Abstract
Rationale
Understanding naltrexone’s effect on motivation to drink and pattern of drinking is important for better treatment outcomes and for comparison with novel medications.
Objectives
Naltrexone’s effects on number and pattern of seeking, self-administration, and extinction responses were evaluated in two groups of baboons trained under a 3 component chained schedule of reinforcement (CSR).
Methods
Alcohol (4% w/v; n=4; Alcohol Group) or a preferred non-alcoholic beverage (n=4; Control Group) was available for self-administration only in Component 3 of the CSR. Responses in Component 2 provided indices of motivation to drink (seeking). Naltrexone (0.32 – 3.2 mg/kg) and saline were administered before drinking and Component 2 extinction sessions.
Results
Acute doses of naltrexone significantly decreased total self-administration responses (p<0.01), intake volume (p<0.001) and g/kg of alcohol (p<0.01) in the Alcohol Group only. Pattern of drinking did not change, but number of drinks during the initial drinking bout was decreased significantly by naltrexone for both groups (P<0.05). During within-session extinction tests, acute naltrexone significantly decreased time to reach extinction (p<0.01) and number of seeking responses (p<0.05), particularly early in the extinction period in the Alcohol Group only. When administered chronically, naltrexone did not decrease progressive-ratio breaking points to gain access to alcohol, but dose-dependently reduced alcohol self-administration (p<0.05) by decreasing the magnitude of the initial drinking bout.
Conclusions
The results support clinical observations that naltrexone may be most effective at reducing self-administration in the context of ongoing alcohol availability and may reduce motivation to drink in the presence of alcohol-related cues.
Keywords: Alcohol, Naltrexone, Self-Administration, Extinction, Progressive-Ratio, Baboon
Introduction
The opioid receptor antagonist naltrexone is one of the three current Food and Drug Administration (FDA)-approved medications for treatment of alcoholism. Numerous clinical trials have demonstrated its efficacy for treatment of alcohol dependence, although the effect size is typically modest (Johnson 2008). Naltrexone decreased alcohol consumption in animal and human laboratory studies (see Rosner et al. 2010; Ulm et al. 1995). Both clinical trials and controlled laboratory studies suggest it may be most effective at reducing heavy drinking in patients who continue to drink during treatment (Anton et al. 2006; Bouza et al. 2004; Killeen et al. 2004; Pettinati et al. 2006; Ray et al. 2010; Rosner et al. 2010). In addition, the reduction in alcohol drinking is greatest in patients reporting high levels of craving prior to naltrexone treatment (Monterosso et al. 2001; Richardson et al. 2008; Unterwald 2008).
Naltrexone is thought to decrease consumption by reducing alcohol’s positive reinforcing effects (Davidson et al 1999) and/or decreasing motivation to drink (Johnson et al. 2004; Johnson 2010), although specific effects on urges to drink in clinical trials are unclear. Animal models which include responses directed at obtaining alcohol (seeking responses) provide a laboratory measure of motivation to drink (Krank 2003; Markou et al. 1993). In some studies, the “seeking” response has been defined as the previously reinforced self-administration response under conditions of extinction. In rats, naltrexone attenuated cue-induced reinstatement of extinguished lever responses (Katner et al. 1999; Ciccocioppo et al. 2002) and facilitated extinction of lever responses previously maintained by alcohol delivery (Bienkowski et al.1999). Resistance to extinction has been used to measure persistence of operant responding maintained by alcohol (Jimenez and Shahan 2007; Shahan and Burke 2004) and other drugs (e.g., Cohen et al. 2005; Gracy et al. 2000) and is useful for understanding how drug-associated contexts contribute to the persistence of drug seeking and relapse (Weiss 2010). Persistence of “seeking” responses leading to alcohol access, and changes in that responding as a function of naltrexone administration, provides a measure of naltrexone’s effects on motivation to drink.
Our laboratory developed a nonhuman primate (baboon) procedure (Kaminski et al. 2008; Weerts et al. 2006) designed to model alcohol drinking in humans. The chained schedule of reinforcement (CSR) reported was composed of distinct, sequential contingencies (“components”), each of which was correlated with a different stimulus. Fulfilling the schedule requirement in each successive component was necessary to progress to the next component, with alcohol available only in the final component. Responding in the early components must be completed in order to produce alcohol availability, and thus provide a measure of seeking which can be separated from self-administration responding that occurs later in the session.
