Abstract
Cytokines are critical for normal cell growth and immunoregulation but also contribute to growth of malignant cells and drive immune-mediated disease. A major subset of immunoregulatory cytokines, roughly 60, use the type I and type II cytokine receptors and pharmacological targeting of these cytokines/cytokines receptors has proven to be efficacious in treating immune and inflammatory diseases. These receptors rely on Janus family of kinases (Jaks) for signal transduction and recently the first Jak inhibitor has been approved by the FDA. Many other Jakinibs are likely to follow and in this brief review, we will discuss the state-of-the art of this new class of pharmacological agents.
Introduction
Enabled by advances in molecular biology, it is now clear that an array of cytokines controls the growth and differentiation of hematopoietic cells and orchestrate all aspects of immune response. [1] From the differentiation of stem cells to the inciting events precipitated by activation of innate immune cells and the fine-tuning of helper T cell responses, cytokines play pivotal roles. However, cytokines are also fundamentally important for immune-mediated disease. A large segment of the population of industrialized countries suffers from asthma and allergy and a range of autoimmune diseases. In addition though, it is increasingly recognized that inflammation and dysregulation of cytokine production are directly involved in the pathophysiology of many other diseases including atherosclerosis and metabolic syndrome, degenerative neurologic disease and cancer. For these reasons, therapeutic targeting of cytokines has immense potential.
The advent of monoclonal antibody technology and the ability to create therapeutically useful recombinant cytokine receptors has dramatically changed the therapeutic landscape of a wide variety of diseases. Thanks to “biologics” debilitating diseases like rheumatoid arthritis which were previously associated with inexorable joint destruction, can be effectively treated. The question then arises: can the actions of cytokines be blocked by targeting intracellular signal transduction? In other words, might a pill be as efficacious as a parenteral biologic?
