Abstract
Background
Growing evidence suggests platelets are essential in post-traumatic acute lung injury (ALI). Halogenated ethers interfere with platelet-granulocyte aggregate formation. The potential benefit of halogenated ethers has not been investigated in trauma/hemorrhagic shock (T/HS) models. Therefore, we hypothesized that isoflurane decreases T/HS-mediated ALI through platelet inhibition.
Methods
Sprauge-Dawley rats (n=47) were anesthetized by either pentobarbital or inhaled isoflurane, and placed into groups: control, trauma (laparotomy) sham shock, T/HS (MAP of 30 mmHg × 45 min), pre-treatment with an ADP receptor antagonist, or T/HS with isoflurane initiated during resuscitation. ALI was determined by BALF protein and pulmonary immunofluorescence. PlateletMapping™ specifically evaluated thrombin-independent inhibition of the ADP and AA pathways of platelet activation.
Results
Pre-treatment with isoflurane abrogated ALI as measured by both BAL fluid protein and pulmonary immunofluorescence (p<0.001). PlateletMapping™, revealed specific platelet ADP-pathway inhibition with isoflurane (p<0.001). Pre-treatment with an ADP receptor antagonist decreased ALI to sham levels, confirming that specific platelet ADP inhibition decreases ALI. Isoflurane initiated during resuscitation also decreased ALI (p<0.001).
Conclusion
Isoflurane attenuates ALI through an anti-platelet mechanism, in part, through inhibition of the platelet ADP pathway. Isoflurane given post-injury also protects against ALI, and highlights the potential applications of this therapy in various ischemia/reperfusion clinical scenarios.
Keywords: Isoflurane, Trauma and Hemorrhagic Shock, Acute Lung Injury, Microthrombi, Platelets, Adenosine Diphosphate (ADP)
INTRODUCTION
Acute lung injury (ALI), and the more severe form, acute respiratory distress syndrome (ARDS), continue to have high morbidity and mortality in severely injured trauma patients, without any known effective pharmacologic therapy.1,2 Neutrophils are believed to be the principle cellular mediators in ALI/ARDS, but emerging evidence supports a strong coupling of both coagulation and innate immunity in inflammatory processes.3 This relationship is particularly compelling in ALI, since pre-clinical sepsis,4 transfusion-related acute lung injury (TRALI),5 and trauma/hemorrhagic shock (T/HS)6 models demonstrate improved outcomes with platelet inhibition.
Halogenated ethers (i.e. isoflurane, sevoflurane) are known to possess anti-inflammatory properties and have been shown to prevent sepsis-mediated ALI,7 as well as ischemia/reperfusion injury following cardiac bypass (CBP) procedures.8 These volatile gasses are known to prevent neutrophil adhesion to the endothelium,9,10 but the exact mechanism remains unclear. Recently, sub-anesthetic doses of sevoflurane have been shown to inhibit granulocyte-platelet interactions,11 suggesting a possible reagent to uncouple coagulation and the maladaptive innate immunity in ALI/ARDS. Thus, platelets appear to have a role in the pathogenesis of ALI via a response to the initial insult resulting in neutrophil activation and release of inflammatory mediators. Therefore, we hypothesized that isoflurane would attenuate T/HS-mediated ALI through platelet inhibition.
METHODS
Adult male Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN) weighing 350–425 g were housed under barrier-sustained conditions with 12 hr light/dark cycles and allowed free access to food and water for a minimum of one week before use. All animals were maintained in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals, and this study was approved by the University of Colorado Denver Animal Care and Use Committee.
Unless otherwise specified, all reagents were purchased from Sigma-Aldrich Corp. (St. Louis, MO). Platelet function assay equipment and supplies were provided by Haemonetics Corporation (Niles, IL); 0.9% injection grade normal saline (NS) was purchased from Baxter Healthcare (Deerfield, IL); Sodium pentobarbital was purchased from Abbott Labs (Chicago, IL); Isoflurane inhalant was purchased from VET One (Meridian, ID); Polyethylene tubing was acquired from Fisher Scientific (Pittsburgh, PA). Continuous blood pressure measurement was performed using a ProPaq invasive monitoring device (Welch-Allyn, Skaneateles Falls, NY).
Trauma/Hemorrhagic Shock Model
Animals were anesthetized with 50 mg/kg sodium pentobarbital (n=7) or 0.5% continuous inhaled isoflurane (n=7), and were given a subcutaneous injection of 1% lidocaine for local anesthesia. The femoral artery and vein were cannulated with polyethylene (PE-50) tubing for continuous invasive pressure monitoring and to establish venous access. A tracheotomy was performed, at which point the animal was placed on 30% FiO2 using an air-oxygen mixer (Sechrist, Anaheim, CA) at a flow rate of 2 liters/minute. The animal’s body temperature was measured rectally, and kept euthermic with a heating table. After a 45-minute observation period, a laparotomy was performed to simulate trauma, and closed in a two-layer fashion using a 3-0 non-absorbable monofilament suture. Controlled hemorrhage was then induced over a period of 10-minutes through the arterial catheter to maintain a MAP of 30 mmHg for 45 minutes. The shed blood was collected into a conical containing 80 Units/kg heparin. At the end of shock, to simulate clinical practice, animals were resuscitated with twice their shed blood (SB) volume in NS over a 30-minute period, followed by ½ of the SB volume over 30 minutes, and an additional hour of resuscitation with twice the SB volume in NS. The animals were observed for another hour prior to being euthanized with a pentobarbital overdose.
