Abstract
Objective and design
To determine whether repetitive airway Pseudomonas aeruginosa (Pa) infection results in lung inflammation and injury and, if so, whether these responses are affected by Muc1 mucin. Muc1 wild type (WT) and knockout (KO) mice were compared for body weights, lung inflammatory responses, and airspace enlargement using a chronic lung infection model system.
Material
Mice were treated intranasally with Pa (107 CFU) on days 0, 4, 7 and 10. On day 14, body weights, inflammatory cell numbers in bronchoalveolar lavage fluid (BALF), and airspace enlargement were measured. Differences in inflammatory responses between groups were statistically analyzed by the Student’s t test and ANOVA.
Results
Muc1 WT mice exhibited mild degrees of both inflammation and airspace enlargement following repetitive airway Pa infection. However, Muc1 KO mice exhibited significantly decreased body weights, greater macrophage numbers in the BALF, and increased airspace enlargement compared with Muc1 WT mice.
Conclusions
This is the first report demonstrating that Muc1 deficiency can lead to lung injury during chronic Pa infection in mice. These results suggest that MUC1 may play a crucial role in the resolution of inflammation during chronic respiratory infections and that MUC1 dysfunction likely contributes to the pathogenesis of chronic inflammatory respiratory disease.
Keywords: Respiratory, MUC1 mucin, Pseudomonas aeruginosa, inflammation, airspace enlargement
Introduction
Pseudomonas aeruginosa (Pa) is an opportunistic Gram-negative bacillus causing acute and chronic infections (1–3). In addition to being a primary pathogen in individuals with cystic fibrosis and bronchiectasis, Pa has also been implicated in stable chronic obstructive pulmonary disease (COPD) as well as during COPD exacerbations (4–8). Whilst many of these patients suffer from severe tissue-debilitating lung in ammation that is induced by exposure to environmental contaminants, such as cigarette smoke (CS) and bacterial infections, the molecular and cellular mechanisms underlying chronic airway inflammation are unknown. Many recently developed experimental models of emphysema, whether smoking-related or not, show increased numbers of inflammatory cells of varying compositions in the airspaces (9,10). Clinically, both the severity of airway limitation and the rate of decline in pulmonary function have been associated with the degree of airway inflammation in smokers (11), the numbers of macrophages and neutrophils in bronchoalveolar lavage fluid (BALF) of smokers with early emphysema (12,13), and the expression of matrix metalloproteases (MMPs) that may be responsible for tissue damage (14,15).
MUC1 (MUC in human, Muc in animals) is a transmembrane glycoprotein expressed in mucosal epithelial cells as well as hematopoietic cells (16), and has been postulated to be involved in the regulation of cell growth (17), differentiation, apoptosis, and inflammation (18). Recently we showed that MUC1/Muc1 expressed on the surface of airway epithelial cells is an adhesion site for Pa (19,20), and that binding of Pa or its flagellin to Muc1 resulted in phosphorylation of its cytoplasmic tail (CT) and activation of the ERK1/2 mitogen-activated protein kinase (21). These results suggested a possible role for MUC1/Muc1 as a receptor for Pa. Our subsequent studies revealed that Muc1 knockout (KO) mice exhibited hyper- inflammatory response in the airways during acute experimental Pa lung infection, as evidenced by higher levels of BALF inflammatory cytokines and chemokines, and increased numbers of lung neutrophils, coincident with reduced levels of viable Pa in the lung (18,22). However, the possible involvement of MUC1/Muc1 in the pathogenesis of chronic inflammatory respiratory disease is unknown. Therefore, in the present study, we sought to determine whether deficiency of Muc1 expression results in more severe lung injury in a mouse model of chronic Pa infection.
Materials and methods
Materials
All reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise indicated.
Animals
Muc1 KO and Muc1 wild-type (WT) mice (C57BL/6, female, 12–14 weeks of age) were used. Details of Muc1 KO mice were previously described (22,23). Muc1 WT mice were purchased from Jackson Laboratories (Bar Harbor, ME). There were no significant differences in the inflammatory responses to Pa infection between the WT littermates bred at our facility and those purchased from Jackson Laboratories (data not shown). Mice were housed in an air-filtered, temperature controlled (24°C), and pathogen-free environment with free access to food and water. All animal experiments were conducted in accordance with the guidelines provided by the Institutional Animal Care and Use Committees of the Temple University School of Medicine.
Pa lung infection
Pa strain K (PAK) was cultured in Luria Broth at 37ºC for 16 hr and an aliquot of the bacterial culture was cultured for another 2 hr to produce bacteria at log phase. Bacteria were collected by centrifugation for 10 min at 600 × g and resuspended in sterile PBS to 1.0 × 107 colony forming units (CFU)/40 μl. Mice were anesthetized by1 min inhalation of Isoflurane (Vedco, Inc., St. Joseph, MO), and instilled intranasally (i.n.) with 1.0 × 107 CFU/40 μl. At various times post-infection, mice were sacrificed by CO2 asphyxiation, the left lung was tied with surgical suture, and BALF was collected from the right lung using 3 × 0.6 ml of sterile saline containing 0.6 mM EDTA. The left lung was removed for homogenization and measurement of Muc1 protein levels, whereas the right lung was used for morphological and histological analyses. In experiments designed to measure airspace enlargement, the right lung was fixed without BALF collection as described below.