Janus kinases and signaling by Type I/II cytokine receptors
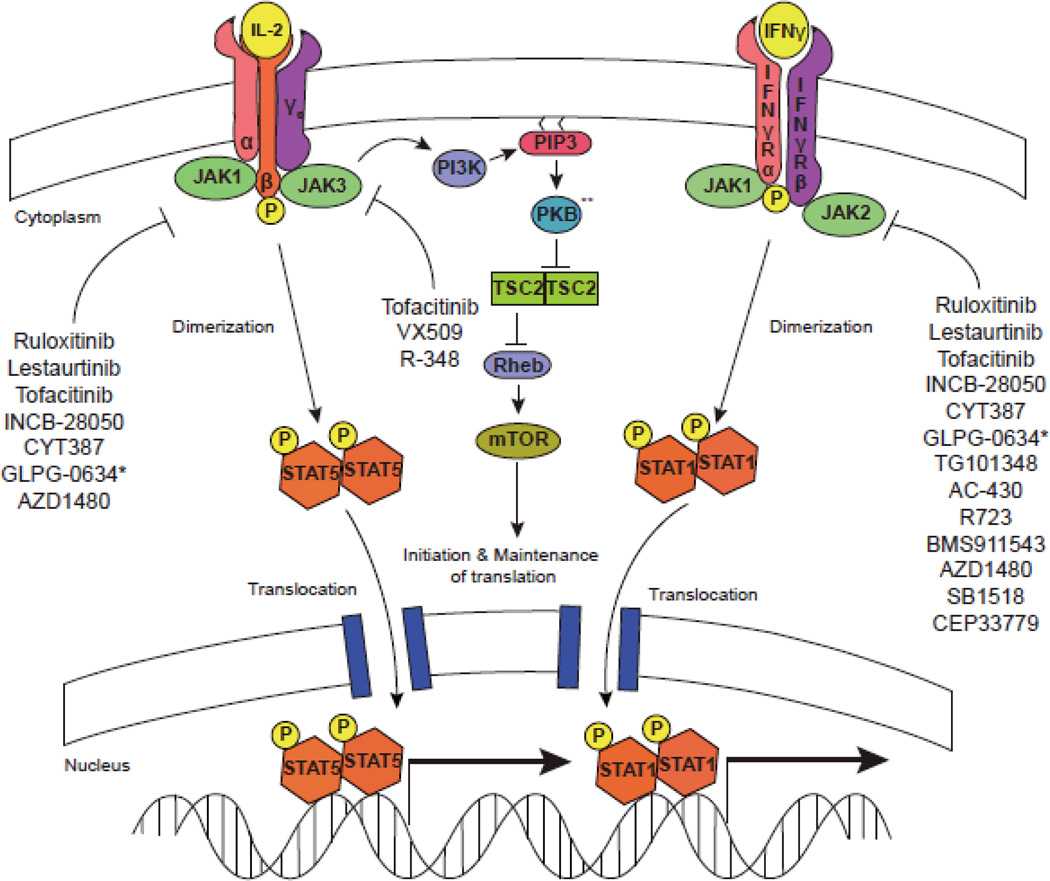
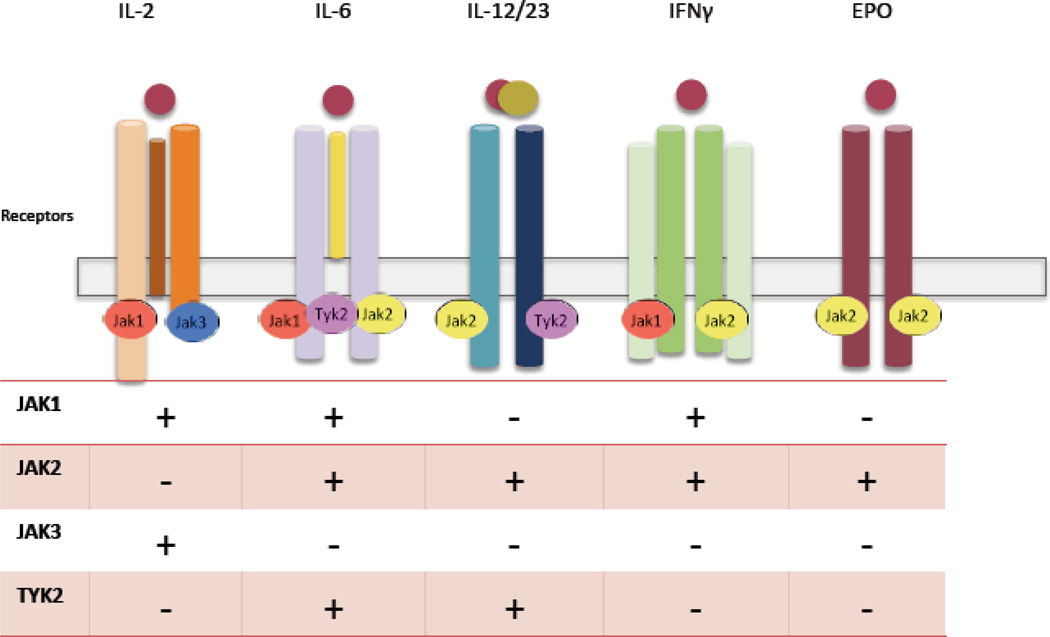
The family of cytokines that bind type I and type II cytokine receptors includes interleukins, interferons, and colony stimulating factor, as well as classic hormones such erythropoietin, prolactin and growth hormone. [2] Signaling via these receptors is dependent upon a small family of structurally distinct kinases with apparently circumscribed function. (Figure 1) Janus family of kinases (Jaks) comprises four members Tyk2, Jak1, Jak2 and Jak3 [3], which selectively associate with membrane proximal domains of type I and II receptors in different combinations. Upon ligand binding, Jaks phosphorylate cytokine receptors. In this way, they induce recruitment of various signaling intermediates including the Stat family of transcription factors, which directly modulate gene transcription. [4, 5] (Figure 2)
Figure 1.
Jakinibs block multiple aspects of cytokine signaling. Cytokine binding to its cognate receptor leads to phosphorylation of the intracellular domain of the tyrosine kinase receptor by specific Jaks. STATs are then recruited, bind to the receptor and become phosphorylated by Jaks. This results in STAT dimerization, translocation, and regulation of gene transcription. Cytokines also activate the PKB (Akt) and mTOR. Though not carefully studied, it is highly likely that blocking proximal cytokine signals will disrupt all downstream pathways. ** Also referred to as AKT.
Figure 2.
Impact of inhibiting various Jaks on signaling by different cytokines
The importance of Jaks in cytokine signaling was initially recognized in a series of mutant cell lines. [1, 4, 6], but the first evidence of the non-redundant, essential function of the Jaks in vivo came from patients with primary immunodeficiency.
