Highlights
► Conditional mouse models illuminated the role of hepatic GH-STAT5 and GC-GR signaling in liver function. ► We provide an overview of overlapping and distinct functions of hepatic GH-STAT5 and GC-GR signaling in growth/metabolism. ► Impaired hepatic GH-STAT5 signaling sensitizes hepatocytes to injury and tumorigenic transformation. ► Loss of hepatic GR function causes chronic stress, thereby aggravating liver cancer formation upon impaired STAT5 function.
Keywords: STAT5, Non-alcoholic fatty liver disease, Glucose metabolism, Glucocorticoids, Growth hormone
Abstract
Growth hormone (GH) and glucocorticoids (GCs) are involved in the control of processes that are essential for the maintenance of vital body functions including energy supply and growth control. GH and GCs have been well characterized to regulate systemic energy homeostasis, particular during certain conditions of physical stress. However, dysfunctional signaling in both pathways is linked to various metabolic disorders associated with aberrant carbohydrate and lipid metabolism. In liver, GH-dependent activation of the transcription factor signal transducer and activator of transcription (STAT) 5 controls a variety of physiologic functions within hepatocytes. Similarly, GCs, through activation of the glucocorticoid receptor (GR), influence many important liver functions such as gluconeogenesis. Studies in hepatic Stat5 or GR knockout mice have revealed that they similarly control liver function on their target gene level and indeed, the GR functions often as a cofactor of STAT5 for GH-induced genes. Gene sets, which require physical STAT5–GR interaction, include those controlling body growth and maturation. More recently, it has become evident that impairment of GH-STAT5 signaling in different experimental models correlates with metabolic liver disease, ranging from hepatic steatosis to hepatocellular carcinoma (HCC). While GH-activated STAT5 has a protective role in chronic liver disease, experimental disruption of GC-GR signaling rather seems to ameliorate metabolic disorders under metabolic challenge. In this review, we focus on the current knowledge about hepatic GH-STAT5 and GC-GR signaling in body growth, metabolism, and protection from fatty liver disease and HCC development.
1. Introduction
The liver plays an important role in whole-body metabolism and energy homeostasis. Depending on the body’s needs, hepatocytes coordinate these processes by regulating gene expression programs in response to various humoral signals. Imbalances in this control system are associated with a variety of liver pathologies ranging from hepatic steatosis to end-stage liver disease including hepatocellular carcinoma (HCC) (Feldstein, 2010; Bechmann et al., 2011).
Growth hormone (GH) and glucocorticoids (GCs) are necessary for normal growth and development, as well as immune functions (Sapolsky et al., 2000; Jeay et al., 2002). Further, both factors are important regulators of whole body energy homeostasis (Vegiopoulos and Herzig, 2007; Moller and Jorgensen, 2009; Vijayakumar et al., 2010). At the cellular level, GH action is mediated amongst others by the signal transducer and activator of transcription (STAT) 5 pathway. Through STAT5, GH controls many features of liver physiology, including regulation of genes associated with somatic growth and maturation (Hennighausen and Robinson, 2008). GCs exert their functions through cell-specific action of the glucocorticoid receptor (GR). The GR acts as a transcriptional regulator of distinct target genes via direct DNA binding or through protein–protein interactions with other transcription factors (Kassel and Herrlich, 2007).
Whole-body STAT5- or GR-knockout mice are not viable, demonstrating the importance of both transcription factors for development and survival (Cole et al., 1995; Cui et al., 2004). Therefore, conditional knockouts were key to explore the functions of both transcription factors in liver physiology. Hepatic deficiency of STAT5 or the GR results in a comparable retardation of postnatal body growth (Tronche et al., 2004; Engblom et al., 2007). From these studies, it became evident that transcription of distinct STAT5 target-gene subsets requires binding of the GR onto the STAT5B N-terminus, the major STAT5 isoform expressed in the liver. This interaction preferentially affects gene sets involved in somatic growth and maturation (Engblom et al., 2007). Yet, although both transcription factors are important for the maintenance of energy homeostasis, the phenotypes obtained upon conditional deletion of either gene in mice with regard to lipid and glucose metabolism are rather distinct. Interference with hepatic GC-GR signaling is associated with defects in gluconeogenesis leading to fasting hypoglycemia, with no effects on hepatic lipid homeostasis under basal conditions (Opherk et al., 2004; Mueller et al., 2011). In contrast, impairment of hepatic GH-STAT5 signaling is associated with aberrant glucose metabolism, fatty liver disease and it sensitizes hepatocytes to injury and tumorigenic transformation (Cui et al., 2007; Fan et al., 2009; Hosui et al., 2009; Mueller et al., 2011; Friedbichler et al., 2012).
In the following, we will summarize the consequences of genetic GR and/or Stat5 deletion for body growth, metabolic homeostasis, hepatic steatosis and HCC development.
2. Mechanism of GH-STAT5 and GC-GR action
2.1. GH signaling and STAT5
GH is an important regulator of postnatal body growth. In contrast to a nuclear hormone receptor steroid ligand it is a peptide and belongs to the superfamily of cytokines. GH controls regeneration and cellular reproduction. In addition, GH exerts important functions in energy metabolism (Moller and Jorgensen, 2009; Vijayakumar et al., 2010) and influences the immune system (Jeay et al., 2002). GH is part of the somatotropic axis and it is synthesized and secreted by the anterior pituitary gland. Its secretion is under strict hormonal control. Hypothalamic growth hormone-releasing hormone (GHRH) is the central stimulator of GH synthesis, while hypothalamic somatostatin exerts strong inhibitory effects (Schneider et al., 2003). Other factors stimulating GH release are acute stress and energy deprivation (Moller and Jorgensen, 2009), whereas overnutrition and obesity inhibit its secretion (Scacchi et al., 1999; Flores-Morales et al., 2006).
At the cellular level, GH action is mediated via the GH receptor (GHR), which is widely expressed in many tissues such as liver, muscle and adipose tissue. GH signaling is very similar both from receptor binding and signal transduction to prolactin, erythropoietin and thrombopoietin signaling. These four cytokine signaling receptors all make homodimers upon cytokine binding, and their main tyrosine kinase responsible for signal transduction is the cytoplasmic Janus kinase 2 (JAK2). GHR binding leads to the activation of multiple signaling pathways, including the RAS/RAF/ERK, the PI3K and the JAK/STAT pathways (Lanning and Carter-Su, 2006; Waters et al., 2006). Although STAT1 and STAT3 can be activated through GHR signaling, STAT5 activation is the major target (Zhu et al., 2001) (Fig. 1). Yet, in the absence of STAT5 expression, increased STAT1 and STAT3 activation was reported (Cui et al., 2007; Mueller et al., 2011). STAT5 consists of two different but highly homologous isoforms STAT5A and STAT5B (referred to as STAT5), which are encoded by two juxtaposed genes on mouse chromosome 11 and human chromosome 17. Both STAT5 isoforms differ in their tissue distribution (Hennighausen and Robinson, 2008). Activated GHR brings two JAK2 molecules into proximity which causes auto activation, and subsequent tyrosine phosphorylation of predimerized STAT5 molecules. Subsequently, activated STAT5 translocates to the nucleus, where it binds specific DNA binding response elements (REs), usually an inverted repeat of TTCN3GAA, to modulate target gene transcription (Zhu et al., 2001; Kornfeld et al., 2008). STAT5 was also shown to be an efficient chromatin regulator and it can induce loop formation through oligomerization via the N-terminus (Moriggl et al., 2005; Kornfeld et al., 2008). Whether hepatic STAT5B participates in oligomerization is controversial, yet, multiple STAT5 REs in target genes such as in Socs2 and Igf-1 suggests that possibility (Laz et al., 2009). Amongst other functions, the classical STAT5 target genes Igf1 and Socs2 serve to down-regulate GH signaling, thereby establishing a negative feedback loop (Flores-Morales et al., 2006; Cui et al., 2007).
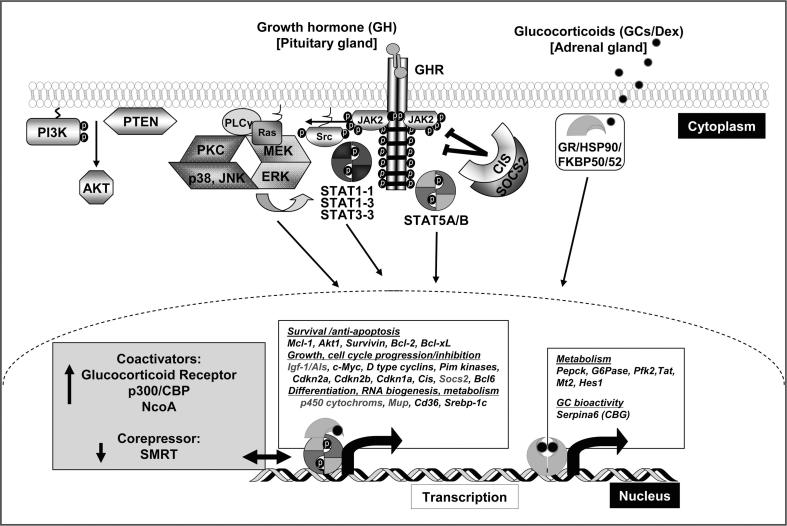
Fig. 1.
Signal transduction pathways induced by GH and GCs. GH binding to a GHR dimer induces a conformational change that activates two JAK2 molecules which results in phosphorylation of multiple tyrosine residues in the cytoplasmic domain of the GHR. The activated GHR-JAK2 complex facilitates activation of STAT5 proteins by tyrosine phosphorylation. Activation of STAT5 allows GH to elicit diverse biological and physiological effects. STAT5 targets include anti-apoptotic genes and genes that promote cell cycle progression as well as inhibition. STAT5 proteins also regulate expression of genes involved in growth, differentiation, RNA biogenesis and metabolism. In addition, GH-activated JAK2 activates multiple signaling proteins and pathways including STAT1/STAT3, MAPK and PI3K signaling. The binding of GH to GHR may also activate Src tyrosine kinase, initiating other signaling pathways. The GR is retained in the cytosol as part of a chaperone-containing multiprotein complex. GCs can diffuse freely across the plasma membrane. Upon ligand binding, GR dissociates from its chaperoning complex and translocates into the nucleus, where it exerts transcriptional effects via direct DNA binding at GREs or through a GRE-independent distinct protein–protein interaction mechanism. GR target genes include amongst others rate limiting enzymes of gluconeogenesis. Additionally, the GR regulates GH-STAT5-dependent transcription of gene sets involved in postnatal body growth and maturation in a cofactor-dependent manner (displayed in gray). GH, Growth hormone; GCs, glucocorticoids; GHR, GH receptor; JAK2, janus kinase 2; STAT, signal transducer and activator of transcription; SOCS2, suppressor of cytokine signaling 2; CIS, Cytokine Inducible SH2 containing protein; MAPK, mitogen-activated protein kinase; GR, glucocorticoid receptor; GRE, glucocorticoid responsive elements; HSP90, heat-shock protein 90; Src, sarcoma kinase; MEK, mitogen-activated protein kinase kinase; PLCγ, phosphoinositide-specific phospholipase γ; JNK, c-Jun N-terminal kinase; ERK, extracellular signal-regulated kinases; PKC, protein kinase C; PI3K, phosphoinositide 3-kinase; AKT, PTEN, phosphatase and tensin homolog.
