Abstract
OBJECTIVES
Biochemical evidence of low vitamin B-12 status is common in seniors, but its clinical relevance is unclear. Vitamin B-12 deficiency can result in rapid, irreversible cognitive decline – a phenomenon that has been linked to high folate status. Our objective was to investigate the cognitive significance of low to low-normal plasma vitamin B-12 concentrations. Secondarily, we sought to shed light on the role that folate status plays in the association between vitamin B-12 status and cognitive decline.
DESIGN
We evaluated associations between plasma vitamin B-12 and folate and 8-year cognitive decline. We also assessed interactions between vitamin B-12 status and both folate status and supplemental folate use in relation to cognitive decline.
SETTING
The Framingham Heart Study -- a prospective epidemiologic study
PARTICIPANTS
Five hundred forty-nine community-dwelling seniors (mean age 74.8±4.6 years).
MEASUREMENTS
Mini-Mental State Examination (MMSE), plasma folate, vitamin B-12, methylmalonic acid, homocysteine, demographic factors, and body mass index.
RESULTS
MMSE scores declined by 0.24 points/year over the 8-year follow-up period. Decline was significantly accelerated among cohort members in the bottom two plasma vitamin B-12 quintile categories, and no apparent cognitive advantage was associated with plasma vitamin B-12 from 187–256.8 pmol/L versus <186 pmol/L. Among cohort members with plasma vitamin B-12<258 pmol/L, having a plasma folate concentration>20.2 nmol/L was associated with an approximate 1-point/year decline, as was use of supplemental folate.
CONCLUSION
Plasma vitamin B-12 from 187–256.8 pmol/L predicts cognitive decline. High plasma folate and supplemental folate use identify subgroups in this vitamin B-12 range and below who are prone to especially rapid cognitive decline.
Keywords: aged, cognition/*physiology, folic acid/blood, humans, methylmalonic acid/blood, vitamin B 12/*blood
INTRODUCTION
Biochemical evidence of vitamin B-12 deficiency is highly prevalent among seniors,1–12 but the clinical relevance of this condition remains unclear.13 The classic presentation of vitamin B-12 deficiency features potentially serious hematologic, neurologic, and psychiatric disturbances.14 However, that picture was gleaned from studies of patients diagnosed with pernicious anemia (PA), which affects 2–4% of Americans and is caused by the auto-immune destruction of the gastrointestinal cells that produce intrinsic factor.15 High folic acid intake has been observed to improve hematologic indices, thus masking the deficiency, and to precipitate a life-threatening neuropathy.16 A causal interpretation of the link between the treatment and the neuropathy is controversial, however, considering the “explosive” nature of neurological deterioration in vitamin B-12 deficiency.17
Most people with so-called “biochemical” vitamin B-12 deficiency do not lack intrinsic factor, which is needed for vitamin B12 absorption.13 Instead, they have diminished ability to detach vitamin B-12 from food protein because of age-related achlorydia.15 Since classic clinical features of vitamin B-12 deficiency are usually lacking in such cases, most of this biochemical deficiency escapes medical attention.13 This is appropriate if, as Carmel has speculated, the condition is benign, non-progressive, and impervious to high folic acid intake.13 However, a high prevalence of anemia and impaired mental functioning noted in a study of seniors with established food-cobalamin malabsorption18 raises the hypothesis that common forms of vitamin B-12 deficiency have clinical consequences similar to PA. This view is reinforced by some recent studies linking biochemical vitamin B-12 deficiency to poor cognitive test performance19–21 and cognitive decline.12, 22 Recent studies by our group have also raised the hypothesis that high folate status interacts with biochemical deficiency in a manner consistent with the documented effects of folic acid treatment on the clinical consequences of PA.23, 24
Considering that biochemical vitamin B-12 deficiency is common, largely undiagnosed, and potentially susceptible to adverse effects of high folic acid intakes, studies aimed at establishing the clinical relevance of this condition are vitally important. Results of such studies will inform recommendations for B-vitamin intakes and decisions about whether and how to screen for vitamin B-12 deficiency.
Our main goal in this investigation was to evaluate the hypothesis that low baseline plasma vitamin B-12 concentrations were associated with cognitive impairment and accelerated cognitive decline among participants in the Framingham Heart Study. To shed light on the interrelationship among folate, vitamin B-12, and cognition, we also evaluated interactions between folate status and vitamin B-12 status in relation to cognition and cognitive decline.
METHODS
Study Population
Subjects in this investigation were members of the original Framingham cohort assembled in 1948 and numbering 5,209 at that time.25 Approximately every 2 years, cohort members have undergone an examination that includes phlebotomy and the Mini-mental State Examination (MMSE). Survivors who attended examination 20 (1986–1990), the baseline examination for this investigation, numbered 1,401. We focus here on those survivors who scored >18 on the 30-point MMSE at examination 20,26 and met the following additional criteria at baseline: 1) not diabetic, 2) serum creatinine concentration below cut-off points for frank kidney dysfunction (i.e., men, 131 μmol/L; women, 115 μmol/L), 4) valid food frequency questionnaire results, and 5) measurements for plasma vitamin B-12, plasma folate, and covariates controlled for in the data analyses. Rather than signifying good vitamin B-12 status, very high plasma vitamin B-12 concentrations reflect malignant hemopathies or other serious conditions.27, 28 Consequently, from the 588 cohort members who met the above criteria, we excluded 27 with plasma vitamin B-12 concentrations above the reference range (i.e., 118–701 pmol/L).29 Twelve cohort members who had experienced a stroke before or during the follow-up period were excluded from the remainder, leaving 549 subjects for the data analyses. When compared to the included cohort members, those who were not included in the data analyses for these reasons were older, less well educated, and more likely to have been active smokers at the time of the baseline examination (data not shown).