Leonard and colleagues had recognized that absence of the receptor subunit denoted the common gamma chain, γc (encoded by IL2RG), results in the disorder X-linked severe combined immunodeficiency (X-SCID). [7] Jak3 was found to be uniquely associated with this receptor subunit and it was quickly recognized that mutations of JAK3 cause autosomal recessive SCID. [8–10]Shortly after this initial discovery, mouse knockout models were generated for the various JAKS and STATs, establishing their essential, non-redundant functions. [11] Because of this essential, but discrete functionality, it was predicted at this time that Jak antagonists would represent a new class of immunomodulatory drugs. [8]
Feasibility of kinases as therapeutic targets
At that time though, it was by no means a given that kinases were good therapeutic targets. Recall that this work preceded our present understanding of the human kinome. Of course, we now know that there are 518 kinases that can be divided into eight distinct families. Jaks belong to the tyrosine protein kinase family of which there are 90 other members. Because of the conserved kinase domain structure, it might be assumed that attaining the needed specificity to inhibit a certain kinase would be an insurmountable task. Moreover, most of the commonly used kinase inhibitors are competitive ATP inhibitors and for these reasons, the prospect of developing specific kinase inhibitors as therapeutic targets seemed tenuous. Remarkably though, kinases have turned out to be excellent targets and there are now 13 FDA-approved kinase inhibitors.
Once kinase inhibitors entered the clinic, it became apparent that the drugs did not target a single kinase; in fact, none of the currently approved kinase inhibitor fit this profile. [12, 13] Imatininib, which inhibits BCR-Abl in chronic myelogenous leukemia (CML) induces remission more than 90% of patients in the early stages of the disease. [14, 15] However, imatinib also inhibits the PDGFR kinase and KIT receptor tyrosine kinase. [12, 13] This is beneficial as it expands imatinib’s therapeutic utility to gastrointestinal stromal tumors (GISTs) [16] and idiopathic hypereosinophilic syndrome. [17] An issue with the use of kinase inhibitors in cancer was the emergence of resistance. This led to generation of “multikinase” inhibitors such as sunitinib, dasatinib [18] and nilotinib [19]. This established the principle that kinase inhibitors need not be absolutely specific to be clinically useful.
Targeting Janus kinases in autoimmune diseases and transplant rejection
The first selective Jak inhibitor to be tested in humans was tofacitinib (formerly designated CP-690,550). Tofacitinib potently inhibits Jak3 (IC 50, 2.2nM) and Jak1 and to a lesser extent Jak2 (IC 50, 5.0nM). It has little effect on Tyk2 (IC 50 260nM). [20] Remarkably, tofacitinib has potent activity against Jaks with little effect on other kinases. [21]
The utility of tofacitinib as an immunomodulatory drug was established in a variety of transplant models (murine and nonhuman primates) [22, 23] as well as in rheumatoid arthritis models. [24] The efficacy and safety of tofacitinib has been studied in multiple Phase II and Phase III trials in rheumatoid arthritis (RA), inflammatory bowel disease, psoriasis and transplant rejection. [25–28] Phase III trials in RA assessing efficacy of tofacitinib both as monotherapy [29] and combined with methotrexate in patients who failed DMARDs [30] met their primary endpoint, confirming results from the respective phase II trials. [27, 31] Importantly, structural damage was also prevented. [32] Of note, tofacitinib was found to be non-inferior to adalimumab, a TNF inhibitor, in the background of methotrexate [26] and was also efficacious in patients who had failed multiple biologics. [33]
With respect to tofacitinib’s mechanism of action, as a potent Jak3 inhibitor, it efficiently blocks common γc cytokines including IL-2, IL-4, IL-15 and IL-21. Consequently, Th2 differentiation is blocked and the drug is efficacious in models of allergic disease. [34] Tofacitinib’s ability to block Jak1 and Jak2 inhibits signaling by IFN-γ, IL-6 and to a lesser extent IL-12 and IL-23. [20] Th1 differentiation is therefore blocked, as is the generation of pathogenic Th17 cells [20, 35] Tofacitinib also abrogates innate responses limiting the production of tumor necrosis factor and other proinflammatory cytokines in an LPS model. [20] Tofacitinib also blocks the effects IL-6 and Type I interferons on synovial fibroblasts, inhibiting chemokine expression. [36]
Tofacitinib’s side effects appear to be directly related to its mode of action. (Table 1) Upper respiratory and other infections are among the common adverse effects, but opportunistic infections are uncommon. Anemia and neutropenia, presumably related to Jak 2 inhibition and interference with signaling by erythropoietin and other colony-stimulating factors. High LDL was also noted, as has been seen with tocilizumab that blocks IL-6. In nonhuman primates treated with tofacitinib, numbers of CD4+ T cells do not change, but NK cells and CD8+ T cells can decline. [37, 38] It remains to be determined whether this will be problematic in humans treated with doses of tofacitinib that control autoimmune disease. Although IL-2 is thought to be important for regulatory T cell homeostasis, a decline in functional T reg cells has not been noted. [39]
Table 1.