Sham Animals
Trauma/Sham Shock (T/SS) animals were anesthetized with 50 mg/kg sodium pentobarbital (n=7) or 0.5% inhaled isoflurane (n=3), and were given a subcutaneous injection of 1% lidocaine for local anesthesia. The femoral artery and vein were then cannulated. A tracheotomy was performed, and the animal was placed on 30% FiO2 using an air-oxygen mixer at a flow rate of 2 liters/minute. The animal was kept euthermic with a heating table. After a 45-minute observation period, a laparotomy was performed to simulate trauma. No hemorrhagic shock was performed and T/SS animals were observed for 3 hours prior to being euthanized with a pentobarbital overdose.
Control Animals
Control animals were anesthetized with 50 mg/kg sodium pentobarbital (n=7) or 0.5% inhaled isoflurane (n=4). The femoral artery was then cannulated, and the animal was euthanized.
Platelet Inhibition
Animals (n=5) were pre-treated with a 20 mg/kg oral dose of a P2Y12 receptor antagonist (clopidogrel) (Sanofi-Aventis Pharmaceuticals, Bridgewater, NJ) daily for 3 consecutive days prior to undergoing T/HS. Animals were then subjected to the identical T/HS described above with the shed blood collected on 80 Units/kg of heparin.
Isoflurane Resuscitation
Animals (n=7) were anesthetized with 50 mg/kg sodium pentobarbital, and were subjected to the standard T/HS model described above. However, at the start of resuscitation, animals were given 0.5% inhaled isoflurane for the remainder of the resuscitation and observation periods.
Platelet Function Assay
Thrombelestography (TEG) has been used in rodent models to evaluate the individual components of coagulation.12 Therefore, platelet function was determined by utilizing a TEG-derived platelet function assay - PlateletMapping™.13 Briefly, a 900 µl blood sample was collected in a vial containing 100 µl of 0.3% heparin or 100 µl of 4% citrate. Next, 360 µl of the blood sample was placed into the pre-warmed cup of the TEG analyzer containing 10µl of the prepared activator (Reptilase, Factor XIII, and phospholipids). Next, 10µl of either prepared arachidonic acid (AA) or adenosine diphosphate (ADP) was added as a platelet agonist, and AA (MAAA) an ADP (MAADP) tracings were generated. To determine the independent fibrin contribution to the clot, the activator solution was added to 360 µl of heparinized blood (MAFibrin). The maximum hemostatic activity (MAThrombin) was measured using a kaolin activated whole blood sample collected in citrate, rather than heparin. The citrated whole blood sample was inverted gently 5 times and set on its side, undisturbed, for 30 min. Samples were analyzed within 2 hours of blood sample collection. The percent platelet inhibition in response to either the ADP or AA agonist was calculated using the following equation: 100 − [(MAADP/AA − MAFibrin)/ (MAThrombin ™ MAFibrin) × 100].
Lung Vascular Permeability
Following pentobarbital euthanasia, a BAL was performed through the tracheotomy tube. Normal saline (5 ml) was injected into the trachea, aspirated, and collected. This procedure was repeated three times. The return of bronchoalveolar lavage fluid (BALF) was consistently greater than 12 mL. The BAL fluid was frozen, and stored at −80°C until the assay was performed. The Pierce BCA protein assay kit (Thermo Scientific) was used to measure BAL protein, reflecting lung vascular permeability. The assay was performed per the manufacturers instructions, and the absorbance at 562 nm was then measured using a spectrophotometer (Molecular Devices Corp, Sunnyvale, CA) to determine the protein concentration.
Immunofluorescence
At the end of the experiment, the left lung was inflated with 4% paraformaldehyde in PBS and fixed overnight. The lung was then inflated with 1.5 ml Optimal Cutting Temperature (OCT) medium in 4% sucrose, sectioned, and embedded in OCT prior to freezing. Frozen sections (5 µm) were cut, and placed on glass slides. The sections were then washed in phosphate-buffered saline (PBS) pH 7.4, and permeabilized with a 70% acetone/30% methanol solution. The slides were then treated with a 1:400 dilution of Re-Blot™ (Millipore, Billerica, MA) in distilled H2O, followed by three washes in PBS. A 10% normal donkey serum in PBS blocking solution was applied to the sections for 1 hour. The primary antibodies, as well as isotype controls, were applied and incubated overnight at 4°C. The sections were again washed in PBS, and anti-species fluorescent antibodies were applied for 1 hour in a dark room. Three final washings with PBS occurred, and Prolong Plus solution containing DAPI was added prior to the placing of a cover slip.
Fibrin was labeled with a sheep polyclonal anti-fibrinogen antibody (Serotec, Raleigh, NC). Platelets were labeled with a mouse polyclonal anti-integrin αIIb (CD41) antibody (Santa Cruz Biotechnology, Santa Cruz, CA), and neutrophils were labeled with rabbit polyclonal anti-PMN serum (Accurate Chemical, Westbury, NY). As a secondary label for the fibrinogen, a 488-nm (green) labeled donkey anti-sheep IgG was used. A 647-nm (far red) labeled donkey anti-rabbit IgG was used to label neutrophils, and a Cy3 (red) donkey anti-mouse IgG was used to label platelets. Normal sheep IgG (Jackson Immuno Research, West Grove, PA), normal mouse IgG (Jackson Immuno Research), and normal rabbit serum (Jackson Immuno Research) were used as isotype controls.
Statistical Analysis
BAL protein, MPO, and immunofluorescence data are reported as the mean ± standard error of the mean and were compared by analysis of variance using the Bonferroni/Dunn test for post hoc comparisons. The results of the PlateletMapping™ assay are reported as the mean ± standard error of the mean and were compared by a paired student’s t-test. A p-value <0.001 was considered statistically significant.