Dietary Data
Diet was assessed using a 126-item, semi-quantitative food frequency questionnaire (FFQ) based on the Willett FFQ.30 The FFQ queried subjects about how frequently, on average, they had consumed each food item during the previous year, and subjects responded by selecting from several possible frequencies ranging from never to ≥6 times/day. The subjects’ responses for food items were converted to nutrient intakes using standard nutrient database information. The FFQ also contained items about supplement use (i.e., multivitamins, brand and frequency of use; single-vitamin supplements, doasages), and folate intakes were computed both with and without supplements.
Plasma Measures
Plasma concentrations of folate and vitamin B-12 were measured in non-fasting blood samples. Folate concentration was determined by using a microbial (Lactobacillus casei) assay with a 96-well plate and manganese supplementation as described by Tamura et al.31 Vitamin B-12 concentration was determined using a radioassay kit from Ciba-Corning (Medfield, MA). Plasma total homocysteine was measured by the HPLC method of Araki and Sako.32 Lindenbaum et al. measured the plasma methylmalonic acid (MMA) concentrations of 260 cohort members by capillary gas chromatography/mass spectrometry.5
The Mini-mental State Examination (MMSE)
The MMSE is a brief, crude dementia-screening instrument consisting of 16 individual questions or simple tasks. Tasks involve naming objects, repeating and remembering a series of three common words, copying a figure, writing a sentence, repeating a phrase, spelling a word backwards, and folding a piece of paper and placing it on a desk, table, or floor. Functions assessed include orientation (10 points), registration (3 points), attention and calculation (5 points), recall (3 points), and language/praxis (9 points).26
Follow-Up Study Design
Examination 20, the first examination cycle at which dietary data were collected, was considered the baseline examination for the prospective analyses. The subjects brought their completed FFQs to the baseline examination, where blood was drawn, the MMSE was administered, and FFQ responses were reviewed and finalized. In the prospective data analyses, the baseline values obtained from the FFQ responses and biochemical analyses were related to decline over time in the MMSE scores that were obtained at baseline and every 2 years thereafter.
Statistical Analyses
All statistical analyses were performed using SAS version 9 software (SAS Institute, Inc., Cary, NC), and P<0.05 was considered statistically significant for all tests.
Subject Description
Using means (±SD), percentages, and for highly skewed variables, geometric means (95% CI), we described the subjects as a group, and also after dividing them according to fifths of the plasma vitamin B-12 and folate distribuitions. We also used linear regression analysis with the plasma vitamin B-12 concentration (continuous) or the log of the plasma folate concentration (continuous) as the outcome variable to evaluate bivariate associations between each characteristic and B-vitamin status. When associations were found, we also used multiple linear regression analysis to evaluate the independence of the associations from those between B-vitamin status and other characteristics.
Subject Characteristics and Cognitive Function
We used repeated measures linear regression analysis as performed by SAS PROC MIXED to evaluate associations between subject characteristics and cognitive function. We created a multivariate model for this purpose with the MMSE score as the continuous outcome variable and the following predictors: sex, educational achievement, examination cycle, and baseline status for age, smoking, alcohol use, plasma folate, body mass index (BMI), serum creatinine, and plasma vitamin B-12. In the modeling, each subject was represented by up to 5 records, depending upon the number of examinations between 20 and 24 for which an MMSE score had been obtained for him/her. However, the modeling accounted for the correlation among the multiple observations made on a given subject. For plasma vitamin B-12 and plasma folate, as well as other subject characteristics related to the MMSE score at P≤0.2, we created exposure categories and estimated the least squares mean (95% CI) MMSE score for each category after controlling for the other characteristics also associated with the MMSE score at P≤0.2. Except for alcohol use, which was only related to the MMSE score when dichotomized as use versus non-use, naturally continuous characteristics were controlled for using continuously scaled terms.
B-vitamin Status and 8-Year Decline in Cognitive Function
Our main hypotheses concerned relationships between baseline B-vitamin status and MMSE-score decline over the 8-year average follow-up period. We assessed these associations by adding terms for interactions between baseline B-vitamin status and time since baseline (i.e., 0 for examination 20, 2 for examination 21, 4 for examination 22, 6 for examination 23, and 8 for examination 24) to the final model for cognitive function. The values for time reflected the approximate 2-year gap between examinations. Because our a priori hypothesis was that any enhancement of cognitive decline related to higher folate status would be restricted to subjects with lower vitamin B-12 status, we also tested the 3-way interaction among time, vitamin B-12 status, and folate status. From separate models, we reported least squares mean (95% CI) annual change in the MMSE score for fifths of the plasma vitamin B-12 and folate distributions. When we examined the 3-way interaction among time, vitamin B-12 status, and folate status, we modeled vitamin B-12 status as plasma vitamin B-12<258 pmol/L versus a higher value. This cut-off point was proposed by Lindenbaum et al., and endorsed by Allen and Casterline,33 after Lindenbaum et al. found a high prevalence of functional vitamin B-12 deficiency in the Framingham Original Cohort at plasma vitamin B-12 concentrations below this cut-off point, which is well above the traditionally used cut-off point of 148 pmol/L.5
Use of Supplements Containing Folic Acid and 8-year Cognitive Decline
Because hypotheses concerning cognitive harm from high folate status relate specifically to the effect of consuming folic acid, the synthetic form of folate, on people who are deficient in vitamin B-12, we also considered the interaction between time and the use of supplements containing folic acid at examination 20 (versus non-use of such supplements) in relation to cognitive decline among cohort members stratified by vitamin B-12 status (dichotomous classification).
RESULTS
Subject Characteristics and Their Relationship to B-vitamin Status
At baseline, the mean age of the surviving cohort members was 74.8 years (Table 1). Women comprised about 2/3 of the survivors, and the vast majority were high school graduates. About 12% were cigarette smokers at baseline, and most consumed alcohol. On average, intakes of vitamin B-12 were more than adequate and intakes of folate were inadequate, given the Recommended Dietary Intakes of 2.4 μg/day of vitamin B-12 and 400 μg/day of folate.34 Nevertheless, most cohort members had plasma concentrations of both vitamins that exceeded the traditional cut-off points for deficiency of 7 nmol/L for folate and 120–180 pmol/L for vitamin B-12.34
Table 1.