2.2. GC signaling and GR
Main biological functions of the GC-GR pathway include the suppression of inflammation (Webster et al., 2002) and the control of energy metabolism in metabolically active organs (Vegiopoulos and Herzig, 2007). Secretion of GCs by the adrenal cortex is under control of the hypothalamic–pituitary–adrenal (HPA) axis, a neuroendocrine feedback system. Activation of the HPA axis is influenced by various stressors, such as energy deprivation, by the circadian clock and inflammation (Tsigos and Chrousos, 2002; Buckley and Schatzberg, 2005; Lamia et al., 2011). The subsequent release of hypothalamic corticotropin releasing hormone (CRH) stimulates synthesis and secretion of pituitary adrenocorticotropic hormone (ACTH) and the following ACTH-induced stimulation of adrenal GC synthesis. GCs, in turn, control the regulation of basal HPA axis activity, thereby establishing a regulatory feedback loop.
Cellular action of GCs is attributed to their binding to intracellular GR, a member of the nuclear hormone receptor family (Kassel and Herrlich, 2007; Beck et al., 2011). The inactive GR is retained in the cytoplasm complexed with chaperones (e.g. Heat Shock Protein-90 and Heat Shock Protein-70) until binding to its ligand. After ligand binding, GR dissociates from the multi-protein complex and either interferes with signal transduction components in the cytoplasm or translocates to the nucleus (Rauch et al., 2010). In the nucleus the GR acts as a transcriptional regulator of distinct GC-responsive target genes via direct DNA binding at glucocorticoid response elements (GREs) as a dimer. Alternatively, GR can also modulate the expression of genes through a GRE-independent mechanism, which is mediated in part through protein–protein interactions with other transcription factors or coactivators (Fig. 1) (Kassel and Herrlich, 2007; Beck et al., 2011). The degree to which extent the GR dimerization or the monomeric activity contributes to physiological effects of GCs varies dependent on the type of process studied (Rauch et al., 2010; Baschant et al., 2011; Kleiman et al., 2011).
2.3. STAT5 and GR synergism in hepatocytes
DNA binding of the GR is believed to mediate most of its activating function, while cross-talk with other transcription factors is thought to mediate the repressive actions (Reichardt et al., 1998, 2001). One of the exceptions, in which GR activates transcription without classic DNA binding, is its functional interaction with STAT5. This synergism was first shown for STAT5-dependent transcription of the β-casein gene, which requires the GR as a transcriptional coactivator (Stoecklin et al., 1996, 1997). It became evident, that activated STAT5 and GR form complexes which bind DNA and regulate gene expression independently of GREs. The idea that the GR interacts with DNA-bound STAT5 as a cofactor was supported by the finding that GR mutants deficient for dimerization and DNA binding (GRdim) still synergize with STAT5 (Stoecklin et al., 1997). More recently, it was shown that protein–protein interaction of hepatic STAT5 and GR is essential for many of the functions exerted by either transcription factor in vivo (Engblom et al., 2007). Many functions of the GR depend on its cofactor interaction as revealed through the study of mice with a knock-in of the DBD-defective GRdim, which display relatively normal GH target gene expression pattern in liver (Reichardt et al., 1998; Tronche et al., 2004). Whole genome expression analysis of STAT5, GR and STAT5/GR knockout livers revealed that genes positively regulated by either transcription factor overlap to a large extent (Engblom et al., 2007). More than 40% of genes significantly downregulated in GR-deficient livers were also downregulated in absence of STAT5. Correspondingly, almost 30% of genes downregulated in STAT5-deficient livers were also downregulated in the absence of GR. The magnitudes of expression changes highly correlated between all three genotypes and it became evident that STAT5–GR synergism preferentially affects gene sets involved in growth and maturation. Noteworthy, the expression changes elicited by STAT5 and the GR correlate closely with those found for various truncations of the GHR (Rowland et al., 2005). Moreover, observed changes for male-predominant genes were in line with earlier reports (Clodfelter et al., 2006) which reported maturation-related sexual dimorphic liver gene expression to be dependent on GH action and hepatic STAT5. Further, whole genome expression analysis of livers from mice expressing hypomorphic N-terminally truncated STAT5A and STAT5B proteins (referred to as STAT5ΔN mice) suggests that many of GH target genes depend on an intact STAT5N-terminus (Engblom et al., 2007). Indeed, in accordance with earlier studies STAT5–GR synergism in hepatocytes was found to be independent of DNA-bound GR, but highly dependent on functional STAT5–GR protein–protein interaction (Figs. 1 and 2A). This interaction requires the STAT5N-terminus and the AF-1 domain of the GR as the protein-binding interfaces (Stoecklin et al., 1997; Engblom et al., 2007). To date, there is little information whether other cell types or other cytokines and growth factors which signal through STAT5 display a similar STAT5–GR cofactor interaction and synergistic gene transcription apart from GH signaling. Future research is required to elucidate if STAT5–GR interaction is a selective requirement for the activation of specific gene sets in hepatocytes or if that concept is of general importance for other cell types.
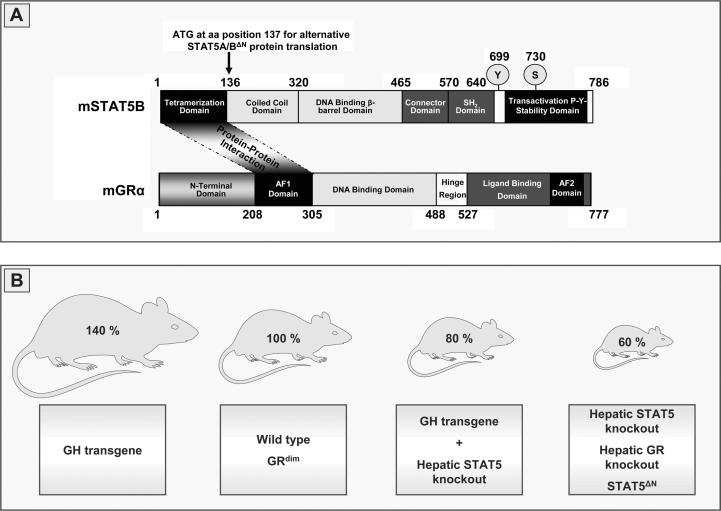
Fig. 2.
STAT5 and GR protein–protein interaction in postnatal body growth. (A) Structural properties of murine STAT5B and murine GR. The STAT5N-terminal domain (tetramerization domain) and the AF1 domain of the GR are the protein-binding interfaces for the GR as a cofactor on DNA bound STAT5. (B) Schematic illustration of postnatal body growth in GH transgenic mice, mice harboring a hepatic deletion of STAT5 in presence of the GH transgene, STAT5ΔN mice, GRdim mice and mice with a hepatocyte-specific deletion of STAT5 or GR compared to wild type mice. STAT5, signal transducer and activator of transcription 5; GR, glucocorticoid receptor; SH2, Src homology 2; AF-1, hormone-independent transactivation function domain; AF-2, transactivation domain; GH, growth hormone.
3. STAT5 and GR function in body growth
As evidenced by patients with abnormally low circulating GH levels or mutations in core genes of the GH pathway disrupting GH-signaling (Laron’s syndrome) (Laron et al., 1965; Rosenfeld et al., 2007; Brooks and Waters, 2010), a major function of GH is regulation of longitudinal body growth as well as of all internal organs excluding the brain. Here, mutations have been identified in core genes of GH signaling, which affect the GHR itself, STAT5B, as well as IGF-1 (Rosenfeld et al., 2007; Brooks and Waters, 2010). As verified by mouse knockout models, the regulation of body size by GH is mainly executed by the activation of STAT5B which, in turn, mediates the transcription of Igf-1 and acid labile subunit (Als) (Ooi et al., 1998; Woelfle et al., 2003a,b). IGF-1 and ALS form a trimeric complex together with IGF-1 binding protein 3, termed bioactive IGF-1, in the serum (Dai and Baxter, 1994; Jones and Clemmons, 1995) to promote cellular growth and to control neuroendocrine functions. The first indication that STAT5B is an essential mediator of postnatal body growth came from mice which either lack the Stat5a or Stat5b gene. STAT5B-deficient males but not females suffer from impaired growth (Udy et al., 1997), while STAT5A-deficient mice exhibit normal body stature (Liu et al., 1997). The expression of N-terminally truncated STAT5A and STAT5B proteins in STAT5ΔN mice affects postnatal body growth in either sex. However, STAT5ΔN females displayed more pronounced dwarfism (Teglund et al., 1998). To evaluate the impact of hepatic GH-STAT5 signaling and liver-derived IGF-1 on body growth, hepatocyte-specific knockout mice targeting the core components of GHR-STAT5 signaling, the GHR (Fan et al., 2009), JAK2 (Sos et al., 2011) and STAT5 (Cui et al., 2007; Engblom et al., 2007; Friedbichler et al., 2012) were generated. All mice deficient in hepatic GHR-JAK2-STAT5 signaling are GH insensitive, with blunted hepatic Igf-1 mRNA expression and severely reduced bioactive IGF-1 resulting in elevated plasma GH. Interestingly, despite comparably reduced levels of circulating IGF-1, the phenotypes of the different mouse lines are quite diverse in regard to postnatal body growth. Liver-specific deletion of STAT5 (Cui et al., 2007) or the GHR (Fan et al., 2009) using a Cre recombinase under the control of the albumin gene promoter (Alb-Cre (Yakar et al., 1999)) did not lead to a reduction in body growth. Yet, deletion of STAT5 mediated by a hepatocyte-specific Cre recombinase under the albumin gene promoter and the α-fetoprotein gene enhancer (Alfp-Cre (Kellendonk et al., 2000)) results in stunted body growth (Engblom et al., 2007), while Alb-Cre-mediated deletion of JAK2 causes a modest but significant decrease in body weight and length (Sos et al., 2011). The observed variations in growth phenotypes remains poorly understood and may partly reflect differences in gene deletion efficiency and mouse genetic backgrounds.