Expected impact of Jak inhibition on cytokine signaling based on their functional role
| Jaks | Cytokines affected upon inhibition |
Knock-out phenotype |
Impaired functionality |
Predicted side effects |
|---|---|---|---|---|
| Jak1 |
|
Perinatal lethality due to neurologic defects Severe combined immunodeficiency |
|
|
| Jak2 |
|
In utero lethality due to absence of erythropoiesis |
|
|
| Jak3 |
|
Severe combined immunodeficiency |
|
|
| Tyk2 |
|
Normal development but increased viral and bacterial susceptibility |
|
|
Not shown in clinical trials with Jak 3 inhibitors such as tofacitinib
Additional Jak inhibitors are rapidly moving ahead in clinical trials of RA and other autoimmune diseases. Ruxolitinib, which inhibits Jak1 and Jak2, has been beneficial in a highly active RA patient group in a double-blind, placebo controlled Phase IIa trial with superior results in higher doses up to 50 mg twice daily. [40] A topical formulation of ruxolitinib, has also been used in psoriasis with promising results. [41] The Jak1 and Jak2 inhibitor, LY3009104 (formerly INCB028050) demonstrated dose dependent efficacy in active RA patients refractory to disease modifying drugs and biologics. [42] VX-509 is a reportedly specific JAK3 inhibitor, which has shown efficacy in a Phase IIa study in RA. Interestingly though, a selective Jak1 inhibitor, GLPG0634, also showed efficacy in a Phase IIa RA trial. CEP-33779, a selective Jak2 inhibitor demonstrated efficacy in two preclinical models of RA [43]. It also improved SLE nephritis in mice by depleting auto-reactive plasma cells further expanding the therapeutic spectrum. [44] Thus, the relative merits of inhibiting one Jak versus another remains to be determined. At present, there are no selective Tyk2 inhibitors in clinical trials.
Targeting JAKs in myelofibrosis and polycythemia vera
A breakthrough in understanding the pathogenesis of the myeloproliferative diseases, polycythemia vera (PV), essential thrombocythemia (ET) and myelofibrosis (MF) was the discovery of gain-of-function JAK2 mutations. [45] All of these mutations reside in the regulatory kinase-like domain, which has recently been found to have enzymatic activity. [46] In view of the success of imatinib in the treatment of CML, it was logical to consider that the development of a Jak2 inhibitor would be similarly successful.
A Jak1/2 blocker, ruxolitinib, is now the first FDA approved Jak inhibitor [47]. In MF, ruxolitinib reduces splenomegaly and effectively treats systemic disease. Leukemic transformation is an important cause of mortality in MF. It remains to be determined whether ruxolitinib, analogously to imatinib, will reduce this outcome. In addition to anemia and thrombocytopenia a withdrawal syndrome can occur, manifested by exacerbated splenomegaly, cytopenias and occasional hemodynamic decompensation. [48] Interestingly, ruxolitinib and CYT 387 are efficacious even in MF patients with no JAK2 mutations, presumably indicating that these inhibitors act on kinases besides Jak2, re-emphasizing the potential of multikinase inhibitors.
Other Jakinibs that target Jak2 are in development for myeloproliferative disorders. (Table 2) In addition, potential importance of the JAK-STAT pathway in a wide variety of cancers beyond myelofibrosis has long been recognized. [49] Various types of mutations and fusion proteins affecting JAKs have been noted in a range of different leukemias. [50–52]. Furthermore, there is evidence of activation of the Jak/STAT pathway in a variety of solid tumors including head and neck, breast and prostate cancer. [53–55]Constitutive activation of Jaks and Stats can also be due to autocrine cytokine production. [56–59] These findings argue for the testing of Jak inhibitors in these various settings. [49, 60]
Table 2.
