Abstract
Aquaporins have been assumed to be selective for water alone, and aquaglyceroporins are accepted as carrying water and small uncharged solutes including glycerol. This review presents an expanded view of aquaporins as channels with more complex mechanisms of regulation and diverse repertoires of substrate permeabilities than were originally appreciated in the early establishment of the field. The role of aquaporins as dual water and gated ion channels is likely to have physiological and potentially translational relevance, and can be evaluated with newly developed molecular and pharmacological tools. Ion channel activity has been shown for Aquaporins -0, -1, and -6, Drosphila Big Brain, and plant Nodulin-26. Although the concept of ion channel function in aquaporins remains controversial, research advances are beginning to define not only the ion channel function but also the detailed molecular mechanisms that govern and mediate the multifunctional capabilities. With regard to physiological relevance, the adaptive benefit of expression of ion channel activity in aquaporins, implied by amino acid sequence conservation of the ion channel gating domains, suggests they provide more than water or glycerol and solute transport. Dual ion and water channels are of interest for understanding the modulation of transmembrane fluid gradients, volume regulation, and possible signal transduction in tissues expressing classes of aquaporins that have the dual function capability. Other aquaporin classes might be found in future work to have ion channel activities, pending identification of the possible signaling pathways that could govern activation.
Keywords: MIP, arylsulfonamide, nonselective cation channel, cyclic GMP, AQP, fluid transport
Reviews of aquaporin research traditionally begin with a simple binary classification of the channel families into the aquaporins and the aquaglyceroporins. Aquaporins are assumed to be selective for water alone, and aquaglyceroporins are accepted as carrying a broader range of other solutes including glycerol (King et al., 2004). This view has been useful for framing the early stages of aquaporin research, but a comprehensive view of recent progress shows that a broader view of aquaporins is timely. The full perspective must account for a higher degree of complexity in mechanisms of regulation and a more diverse repertoire of substrate permeability in the aquaporin classes than could have been appreciated during the early establishment of the field (Gomes et al., 2009; Hachez and Chaumont, 2010; Yool, 2007a). In particular, the role of aquaporins as gated ion channels, in addition to their roles as water channels, is likely to have physiological and potentially translational relevance (Yool, 2007a; Yool, 2007c). Although the concept of ion channel function in aquaporins remains controversial, research advances are beginning to define not only the ion channel function but also the detailed molecular mechanisms that govern and mediate the multifunctional capabilities. With regard to physiological relevance, the adaptive benefit of expression of aquaporins is not obvious in all cell types, and suggests they provide more than water or glycerol transport alone. In tissues expressing aquaporin classes that have dual ion and water channel, modulation of transmembrane gradients might augment water channel function, or as in the case of the aquaporin-related channel Drosophila Big Brain might be the primary function, since no osmotic water channel activity is apparent in the wild type channel (Yanochko and Yool, 2002).
1. Aquaporin ion channels
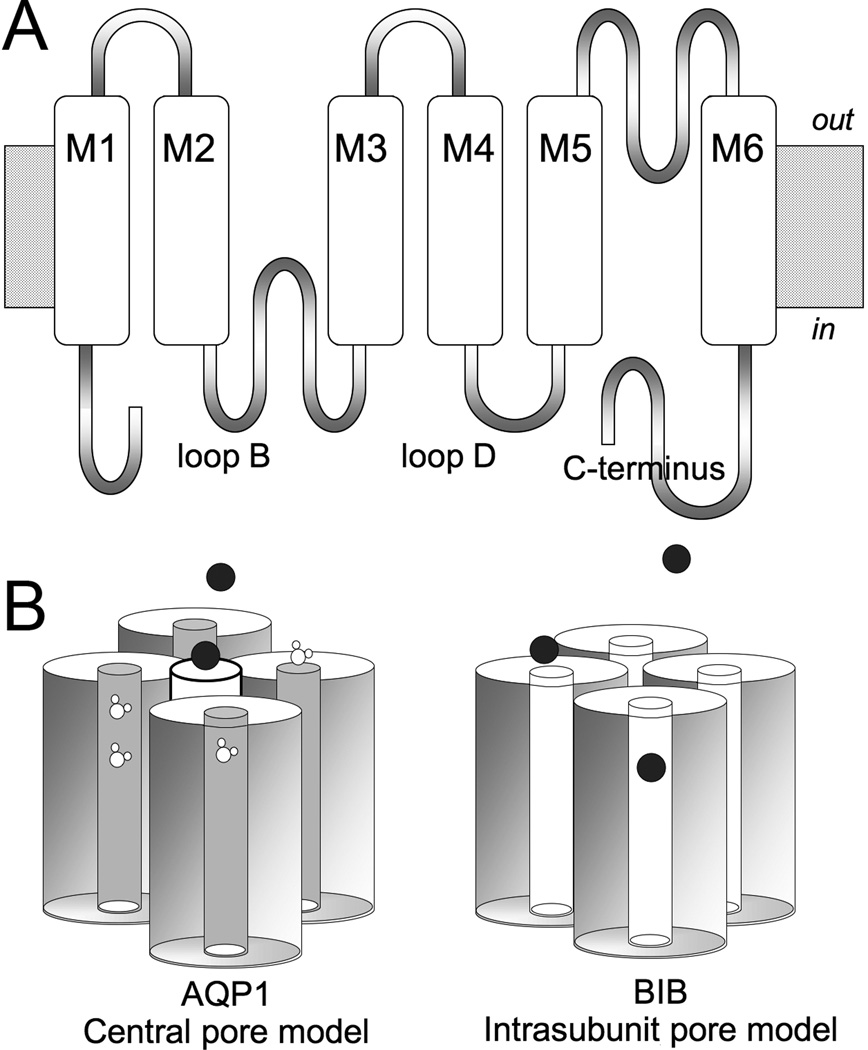
Aquaporins (AQPs) are part of a large family of major intrinsic proteins, MIPs (Reizer et al., 1993). Over the past two decades, ion channel activity has been shown for several classes of MIPs, although the physiological role of aquaporin ion channels in vivo remains to be determined (Yool and Stamer, 2004). The mechanisms of activation, molecular determinants of functional properties and ionic selectivities differ between aquaporin ion channel types, arguing against explanation of the findings as simply a generic leak effect due to the presence of exogenous protein. A combination of electrophysiology and site-directed mutagenesis has proven useful in defining the molecular domains involved in gating and modulation of AQP ion channel activity, within the subset found thus far to have ionic conductance capacity. In AQP1, the central pore at the four-fold axis of symmetry in the tetramer has been proposed as the most likely pathway for cation conduction (Yool and Weinstein, 2002; Yu et al., 2006). In contrast, the water fluxes are accepted as being mediated by separate distinct pores that are located in each of the subunits of the tetramer (Jung et al., 1994; Preston et al., 1994). In other AQPs such as AQP6 and Drosophila Big Brain, the possible role of the intrasubunit pores as ionic conductance pathways is consistent with the idea that a diverse multifunctional repertoire is expressed across the MIP channel family (Fig. 1).