A recent study by our group using an Alfp-Cre-mediated STAT5 knockout in the settings of systemic GH overexpression provided an additional hint that STAT5 signaling in liver is essential for GH-stimulated body growth (Friedbichler et al., 2012). Overexpression of GH in mice leads to an alteration of body proportions resulting in an acromegaly-like phenotype and differential enlargement of internal organs (Eisen et al., 1998; Iida et al., 2004). In contrast, up to 9 weeks of age mice overexpressing GH but lacking hepatic STAT5 display body growth identical to that of wild type (wt) littermates. Thereafter, STAT5 deficiency in liver, despite the presence of GH overexpression, results in growth retardation similar to that caused by STAT5 deficiency alone (Fig. 2). Noteworthy, despite the obvious reduction in body size, the bone length of animals systemically overexpressing GH yet lacking STAT5 in hepatocytes is 5% above that of wt littermates (Friedbichler et al., 2012). These observations indicate that a direct action of GH on bone and muscle and a paracrine function of IGF-1 (Yakar et al., 1999; Klover and Hennighausen, 2007) are not fully sufficient to compensate for the loss of hepatic GH-STAT5 signaling. Of note, liver-specific ablation of the GR leads to similar growth retardations as observed for STAT5-deficient mice while combined mutations do not add significantly to the growth impairment caused by single mutations (Tronche et al., 2004; Engblom et al., 2007). Accordingly, the growth retardation of STAT5ΔN mice is in line with a defect in GR cofactor recruitment, since the N-terminus of STAT5 needs to physically recruit the GR for protein–protein interaction and gene regulation (Fig. 2) (Engblom et al., 2007).
4. GH-STAT5 and GR function in metabolism and hepatic steatosis
The liver plays a central role in the control of glucose and lipid metabolism as it is the major site for interconversion, distribution and storage of energy metabolites. Imbalances in hepatic metabolic function are tightly linked to non-alcoholic fatty liver disease (NAFLD) and associated metabolic disorders (Feldstein, 2010; Bechmann et al., 2011). At this, GH and GC action is not only essential for normal development and survival; it is also required for maintenance of the body’s overall metabolic homeostasis (Vegiopoulos and Herzig, 2007; Moller and Jorgensen, 2009). Further, whole genome expression analysis of gene networks affected by GH and GC signaling in liver has shown that STAT5 and GR control many aspects of hepatocyte metabolism (Phuc Le et al., 2005; Cui et al., 2007; Engblom et al., 2007; Schirra et al., 2008). In the following paragraphs we discuss the current insights into the regulation of glucose and lipid metabolism by GH-STAT5 and GC-GR signaling. In particular, we highlight those insights gained from hepatocyte-specific deletions of both transcription factors in mice.
4.1. Glucose metabolism
The liver is the main site for glucose storage in form of glycogen and glucose synthesis via gluconeogenesis. The liver provides this energy metabolite to maintain blood glucose level in times of need and to provide glucose to extrahepatic tissues such as the brain, which uses approximately 25% of total body glucose. Hepatic GC-GR signaling plays a critical role in maintaining blood glucose level, particularly in states of energy deprivation, by directly controlling rate limiting enzymes of gluconeogenesis such as PEPCK and G6Pase (Hanson and Reshef, 1997; van Schaftingen and Gerin, 2002; Opherk et al., 2004). In turn, GC signaling impairs glucose uptake in peripheral tissues, such as skeletal muscle and adipose tissues. Hence, GCs oppose insulin action, which suppresses glucose production by inhibiting hepatic glycogenolysis and gluconeogenesis, and stimulates glucose uptake, storage, and utilization by other tissues (Andrews and Walker, 1999). Thus, it is not surprising that pathologic conditions of elevated GC levels are linked to defects in glucose metabolism characterized by insulin resistance and hyperglycemia (Andrews and Walker, 1999; Vegiopoulos and Herzig, 2007). In contrary, mice which are GR-deficient specifically in liver show a mild decrease in blood glucose level (Mueller et al., 2011) and suffer from profound hypoglycemia after prolonged fasting (Opherk et al., 2004). The inability to perform de novo glucose synthesis is associated with a reduced induction of rate limiting enzymes of gluconeogenesis such as PEPCK (Opherk et al., 2004). Plasma GC levels were found to be markedly increased in response to fasting (Opherk et al., 2004), while we found an elevation of ACTH and GC levels in these mice already under basal conditions (Mueller et al., 2011). This elevation of systemic GCs in mutant animals might reflect the body’s attempt to sustain gluconeogenesis upon GR deficiency. Interestingly, a similar compensatory hypercortisolism is also reported in mice deficient for hexose-6-phosphate dehydrogenase. These mice lack 11β-hydroxysteroid dehydrogenase type 1 reductase activity which leads to decreased intracellular levels of corticosterone and subsequent defects in glucose metabolism as well as impaired responses of hepatic enzymes to fasting (Rogoff et al., 2007). A second possible cause for the compensatory activation of the HPA axis is an increased expression and release of liver-derived corticosteroid binding globulin (CBG), which binds the majority of GCs in the circulation to retain them in a biologically inactive form (Rosner, 1990). GCs suppress CBG expression, hence, GR knockout mice show increased hepatic CBG expression (Mueller et al., 2011) and display high basal CBG levels that are not suppressed by the synthetic glucocorticoid Dexamethasone (Cole et al., 1999). The increase in CBG and a decrease in free GCs might play a role in elevated HPA activity due to decreased GC bioavailability, as observed in studies with gonadectomized rats and in a porcine model (Ousova et al., 2004; Viau and Meaney, 2004).
Further, in response to hepatic GR deficiency and the concomitant impairment of the liver to counteract low energy levels by gluconeogenesis, a compensatory increase in glucagon (Opherk et al., 2004) and a parallel decrease in insulin were observed (Opherk et al., 2004; Mueller et al., 2011). Elevated hepatic gluconeogenesis is a major contributor to hyperglycemia in type 2 diabetes. Here, the states of insulin resistance or deficiency favor glucose synthesis by increased GC levels. Indeed, enhanced GR-dependent activation of Pepck expression is reported in some murine models of obesity and diabetes, which can be limited by GR antagonism (Liu et al., 2006, 2008). In addition, different approaches using antisense oligonucleotide-mediated downregulation of GR expression show that this improves fasting hyperglycemia and systemic glucose homeostasis in diabetic mice without affecting blood GC levels (Liang et al., 2005; Watts et al., 2005). Systemic GR antagonism and the antisense oligonucleotide approach does not allow to determine inhibition of hepatic GC-GR signaling on diabetes related hyperglycemia, as GR signaling in other organs and concomitant inhibition of glucose uptake, is most likely also impaired. An indication for hepatic GR as a critical inducer of diabetic hyperglycemia came from streptozotocin-induced diabetes in hepatocyte-specific GR knockout mice, which display less severe hyperglycemia and lack hepatic Pepck mRNA expression upon diabetic challenge (Opherk et al., 2004).
GH has both chronic and acute effects on glucose metabolism. The latter are designated as temporary insulin-like effects, and their physiological significance is not clear. The well studied chronic effects of GH oppose, like those of GCs, insulin action on glucose metabolism (Davidson, 1987). Excess GH is associated with impaired glucose tolerance, compensatory hyperinsulinemia, insulin resistance and fasting hyperglycemia. GH deficiency, on the other hand, is linked to enhanced insulin sensitivity, decreased fasting glucose levels, decreased insulin secretion, and lowered hepatic glucose production (Gorin et al., 1990; Dominici and Turyn, 2002; Moller and Jorgensen, 2009). Upon systemic GHR deficiency, as found in Ghr-null mice, GH is secreted in large quantities, but it lacks biological effects due to deficiency of its receptor (Zhou et al., 1997). In contrast, Ames dwarf mice (Prop1df/df) suffer from primary pituitary deficiency and lack GH secretion from the anterior pituitary (Sornson et al., 1996). However, both Ghr-null and Ames dwarf mice exhibit a state of hypersensitivity to insulin and an increased hypoglycemic response to exogenous insulin (Dominici et al., 2002; Dominici and Turyn, 2002). The counterpart to the studies described above is the bovine GH transgenic mouse model, which displays hyperinsulinemia and insulin resistance in response to excess GH exposure, but nearly normal glucose tolerance (Valera et al., 1993; Balbis et al., 1996). Additionally, hepatocyte-specific impairment of GH-STAT5 signaling by targeting the GHR or STAT5 induces a state of insulin resistance, which manifests itself in profound hyperinsulinemia and glucose intolerance (Cui et al., 2007; Fan et al., 2009; Mueller et al., 2011). In regard to the induction of an insulin resistant state, interference with hepatic GH-STAT5 signaling closely resembles the anti-insulin actions described in GH transgenic mice. Here, chronic GH excess results in a diminished response to insulin injection particularly in skeletal muscle at the level of IRS-1 phosphorylation and downstream signaling events such as PI3K activation (Dominici and Turyn, 2002). More recently, excess GH exposure in mice and the acquired insulin resistance was linked to enhanced expression of p85α regulatory subunit of PI3K accompanied by a decrease in IRS-1-associated PI3K activity in the skeletal muscle and white adipose tissue (Barbour et al., 2005; del Rincon et al., 2007). Hepatocyte-specific impairment of GH-STAT5 signaling induces GH insensitivity of the liver and a corresponding elevation of pituitary GH secretion. As GH signaling in other tissues remains intact, it is tempting to speculate that a defective insulin receptor signaling in muscle and adipose tissue as observed in GH overexpressing mice partly accounts for insulin resistance. Moreover, deletion of hepatic STAT5 markedly impairs downstream insulin signaling in the liver (Mueller et al., 2011) and favors an upregulation of hepatic p85α mRNA expression (Mueller et al., unpublished observation). A predominant role for defective hepatic insulin signaling is also described in mice with liver-specific knockout of IR, where impaired IR signal transduction contributes to fasting hyperglycemia (Michael et al., 2000). Interestingly, in the settings of hepatic STAT5 deficiency and the associated GH insensitivity of the liver, the additional lack of GR in hepatocytes is not sufficient to ameliorate hyperglycemia. In summary, STAT5 and STAT5/GR knockout mice are equally affected by hyperinsulinemia and insulin resistance (Mueller et al., 2011).
4.2. Lipid metabolism and hepatic steatosis
Hepatic triglyceride (TG) stores are determined by the balance of fatty acid (FA) uptake and release, de novo lipogenesis, and oxidative clearance, a complex process regulated at pre-receptor, transcriptional and posttranscriptional levels (Browning and Horton, 2004; Desvergne et al., 2006). Defects in GH and GC signaling pathways have been implicated in NAFLD development. Chronically increased GC levels are associated with a fatty liver phenotype in NAFLD (Targher et al., 2006). Moreover, fatty degeneration of hepatocytes was demonstrated in patients with Cushing’s syndrome (Shibli-Rahhal et al., 2006) and fatty liver disease is also a typical side effect of long-term systemic GC treatment, e.g. immunosuppressive therapy (Schacke et al., 2002). GH has pronounced lipolytic effects and under conditions of food deprivation provides peripheral tissues with ketone bodies as an alternative energy source to glucose. Excess GH, as observed in untreated acromegaly, is associated with increased lipolysis, decreased fat mass and an abnormal lipid profile (Moller and Jorgensen, 2009). GHR loss of function mutations, on the other hand, causes NAFLD in adults (Laron et al., 2008) and patients who are GH deficient also display fatty degeneration of the liver more frequently than those with normal GH levels (Ichikawa et al., 2003; Adams et al., 2004). Indeed, a recent case report has shown that administration of recombinant GH can improve NAFLD in a GH-deficient patient accompanied by a reduction in fibrosis and reversion of hepatocyte ballooning (Takahashi et al., 2007).