Jakinibs in development and testing for autoimmunity and cancer
| Agent | Targeted Jak |
Indication | Stage of Development |
|---|---|---|---|
| Tofacitinib | 3, 1, 2 | RA Psoriasis IBD |
III III III |
| VX-509 | 3 | RA | II |
| R-348 | 3 | RA | I |
| Ruxolitinib | 1, 2 | MF Polycythemia vera Essential thrombocythemia |
FDA approved III II |
| INCB18424 (topical formulation) |
1,2 | Psoriasis | II |
| LY3009104 (formerly INCB-28050) |
1, 2 | RA Psoriasis |
II IIb |
| CYT387 | 1, 2 | MF | I/II |
| GLPG-0634 | 1, 2, Tyk2 | RA | II |
| SAR302503 (TG101348) |
1, 2 | MF | I/II |
| Pacritinib (SB1518) |
2 | MF | II |
| AC-430 | 2 | RA/Lymphoma | Pre-clinical |
| R723 | 2 | Myeloproliferative neoplasias |
Pre-clinical |
| BMS911543 | 2 | MF | Pre-clinical |
| AZD1480 | 1, 2 | Glioblastoma | Pre-clinical |
| CEP-33779 | 2 | RA SLE |
Pre-clinical Pre-clinical |
Bold characters indicate primary target
Abbreviations: RA: rheumatoid arthritis; IBD: inflammatory bowel disease; MF: myelofibrosis; SLE: systemic lupus erythematosus
Conclusions
Despite potential challenges, kinase inhibitors have emerged as an exciting new class of drugs. Given the key role of cytokines in many disorders ranging from malignancy to autoimmunity, Jak inhibitors or Jakinibs have the potential for wide utility in a range of diseases. They have demonstrated efficacy in PV/MF and an array of common autoimmune disorders. The extent to which JAK inhibitors will be used as steroid-sparing agents or even supplant the use of steroids in diseases like the vasculitides or systemic lupus erythematosus remains to be determined. Given the range of tumors that exhibit constitutive Jak/Stat activation, it seems like that Jakinibs will have utility in these disorders too. A surprise in the field is that targeting multiple kinases may not be detrimental, especially in circumstances where multiple cytokines drive pathogenesis. Multi-kinase inhibitors appear to be especially useful in the treatment of cancer. Conversely though, it is conceivable that more selective Jak inhibitors (e.g. selective Jak1, Jak3 and TYK2 inhibitors) might be efficacious with reduced adverse effects related to Jak2 inhibition. It is likely that we will soon see if this is the case given the intense interest in Jakinibs.
References
- 1.O'Shea JJ, Gadina M, Kanno Y. Cytokine signaling: birth of a pathway. Journal of immunology. 2011;187(11):5475–5478. doi: 10.4049/jimmunol.1102913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boulay JL, O'Shea JJ, Paul WE. Molecular phylogeny within type I cytokines and their cognate receptors. Immunity. 2003;19(2):159–163. doi: 10.1016/s1074-7613(03)00211-5. [DOI] [PubMed] [Google Scholar]
- 3.Leonard WJ, O'Shea JJ. Jaks and STATs: biological implications. Annu Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 4.Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264(5164):1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 5.Levy DE, Darnell JE., Jr Stats: transcriptional control and biological impact. Nature reviews. Molecular cell biology. 2002;3(9):651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 6.Velazquez L, et al. A protein tyrosine kinase in the interferon alpha/beta signaling pathway. Cell. 1992;70(2):313–322. doi: 10.1016/0092-8674(92)90105-l. [DOI] [PubMed] [Google Scholar]
- 7.Leonard WJ, Noguchi M, Russell SM. Sharing of a common gamma chain, gamma c, by the IL-2, IL-4, and IL-7 receptors: implications for X-linked severe combined immunodeficiency (XSCID) Adv Exp Med Biol. 1994;365:225–232. doi: 10.1007/978-1-4899-0987-9_23. [DOI] [PubMed] [Google Scholar]
- 8.Russell SM, et al. Mutation of Jak3 in a patient with SCID: essential role of Jak3 in lymphoid development. Science. 1995;270(5237):797–800. doi: 10.1126/science.270.5237.797. [DOI] [PubMed] [Google Scholar]
- 9.Macchi P, et al. Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID) Nature. 1995;377(6544):65–68. doi: 10.1038/377065a0. [DOI] [PubMed] [Google Scholar]
- 10.Buckley RH, et al. Human severe combined immunodeficiency: genetic, phenotypic, and functional diversity in one hundred eight infants. J Pediatr. 1997;130(3):378–387. doi: 10.1016/s0022-3476(97)70199-9. [DOI] [PubMed] [Google Scholar]