Differential cell counting of BALF
BALF was subjected to cytocentrifugation and cells were stained with a modified Giemsa stain protocol using the Diff-Quick staining kit (Polysciences, Inc., Warrington, PA). Total leukocytes, neutrophils, macrophages, and lymphocytes were counted with a hemocytometer and expressed as the number of cells per high power field (HPF).
Immunohistochemistry
Paraffin-embedded 5 μm thick sections of the right lung were prepared as previously described (22) and subjected to immunohistochemistry for either alveolar and interstitial macrophages using rat anti-mouse Mac-3 antibody (24,25) (1:250 dilution, BD Biosciences) or alveolar type II pneumocytes using hamster anti-Muc1 antibody CT2 (26). The Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA) was used for visualization with 3, 3′-diaminobenzidine as the chromogenic substrate. Five images per section were evaluated at random using two separate sections from each mouse. Results are presented as the average cell counts from 10 separate HPFs. Fluorescence immunohistochemistry for the localization of Muc1 in mouse alveolar epithelial cells was performed as described (26).
Morphometry
Lung were perfused via cardiac puncture with 5 ml PBS followed by 10% paraformaldehyde, inflated in situ by instilling 10% formalin at a constant pressure of 25 cm H2O for 15 min, ligated and removed. The inflated right lungs were kept in 10% formalin for 24 hr before embedding in paraffin. Midsagittal sections were stained with H&E and mean linear intercepts (MLIs) were measured (27). Ten images (X10 magnification) per section were evaluated at random using two separate sections from each mouse. Major airways and vasculature were avoided to focus on peripheral parenchyma.
Western blot analysis
Lungs were homogenized in PBS (0.4 ml/lung) and cells were lysed for 60 min on ice with the RIFA buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1.0% Nonidet P-40, 0.1% sodium deoxycholate, and 1.0% protease inhibitor cocktail). The lysates were subjected to Muc1 Western blot analysis as previously described with a slight modification (28).
Statistics
Differences between groups were assessed using Student’s t-test for unpaired samples or one-way analysis of variance and p < 0.05 was considered significantly different.
Results
Muc1 KO mice exhibit greater Pa-induced body weight loss compared with Muc1 WT mice
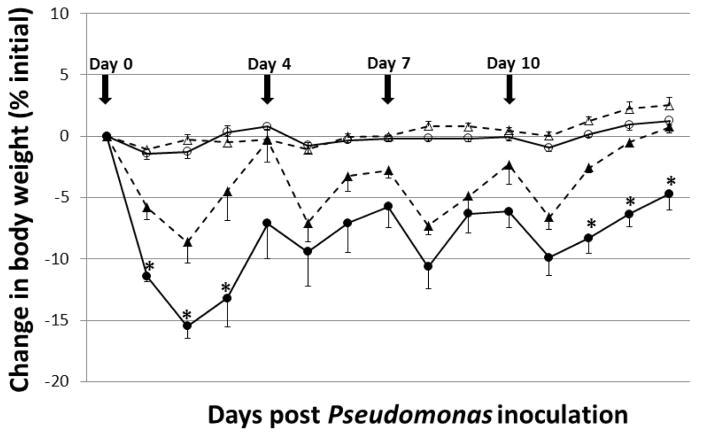
Chronic airway Pa infection in mice results in body weight loss which has been shown to be associated with inflammation and not with pulmonary responsiveness (29). Therefore, we initially evaluated body weights in Pa-infected and uninfected Muc1 KO and Muc1 WT mice. Animals were treated i.n. with PBS, or were infected with 1.0 × 107 CFU/mouse of PAK, on days 0, 4, 7, and 10 and body weights were measured daily between days 0 and 14. No significant differences in body weights between uninfected Muc1 KO and WT mice (Fig. 1). Animals infected with PAK displayed visible responses within the first 4 hr of infection, including lethargy and breathing difficulties. These symptoms were manifested in Muc1 KO and WT mice, but gradually disappeared in both groups by 48 hr post-infection. Animals treated with repetitive PAK infection showed an initial loss of body weight in both groups, with Muc1 KO mice exhibiting significantly reduced body weights following PAK infections at days 1, 2, and 3 post-infection (Fig. 1). Muc1 KO mice also had significantly reduced body weights at days 12, 13, and 14 post-infection compared with Muc1 WT animals. No significant differences in body weights between Muc1 KO and WT mice following PAK infections were evident at any other time points. Reduced body weights following infections on days 1, 2, 3, 12, 13, and 14 in Muc1 KO mice compared with WT mice appeared to be due to less food intake and the effect on day 14 was correlated with greater lung inflammation and increased airspace enlargement at this time point (see below).
Fig. 1.
Muc1 KO mice exhibit greater Pa-induced body weight loss compared with Muc1 WT mice. Daily percentage change in mean body weights following PAK i.n. infections (1.0 × 107 CFU/mouse) on days 0, 4, 7 and 10 (arrows) were determined daily for 14 days post-infection. Values are means ± ½ SEM of percent changes of body weight from pre-infection. The total number of mice in each group were 5 (Pa-infected Muc1 KO; closed circles), 5 (Pa-infected Muc1 WT; closed triangles), 3 (PBS-treated Muc1 KO; open circles), and 3 (PBS-treated Muc1 WT; open triangles). Percent changes of body weight between Muc1 KO and MUC1 WT mice were significantly different at days 1, 2, 3, 12, 13, and 14 days post-infection. *, p < 0.05.