Figure 1.
Diagram illustrating the Aquaporin channel structural organisation and candidate permeation pathways.
(A) Transmembrane topology of an aquaporin subunit, with six transmembrane domains (M1-6), intracellular amino and carboxyl terminal domains, and two folded loops (B and E) that meet within the transmembrane region of the channel to create the intrasubunit pore of each subunit.
(B) Two models for ion permeation. Ions can pass through the central pore in the middle of the tetramer of subunits (as has been shown for Aquaporin-1, AQP1), or might through the individual intrasubunit pore pathways of aquaporin channels (as has been proposed for Drosophila Big Brain, BIB, and others). Water flux in AQP1 is mediated primary by the intrasubunit pores. BIB has no appreciable osmotic water permeability.
1.1 Aquaporin-0, the lens MIP channel
AQP0, the major protein component of isolated lens junctions, when reconstituted in bilayers has been shown to have ion channel activity (Modesto et al., 1990; Shen et al., 1991; Zampighi et al., 1985). However, other groups have reported absence of a change in ionic conductance with lens MIP expression in Xenopus oocytes (Kushmerick et al., 1995; Mulders et al., 1995). Data supporting ion channel activity have shown that bovine MIP26 has a conductance of 200 pS in unilamellar vesicles with 100 mM saline. The ion channel is voltage- and pH-sensitive, open at acidic pH and tending to close permanently at neutral pH to a current amplitude comparable to that of control bilayers (Zampighi et al., 1985). Chicken MIP28 has a single channel conductance of approximately 230 pS in 150 mM KCl (Modesto et al., 1996). Evidence against ion channel activity came from impedance studies used to determine lens fiber cell membrane conductances, comparing cells from wild-type and a heterozygous mutant mouse strain (CatFr) that has reduced translocation of the MIP channel to the membrane; the coupling conductance was slightly less in the heterozygous mutant lens (n=2) as compared to wild type (n=1), but the differences were not statistically significant (Varadaraj et al., 1999). This finding might challenge the data from bilayer assays; alternatively, the lack of a difference could be the low n values used, the fact that the heterozygote mutant still had some MIP expression in the membrane albeit at a reduced level, or the possibility that ion channel activity of AQP0 is regulated and the putative ion channel activity might more apparent in conditions other than those used in the collagenase-dissociated cell preparation from rabbit lens. The homozygous CatFr mutant shows the diagnostic cataract formation, whereas the heterozygote used for the conductance measures is normal in appearance. The observation by Varadaraj and colleagues that the heterozygote CatFr lens fiber water permeability is significantly reduced does not justify the assumption that the ionic conductance should be comparably reduced, since aquaporin channel mutations can differentially affect ionic or water fluxes (as discussed below for AQP1).
The single channel water permeability of AQPO is about l/40th that of AQP1 (Chandy et al., 1997). Regulation by pH ranging 7.5 to 6.5 is mediated by a histidine in the extracellular loop A (Nemeth-Cahalan and Hall, 2000). One proposed role for MIP in lens is in volume regulation, preventing extracellular fluid accumulation via uptake of water into the crystalline-rich cytoplasm of the fiber cell, which minimises the extracellular space to enhance light transmission properties (Modesto et al., 1996).
1.2 Soybean Nodulin-26
The soybean MIP, nodulin (nod26), reconstituted in bilayers is a voltage-sensitive channel with a large single channel conductance and weak anion selectivity (Weaver et al., 1994). In the carboxyl terminal domain, ser 262 of nodulin 26 is phosphorylated by calmodulin-like domain protein kinase, which increases voltage-dependent gating and preferential occupancy of subconductance states (Lee et al., 1995). Nod26 is the major membrane protein component in root nodules which enclose symbiotic nitrogen-fixing bacteroids. When reconstituted into proteoliposomes, nod26 facilitates mercury-sensitive ammonium transport, suggesting an important role in fixed nitrogen transport out of the symbiosome (Hwang et al., 2010; Niemietz and Tyerman, 2000).
1.3 Aquaporin-1
Human AQP1 is a water channel (Benga et al., 1986a; Benga et al., 1986b; Preston et al., 1992) and functions as a non-selective monovalent cation channel when activated by intracellular cGMP, with a large single channel conductance of approximately 150 pS in standard physiological saline conditions (Anthony et al., 2000) and apparently smaller subconductance states when reconstituted in lipid bilayers (Saparov et al., 2001). The wild type AQP1 ion conductance carries monovalent cations (K+ ≈ Cs+ > Na+ > tetraethylammonium+), but not anions, protons, or the divalent cations Ca2+ or Mg2+ as determined from measured reversal potentials in ion substitution experiments (Yool et al., 1996). The AQP1 central pore is lined by hydrophobic barrier residues (valine 50, and leucines 54, 170, and 174; human AQP1) located in the 2nd and 5th transmembrane domains, M2 and M5 (Yu et al., 2006). Substitution of all four barrier residues by alanine increased the relative permeability of tetraethylammonium (Campbell et al., 2012), a large monovalent cation that blocks the monomeric water pores but carries an ionic current through the large cGMP-activated channel pore (Brooks et al., 2000; Yool et al., 2002). Substitution of cysteine for lys 51 in the central pore domain a cysteine-less AQP1 background created a new site for inhibition of the ionic conductance by mercury (Campbell et al., 2012), indicating the central pore is the ion conduction pathway. The AQP1-mediated cationic conductance has been implicated in influencing rates of net fluid transport in primary cultures of choroid plexus (Boassa et al., 2006). The main point of uncertainty in the field is the variability in response amplitudes between different experimental models (Saparov et al., 2001; Tsunoda et al., 2004). In light of recent findings, it seems likely that the differences between preparations results from differences in intracellular regulatory pathways that govern AQP1 ion channel availability. Phosphorylation of tyrosine Y253 in the carboxyl terminal domain, confirmed by western blot, could be one of the master switches regulating responsiveness of AQP1 ion channels to cGMP (Campbell et al., 2012). Threonine and serine kinase activity also regulates AQP1 ion channel activity (Zhang et al., 2007).