Even though, aberrant GC and GH action is frequently associated with steatosis and associated metabolic disorders, the impact of GH-STAT5 and GC-GR signaling on liver lipid metabolism, particularly in humans, is less well characterized. However, several recent studies in mice have provided new mechanistic insights. Adult mice with a hepatocyte-specific GR knockout display an increase in body fat, while plasma TG levels are substantially lower (Opherk et al., 2004). The relative size of GR-deficient livers as well as liver histology is comparable to those of wt mice (Mueller et al., 2011). Yet, an additional study of these mice has shown that GR signaling promotes transient hepatic fat accumulation following partial hepatectomy (Shteyer et al., 2004). Interestingly, Herzig and colleagues (Lemke et al., 2008) have shown that liver specific knockdown of the GR, mediated by adenoviral shRNA, improves the steatotic phenotype in mouse models of fatty liver disease. Here, hepatic GR signaling upon metabolic challenge was linked to downregulation of genes involved in hepatic TG lipolysis and β-oxidation, which is accompanied by upregulation of fatty acid uptake and storage. Moreover, the GR-dependent hepatic lipid accumulation was shown to depend on inhibition of expression of Hes-1, a known anti-lipogenic factor (Herzig et al., 2003). Restoration of Hes-1 expression favorable changes hepatic lipid metabolism including the downregulation of Pparγ, Cd36 and caveolin expression, which results in lowered hepatic TG content (Lemke et al., 2008).
In contrast, mouse models of GH excess are not steatotic and they have lower hepatic TG content (Wang et al., 2007; Friedbichler et al., 2012), whereas impaired GH-STAT5 signaling in liver perturbs lipid metabolism resulting in liver steatosis even upon excess GH exposure (Friedbichler et al., 2012). Several studies have shed light on the underlying mechanisms how hepatic GHR–STAT5 signaling maintains hepatic lipid homeostasis. Hepatocyte-specific ablation of the GHR (Fan et al., 2009), JAK2 (Sos et al., 2011) and STAT5 (Cui et al., 2007; Mueller et al., 2011; Friedbichler et al., 2012) results in progressive steatosis accompanied by elevated liver damage parameters. The increase in TG accumulation in the absence of hepatic GH-STAT5 signaling probably results from an upregulation of genes involved in hepatic fatty acid uptake and/or de novo synthesis. Genetic alterations shared by the before mentioned models include enhanced expression of Pparγ (FA uptake and synthesis) and its target gene Cd36 (FA uptake), which are both frequently associated with development of fatty liver disorders (Fig. 3) (Browning and Horton, 2004; Bechmann et al., 2011). It was shown that treatment with a PPARγ-specific antagonist leads to reduced Cd36 expression and decreased lipid load in JAK2-deficient livers (Sos et al., 2011). A further study has established that STAT5 binds to the Cd36 gene promoter which might suppress transcription (Barclay et al., 2011). Yet, upregulation of Pparγ itself is presumably not due to loss of a STAT5-mediated inhibition. It is rather a consequence of enhanced GH-dependent STAT1 activation upon hepatic STAT5 deficiency (Cui et al., 2007; Barclay et al., 2011). Secondly, enhanced expression of the prolipogenic transcription factor SREBP-1c (Browning and Horton, 2004; Bechmann et al., 2011) is a possible cause for steatosis in the absence of GH-STAT5 signaling (Fig. 3). Hepatic GHR and STAT5 deficiency was shown to result in enhanced expression of Srebp-1c and lipogenic downstream targets such as Fas (Fan et al., 2009; Mueller et al., 2011). Vice versa, overexpression of bovine GH reduces expression of Srebp-1c and several lipogenic downstream target genes in liver, despite overt hyperinsulinemia, a well known cause of increased Srebp-1c transcription (Olsson et al., 2003). Consistently, GH treatment of wt mice leads to decreased hepatic Srebp-1c expression, and GH-activated STAT5 was found to interact with the Srebp-1c gene promoter. Thereby, it might contribute to a transcriptional inhibition (Mueller et al., 2011). Further, immature SREBP-1c can be activated in response to decreased expression of Fgf21 and Insig2 (Osborne and Espenshade, 2009; Xu et al., 2009), the transcription of which is severely decreased in liver upon hepatocyte-specific STAT5 deletion and upon impairment of GHR signaling (Barclay et al., 2011; Mueller et al., 2011). Overall, the precise mechanisms leading to deregulation of PPARγ and SREBP-1c signaling upon impaired hepatic GH-STAT5 signaling are not completely understood.
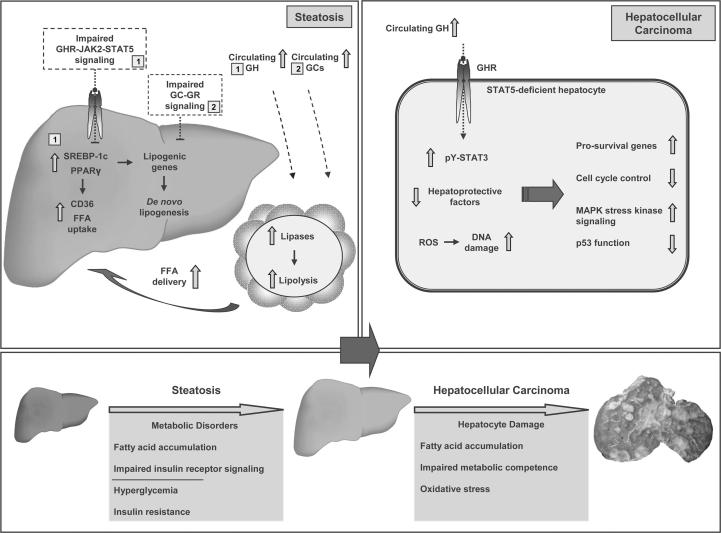
Fig. 3.
Schematic illustrations demonstrating the development of liver phenotypes following combined hepatic STAT5/GR deletion and STAT5 deletion in GH transgenic mice. Loss of hepatic GH-STAT5 signaling, in presence and absence of hepatic GR, causes defects in lipid and glucose metabolism leading to steatosis, hyperglycemia, insulin resistance and impaired hepatic insulin receptor signal transduction. On the molecular level, impairment of hepatic GH-STAT5 signaling is associated with enhanced pro-lipogenic PPARγ and SREBP-1c signaling leading to increased lipogenesis and/or increased hepatic FFA uptake (1; see text for details). Further, loss of STAT5 results in hepatic GH insensitivity resulting in high endogenous GH levels, while hepatic GR deficiency leads to hypercortisolism. Consequently, high GH levels (endogenous and transgene expression; 1) or high endogenous GH level in combination with elevated GCs (1 and 2) lead to lipolysis of adipose tissues and enhanced FFA release. The increase in circulating FFA in combination with enhanced expression of the fatty acid transporter CD36 results in additional FA accumulation in the liver. The increased hepatic FA load and associated metabolic dysfunctions upon STAT5/GR deficiency as well as STAT5 deficiency in GH transgenic mice results in oxidative stress and chronic liver damage. These metabolic changes additionally activate the stress kinase signaling (JNK1 and/or p38). Moreover, increased lipid synthesis, deregulated expression of hepatoprotective factors (EGFR, PRLR, LIFR; HNF6), accumulation of mutations (increased DNA damage) following loss of cell cycle control (reduced p53 levels), and enhanced activity of tumor-promoting STAT3 and c-JUN (elevated protein levels induced by STAT3 and increased phosphorylation mediated by JNK1 and p38 stress kinases) within hepatocytes contribute to severe tissue damage and the development of HCC. STAT, signal transducer and activator of transcription; GR, glucocorticoid receptor; GH, growth hormone; GCs, glucocorticoids; GHR, GH receptor; PPARγ, peroxisome proliferator-activated receptor gamma; SREBP-1c, sterol regulatory element-binding protein 1c; FFA, free fatty acids; CD36, cluster of differentiation 36; JNK1, c-JUN-N-terminal kinase; EGFR, epithelial growth factor receptor; PRLR, prolactin receptor; LIFR, leukemia inhibitory factor receptor; HNF6, hepatocyte nuclear factor 6; ROS, reactive oxygen species.
Interestingly, the additional deletion of hepatic GR neither ameliorates the steatosis phenotype caused by STAT5 deficiency nor does it improves the gene expression profile of altered hepatic lipid metabolism, e.g. by downregulation of Pparγ and Cd36 transcription. A combined STAT5/GR deficiency rather results in an even more pronounced hepatocyte TG accumulation (Mueller et al., 2011). The increased hepatic TG load in hepatic STAT5/GR-deficient animals compared to STAT5-deficient animals results from a combination of elevated plasma GH and GC levels, which, in turn, activate STAT5 and GR signaling in adipocytes. Both transcription factors drive the upregulation of adipose tissue triglyceride lipase and hormone sensitive lipase, which are essential for lipolysis (Fain et al., 2008; Mueller et al., 2011). The subsequent increase in available plasma FFA in combination with enhanced expression of the fatty acid transporter CD36 in hepatocytes facilitates additional TG accumulation in the liver (Fig. 3).
In conclusion, hepatic GH-STAT5 signaling protects the liver from steatosis by ensuring proper transcriptional control of genes involved in regulation of hepatic de novo lipogenesis and FA uptake.
5. GH-STAT5 signaling and HCC
Hepatocellular carcinoma (HCC) is a common complication of chronic liver disease and in most cases carcinogenesis follows a sequential process, with cirrhosis as an intermediate key step (Feldstein, 2010; Bechmann et al., 2011). However, development of HCC is increasingly observed in absence of advanced liver injury and cirrhosis (Paradis et al., 2009; Starley et al., 2010; Bechmann et al., 2011). In this regard, independent risk factors for HCC include obesity and diabetes (Calle et al., 2003; Paradis et al., 2009).