- 11.Ghoreschi K, Laurence A, O'Shea JJ. Janus kinases in immune cell signaling. Immunological reviews. 2009;228(1):273–287. doi: 10.1111/j.1600-065X.2008.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Druker BJ, Lydon NB. Lessons learned from the development of an abl tyrosine kinase inhibitor for chronic myelogenous leukemia. J Clin Invest. 2000;105(1):3–7. doi: 10.1172/JCI9083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buchdunger E, et al. Abl protein-tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-kit and platelet-derived growth factor receptors. J Pharmacol Exp Ther. 2000;295(1):139–145. [PubMed] [Google Scholar]
- 14.Schindler T, et al. Structural mechanism for STI-571 inhibition of abelson tyrosine kinase. Science. 2000;289(5486):1938–1942. doi: 10.1126/science.289.5486.1938. [DOI] [PubMed] [Google Scholar]
- 15.Druker BJ, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 16.Demetri GD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347(7):472–480. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 17.Cools J, et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med. 2003;348(13):1201–1214. doi: 10.1056/NEJMoa025217. [DOI] [PubMed] [Google Scholar]
- 18.Hochhaus A, et al. Dasatinib induces notable hematologic and cytogenetic responses in chronic-phase chronic myeloid leukemia after failure of imatinib therapy. Blood. 2007;109(6):2303–2309. doi: 10.1182/blood-2006-09-047266. [DOI] [PubMed] [Google Scholar]
- 19.Rosti G, et al. Dasatinib and nilotinib in imatinib-resistant Philadelphia-positive chronic myelogenous leukemia: a 'head-to-head comparison'. Leuk Lymphoma. 51(4):583–591. doi: 10.3109/10428191003637282. [DOI] [PubMed] [Google Scholar]
- 20.Ghoreschi K, et al. Modulation of innate and adaptive immune responses by tofacitinib (CP-690,550) Journal of immunology. 2011;186(7):4234–4243. doi: 10.4049/jimmunol.1003668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karaman MW, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26(1):127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 22.Changelian PS, et al. Prevention of organ allograft rejection by a specific Janus kinase 3 inhibitor. Science. 2003;302(5646):875–878. doi: 10.1126/science.1087061. [DOI] [PubMed] [Google Scholar]
- 23.Kudlacz E, et al. The novel JAK-3 inhibitor CP-690550 is a potent immunosuppressive agent in various murine models. Am J Transplant. 2004;4(1):51–57. doi: 10.1046/j.1600-6143.2003.00281.x. [DOI] [PubMed] [Google Scholar]
- 24.Milici AJ, et al. Cartilage preservation by inhibition of Janus kinase 3 in two rodent models of rheumatoid arthritis. Arthritis Res Ther. 2008;10(1):R14. doi: 10.1186/ar2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boy MG, et al. Double-blind, placebo-controlled, dose-escalation study to evaluate the pharmacologic effect of CP-690,550 in patients with psoriasis. The journal of investigative dermatology. 2009;129(9):2299–2302. doi: 10.1038/jid.2009.25. [DOI] [PubMed] [Google Scholar]
- 26.Fleischmann R, et al. Phase 2B dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) or adalimumab monotherapy versus placebo in patients with active rheumatoid arthritis with an inadequate response to DMARDs. Arthritis and rheumatism. 2011 doi: 10.1002/art.33383. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka Y, et al. Phase II study of tofacitinib (CP-690,550) combined with methotrexate in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis care & research. 2011;63(8):1150–1158. doi: 10.1002/acr.20494. [DOI] [PubMed] [Google Scholar]
- 28.Wojciechowski D, Vincenti F. Targeting JAK3 in kidney transplantation: current status and future options. Current opinion in organ transplantation. 2011;16(6):614–619. doi: 10.1097/MOT.0b013e32834c23ce. [DOI] [PubMed] [Google Scholar]
- 29.Wollenhaupt J, SJC, Lee EB, Wood S, Soma K, Wang L, Nakamura H, Komuro Y, Nduaka CI, Gruben D, Zwillich SH, Bradley JD. Tofacitinib (CP-690,550), an oral Janus kinase inhibitor, in the treatment of Rheumatoid arthritis: open label, long-term extension studies up to 36 months. Arthritis & rheumatism. 2011;63(10 Suppl):161–162. [Google Scholar]
