Highlights
► We examined the role of the TRAP enzyme during endochondral bone formation in mice. ► TRAP does not impair vascularization as well as ossification of the long bone epiphysis. ► TRAP−/− mice show an expansion of the cartilaginous growth plate. ► This malformation is first detected at week three and during further development. ► TRAP appears to be critical for the formation of the metaphysis but not of the epiphysis.
Keywords: TRAP, Long bones, Endochondral bone development, Epiphysis, Growth plate
Abstract
Tartrate resistant acid phosphatase (TRAP) was shown to be critical for skeleton development, and TRAP deficiency leads to a reduced resorptive activity during endochondral ossification resulting in an osteopetrotic phenotype and shortened long bones in adult mice. A proper longitudinal growth depends on a timely, well-coordinated vascularization and formation of the secondary ossification center (SOC) of the long bones epiphysis. Our results demonstrate that TRAP is not essential for the formation of the epiphyseal vascular network. Therefore, in wild type (Wt) controls as well as TRAP deficient (TRAP−/−) mutants vascularised cartilage canals are present from postnatal day (P) five. However, in the epiphysis of the TRAP−/− mice cartilage mineralization, formation of the marrow cavity and the SOC occur prematurely compared with the controls. In the mutant mice the entire growth plate is widened due to an expansion of the hypertrophic zone. This is not seen in younger animals but first detected at week (W) three and during further development. Moreover, an enhanced number of thickened trabeculae, indicative of the osteopetrotic phenotype, are observed in the metaphysis beginning with W three. Epiphyseal excavation was proposed as an important function of TRAP, and we examined whether TRAP deficiency affects this process. We therefore evaluated the marrow cavity volume (MCV) and the epiphyseal volume (EV) and computed the MCV to EV ratio (MCV/EV). We investigated developmental stages until W 12. Our results indicate that both epiphyseal excavation and establishment of the SOC are hardly impaired in the knockouts. Furthermore, no differences in the morphology of the epiphyseal bone trabeculae and remodeling of the articular cartilage layers are noted between Wt and TRAP−/− mice. We conclude that in long bones, TRAP is critical for the development of the growth plate and the metaphysis but apparently not for the epiphyseal vascularization, excavation, and establishment of the SOC.
1. Introduction
In mammals, long bones are formed via a cartilage model by a process referred to as endochondral bone formation. In the course of which the primary ossification center (POC) initially develops within the diaphysis, followed by the establishment of the secondary ossification center (SOC) within the epiphysis. Basically, the development of the two ossification centers is quite similar, requiring cartilage matrix mineralization, its resorption, angiogenesis and eventually deposition of the bone matrix. The overall bone growth is, on the one hand, governed by the growth plate located between the two sites of ossification, and responsible for the length growth of the diaphysis. On the other hand, the longitudinal, radial and lateral growth of the epiphysis is controlled by the articular cartilage layers that act as a surface growth plate during the early postnatal period (Alvarez et al., 2005a; Holmbeck and Szabova, 2006; Hunziker et al., 2007; Stempel et al., 2011).
A timely well-coordinated epiphyseal vascularization is essential for a proper histology and function of the growth plate in the long bones (Brashear, 1963; Holmbeck et al., 1999; Maes et al., 2004). Accordingly, in murine species cartilage canals are generated at a certain point of time, shortly after birth (Alvarez et al., 2005b; Blumer et al., 2008a,b; Holmbeck and Szabova, 2006; Kugler et al., 1979). The canals erode the non-mineralized cartilage matrix and thus give blood vessels and bone-forming cells access to the epiphysis for the subsequent development of the SOC. Canal formation is governed by several matrix metalloproteinases (MMPs) and, most notably, membrane-bound type-1 matrix metalloproteinase (MT1-MMP = MMP 14) is critical for this process. As a result, MT1-MMP−/− mice reveal a vascular defect accompanied by delayed ossification (Davoli et al., 2001; Holmbeck et al., 1999; Lee et al., 2009; Zhou et al., 2000). In mice lacking both MMP 9 and MMP 13 epiphyseal development is affected likewise (Ortega et al., 2004; Stickens et al., 2004). Apart from the importance of the MMPs, the establishment of the epiphyseal vascular network is triggered by the vascular endothelial growth factor (VEGF), and VEGF deficiency causes a delayed vascularization and formation of the SOC (Allerstorfer et al., 2010; Maes et al., 2004; Petersen et al., 2002). Taken together, failures in early epiphyseal development result in impaired bone growth leading to dwarfism (Blumer et al., 2008b; Holmbeck et al., 1999; Maes et al., 2004; Stickens et al., 2004; Zhou et al., 2000).
Tartrate-resistant acid phosphatase activity type 5 (TRAP or Acp5) is an iron-containing enzyme that is found in humans and murine species. It occurs in diverse tissues including bone and cartilage (Hayman et al., 2000; Hayman and Cox, 2003). TRAP is, at first, synthesized as a latent proenzyme with low activity, and proteolytic processing generates two subunits of about 16 and 20–23 kDa with enhanced enzymatic activity. The cysteine proteinase cathepsin K has been suggested to be responsible for the proteolytic activation of TRAP (Hollberg et al., 2002, 2005; Yamaza et al., 1998). TRAP is highly expressed in chondroclasts as well as osteoclasts and, therefore, used as a specific histochemical marker for these cells (Burstone, 1959; Minkin, 1982). Both are polynucleated, having the same ultrastructural features. However, chondroclasts attack the mineralized cartilage matrix whereas osteoclasts participate in the resorption of the mineralized bone matrix (Hayman et al., 2000). TRAP prompts the dephosphorylation of bone matrix phosphoproteins like osteopontin and bone sialoprotein and was originally shown to be important for a normal endochondral bone formation (Ek-Rylander et al., 1994; Hayman et al., 1996; Hollberg et al., 2002; Suter et al., 2001). Mice lacking TRAP develop normally, but adults reveal a malformation of the skeleton. Specifically, they have a mild osteopetrotic phenotype with increased bone tissue and mineral density. The bones are brittle and shortened due to a malfunction of the growth plate (Hayman and Cox, 2003; Hayman et al., 1996; Hollberg et al., 2002; Roberts et al., 2007; Suter et al., 2001).
The importance of TRAP during early epiphyseal development of the long bones has not been elucidated. However, the occurrence of the enzyme in the cartilage canals exactly at the onset of their formation (Allerstorfer et al., 2010; Alvarez et al., 2005b; Blumer et al., 2008a) suggests a possible role in the establishment of the vascular network. Loss of TRAP may impair this process, thus altering the epiphyseal development and growth plate structure. To delineate the long-range impact of TRAP during bone development, we investigated the femur in TRAP deficient mice from postnatal day (P) 5 until week (W) 12 and compared the results with wild type (Wt) mice.
2. Materials and methods
2.1. Experimental animals
Mice (129SvEv) with a targeted disruption of the single Acp5 gene that maps to murine chromosome 9 were generated as previously described by Hayman et al. (1996). In brief, homologous recombination was used to disrupt exon 2 of the murine TRAP gene. These mutant mice and their Wt littermates aged 5, 6, 7, 8, 9, 10, and 13 days were obtained from the School of Clinical Veterinary Science, University of Bristol, Langford BS40 5DU, UK. Wt mice of the same inbred strain 129SvEv aged 3, 4, 6, 8, and 12 weeks were obtained from the Central Laboratory Animal Facilities of the Innsbruck Medical University. TRAP−/− mice of corresponding age were obtained from the School of Clinical Veterinary Science, University of Bristol, Langford BS40 5DU, UK. Three animals per age group were used; the sex of the mice was not recorded. The animals were anesthetized with CO2, and killed by cervical displacement. Subsequently the legs were amputated, the soft tissue was carefully removed, and the distal part of the right and left femur examined. All mice were killed between 9 and 11 a.m. for better comparability at early stages.
2.2. Tissue preparation for histology, histochemistry and immunohistochemistry
The bones were fixed with 4% PFA in phosphate buffer saline (PBS, 0.1 M) for 4 h at room temperature and rinsed in PBS. Until P 13 the femurs were decalcified in 3% ascorbic acid in sodium chloride (0.15 M) for 1–2 days at room temperature, and from each age group only one femur/animal was decalcified. Non-decalcified bones were used for von Kossa staining. The femurs of mice aged 3, 6, 8, and 12 weeks were decalcified for up to 5 days. Subsequently, all samples were dehydrated in graded isopropanol and xylene series and embedded in paraffin. Serial sections (6 μm) were made on a HM 355S microtome (Microm, Walldorf, Germany), and three sections per slide mounted on SuperFrost®Plus slides. For the histological purpose only, the sections were stained with hematoxylin/eosin (HE) (Shandon Varistain 24-4, Histocom Vienna, Austria).