Characteristics of Framingham Heart Study Participants at Baseline (1986–1990) by Vitamin B-12 Status
| Characteristic | All subjects | Plasma vitamin B-12 (pmol/L)
|
P | ||||
|---|---|---|---|---|---|---|---|
| 18.6–186 | 187–256.8 | 257–342.8 | 342.9–435 | 435.4–695 | |||
| n | 549 | 109 | 110 | 110 | 110 | 110 | |
| Age (years)a | 74.8± 4.6 | 75.1±4.7 | 74.7±4.3 | 74.2±4.6 | 74.9±4.7 | 75.3±5.0 | 0.723 |
| Female (%) | 62 | 52 | 53 | 67 | 68 | 72 | <0.001 |
| High school graduate (%) | 72 | 62 | 62 | 76 | 81 | 78 | <0.001 |
| Smoker (%) | 12 | 16 | 8.2 | 14 | 14 | 10 | 0.288 |
| Alcohol user (%) | 60 | 60 | 63 | 56 | 59 | 63 | 0.682 |
| Body mass index (kg/m2)a | 26.5 ±4.5 | 26.1 ±4.4 | 27.3± 4.7 | 26.6 ±4.7 | 26.1± 3.7 | 25.9 ±4.7 | 0.255 |
| Creatinine (μmol/L)b | |||||||
| Men | 86 (84–88) | 88 (84–94) | 83 (79–87) | 85 (80–90) | 84 (79–88) | 95 (88–102) | 0.084 |
| Women | 73 (71–74) | 70 (66–74) | 73 (69–77) | 72 (69–76) | 73 (70–76) | 75 (72–79) | 0.079 |
| Vitamin user (%)c | 26 | 12 | 17 | 25 | 31 | 43 | <0.001 |
| Vitamin B12 intake (μg/d)b | 6.6 (6.2–7.1) | 5.3 (4.5–6.2) | 6.3 (5.5–7.3) | 6.4 (5.5–7.4) | 7.0 (5.9–8.2) | 8.7 (7.3–10.3) | <0.001 |
| Folate intake (μg/d)b | 320 (306–336) | 251 (227–278) | 288 (264–314) | 306 (273–343) | 365 (331–403) | 416 (378–458) | <0.001 |
| MMSE scorea,d | 28.2 ±2.1 | 27.8 ±2.3 | 28.2±1.9 | 28.2 ±2.2 | 28.4 ±1.9 | 28.2 ±2.0 | 0.122 |
Mean (SD).
Geometric mean (95% CI).
User of a vitamin supplemement containing vitamin B-12.
MMSE = Mini-mental State Examination.
Higher vitamin B-12 status was significantly related to female sex, more education, use of vitamin supplements containing vitamin B-12, and higher total intakes of vitamin B-12 and folate. Despite the exclusion of cohort members with very high plasma vitamin B-12 concentrations, a significant positive association between serum creatinine and plasma vitamin B-12 was revealed by an analysis that was controlled for vitamin B-12 intake (data not shown). Female sex and higher educational status also remained significantly related to plasma vitamin B-12 in that model. In fact, men consumed about 1 μg/day more vitamin B-12 than women (P=0.046) but had significantly lower plasma vitamin B-12 concentrations. In multivariate modeling, the positive association between folate intake and vitamin B-12 status was statistically independent of vitamin B-12 intake.
Other than total folate intake and use of supplements containing folic acid, few subject characteristics were associated with folate status (Table 2). Smokers had low status, but not after the low folate intakes of that subgroup were accounted for (data not shown). On the other hand, education and BMI remained positively associated with folate status in an analysis that was controlled for total folate intake (data not shown). When a term for total vitamin B-12 intake was added to this model, that exposure was not related to plasma folate at all (P=0.81) despite the highly significant association revealed by the bivariate analysis.
Table 2.
Characteristics of Framingham Heart Study Participants at Baseline (1986–1990) by Folate Status
| Characteristic | Plasma folate (nmol/L)
|
P | ||||
|---|---|---|---|---|---|---|
| 0.54–4.8 | 4.9–7.5 | 7.52–11.49 | 11.5–20.14 | 20.2–149 | ||
| n | 110 | 109 | 110 | 110 | 110 | |
| Age (years)a | 74.3±4.3 | 74.6±4.8 | 75.4±5.0 | 75.2±4.8 | 74.6±4.4 | 0.229 |
| Female (%) | 60 | 64 | 60 | 60 | 68 | 0.163 |
| High school graduate (%) | 57 | 70 | 71 | 76 | 84 | <0.001 |
| Smoker (%) | 25 | 6.4 | 7.3 | 11 | 12 | 0.038 |
| Alcohol user (%) | 56 | 60 | 65 | 62 | 58 | 0.693 |
| Body mass index (kg/m2)a | 27.1±5.1 | 27.2±4.8 | 26.4± 4.4 | 25.7±3.7 | 25.6±3.9 | <0.001 |
| Creatinine (μmol/L)b | ||||||
| Men | 86 (81–91) | 87 (82–92) | 84 (79–89) | 87 (82–92) | 88 (82–93) | 0.643 |
| Women | 73 (70–77) | 74 (70–78) | 73 (69–77) | 72 (68–76) | 72 (68–75) | 0.2 |
| Vitamin user (%)c | 4.9 | 5.5 | 10 | 23 | 50 | <0.001 |
| Vitamin B-12 intake (μg/d)b | 4.3 (3.7–5) | 5.8 (5–6.7) | 6.6 (5.5–7.8) | 8.2 (6.9–9.6) | 9.8 (8.6–11) | <0.001 |
| Folate intake (μg/d)b | 210 (190–232) | 269 (248–291) | 322 (296–350) | 381 (348–416) | 488 (445–536) | <0.001 |
| MMSE scorea,d | 28.1±2.0 | 28.2± 2.1 | 28.3±1.9 | 27.9±2.2 | 28.3±2.1 | 0.695 |
Mean±SD.