- 30.Kremer J, LZ-G, Hall S, Fleischmann R, Genovese M, Martin-Mola E, Isaacs J, Gruben D, Wallenstein G, Krishnaswami S, Zwillich S, Koncz T, Riese R, Bradley J. TOFACITINIB (CP-690,550), AN ORAL JAK INHIBITOR, IN COMBINATION WITH TRADITIONAL DMARDS: PHASE 3 STUDY IN PATIENTS WITH ACTIVE RHEUMATOID ARTHRITIS WITH INADEQUATE RESPONSE TO DMARDS. Ann Rheum Dis. 2011;70(Suppl3):170. [Google Scholar]
- 31.Kremer JM, et al. A Phase 2B dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) versus placebo in combination with background methotrexate in patients with active rheumatoid arthritis and inadequate response to methotrexate alone. Arthritis and rheumatism. 2011 doi: 10.1002/art.33419. [DOI] [PubMed] [Google Scholar]
- 32.Désirée van der Heijde YT, Fleischmann Roy, Keystone Edward C, Kremer JM, Zerbini CAF, Cardiel M, Cohen SB, Nash PT, Song Yeong Wook, Tegzova D, Wyman B, Gruben D, Benda B, Wallenstein G, Zwillich SH, Bradley JD, Connell CA the ORAL Scan investigators. Tofacitinib (CP-690,550), An Oral Janus Kinase Inhibitor, in Combination with Methotrexate Reduced the Progression of Structural Damage in Patients with Rheumatoid Arthritis: a 24-Month Phase 3 Study. Arthritis & rheumatism. 2011:2592. [Google Scholar]
- 33.Burmester Gerd-Reudiger BR, Charles-Schoeman C, Wollenhaupt J, Zerbini B, Benda CAF, Gruben D, Wallenstein G, Krishnaswami S, Zwillich SH, Koncz T, Bradley JD, Mebus CA. Tofacitinib (CP-690,550), an oral Janus kinase inhibitor, in combination with methotrexate, in patients with active rheumatoid arthritis with an inadequate response to tumor necrosis factor inhibitors: A 6-month phase 3 study. Arthritis & rheumatism. 2011;63(10 Suppl):288. [Google Scholar]
- 34.Kudlacz E, et al. The JAK-3 inhibitor CP-690550 is a potent anti-inflammatory agent in a murine model of pulmonary eosinophilia. European journal of pharmacology. 2008;582(1–3):154–161. doi: 10.1016/j.ejphar.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 35.Rosengren S, et al. The JAK inhibitor CP-690,550 (tofacitinib) inhibits TNF-induced chemokine expression in fibroblast-like synoviocytes: autocrine role of type I interferon. Annals of the Rheumatic Diseases. 2011 doi: 10.1136/ard.2011.150284. [DOI] [PubMed] [Google Scholar]
- 36.Maeshima K, et al. A JAK inhibitor tofacitinib regulates synovitis through inhibition of IFN-gamma and IL-17 production by human CD4(+) T cells. Arthritis and rheumatism. 2011 doi: 10.1002/art.34329. [DOI] [PubMed] [Google Scholar]
- 37.Conklyn M, et al. The JAK3 inhibitor CP-690550 selectively reduces NK and CD8+ cell numbers in cynomolgus monkey blood following chronic oral dosing. J Leukoc Biol. 2004;76(6):1248–1255. doi: 10.1189/jlb.0504282. [DOI] [PubMed] [Google Scholar]
- 38.Paniagua R, et al. Effects of JAK3 inhibition with CP-690,550 on immune cell populations and their functions in nonhuman primate recipients of kidney allografts. Transplantation. 2005;80(9):1283–1292. doi: 10.1097/01.tp.0000177643.05739.cd. [DOI] [PubMed] [Google Scholar]
- 39.Sewgobind VD, et al. The Jak inhibitor CP-690,550 preserves the function of CD4CD25FoxP3 regulatory T cells and inhibits effector T cells. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10(8):1785–1795. doi: 10.1111/j.1600-6143.2010.03200.x. [DOI] [PubMed] [Google Scholar]
- 40.Williams W, SP, Shi J, et al. A randomized placebocontrolled study of INCB018424, a selective Janus kinase 1& 2 (JAK1&2) inhibitor in rheumatoid arthritis. Arthritis & rheumatism. 2008;58(9):S431. [Google Scholar]
- 41.Mesa RA. Ruxolitinib, a selective JAK1 and JAK2 inhibitor for the treatment of myeloproliferative neoplasms and psoriasis. IDrugs : the investigational drugs journal. 2010;13(6):394–403. [PubMed] [Google Scholar]
- 42.Greenwald MW, Fidelus-Gort, Rosanne, Levy, Rich, Liang, Jinjin, Vaddi, Kris, Williams, William V, et al. A Randomized Dose-Ranging, Placebo-Controlled Study of INCB028050, a Selective JAK1 and JAK2 Inhibitor in Subjects with Active Rheumatoid Arthritis. Arthritis & rheumatism. 2010;62(Suppl 10):2172. [Google Scholar]