STAT5 activation is oncogenic in hematopoietic cancers and it correlates with poor prognosis in myeloid leukemia. However, the role of STAT5 protein activation in carcinomas is more complicated and cell type specific. Persistent STAT5 activation correlates with a good prognosis in breast cancer, while in prostate cancer the opposite was reported (reviewed in Ferbeyre and Moriggl (2011)). So far, there is little information regarding the impact of hepatic GH-STAT5 signaling on HCC development. Increased STAT5B activity was reported to correlate with more aggressive tumors and poor clinical outcomes in hepatitis B virus-related HCC due to increased cell motility and concomitant tumor spread (Lee et al., 2006). In patients with liver cirrhosis, a premalignant condition, the GH-IGF-1 axis is known to be severely impaired caused by a state of acquired hepatic GH resistance (Picardi et al., 2006), which is indicative of low STAT5 activity. In parallel, a mouse model of cholestatic liver disease with ablated hepatic GH-STAT5 signaling displayed an early and more severe liver fibrosis phenotype. This was attributed to the lack of IGF-1 and down-regulation of hepatoprotective factors such as Egfr, Lifr, Plr and Hnf-6 (Fig. 3) (Blaas et al., 2010). Upon CCl4 challenge, hepatic STAT5 deficiency promotes the development of liver fibrosis and, in some cases, HCC as a result of increased STAT3 activation and TGF-β stabilization (Hosui et al., 2009). STAT5 activity in hepatocytes was suggested to promote cell cycle arrest upon chronic hepatocyte injury, while loss of STAT5 signaling favors activation of pro-survival and proliferation pathways. This was linked to (1) reduced expression of the cell cycle inhibitors and STAT5 target genes Cdkn1a and Cdkn2b, and (2) excessive GH-dependent activation of STAT3 in absence of STAT5 (Hosui et al., 2009; Yoo et al., 2011; Yu et al., 2011). Upon additional challenge, in form of increased adipose tissue derived FA influx due to combined GH resistance and hypercortisolism (Mueller et al., 2011) or GH overexpression (Friedbichler et al., 2012), STAT5-deficient livers develop HCC in the presence of progressive steatosis, despite minor inflammation and fibrotic degeneration. Steatosis combined with elevated plasma FFAs was shown to coincide with increased plasma and liver TNF-α level and oxidative stress in hepatocytes (Mueller et al., 2011). An elevation of FFA, TNF-α, and ROS levels are typically observed in NAFLD and are associated with persistent hepatocyte damage (Bechmann et al., 2011). These conditions support the accumulation of DNA damage and sustained stress-dependent JNK1 activity (Mueller et al., 2011), which are both recognized as critical factors in the promotion and progression of human and murine HCC (Luedde et al., 2007; Park et al., 2010; Starley et al., 2010).
GH overexpression in mice is linked to a severe systemic inflammatory phenotype, reduced life expectancy and the development of liver tumors (Orian et al., 1990; Bartke et al., 2002). Intriguingly, hepatic STAT5 deficiency reverses all pathologic alterations induced by high GH levels, but leads to a more aggressive form of HCC at earlier time points (Friedbichler et al., 2012). As in the former model, hepatic STAT5 deficiency attributes to increased adipose tissue derived FA accumulation in hepatocytes, subsequent chronic liver damage and accumulation of DNA damage. Accordingly, JNK1 activity and a related increase in c-JUN expression and activity were also present in this transgenic HCC model. Further, following c-JUN activation (Eferl et al., 2003) or other oncogenic mechanisms potentially involving STAT3 (Niu et al., 2005) p53 activity was abolished in STAT5-deficient livers. Thereby, diminished activity of p53 downstream signaling might impair clearance of cells harboring DNA damage and favor subsequent fixation of mutations. In this regard, it was shown that persistent activation of STAT5A triggers a permanent cell cycle arrest with characteristics of cellular senescence including activation of p53 and a constitutive activation of the DNA damage response in non-hepatic cells (Mallette et al., 2007a,b; Calabrese et al., 2009).
Based on the current state of knowledge, HCC development in the absence of STAT5 might be the result of several direct and indirect mechanisms: (1) Deregulation of STAT5 target genes involved in the protection of hepatocyte integrity and cell cycle inhibition. (2) Increased STAT3 signaling most likely due to misrecruitment to the GHR in absence of STAT5 accelerates progression of chronic liver disease. (3) Metabolic dysfunctions contribute to liver cell and DNA damage and subsequent activation of stress-kinase signaling. (4) An accumulation of mutations is most likely facilitated by a loss of cell-cycle control and tumor-suppressive functions of p53 (Fig. 3). In summary, while some mechanisms by which GH-activated STAT5 contributes to safeguard mechanisms that protect hepatocytes from chronic injury and tumorigenic transformation have been revealed, further work is clearly required to better understand the consequences and possible connection to development of human disease.
6. Concluding remarks
Conditional knockouts targeting the core components of murine hepatic GH-STAT5 signaling and the GR have provided an important tool to obtain mechanistic insights into their role in liver function. We provided an overview of overlapping and distinct functions of hepatic GH-STAT5 and GC-GR signaling in postnatal body growth, glucose/lipid homeostasis and metabolic diseases.
Aberrant hepatic lipid and glucose metabolism are closely related to the pathogenesis of the most common liver diseases including their progression to hepatocarcinogenesis. Better understanding of the underlying molecular mechanisms is thus crucial, particularly in light of the increasing prevalence of obesity and its pathological consequences.
A characteristic of metabolic liver disease in the absence of hepatic GH-STAT5/GR signaling is the activation of both signaling pathways in peripheral tissues such as the adipose compartments. Thus, the use of transgenic mice will greatly help to understand the contribution of STAT5 and GR signaling in extra-hepatic tissues to the aberrations in carbohydrate and lipid metabolism. Additionally, the downstream molecular mechanisms of GH-STAT5 and GC-GR signaling particularly in hepatic lipid metabolism and steatosis needs to be further analyzed. While interference with hepatic GR signaling seems to ameliorate TG accumulation in mouse models of fatty liver disease, a protective role for GH-STAT5 signaling has emerged in chronic liver disease including HCC development. HCC is a complex, heterogeneous cancer, which develops in a multistep process involving deregulation of multiple cellular signaling pathways and impaired tissue homeostasis. Hence, future studies on global gene expression profiling of HCCs will help to pursue the synergism of impaired GH-STAT5 signaling and potentially deregulated tumor suppressor as well as oncogenic pathways in hepatocarcinogenesis.
Acknowledgments
Recent work by the authors was supported by Grant SFB F28 from the Austrian Science Funds (FWF; Project F2807-B20) to R.M., K.M., M.T., K.F. and J.W.K. H.E. was supported by grants of the Vienna Science and Technology Fund (WWTF Project LS07-058). J.P.T. was supported by the Boehringer Stiftung, the Deutsche Forschungsgemeinschaft (TU220/3 and TU220/6 and by the European Union FP7-BrainAge).
References
- Adams L.A., Feldstein A., Lindor K.D., Angulo P. Nonalcoholic fatty liver disease among patients with hypothalamic and pituitary dysfunction. Hepatology. 2004;39:909–914. doi: 10.1002/hep.20140. [DOI] [PubMed] [Google Scholar]
- Andrews R.C., Walker B.R. Glucocorticoids and insulin resistance: old hormones, new targets. Clin. Sci. (Lond.) 1999;96:513–523. doi: 10.1042/cs0960513. [DOI] [PubMed] [Google Scholar]
- Balbis A., Bartke A., Turyn D. Overexpression of bovine growth hormone in transgenic mice is associated with changes in hepatic insulin receptors and in their kinase activity. Life Sci. 1996;59:1363–1371. doi: 10.1016/0024-3205(96)00462-6. [DOI] [PubMed] [Google Scholar]
- Barbour L.A., Mizanoor Rahman S., Gurevich I., Leitner J.W., Fischer S.J., Roper M.D., Knotts T.A., Vo Y., McCurdy C.E., Yakar S., Leroith D., Kahn C.R., Cantley L.C., Friedman J.E., Draznin B. Increased P85alpha is a potent negative regulator of skeletal muscle insulin signaling and induces in vivo insulin resistance associated with growth hormone excess. J. Biol. Chem. 2005;280:37489–37494. doi: 10.1074/jbc.M506967200. [DOI] [PubMed] [Google Scholar]
- Barclay J.L., Nelson C.N., Ishikawa M., Murray L.A., Kerr L.M., McPhee T.R., Powell E.E., Waters M.J. GH-dependent STAT5 signaling plays an important role in hepatic lipid metabolism. Endocrinology. 2011;152:181–192. doi: 10.1210/en.2010-0537. [DOI] [PubMed] [Google Scholar]
- Bartke A., Chandrashekar V., Bailey B., Zaczek D., Turyn D. Consequences of growth hormone (GH) overexpression and GH resistance. Neuropeptides. 2002;36:201–208. doi: 10.1054/npep.2002.0889. [DOI] [PubMed] [Google Scholar]
- Baschant U., Frappart L., Rauchhaus U., Bruns L., Reichardt H.M., Kamradt T., Brauer R., Tuckermann J.P. Glucocorticoid therapy of antigen-induced arthritis depends on the dimerized glucocorticoid receptor in T cells. Proc. Natl. Acad. Sci. USA. 2011;108:19317–19322. doi: 10.1073/pnas.1105857108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechmann, L.P., Hannivoort, R.A., Gerken, G., Hotamisligil, G.S., Trauner, M., Canbay, A., 2011. The interaction of hepatic lipid and glucose metabolism in liver diseases. J. Hepatol. [DOI] [PubMed]