Muc1 KO mice exhibit greater Pa-induced lung macrophage numbers and greater airspace enlargement compared with Muc1 WT mice
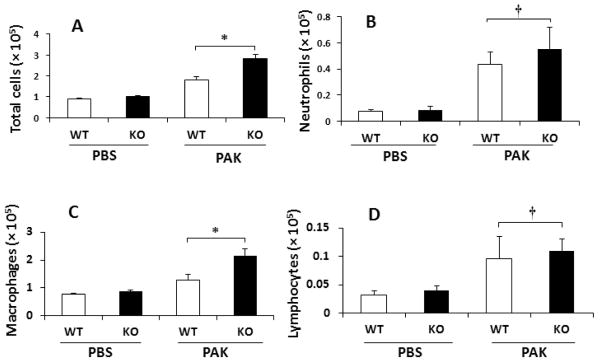
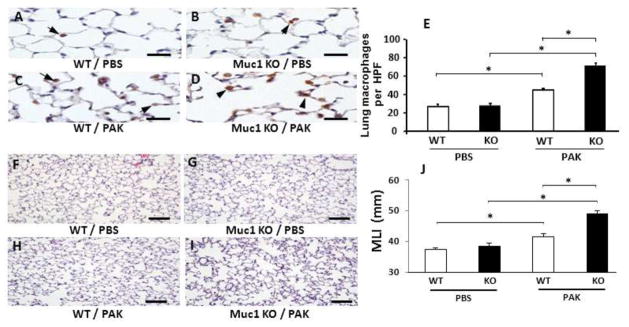
Our prior report demonstrated increased leukocyte numbers in the airways of Muc1 KO mice following acute Pa lung infection compared with Muc1 WT mice (18). To extend these studies to the chronic Pa lung infection system, the numbers of inflammatory cells in BALF were determined in Muc1 KO and WT mice at day 14 post-treatment with PBS or PAK. No significant differences in total leukocytes, neutrophils, macrophages, or lymphocytes were seen between Muc1 KO and WT mice following PBS treatment (Fig. 2). Following repetitive PAK infection, however, total leukocyte numbers were greater in Muc1 KO mice compared with Muc1 WT mice, which was primarily attributed to increased numbers of macrophages. BALF neutrophil and lymphocyte numbers were comparable in the two mouse strains. To confirm the increased number of BALF macrophages in PAK-infected Muc1 KO vs. WT mice, lung sections were stained with an antibody against the macrophage marker, Mac-3 (Figs. 3A–3D), and positively stained cells were enumerated. Greater numbers of Mac-3-staining cells were observed in Muc1 KO mice compared with Muc1 WT mice following PAK infection (Fig. 3E).
Fig. 2.
Muc1 KO mice exhibit greater Pa-induced airway macrophage numbers compared with Muc1 WT mice. PAK i.n. infections (1.0 × 107 CFU/mouse) were performed on days 0, 4, 7 and 10 and bronchoalveolar lavage fluid was isolated for enumeration of (A) total leukocytes, (B) neutrophils, (C) macrophages, and (D) lymphocytes on day 14 post-infection. Each bar represents the mean ± SEM value (n = 5). WT, wild type; KO, knockout; PAK, Pseudomonas aeruginosa K strain. *, p < 0.01; †, p > 0.05.
Fig. 3.
Muc1 KO mice exhibit greater Pa-induced alveolar macrophage staining and airspace enlargement compared with Muc1 WT mice. PAK i.n. infections (1.0 × 107 CFU/mouse) were performed on days 0, 4, 7 and 10. On day 14, lungs were isolated for immunochemical staining to measure the numbers of macrophages using anti-Mac-3 antibody (A–D) as well as for H&E staining to measure airspace enlargement using MLI (mean linear intercept) (F–I) as described in Methods. Results of quantitative analyses are shown in 3E and 3J. Numbers of mice used for the representative sections were 5 for A–D, 3 for F–G, and 5 for H–I. Each bar represents the mean ± SEM value. *, p < 0.01. Scale bars = 20 μm for A–D and 100 μm for F–I. The results were reproduced in a separate experiment.
Published evidence exists to suggest that MMPs released by macrophages during chronic airway inflammation, such as emphysema, degrades the extracellular matrix leading to alveolar destruction and airspace enlargement (14,30). Therefore, we hypothesized that increased macrophages in Muc1 KO mice following repetitive Pa infection would be correlated with greater airspace enlargement compared with Muc1 WT animals. The mean linear intercept (MLI) values between alveolar walls have been used as a measure of airspace enlargement in chronic inflammatory lung diseases (27). Therefore, MLI values were determined in Muc1 KO and Muc1 WT mice at day 14 following repetitive treatments with PBS or PAK infections. Shown in Figs. 3F–3I are representative H&E-stained lung sections from the 4 treatment groups. Results of morphometric analysis are shown in Fig. 3J. No difference in MLI values were observed between Muc1 KO and WT mice following PBS treatment. Greater MLI values were seen in Pa-infected Muc1 KO mice compared with PBS-treated Muc1 KO mice. Similarly, increased MLI values were apparent in Pa-infected Muc1 WT mice compared with PBS-treated WT mice. Further, increased MLI values were observed in Muc1 KO mice vs. Muc1 WT mice following PAK infection. Taken together, these results indicate that Muc1 KO mice have greater airspace enlargement, concomitant with greater airway macrophage numbers, compared with Muc1 WT mice in response to the repetitive PAK infections.