1.4 Drosophila Big Brain
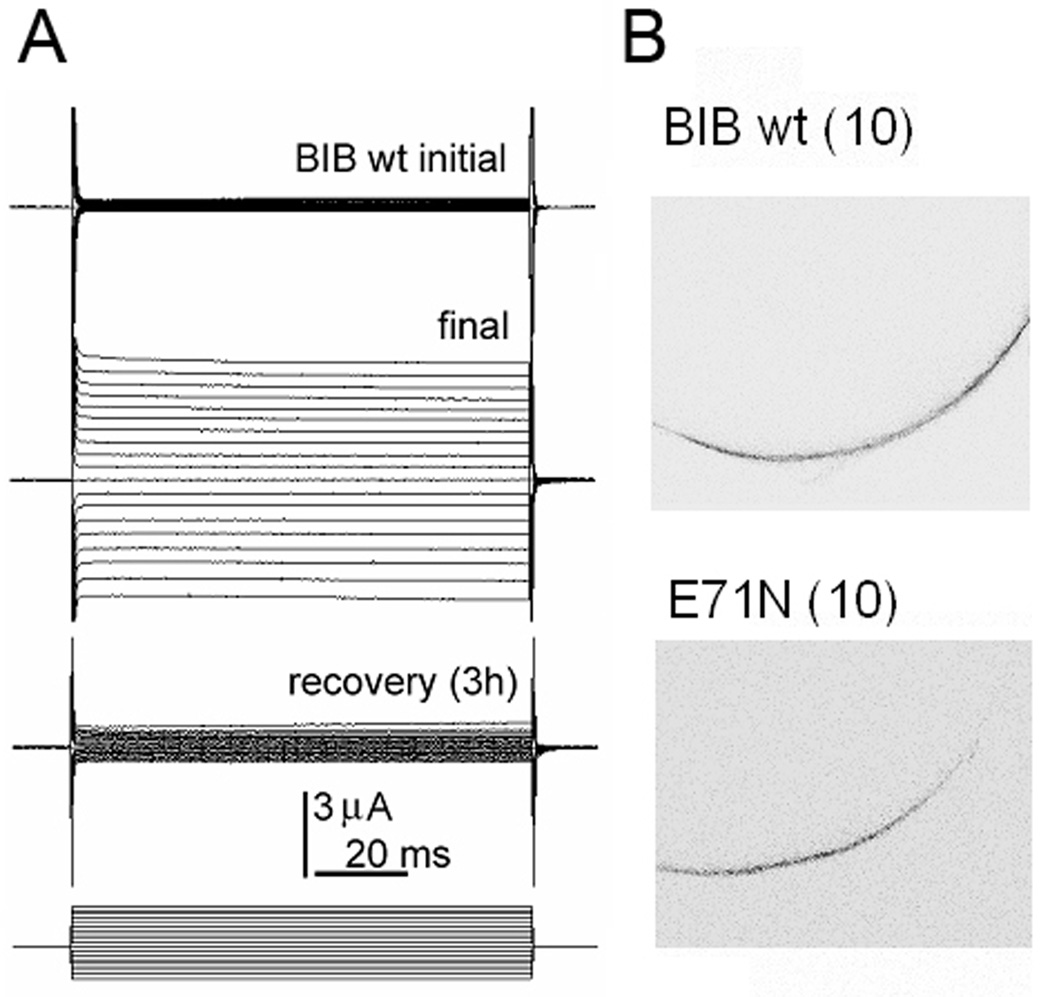
Drosophila Big Brain (BIB) expressed in oocytes is a monovalent cation channel reversibly activated by tyrosine kinase signaling (Fig. 2), without any appreciable water channel activity (Yanochko and Yool, 2002; Yanochko and Yool, 2004). Loss-of-function mutations of BIB result in an excess of neuronal precursors in the embryonic nervous system and a reduction in the number of epidermal cells, classifying it as one of the neurogenic genes in addition to Notch and Delta and others (Rao et al., 1992). However, its mechanism of action in cell fate determination remains unknown. BIB was suggested to be a channel protein that participates in the lateral inhibition signal controlling the fate of precursor cells (Doherty et al., 1997), and also has been proposed to play a structural role in cell aggregration based on assays of BIB-transfected L-cells (Tatsumi et al., 2009). Yanochko and colleagues showed that the BIB channel expressed in oocytes mediates a nonselective monovalent cation channel conductance that is modulated by pharmacological agents which alter endogenous tyrosine kinase signaling pathways in oocytes, and BIB protein analyzed by western blot shows tyrosine phosphorylation in the carboxyl terminal domain. Membrane depolarization could in theory be involved in the neurogenic function of BIB in early development (Yanochko and Yool, 2002), but pharmacological tools for testing this hypothesis currently are lacking.
Figure 2.
Reversible ion channel activation of Drosophila Big Brain aquaporins expressed in Xenopus oocytes.
(A) Reversible activation of the Big Brain (BIB) ionic conductance measured by twoelectrode voltage clamp of an oocyte expressing hemagglutinin (HA) epitope-tagged BIB channels.
(B) Confocal imaging with anti-HA antibody labeling of BIB protein, showing expression in Xenopus oocyte plasma membranes for wild type channels and for a non-ion conducting mutant (glutamate 71 to asparagine) which exerts a dominant negative effect on ionic conductance activity.
1.5 Aquaporin-6
Mammalian AQP6 expressed in oocytes shows an intermediate single channel conductance (49 pS in 100 mM NaCl) induced by treatment with 10 mM HgCl2 (Hazama et al., 2002). Rat AQP6 is found in intracellular vesicles in renal epithelia. At acidic pH (<5.5), the water and anion permeability of AQP6-expressing oocytes was increased. Site-directed mutation of lys 72 to glu at the internal side of the intrasubunit pore altered the cation to anion selectivity ratio without impairing pH-dependent activation (Yasui et al., 1999). The high nitrate permeability was reduced by site-directed mutation of a pore-lining thr 63 to ile (Ikeda et al., 2002). Anion permeability of AQP6 was eliminated by the mutation of asn 60 to gly (located at the crossover point of the 2nd and 5th transmembrane domains M2 and M5), whereas basal osmotic water permeability was increased (Liu et al., 2005). The research group that presented AQP6 as an ion channel has dismissed the ionic conductances of AQP1 as “rare and aberrant” (Ikeda et al., 2002). However, it is interesting to note that the similarities in relative levels of water and ion channel activities between AQP1- and AQP6-expressing oocytes, as well as similar properties of ionic currents measured by voltage clamp such as current amplitude, kinetics and low sensitivity to voltage, and the importance of loop B, M2 and M5 structures for ion channel function, all suggest that a common theme extends across multiple classes of AQP ion channels.
Other aquaporins besides those summarized above might be found in future work to have ion channel activities, pending identification of the possible signaling pathways that could govern activation.
2. Molecular basis of Aquaporin ion channel gating and conductance
2.1 The ionic pore
The central pore might not be the only ion permeation pathway for the classes of ion-conducting AQP channels (Fig 1). For example, in AQP6, amino acid residues that affect ion channel properties when mutated are located in loop B, an intracellular linker between the 2nd and 3rd transmembrane domains M2 and M3 (Ikeda et al., 2002; Yasui et al., 1999). Loop B forms part of the intracellular half of the intrasubunit pore that typically is associated with water channel function in other AQPs (Jung et al., 1994). Thus, the intrasubunit pores might be the ionic conduction pathways in AQP6, in contrast to the central pore model proposed for AQP1 (Yu et al., 2006). Similarly, the Big Brain-mediated ionic conductance is affected by the mutation of a conserved residue Glu 71 in the first transmembrane domain M1, which is a site that based on crystal structures of other AQPs is more likely to affect the intrasubunit pore than the central pore (Yool, 2007b). Mutation of glu 71 to asn (E71N) in BIB abolished ion channel function, whereas the equivalent mutation in AQP1 E17N did not prevent ion channel activity, but blocked AQP1 water channel function. The magnitude of the knockdown effect of the BIB mutant correlated with the ratio of mutant to wild type cRNA injected into the Xenopus oocytes. Coexpression of AQP1 wild type and E17N significantly decreased osmotic water permeability as compared with AQP1 wild type alone, without creating a dominant negative effect on AQP1 water channel function. The differential sensitivity of BIB and AQP1 to mutation of the M1 glutamate suggests that ion permeation pathways could involve the central pore or the intrasubunit pores, depending on the channel type (Yool, 2007b).