2.3. Tissue preparation for semithin sections
The bones of the mice aged 3 and 4 weeks were fixed in 2.5% glutaraldehyde, 2% paraformaldehyde (PFA) buffered in sodium cacodylate (0.1 M, pH 7.4) for 4 h at room temperature and rinsed in the same buffer. They were postfixed in 0.5% osmium tetroxide, 1% potassium hexacyanoferrat III in distilled water over night at 4 °C, rinsed, and decalcified as described before. This procedure was followed by dehydration in graded ethanol series and embedding in Spurr’s epoxy resin (Spurr, 1969). Ribbons of consecutive semithin sections (2 μm) were cut on a Reichert Ultracut S microtome (Leica Microsystem, Wetzlar, Germany) with a histo-jumbo-diamond knife (Diatome, Biel, Switzerland) (Blumer et al., 2002) and stained with toluidine blue (0.1% toluidine blue, 3% borax in distilled water) for 20 s at 60 °C.
2.4. Histochemistry (HC)
Tartrate-resistant acid phosphatase (TRAP) is a well-established marker for the chondro-/osteoclast lineage. To show TRAP activity, sections were deparaffinized, rinsed in PBS and incubated with a solution containing 50 mM sodium acetate (pH 5.2), 0.15% Naphtol-AS-TR-phosphate, 50 mM sodium tartrate, and 0.1% Fast Red T.R. (Sigma Aldrich Chemie Gmbh, Taufkirchen, Germany) for 30–40 min at room temperature. Subsequently, the sections were rinsed in PBS and counterstained.
Von Kossa staining was performed to visualize the mineralized cartilage matrix. Sections were deparaffinized, rinsed in PBS, incubated in 5% silver nitrate and exposed to light (60 Watt lamp) for 1 h. Subsequently the sections were rinsed in distilled water, briefly incubated with 2% ascorbic acid (pH 4.0) and fixed with 5% sodium thiosulfate. The sections were rinsed in PBS and counterstained.
2.5. Immunohistochemistry (IHC)
For detection of cathepsin K, a rabbit anti-mouse [10 μg/ml in antibody diluents (AB); ab19027, Abcam, Cambridge, UK], for type I collagen, a rabbit anti-human (1:200 in antibody diluents; LF-68 donated by Prof. L. Fisher, National Institutes of Health, Bethesda MD, USA), for Runx2, a rabbit anti-mouse Runx2 (1:20 in AB diluents; sc-10758, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), and for osteopontin, a goat anti-mouse osteopontin antibody (1:300 in AB-diluents; AF808 R&D Minneapolis, Wisconsin, USA) was used.
The sections were deparaffinized and rinsed in PBS. For antigen retrieval a citrate buffer (10 mM, pH 6.0) and a micro-wave (400 Watt, 7 min for cathepsin K and 20 min for Runx2) were used. Subsequently endogenous peroxidase activity was blocked with 1% H2O2 in 30% methanol for 30 min in the dark. The following protocol comprised primary antibody incubation overnight at 4 °C and saturation of unspecific sites with either 10% normal goat serum (NGS) for cathepsin K, type I collagen, and Runx2 or 10% normal rabbit serum (NRS) for osteopontin for 20 min. This was followed by a 4-h incubation with a secondary antibody in AB at room temperature [goat anti-rabbit IgG/HRP-conjugated (P0448, DakoCytomation, Glostrup, Denmark) (1:250 for cathepsin K and 1:500 for type I collagen and Runx2, respectively), rabbit anti-goat IgG/HRP-conjugated (P0449, DakoCytomation, Glostrup, Denmark) (1:1000 for Osteopontin)]. Afterwards the antigen–antibody complex was visualized by 0.05% 3, 3′-diaminobenzidine (DAB), 0.01% H202 in distilled water (10–15 min in the dark).
Negative controls were obtained by substituting the primary antibodies with antibody diluents. Negative control yielded no labeling.
2.6. Double labeling (type I collagen and TRAP, osteopontin and TRAP)
For double labeling, TRAP reaction was performed first, followed by IHC. As a control for TRAP deficiency, HC was also performed on the sections from TRAP−/− mice.
All sections were counterstained with Gils’ hematoxylin (1:1 in distilled water) and examined with a Zeiss Axioplan 2 (Zeiss, Oberkochen, Germany) and photographed as color images using a Zeiss AxioCam HR and AxioVision 4.1. software running on a Pentium 4 (Intel Inc. Santa Cruz, CA, USA) with WindowsXP (Microsoft Inc., Redmond, WA, USA).
2.7. Morphometric analysis
Volume measurements of the epiphysis, its mineralized cartilage matrix and its marrow cavity were performed in Wt mice and TRAP−/− mice. Every fifth slide of serially sectioned femurs was von Kossa or HE stained. Then, every fifteenth section (distance: 90 μm) was photographed, and the area of the epiphysis, the mineralized cartilage matrix and the marrow cavity was measured. The upper border of the epiphysis was defined by the boundary between the proliferative and the hypertrophic zone of the growth plate. For volume measurements of the epiphysis and its mineralized cartilage matrix, mice aged 6, 7, 8, 10, and 13 days were analyzed. For volume measurements of the epiphysis and its marrow space, mice aged 8, 10, and, 13 days as well as 3, 6, and 8 weeks were investigated. Three animals per age group were used.
For measurements of the total height of the growth plate and its individual zones longitudinal sections through the mid region of both condoyles and the intercondylar section were examined. The sections were HE stained. The upper border of the growth plate was defined by the chondro-osseous junction of the metaphyseal cartilage and the SOC, the lower border by the chondro-osseous junction of the metaphyseal cartilage and the POC.
For all measurements MetaMorph® (version 4.6, Visitron Systems) was used. For volume analysis, GraphPad Prism® 5 software was applied. Statistical analysis was made using a 2-way ANOVA with Bonferroni’s post tests.
3. Results
3.1. Formation of the cartilage canals and the epiphyseal marrow cavity
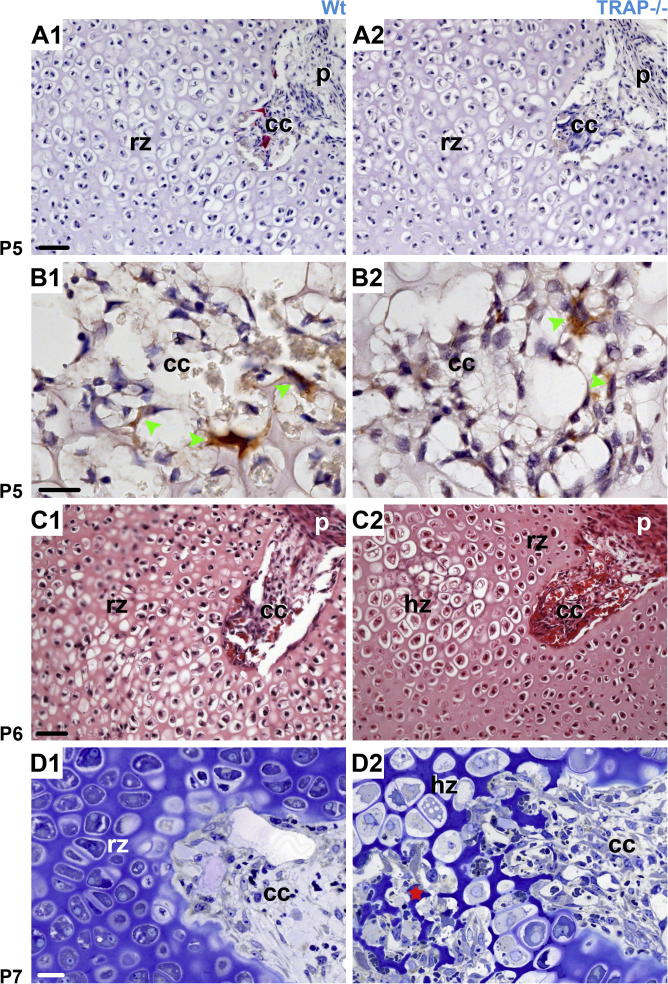
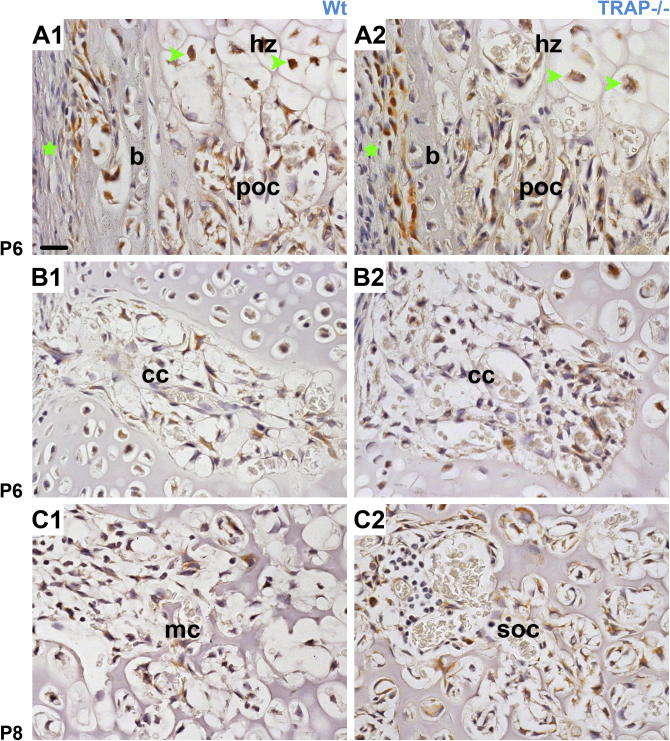