RESULTS
Trauma/Hemorrhagic Shock
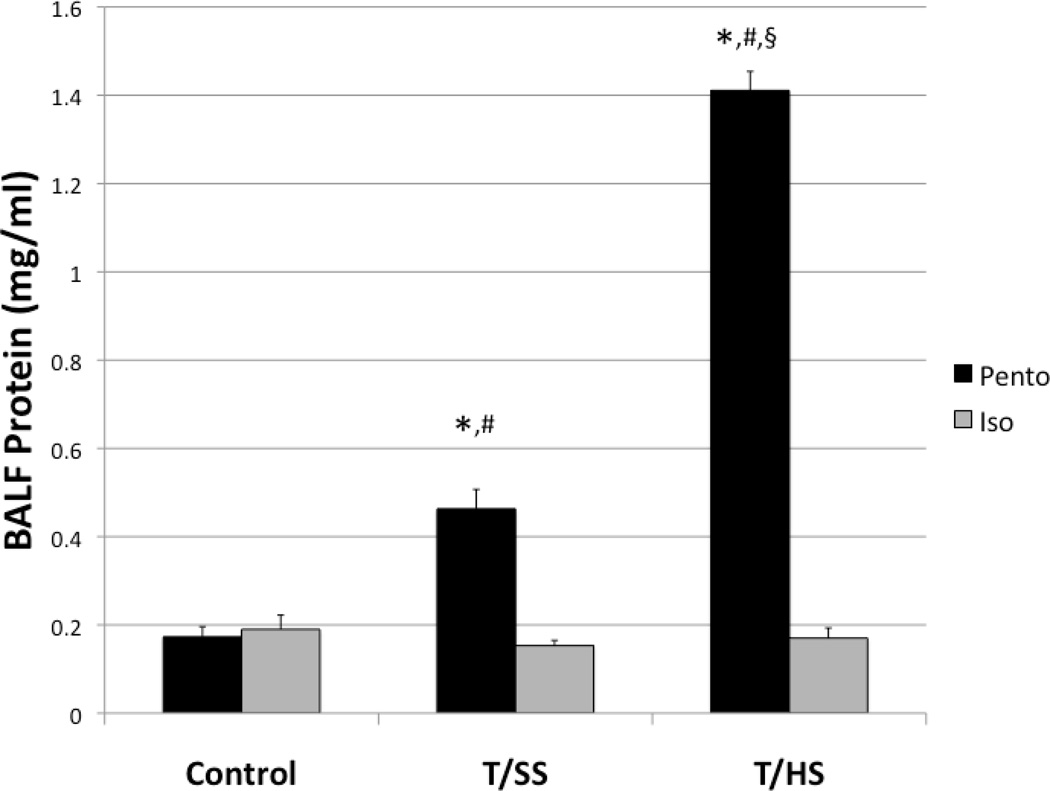
Animals were subjected to control, trauma/sham shock (T/SS), and trauma/hemorrhagic shock (T/HS) arms. In animals anesthetized with pentobarbital, T/HS was sufficient to cause ALI and pulmonary capillary leak as measured by BALF protein compared to both T/SS and control animals (1.41±0.04 vs. 0.46±0.04 and 0.17±0.02) (p<0.001) (Figure 1). T/SS animals also had a modest but significant increase in pulmonary capillary leak compared to controls (p<0.001) (Figure 1). In animals anesthetized with isoflurane, the control group had similar pulmonary capillary leak as the pentobarbital control group (0.19±0.03 vs. 0.17±0.02) (Figure 1). However, the T/HS Iso group had significantly lower pulmonary capillary leak compared to the pentobarbital T/HS group (0.17±0.02 vs. 1.41±0.04), attenuating lung injury to control levels. (Figure 1). Isoflurane also attenuated pulmonary capillary leak in the T/SS group compared to the T/SS Pento group (0.15±0.01 vs 0.46±0.04).
Figure 1.
T/SS modestly increases pulmonary capillary leak, and T/HS greatly increases pulmonary capillary leak in animals anesthetized with pentobarbital. Pre-treatment with isoflurane diminishes pulmonary capillary leak in both the T/SS and T/HS groups to control levels. *=p<0.001 compared to control group. #=p<0.001 compared corresponding isoflurane group. §=p<0.001 compared to T/SS group.