2.2 The role of the carboxyl terminal as a modulatory domain
The carboxyl terminal domain has been suggested to modulate aquaporin ion channel activity in AQP1 and BIB (Boassa and Yool, 2002; Campbell et al., 2012; Yanochko and Yool, 2002). In AQP1, a pattern of amino acids in the carboxyl terminal domain appears to mimic some key residues that have been associated with cGMP binding selectivity of cGMP phosphodiesterases. Site-directed mutagenesis of these residues in AQP1 decreased the magnitude of the ionic conductance activated by cGMP (Boassa and Yool, 2003). Phosphorylation of a carboxyl terminal tyrosine (tyr 253, human AQP1) enhanced the availability of AQP1 to be gated as ion channels in response to cGMP (Campbell et al., 2012). Pharmacological agents inducing tyrosine dephosphorylation prevented AQP1 ion channel activation, whereas agents increasing the tyrosine phosphorylated state promoted AQP1 ion channel activation. Mutation of the tyrosine phosphorylation site to cysteine (Y253C) prevented ion channel activation, but covalent addition of a negatively charged alkylthiosulfonate agent (sodium (2-sulfonatoethyl) methanethiosulfonate; MTSES) to the intracellular side of AQP1 Y253C rescued the cGMP-activated conductance response. The MTSES effect was reversed by the reducing agent dithiothreitol. These results support the proposal that phosphorylation of tyrosine Y253 in the carboxyl terminal domain, confirmed by western blot, acts as a master switch regulating responsiveness of AQP1 ion channels to cGMP (Campbell et al., 2012). Tyrosine phosphorylation of BIB in the carboxyl terminal domain conversely has been shown to negatively modulate ion channel activity, resulting in a decreased amplitude of the activated ionic conductance in BIB channels expressed in Xenopus oocytes (Yanochko and Yool, 2002).
2.3 The role of loop D as a gating domain
Loop D between the 4th and 5th transmembrane domains M4 and M5 has been linked with gating of the ionic conductance in AQP1 (Campbell et al., 2012; Yu et al., 2006). The equivalent loop in plant AQPs is essential for gating water channel activity in response to environmental stressors (Tornroth-Horsefield et al., 2006). Loop D in rat AQP4 has been proposed as the target for regulation of water channel activity by phosphorylation of Ser 180 (Zelenina et al., 2002).
Amino acid sequence alignments of the loop D regions of the known aquaporin ion channels, AQPs -0, -1, -6, Big Brain and nodulin, show the domain is highly conserved for a given class of channels expressed in diverse species, but the patterns within each of the different aquaporin classes are unique and distinctive from those of other AQP channel classes (Fig. 3). The high degree of conservation of amino acid sequences in the loop D domains in a class of channels from a variety of organisms implies an adaptive advantage is conferred by the loop D region that involves channel class-specific functional roles. Loop D has been implicated in plant and animal aquaporins as important in the gating of channel functions by various factors such as intracellular ligand binding, phosphorylation, and pH. In AQP1, mutations in loop D that interfere with ion channel activation have comparatively little effect on water channel activity (Yu et al., 2006), suggesting that the ion channel function in AQP1 has been positively selected and is likely to be physiologically relevant.
Figure 3.
Amino acid sequence alignments of the loop D domain and adjacent 5’ and 3’ flanking regions of the known ion channel aquaporins. Loop D regions for human AQP0, AQP1, AQP6, soybean NOD26 and Drosophila BIB were determined by structural prediction software (www.cbs.dtu.dk/services/TMHMM/) and by examining crystal structures. Loop D (underlined, bold) and flanking transmembrane residues from each cohort of aquaporin were mined from Genbank and aligned using ClustalW (v1.83). Asterisks (*) show residues that are identical in all aligned sequences, colons (:) show conserved substitutions, and periods (.) show semi-conserved substitutions. Colours (on-line figure version only) represent physiochemical properties of each residue; basic (magenta), acidic (blue), small hydrophobic (red) and hydroxyl, sulfhydryl or amine groups (green). Accession numbers are listed to the right of each partial sequence.
3. Physiological roles of aquaporin ion channels
3.1 Unexpected roles of aquaporin channels
AQP1 is expressed in barrier epithelia at which the maintenance of fluid homeostatic balance is essential, and their roles in facilitating water flux in these tissues is evident. In choroid plexus in brain ventricles, AQP1 enables the production of cerebral spinal fluid (Boassa and Yool, 2005; Johansson et al., 2005). In kidney proximal tubule and descending thin limb of Henley, AQP1 enables efficient fluid reabsorption (Ma et al., 1998). In the eye, expression of AQP1 in ciliary epithelia is important for aqueous humour production, and its expression in retinal pigment epithelium is thought to be important for fluid removal from the subretinal space (Levin and Verkman, 2006; Stamer et al., 2008). However, AQP1 channels also are localized in cells in which the need for having high rates of transmembrane water flux is not readily apparent. The functional purposes of AQP1 expression in these cases remain open to speculation. Examples include: neural crest derivatives such as enteric neurons and dorsal root ganglia; vascular endothelial cells of the peripheral (but not central) vasculature; red blood cells; trabecular meshwork in the fluid outflow pathway of the eye; reactive astrocytes and glioblastomas; breast cancer; retinal photoreceptors; pancreatic cells; cardiac muscle; and other tissues (Arciszewski et al., 2010; Baetz et al., 2009; Bondy et al., 1993; Burghardt et al., 2003; Endo et al., 1999; Hayashi et al., 2007; Iandiev et al., 2005; Markert et al., 2001; Nagahama et al., 2006; Nielsen et al., 1993; Oshio et al., 2005; Oshio et al., 2006; Page et al., 1998; Pannabecker et al., 2000; Stamer et al., 1996; Yang et al., 2001). Some possible roles include signal transduction (potentially in association with other ion channels, receptors and transporters); increased mechanical compliance in tissues subject to sudden changes in pressure; neurite or cell process outgrowth; organelle volume regulation; and cell volume regulation for example for enabling migration through restricted extracellular spaces (Arnaoutova et al., 2008; Baetz et al., 2009; Cowan et al., 2000; McCoy and Sontheimer, 2007; Oshio et al., 2006).