Geometric mean (95% CI).
User of a vitamin supplemement containing vitamin folic acid.
MMSE = Mini-mental State Examination.
There was no hint of a cross-sectional association between B-vitamin status and the MMSE score at baseline.
Subject Characteristics and Cognitive Function
By examination 24, the mean MMSE score had dropped to 26.3 from the baseline value of 28.1. In the repeated measures multivariate analysis relating subject characteristics to cognitive function, P-values relating smoking status and serum creatinine to the MMSE score were both >0.97. Relationships between most other subject characteristics and cognitive function were statistically significant. The only exceptions were plasma folate and BMI. Not surprisingly, high school graduates and younger subjects had higher MMSE scores than less educated and older subjects. Female sex and alcohol use were also associated with higher MMSE scores. Finally, being in any of the top three fifths of the plasma vitamin B-12 distribution was associated with significantly better MMSE performance than having plasma vitamin B-12 below the bottom quintile.
Among the 260 cohort members whose MMA concentrations had been measured by Lindenbaum et al.,5 plasma total homocysteine was significantly inversely related to the MMSE-score, whether or not a term for plasma folate was in the model, after controlling for sex, years of education, examination cycle, and baseline status for age, smoking, alcohol use, BMI, and serum creatinine, (−0.067≤β≤−0.064, P<0.01). However, the association was attenuated and not statistically significant (β=−0.04, P=0.165) when a term for plasma MMA was in the model.
B-Vitamin Status and 8-Year Decline in Cognitive Function
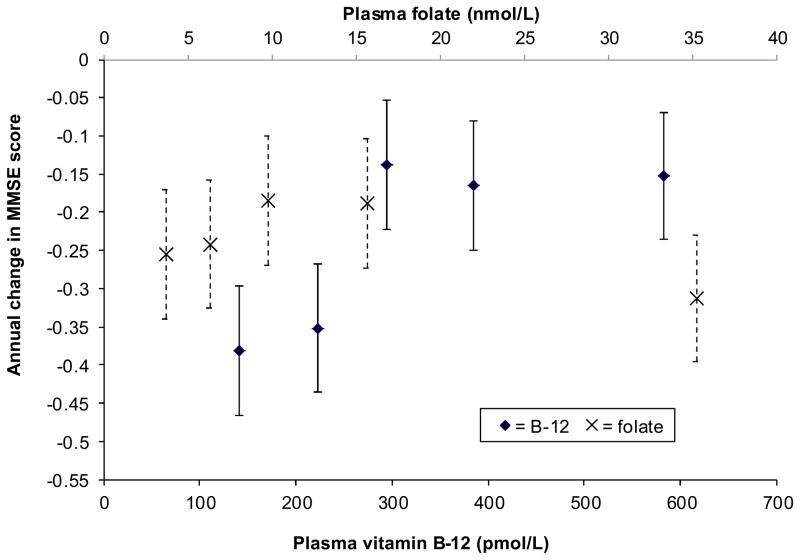
After multivariate adjustment, MMSE scores decreased by 0.24 points/year (P<0.001) on average. However, the MMSE scores of cohort members in the bottom two fifths of the plasma vitamin B-12 distribution declined by >0.35 points/year, as compared to 0.13–0.17 points/year for subjects in the top three fifths (P<0.001) (Figure 1). The bottom two fifths included subjects with plasma vitamin B-12 concentrations <257 pmol/L and thus corresponded well to Lindenbaum et al.’s preferred definition of low vitamin B-12 status.5
Figure 1.
Annual change in MMSE score during the 8 years between examinations 20 (baseline) and 24 of the Framingham Original Cohort (n = 549) by baseline plasma vitamin B-12 and plasma folate quintile categories. Results are controlled for age, sex, educational achievement, body mass index, and baseline status for plasma vitamin B-12 or folate. Points represent β-coefficients, which are plotted at quintile category medians. Error bars represent 95% CI for the β-coefficients. For vitamin B-12, quintile categories 1–3 do not differ from each other, and quintile categories 4 and 5 do not differ from each other, but 1–3 and 4/5 differ significantly. P<0.001 for the interaction between time and plasma vitamin B-12. For folate, quintile category 5 differs significantly from quintile categories 2 and 3; quintile categories 1 and 2 do not differ from any of the other categories. P = 0.019 for the interaction between time and plasma folate.
After controlling for vitamin B-12 status and the other covariates, the trend in MMSE-score decline across plasma folate categories did not suggest a consistent decrease in the rate of cognitive decline over time with increasing folate status. However, the rate of MMSE-score decline associated with having a plasma folate concentration above the top quintile was accelerated when compared to the rate of decline associated with being in the bottom two fifths of the distribution (Pinteraction=0.019). The least squares mean rate of decline for subjects in the top fifth of the distribution was comparable to the rates estimated for the bottom two, for which the least squares mean plasma folate concentrations were in the deficient range.
Interaction between Vitamin B-12 Status and Folate Status in Relation to Cognitive Decline
Including in the model a term for the 3-way interaction among time, plasma folate, and lower versus higher vitamin B-12 status revealed that the cognitive disadvantage associated with having low vitamin B-12 status was amplified at high folate status (Pinteraction<0.001) (Table 3).
Table 3.