Because previous research suggests that naltrexone may be most effective at reducing heavy drinking in patients who continue to drink during treatment, a series of experiments evaluated the effects of a range of naltrexone doses (0.32 – 3.2 mg/kg, IM) in groups of baboons that self-administered either alcohol or preferred, non-alcoholic beverage under a CSR.
METHODS AND MATERIALS
Subjects
Eight adult male baboons (Papio anubis; Southwest Foundation for Biomedical Research, San Antonio, TX), weighing 20.1 to 37.4 kg (mean = 26.9 ± 5.3 SD), were housed in a room under a 12-hr light/dark cycle and with natural light from windows. For the Alcohol Group (N=4), the reinforcer delivered was 4% alcohol w/v. For the Control Group (N=4), the reinforcer delivered was a preferred non-alcohol beverage (orange-flavored, sugar-free Tang®), diluted to a concentration that functioned as a comparable reinforcer. All baboons had a history of self-administration of the reinforcer under the CSR. Food deprivation was not a part of the protocol. Food was controlled in that baboons received standard primate chow (50–73 kcals/kg) to maintain normal weights for baboons of their size and age, and to allow weights to increase gradually. The baboons also received fresh fruit or vegetables and a children’s chewable multivitamin daily. Drinking water was available ad libitum except during sessions. Facilities were maintained in accordance with USDA and AAALAC standards. The protocol was approved by the JHU Animal Care and Use Committee and followed the Guide for the Care and Use of Laboratory Animals (1996).
Apparatus
Baboons were housed singly in standard primate cages, modified to also function as the experimental chamber (for details, see Weerts et al. 2006). Briefly, each cage contained a panel with three colored “cue” lights as well as an intelligence panel with 2 vertically operated levers, 2 different colored “jewel” lights, and a “drinkometer” connected to a calibrated 1000-ml bottle. A speaker mounted above the cage presented auditory tones. Experimental conditions and data collection were controlled remotely using a computer interfaced with MED Associates hardware and software.
Drugs
All solutions for oral consumption were mixed using reverse osmosis (RO) purified drinking water. Ethyl alcohol (190 Proof, Pharmco-AAPER, Brookville CT) was diluted with RO water to 4% w/v alcohol. Orange-flavored, sugar-free, Tang® powder (Kraft Foods) was dissolved in RO water following package instructions and then diluted from full strength to concentrations of 25% (two baboons) and 50% (two baboons).
Naltrexone hydrochloride (Sigma-Aldrich, St. Louis MO) was dissolved in 2 ml of 0.09% saline and administered via intramuscular (IM) injection. Naltrexone doses (0.3–3.2 mg/kg), based on the salt, were administered under acute and chronic conditions depending on the procedure, as detailed below.
Chained Schedule of Reinforcement (CSR) Procedure
The CSR procedure has been described in detail previously (Weerts et al. 2006). Sessions were conducted 7 days/week. The drinking water spout was disabled for the duration of the session. The start of each session was signaled by a 3-s tone, followed by the onset of Component 1 (C1), and illumination of the red cue light. All responses (i.e., lever presses; spout contacts) were recorded but had no consequence. C1 ended (and the red cue light was extinguished) automatically after 20 min and Component 2 (C2) was initiated.
During C2, a yellow cue light was illuminated throughout, and a 2-link schedule was in effect. During the first link (C2-Link 1), a yellow jewel light over the left lever was continuously illuminated and a concurrent Fixed Interval 10 min Fixed Time 20 min schedule was in effect for transition to the second link (C2-Link 2). That is, C2-Link 2 was initiated after either the first left lever response after 10 min elapsed (completion of the FI requirement) or after 20 min (completion of the FT requirement), whichever occurred first. In C2-Link 2, the jewel light over the lever flashed and a Fixed Ratio (FR) (X) schedule was in effect on the left lever. Under baseline, the FR(X) =10. Upon completion of the FR (X), the yellow cue and jewel light were turned off, ending C2-Link 2, and Component 3 (C3) was initiated. If the FR (X) ratio in C2-Link 2 was not completed the session terminated without transitioning to C3.