Repetitive Pa infection upregulates Muc1 protein expression
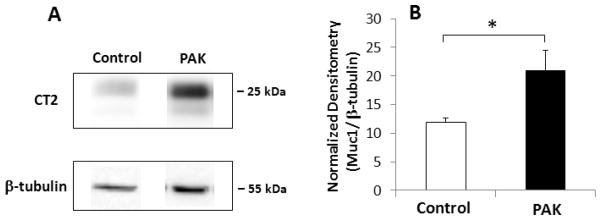
Our previous study using a single PAK infection of Muc1 WT mice revealed increased Muc1 protein levels in whole lung homogenates, reaching maximum levels between days 2 and 7 post-infection (22). These results suggested that increased Muc1 expression in the setting of an acute Pa lung infection attenuated airway inflammation, allowing the return to a condition of preinfection homeostasis (31). To determine if a similar mechanism may be operative in the chronic Pa airway infection model system, Muc1 protein levels were determined at day 14 following repetitive PBS treatments or PAK infections by a quantitative Western blotting procedure. As shown in Fig. 4, Muc1 protein expression was increased by 80% following PAK infection compared with PBS treatment. Tyrosine phosphorylation of Muc1 was not detectable in both samples (data not shown).
Fig. 4.
Pa infection induces Muc1 protein expression. (A) Western blotting using anti-Muc1 antibody (CT2) was performed using whole lung homogenates from Day 14 Muc1 WT mice following treatment with PBS (control) or PAK i.n. infections (1.0 × 107 CFU/mouse) on days 0, 4, 7 and 10. As a control, the blots were probed with anti-β-tubulin antibody. The molecular weight of prestained protein markers is shown on the right. (B) The density of each band was measured and the density of the Muc1 band was normalized to the density of the corresponding β-tubulin band. Each bar represents the mean ± SEM value (n=3). *, p <0.05.
Expression of Muc1 in alveolar type II cells
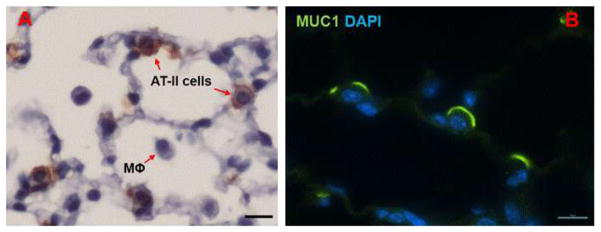
Immunohistochemistry of paraffin sections of an untreated mouse lung with Muc1 antibody CT2 revealed the presence of Muc1 in Type II pneumocytes (Fig. 5A). Confocal fluorescence microscopy localized Muc1 on the cell surface (Fig. 5B). There was no reactivity with either Type I pneumocytes or macrophages.
Fig. 5.
Expression of Muc1 in alveolar Type II cells. Paraffin embedded mouse lung sections were processed for immunostaining with anti-Muc1 antibody CT2 followed by incubation with peroxidase- (A) or fluorescein-conjugated secondary antibodies and DAPI to counterstain nuclei (B) as described in the Methods. Scale bars represent 20 μm.
Discussion
Although instillation of agar beads or alginate containing mucoid Pa is an ideal model for Pa infection because of its relevance to cystic fibrosis airways (32–34), we chose to use repetitive PAK inhalation in this study because we thought this simple method should be sufficient to provide an answer to the key question – whether or not mice deficient in Muc1 exhibit greater susceptibility than WT mice to excessive inflammation. The results from this study clearly demonstrated that mice deficient in Muc1 expression have greater BALF macrophage numbers and increased airspace enlargement following multiple Pa infections compared with Muc1 WT mice. Muc1 KO mice also had decreased body weights at days 1, 2, 3, 12, 13, and 14 post-infection, compared with the WT mice. Thus, the results are identical with those from our previously described acute Pa infection model system (18,22), and support the existing theory that Muc1 plays an anti-inflammatory role during airway infection (31).
In the present chronic Pa infection model, the levels of both KC and TNF-α in BALF were consistently greater, whereas the numbers of live bacteria in lung homogenates were consistently less in Muc1 KO mice compared with those of WT mice at 8 hr but decreased to undetectable levels at 2 days following each Pa infection (i.e., Days 1, 4, 7, and 10) (data not shown). These results are almost identical with our recent report (22) with acute Pa infection. However, on Day 14, the levels of major chemo/cytokines (KC, TNF-α, IFN-γ, IL-4, and IL-12) in BALF were not significantly different (p > 0.05) between WT and Muc1 KO mice (data not shown) although the numbers of macrophages in BALF were significantly greater in Muc1 KO mice (Fig. 2) apparently due to an increase in influx and/or a decrease in clearance of these cells, possibly efferocytosis. Further study is needed to fully understand the mechanism for the increased numbers of macrophages in Muc1 KO mice.