Annual Change in MMSEa Score over 8 Years of Follow-Up by Quintile Category of Plasma Folate at Baseline (1986–1990) among Members of the Framingham Original Cohort Stratified by Vitamin B-12 Status at Baselineb
| Plasma folate (nmol/L)
|
Ptrend | |||||
|---|---|---|---|---|---|---|
| <5 | 5–7.69 | 7.7–12 | 12.01–21.7 | ≥21.75 | ||
| Plasma B-12 ≥258 pmol/L (n) | 57 | 53 | 75 | 79 | 93 | |
| β-coefficient | −0.18 | −0.14 | −0.17 | −0.14 | −0.14 | |
| 95% CI | (−0.305, −0.06) | (−0.27, −0.01) | (−0.27, −0.06) | (−0.24, −0.03) | (−0.23, −0.05) | |
| P-value | referent | 0.623 | 0.85 | 0.575 | 0.589 | 0.477 |
| Plasma B-12 <258 pmol/L (n) | 60 | 64 | 44 | 39 | 24 | |
| β-coefficient | −0.32 | −0.32 | −0.22 | −0.28 | −0.92 | |
| 95% CI | (−0.44, −0.21) | (−0.42, −0.21) | (−0.35, −0.08) | (−0.42, −0.14) | (−1.09, −0.74) | |
| P-value | referent | 0.941 | 0.254 | 0.668 | <0.001 | <0.001 |
| Differencec | −0.14 | −0.18 | −0.05 | −0.14 | −0.78 | |
| 95% CI | (−0.31, 0.03) | (−0.34, −0.009) | (−0.22, 0.13) | (−0.32, 0.03) | (−0.98, −0.58) | |
| P-valued | 0.108 | 0.039 | 0.588 | 0.111 | <0.001 | |
MMSE = Mini-mental state examination.
Results were obtained using a mixed multivariate model adjusted for age, sex, and educational achievement, as well as baseline status for BMI and alcohol use versus non-use.
Difference in the annual change in the MMSE score between those with low and higher vitamin B-12 concentrations in the plasma folate quintile category.
P-valuecomparing the annual change in the MMSE score between those with low and higher plasma vitamin B-12 concentrations in that plasma folate quintile category.
Interaction between Vitamin B-12 Status and Supplement Use in Relation to Cognitive Decline
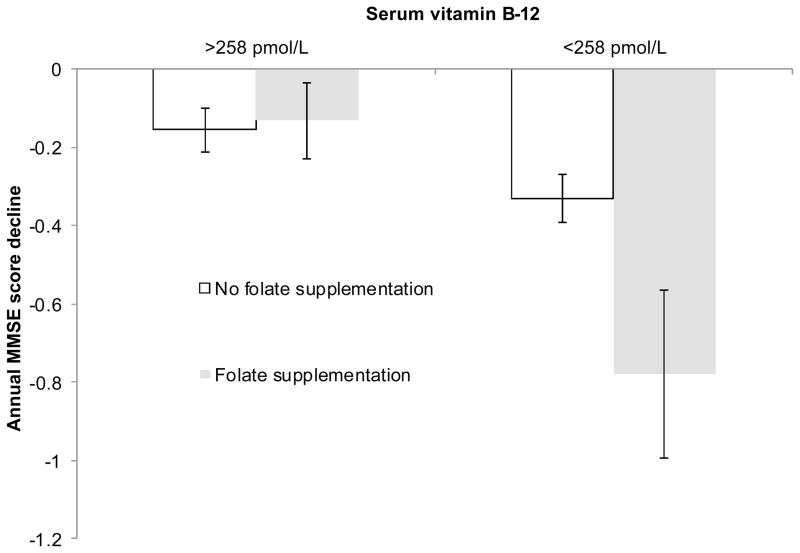
We also found a significant 3-way interaction among time, low versus higher vitamin B-12 status, and use versus non-use of vitamin supplements containing folic acid (Pinteraction=0.002) (Figure 2). Consistent with the results we obtained for plasma folate, consuming vitamin supplements that contained folic acid was not associated with cognitive decline in subjects with plasma vitamin B-12≥258 pmol/L. However, among subjects with lower vitamin B-12 status, the MMSE scores of users of such supplements (mainly multivitamins) declined by nearly 1 point/year, as compared to <1/2 point per year for non-consumers of supplemental folic acid.
Figure 2.
Annual change in MMSE scores during the 8 years between examinations 20 (baseline) and 24 of the Framingham Original Cohort (n = 549) for users and non-users of supplements containing folic acid, stratified by vitamin B-12 status. Results are controlled for age, sex, educational achievement, and baseline status for serum creatinine, BMI, and smoking status. Points represent β-coefficients. Error bars represent 95% CI for the β-coefficients. Subgroups indicated by the same letter do not differ significantly.
DISCUSSION
Based on Lindenbaum et al.’s demonstration of elevated circulating levels of MMA and total homocysteine in association with plasma vitamin B-12 concentrations <258 pmol/L,5 we separated cohort members with plasma vitamin B-12 concentrations between 187 pmol/L and 257 pmol/L, the second quintile, from those with lower values and found that cognitive decline was similarly accelerated in both groups.
Many previous studies have found cross-sectional associations between either low circulating folate levels or hyperhomocysteinemia and low MMSE scores, but results of prospective studies have been mixed.35 We found no evidence of significant protection by high plasma folate against poor MMSE performance or 8-year MMSE-score decline. The plasma total homocysteine concentration, which can reflect folate status, was significantly inversely related to MMSE performance, but not when a term for MMA was in the multivariate model. Since MMA is a sensitive measure of vitamin B-12 status,36 this finding may suggest that, at least in this cohort, the association between homocysteine and MMSE performance reflects the importance of replete vitamin B-12 status to cognition in the elderly. This is not to say that folate status is irrelevant to cognition. The MMSE score is a crude measure of cognitive function, and lower folate status has been linked to poor performance on more sensitive tests.35 However, since a low total MMSE score indicates a severe cognitive deficit,37 our findings reinforce the importance of identifying people with biochemical evidence of vitamin B-12 deficiency.