Onset of C3 was signaled by illumination of the blue cue light and a blue jewel light over the right lever. During C3, “drinks” were available under an FR 10 schedule on the right lever and contact with the drink spout. For each drink, fluid was delivered for the duration of spout contact or 5 s, whichever came first. The volume of each drink, within the constraints described, was under the control of the baboon and averaged 35 ml/drink. Drinks were available for 120 min, then all stimuli were terminated and C3 (and the session) ended.
Within-Session Extinction Procedure
A within-session extinction procedure was used to examine the resistance of seeking behavior to extinction within a single daily session. All stimuli were the same for C1 and C2 of the CSR, but the FR (X) schedule was not in effect for C2-Link 2. Instead, the session terminated (i.e., no C3 and no access to alcohol or the non-alcoholic beverage) after 30 consecutive min with no left lever responding (criterion for extinction).
Between-Session Progressive-Ratio Procedure
The between-session progressive ratio (PR) procedure was described in detail by Kaminski et al. (2008). Briefly, the FR (X) requirement in C2-Link 2 was increased for each daily session (x 2) until the baboon failed to complete the requirement within 90 min. Breaking point (BP) was defined as the last response requirement completed that resulted in transition to C3. Before naltrexone testing was initiated, BPs were determined in the Alcohol and Control Groups (n=5 determinations in each baboon). Tang concentration was titrated (25–50%) for each baboon, so that Control Group BPs matched BPs for 4% w/v alcohol in the Alcohol Group.
Naltrexone Tests
For all experiments, doses were given in mixed order. Two different pretreatment times were used to target the onset of C2 and C3 of the CSR. This was to control for possible differences in drug effects on seeking vs. self-administration due to the sequential order of C2 and C3 within the CSR.
For Experiments 1 and 2 (acute dose tests), baseline self-administration under the CSR was first established and stable (i.e., ± 20% intake volume) for 3 consecutive sessions (defined as CSR baseline criterion) before tests with naltrexone (0.32–3.2 mg/kg) or vehicle (saline). For Experiment 1, acute test doses were administered 5 min before CSR sessions (i.e., approximately 50 min before the onset of C3) to examine naltrexone effects on self-administration. For Experiment 2, test doses were administered 30 min before CSR sessions (i.e., 50 min before the onset of C2) to examine naltrexone effects on seeking. For both, each dose was tested once; some doses were retested due to equipment malfunction and only the second is reported. Some randomly selected doses were retested for stability; data reported is the average of the tests.
For Experiment 3, the resistance of seeking responses to extinction within a single session was of interest. Acute doses of naltrexone or its vehicle were administered 30 min before sessions using the within-session extinction procedure. Following each within-session extinction test, the CSR procedure was reinstated and the CSR baseline criterion was met. To reduce habituation to repeated extinction testing (Bullock 1960, Bullock and Smith 1953, Clark and Taylor 1960, Zarcone and Ator 2000), the between-session PR procedure was implemented between tests using an abbreviated schedule (the starting ratio was set at 160). Naltrexone was not administered prior to these PR sessions. Thus, for each test, the sequence was: 1) criterion performance under the CSR baseline, 2) abbreviated PR sessions until BP, 3) criterion performance under CSR baseline, and 4) within-session extinction test for a single session. This sequence was repeated until all doses, plus matched vehicle determinations, were tested.
For Experiment 4, the motivation to obtain alcohol (as measured by BP) under chronic naltrexone dosing was of primary interest. Doses of naltrexone or vehicle were administered 30 min before sessions using the between-session PR procedure. The same dose of naltrexone was administered daily until a BP was reached. The baseline CSR procedure was then re-established (FR X=0; no naltrexone) and in effect for at least 7 days and until the CSR baseline criterion was met, before beginning the next BP determination. BPs were determined for each chronic dose (0.32–3.2 mg/kg) of naltrexone, as well as for matched vehicle tests (i.e., 3 vehicle determinations). Based on the negative findings for BP with alcohol (see results), chronic naltrexone effects on BP were not evaluated in the Control Group.