- 43.Stump KL, et al. A highly selective, orally active inhibitor of Janus kinase 2, CEP-33779, ablates disease in two mouse models of rheumatoid arthritis. Arthritis research & therapy. 2011;13(2):R68. doi: 10.1186/ar3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu LD, et al. Depletion of autoreactive plasma cells and treatment of lupus nephritis in mice using CEP-33779, a novel, orally active, selective inhibitor of JAK2. Journal of immunology. 2011;187(7):3840–3853. doi: 10.4049/jimmunol.1101228. [DOI] [PubMed] [Google Scholar]
- 45.Tefferi A, Pardanani A. JAK inhibitors in myeloproliferative neoplasms: rationale, current data and perspective. Blood reviews. 2011;25(5):229–237. doi: 10.1016/j.blre.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 46.Ungureanu D, et al. The pseudokinase domain of JAK2 is a dual-specificity protein kinase that negatively regulates cytokine signaling. Nature structural & molecular biology. 2011;18(9):971–976. doi: 10.1038/nsmb.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Verstovsek S, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. The New England journal of medicine. 2010;363(12):1117–1127. doi: 10.1056/NEJMoa1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tefferi A, Litzow MR, Pardanani A. Long-term outcome of treatment with ruxolitinib in myelofibrosis. The New England journal of medicine. 2011;365(15):1455–1457. doi: 10.1056/NEJMc1109555. [DOI] [PubMed] [Google Scholar]
- 49.Quintas-Cardama A, et al. Janus kinase inhibitors for the treatment of myeloproliferative neoplasias and beyond. Nature reviews. Drug discovery. 2011;10(2):127–140. doi: 10.1038/nrd3264. [DOI] [PubMed] [Google Scholar]
- 50.Zenatti PP, et al. Oncogenic IL7R gain-of-function mutations in childhood T-cell acute lymphoblastic leukemia. Nature genetics. 2011;43(10):932–939. doi: 10.1038/ng.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang J, et al. The genetic basis of early T-cell precursor acute lymphoblastic leukaemia. Nature. 2012;481(7380):157–163. doi: 10.1038/nature10725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blink M, et al. Frequency and prognostic implications of JAK 1–3 aberrations in Down syndrome acute lymphoblastic and myeloid leukemia. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, U.K. 2011;25(8):1365–1368. doi: 10.1038/leu.2011.86. [DOI] [PubMed] [Google Scholar]
- 53.Hedvat M, et al. The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and oncogenesis in solid tumors. Cancer Cell. 2009;16(6):487–497. doi: 10.1016/j.ccr.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xin H, et al. Antiangiogenic and antimetastatic activity of JAK inhibitor AZD1480. Cancer Research. 2011;71(21):6601–6610. doi: 10.1158/0008-5472.CAN-11-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stransky N, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333(6046):1157–1160. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Migone TS, et al. Constitutively activated Jak-STAT pathway in T cells transformed with HTLV-I. Science. 1995;269(5220):79–81. doi: 10.1126/science.7604283. [DOI] [PubMed] [Google Scholar]
- 57.Yu CL, et al. Enhanced DNA-binding activity of a Stat3-related protein in cells transformed by the Src oncoprotein. Science. 1995;269(5220):81–83. doi: 10.1126/science.7541555. [DOI] [PubMed] [Google Scholar]
- 58.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nature reviews. Cancer. 2009;9(11):798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee H, et al. STAT3-induced S1PR1 expression is crucial for persistent STAT3 activation in tumors. Nature medicine. 2010;16(12):1421–1428. doi: 10.1038/nm.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang F, et al. Sorafenib induces growth arrest and apoptosis of human glioblastoma cells through the dephosphorylation of signal transducers and activators of transcription 3. Molecular cancer therapeutics. 2010;9(4):953–962. doi: 10.1158/1535-7163.MCT-09-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]