Differences across populations in factors that affect MMSE performance (e.g., age and education) likely influence the ability to find associations between exposures and this outcome, since there is a known ceiling effect in relatively cognitively intact and highly educated but impaired people.37 For the same reason, a long follow-up period might be needed to detect associations in studies of MMSE-score decline. Indeed, though Tucker et al. demonstrated associations between B-vitamin status and 3-year decline in scores on some sensitive cognitive tests, they found no link between B-vitamin status and MMSE-score decline over the same time period.38 Furthermore, Seshadri et al. found an association between plasma homocysteine concentrations measured at examination 20 of the Framingham Original Cohort and MMSE-score decline, but only after ≥4 years of follow-up.39
A population’s nutritional status should also be considered when interpreting results. For example, as suggested by Kado et al.’s findings,40 a relationship between folate status and cognitive decline might not be demonstrable in the US in the era of food folic acid fortification, when folate deficiency is virtually non-existent.41 All of these factors could be relevant to the difference between Durga et al.’s successful trial of the effect folic acid supplementation on cognitive decline in hyperhomocysteinemic 50–70-year-olds and McMahon et al.’s failure to slow cognitive decline among hyperhomocysteinemic seniors with a B-vitamin supplement.42 Specifically, the baseline folate status of the subjects in the latter trial was quite high, and MMSE scores were nearly perfect and did not decline during the 1–2 years of follow-up. Despite the methodologic features that limited that trial’s ability to detect differences, there was some evidence that the treatment had an exacerbating effect on cognitive decline. It is important to note in this regard that the treatment boosted the mean plasma folate concentration to 75 nmol/L (a level achieved by <1% of the Framingham Original Cohort members), and that it may not have cured all cases of vitamin B-12 deficiency.43
Exacerbation of the neuropsychiatric effects of vitamin B-12 deficiency by folic acid treatment is not an isolated finding. Specifically, intervention studies carried out in the 1940s and 1950s demonstrated this effect of folic acid on PA cases.16 Furthermore, increased prevalence of cognitive impairment due to vitamin B-12 deficiency is a feared adverse consequence of the increased folic acid intakes that characterize the modern era of food folic acid fortification and heavy supplement use.16, 44 Shedding light on this hypothesis was, in fact, the motivation behind our group’s previous studies of the interaction between folate status and vitamin B-12 status in relation to cognition.23, 24 Results of those studies showed that serum folate>59 nmol/L and exposure to circulating unmetabolized folic acid were related to worse cognitive function among people with low vitamin B-12 status. Perhaps consistent with those findings, the Chicago Health and Aging Project (CHAP) demonstrated accelerated cognitive decline over 6 years of follow-up in association with high folate intakes from foods and supplements in the era of mandatory food folic acid fortification.45 A later study documented a high prevalence of biochemical evidence of low vitamin B-12 status in the CHAP cohort.12
In our current study, we found especially rapid cognitive decline in association with low vitamin B-12 status combined with high plasma folate or use of supplements containing folic acid. Although it may be tempting to attribute these findings to exacerbation of the cognitive effects of vitamin B-12 deficiency by folic acid, they likely have a different explanation. Specifically, the Framingham subjects were studied entirely before mandatory fortification went into effect. Consequently, they had rather low folate intakes. Furthermore, plasma folate was >59 nmol/L in only one subject with plasma vitamin B-12<258 pmol/L. A lack of overlap between the two subgroups that experienced accelerated cognitive decline may suggest that, rather than causing the decline, the folate variables selected for subgroups of vitamin B-12 deficient subjects at particularly high risk. Specifically, folate deficiency was highly prevalent (i.e., 30%) in the subjects whose low vitamin B-12 status persisted despite supplement use, and folate intakes of the subjects with both low vitamin B-12 status and high plasma folate were not very high (mean<400 μg/d). Consistent with our results, Kang et al. found particularly poor cognition among Nurses’ Health Study participants with low plasma vitamin B-12 despite supplement use.46 The persistence of low combined B-vitamin status among the supplemented Framingham subjects suggests a mal absorption syndrome like celiac disease,47 which affects about 1% of the population48 and has been linked to progressive cognitive impairment.49 Elevated serum folate has been noted in classic PA50 – perhaps explaining the accelerated cognitive decline associated with high plasma folate combined with low plasma B-12.
Among the strengths of our study was its longitudinal design featuring long-term follow-up and multiple assessments through the age range of rapid cognitive decline. The ability of the MMSE to detect cognitive impairment and cognitive decline in well-educated populations like Framingham is limited.37 However, the cohort members were old enough at baseline that significant decline in the MMSE scores was observed over the follow-up period. On the other hand, because the MMSE is a general screening tool, our study was incapable of identifying the specific cognitive domains affected by low vitamin B-12 status. Finally, our findings may not be generalizable to racial/ethnic minorities or poorly educated populations.
In conclusion, despite the high prevalence of biochemical evidence of low vitamin B-12 status in the elderly, steps should be taken to identify this condition, and to discover and treat its cause to avoid rapid cognitive decline.
Acknowledgments
Funding sources: USDA agreement No. 58-1950-7-707, NIH grant 1 R01 NS062877-01A2
Sponsor’s Role: None.
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions: Martha Morris, Paul Jacques, and Jacob Selhub conceived the research questions addressed in the study. Martha Morris designed and conducted the data analyses and drafted the manuscript. All authors contributed to interpretation of data and reviewed and revised the manuscript for important intellectual content. This work was supported by USDA agreement No. 58-1950-7-707, and by NIH grant 1 R01 NS062877-01A2. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Dept of Agriculture.