Platelet Function Assay
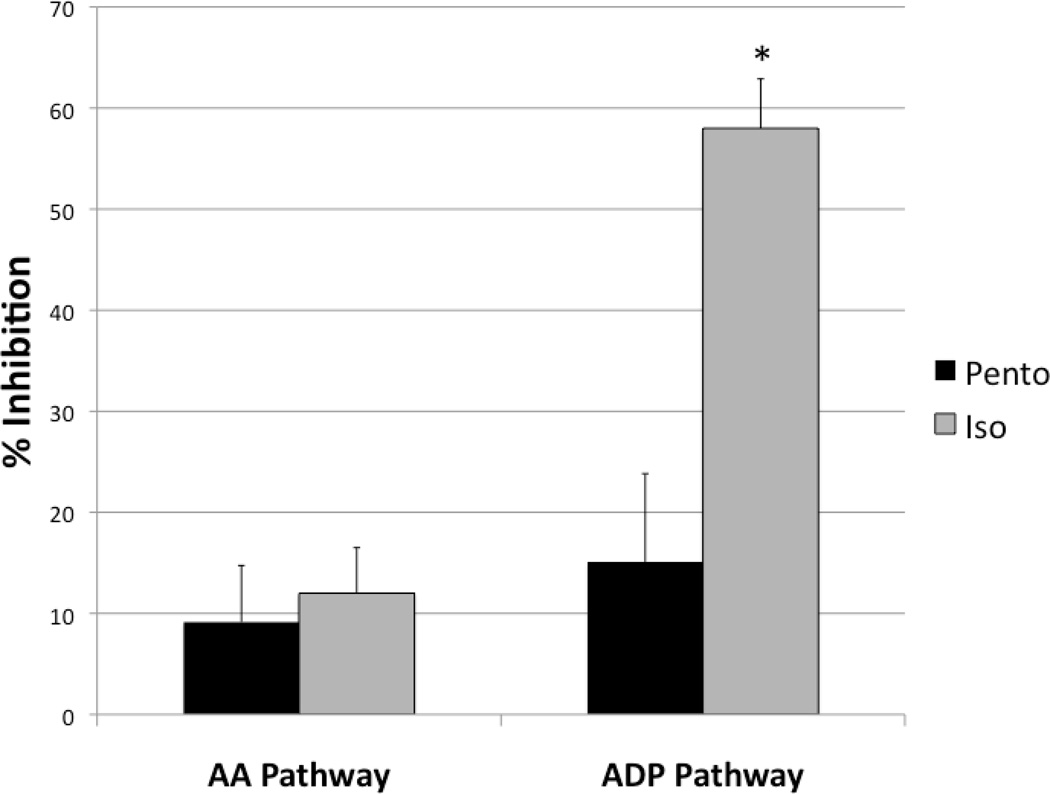
Platelet inhibition was measured from baseline blood draws in animals anesthetized with either pentobarbital or isoflurane. The percent inhibition of platelet activation via Thromboxane A2, a metabolite of arachidonic acid (the AA pathway) between the pentobarbital and isoflurane groups were (9.14±5.60 vs. 12.00±4.50) respectively (Figure 2). The percent inhibition of platelet activation through ADP stimulation of P2Y receptors (the ADP pathway) in the isoflurane group was significantly higher than the pentobarbital group (58.00±4.89 vs. 15.10± 8.73), reflecting specific ADP pathway inhibition (Figure 2).
Figure 2.
PlateletMapping™ results of whole blood taken from animals anesthetized with pentobarbital or isoflurane. The percent inhibition of maximal platelet function as measured by thrombelastography remained similar after platelet stimulation with AA between the pentobarbital and isoflurane groups. However, maximal platelet function was greatly inhibited after stimulation with ADP in the isoflurane group indicating isoflurane specifically inhibits the platelet ADP pathway. *=p<0.001 compared to the Pento ADP group.
Isoflurane Resuscitation
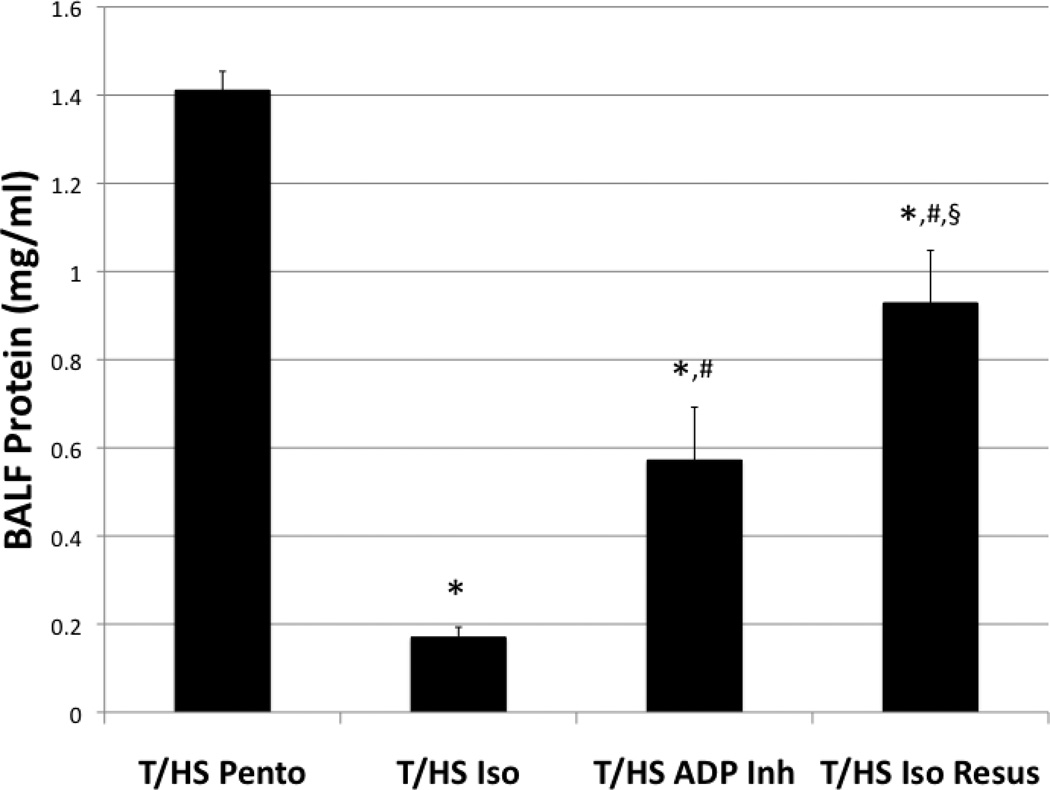
To determine if isoflurane had any effect post-injury, animals were anesthetized with pentobarbital, underwent T/HS, and were given 0.5% isoflurane at the initiation of resuscitation. The T/HS Iso Resus group had a significant decrease in pulmonary capillary leak compared to the T/HS Pento group (0.92±0.11 vs. 1.41±0.04) (Figure 3).
Figure 3.