Based on published data (18,22,35), we postulate that increased airspace enlargement and greater macrophage numbers in Muc1 KO mouse airways occur through the following mechanism following chronic Pa exposure. Inhaled Pa activate innate immune pattern recognition receptors, primarily Toll-like receptors (TLRs) (36), which in turn induce inflammatory responses through the release of soluble mediators, including KC (murine interleukin-8 [IL-8]), and tumor necrosis factor- (TNF- ). As a result, macrophages are recruited to the lumen of the lungs where they clear the infectious bacteria by phagocytosis and release of MMPs and other degradative enzymes as well as reactive free radicals. Normally, in response to heightened TNF- levels, Muc1 levels are increased (22,35), that suppress TLR-driven inflammation (37–39) and return macrophage numbers to their preinfection levels. In the absence of Muc1 expression, however, macrophage influx persists, leading to excessive release of MMPs, destruction of the extracellular matrix, and airspace enlargement. Since Muc1 is also expressed in alveolar type II cells (Fig. 5) (40), the same sequence of events that were originally described at the level of airways (31) may be equally applicable at the level of alveoli in explaining airspace enlargement in Muc1 deficient mice.
Airspace enlargement is the major phenotype of COPD such as emphysema (41,42) and is believed to result from an imbalance between the levels of proteases and anti-proteases, more specifically MMPs and tissue inhibitors of metalloproteases (TIMPs) (42). Proteases that are produced by infiltrating leukocytes include elastase, MMP-8, and MMP-9 from neutrophils (43), and macrophage elastase (MMP-12) and cysteine and serine MMPs from macrophages (44). Shapiro et al. (14,45) demonstrated that CS-induced emphysema in mice does not develop in the absence of either neutrophil elastase or MMP-12. Thus, it is possible that one of these proteases, more likely MMP-12, may have been responsible for the airspace enlargement observed in the present study. On the other hand, it is also possible that airspace enlargement may have been due to the decreased production of TIMPs in the lung. The important role of TIMPs in the development of airspace enlargement has been demonstrated in a number of experiments including those using TIMP-3 null mice, mice exposed to CS with an increase ratio of MMP-12/TIMP-2, and alveolar macrophages from COPD patients showing lower levels of TIMP-1 (46–48). Detailed analysis of MMPs and TIMPs in conjunction with airspace enlargement in Muc1 KO mice is currently under way in our laboratory.
In addition to the protease-antiprotease theory, apoptosis has recently been implicated as a central mechanism for airspace enlargement in COPD patients (49). Airspace enlargement has been demonstrated in mice treated to induce lung cell apoptosis, such as intratracheal instillation of active caspase, or vascular endothelial growth factor (VEGF) receptor blockade (50,51). It is thought that ineffective removal of apoptotic cells on the alveolar wall induces autoimmune disease resulting in alveolar septal destruction (52). Given the anti-apoptotic role of MUC1/Muc1 (53), the enhanced airspace enlargement in Muc1 KO mice in the present study might be due to an increase in lung cell apoptosis as a result of Muc1 deficiency. Further research is required to test this hypothesis.
In summary, the present study clearly demonstrates that mice deficient in Muc1 expression develop greater lung injury following multiple Pa infections as a result of greater inflammatory responses. Given that COPD is a chronic inflammatory disease mainly among smokers resulting from a failure to control inflammation, these results suggest the possible role of MUC1 in the genesis of COPD or other chronic inflammatory lung diseases. We postulate that either failure to upregulate MUC1 expression during inflammation (22,35,54) and/or a functional defect due to mutation(s) in the MUC1 CT domain may be responsible for the development of human airway inflammatory disease states such as COPD. Given that the anti-inflammatory effect of MUC1 requires its intact CT domain (18,26,37), it may be possible that the MUC1 gene sequence corresponding to this region may be susceptible to mutation by chronic CS exposure (55,56). Should this hypothesis be proven correct, pharmacological manipulations of MUC1 or its cytoplasmic domain sequence might be a valuable approach toward the prevention and treatment of chronic inflammatory respiratory diseases such as COPD and cystic fibrosis.
Acknowledgments
This work was supported by grants from National Institute of Health, HL-47125 and HL-81825 (KCK).