In Wt- and TRAP−/− mice 4–5 short cartilage canals, containing blood vessels and perivascular cells were present in the epiphysis at P 5. Cartilage canals originated from the perichondrium, were non-branched and terminated blindly in the resting cartilage. The canals had similar length in controls and TRAP deficient mice, and in Wt mice small TRAP positive chondroclasts were seen in the canal lumen (Fig. 1A1 and A2). In the knockouts, of course, histochemical staining for the enzyme was absent. However, immunohistochemistry using an anti-cathepsin K antibody demonstrated that chondroclasts were also present in these mice (Fig. 1B1 and B2). During advancing development the cartilage canals penetrated deeper into the epiphysis. In Wt mice they remained non-branched until P 7, and the epiphysis was still exclusively composed of resting cartilage (Fig. 1C1 and D1). In TRAP−/− mice the canals were non-branched until P 6, yet at this point hypertrophic chondrocytes were already present (Fig. 1C2). At P 7 the canals and their vessels were highly branched at their endings, giving rise to the formation of several small marrow cavities (Fig. 1D2). In control littermates, the development of the marrow spaces coupled with cartilage hypertrophy occurred at P 8, and the spaces were clearly smaller compared with those of the knockouts (Fig. 2A1 and A2). Our sections revealed that from P 10 onward, after the coalescence of the marrow spaces into one large single cavity, the area of the marrow cavity appeared similar in Wt vs. TRAP−/− littermates (Fig. 2B1–F2). However, in order to ascertain whether or not TRAP deficiency had an impact on the excavation of the entire epiphysis, we measured the marrow cavity volume (MCV) and the epiphyseal volume (EV) and calculated the ratio of MCV to EV (MCV/EV). In the present study, the upper border of the developing epiphysis was defined by the boundary between the proliferative and the hypertrophic zone of the growth plate, and this transition zone could easily be distinguished in the histological sections (Fig. 2B1). At P 8 and P 10 the MCV/EV was similar in Wt and TRAP−/− mice (Table 1). At P 13 the volume of the marrow cavity had further expanded in radial direction, and an increased MCV/EV was noted in the Wt mice compared to the TRAP−/− mice (Table 1). From W 3 onward the epiphysis was largely excavated, and the MCV/EV was similar in Wt vs. TRAP−/− mice (Table 1). The MCV/EV contrast at W 6 was due to an increased height of the proliferative zone and resting zone in TRAP−/− mice (Fig. 9). At P 10, P 13, W 3, and W 8 the height of the proliferative together with the resting zone was similar in both genotypes (Fig. 2B1, B2 and Fig. 9).
Fig. 1.
Sections through the femoral epiphysis at different developmental stages. (A1–B2 paraffin sections counterstained with hematoxylin; C1 and C2 paraffin sections HE stained; D1, D2 semithin resin sections counterstained with toluidine blue). A1–B2 At P 5 the epiphysis is solely composed of resting cartilage (rz). Short cartilage canals (cc) originating from the perichondrium (p) are seen in Wt as well as TRAP−/− mice. B1–B2 Immunohistochemistry (cathepsin K) reveals that small chondroclasts (brown staining, green arrowheads) are present in both genotypes. However, labeling for TRAP (red staining) is only detectable in Wt mice. C1–D2 In Wt controls the cartilage canals (cc) are non-branched until P 7 and the epiphysis still consists of resting cartilage (rz). In TRAP deficient mice chondrocytes undergo hypertrophy (Hz) at P 6, and at P 7 the canals tip is highly branched within the hypertrophic zone (Hz, red asterisk). The micrographs in A1, A2 and C1, C2, respectively have the same magnification; bar = 50 μm. B1, B2 same magnification; bar = 20 μm. D1 and D2 same magnification; bar = 20 μm.
Fig. 2.
HE A1–F2 Longitudinal sections through the mid-region of the condoyles from P 8 until W 8. The sections demonstrate the epiphysis, the growth plate with the resting (rz), proliferating (pz) and hypertrophic zone (Hz), and the POC (poc). In A1 and A2 the perimeter of the marrow cavity (mc) and the epiphysis are depicted with yellow ink. For each developmental stage and genotype, the areas were measured and the MCV/EV was calculated. At P 8 the epiphyseal marrow space is larger in TRAP−/− mice compared to the Wt mice but in older animals the extension of the marrow cavity is quite similar in Wt vs. TRAP−/− mice. In addition, the epiphyseal trabecular pattern is similar in the two genotypes. In the knockouts extensive trabeculation of the metaphysis is seen beginning with W 3. At this moment the entire height of the growth plate is more expanded in the TRAP deficient mice compared to Wt mice. In B1 the individual zones of the growth plate are depicted. The yellow arrowheads in D1 and D2 point to the subepiphyseal bone plate separating the marrow cavity from the growth plate. All sections have the same magnification; bar = 500 μm.
Table 1.
Measurements of the marrow cavity volume to epiphysis volume (MCV/EV) and the mineralized cartilage volume to epiphysis volume (minCV/EV).
| MCV/EV (%) |
minCV/EV (%) |
|||
|---|---|---|---|---|
| Wt | TRAP−/− | Wt | TRAP−/− | |
| P 6 | 0 | 0 | 0 | 0.3 (±0.2) n.s. |
| P 7 | 0 | 1.0 (±0.3) n.s. | 0 | 2.8 (±0.8) n.s. |
| P 8 | 1.0 (±0.1) | 2.4 (±0.5) n.s. | 4.2 (±1.0) | 8.1 (±2.3) ∗∗ |
| P 10 | 13.0 (±1.0) | 12.3 (±1.5) n.s. | 25.9 (±2.6) | 26.9 (±1.6) n.s. |
| P 13 | 38.4 (±1.9) | 33.4 (±2.3) ∗∗ | 45.7 (±0.9) | 44.9 (±0.6) n.s. |
| W 3 | 56.1 (±0.3) | 55.5 (±2.3) n.s. | n.m. | n.m. |
| W 6 | 67.7 (±0.4) | 61.5 (±1.8) (∗∗) | n.m. | n.m. |
| W 8 | 70.5 (±1.6) | 67.7 (±0.6) n.s. | n.m. | n.m. |
P, postnatal day; W, week; n = 3; n.m., no measurements; n.s., not significant; ∗∗ indicate significant differences between TRAP deficient mice and Wt mice. (∗∗) the MCV/EV contrast at W 6 can be explained by an increased height of the proliferative zone and resting zone in TRAP−/− mice. p < 0.01, two-way ANOVA with Bonferroni’s post test.
Fig. 9.
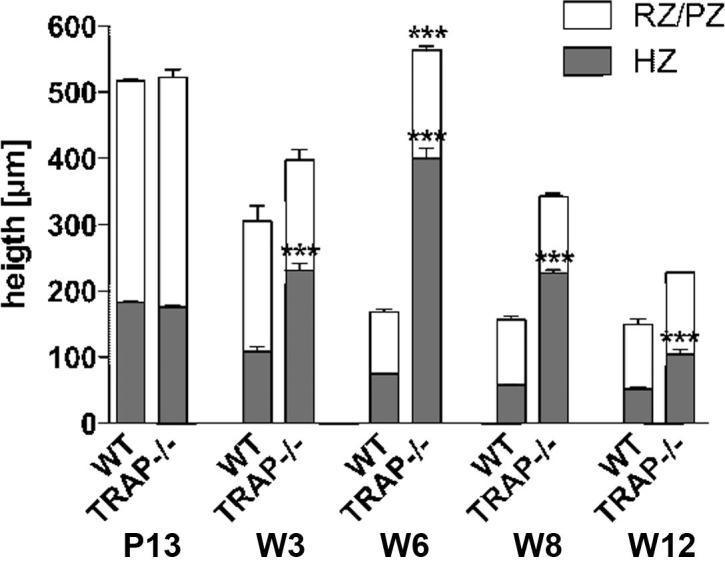
Measurement of the growth plate. The diagram shows the entire height of the growth plate, and the portions of individual zones from P 13 until W 12. n = 3. hypertrophic zone (Hz), resting and proliferating zone (rz/pz). ∗∗∗ indicate significant differences between TRAP deficient mice and Wt mice. p < 0.001, two-way ANOVA with Bonferroni’s post test.