Data Analysis
The grand mean of the 3 days that preceded each test condition for each baboon was used as the baseline (BL) for comparison with vehicle and doses of naltrexone. Unless otherwise noted, data were analyzed using separate statistical analysis of variance (ANOVA) for each Group (Alcohol or Control) with naltrexone dose (BL, 0–3.2 mg/kg) as a repeated measure. Dunnett’s t-tests were used for pair-wise comparisons of BL with vehicle and naltrexone doses. Total g/kg of alcohol was calculated based on individual body weights and total volume of alcohol consumed. Change in volume consumed and g/kg of alcohol consumed was calculated as test dose (vehicle or naltrexone) – BL.
The pattern of drinks were analyzed in 20-min bins using a two-way repeated measures ANOVA (Time x Dose) for each group under acute (Experiment 1) and chronic (Experiment 4) naltrexone dosing. The pattern of left lever responses for the within-session extinction tests (Experiment 3) were analyzed in 10-min bins using a two-way repeated measures ANOVA (Time x Dose) for each group. Bonferroni pairwise comparisons examined differences between vehicle and doses of naltrexone.
In Experiment 4, the primary dependent variable was BP, which was transformed to number of steps completed for analysis as described previously (Kaminski et al. 2008). C2 and C3 data were also analyzed as the mean of the first 5 days of chronic naltrexone administration. One baboon did not complete the lowest ratio at 3.2 mg/kg and did not have further chronic administration under this dose; this baboon’s 3.2 mg/kg data is not included in these analyses.
RESULTS
During baseline (BL) sessions preceding Experiments 1 and 2, baboons in both groups reliably completed the left lever FI requirement in C2-Link 1 and FR 10 requirement in C2-Link 2, with few or no responses on the inactive operanda (right lever and drinkometer). Systematic differences between the Alcohol Group and Control Group were not observed. In C3, both alcohol and the non-alcoholic beverage maintained self-administration responses (right lever responses; drink contacts) and high intake (ml). Little or no responding occurred on the inactive (left) lever in C3. During the BL sessions preceding all Experiment 1 and 2 tests, the grand mean (± SEM) alcohol intake