Pre-treatment with isoflurane significantly reduced pulmonary capillary leak following T/HS. Animals pre-treated with a platelet ADP-receptor antagonist (T/HS ADP Inh) also significantly decreased pulmonary capillary leak indicating the role of platelets in the pathogenesis of ALI. Animals anesthetized with pentobarbital, who also underwent T/HS and were given isoflurane at the initiation of resuscitation (T/HS Iso Resus), had a significant reduction of pulmonary capillary leak suggesting a post-injury clinical application of isoflurane administration. *p<0.001 compared to T/HS Pento group. #=p<0.001 compared to T/HS Iso group. §=p<0.001 compared to T/HS ADP Inh group.
P2Y12 (ADP Receptor) Inhibition
To determine if specific ADP pathway inhibition was responsible for the attenuation of lung injury in the isoflurane-anesthetized group, animals were pre-treated with a P2Y12 (ADP) receptor antagonist (clopidogrel) and anesthetized with pentobarbital. The pulmonary capillary leak in this group (T/HS ADP Inh) was attenuated compared to T/HS (0.57±0.12 vs. 1.41±0.04) (Figure 3). Although pre-treatment with a P2Y12 receptor antagonist reduced pulmonary capillary leak to T/SS levels (0.57±0.12 vs. 0.46±0.04), did not reduce pulmonary capillary leak to control levels as did isoflurane anesthesia.
Pulmonary Immunofluorescence
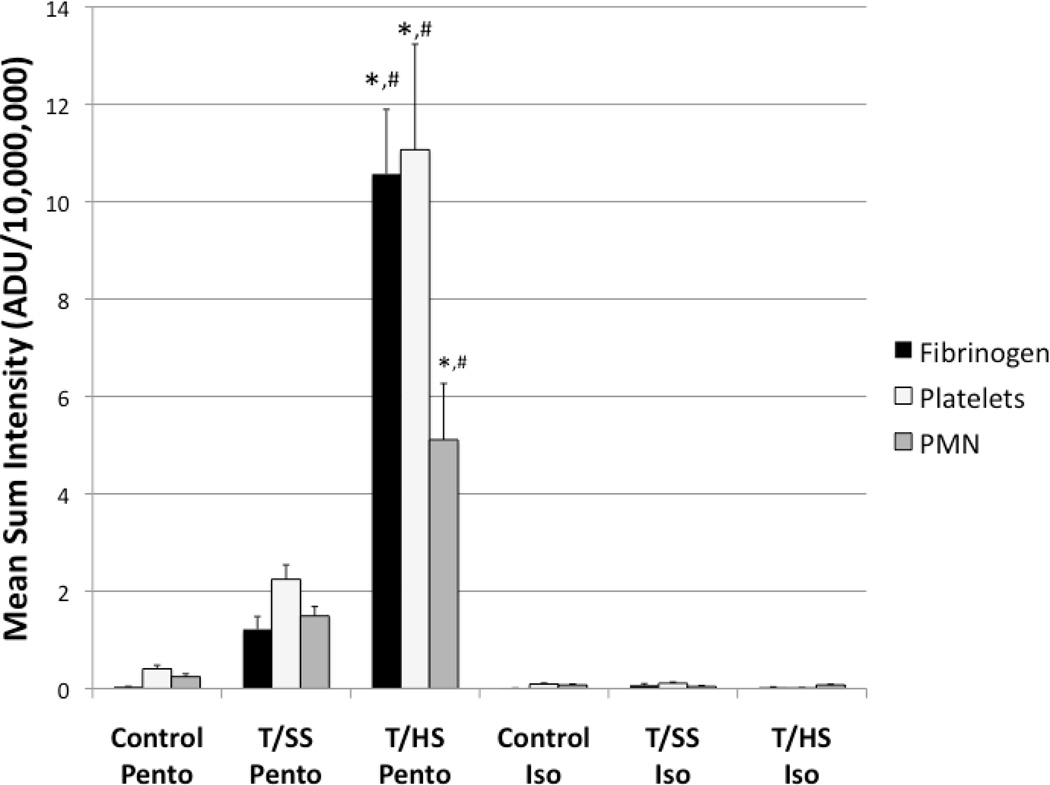
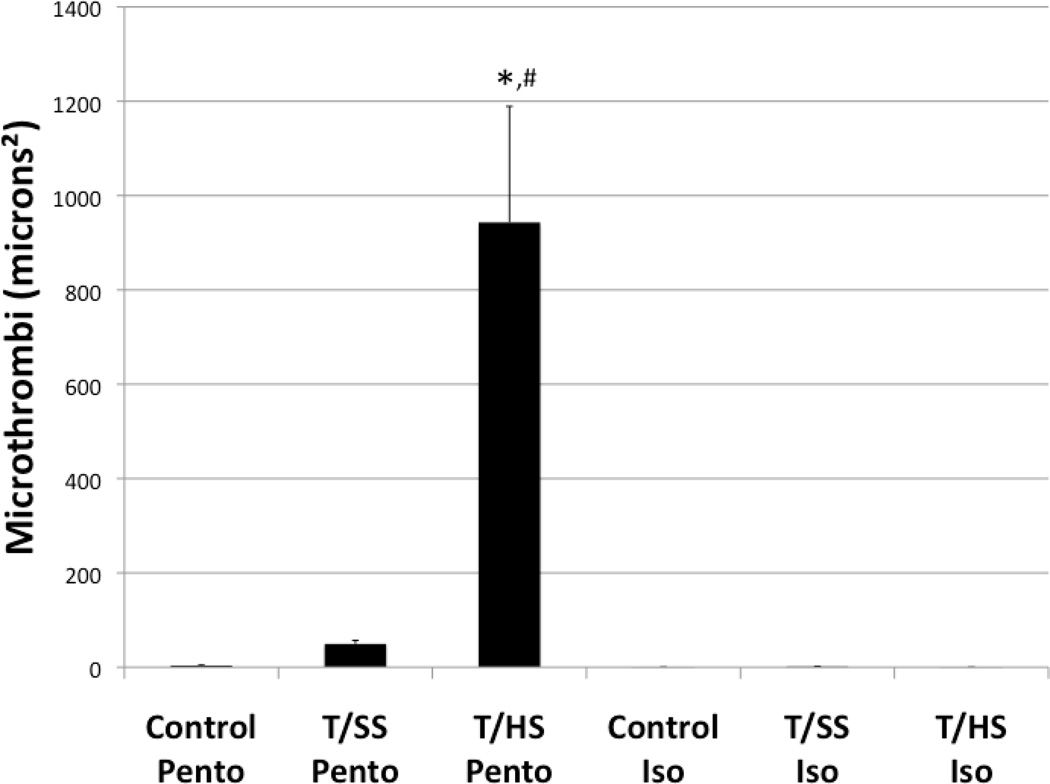
Pulmonary immunofluorescence was employed to confirm lung injury by the presence of microthrombi, and to evaluate neutrophil sequestration in the lung. Microthrombi have been implicated in the development of ALI, and are composed of fibrinogen, platelets, and neutrophils.14–16 The colocalization of these components were examined by immunofluorescence in pulmonary histological sections, and the mean sum intensity of fluorescently labeled fibrinogen, platelets, and neutrophils where measured for each group (Figure 4). The pentobarbital anesthetized T/HS group had the highest immunofluorescence of fibrinogen (10.57×107±1.33×107), platelets (11.15×107±2.17×107), and neutrophils (5.11×107±1.16×107) compared to T/SS Pento and Control Pento groups (p<0.0001). Although there was a modest increase in fibrinogen (1.22×107±0.26×107), platelet (2.24×107±0.30 ×107), and neutrophil (1.49 ×107±0.19 ×107) immunofluorescence in the T/SS Pento group compared to the Control Pento group (0.03×107±0.006×107, 0.4×107±0.08×107, 0.24×107±0.05×107), there was no statistical significance among any of these treatment groups (Figure 4). Microthrombi, colocalization of these components, were significantly increased in the T/HS Pento group (943.30±246.22), compared to the T/SS Pento (49.42±7.47) and Control Pento (3.40±1.05) groups (Figure 5).
Figure 4.