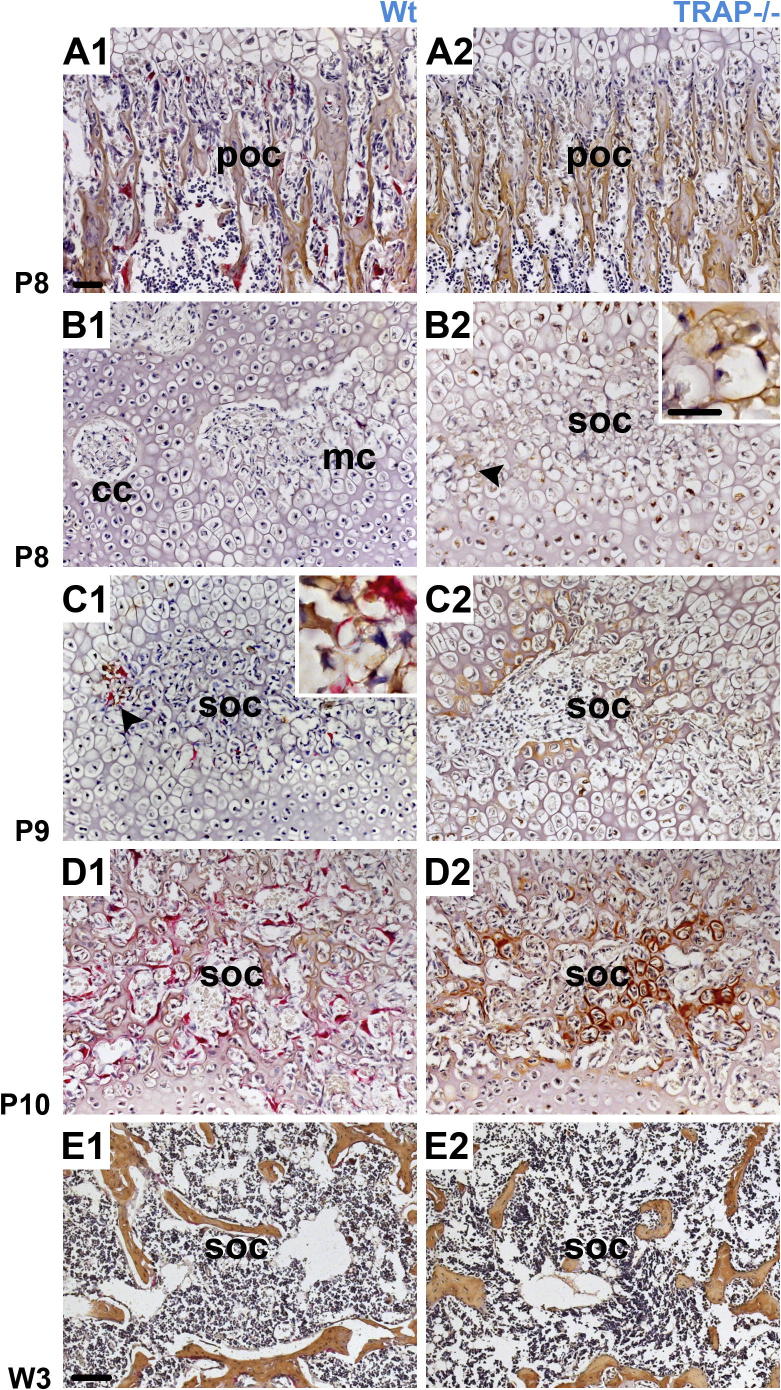
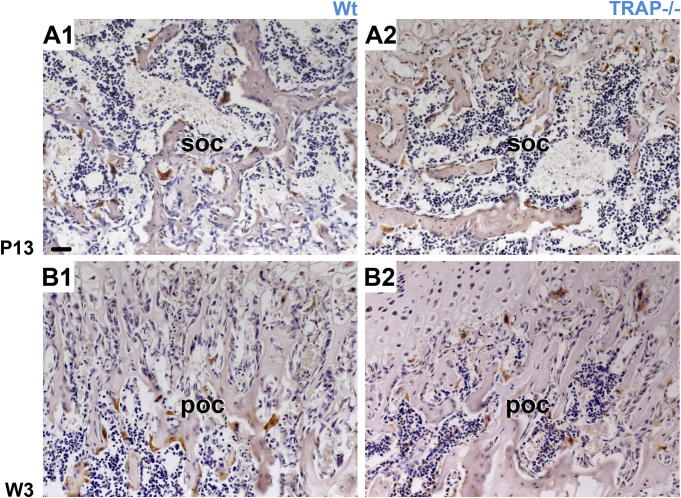
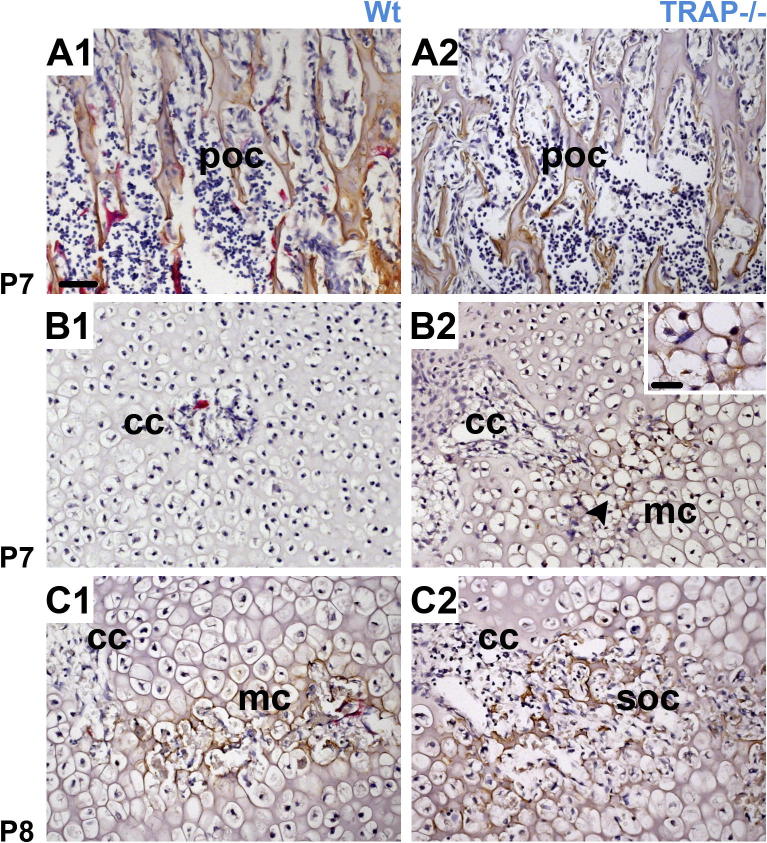
In the epiphyseal marrow cavity, no difference in the morphology of the bone trabeculae was noted between Wt animals and TRAP−/− mice in the course of development (Fig. 2A1–F2, Fig. 4E1 and E2). In the metaphysis of TRAP deficient mice, the trabeculae became thicker, and their number increased in the course of development compared to Wt mice. This was initially observed at W 3 and also seen in older animals (Fig. 2D2–F2 and Fig. 5B2).
Fig. 4.
HC and IHC. Type I collagen (brown staining) and TRAP positive cells (red staining). In Wt mice, numerous TRAP positive chondro/osteoclasts border the cartilage and the bone matrix, respectively during the formation of the SOC. The sections are counterstained with hematoxylin. A1 and A2 Longitudinal sections through the POC (poc). At P 8 the bone matrix is strongly stained, and numerous chondro/osteoclasts are visible in the Wt mice. B1–E2 Longitudinal sections through the developing epiphysis show the cartilage canals (cc), the marrow cavity (mc) and the SOC (soc). B1–C2 In TRAP deficient mice the SOC is formed at P 8. In the controls it emerges at P 9. The arrowheads indicate the staining for type I collagen. Insets show a higher magnification of the type I collagen layer and the micrographs have the same magnification. Bar = 20 μm. D1–E2 At P 10 and W 3 the SOC (soc) is of similar size. Note that at W 3 the bone trabeculae have a similar morphological appearance in Wt controls and TRAP mutants. A1–D2 The micrographs have the same magnification; bar = 50 μm. E1 and E2 The micrographs have the same magnification; bar = 100 μm.
Fig. 5.
IHC: (Cathepsin K) A1–B2 Section through the epiphysis and metaphysis show cathepsin K positive chondro/osteoclasts. In Wt and TRAP−/− mice chondro/osteoclasts develop to the same extent in the SOC (soc) as well as the POC (poc) and their distribution is depicted at P 13 and W 3, respectively. Within the POC numerous thickened trabeculae, indicative for the osteopetrotic phenotype, are found in the knockouts. The sections have the same magnification and are counterstained with hematoxylin; bar = 50 μm.
Taken together, the formation of cartilage canals was not affected in TRAP−/− mice. In the knockouts, however, cartilage hypertrophy and formation of the marrow cavity occurred prior to the controls. At W 3 the expansion of the marrow cavity was similar in Wts and TRAP deficient mice, and this did not alter throughout development.