3.2 New research strategies
Understanding the full spectrum of functional roles and regulatory pathways is essential for determining the range of physiological roles that channels such as AQP1 might serve, as well as their potential value as targets for therapeutic treatments (Frigeri et al., 2007; Yool et al., 2009). Tools for differentially probing the ion and the water channel activities of AQPs are beginning to emerge from ongoing drug discovery projects, and from the molecular analyses of candidate gating and regulatory domains. For example, with information now available it is possible in cell cultures to evaluate the role of AQP1-mediated ion channel activity without the water channel activity, by transfecting cells with an AQP1 mutant construct in which glu17 is substituted with asn (E17N, human AQP1). With this construct, the cGMP-induced ionic conductance response remains intact, but AQP1 no longer shows any appreciable osmotic water permeability (Yool, 2007b). Conversely, to evaluate the role of AQP1 water fluxes without the capacity for cGMP activation of the cationic conductance, a strategy would be to transfect cells with the AQP1 double mutant construct in which arg 159 and arg 160 are substituted with alanines (R159A,R160A; human AQP1), a construct in which the water channel but not the ion channel activity remains intact (Campbell et al., 2012; Yu et al., 2006). For these approaches, the cells chosen for transfection would presumably need to be free of native wild type AQP1 expression, since neither of the selective functionally deficient mutants described above exerts a dominant negative effect. In a dominant negative condition, expression of the non-functional mutant also would decrease activity of the wild type because of co-assembly of the subunits in the channel multimer. It will be valuable in ongoing studies to continue to screen new mutant constructs for an ability to reduce or eliminate either water or ion channel activity via a dominant negative action, which could open possibilities for evaluating the differential contributions of water and ion channel activities in native cells expressing wild type AQP1. The dominant negative mechanism is a phenomenon that has been used to advantage for example in studies of the physiological roles of voltage-gated channels and for defining etiologies of inherited ionchannelopathy diseases (Jurkat-Rott et al., 2010; Nerbonne et al., 2001).
Identification of a diverse panel of selective pharmacological blockers for AQP channels could be realized within the next decade. While Cd2+ has been useful as an experimental tool for blocking AQP1 ion channels (without affecting the parallel water pores), problems with toxicity as well as a lack of specificity for AQP channels limit its value as a probe for physiological contributions of AQP1 ion channels in vivo. Preliminary data from a library of synthetic bumetanide derivatives that has been found to show AQP pharmacological activity (Migliati et al., 2009) offers promise that at least one of the agents is an effective blocker of the cGMP-dependent AQP1 ionic conductance at micromolar concentration, and has no effect on AQP1 water channel activity at doses up to 100 micromolar (Yool and Campbell, unpublished data). Other arylsulfonamide agents being developed independently hold promise for creation of an array of blocking compounds for AQPs (Huber et al., 2009; Huber et al., 2007). An acute selective blocker of the ionic conductance will be a powerful tool for addressing the translational relevance of the dual water and ion channel function of AQP1. Once the functional roles of the dual pathways are understood, it is conceivable that the selective AQP1 ion channel blockers might have therapeutic applications in medicine that would never have been envisioned when viewing AQP1 as “nothing more than a water channel”. Similar explorations of the multifunctional capacities of other multifunctional aquaporins offer exciting challenges and opportunities for research and translational advances.
Acknowledgements
This work was supported in part by the Adelaide Centre for Neuroscience Research and the Channel 7 Children’s Research Foundation.
Biographies
Andrea J Yool, PhD, is Professor and Head of Physiology at the University of Adelaide in South Australia. She earned her PhD degree in 1985 in Physiology at the University of California Santa Barbara, studying the neural control of metamorphosis in marine invertebrates. Postdoctoral training at Scripps Clinic and Stanford University focused on potassium channel structure, function, and developmental expression. Her research program at the University of Arizona (1992–2007) and University of Adelaide (2007-present) has contributed innovative advances in aquaporin physiology and drug discovery as evidenced by highly cited research papers, and invited presentations at national and international conferences.
Ewan M Campbell, PhD, is a postdoctoral fellow in Physiology at the University of Adelaide. He earned his PhD degree in in 2008 in Molecular Biology at the University of Aberdeen, studying the molecular basis for annelid and invertebrate osmoregulation. His postdoctoral training at University of Aberdeen primarily focused on blood-feeding physiology of ectoparasites and resulted in the development of RNAi techniques in a range of economically important pest species including varroa mite, sea lice and ticks. In 2009 he moved to Adelaide, where he currently researches human and protozoan aquaporin physiology and drug discovery.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Andrea J Yool, Email: Andrea.Yool@adelaide.edu.au.
Ewan M Campbell, Email: Ewan.Campbell@adelaide.edu.au.
References cited
- Anthony TL, Brooks HL, Boassa D, Leonov S, Yanochko GM, Regan JW, Yool AJ. Cloned human aquaporin-1 is a cyclic GMP-gated ion channel. Mol Pharmacol. 2000;57(3):576–588. doi: 10.1124/mol.57.3.576. [DOI] [PubMed] [Google Scholar]