Pulmonary Immunofluorescence of fibrinogen, platelets, and neutrophils. All components are significantly increased in the T/HS Pento group. Pre-treatment with Isoflurane decreased the immunofluorescence of fibrinogen, platelets, and neutrophils to control levels. *=p<0.001 compared to the Control Pento group. #=p<0.001 compared to the T/SS Pento group.
Figure 5.
Pulmonary microthrombi as measured by the colocalization of fibrinogen, platelet, and neutrophil immunofluorescence. The T/HS Pento group had a significant increase in pulmonary microthrombi following T/HS. Pre-treatment with isoflurane prevented microthrombi formation including the group subjected to T/HS. *=p<0.001 compared to Control Pento group. #=p<0.001 compared to T/SS Pento group.
Isoflurane anesthesia decreased fibrinogen, platelet, and neutrophil immunofluorescence in the T/HS Iso group (0.03×107±0.008×107, 0.09×107±0.004×107, 0.07×107±0.01×107) compared to the pentobarbital T/HS group, and had a modest, but not statistically significant, decrease in the T/SS Iso (0.06×107±0.03×107, 0.19×107±0.02×107, 0.04×107±0.08×107) and Control Iso (0.05×107±0.01×107, 0.09×107±0.02×107, 0.07×107±0.02×107) groups compared to the groups anesthetized with pentobarbital (Figure 4). There were minimal microthrombi in the T/HS Iso (0.01±0.01), T/SS Iso (1.14±0.77), and Control Iso (0.08±0.07) groups (Figure 5).
DISCUSSION
These data show that isoflurane anesthesia prevents the development of ALI, in part, through inhibition of the platelet ADP pathway. Our standard T/HS model was shown to induce ALI as evidenced by BALF protein levels, and histologically, by imunofluorescence. However, animals pre-treated with isoflurane prior to T/HS developed no lung injury. Platelet Mapping was employed on whole blood obtained from animals pre-treated with isoflurane to determine isoflurane’s in-vivo effect on platelets. Platelet Mapping confirmed a specific ADP-pathway inhibition, and no change was observed in the AA-pathway. To determine if ADP-pathway inhibition was involved in the mechanism of protection, animals were pre-treated with a platelet ADP-receptor antagonist (clopidogrel) and subjected to T/HS. This resulted in protection from developing ALI, suggesting that isoflurane’s antiplatelet effects are significant in its post-injury protective properties.
Since these studies are performed at intermediate altitude, with a lower partial pressure of oxygen, our model incorporates the return of heparinized shed blood during resuscitation to prevent critical anemia and early mortality. Our prior work has shown that heparinized shed blood contains activated platelet microaggregates, and thus, exacerbates ALI compared to other models which do not use the return of heparinized shed blood.6 In our prior study, thrombelastography-based platelet mapping demonstrated thrombin-independent platelet activation without the addition of AA or ADP. This current study demonstrated the capability of isoflurane to overcome the platelet activation observed in heparinized shed blood, and specifically inhibit platelets through the ADP pathway.