3.2. Mineralization of the epiphyseal cartilage matrix
Hypertrophy of the epiphyseal chondrocytes triggers the mineralization of the surrounding cartilage (Allerstorfer et al., 2010; Alvarez et al., 2005b). We therefore examined whether the premature development of the hypertrophic chondrocytes in TRAP−/− mice was accompanied by calcification of the cartilage matrix. Calcified regions were visualized by von Kossa staining. In Wt mice there were no signs of mineralization at P 6 and 7 (Fig. 3A1 and B1). In contrast, in the TRAP−/− mice examination of the serial sections revealed that a few consecutive sections labeled positively at P 6, and the dark reaction product was associated with the hypertrophic cartilage. At P 7 the number of von Kossa positive sections as well as the area of calcification had increased (Fig. 3A2 and B2). We want to emphasize that in Wt as well as TRAP−/− mice at P 6 and 7, respectively, every section was subjected to von Kossa staining in order to be certain that no areas of mineralization were overlooked. In Wt mice mineralization started at P 8, and a large number of consecutive sections stained positively. However, these areas were small compared to those of the TRAP deficient mice at this point of time (Fig. 3C1 and C2). This was confirmed by the calculations of the mineralized cartilage volume (minCV) to the EV (Table 1). At P 10 and 13 the minCV/EV revealed no difference between Wt and TRAP−/− mice (table l and Fig. 3D1, D2, E1 and E2). We could not calculate the minCV/EV in older animals because we were unable to cut complete series of proper sections through the bones due to the increased mineralization of the tissue in the course of development.
Fig. 3.
HC (von Kossa reaction) A1–E2 Longitudinal sections through mid-region of the condoyles reveal the presence of mineralization (P 6–P 13). The sections demonstrate the epiphysis, the growth plate and the POC (poc) that is highly mineralized from P 6 onward. A1–C2 In TRAP−/− mice small areas of the cartilage matrix are mineralized at P 6. In the Wt mice mineralization occurs at P 8, and at this moment the area is smaller compared to the knockouts. D1–E2 During further development the epiphyseal cartilage is mineralized to the same extent. In C1 and C2 the perimeter of the mineralized area and the epiphysis are depicted with yellow ink. For each developmental stage and genotype, the areas were measured and the minCV/EV was calculated. All sections are counterstained with hematoxylin and have the same magnification; bar = 500 μm.
3.3. Localization of osteoblast markers
We further explored whether the premature formation of the epiphyseal marrow cavity in TRAP−/− mice had an effect on the moment of the bone matrix formation. Thus, we investigated the localization of type I collagen, the major protein of the bone. In knockouts, a thin layer of type I collagen was laid down by the osteoblasts onto non-resorbed cartilage spicules at P 8. It was irregularly distributed within the marrow cavity since some zones exhibited no labeling (Fig. 4B2), suggesting that an initial SOC was present at this very moment. In Wt littermates type I collagen could not be detected at P 8 (Fig. 4B1). Sections through the metaphysis of the femur served as a control, and an identical labeling pattern was visible at P 8 (Fig. 4A1 and A2). In Wt controls bone matrix was noticed in small regions of the marrow space at P 9. Collagen localization had increased at P 10 but some areas still displayed no staining (Fig. 4C1 and D1). In the TRAP−/− mice the whole marrow space was lined by type I collagen at P 9, and immunoreactivity was remarkably intense at P 10 (Fig. 4C2 and D2). The expansion of the SOC ran parallel to that of the marrow cavity. At P 13 staining for type I collagen remained stronger in the TRAP−/− mice than in Wts (data not shown) but at W 3 the staining intensity was alike in both genotypes (Fig. 4E1 and E2). In Wt littermates, numerous TRAP-positive cells were noted during the formation of the marrow cavity and the SOC. Prior to bone matrix formation, these cells should be referred to as chondroclasts. After bone matrix was laid down onto the cartilage remnants, TRAP-positive cells should rather be termed osteoclasts. However, in the present study, for reasons of simplification, we referred to the TRAP-positive cells as chondro/osteoclasts. We want to emphasize that throughout development the chondro/osteoclasts developed to the same extent in Wt controls as in TRAP−/− mice and as an example we showed their distribution at P 13 and W 3 (Fig. 5A1–B2).
To get an indication whether other bone relevant proteins were affected in the knockouts we examined the localization of osteopontin, which was proposed to be dephosphorylated by the TRAP enzyme (Ek-Rylander et al., 1994). Osteopontin is important for the adhesion, spreading and migration of the chondro/osteoclasts (Franzen et al., 2008; Ishizeki et al., 1998; Natasha et al., 2006; Reinholt et al., 1990). In TRAP−/− mice osteopontin was localized at P 7, but it was not seen in the controls. Osteopontin was restricted to the branched segment of the cartilage canals where a continuous seam surrounded the non-resorbed mineralized cartilage matrix (Fig. 6B1 and B2). Sections through the metaphysis served as a control, and a similar staining pattern was encountered at P 7 (Fig. 6A1 and A2). In Wt littermates a distinct layer of osteopontin was visible in the marrow space at P 8 and no differences in the staining intensity were observed between control and knockout mice (Fig. 6C1 and C2).
Fig. 6.
HC and IHC. TRAP positive cells (red staining) and osteopontin (brown staining). A1, A2 Longitudinal sections through the POC (poc). At P 7, a distinct layer of osteopontin lines the trabeculae of the POC, and numerous chondro/osteoclasts are visible in the Wt mice. B1–C2 Longitudinal sections through the developing epiphysis show the cartilage canals (cc), the marrow cavity (mc) and the SOC (soc). B1–C2 Localization of osteopontin is coupled with the onset of the marrow space formation. In TRAP−/− mice labeling for the protein is detected in the branched segment of the cartilage canal (cc) at P 7 whereas in the Wt mice it is observed at P 8. Inset in B2 shows a higher magnification of the area indicated by the arrowhead; bar = 20 μm. All sections have the same magnification and are counterstained with hematoxylin; bar = 50 μm.
In summary, these findings indicated that bone relevant proteins were noticed earlier in TRAP−/− mice.
For the osteoblast differentiation the transcription factor, Runt-related transcription factor 2 (Runx2), is essential and Runx2 induces the expression of type I collagen and osteopontin (Maruyama et al., 2007). Surprisingly, in Wt and TRAP−/− mice expression of Runx2 was observed at the same moment, at P 6. The protein was localized in several cells of the cartilage canals, and we considered these cells as immature osteoblasts or preosteoblasts. In addition, the protein was expressed by several chondrocytes (Fig. 7B1 and B2). Sections through the metaphysis served as positive controls, and an identical labeling pattern was observed at P 6. Runx2 was expressed in the cells of the inner layer of the periosteum, the hypertrophic cells of the growth plate, and the osteoblasts of the POC (Fig. 7A1 and A2). In rats aged one week, a similar expression pattern has been noted in the metaphysis of the femur (Maruyama et al., 2007). In Wt mice at P 8, Runx2 was present in a few preosteoblasts of the marrow spaces whereas in the TRAP deficient mice the majority of the cells (preosteoblasts and osteoblasts) within the SOC labeled positively (Fig. 7C1 and C2). In both genotypes all osteoblasts of the SOC expressed Runx2 at P 13 (data not shown).
Fig. 7.
IHC. The protein expression of Runx2 is shown. A1 and A2 Longitudinal sections through the metaphysis. Runx2 is located in the preosteoblasts as well as osteoblasts of the inner layer of the periosteum (green asterisks). In addition, protein expression is noted in the hypertrophic chondrocytes (Hz, green arrowheads) of the growth plate and the osteoblasts of the POC (poc). B1–C2 Longitudinal sections through the developing epiphysis show the cartilage canals (cc), the marrow cavity (mc) and the SOC (soc). B1 and B2 At P 6 no difference in Runx2 expression is observed. Runx2 positive cells are seen in the lumen of the cartilage canals (cc), and, furthermore, several chondrocytes stained positively. C1 and C2 At P 8, Runx2 expression is encountered in several preosteoblasts within the marrow cavity (mc) of Wt controls. In the knockouts the SOC (soc) is already formed and numerous osteoblasts are Runx2 positive. The sections have the same magnification and are counterstained with hematoxylin; bar = 20 μm.