References
- 1.Levison ME, Kaye D. Pneumonia caused by gram-negative bacilli: an overview. Rev Infect Dis. 1985 Nov-Dec;7(Suppl 4):S656–65. doi: 10.1093/clinids/7.supplement_4.s656. [DOI] [PubMed] [Google Scholar]
- 2.Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med. 2003 Oct 15;168(8):918–951. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- 3.Afessa B, Green B. Bacterial pneumonia in hospitalized patients with HIV infection: the Pulmonary Complications, ICU Support, and Prognostic Factors of Hospitalized Patients with HIV (PIP) Study. Chest. 2000 Apr;117(4):1017–1022. doi: 10.1378/chest.117.4.1017. [DOI] [PubMed] [Google Scholar]
- 4.Eller J, Ede A, Schaberg T, Niederman MS, Mauch H, Lode H. Infective exacerbations of chronic bronchitis: relation between bacteriologic etiology and lung function. Chest. 1998 Jun;113(6):1542–1548. doi: 10.1378/chest.113.6.1542. [DOI] [PubMed] [Google Scholar]
- 5.Soler N, Ewig S, Torres A, Filella X, Gonzalez J, Zaubet A. Airway inflammation and bronchial microbial patterns in patients with stable chronic obstructive pulmonary disease. Eur Respir J. 1999 Nov;14(5):1015–1022. doi: 10.1183/09031936.99.14510159. [DOI] [PubMed] [Google Scholar]
- 6.Miravitlles M, Espinosa C, Fernandez-Laso E, Martos JA, Maldonado JA, Gallego M. Relationship between bacterial flora in sputum and functional impairment in patients with acute exacerbations of COPD. Study Group of Bacterial Infection in COPD. Chest. 1999 Jul;116(1):40–46. doi: 10.1378/chest.116.1.40. [DOI] [PubMed] [Google Scholar]
- 7.Sethi S, Evans N, Grant BJ, Murphy TF. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002 Aug 15;347(7):465–471. doi: 10.1056/NEJMoa012561. [DOI] [PubMed] [Google Scholar]
- 8.Groenewegen KH, Wouters EF. Bacterial infections in patients requiring admission for an acute exacerbation of COPD; a 1-year prospective study. Respir Med. 2003 Jul;97(7):770–777. doi: 10.1016/s0954-6111(03)00026-x. [DOI] [PubMed] [Google Scholar]
- 9.Eidelman D, Saetta MP, Ghezzo H, Wang NS, Hoidal JR, King M, et al. Cellularity of the alveolar walls in smokers and its relation to alveolar destruction. Functional implications. Am Rev Respir Dis. 1990 Jun;141(6):1547–1552. doi: 10.1164/ajrccm/141.6.1547. [DOI] [PubMed] [Google Scholar]
- 10.Finkelstein R, Fraser RS, Ghezzo H, Cosio MG. Alveolar inflammation and its relation to emphysema in smokers. Am J Respir Crit Care Med. 1995 Nov;152(5 Pt 1):1666–1672. doi: 10.1164/ajrccm.152.5.7582312. [DOI] [PubMed] [Google Scholar]
- 11.Di Stefano A, Capelli A, Lusuardi M, Balbo P, Vecchio C, Maestrelli P, et al. Severity of airflow limitation is associated with severity of airway inflammation in smokers. Am J Respir Crit Care Med. 1998 Oct;158(4):1277–1285. doi: 10.1164/ajrccm.158.4.9802078. [DOI] [PubMed] [Google Scholar]
- 12.Betsuyaku T, Nishimura M, Takeyabu K, Tanino M, Venge P, Xu S, et al. Neutrophil granule proteins in bronchoalveolar lavage fluid from subjects with subclinical emphysema. Am J Respir Crit Care Med. 1999 Jun;159(6):1985–1991. doi: 10.1164/ajrccm.159.6.9809043. [DOI] [PubMed] [Google Scholar]
- 13.Tetley TD. Macrophages and the pathogenesis of COPD. Chest. 2002 May;121(5 Suppl):156S–159S. doi: 10.1378/chest.121.5_suppl.156s. [DOI] [PubMed] [Google Scholar]
- 14.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 1997 Sep 26;277(5334):2002–2004. doi: 10.1126/science.277.5334.2002. [DOI] [PubMed] [Google Scholar]
- 15.Owen CA. Roles for proteinases in the pathogenesis of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2008;3(2):253–268. doi: 10.2147/copd.s2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gendler SJ. MUC1, the renaissance molecule. J Mammary Gland Biol Neoplasia. 2001 Jul;6(3):339–353. doi: 10.1023/a:1011379725811. [DOI] [PubMed] [Google Scholar]
- 17.Hirasawa Y, Kohno N, Yokoyama A, Inoue Y, Abe M, Hiwada K. KL-6, a human MUC1 mucin, is chemotactic for human fibroblasts. Am J Respir Cell Mol Biol. 1997 Oct;17(4):501–507. doi: 10.1165/ajrcmb.17.4.2253. [DOI] [PubMed] [Google Scholar]
- 18.Lu W, Hisatsune A, Koga T, Kato K, Kuwahara I, Lillehoj EP, et al. Cutting edge: enhanced pulmonary clearance of Pseudomonas aeruginosa by Muc1 knockout mice. J Immunol. 2006 Apr 1;176(7):3890–3894. doi: 10.4049/jimmunol.176.7.3890. [DOI] [PubMed] [Google Scholar]
- 19.Lillehoj EP, Hyun SW, Kim BT, Zhang XG, Lee DI, Rowland S, et al. Muc1 mucins on the cell surface are adhesion sites for Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol. 2001 Jan;280(1):L181–7. doi: 10.1152/ajplung.2001.280.1.L181. [DOI] [PubMed] [Google Scholar]