References
- 1.Flood VM, Smith WT, Webb KL, et al. Prevalence of low serum folate and vitamin B12 in an older Australian population. Aust N Z J Public Health. 2006;30:38–41. doi: 10.1111/j.1467-842x.2006.tb00084.x. [DOI] [PubMed] [Google Scholar]
- 2.Clarke R, Refsum H, Birks J, et al. Screening for vitamin B-12 and folate deficiency in older persons. Am J Clin Nutr. 2003;77:1241–1247. doi: 10.1093/ajcn/77.5.1241. [DOI] [PubMed] [Google Scholar]
- 3.Pennypacker LC, Allen RH, Kelly JP, et al. High prevalence of cobalamin deficiency in elderly outpatients. J Am Geriatr Soc. 1992;40:1197–1204. [PubMed] [Google Scholar]
- 4.Morris MS, Jacques PF, Rosenberg IH, et al. Elevated serum methylmalonic acid concentrations are common among elderly americans. J Nutr. 2002;132:2799–2803. doi: 10.1093/jn/132.9.2799. [DOI] [PubMed] [Google Scholar]
- 5.Lindenbaum J, Rosenberg IH, Wilson PW, et al. Prevalence of cobalamin deficiency in the Framingham elderly population. Am J Clin Nutr. 1994;60:2–11. doi: 10.1093/ajcn/60.1.2. [DOI] [PubMed] [Google Scholar]
- 6.Carmel R, Green R, Jacobsen DW, et al. Serum cobalamin, homocysteine, and methylmalonic acid concentrations in a multiethnic elderly population: Ethnic and sex differences in cobalamin and metabolite abnormalities. Am J Clin Nutr. 1999;70:904–910. doi: 10.1093/ajcn/70.5.904. [DOI] [PubMed] [Google Scholar]
- 7.Wolters M, Hermann S, Hahn A. B vitamin status and concentrations of homocysteine and methylmalonic acid in elderly German women. Am J Clin Nutr. 2003;78:765–772. doi: 10.1093/ajcn/78.4.765. [DOI] [PubMed] [Google Scholar]
- 8.Yao Y, Yao SL, Yao SS, et al. Prevalence of vitamin B12 deficiency among geriatric outpatients. J Fam Pract. 1992;35:524–528. [PubMed] [Google Scholar]
- 9.Clarke R, Grimley Evans J, Schneede J, et al. Vitamin B12 and folate deficiency in later life. Age Ageing. 2004;33:34–41. doi: 10.1093/ageing/afg109. [DOI] [PubMed] [Google Scholar]
- 10.Pfeiffer CM, Caudill SP, Gunter EW, et al. Biochemical indicators of B vitamin status in the US population after folic acid fortification: Results from the National Health and Nutrition Examination Survey 1999–2000. Am J Clin Nutr. 2005;82:442–450. doi: 10.1093/ajcn.82.2.442. [DOI] [PubMed] [Google Scholar]
- 11.Rajan S, Wallace JI, Beresford SA, et al. Screening for cobalamin deficiency in geriatric outpatients: Prevalence and influence of synthetic cobalamin intake. J Am Geriatr Soc. 2002;50:624–630. doi: 10.1046/j.1532-5415.2002.50155.x. [DOI] [PubMed] [Google Scholar]
- 12.Tangney CC, Tang Y, Evans DA, et al. Biochemical indicators of vitamin B12 and folate insufficiency and cognitive decline. Neurology. 2009;72:361–367. doi: 10.1212/01.wnl.0000341272.48617.b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carmel R. Mean corpuscular volume and other concerns in the study of vitamin B-12 deficiency: Epidemiology with pathophysiology. Am J Clin Nutr. 2008;87:1962–1963. doi: 10.1093/ajcn/87.6.1962. author reply 1963–1964. [DOI] [PubMed] [Google Scholar]
- 14.Toh BH, van Driel IR, Gleeson PA. Pernicious anemia. N Engl J Med. 1997;337:1441–1448. doi: 10.1056/NEJM199711133372007. [DOI] [PubMed] [Google Scholar]
- 15.Allen LH. How common is vitamin B-12 deficiency? Am J Clin Nutr. 2009;89:693S–696S. doi: 10.3945/ajcn.2008.26947A. [DOI] [PubMed] [Google Scholar]
- 16.Reynolds E. Vitamin B12, folic acid, and the nervous system. Lancet Neurol. 2006;5:949–960. doi: 10.1016/S1474-4422(06)70598-1. [DOI] [PubMed] [Google Scholar]
- 17.Dickinson CJ. Does folic acid harm people with vitamin B12 deficiency? QJM. 1995;88:357–364. [PubMed] [Google Scholar]
- 18.Andres E, Affenberger S, Vinzio S, et al. Food-cobalamin malabsorption in elderly patients: Clinical manifestations and treatment. Am J Med. 2005;118:1154–1159. doi: 10.1016/j.amjmed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 19.Jelicic M, Jonker C, Deeg DJ. Effect of low levels of serum vitamin B12 and folic acid on cognitive performance in old age: A population-based study. Dev Neuropsychol. 2001;20:565–571. doi: 10.1207/S15326942DN2003_1. [DOI] [PubMed] [Google Scholar]
- 20.Hin H, Clarke R, Sherliker P, et al. Clinical relevance of low serum vitamin B12 concentrations in older people: the Banbury B12 study. Age Ageing. 2006;35:416–422. doi: 10.1093/ageing/afl033. [DOI] [PubMed] [Google Scholar]
- 21.Lildballe DL, Fedosov S, Sherliker P, et al. Association of cognitive impairment with combinations of vitamin B12-related parameters. Clin Chem. 2011 doi: 10.1373/clinchem.2011.165944. [DOI] [PubMed] [Google Scholar]
- 22.Clarke R, Birks J, Nexo E, et al. Low vitamin B-12 status and risk of cognitive decline in older adults. Am J Clin Nutr. 2007;86:1384–1391. doi: 10.1093/ajcn/86.5.1384. [DOI] [PubMed] [Google Scholar]
- 23.Morris MS, Jacques PF, Rosenberg IH, et al. Folate and vitamin B-12 status in relation to anemia, macrocytosis, and cognitive impairment in older Americans in the age of folic acid fortification. Am J Clin Nutr. 2007;85:193–200. doi: 10.1093/ajcn/85.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris MS, Jacques PF, Rosenberg IH, et al. Circulating unmetabolized folic acid and 5-methyltetrahydrofolate in relation to anemia, macrocytosis, and cognitive test performance in American seniors. Am J Clin Nutr. 2010 Mar 31; doi: 10.3945/ajcn.2009.28671. [Epub ahead of print] PubMed PMID: 20357042. [DOI] [PubMed] [Google Scholar]