3.4. Formation of the growth plate and the articular cartilage
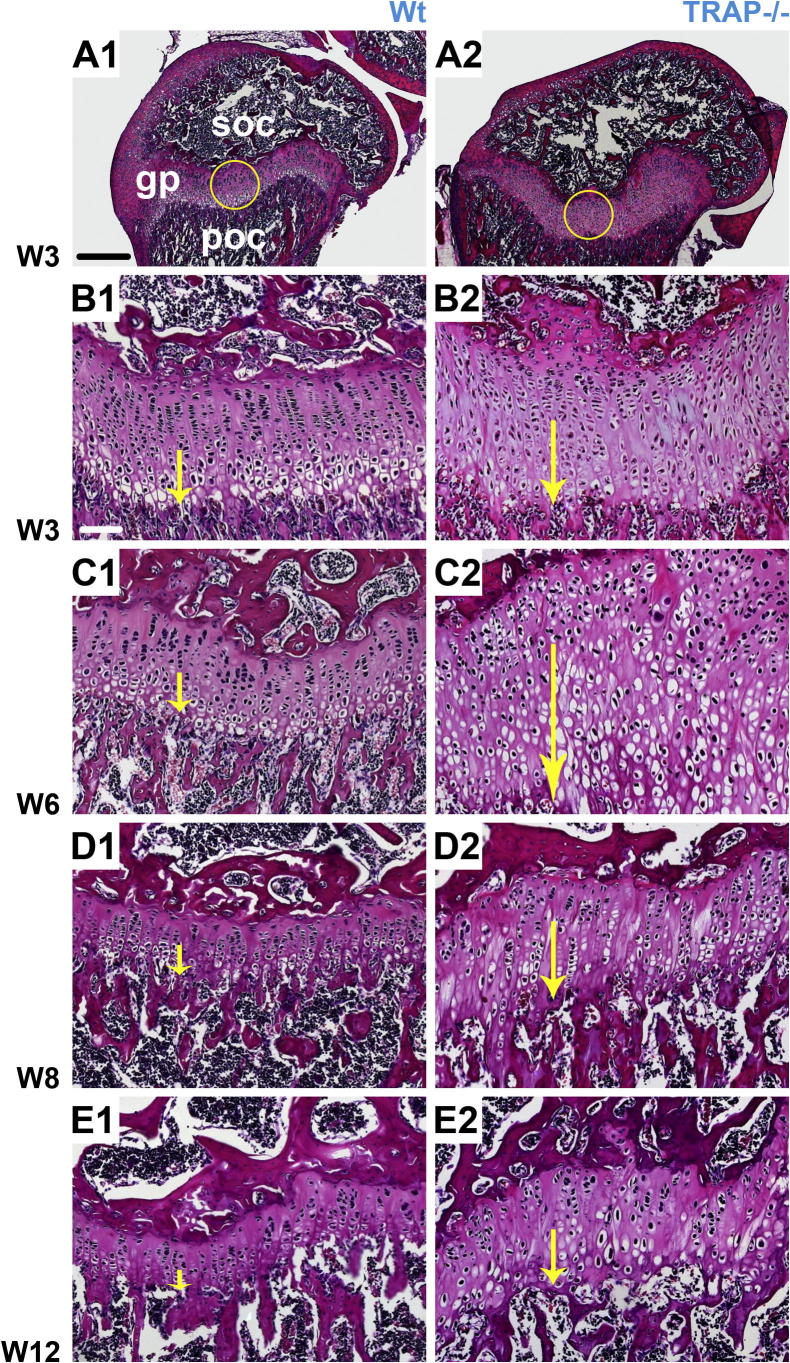
In Wt and TRAP−/− mice the growth plate is a disc that comprises columns of resting, proliferative and hypertrophic chondrocytes. For measurements sections were taken from the mid region of both condoyles and the intercondylar section. The height of the entire growth plate and its hypertrophic zone was measured in triplicates in the midst of the growth plate (Fig. 8A1 and A2). No differences were noted between the single regions of the bone. Our results demonstrated that until P 13 the growth plates were identically structured, and in Wt mice the height of the individual zones clearly decreased from P 13 onward (Fig. 8A1–E1; Fig. 9). However, in the knockouts an increase in both total growth plate height and height of the hypertrophic zone was seen from W 3 until W 12. In addition, the growth plate histology appeared to be altered compared with the controls (Fig. 8B2–E2; Fig. 9). These differences were most conspicuous at W 6 when the entire height of the growth plate was approximately 3-fold and its hypertrophic zone 5-fold higher in comparison to the Wt mice. Furthermore, an increased height of the proliferative zone was noted (Fig. 8 C1 and C2; Fig. 9).
Fig. 8.
HE A1 and A2 Longitudinal sections through the mid-region of the condoyles at W 3 show the SOC, the growth plate (gp), and the POC. The yellow circles indicate the area where the height of the growth plate as well as the hypertrophic zone was measured. The sections have the same magnification; bar 500 μm. B1–E2 Longitudinal sections through the growth plate from W 3 until W 12. TRAP−/− mice show both an increased height of the entire growth plate and the hypertrophic zone (yellow arrow). This is most evident at W 6. The beginning of the hypertrophic zone is defined by the region where the chondrocytes are shaped in such a way that their height equals the width of the cells. In the knockouts the histology of the growth plate appears less organized compared to the controls. The sections have the same magnification; bar 100 μm.
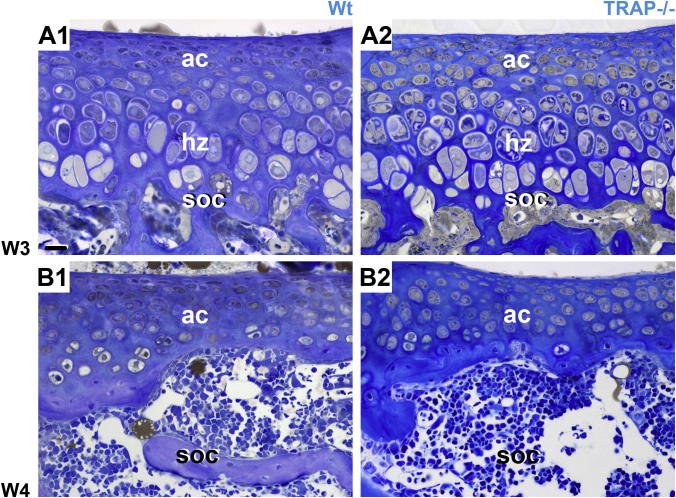
Next, we examined whether the articular cartilage layers were also affected in TRAP−/− mice. In both genotypes these layers were equally remodeled during advancing development. Up to W 3 a zone of mineralized hypertrophic cartilage followed the articular cartilage, but at W 4 hypertrophic cells were not detected anymore (Fig. 10A1, A2, B1 and B2). No differences concerning the height of the articular cartilage layers were observed between Wt controls and TRAP−/− mice throughout development (Fig. 2).
Fig. 10.
Longitudinal semithin resin sections through the epiphysis show the articular cartilage layers and the SOC (soc) at W 3, and W 4. No differences during remodeling of the articular cartilage layers are visible between the genotypes. A1–B2 At W 3 the articular cartilage has not yet gained structural maturity since it comprises layers of hypertrophic cells (Hz). At W 4 hypertrophic layers are not detected anymore, and the articular cartilage has attained its mature structural organization that does not significantly alter with increasing development. The sections counterstained with toluidine blue. The micrographs have the same magnification; bar = 20 μm.
4. Discussion
TRAP has shown to be essential for skeleton formation, and mice lacking the enzyme have altered bone architecture and develop shortened bones (Dai et al., 2004; Hayman et al., 2000; Hayman et al., 1996; Hollberg et al., 2002; Roberts et al., 2007; Suter et al., 2001). According to former studies, a disturbed longitudinal growth of human and murine long bones is due to an interrupted vascularization and ossification of the epiphysis, eventually impairing growth plate function (Brashear, 1963; Holmbeck et al., 1999; Maes et al., 2004; Stickens et al., 2004; Zhou et al., 2000). Thus, we hypothesized an involvement of TRAP in these processes.
In the present investigation we could initially demonstrate that TRAP was clearly not essential for the formation of the vascular network. In Wt controls and TRAP deficient mice, respectively, vascularised cartilage canals appeared at the same developmental stage within the epiphysis, and no differences in their length were discernable. The canals gave rise to the formation of the marrow spaces, and in the TRAP−/− mice they occurred at P 7. In the control littermates the spaces appeared at P 8, being smaller at this moment compared to the knockouts. Formation and advancement of the vascularised cartilage canals into the epiphysis is triggered by VEGF that is expressed in the chondrocytes adjacent to the cartilage canals (Allerstorfer et al., 2010). No differences in VEGF expression were detected between Wt and TRAP−/− mice (data not shown). Thus, VEGF appears not to be involved in the premature development of the marrow spaces in the knockouts. Unlike the metaphysis (Gerber et al., 1999; Harper and Klagsbrun, 1999) hypertrophy and mineralization of the epiphysis occurred after the invasion of the vessels. In the knockouts both were present at P 6, two days earlier compared with the controls but at P 10 and 13, respectively the epiphysis was mineralized to the same extent in both genotypes. Bone forming cells gain access to the epiphysis via cartilage canals, thereby establishing the SOC (Blumer et al., 2007; Holmbeck and Szabova, 2006). We could demonstrate that in TRAP deficient mice, the formation of distinct bone relevant proteins was initiated at an earlier point of time, and consistently type I collagen was present at P 8, whereas it was first noted at P 9 in their control littermates. Osteopontin, another bone specific marker (Franz-Odendaal et al., 2006; Franzen et al., 2008; Reinholt et al., 1990), was associated with the onset of the marrow space formation, and accordingly in TRAP−/− mice the protein was located before it was distinguishable in Wt mice. Runx2 is crucial for osteoblast differentiation, but interestingly its expression occurred at the same point of time in Wt- and TRAP−/− mice, that is at P 6. Taken together, in TRAP deficient mice formation of the marrow spaces, cartilage mineralization and expression of bone relevant proteins occurred prior in comparison to their Wt littermates. The latter finding suggests a premature maturation of the osteoblasts in the knockouts, this being in line with preliminary observations that osteoblasts in culture differentiate earlier when TRAP is absent (Hayman, personal communication).