- Arciszewski MB, Stefaniak M, Zacharko-Siembida A, Calka J. Aquaporin 1 water channel is expressed on submucosal but not myenteric neurons from the ovine duodenum. Ann Anat. 2010;193(2):81–85. doi: 10.1016/j.aanat.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Arnaoutova I, Cawley NX, Patel N, Kim T, Rathod T, Loh YP. Aquaporin 1 is important for maintaining secretory granule biogenesis in endocrine cells. Mol Endocrinol. 2008;22(8):1924–1934. doi: 10.1210/me.2007-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baetz NW, Hoffman EA, Yool AJ, Stamer WD. Role of aquaporin-1 in trabecular meshwork cell homeostasis during mechanical strain. Exp Eye Res. 2009;89(1):95–100. doi: 10.1016/j.exer.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benga G, Popescu O, Borza V, Pop VI, Muresan A, Mocsy I, Brain A, Wrigglesworth JM. Water permeability in human erythrocytes: identification of membrane proteins involved in water transport. Eur J Cell Biol. 1986a;41(2):252–262. [PubMed] [Google Scholar]
- Benga G, Popescu O, Pop VI, Holmes RP. p-(Chloromercuri)benzenesulfonate binding by membrane proteins and the inhibition of water transport in human erythrocytes. Biochemistry. 1986b;25(7):1535–1538. doi: 10.1021/bi00355a011. [DOI] [PubMed] [Google Scholar]
- Boassa D, Stamer WD, Yool AJ. Ion channel function of aquaporin-1 natively expressed in choroid plexus. J Neurosci. 2006;26(30):7811–7819. doi: 10.1523/JNEUROSCI.0525-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boassa D, Yool AJ. A fascinating tail: cGMP activation of aquaporin-1 ion channels. Trends Pharmacol Sci. 2002;23(12):558–562. doi: 10.1016/s0165-6147(02)02112-0. [DOI] [PubMed] [Google Scholar]
- Boassa D, Yool AJ. Single amino acids in the carboxyl terminal domain of aquaporin-1 contribute to cGMP-dependent ion channel activation. BMC Physiol. 2003;3:12. doi: 10.1186/1472-6793-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boassa D, Yool AJ. Physiological roles of aquaporins in the choroid plexus. Curr Top Dev Biol. 2005;67:181–206. doi: 10.1016/S0070-2153(05)67005-6. [DOI] [PubMed] [Google Scholar]
- Bondy C, Chin E, Smith BL, Preston GM, Agre P. Developmental gene expression and tissue distribution of the CHIP28 water-channel protein. Proc Natl Acad Sci U S A. 1993;90(10):4500–4504. doi: 10.1073/pnas.90.10.4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks HL, Regan JW, Yool AJ. Inhibition of aquaporin-1 water permeability by tetraethylammonium: involvement of the loop E pore region. Mol Pharmacol. 2000;57(5):1021–1026. [PubMed] [Google Scholar]
- Burghardt B, Elkaer ML, Kwon TH, Racz GZ, Varga G, Steward MC, Nielsen S. Distribution of aquaporin water channels AQP1 and AQP5 in the ductal system of the human pancreas. Gut. 2003;52(7):1008–1016. doi: 10.1136/gut.52.7.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell EM, Birdsell DN, Yool AJ. The activity of human aquaporin 1 as a cGMP-gated cation channel is regulated by tyrosine phosphorylation in the carboxyl terminal domain. Mol Pharmacol. 2012;81:1–9. doi: 10.1124/mol.111.073692. [DOI] [PubMed] [Google Scholar]
- Chandy G, Zampighi GA, Kreman M, Hall JE. Comparison of the water transporting properties of MIP and AQP1. J Membr Biol. 1997;159(1):29–39. doi: 10.1007/s002329900266. [DOI] [PubMed] [Google Scholar]
- Cowan CA, Yokoyama N, Bianchi LM, Henkemeyer M, Fritzsch B. EphB2 guides axons at the midline and is necessary for normal vestibular function. Neuron. 2000;26(2):417–430. doi: 10.1016/s0896-6273(00)81174-5. [DOI] [PubMed] [Google Scholar]
- Doherty D, Jan LY, Jan YN. The Drosophila neurogenic gene big brain, which encodes a membrane-associated protein, acts cell autonomously and can act synergistically with Notch and Delta. Development. 1997;124(19):3881–3893. doi: 10.1242/dev.124.19.3881. [DOI] [PubMed] [Google Scholar]
- Endo M, Jain RK, Witwer B, Brown D. Water channel (aquaporin 1) expression and distribution in mammary carcinomas and glioblastomas. Microvasc Res. 1999;58(2):89–98. doi: 10.1006/mvre.1999.2158. [DOI] [PubMed] [Google Scholar]
- Frigeri A, Nicchia GP, Svelto M. Aquaporins as targets for drug discovery. Curr Pharm Des. 2007;13(23):2421–2427. doi: 10.2174/138161207781368738. [DOI] [PubMed] [Google Scholar]
- Gomes D, Agasse A, Thiebaud P, Delrot S, Geros H, Chaumont F. Aquaporins are multifunctional water and solute transporters highly divergent in living organisms. Biochim Biophys Acta. 2009;1788(6):1213–1228. doi: 10.1016/j.bbamem.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Hachez C, Chaumont F. Aquaporins: a family of highly regulated multifunctional channels. Adv Exp Med Biol. 2010;679:1–17. doi: 10.1007/978-1-4419-6315-4_1. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Edwards NA, Proescholdt MA, Oldfield EH, Merrill MJ. Regulation and function of aquaporin-1 in glioma cells. Neoplasia. 2007;9(9):777–787. doi: 10.1593/neo.07454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazama A, Kozono D, Guggino WB, Agre P, Yasui M. Ion permeation of AQP6 water channel protein. Single channel recordings after Hg2+ activation. J Biol Chem. 2002;277(32):29224–29230. doi: 10.1074/jbc.M204258200. [DOI] [PubMed] [Google Scholar]
- Huber VJ, Tsujita M, Kwee IL, Nakada T. Inhibition of aquaporin 4 by antiepileptic drugs. Bioorg Med Chem. 2009;17(1):418–424. doi: 10.1016/j.bmc.2007.12.038. [DOI] [PubMed] [Google Scholar]
- Huber VJ, Tsujita M, Yamazaki M, Sakimura K, Nakada T. Identification of arylsulfonamides as Aquaporin 4 inhibitors. Bioorg Med Chem Lett. 2007;17(5):1270–1273. doi: 10.1016/j.bmcl.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Hwang JH, Ellingson SR, Roberts DM. Ammonia permeability of the soybean nodulin 26 channel. FEBS Lett. 2010;584(20):4339–4343. doi: 10.1016/j.febslet.2010.09.033. [DOI] [PubMed] [Google Scholar]
- Iandiev I, Pannicke T, Reichel MB, Wiedemann P, Reichenbach A, Bringmann A. Expression of aquaporin-1 immunoreactivity by photoreceptor cells in the mouse retina. Neurosci Lett. 2005;388(2):96–99. doi: 10.1016/j.neulet.2005.06.046. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Beitz E, Kozono D, Guggino WB, Agre P, Yasui M. Characterization of aquaporin-6 as a nitrate channel in mammalian cells. Requirement of pore-lining residue threonine 63. J Biol Chem. 2002;277(42):39873–39879. doi: 10.1074/jbc.M207008200. [DOI] [PubMed] [Google Scholar]