Although most of the protective effects of isoflurane in sepsis17,18 and ischemia/reperfusion8 injuries have been attributed to the neutrophil, the effect on the platelet has been largely overlooked. The emerging role of platelets as a key mediator of ALI has been shown in TRALI, sepsis, and T/HS models, and continues to gain clinical support.4–6 Moreover, there is substantial evidence that a coupling of both the inflammatory and innate immune systems are necessary for the development of ALI.19–21 Thus, platelets may be the principle link, since uncoupling this system through platelet inhibition prevents the neutrophil-mediated injury. Recent evidence has shown that the platelet-endothelium interaction mediated through platelet P-selectin is vital in the pathogenesis of ALI.22 Platelet activation results in platelet P-selectin expression and platelet-leukocyte aggregations through P-selectin glycoprotein ligand-I (PSGL-1) expression on the leukocyte, and this activation may induce a pro-thrombotic state in the microcirculation through the release of leukocyte tissue factor.23 Since pulmonary capillary microthrombi have been implicated in the development of ALI/ARDS,14,15 this phenomenon could explain the microthrombi observed in patients who develop ALI/ARDS. In this animal model, isoflurane eliminated pulmonary microthrombi observed following T/HS.
Halogenated ethers, therefore, with known anti-inflammatory properties, and now with known anti-platelet effects could be a potential therapeutic in the prevention of post-traumatic ALI through this uncoupling effect. Animals pre-treated with an ADP-receptor antagonist had a greater BALF protein leak compared to animals pre-treated with isoflurane. This may be attributed to the dual inhibitory effects of isoflurane on both coagulation and inflammation. Most studies exploring the protective effects of halogenated ethers have involved cardiothoracic surgery patients, since volatile anesthetics have been shown to protect the myocardium against ischemic-reperfusion injury.8 These studies have demonstrated that isoflurane reduces neutrophil adherence to the endothelium,9,10 and attenuates the neutrophil-mediated injury.24,25 In addition, isoflurane reduces the release of tumor necrosis factor-α, and inhibits cytokine-induced death of endothelial and smooth muscle cells.26 These factors, along with anti-platelet properties, make halogenated ethers a potential therapeutic against the development of ALI.
Clinically, since patients cannot be pre-treated with isoflurane prior to their injury, we explored if isoflurane given post-injury could offer protection. Animals were, therefore, given isoflurane at the initiation of resuscitation. Although this did not decrease pulmonary capillary leak to T/SS levels, there was a significant reduction observed, further implicating isoflurane as a potential post-injury therapeutic. Full anesthetic doses of halogenated ethers may not be required to see these effects, since sub-anesthetic doses can decrease platelet-neutrophil interactions in human subjects.11
With the use of any drug in in-vivo models, unintentional effects must be assumed, and controlling for these effects is difficult. Animals not receiving isoflurane received sodium pentobarbital as an anesthetic. We attempted to minimize possible confounding effects by studying both isoflurane and pentobarbital control and T/SS groups. Other limitations of this study include the lack of both dose-response and timing relationships. Our primary purpose was to determine if isoflurane would decrease ALI in a clinically relevant animal model, and to determine if isoflurane had anti-platelet properties supporting other models of lung injury. In fact, increasing the isoflurane may have additional protective effects in the lung, as well as in other organs. On the other hand, further derangement in platelet function may have adverse effects, particularly in the context of uncontrolled cavitary bleeding or intracranial hemorrhage.
In spite of these acknowledged limitations, we have shown that pre-treatment with isoflurane abolishes T/HS-induced ALI, inhibits platelets specifically through the ADP pathway, and that treatment initiated at the start of resuscitation has a significant reduction of pulmonary capillary leak. Due to what is known about the pathogenesis of ALI and the mechanistic effects of halogenated ethers, further investigation is warranted to determine if volatile anesthetics have any clinical therapeutic potential. A large body of clinical evidence supports their use in cardiothoracic surgery, and therefore, these volatile anesthetics may be a viable option in post-injury surgeries or may be given in sub-anesthetic doses by paramedics prior to arrival to the emergency department.