In long bones, the excavation of the epiphysis is mainly achieved by chondro/osteoclasts that bind to the substrate via their ruffled border. They create a highly acidic milieu, enabling disintegration and finally resorption of the mineralized cartilage and bone matrix (Holmbeck and Szabova, 2006; Reinholt et al., 1990; Suter et al., 2001). We could show that in Wt controls and TRAP−/− mice chondro/osteoclasts were formed to the same extent throughout development, hence the absence of TRAP did not impair their genesis. Moreover, their differentiation is normal, even though several ultrastructural subdomains are altered, resulting in a moderately reduced lysosomal degradation of the mineral crystallites compared to Wt littermates (Hayman et al., 1996; Hollberg et al., 2002; Suter et al., 2001). Apart from TRAP, chondro/osteoclasts secrete tartrate-sensitive lysosomal acid phosphatase (LAP) during the resorptive process. LAP mutants show almost no bone abnormalities but in LAP/TRAP doubly deficient mice, bone malformations are severe, and long bones are distinctly shorter compared to the TRAP knockouts (Hollberg et al., 2002; Suter et al., 2001). Furthermore, in the double knockout mice a distinct enlargement of the liver and spleen has been observed whereas hepatosplenomegaly has never been noted in LAP- and TRAP single knockout mice. This suggests that in distinct tissues the two phosphatases are capable to complement for each other thereby maintaining lysosomal function (Suter et al., 2001). We could demonstrate that after examination of a long postnatal period, epiphyseal excavation and bone formation was hardly impaired in the femur of mice lacking TRAP. Furthermore, trabeculation and reorganization of the articular cartilage layers was not altered in the knockouts. Thus, our data collectively indicate that the absence of TRAP does not affect marrow cavity formation, bone morphology and formation of the articular cartilage, and we suggest that LAP largely compensates for the loss of TRAP. In the knockouts enhanced trabeculation of the metaphysis was observed, but not before W 3. Similarly, in the tibia the osteopetrotic phenotype is manifested 4 weeks after birth, and it was concluded that the resorptive defect in osteoclasts, although modest, is responsible for the increase of the metaphyseal trabecular bone (Hollberg et al., 2002). However, it remains puzzling why the osteopetrotic phenotype in knockouts is only present in the metaphysis but not in the epiphysis.
A timely well-coordinated epiphyseal ossification is a prerequisite for a proper development of the growth plate (Holmbeck and Szabova, 2006; Maes et al., 2004). In MT1-MMP−/− mice, formation of the SOC is delayed, and this is accompanied by growth plate abnormalities, including disorganization and progressive thinning of the proliferative zone. The growth plate function is impaired, resulting in a reduced longitudinal growth of the long bones (Holmbeck et al., 1999). In the femur of TRAP−/− mice the growth plate structure was likewise disorganized and its entire height was expanded. Measurement of the individual zones indicated that the height of the proliferative zone was markedly increased compared to Wt mice at W 6. In contrast, an expansion of the hypertrophic zone was observed from W 3 onwards, this being mainly responsible for the growth plate widening. In Wt mice the height of the hypertrophic zone continuously decreased in the course of development. In TRAP−/− mice the hypertrophic zone height peaked at W 6, declining from then onwards. This is in line with the data obtained from the tibia of TRAP deficient mice aged 1 and 4 months (Hollberg et al., 2002). Hollberg et al. (2002) hypothesized that an impaired function of the chondroclasts, which less effectively resorb the terminal layers of the hypertrophic zone, might be responsible for this phenotype. Despite this failure, a disturbed maturation of the chondrocytes may also contribute to the increased width of the hypertrophic zone.
In summary, our data provide evidence that in TRAP−/− mice the mineralization of the epiphyseal cartilage, formation of the marrow cavity, and localization of bone relevant proteins occur prematurely. Despite these early events, TRAP deficiency does not impair epiphyseal excavation and development of the SOC when a longer postnatal period is examined. In TRAP−/− mice the histology of the growth plate is altered and this is accompanied, above all, by an expansion of the hypertrophic zone. In the knockouts, the osteopetrotic phenotype is only detectable in the metaphysis. We conclude that in long bones, TRAP is critical for the development of the metaphysis and the growth plate. It is, however, remarkable that these histological alterations are not seen in young animals but manifest themselves 3 weeks after birth. In spite of its importance in distinct anatomical sites, TRAP is not essential for epiphyseal vascularization, excavation, and establishment of the SOC.
Acknowledgment
This study was supported by the Austrian Science Fund (FWF) (Grant P20758-B05). The authors thank Prof. H. Dietrich, VMD, of the Central Laboratory Animal Facility, Innsbruck Medical University for helping to raise the wild type animals. We specially thank Mag. H. Hübl for technical assistance, Prof. K. Pfaller, PhD, for his helpful discussion, and C. Siemon and Travieso for carefully reading and correcting the manuscript.
References
- Allerstorfer D., Longato S., Schwarzer C., Fischer-Colbrie R., Hayman A.R., Blumer M.J. VEGF and its role in the early development of the long bone epiphysis. Journal of Anatomy. 2010;216:611–624. doi: 10.1111/j.1469-7580.2010.01223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez J., Costales L., Lopez-Muniz A., Lopez J.M. Chondrocytes are released as viable cells during cartilage resorption associated with the formation of intrachondral canals in the rat tibial epiphysis. Cell and Tissue Research. 2005;320:501–507. doi: 10.1007/s00441-004-1034-z. [DOI] [PubMed] [Google Scholar]
- Alvarez J., Costales L., Serra R., Balbin M., Lopez J.M. Expression patterns of matrix metalloproteinases and vascular endothelial growth factor during epiphyseal ossification. Journal of Bone and Mineral Research: the Official Journal of the American Society for Bone and Mineral Research. 2005;20:1011–1021. doi: 10.1359/JBMR.050204. [DOI] [PubMed] [Google Scholar]
- Blumer M.J., Gahleitner P., Narzt T., Handl C., Ruthensteiner B. Ribbons of semithin sections: an advanced method with a new type of diamond knife. Journal of Neuroscience Methods. 2002;120:11–16. doi: 10.1016/s0165-0270(02)00166-8. [DOI] [PubMed] [Google Scholar]
- Blumer M.J., Longato S., Schwarzer C., Fritsch H. Bone development in the femoral epiphysis of mice. the role of cartilage canals and the fate of resting chondrocytes. Developmental dynamics: An Official Publication of the American Association of Anatomists. 2007;236:2077–2088. doi: 10.1002/dvdy.21228. [DOI] [PubMed] [Google Scholar]
- Blumer M.J., Longato S., Fritsch H. Localization of tartrate-resistant acid phosphatase (TRAP), membrane type-1 matrix metalloproteinases (MT1-MMP) and macrophages during early endochondral bone formation. Journal of Anatomy. 2008;213:431–441. doi: 10.1111/j.1469-7580.2008.00958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumer, M.J., Longato, S., Fritsch, H., 2008b. Structure, formation and role of cartilage canals in the developing bone. Annals of anatomy = Anatomischer Anzeiger: official organ of the Anatomische Gesellschaft 190, 305–315. [DOI] [PubMed]
- Brashear H.R., Jr. Epiphyseal avascular necrosis and its relation to longitudinal bone growth. The Journal of Bone and Joint Surgery American Volume. 1963;45:1423–1438. [PubMed] [Google Scholar]
- Burstone M.S. Histochemical demonstration of acid phosphatase activity in osteoclasts. The journal of Histochemistry and Cytochemistry: Official Journal of the Histochemistry Society. 1959;7:39–41. doi: 10.1177/7.1.39. [DOI] [PubMed] [Google Scholar]
- Dai X.M., Zong X.H., Akhter M.P., Stanley E.R. Osteoclast deficiency results in disorganized matrix, reduced mineralization, and abnormal osteoblast behavior in developing bone. Journal of Bone And Mineral Research: the Official Journal of the American Society for Bone and Mineral Research. 2004;19:1441–1451. doi: 10.1359/JBMR.040514. [DOI] [PubMed] [Google Scholar]
- Davoli M.A., Lamplugh L., Beauchemin A., Chan K., Mordier S., Mort J.S., Murphy G., Docherty A.J., Leblond C.P., Lee E.R. Enzymes active in the areas undergoing cartilage resorption during the development of the secondary ossification center in the tibiae of rats aged 0–21 days: II. Two proteinases, gelatinase B and collagenase-3, are implicated in the lysis of collagen fibrils. Developmental dynamics: An Official Publication of the American Association of Anatomists. 2001;222:71–88. doi: 10.1002/dvdy.1160. [DOI] [PubMed] [Google Scholar]