- Johansson PA, Dziegielewska KM, Ek CJ, Habgood MD, Mollgard K, Potter A, Schuliga M, Saunders NR. Aquaporin-1 in the choroid plexuses of developing mammalian brain. Cell Tissue Res. 2005;322(3):353–364. doi: 10.1007/s00441-005-1120-x. [DOI] [PubMed] [Google Scholar]
- Jung JS, Preston GM, Smith BL, Guggino WB, Agre P. Molecular structure of the water channel through aquaporin CHIP. The hourglass model. J Biol Chem. 1994;269(20):14648–14654. [PubMed] [Google Scholar]
- Jurkat-Rott K, Lerche H, Weber Y, Lehmann-Horn F. Hereditary channelopathies in neurology. Adv Exp Med Biol. 2010;686:305–334. doi: 10.1007/978-90-481-9485-8_18. [DOI] [PubMed] [Google Scholar]
- King LS, Kozono D, Agre P. From structure to disease: the evolving tale of aquaporin biology. Nat Rev Mol Cell Biol. 2004;5(9):687–698. doi: 10.1038/nrm1469. [DOI] [PubMed] [Google Scholar]
- Kushmerick C, Rice SJ, Baldo GJ, Haspel HC, Mathias RT. Ion, water and neutral solute transport in Xenopus oocytes expressing frog lens MIP. Exp Eye Res. 1995;61(3):351–362. doi: 10.1016/s0014-4835(05)80129-0. [DOI] [PubMed] [Google Scholar]
- Lee JW, Zhang Y, Weaver CD, Shomer NH, Louis CF, Roberts DM. Phosphorylation of nodulin 26 on serine 262 affects its voltage-sensitive channel activity in planar lipid bilayers. J Biol Chem. 1995;270(45):27051–27057. doi: 10.1074/jbc.270.45.27051. [DOI] [PubMed] [Google Scholar]
- Levin MH, Verkman AS. Aquaporins and CFTR in ocular epithelial fluid transport. J Membr Biol. 2006;210(2):105–115. doi: 10.1007/s00232-005-0849-1. [DOI] [PubMed] [Google Scholar]
- Liu K, Kozono D, Kato Y, Agre P, Hazama A, Yasui M. Conversion of aquaporin 6 from an anion channel to a water-selective channel by a single amino acid substitution. Proc Natl Acad Sci U S A. 2005;102(6):2192–2197. doi: 10.1073/pnas.0409232102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Severely impaired urinary concentrating ability in transgenic mice lacking aquaporin-1 water channels. J Biol Chem. 1998;273(8):4296–4299. doi: 10.1074/jbc.273.8.4296. [DOI] [PubMed] [Google Scholar]
- Markert JM, Fuller CM, Gillespie GY, Bubien JK, McLean LA, Hong RL, Lee K, Gullans SR, Mapstone TB, Benos DJ. Differential gene expression profiling in human brain tumors. Physiol Genomics. 2001;5(1):21–33. doi: 10.1152/physiolgenomics.2001.5.1.21. [DOI] [PubMed] [Google Scholar]
- McCoy E, Sontheimer H. Expression and function of water channels (aquaporins) in migrating malignant astrocytes. Glia. 2007;55(10):1034–1043. doi: 10.1002/glia.20524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliati E, Meurice N, DuBois P, Fang JS, Somasekharan S, Beckett E, Flynn G, Yool AJ. Inhibition of aquaporin-1 and aquaporin-4 water permeability by a derivative of the loop diuretic bumetanide acting at an internal pore-occluding binding site. Mol Pharmacol. 2009;76(1):105–112. doi: 10.1124/mol.108.053744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modesto E, Barcellos L, Campos-de-Carvalho AC. MIP 28 forms channels in planar lipid bilayers. Braz J Med Biol Res. 1990;23(10):1029–1032. [PubMed] [Google Scholar]
- Modesto E, Lampe PD, Ribeiro MC, Spray DC, Campos de Carvalho AC. Properties of chicken lens MIP channels reconstituted into planar lipid bilayers. J Membr Biol. 1996;154(3):239–249. doi: 10.1007/s002329900148. [DOI] [PubMed] [Google Scholar]
- Mulders SM, Preston GM, Deen PM, Guggino WB, van Os CH, Agre P. Water channel properties of major intrinsic protein of lens. J Biol Chem. 1995;270(15):9010–9016. doi: 10.1074/jbc.270.15.9010. [DOI] [PubMed] [Google Scholar]
- Nagahama M, Ma N, Semba R, Naruse S. Aquaporin 1 immunoreactive enteric neurons in the rat ileum. Neurosci Lett. 2006;395(3):206–210. doi: 10.1016/j.neulet.2005.10.092. [DOI] [PubMed] [Google Scholar]
- Nemeth-Cahalan KL, Hall JE. pH and calcium regulate the water permeability of aquaporin 0. J Biol Chem. 2000;275(10):6777–6782. doi: 10.1074/jbc.275.10.6777. [DOI] [PubMed] [Google Scholar]
- Nerbonne JM, Nichols CG, Schwarz TL, Escande D. Genetic manipulation of cardiac K(+) channel function in mice: what have we learned, and where do we go from here? Circ Res. 2001;89(11):944–956. doi: 10.1161/hh2301.100349. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Smith BL, Christensen EI, Agre P. Distribution of the aquaporin CHIP in secretory and resorptive epithelia and capillary endothelia. Proc Natl Acad Sci U S A. 1993;90(15):7275–7279. doi: 10.1073/pnas.90.15.7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemietz CM, Tyerman SD. Channel-mediated permeation of ammonia gas through the peribacteroid membrane of soybean nodules. FEBS Lett. 2000;465(2–3):110–114. doi: 10.1016/s0014-5793(99)01729-9. [DOI] [PubMed] [Google Scholar]
- Oshio K, Binder DK, Liang Y, Bollen A, Feuerstein B, Berger MS, Manley GT. Expression of the aquaporin-1 water channel in human glial tumors. Neurosurgery. 2005;56(2):375–381. doi: 10.1227/01.neu.0000148904.57841.6b. discussion 375–381. [DOI] [PubMed] [Google Scholar]
- Oshio K, Watanabe H, Yan D, Verkman AS, Manley GT. Impaired pain sensation in mice lacking Aquaporin-1 water channels. Biochem Biophys Res Commun. 2006;341(4):1022–1028. doi: 10.1016/j.bbrc.2006.01.062. [DOI] [PubMed] [Google Scholar]
- Page E, Winterfield J, Goings G, Bastawrous A, Upshaw-Earley J. Water channel proteins in rat cardiac myocyte caveolae: osmolarity-dependent reversible internalization. Am J Physiol. 1998;274(6 Pt 2):H1988–H2000. doi: 10.1152/ajpheart.1998.274.6.H1988. [DOI] [PubMed] [Google Scholar]
- Pannabecker TL, Dahlmann A, Brokl OH, Dantzler WH. Mixed descending- and ascending-type thin limbs of Henle's loop in mammalian renal inner medulla. Am J Physiol Renal Physiol. 2000;278(2):F202–F208. doi: 10.1152/ajprenal.2000.278.2.F202. [DOI] [PubMed] [Google Scholar]
- Preston GM, Carroll TP, Guggino WB, Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science. 1992;256(5055):385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- Preston GM, Jung JS, Guggino WB, Agre P. Membrane topology of aquaporin CHIP. Analysis of functional epitope-scanning mutants by vectorial proteolysis. J Biol Chem. 1994;269(3):1668–1673. [PubMed] [Google Scholar]
- Rao Y, Bodmer R, Jan LY, Jan YN. The big brain gene of Drosophila functions to control the number of neuronal precursors in the peripheral nervous system. Development. 1992;116(1):31–40. doi: 10.1242/dev.116.1.31. [DOI] [PubMed] [Google Scholar]