- 20.Kato K, Lillehoj EP, Kai H, Kim KC. MUC1 expression by human airway epithelial cells mediates Pseudomonas aeruginosa adhesion. Front Biosci (Elite Ed) 2010 Jan 1;2:68–77. doi: 10.2741/e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lillehoj EP, Kim H, Chun EY, Kim KC. Pseudomonas aeruginosa stimulates phosphorylation of the airway epithelial membrane glycoprotein Muc1 and activates MAP kinase. Am J Physiol Lung Cell Mol Physiol. 2004 Oct;287(4):L809–15. doi: 10.1152/ajplung.00385.2003. [DOI] [PubMed] [Google Scholar]
- 22.Choi S, Park YS, Koga T, Treloar A, Kim KC. TNF-alpha is a key regulator of MUC1, an anti-inflammatory molecule, during airway Pseudomonas aeruginosa infection. Am J Respir Cell Mol Biol. 2011 Feb;44(2):255–260. doi: 10.1165/rcmb.2009-0323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spicer AP, Rowse GJ, Lidner TK, Gendler SJ. Delayed mammary tumor progression in Muc-1 null mice. J Biol Chem. 1995 Dec 15;270(50):30093–30101. doi: 10.1074/jbc.270.50.30093. [DOI] [PubMed] [Google Scholar]
- 24.Ho MK, Springer TA. Tissue distribution, structural characterization, and biosynthesis of Mac-3, a macrophage surface glycoprotein exhibiting molecular weight heterogeneity. J Biol Chem. 1983 Jan 10;258(1):636–642. [PubMed] [Google Scholar]
- 25.Flotte TJ, Springer TA, Thorbecke GJ. Dendritic cell and macrophage staining by monoclonal antibodies in tissue sections and epidermal sheets. Am J Pathol. 1983 Apr;111(1):112–124. [PMC free article] [PubMed] [Google Scholar]
- 26.Kato K, Lillehoj EP, Park YS, Umehara T, Hoffman NE, Madesh M, et al. Membrane-Tethered MUC1 Mucin Is Phosphorylated by Epidermal Growth Factor Receptor in Airway Epithelial Cells and Associates with TLR5 To Inhibit Recruitment of MyD88. J Immunol. 2012 Jan 16;188:2014–2022. doi: 10.4049/jimmunol.1102405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunnill MS. Quantitative methods in the study of pulmonary pathology. Thorax. 1962;17:320–328. doi: 10.1136/thx.17.4.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Dinwiddie DL, Harrod KS, Jiang Y, Kim KC. Anti-inflammatory effect of MUC1 during respiratory syncytial virus infection of lung epithelial cells in vitro. Am J Physiol Lung Cell Mol Physiol. 2010 Apr;298(4):L558–63. doi: 10.1152/ajplung.00225.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Heeckeren AM, Tscheikuna J, Walenga RW, Konstan MW, Davis PB, Erokwu B, et al. Effect of Pseudomonas infection on weight loss, lung mechanics, and cytokines in mice. Am J Respir Crit Care Med. 2000 Jan;161(1):271–279. doi: 10.1164/ajrccm.161.1.9903019. [DOI] [PubMed] [Google Scholar]
- 30.Lagente V, Le Quement C, Boichot E. Macrophage metalloelastase (MMP-12) as a target for inflammatory respiratory diseases. Expert Opin Ther Targets. 2009 Mar;13(3):287–295. doi: 10.1517/14728220902751632. [DOI] [PubMed] [Google Scholar]
- 31.Kim KC, Lillehoj EP. MUC1 mucin: a peacemaker in the lung. Am J Respir Cell Mol Biol. 2008 Dec;39(6):644–647. doi: 10.1165/rcmb.2008-0169TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cash HA, Woods DE, McCullough B, Johanson WG, Jr, Bass JA. A rat model of chronic respiratory infection with Pseudomonas aeruginosa. Am Rev Respir Dis. 1979 Mar;119(3):453–459. doi: 10.1164/arrd.1979.119.3.453. [DOI] [PubMed] [Google Scholar]
- 33.Starke JR, Edwards MS, Langston C, Baker CJ. A mouse model of chronic pulmonary infection with Pseudomonas aeruginosa and Pseudomonas cepacia. Pediatr Res. 1987 Dec;22(6):698–702. doi: 10.1203/00006450-198712000-00017. [DOI] [PubMed] [Google Scholar]
- 34.Heeckeren A, Walenga R, Konstan MW, Bonfield T, Davis PB, Ferkol T. Excessive inflammatory response of cystic fibrosis mice to bronchopulmonary infection with Pseudomonas aeruginosa. J Clin Invest. 1997 Dec 1;100(11):2810–2815. doi: 10.1172/JCI119828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koga T, Kuwahara I, Lillehoj EP, Lu W, Miyata T, Isohama Y, et al. TNF-alpha induces MUC1 gene transcription in lung epithelial cells: its signaling pathway and biological implication. Am J Physiol Lung Cell Mol Physiol. 2007 Sep;293(3):L693–701. doi: 10.1152/ajplung.00491.2006. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Z, Louboutin JP, Weiner DJ, Goldberg JB, Wilson JM. Human airway epithelial cells sense Pseudomonas aeruginosa infection via recognition of flagellin by Toll-like receptor 5. Infect Immun. 2005 Nov;73(11):7151–7160. doi: 10.1128/IAI.73.11.7151-7160.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ueno K, Koga T, Kato K, Golenbock DT, Gendler SJ, Kai H, et al. MUC1 mucin is a negative regulator of toll-like receptor signaling. Am J Respir Cell Mol Biol. 2008 Mar;38(3):263–268. doi: 10.1165/rcmb.2007-0336RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kato K, Lu W, Kai H, Kim KC. Phosphoinositide 3-kinase is activated by MUC1 but not responsible for MUC1-induced suppression of Toll-like receptor 5 signaling. Am J Physiol Lung Cell Mol Physiol. 2007 Sep;293(3):L686–92. doi: 10.1152/ajplung.00423.2006. [DOI] [PubMed] [Google Scholar]