- Ek-Rylander B., Flores M., Wendel M., Heinegard D., Andersson G. Dephosphorylation of osteopontin and bone sialoprotein by osteoclastic tartrate-resistant acid phosphatase. Modulation of osteoclast adhesion in vitro. The Journal of Biological Chemistry. 1994;269:14853–14856. [PubMed] [Google Scholar]
- Franzen A., Hultenby K., Reinholt F.P., Onnerfjord P., Heinegard D. Altered osteoclast development and function in osteopontin deficient mice. Journal of Orthopaedic Research: Official Publication of the Orthopaedic Research Society. 2008;26:721–728. doi: 10.1002/jor.20544. [DOI] [PubMed] [Google Scholar]
- Franz-Odendaal T.A., Hall B.K., Witten P.E. Buried alive: how osteoblasts become osteocytes. Developmental dynamics: An Official Publication of the American Association of Anatomists. 2006;235:176–190. doi: 10.1002/dvdy.20603. [DOI] [PubMed] [Google Scholar]
- Gerber H.P., Vu T.H., Ryan A.M., Kowalski J., Werb Z., Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nature medicine. 1999;5:623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- Harper J., Klagsbrun M. Cartilage to bone–angiogenesis leads the way. Nature medicine. 1999;5:617–618. doi: 10.1038/9460. [DOI] [PubMed] [Google Scholar]
- Hayman A.R., Cox T.M. Tartrate-resistant acid phosphatase knockout mice. Journal of Bone And Mineral Research: the Official Journal of the American Society for Bone and Mineral Research. 2003;18:1905–1907. doi: 10.1359/jbmr.2003.18.10.1905. [DOI] [PubMed] [Google Scholar]
- Hayman A.R., Jones S.J., Boyde A., Foster D., Colledge W.H., Carlton M.B., Evans M.J., Cox T.M. Mice lacking tartrate-resistant acid phosphatase (Acp 5) have disrupted endochondral ossification and mild osteopetrosis. Development. 1996;122:3151–3162. doi: 10.1242/dev.122.10.3151. [DOI] [PubMed] [Google Scholar]
- Hayman A.R., Bune A.J., Cox T.M. Widespread expression of tartrate-resistant acid phosphatase (Acp 5) in the mouse embryo. Journal of Anatomy. 2000;196(Pt 3):433–441. doi: 10.1046/j.1469-7580.2000.19630433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollberg K., Hultenby K., Hayman A., Cox T., Andersson G. Osteoclasts from mice deficient in tartrate-resistant acid phosphatase have altered ruffled borders and disturbed intracellular vesicular transport. Experimental Cell Research. 2002;279:227–238. doi: 10.1006/excr.2002.5612. [DOI] [PubMed] [Google Scholar]
- Hollberg K., Nordahl J., Hultenby K., Mengarelli-Widholm S., Andersson G., Reinholt F.P. Polarization and secretion of cathepsin K precede tartrate-resistant acid phosphatase secretion to the ruffled border area during the activation of matrix-resorbing clasts. Journal of Bone and Mineral Metabolism. 2005;23:441–449. doi: 10.1007/s00774-005-0626-3. [DOI] [PubMed] [Google Scholar]
- Holmbeck K., Szabova L. Aspects of extracellular matrix remodeling in development and disease. Birth defects research. Part C, Embryo Today: Reviews. 2006;78:11–23. doi: 10.1002/bdrc.20064. [DOI] [PubMed] [Google Scholar]
- Holmbeck K., Bianco P., Caterina J., Yamada S., Kromer M., Kuznetsov S.A., Mankani M., Robey P.G., Poole A.R., Pidoux I., Ward J.M., Birkedal-Hansen H. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- Hunziker E.B., Kapfinger E., Geiss J. The structural architecture of adult mammalian articular cartilage evolves by a synchronized process of tissue resorption and neoformation during postnatal development. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2007;15:403–413. doi: 10.1016/j.joca.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Ishizeki K., Nomura S., Takigawa M., Shioji H., Nawa T. Expression of osteopontin in Meckel’s cartilage cells during phenotypic transdifferentiation in vitro, as detected by in situ hybridization and immunocytochemical analysis. Histochemistry and Cell Biology. 1998;110:457–466. doi: 10.1007/s004180050307. [DOI] [PubMed] [Google Scholar]
- Kugler J.H., Tomlinson A., Wagstaff A., Ward S.M. The role of cartilage canals in the formation of secondary centres of ossification. Journal of Anatomy. 1979;129:493–506. [PMC free article] [PubMed] [Google Scholar]
- Lee E.R., Lamplugh L., Kluczyk B., Leblond C.P., Mort J.S. Neoepitopes reveal the features of type II collagen cleavage and the identity of a collagenase involved in the transformation of the epiphyses anlagen in development. Developmental Dynamics: An Official Publication of the American Association of Anatomists. 2009;238:1547–1563. doi: 10.1002/dvdy.21960. [DOI] [PubMed] [Google Scholar]
- Maes C., Stockmans I., Moermans K., Van Looveren R., Smets N., Carmeliet P., Bouillon R., Carmeliet G. Soluble VEGF isoforms are essential for establishing epiphyseal vascularization and regulating chondrocyte development and survival. The Journal of Clinical Investigation. 2004;113:188–199. doi: 10.1172/JCI19383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama Z., Yoshida C.A., Furuichi T., Amizuka N., Ito M., Fukuyama R., Miyazaki T., Kitaura H., Nakamura K., Fujita T., Kanatani N., Moriishi T., Yamana K., Liu W., Kawaguchi H., Komori T. Runx2 determines bone maturity and turnover rate in postnatal bone development and is involved in bone loss in estrogen deficiency. Developmental Dynamics: An Official Publication of the American Association of Anatomists. 2007;236:1876–1890. doi: 10.1002/dvdy.21187. [DOI] [PubMed] [Google Scholar]
- Minkin C. Bone acid phosphatase: tartrate-resistant acid phosphatase as a marker of osteoclast function. Calcified Tissue International. 1982;34:285–290. doi: 10.1007/BF02411252. [DOI] [PubMed] [Google Scholar]
- Natasha T., Kuhn M., Kelly O., Rittling S.R. Override of the osteoclast defect in osteopontin-deficient mice by metastatic tumor growth in the bone. The American Journal of Pathology. 2006;168:551–561. doi: 10.2353/ajpath.2006.050480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega N., Behonick D.J., Werb Z. Matrix remodeling during endochondral ossification. Trends in Cell Biology. 2004;14:86–93. doi: 10.1016/j.tcb.2003.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen W., Tsokos M., Pufe T. Expression of VEGF121 and VEGF165 in hypertrophic chondrocytes of the human growth plate and epiphyseal cartilage. Journal of Anatomy. 2002;201:153–157. doi: 10.1046/j.1469-7580.2002.00085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinholt F.P., Hultenby K., Oldberg A., Heinegard D. Osteopontin–a possible anchor of osteoclasts to bone. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:4473–4475. doi: 10.1073/pnas.87.12.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts H.C., Knott L., Avery N.C., Cox T.M., Evans M.J., Hayman A.R. Altered collagen in tartrate-resistant acid phosphatase (TRAP)-deficient mice. a role for TRAP in bone collagen metabolism. Calcified Tissue International. 2007;80:400–410. doi: 10.1007/s00223-007-9032-2. [DOI] [PubMed] [Google Scholar]
- Spurr A.R. A low-viscosity epoxy resin embedding medium for electron microscopy. Journal of Ultrastructure Research. 1969;26:31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Stempel J., Fritsch H., Pfaller K., Blumer M.J. Development of articular cartilage and the metaphyseal growth plate: the localization of TRAP cells, VEGF, and endostatin. Journal of Anatomy. 2011;218:608–618. doi: 10.1111/j.1469-7580.2011.01377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickens D., Behonick D.J., Ortega N., Heyer B., Hartenstein B., Yu Y., Fosang A.J., Schorpp-Kistner M., Angel P., Werb Z. Altered endochondral bone development in matrix metalloproteinase 13-deficient mice. Development. 2004;131:5883–5895. doi: 10.1242/dev.01461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter A., Everts V., Boyde A., Jones S.J., Lullmann-Rauch R., Hartmann D., Hayman A.R., Cox T.M., Evans M.J., Meister T., von Figura K., Saftig P. Overlapping functions of lysosomal acid phosphatase (LAP) and tartrate-resistant acid phosphatase (Acp5) revealed by doubly deficient mice. Development. 2001;128:4899–4910. doi: 10.1242/dev.128.23.4899. [DOI] [PubMed] [Google Scholar]
- Yamaza T., Goto T., Kamiya T., Kobayashi Y., Sakai H., Tanaka T. Study of immunoelectron microscopic localization of cathepsin K in osteoclasts and other bone cells in the mouse femur. Bone. 1998;23:499–509. doi: 10.1016/s8756-3282(98)00138-0. [DOI] [PubMed] [Google Scholar]
- Zhou Z., Apte S.S., Soininen R., Cao R., Baaklini G.Y., Rauser R.W., Wang J., Cao Y., Tryggvason K. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:4052–4057. doi: 10.1073/pnas.060037197. [DOI] [PMC free article] [PubMed] [Google Scholar]