- Reizer J, Reizer A, Saier MH., Jr The MIP family of integral membrane channel proteins: sequence comparisons, evolutionary relationships, reconstructed pathway of evolution, and proposed functional differentiation of the two repeated halves of the proteins. Crit Rev Biochem Mol Biol. 1993;28(3):235–257. doi: 10.3109/10409239309086796. [DOI] [PubMed] [Google Scholar]
- Saparov SM, Kozono D, Rothe U, Agre P, Pohl P. Water and ion permeation of aquaporin-1 in planar lipid bilayers. Major differences in structural determinants and stoichiometry. J Biol Chem. 2001;276(34):31515–31520. doi: 10.1074/jbc.M104267200. [DOI] [PubMed] [Google Scholar]
- Shen L, Shrager P, Girsch SJ, Donaldson PJ, Peracchia C. Channel reconstitution in liposomes and planar bilayers with HPLC-purified MIP26 of bovine lens. J Membr Biol. 1991;124(1):21–32. doi: 10.1007/BF01871361. [DOI] [PubMed] [Google Scholar]
- Stamer W, Baetz N, Yool A. Ocular aquaporins and aqueous humor dynamics. In: Civan M, editor. Current Topics In Membranes: The Eye’s Aqueous Humor. Elsevier Inc.; 2008. [Google Scholar]
- Stamer WD, Huang Y, Seftor RE, Svensson SS, Snyder RW, Regan JW. Cultured human trabecular meshwork cells express functional alpha 2A adrenergic receptors. Invest Ophthalmol Vis Sci. 1996;37(12):2426–2433. [PubMed] [Google Scholar]
- Tatsumi K, Tsuji S, Miwa H, Morisaku T, Nuriya M, Orihara M, Kaneko K, Okano H, Yasui M. Drosophila big brain does not act as a water channel, but mediates cell adhesion. FEBS Lett. 2009;583(12):2077–2082. doi: 10.1016/j.febslet.2009.05.035. [DOI] [PubMed] [Google Scholar]
- Tornroth-Horsefield S, Wang Y, Hedfalk K, Johanson U, Karlsson M, Tajkhorshid E, Neutze R, Kjellbom P. Structural mechanism of plant aquaporin gating. Nature. 2006;439(7077):688–694. doi: 10.1038/nature04316. [DOI] [PubMed] [Google Scholar]
- Tsunoda SP, Wiesner B, Lorenz D, Rosenthal W, Pohl P. Aquaporin-1, nothing but a water channel. J Biol Chem. 2004;279(12):11364–11367. doi: 10.1074/jbc.M310881200. [DOI] [PubMed] [Google Scholar]
- Varadaraj K, Kushmerick C, Baldo GJ, Bassnett S, Shiels A, Mathias RT. The role of MIP in lens fiber cell membrane transport. J Membr Biol. 1999;170(3):191–203. doi: 10.1007/s002329900549. [DOI] [PubMed] [Google Scholar]
- Weaver CD, Shomer NH, Louis CF, Roberts DM. Nodulin 26, a nodule-specific symbiosome membrane protein from soybean, is an ion channel. J Biol Chem. 1994;269(27):17858–17862. [PubMed] [Google Scholar]
- Yang B, Ma T, Verkman AS. Erythrocyte water permeability and renal function in double knockout mice lacking aquaporin-1 and aquaporin-3. J Biol Chem. 2001;276(1):624–628. doi: 10.1074/jbc.M008664200. [DOI] [PubMed] [Google Scholar]
- Yanochko GM, Yool AJ. Regulated cationic channel function in Xenopus oocytes expressing Drosophila big brain. J Neurosci. 2002;22(7):2530–2540. doi: 10.1523/JNEUROSCI.22-07-02530.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanochko GM, Yool AJ. Block by extracellular divalent cations of Drosophila big brain channels expressed in Xenopus oocytes. Biophys J. 2004;86(3):1470–1478. doi: 10.1016/S0006-3495(04)74215-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui M, Hazama A, Kwon TH, Nielsen S, Guggino WB, Agre P. Rapid gating and anion permeability of an intracellular aquaporin. Nature. 1999;402(6758):184–187. doi: 10.1038/46045. [DOI] [PubMed] [Google Scholar]
- Yool A, Brokl O, Pannabecker T, Dantzler W, Stamer W. Tetraethylammonium block of water flux in Aquaporin-1 channels expressed in kidney thin limbs of Henle's loop and a kidney-derived cell line. BMC Physiology. 2002;2:4. doi: 10.1186/1472-6793-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yool AJ. Aquaporins: multiple roles in the central nervous system. Neuroscientist. 2007a;13(5):470–485. doi: 10.1177/1073858407303081. [DOI] [PubMed] [Google Scholar]
- Yool AJ. Dominant-negative suppression of big brain ion channel activity by mutation of a conserved glutamate in the first transmembrane domain. Gene Expr. 2007b;13(6):329–337. doi: 10.3727/000000006781510688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yool AJ. Functional domains of aquaporin-1: keys to physiology, and targets for drug discovery. Curr Pharm Des. 2007c;13(31):3212–3221. doi: 10.2174/138161207782341349. [DOI] [PubMed] [Google Scholar]
- Yool AJ, Brown EA, Flynn GA. Roles for novel pharmacological blockers of aquaporins in the treatment of brain oedema and cancer. Clin Exp Pharmacol Physiol. 2009 doi: 10.1111/j.1440-1681.2009.05244.x. [DOI] [PubMed] [Google Scholar]
- Yool AJ, Stamer WD. Novel roles for aquaporins as gated ion channels. In: Maui RA, editor. Molecular and Cellular Insights to Ion Channel Biology. Amsterdam: Elsevier; 2004. pp. 351–357. [Google Scholar]
- Yool AJ, Stamer WD, Regan JW. Forskolin stimulation of water and cation permeability in aquaporin 1 water channels. Science. 1996;273(5279):1216–1218. doi: 10.1126/science.273.5279.1216. [DOI] [PubMed] [Google Scholar]
- Yool AJ, Weinstein AM. New roles for old holes: Ion channel function in aquaporin-1. News Physiological Sciences. 2002;17:68–72. doi: 10.1152/nips.01372.2001. [DOI] [PubMed] [Google Scholar]
- Yu J, Yool AJ, Schulten K, Tajkhorshid E. Mechanism of gating and ion conductivity of a possible tetrameric pore in aquaporin-1. Structure. 2006;14(9):1411–1423. doi: 10.1016/j.str.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Zampighi GA, Hall JE, Kreman M. Purified lens junctional protein forms channels in planar lipid films. Proc Natl Acad Sci U S A. 1985;82(24):8468–8472. doi: 10.1073/pnas.82.24.8468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelenina M, Zelenin S, Bondar AA, Brismar H, Aperia A. Water permeability of aquaporin-4 is decreased by protein kinase C dopamine. Am J Physiol Renal Physiol. 2002;283(2):F309–F318. doi: 10.1152/ajprenal.00260.2001. [DOI] [PubMed] [Google Scholar]
- Zhang W, Zitron E, Homme M, Kihm L, Morath C, Scherer D, Hegge S, Thomas D, Schmitt CP, Zeier M, Katus H, Karle C, Schwenger V. Aquaporin-1 channel function is positively regulated by protein kinase C. J Biol Chem. 2007;282(29):20933–20940. doi: 10.1074/jbc.M703858200. [DOI] [PubMed] [Google Scholar]