- Beck I.M., De Bosscher K., Haegeman G. Glucocorticoid receptor mutants: man-made tools for functional research. Trends Endocrinol. Metab. 2011;22:295–310. doi: 10.1016/j.tem.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Blaas L., Kornfeld J.W., Schramek D., Musteanu M., Zollner G., Gumhold J., van Zijl F., Schneller D., Esterbauer H., Egger G., Mair M., Kenner L., Mikulits W., Eferl R., Moriggl R., Penninger J., Trauner M., Casanova E. Disruption of the growth hormone–signal transducer and activator of transcription 5–insulinlike growth factor 1 axis severely aggravates liver fibrosis in a mouse model of cholestasis. Hepatology. 2010;51:1319–1326. doi: 10.1002/hep.23469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks A.J., Waters M.J. The growth hormone receptor: mechanism of activation and clinical implications. Nat. Rev. Endocrinol. 2010;6:515–525. doi: 10.1038/nrendo.2010.123. [DOI] [PubMed] [Google Scholar]
- Browning J.D., Horton J.D. Molecular mediators of hepatic steatosis and liver injury. J. Clin. Invest. 2004;114:147–152. doi: 10.1172/JCI22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley T.M., Schatzberg A.F. On the interactions of the hypothalamic–pituitary–adrenal (HPA) axis and sleep: normal HPA axis activity and circadian rhythm, exemplary sleep disorders. J. Clin. Endocrinol. Metab. 2005;90:3106–3114. doi: 10.1210/jc.2004-1056. [DOI] [PubMed] [Google Scholar]
- Calabrese V., Mallette F.A., Deschenes-Simard X., Ramanathan S., Gagnon J., Moores A., Ilangumaran S., Ferbeyre G. SOCS1 links cytokine signaling to p53 and senescence. Mol. Cell. 2009;36:754–767. doi: 10.1016/j.molcel.2009.09.044. [DOI] [PubMed] [Google Scholar]
- Calle E.E., Rodriguez C., Walker-Thurmond K., Thun M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. New Engl. J. Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- Clodfelter K.H., Holloway M.G., Hodor P., Park S.H., Ray W.J., Waxman D.J. Sex-dependent liver gene expression is extensive and largely dependent upon signal transducer and activator of transcription 5b (STAT5b): STAT5b-dependent activation of male genes and repression of female genes revealed by microarray analysis. Mol. Endocrinol. 2006;20:1333–1351. doi: 10.1210/me.2005-0489. [DOI] [PubMed] [Google Scholar]
- Cole T.J., Blendy J.A., Monaghan A.P., Krieglstein K., Schmid W., Aguzzi A., Fantuzzi G., Hummler E., Unsicker K., Schutz G. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Develop. 1995;9:1608–1621. doi: 10.1101/gad.9.13.1608. [DOI] [PubMed] [Google Scholar]
- Cole T.J., Harris H.J., Hoong I., Solomon N., Smith R., Krozowski Z., Fullerton M.J. The glucocorticoid receptor is essential for maintaining basal and dexamethasone-induced repression of the murine corticosteroid-binding globulin gene. Mol. Cell. Endocrinol. 1999;154:29–36. doi: 10.1016/s0303-7207(99)00105-7. [DOI] [PubMed] [Google Scholar]
- Cui Y., Riedlinger G., Miyoshi K., Tang W., Li C., Deng C.X., Robinson G.W., Hennighausen L. Inactivation of Stat5 in mouse mammary epithelium during pregnancy reveals distinct functions in cell proliferation, survival, and differentiation. Mol. Cell. Biol. 2004;24:8037–8047. doi: 10.1128/MCB.24.18.8037-8047.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y., Hosui A., Sun R., Shen K., Gavrilova O., Chen W., Cam M.C., Gao B., Robinson G.W., Hennighausen L. Loss of signal transducer and activator of transcription 5 leads to hepatosteatosis and impaired liver regeneration. Hepatology. 2007;46:504–513. doi: 10.1002/hep.21713. [DOI] [PubMed] [Google Scholar]
- Dai J., Baxter R.C. Regulation in vivo of the acid-labile subunit of the rat serum insulin-like growth factor-binding protein complex. Endocrinology. 1994;135:2335–2341. doi: 10.1210/endo.135.6.7527331. [DOI] [PubMed] [Google Scholar]
- Davidson M.B. Effect of growth hormone on carbohydrate and lipid metabolism. Endocr. Rev. 1987;8:115–131. doi: 10.1210/edrv-8-2-115. [DOI] [PubMed] [Google Scholar]
- del Rincon J.P., Iida K., Gaylinn B.D., McCurdy C.E., Leitner J.W., Barbour L.A., Kopchick J.J., Friedman J.E., Draznin B., Thorner M.O. Growth hormone regulation of p85alpha expression and phosphoinositide 3-kinase activity in adipose tissue: mechanism for growth hormone-mediated insulin resistance. Diabetes. 2007;56:1638–1646. doi: 10.2337/db06-0299. [DOI] [PubMed] [Google Scholar]
- Desvergne B., Michalik L., Wahli W. Transcriptional regulation of metabolism. Physiol. Rev. 2006;86:465–514. doi: 10.1152/physrev.00025.2005. [DOI] [PubMed] [Google Scholar]
- Dominici F.P., Turyn D. Growth hormone-induced alterations in the insulin-signaling system. Exp. Biol. Med. (Maywood) 2002;227:149–157. doi: 10.1177/153537020222700301. [DOI] [PubMed] [Google Scholar]
- Dominici F.P., Hauck S., Argentino D.P., Bartke A., Turyn D. Increased insulin sensitivity and upregulation of insulin receptor, insulin receptor substrate (IRS)-1 and IRS-2 in liver of Ames dwarf mice. J. Endocrinol. 2002;173:81–94. doi: 10.1677/joe.0.1730081. [DOI] [PubMed] [Google Scholar]
- Eferl R., Ricci R., Kenner L., Zenz R., David J.P., Rath M., Wagner E.F. Liver tumor development: c-Jun antagonizes the proapoptotic activity of p53. Cell. 2003;112:181–192. doi: 10.1016/s0092-8674(03)00042-4. [DOI] [PubMed] [Google Scholar]
- Eisen E.J., Peterson C.B., Parker I.J., Murray J.D. Effects of zinc ion concentration on growth, fat content and reproduction in oMT1a-oGH transgenic mice. Growth Devlop. Aging. 1998;62:173–186. [PubMed] [Google Scholar]
- Engblom D., Kornfeld J.W., Schwake L., Tronche F., Reimann A., Beug H., Hennighausen L., Moriggl R., Schutz G. Direct glucocorticoid receptor-Stat5 interaction in hepatocytes controls body size and maturation-related gene expression. Genes Devlop. 2007;21:1157–1162. doi: 10.1101/gad.426007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain J.N., Cheema P., Tichansky D.S., Madan A.K. Stimulation of human omental adipose tissue lipolysis by growth hormone plus dexamethasone. Mol. Cell. Endocrinol. 2008;295:101–105. doi: 10.1016/j.mce.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Fan, Y., Menon, R.K., Cohen, P., Hwang, D., Clemens, T., Digirolamo, D.J., Kopchick, J.J., Leroith, D., Trucco, M., Sperling, M.A., 2009. Liver-specific deletion of the growth hormone receptor reveals essential role of GH signaling in hepatic lipid metabolism. J. Biol. Chem. [DOI] [PMC free article] [PubMed]
- Feldstein A.E. Novel insights into the pathophysiology of nonalcoholic fatty liver disease. Semin. Liver Dis. 2010;30:391–401. doi: 10.1055/s-0030-1267539. [DOI] [PubMed] [Google Scholar]
- Ferbeyre G., Moriggl R. The role of Stat5 transcription factors as tumor suppressors or oncogenes. Biochim. Biophys. Acta. 2011;1815:104–114. doi: 10.1016/j.bbcan.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Flores-Morales A., Greenhalgh C.J., Norstedt G., Rico-Bautista E. Negative regulation of growth hormone receptor signaling. Mol. Endocrinol. 2006;20:241–253. doi: 10.1210/me.2005-0170. [DOI] [PubMed] [Google Scholar]
- Friedbichler, K., Themanns, M., Mueller, K.M., Schlederer, M., Kornfeld, J.W., Terracciano, L.M., Kozlov, A.V., Haindl, S., Kenner, L., Kolbe, T., Mueller, M., Snibson, K.J., Heim, M.H., Moriggl, R., 2012. Growth hormone-induced STAT5 signaling causes gigantism, inflammation and premature death but protects mice from aggressive liver cancer. Hepatology. [DOI] [PubMed]
- Gorin E., Tai L.R., Honeyman T.W., Goodman H.M. Evidence for a role of protein kinase C in the stimulation of lipolysis by growth hormone and isoproterenol. Endocrinology. 1990;126:2973–2982. doi: 10.1210/endo-126-6-2973. [DOI] [PubMed] [Google Scholar]
- Hanson R.W., Reshef L. Regulation of phosphoenolpyruvate carboxykinase (GTP) gene expression. Annu. Rev. Biochem. 1997;66:581–611. doi: 10.1146/annurev.biochem.66.1.581. [DOI] [PubMed] [Google Scholar]
- Hennighausen L., Robinson G.W. Interpretation of cytokine signaling through the transcription factors STAT5A and STAT5B. Genes Devlop. 2008;22:711–721. doi: 10.1101/gad.1643908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig S., Hedrick S., Morantte I., Koo S.H., Galimi F., Montminy M. CREB controls hepatic lipid metabolism through nuclear hormone receptor PPAR-gamma. Nature. 2003;426:190–193. doi: 10.1038/nature02110. [DOI] [PubMed] [Google Scholar]
- Hosui, A., Kimura, A., Yamaji, D., Zhu, B.M., Na, R., Hennighausen, L., 2009. Loss of STAT5 causes liver fibrosis and cancer development through increased TGF-{beta} and STAT3 activation. J. Exp. Med. [DOI] [PMC free article] [PubMed]
- Ichikawa T., Hamasaki K., Ishikawa H., Ejima E., Eguchi K., Nakao K. Non-alcoholic steatohepatitis and hepatic steatosis in patients with adult onset growth hormone deficiency. Gut. 2003;52:914. doi: 10.1136/gut.52.6.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida K., Del Rincon J.P., Kim D.S., Itoh E., Nass R., Coschigano K.T., Kopchick J.J., Thorner M.O. Tissue-specific regulation of growth hormone (GH) receptor and insulin-like growth factor-I gene expression in the pituitary and liver of GH-deficient (lit/lit) mice and transgenic mice that overexpress bovine GH (bGH) or a bGH antagonist. Endocrinology. 2004;145:1564–1570. doi: 10.1210/en.2003-1486. [DOI] [PubMed] [Google Scholar]
- Jeay S., Sonenshein G.E., Postel-Vinay M.C., Kelly P.A., Baixeras E. Growth hormone can act as a cytokine controlling survival and proliferation of immune cells: new insights into signaling pathways. Mol. Cell. Endocrinol. 2002;188:1–7. doi: 10.1016/s0303-7207(02)00014-x. [DOI] [PubMed] [Google Scholar]
- Jones J.I., Clemmons D.R. Insulin-like growth factors and their binding proteins: biological actions. Endocr. Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- Kassel O., Herrlich P. Crosstalk between the glucocorticoid receptor and other transcription factors: molecular aspects. Mol. Cell. Endocrinol. 2007;275:13–29. doi: 10.1016/j.mce.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Kellendonk C., Opherk C., Anlag K., Schutz G., Tronche F. Hepatocyte-specific expression of Cre recombinase. Genesis. 2000;26:151–153. doi: 10.1002/(sici)1526-968x(200002)26:2<151::aid-gene17>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Kleiman A., Hubner S., Rodriguez Parkitna J.M., Neumann A., Hofer S., Weigand M.A., Bauer M., Schmid W., Schutz G., Libert C., Reichardt H.M., Tuckermann J.P. Glucocorticoid receptor dimerization is required for survival in septic shock via suppression of interleukin-1 in macrophages. FASEB J. 2011;26:722–729. doi: 10.1096/fj.11-192112. [DOI] [PubMed] [Google Scholar]
