993;37:135–140. [PubMed] [Google Scholar]
- Nagy A, Gertsenstein M, Vintersten K, Behringer R. Manipulating the mouse embryo: A laboratory manual. 3rd edition Cold Spring Harbor Laboratory Press; Cold Spring Harbor: 2003. [Google Scholar]
- Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Scholer H, Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- Ogawa K, Nishinakamura R, Iwamatsu Y, Shimosato D, Niwa H. Synergistic action of Wnt and LIF in maintaining pluripotency of mouse ES cells. Biochem Biophys Res Commun. 2006;343:159–166. doi: 10.1016/j.bbrc.2006.02.127. [DOI] [PubMed] [Google Scholar]
- Pfister S, Steiner KA, Tam PP. Gene expression pattern and progression of embryogenesis in the immediate post-implantation period of mouse development. Gene Expr Patterns. 2007;7:558–573. doi: 10.1016/j.modgep.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Rashbass P, Cooke LA, Herrmann BG, Beddington RS. A cell autonomous function of Brachyury in T/T embryonic stem cell chimaeras. Nature. 1991;353:348–351. doi: 10.1038/353348a0. [DOI] [PubMed] [Google Scholar]
- Ridgeway AG, Wilton S, Skerjanc IS. Myocyte enhancer factor 2C and myogenin up-regulate each other’s expression and induce the development of skeletal muscle in P19 cells. J Biol Chem. 2000;275:41–46. doi: 10.1074/jbc.275.1.41. [DOI] [PubMed] [Google Scholar]
- Rivera-Perez JA, Magnuson T. Primitive streak formation in mice is preceded by localized activation of Brachyury and Wnt3. Dev Biol. 2005;288:363–371. doi: 10.1016/j.ydbio.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- Schambony A, Wedlich D. Wnt-5A/Ror2 regulate expression of XPAPC through an alternative noncanonical signaling pathway. Dev Cell. 2007;12:779–792. doi: 10.1016/j.devcel.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Scholer HR, Ruppert S, Suzuki N, Chowdhury K, Gruss P. New type of POU domain in germ line-specific protein Oct-4. Nature. 1990;344:435–439. doi: 10.1038/344435a0. [DOI] [PubMed] [Google Scholar]
- Semenov MV, Tamai K, Brott BK, Kuhl M, Sokol S, He X. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol. 2001;11:951–961. doi: 10.1016/s0960-9822(01)00290-1. [DOI] [PubMed] [Google Scholar]
- Sennerstam R, Stromberg JO. A comparative study of the cell cycles of nullipotent and multipotent embryonal carcinoma cell lines during exponential growth. Dev Biol. 1984;103:221–229. doi: 10.1016/0012-1606(84)90023-x. [DOI] [PubMed] [Google Scholar]
- Singla DK, Schneider DJ, LeWinter MM, Sobel BE. wnt3a but not wnt11 supports self-renewal of embryonic stem cells. Biochem Biophys Res Commun. 2006;345:789–795. doi: 10.1016/j.bbrc.2006.04.125. [DOI] [PubMed] [Google Scholar]
- Smith DE, Franco del Amo F, Gridley T. Isolation of Sna, a mouse gene homologous to the Drosophila genes snail and escargot: Its expression pattern suggests multiple roles during postimplantation development. Development. 1992;116:1033–1039. doi: 10.1242/dev.116.4.1033. [DOI] [PubMed] [Google Scholar]
- Smith SC, Reuhl KR, Craig J, McBurney MW. The role of aggregation in embryonal carcinoma cell differentiation. J Cell Physiol. 1987;131:74–84. doi: 10.1002/jcp.1041310112. [DOI] [PubMed] [Google Scholar]
- Solter D. From teratocarcinomas to embryonic stem cells and beyond: A history of embryonic stem cell research. Nat Rev Genet. 2006;7:319–327. doi: 10.1038/nrg1827. [DOI] [PubMed] [Google Scholar]
- Takada S, Stark KL, Shea MJ, Vassileva G, McMahon JA, McMahon AP. Wnt-3a regulates somite and tailbud formation in the mouse embryo. Genes Dev. 1994;8:174–189. doi: 10.1101/gad.8.2.174. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Mitsui K, Yamanaka S. Role of ERas in promoting tumour-like properties in mouse embryonic stem cells. Nature. 2003;423:541–545. doi: 10.1038/nature01646. [DOI] [PubMed] [Google Scholar]