- 39.Kyo Y, Kato K, Park YS, Gajhate S, Umehara T, Lillehoj EP, et al. Anti-inflammatory role of MUC1 mucin during nontypeable Haemophilus influenzae infection. Am J Respir Cell Mol Biol. 2012 Aug 25;46(2):149–156. doi: 10.1165/rcmb.2011-0142OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jarrard JA, Linnoila RI, Lee H, Steinberg SM, Witschi H, Szabo E. MUC1 is a novel marker for the type II pneumocyte lineage during lung carcinogenesis. Cancer Res. 1998 Dec 1;58(23):5582–5589. [PubMed] [Google Scholar]
- 41.Nagai A, Inano H, Matsuba K, Thurlbeck WM. Scanning electronmicroscopic morphometry of emphysema in humans. Am J Respir Crit Care Med. 1994 Nov;150(5 Pt 1):1411–1415. doi: 10.1164/ajrccm.150.5.7952569. [DOI] [PubMed] [Google Scholar]
- 42.Snider GL. Emphysema: the first two centuries--and beyond. A historical overview, with suggestions for future research: Part 1. Am Rev Respir Dis. 1992 Nov;146(5 Pt 1):1334–1344. doi: 10.1164/ajrccm/146.5_Pt_1.1334. [DOI] [PubMed] [Google Scholar]
- 43.Barnes PJ. Medicine. Neutrophils find smoke attractive. Science. 2010 Oct 1;330(6000):40–41. doi: 10.1126/science.1196017. [DOI] [PubMed] [Google Scholar]
- 44.Russell REK, Thorley A, Culpitt SV, Dodd S, Donnelly LE, Demattos C, et al. Alveolar macrophage-mediated elastolysis: roles of matrix metalloproteinases, cysteine, and serine proteases. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2002 Oct 01;283(4):L867–L873. doi: 10.1152/ajplung.00020.2002. [DOI] [PubMed] [Google Scholar]
- 45.Shapiro SD, Goldstein NM, Houghton AM, Kobayashi DK, Kelley D, Belaaouaj A. Neutrophil elastase contributes to cigarette smoke-induced emphysema in mice. Am J Pathol. 2003 Dec;163(6):2329–2335. doi: 10.1016/S0002-9440(10)63589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leco KJ, Waterhouse P, Sanchez OH, Gowing KL, Poole AR, Wakeham A, et al. Spontaneous air space enlargement in the lungs of mice lacking tissue inhibitor of metalloproteinases-3 (TIMP-3) J Clin Invest. 2001 Sep;108(6):817–829. doi: 10.1172/JCI12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.da Hora K, Valenca SS, Porto LC. Immunohistochemical study of tumor necrosis factor-alpha, matrix metalloproteinase-12, and tissue inhibitor of metalloproteinase-2 on alveolar macrophages of BALB/c mice exposed to short-term cigarette smoke. Exp Lung Res. 2005 Oct;31(8):759–770. doi: 10.1080/01902140500324828. [DOI] [PubMed] [Google Scholar]
- 48.Pons AR, Sauleda J, Noguera A, Pons J, Barcelo B, Fuster A, et al. Decreased macrophage release of TGF-beta and TIMP-1 in chronic obstructive pulmonary disease. Eur Respir J. 2005 Jul;26(1):60–66. doi: 10.1183/09031936.05.00045504. [DOI] [PubMed] [Google Scholar]
- 49.Taraseviciene-Stewart L, Douglas IS, Nana-Sinkam PS, Lee JD, Tuder RM, Nicolls MR, et al. Is alveolar destruction and emphysema in chronic obstructive pulmonary disease an immune disease? Proc Am Thorac Soc. 2006 Nov;3(8):687–690. doi: 10.1513/pats.200605-105SF. [DOI] [PubMed] [Google Scholar]
- 50.Aoshiba K, Yokohori N, Nagai A. Alveolar wall apoptosis causes lung destruction and emphysematous changes. Am J Respir Cell Mol Biol. 2003 May;28(5):555–562. doi: 10.1165/rcmb.2002-0090OC. [DOI] [PubMed] [Google Scholar]
- 51.Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, et al. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest. 2000 Dec;106(11):1311–1319. doi: 10.1172/JCI10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mahoney JA, Rosen A. Apoptosis and autoimmunity. Curr Opin Immunol. 2005 Dec;17(6):583–588. doi: 10.1016/j.coi.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 53.Agata N, Ahmad R, Kawano T, Raina D, Kharbanda S, Kufe D. MUC1 oncoprotein blocks death receptor-mediated apoptosis by inhibiting recruitment of caspase-8. Cancer Res. 2008 Aug 1;68(15):6136–6144. doi: 10.1158/0008-5472.CAN-08-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuwahara I, Lillehoj EP, Koga T, Isohama Y, Miyata T, Kim KC. The signaling pathway involved in neutrophil elastase stimulated MUC1 transcription. Am J Respir Cell Mol Biol. 2007 Dec;37(6):691–698. doi: 10.1165/rcmb.2007-0072OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guo X, Verkler TL, Chen Y, Richter PA, Polzin GM, Moore MM, et al. Mutagenicity of 11 cigarette smoke condensates in two versions of the mouse lymphoma assay. Mutagenesis. 2011 Mar;26(2):273–281. doi: 10.1093/mutage/geq083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pleasance ED, Stephens PJ, O'Meara S, McBride DJ, Meynert A, Jones D, et al. A small-cell lung cancer genome with complex signatures of tobacco exposure. Nature. 2010 Jan 14;463(7278):184–190. doi: 10.1038/nature08629. [DOI] [PMC free article] [PubMed] [Google Scholar]