- Klover P., Hennighausen L. Postnatal body growth is dependent on the transcription factors signal transducers and activators of transcription 5a/b in muscle: a role for autocrine/paracrine insulin-like growth factor I. Endocrinology. 2007;148:1489–1497. doi: 10.1210/en.2006-1431. [DOI] [PubMed] [Google Scholar]
- Kornfeld J.W., Grebien F., Kerenyi M.A., Friedbichler K., Kovacic B., Zankl B., Hoelbl A., Nivarti H., Beug H., Sexl V., Muller M., Kenner L., Mullner E.W., Gouilleux F., Moriggl R. The different functions of Stat5 and chromatin alteration through Stat5 proteins. Front. Biosci. 2008;13:6237–6254. doi: 10.2741/3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamia K.A., Papp S.J., Yu R.T., Barish G.D., Uhlenhaut N.H., Jonker J.W., Downes M., Evans R.M. Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature. 2011;480:552–556. doi: 10.1038/nature10700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanning N.J., Carter-Su C. Recent advances in growth hormone signaling. Rev. Endocr. Metab. Disorders. 2006;7:225–235. doi: 10.1007/s11154-007-9025-5. [DOI] [PubMed] [Google Scholar]
- Laron Z., Mannheimer S., Guttmann S. Plasma disappearance of various 131-I-labelled growth hormone preparations in man and rabbit. Nature. 1965;207:298–299. doi: 10.1038/207298a0. [DOI] [PubMed] [Google Scholar]
- Laron Z., Ginsberg S., Webb M. Nonalcoholic fatty liver in patients with Laron syndrome and GH gene deletion – preliminary report. Growth Horm. IGF Res. 2008;18:434–438. doi: 10.1016/j.ghir.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Laz E.V., Sugathan A., Waxman D.J. Dynamic in vivo binding of STAT5 to growth hormone-regulated genes in intact rat liver. Sex-specific binding at low- but not high-affinity STAT5 sites. Mol. Endocrinol. 2009;23:1242–1254. doi: 10.1210/me.2008-0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T.K., Man K., Poon R.T., Lo C.M., Yuen A.P., Ng I.O., Ng K.T., Leonard W., Fan S.T. Signal transducers and activators of transcription 5b activation enhances hepatocellular carcinoma aggressiveness through induction of epithelial–mesenchymal transition. Cancer Res. 2006;66:9948–9956. doi: 10.1158/0008-5472.CAN-06-1092. [DOI] [PubMed] [Google Scholar]
- Lemke U., Krones-Herzig A., Berriel Diaz M., Narvekar P., Ziegler A., Vegiopoulos A., Cato A.C., Bohl S., Klingmuller U., Screaton R.A., Muller-Decker K., Kersten S., Herzig S. The glucocorticoid receptor controls hepatic dyslipidemia through Hes1. Cell Metab. 2008;8:212–223. doi: 10.1016/j.cmet.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Liang Y., Osborne M.C., Monia B.P., Bhanot S., Watts L.M., She P., DeCarlo S.O., Chen X., Demarest K. Antisense oligonucleotides targeted against glucocorticoid receptor reduce hepatic glucose production and ameliorate hyperglycemia in diabetic mice. Metabolism. 2005;54:848–855. doi: 10.1016/j.metabol.2005.01.030. [DOI] [PubMed] [Google Scholar]
- Liu X., Robinson G.W., Wagner K.U., Garrett L., Wynshaw-Boris A., Hennighausen L. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Devlop. 1997;11:179–186. doi: 10.1101/gad.11.2.179. [DOI] [PubMed] [Google Scholar]
- Liu Y., Yan C., Wang Y., Nakagawa Y., Nerio N., Anghel A., Lutfy K., Friedman T.C. Liver X receptor agonist T0901317 inhibition of glucocorticoid receptor expression in hepatocytes may contribute to the amelioration of diabetic syndrome in db/db mice. Endocrinology. 2006;147:5061–5068. doi: 10.1210/en.2006-0243. [DOI] [PubMed] [Google Scholar]
- Liu Y., Nakagawa Y., Wang Y., Liu L., Du H., Wang W., Ren X., Lutfy K., Friedman T.C. Reduction of hepatic glucocorticoid receptor and hexose-6-phosphate dehydrogenase expression ameliorates diet-induced obesity and insulin resistance in mice. J. Mol. Endocrinol. 2008;41:53–64. doi: 10.1677/JME-08-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luedde T., Beraza N., Kotsikoris V., van Loo G., Nenci A., De Vos R., Roskams T., Trautwein C., Pasparakis M. Deletion of NEMO/IKKgamma in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell. 2007;11:119–132. doi: 10.1016/j.ccr.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Mallette F.A., Gaumont-Leclerc M.F., Ferbeyre G. The DNA damage signaling pathway is a critical mediator of oncogene-induced senescence. Genes Devlop. 2007;21:43–48. doi: 10.1101/gad.1487307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallette F.A., Gaumont-Leclerc M.F., Huot G., Ferbeyre G. Myc down-regulation as a mechanism to activate the Rb pathway in STAT5A-induced senescence. J. Biol. Chem. 2007;282:34938–34944. doi: 10.1074/jbc.M707074200. [DOI] [PubMed] [Google Scholar]
- Michael M.D., Kulkarni R.N., Postic C., Previs S.F., Shulman G.I., Magnuson M.A., Kahn C.R. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol. Cell. 2000;6:87–97. [PubMed] [Google Scholar]
- Moller N., Jorgensen J.O. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr. Rev. 2009;30:152–177. doi: 10.1210/er.2008-0027. [DOI] [PubMed] [Google Scholar]
- Moriggl R., Sexl V., Kenner L., Duntsch C., Stangl K., Gingras S., Hoffmeyer A., Bauer A., Piekorz R., Wang D., Bunting K.D., Wagner E.F., Sonneck K., Valent P., Ihle J.N., Beug H. Stat5 tetramer formation is associated with leukemogenesis. Cancer Cell. 2005;7:87–99. doi: 10.1016/j.ccr.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Mueller K.M., Kornfeld J.W., Friedbichler K., Blaas L., Egger G., Esterbauer H., Hasselblatt P., Schlederer M., Haindl S., Wagner K.U., Engblom D., Haemmerle G., Kratky D., Sexl V., Kenner L., Kozlov A.V., Terracciano L., Zechner R., Schuetz G., Casanova E., Pospisilik J.A., Heim M.H., Moriggl R. Impairment of hepatic growth hormone and glucocorticoid receptor signaling causes steatosis and hepatocellular carcinoma in mice. Hepatology. 2011;54:1398–1409. doi: 10.1002/hep.24509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu G., Wright K.L., Ma Y., Wright G.M., Huang M., Irby R., Briggs J., Karras J., Cress W.D., Pardoll D., Jove R., Chen J., Yu H. Role of Stat3 in regulating p53 expression and function. Mol. Cell. Biol. 2005;25:7432–7440. doi: 10.1128/MCB.25.17.7432-7440.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson B., Bohlooly Y.M., Brusehed O., Isaksson O.G., Ahren B., Olofsson S.O., Oscarsson J., Tornell J. Bovine growth hormone-transgenic mice have major alterations in hepatic expression of metabolic genes. Am. J. Physiol. Endocrinol. Metab. 2003;285:E504-11. doi: 10.1152/ajpendo.00444.2002. [DOI] [PubMed] [Google Scholar]
- Ooi G.T., Hurst K.R., Poy M.N., Rechler M.M., Boisclair Y.R. Binding of STAT5a and STAT5b to a single element resembling a gamma-interferon-activated sequence mediates the growth hormone induction of the mouse acid-labile subunit promoter in liver cells. Mol. Endocrinol. 1998;12:675–687. doi: 10.1210/mend.12.5.0115. [DOI] [PubMed] [Google Scholar]
- Opherk C., Tronche F., Kellendonk C., Kohlmuller D., Schulze A., Schmid W., Schutz G. Inactivation of the glucocorticoid receptor in hepatocytes leads to fasting hypoglycemia and ameliorates hyperglycemia in streptozotocin-induced diabetes mellitus. Mol. Endocrinol. 2004;18:1346–1353. doi: 10.1210/me.2003-0283. [DOI] [PubMed] [Google Scholar]
- Orian J.M., Tamakoshi K., Mackay I.R., Brandon M.R. New murine model for hepatocellular carcinoma: transgenic mice expressing metallothionein-ovine growth hormone fusion gene. J. Natl. Cancer Inst. 1990;82:393–398. doi: 10.1093/jnci/82.5.393. [DOI] [PubMed] [Google Scholar]
- Osborne T.F., Espenshade P.J. Evolutionary conservation and adaptation in the mechanism that regulates SREBP action: what a long, strange tRIP it’s been. Genes Devlop. 2009;23:2578–2591. doi: 10.1101/gad.1854309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ousova O., Guyonnet-Duperat V., Iannuccelli N., Bidanel J.P., Milan D., Genet C., Llamas B., Yerle M., Gellin J., Chardon P., Emptoz-Bonneton A., Pugeat M., Mormede P., Moisan M.P. Corticosteroid binding globulin: a new target for cortisol-driven obesity. Mol. Endocrinol. 2004;18:1687–1696. doi: 10.1210/me.2004-0005. [DOI] [PubMed] [Google Scholar]
- Paradis V., Zalinski S., Chelbi E., Guedj N., Degos F., Vilgrain V., Bedossa P., Belghiti J. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: a pathological analysis. Hepatology. 2009;49:851–859. doi: 10.1002/hep.22734. [DOI] [PubMed] [Google Scholar]
- Park E.J., Lee J.H., Yu G.Y., He G., Ali S.R., Holzer R.G., Osterreicher C.H., Takahashi H., Karin M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phuc Le P., Friedman J.R., Schug J., Brestelli J.E., Parker J.B., Bochkis I.M., Kaestner K.H. Glucocorticoid receptor-dependent gene regulatory networks. PLoS Genet. 2005;1:e16. doi: 10.1371/journal.pgen.0010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picardi A., D’Avola D., Gentilucci U.V., Galati G., Fiori E., Spataro S., Afeltra A. Diabetes in chronic liver disease: from old concepts to new evidence. Diabetes Metab. Res. Rev. 2006;22:274–283. doi: 10.1002/dmrr.636. [DOI] [PubMed] [Google Scholar]
- Rauch A., Seitz S., Baschant U., Schilling A.F., Illing A., Stride B., Kirilov M., Mandic V., Takacz A., Schmidt-Ullrich R., Ostermay S., Schinke T., Spanbroek R., Zaiss M.M., Angel P.E., Lerner U.H., David J.P., Reichardt H.M., Amling M., Schutz G., Tuckermann J.P. Glucocorticoids suppress bone formation by attenuating osteoblast differentiation via the monomeric glucocorticoid receptor. Cell Metab. 2010;11:517–531. doi: 10.1016/j.cmet.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Reichardt H.M., Kaestner K.H., Tuckermann J., Kretz O., Wessely O., Bock R., Gass P., Schmid W., Herrlich P., Angel P., Schutz G. DNA binding of the glucocorticoid receptor is not essential for survival. Cell. 1998;93:531–541. doi: 10.1016/s0092-8674(00)81183-6. [DOI] [PubMed] [Google Scholar]