- Takaoka K, Yamamoto M, Hamada H. Origin of body axes in the mouse embryo. Curr Opin Genet Dev. 2007;17:344–350. doi: 10.1016/j.gde.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Tam PP, Loebel DA. Gene function in mouse embryogenesis: Get set for gastrulation. Nat Rev Genet. 2007;8:368–381. doi: 10.1038/nrg2084. [DOI] [PubMed] [Google Scholar]
- Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, Gardner RL, McKay RD. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- van der Heyden MA, Defize LH. Twenty one years of P19 cells: What an embryonal carcinoma cell line taught us about cardiomyocyte differentiation. Cardiovasc Res. 2003;58:292–302. doi: 10.1016/s0008-6363(02)00771-x. [DOI] [PubMed] [Google Scholar]
- van der Heyden MA, van Kempen MJ, Tsuji Y, Rook MB, Jongsma HJ, Opthof T. P19 embryonal carcinoma cells: A suitable model system for cardiac electrophysiological differentiation at the molecular and functional level. Cardiovasc Res. 2003;58:410–422. doi: 10.1016/s0008-6363(03)00247-5. [DOI] [PubMed] [Google Scholar]
- Vidricaire G, Jardine K, McBurney MW. Expression of the Brachyury gene during mesoderm development in differentiating embryonal carcinoma cell cultures. Development. 1994;120:115–122. doi: 10.1242/dev.120.1.115. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Fraser SE, Harland RM. Convergent extension: The molecular control of polarized cell movement during embryonic development. Dev Cell. 2002;2:695–706. doi: 10.1016/s1534-5807(02)00197-1. [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Bradley A, McMahon AP, Jones S. A Wnt5a path-way underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999a;126:1211–1223. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Takada S, Yoshikawa Y, Wu N, McMahon AP. T (Brachyury) is a direct target of Wnt3a during paraxial mesoderm specification. Genes Dev. 1999b;13:3185–3190. doi: 10.1101/gad.13.24.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka H, Moriguchi T, Masuyama N, Kusakabe M, Hanafusa H, Takada R, Takada S, Nishida E. JNK functions in the non-canonical Wnt pathway to regulate convergent extension movements in vertebrates. EMBO Rep. 2002;3:69–75. doi: 10.1093/embo-reports/kvf008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ybot-Gonzalez P, Savery D, Gerrelli D, Signore M, Mitchell CE, Faux CH, Greene ND, Copp AJ. Convergent extension, planar-cellpolarity signalling and initiation of mouse neural tube closure. Development. 2007;134:789–799. doi: 10.1242/dev.000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeom YI, Fuhrmann G, Ovitt CE, Brehm A, Ohbo K, Gross M, Hubner K, Scholer HR. Germline regulatory element of Oct-4 specific for the totipotent cycle of embryonal cells. Development. 1996;122:881–894. doi: 10.1242/dev.122.3.881. [DOI] [PubMed] [Google Scholar]
- Yoshikawa Y, Fujimori T, McMahon AP, Takada S. Evidence that absence of Wnt-3a signaling promotes neuralization instead of paraxial mesoderm development in the mouse. Dev Biol. 1997;183:234–242. doi: 10.1006/dbio.1997.8502. [DOI] [PubMed] [Google Scholar]
- Zakany J, Burg K, Rasko I. Spontaneous differentiation in the colonies of a nullipotent embryonal carcinoma cell line (F9) Differentiation. 1984;27:146–151. doi: 10.1111/j.1432-0436.1984.tb01420.x. [DOI] [PubMed] [Google Scholar]
- Zhang W, Yatskievych TA, Cao X, Antin PB. Regulation of Hex gene expression by a Smads-dependent signaling pathway. J Biol Chem. 2002;47:45435–45441. doi: 10.1074/jbc.M208056200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.