- 25.Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: The Framingham Study. Am J Public Health Nations Health. 1951;41:279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rovner BW, Folstein MF. Mini-mental state exam in clinical practice. Hosp Pract (Off Ed) 1987;22:99, 103, 106, 110. [PubMed] [Google Scholar]
- 27.Carmel R, Vasireddy H, Aurangzeb I, et al. High serum cobalamin levels in the clinical setting--clinical associations and holo-transcobalamin changes. Clin Lab Haematol. 2001;23:365–371. doi: 10.1046/j.1365-2257.2001.00134.x. [DOI] [PubMed] [Google Scholar]
- 28.Ermens AA, Vlasveld LT, Lindemans J. Significance of elevated cobalamin (vitamin B12) levels in blood. Clin Biochem. 2003;36:585–590. doi: 10.1016/j.clinbiochem.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 29.Iverson C, Christiansen S, Flanagin A, et al. AMA Manual of Style: A Guide for Authors and Editors. 10. New York: Oxford University Press; 2007. [Google Scholar]
- 30.Willett WC, Hu F. The food frequency questionnaire. Cancer Epidemiol Biomarkers Prev. 2007;16:182. doi: 10.1158/1055-9965.EPI-06-0843. [DOI] [PubMed] [Google Scholar]
- 31.Tamura T, Freeberg LE, Cornwell PE. Inhibition of EDTA of growth of Lactobacillus casei in the folate microbiological assay and its reversal by added manganese or iron. Clin Chem. 1990;36:1993. [PubMed] [Google Scholar]
- 32.Araki A, Sako Y. Determination of free and total homocysteine in human plasma by high-performance liquid chromatography with fluorescence detection. J Chromatogr. 1987;422:43–52. doi: 10.1016/0378-4347(87)80438-3. [DOI] [PubMed] [Google Scholar]
- 33.Allen LH, Casterline J. Vitamin B-12 deficiency in elderly individuals: Diagnosis and requirements. Am J Clin Nutr. 1994;60:12–14. doi: 10.1093/ajcn/60.1.12. [DOI] [PubMed] [Google Scholar]
- 34.Food and Nutrition Board IoM. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin and choline. Washington, D.C: National Academy Press; 2000. [PubMed] [Google Scholar]
- 35.Morris MS, Jacques PF. Folate and neurological function: epidemiological perspective. In: Bailey LB, editor. Folate in Health and Disease. Vol. 2. Boca Raton, FL: CRC Press; 2009. pp. 325–353. [Google Scholar]
- 36.Elin RJ, Winter WE. Methylmalonic acid: A test whose time has come? Arch Pathol Lab Med. 2001;125:824–827. doi: 10.5858/2001-125-0824-MA. [DOI] [PubMed] [Google Scholar]
- 37.Diniz BS, Yassuda MS, Nunes PV, et al. Mini-mental State Examination performance in mild cognitive impairment subtypes. Int Psychogeriatr. 2007;19:647–656. doi: 10.1017/S104161020700542X. [DOI] [PubMed] [Google Scholar]
- 38.Tucker KL, Qiao N, Scott T, et al. High homocysteine and low B vitamins predict cognitive decline in aging men: the Veterans Affairs Normative Aging Study. Am J Clin Nutr. 2005;82:627–635. doi: 10.1093/ajcn.82.3.627. [DOI] [PubMed] [Google Scholar]
- 39.Seshadri S, Beiser A, Selhub J, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 40.Kado DM, Karlamangla AS, Huang MH, et al. Homocysteine versus the vitamins folate, B6, and B12 as predictors of cognitive function and decline in older high-functioning adults: MacArthur Studies of Successful Aging. Am J Med. 2005;118:161–167. doi: 10.1016/j.amjmed.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 41.Jacques PF, Selhub J, Bostom AG, et al. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N Engl J Med. 1999;340:1449–1454. doi: 10.1056/NEJM199905133401901. [DOI] [PubMed] [Google Scholar]
- 42.McMahon JA, Green TJ, Skeaff CM, et al. A controlled trial of homocysteine lowering and cognitive performance. N Engl J Med. 2006;354:2764–2772. doi: 10.1056/NEJMoa054025. [DOI] [PubMed] [Google Scholar]
- 43.Rajan S, Wallace JI, Brodkin KI, et al. Response of elevated methylmalonic acid to three dose levels of oral cobalamin in older adults. J Am Geriatr Soc. 2002;50:1789–1795. doi: 10.1046/j.1532-5415.2002.50506.x. [DOI] [PubMed] [Google Scholar]
- 44.Clarke R, Sherliker P, Hin H, et al. Folate and vitamin B12 status in relation to cognitive impairment and anaemia in the setting of voluntary fortification in the UK. Br J Nutr. 2008;100:1054–1059. doi: 10.1017/S0007114508958001. [DOI] [PubMed] [Google Scholar]
- 45.Morris MC, Evans DA, Bienias JL, et al. Dietary folate and vitamin B12 intake and cognitive decline among community-dwelling older persons. Arch Neurol. 2005;62:641–645. doi: 10.1001/archneur.62.4.641. [DOI] [PubMed] [Google Scholar]
- 46.Kang JH, Irizarry MC, Grodstein F. Prospective study of plasma folate, vitamin B12, and cognitive function and decline. Epidemiology. 2006;17:650–657. doi: 10.1097/01.ede.0000239727.59575.da. [DOI] [PubMed] [Google Scholar]
- 47.Dickey W. Low serum vitamin B12 is common in coeliac disease and is not due to autoimmune gastritis. Eur J Gastroenterol Hepatol. 2002;14:425–427. doi: 10.1097/00042737-200204000-00016. [DOI] [PubMed] [Google Scholar]
- 48.Rubio-Tapia A, Murray JA. Celiac disease. Curr Opin Gastroenterol. 2010;26:116–122. doi: 10.1097/MOG.0b013e3283365263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu WT, Murray JA, Greenaway MC, et al. Cognitive impairment and celiac disease. Arch Neurol. 2006;63:1440–1446. doi: 10.1001/archneur.63.10.1440. [DOI] [PubMed] [Google Scholar]
- 50.Tisman G, Herbert V. B 12 dependence of cell uptake of serum folate: An explanation for high serum folate and cell folate depletion in B 12 deficiency. Blood. 1973;41:465–469. [PubMed] [Google Scholar]