- Reichardt H.M., Tuckermann J.P., Gottlicher M., Vujic M., Weih F., Angel P., Herrlich P., Schutz G. Repression of inflammatory responses in the absence of DNA binding by the glucocorticoid receptor. Embo J. 2001;20:7168–7173. doi: 10.1093/emboj/20.24.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogoff D., Ryder J.W., Black K., Yan Z., Burgess S.C., McMillan D.R., White P.C. Abnormalities of glucose homeostasis and the hypothalamic–pituitary–adrenal axis in mice lacking hexose-6-phosphate dehydrogenase. Endocrinology. 2007;148:5072–5080. doi: 10.1210/en.2007-0593. [DOI] [PubMed] [Google Scholar]
- Rosenfeld R.G., Belgorosky A., Camacho-Hubner C., Savage M.O., Wit J.M., Hwa V. Defects in growth hormone receptor signaling. Trends Endocrinol. Metab. 2007;18:134–141. doi: 10.1016/j.tem.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Rosner W. The functions of corticosteroid-binding globulin and sex hormone-binding globulin: recent advances. Endocr. Rev. 1990;11:80–91. doi: 10.1210/edrv-11-1-80. [DOI] [PubMed] [Google Scholar]
- Rowland J.E., Lichanska A.M., Kerr L.M., White M., d’Aniello E.M., Maher S.L., Brown R., Teasdale R.D., Noakes P.G., Waters M.J. In vivo analysis of growth hormone receptor signaling domains and their associated transcripts. Mol. Cell. Biol. 2005;25:66–77. doi: 10.1128/MCB.25.1.66-77.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky R.M., Romero L.M., Munck A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Scacchi M., Pincelli A.I., Cavagnini F. Growth hormone in obesity. Int. J. Obes. Relat. Metab. Disord. 1999;23:260–271. doi: 10.1038/sj.ijo.0800807. [DOI] [PubMed] [Google Scholar]
- Schacke H., Docke W.D., Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol. Ther. 2002;96:23–43. doi: 10.1016/s0163-7258(02)00297-8. [DOI] [PubMed] [Google Scholar]
- Schirra H.J., Anderson C.G., Wilson W.J., Kerr L., Craik D.J., Waters M.J., Lichanska A.M. Altered metabolism of growth hormone receptor mutant mice: a combined NMR metabonomics and microarray study. PLoS ONE. 2008;3:e2764. doi: 10.1371/journal.pone.0002764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H.J., Pagotto U., Stalla G.K. Central effects of the somatotropic system. Eur. J. Endocrinol. 2003;149:377–392. doi: 10.1530/eje.0.1490377. [DOI] [PubMed] [Google Scholar]
- Shibli-Rahhal A., Van Beek M., Schlechte J.A. Cushing’s syndrome. Clin. Dermatol. 2006;24:260–265. doi: 10.1016/j.clindermatol.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Shteyer E., Liao Y., Muglia L.J., Hruz P.W., Rudnick D.A. Disruption of hepatic adipogenesis is associated with impaired liver regeneration in mice. Hepatology. 2004;40:1322–1332. doi: 10.1002/hep.20462. [DOI] [PubMed] [Google Scholar]
- Sornson M.W., Wu W., Dasen J.S., Flynn S.E., Norman D.J., O’Connell S.M., Gukovsky I., Carriere C., Ryan A.K., Miller A.P., Zuo L., Gleiberman A.S., Andersen B., Beamer W.G., Rosenfeld M.G. Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature. 1996;384:327–333. doi: 10.1038/384327a0. [DOI] [PubMed] [Google Scholar]
- Sos, B.C., Harris, C., Nordstrom, S.M., Tran, J.L., Balazs, M., Caplazi, P., Febbraio, M., Applegate, M.A., Wagner, K.U., Weiss, E.J., 2011. Abrogation of growth hormone secretion rescues fatty liver in mice with hepatocyte-specific deletion of JAK2. J. Clin. Invest. [DOI] [PMC free article] [PubMed]
- Starley B.Q., Calcagno C.J., Harrison S.A. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820–1832. doi: 10.1002/hep.23594. [DOI] [PubMed] [Google Scholar]
- Stoecklin E., Wissler M., Gouilleux F., Groner B. Functional interactions between Stat5 and the glucocorticoid receptor. Nature. 1996;383:726–728. doi: 10.1038/383726a0. [DOI] [PubMed] [Google Scholar]
- Stoecklin E., Wissler M., Moriggl R., Groner B. Specific DNA binding of Stat5, but not of glucocorticoid receptor, is required for their functional cooperation in the regulation of gene transcription. Mol. Cell. Biol. 1997;17:6708–6716. doi: 10.1128/mcb.17.11.6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y., Iida K., Takahashi K., Yoshioka S., Fukuoka H., Takeno R., Imanaka M., Nishizawa H., Takahashi M., Seo Y., Hayashi Y., Kondo T., Okimura Y., Kaji H., Kitazawa R., Kitazawa S., Chihara K. Growth hormone reverses nonalcoholic steatohepatitis in a patient with adult growth hormone deficiency. Gastroenterology. 2007;132:938–943. doi: 10.1053/j.gastro.2006.12.024. [DOI] [PubMed] [Google Scholar]
- Targher G., Bertolini L., Rodella S., Zoppini G., Zenari L., Falezza G. Associations between liver histology and cortisol secretion in subjects with nonalcoholic fatty liver disease. Clin. Endocrinol. (Oxf.) 2006;64:337–341. doi: 10.1111/j.1365-2265.2006.02466.x. [DOI] [PubMed] [Google Scholar]
- Teglund S., McKay C., Schuetz E., van Deursen J.M., Stravopodis D., Wang D., Brown M., Bodner S., Grosveld G., Ihle J.N. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell. 1998;93:841–850. doi: 10.1016/s0092-8674(00)81444-0. [DOI] [PubMed] [Google Scholar]
- Tronche F., Opherk C., Moriggl R., Kellendonk C., Reimann A., Schwake L., Reichardt H.M., Stangl K., Gau D., Hoeflich A., Beug H., Schmid W., Schutz G. Glucocorticoid receptor function in hepatocytes is essential to promote postnatal body growth. Genes Devlop. 2004;18:492–497. doi: 10.1101/gad.284704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsigos C., Chrousos G.P. Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. J. Psychosom. Res. 2002;53:865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- Udy G.B., Towers R.P., Snell R.G., Wilkins R.J., Park S.H., Ram P.A., Waxman D.J., Davey H.W. Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proc. Natl. Acad. Sci. USA. 1997;94:7239–7244. doi: 10.1073/pnas.94.14.7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera A., Rodriguez-Gil J.E., Yun J.S., McGrane M.M., Hanson R.W., Bosch F. Glucose metabolism in transgenic mice containing a chimeric P-enolpyruvate carboxykinase/bovine growth hormone gene. FASEB J. 1993;7:791–800. doi: 10.1096/fasebj.7.9.8330686. [DOI] [PubMed] [Google Scholar]
- van Schaftingen E., Gerin I. The glucose-6-phosphatase system. Biochem. J. 2002;362:513–532. doi: 10.1042/0264-6021:3620513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegiopoulos A., Herzig S. Glucocorticoids, metabolism and metabolic diseases. Mol. Cell. Endocrinol. 2007;275:43–61. doi: 10.1016/j.mce.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Viau V., Meaney M.J. Testosterone-dependent variations in plasma and intrapituitary corticosteroid binding globulin and stress hypothalamic–pituitary–adrenal activity in the male rat. J. Endocrinol. 2004;181:223–231. doi: 10.1677/joe.0.1810223. [DOI] [PubMed] [Google Scholar]
- Vijayakumar A., Novosyadlyy R., Wu Y., Yakar S., LeRoith D. Biological effects of growth hormone on carbohydrate and lipid metabolism. Growth Horm. IGF Res. 2010;20:1–7. doi: 10.1016/j.ghir.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Masternak M.M., Al-Regaiey K.A., Bartke A. Adipocytokines and the regulation of lipid metabolism in growth hormone transgenic and calorie-restricted mice. Endocrinology. 2007;148:2845–2853. doi: 10.1210/en.2006-1313. [DOI] [PubMed] [Google Scholar]
- Waters M.J., Hoang H.N., Fairlie D.P., Pelekanos R.A., Brown R.J. New insights into growth hormone action. J. Mol. Endocrinol. 2006;36:1–7. doi: 10.1677/jme.1.01933. [DOI] [PubMed] [Google Scholar]
- Watts L.M., Manchem V.P., Leedom T.A., Rivard A.L., McKay R.A., Bao D., Neroladakis T., Monia B.P., Bodenmiller D.M., Cao J.X., Zhang H.Y., Cox A.L., Jacobs S.J., Michael M.D., Sloop K.W., Bhanot S. Reduction of hepatic and adipose tissue glucocorticoid receptor expression with antisense oligonucleotides improves hyperglycemia and hyperlipidemia in diabetic rodents without causing systemic glucocorticoid antagonism. Diabetes. 2005;54:1846–1853. doi: 10.2337/diabetes.54.6.1846. [DOI] [PubMed] [Google Scholar]
- Webster J.I., Tonelli L., Sternberg E.M. Neuroendocrine regulation of immunity. Annu. Rev. Immunol. 2002;20:125–163. doi: 10.1146/annurev.immunol.20.082401.104914. [DOI] [PubMed] [Google Scholar]
- Woelfle J., Billiard J., Rotwein P. Acute control of insulin-like growth factor-I gene transcription by growth hormone through Stat5b. J. Biol. Chem. 2003;278:22696–22702. doi: 10.1074/jbc.M301362200. [DOI] [PubMed] [Google Scholar]
- Woelfle J., Chia D.J., Rotwein P. Mechanisms of growth hormone (GH) action. Identification of conserved Stat5 binding sites that mediate GH-induced insulin-like growth factor-I gene activation. J. Biol. Chem. 2003;278:51261–51266. doi: 10.1074/jbc.M309486200. [DOI] [PubMed] [Google Scholar]
- Xu J., Lloyd D.J., Hale C., Stanislaus S., Chen M., Sivits G., Vonderfecht S., Hecht R., Li Y.S., Lindberg R.A., Chen J.L., Jung D.Y., Zhang Z., Ko H.J., Kim J.K., Veniant M.M. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009;58:250–259. doi: 10.2337/db08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakar S., Liu J.L., Stannard B., Butler A., Accili D., Sauer B., LeRoith D. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc. Natl. Acad. Sci. USA. 1999;96:7324–7329. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo K.H., Baik M., Hennighausen L. Context-specific growth hormone signaling through the transcription factor STAT5: implications for the etiology of hepatosteatosis and hepatocellular carcinoma. Genes Cancer. 2011;2:3–9. doi: 10.1177/1947601911405046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J.H., Zhu B.M., Wickre M., Riedlinger G., Chen W., Hosui A., Robinson G.W., Hennighausen L. The transcription factors signal transducer and activator of transcription 5A (STAT5A) and STAT5B negatively regulate cell proliferation through the activation of cyclin-dependent kinase inhibitor 2b (Cdkn2b) and Cdkn1a expression. Hepatology. 2011;52:1808–1818. doi: 10.1002/hep.23882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Xu B.C., Maheshwari H.G., He L., Reed M., Lozykowski M., Okada S., Cataldo L., Coschigamo K., Wagner T.E., Baumann G., Kopchick J.J. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse) Proc. Natl. Acad. Sci. USA. 1997;94:13215–13220. doi: 10.1073/pnas.94.24.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T., Goh E.L., Graichen R., Ling L., Lobie P.E. Signal transduction via the growth hormone receptor. Cell Signal. 2001;13:599–616. doi: 10.1016/s0898-6568(01)00186-3. [DOI] [PubMed] [Google Scholar]