Acknowledgments
This study was supported by the National Institutes of Health (P50 GM049222 and T32 GM008315 grants).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 2.Ciesla DJ, Moore EE, Johnson JL, Burch JM, Cothren CC, Sauaia A. The role of the lung in postinjury multiple organ failure. Surgery. 2005;138:749–758. doi: 10.1016/j.surg.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 3.Massberg S, Grahl L, von Bruehl ML, et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med. 2010;16:887–896. doi: 10.1038/nm.2184. [DOI] [PubMed] [Google Scholar]
- 4.Asaduzzaman M, Lavasani S, Rahman M, et al. Platelets support pulmonary recruitment of neutrophils in abdominal sepsis. Crit Care Med. 2009;37:1389–1396. doi: 10.1097/CCM.0b013e31819ceb71. [DOI] [PubMed] [Google Scholar]
- 5.Looney MR, Nguyen JX, Ju Y, Van Ziffle JA, Lowell CA, Matthay MA. Platelet depletion and aspirin treatment protect mice in a two-event model of transfusion-related acute lung injury. J Clin Invest. 2009;119:3450–3461. doi: 10.1172/JCI38432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harr JN, Moore EE, Wohlauer MV, Fragoso M, Gamboni F, Liang X, Banerjee A, Silliman CC. Activated platelets in heparinized shed blood: the “second-hit“ of acute lung injury in trauma/hemorrhagic shock models. Shock. 2011;36:595–603. doi: 10.1097/SHK.0b013e318231ee76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li QF, Zhu YS, Jiang H, Xu H, Sun Y. Isoflurane preconditioning ameliorates endotoxin-induced acute lung injury and mortality in rats. Anesth Analg. 2009;109:1591–1597. doi: 10.1213/ANE.0b013e3181baf506. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka K, Ludwig LM, Kersten JR, et al. Mechanisms of cardioprotection by volatile anesthetics. Anesthesiology. 2004;100:707–721. doi: 10.1097/00000542-200403000-00035. [DOI] [PubMed] [Google Scholar]
- 9.Hu G, Vinten-Johansen J, Salem MR, Zhao ZQ, Crystal GJ. Isoflurane inhibits neutrophil-endothelium interactions in the coronary circulation: lack of role for adenosine triphosphate-sensitive potassium channels. Anesth Analg. 2002;94:849–856. doi: 10.1097/00000539-200204000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Mobert J, Zahler S, Becker BF, Conzen PF. Inhibition of neutrophil activation by volatile anesthetics decreases adhesion to cultured human endothelial cells. Anesthesiology. 1999;90:1372–1381. doi: 10.1097/00000542-199905000-00022. [DOI] [PubMed] [Google Scholar]
- 11.Wacker J, Lucchinetti E, Jamnicki M, Aguirre J, Harter L, Keel M, Zaugg M. Delayed inhibition of agonist-induced granulocyte-platelet aggregation after low-dose sevoflurane inhalation in humans. Anesth Analg. 2008;106:1749–1758. doi: 10.1213/ane.0b013e318172f9e9. [DOI] [PubMed] [Google Scholar]
- 12.Wohlauer M, Moore EE, Harr JN, Gonzalez E, Fragoso M, Silliman CC. A Standardized Technique for Performing Thrombelastography in Rodents. Shock. 2011;36:524–526. doi: 10.1097/SHK.0b013e31822dc518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Craft RM, Chavez JJ, Bresee SJ, Wortham DC, Cohen E, Carroll RC. A novel modification of the Thrombelastograph assay, isolating platelet function, correlates with optical platelet aggregation. J Lab Clin Med. 2004;143:301–309. doi: 10.1016/j.lab.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Hardaway RM. A brief overview of acute respiratory distress syndrome. World J Surg. 2006;30:1829–1834. doi: 10.1007/s00268-006-0030-8. [DOI] [PubMed] [Google Scholar]
- 15.Conhaim RL, Mangino MJ, Dovi WF, Watson KE, Warner TF, Harms BA. Microthrombus formation may trigger lung injury after acute blood loss. Shock. 2010;34:601–607. doi: 10.1097/SHK.0b013e3181e46e2a. [DOI] [PubMed] [Google Scholar]
- 16.Helset E, Lindal S, Olsen R, Myklebust R, Jorgensen L. Endothelin-1 causes sequential trapping of platelets and neutrophils in pulmonary microcirculation in rats. Am J Physiol. 1996;271:538–546. doi: 10.1152/ajplung.1996.271.4.L538. [DOI] [PubMed] [Google Scholar]
- 17.Perl M, Lomas-Neira J, Venet F, Chung CS, Ayala A. Pathogenesis of indirect (secondary) acute lung injury. Expert Rev Respir Med. 2001;5:115–126. doi: 10.1586/ers.10.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee HT, Emala CW, Joo JD, Kim M. Isoflurane improves survival and protects against renal and hepatic injury in murine septic peritonitis. Shock. 2007;27:373–379. doi: 10.1097/01.shk.0000248595.17130.24. [DOI] [PubMed] [Google Scholar]
- 19.Sebag SC, Bastarache JA, Ware LB. Therapeutic modulation of coagulation and fibrinolysis in acute lung injury and the acute respiratory distress syndrome. Curr Pharm Biotechnol. 2011;12:1481–1496. doi: 10.2174/138920111798281171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gear AR, Camerini D. Platelet chemokines and chemokine receptors: Linking hemostasis, inflammation, and host defense. Microcirculation. 2003;10:335–350. doi: 10.1038/sj.mn.7800198. [DOI] [PubMed] [Google Scholar]
- 21.Weyrich AS, Zimmerman CA. Platelets: signaling cells in the immune continuum. Trends Immunol. 2004;25:489–495. doi: 10.1016/j.it.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Kiefmann R, Heckel K, Schenkat S, Dorger M, Wesierska-Fadek J, Goetz AE. Platelet-endothelial cell interaction in pulmonary micro-circulation: the role of PARS. Thromb Haemost. 2004;91:761–770. doi: 10.1160/TH03-11-0685. [DOI] [PubMed] [Google Scholar]
- 23.Cerletti C, Tamburrelli C, Izzi B, Gianfagna F, de Gaetano G. Platelet-leukocyte interactions in thrombosis. Thromb Res. 2011 doi: 10.1016/j.thromres.2011.10.010. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 24.Hu G, Vasiliauskas T, Salem MR, Rhone DP, Crystal GJ. Neutrophils pretreated with volatile anesthetics lose ability to cause cardiac dysfunction. Anesthesiology. 2003;98:712–718. doi: 10.1097/00000542-200303000-00020. [DOI] [PubMed] [Google Scholar]
- 25.Hu G, Salem MR, Crystal GJ. Isoflurane and sevoflurane precondition against neutrophil-induced contractile dysfunction in isolated rat hearts. Anesthesiology. 2004;100:489–497. doi: 10.1097/00000542-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 26.de Klaver MJ, Manning L, Palmer LA, Rich GF. Isoflurane pretreatment inhibits cytokine-induced cell death in cultrured rat smooth muscle cells and human endothelial cells. Anesthesiology. 2002;97:24–32. doi: 10.1097/00000542-200207000-00005. [DOI] [PubMed] [Google Scholar]