was 628.0 (36.6) ml and 1.07 (0.125) g/kg. The grand mean non-alcoholic beverage intake was 994.9 (2.7) ml. An unpaired t-test confirmed the greater volume of intake of the non-alcohol beverage than of alcohol (t(6) = 10.0, p<.01). Despite these differences in intake, when the motivation to drink was examined under the between-session PR procedure, the mean (+SEM) BPs for the Alcohol Group (mean +SEM = 585.3 + 52.16) were similar to those for the Control Group (574.5 + 65.33), demonstrating comparable reinforcement.
Experiment 1: Naltrexone Effects on Self-Administration Under the CSR
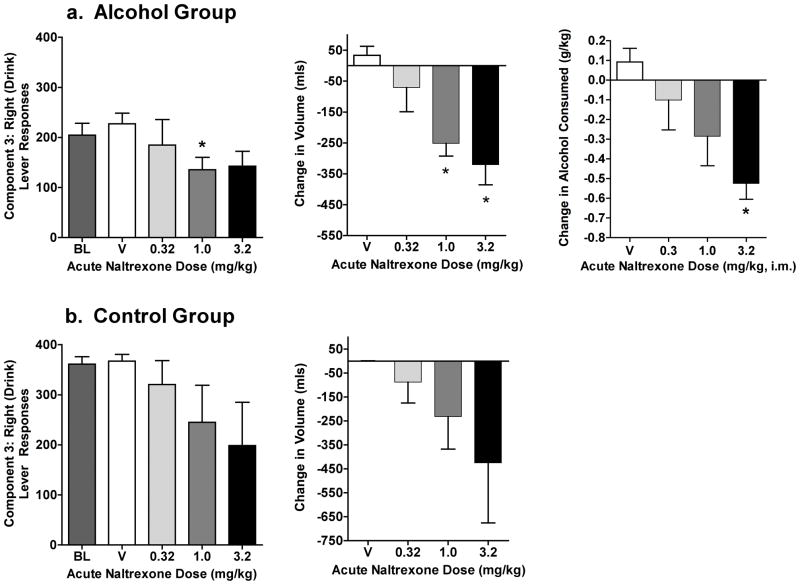
In the Alcohol Group, naltrexone significantly decreased the number of right (FR) lever responses (Fig. 1a; F(4,12) = 12.5) in C3. Although similar decreases were observed at both 1.0 and 3.2 mg/kg, only the decrease at 1.0 was significant in post-hoc tests. Volume of alcohol consumed was dose-dependently decreased by naltrexone (Fig. 1a; F(4,12) = 9.6), with statistically significant decreases at both 1.0 and 3.2 mg/kg. Likewise, when compared to BL intake (mean 0.83 g/kg) naltrexone significantly decreased g/kg alcohol consumed (Fig. 1a; F(4,12) = 4.7), at 3.2 mg/kg. Naltrexone produced nonsignificant decreases in the mean number of right lever responses and volume consumed in the Control Group (Fig. 1b). Individual data showed that naltrexone dose-dependently decreased self-administration in two baboons, with no appreciable change shown in the other two regardless of dose.
Fig. 1.
Experiment 1: the effects of acute naltrexone on self-administration in C3 of the CSR in (a) the Alcohol Group and (b) the Control Group. Data shown are the group means (± SEM) of number of right lever (drink) responses (left panels), change in volume from baseline (middle panels) and for the alcohol group, change in g/kg of alcohol consumed (right panels). Baseline (BL) g/kg intake was the average of the three days of alcohol self-administration that preceded each naltrexone dose or vehicle (V) test session. * indicates p<0.05 for pairwise comparison for each dose vs. baseline
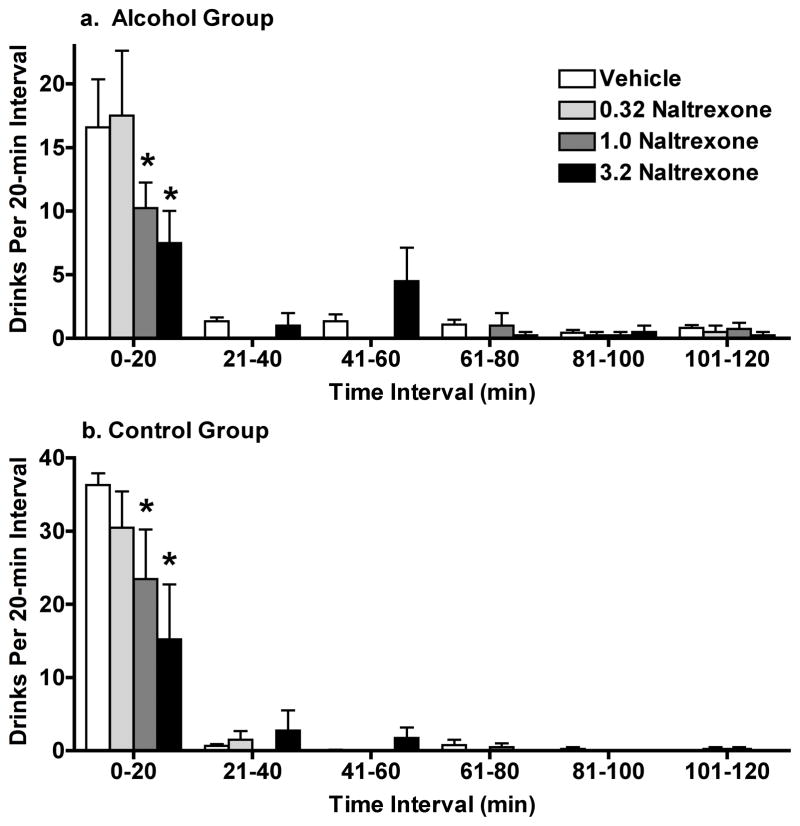
During BL, the temporal pattern of alcohol and non-alcoholic beverage drinking was very similar, with at least 80% of drinks occurred in the first 20 min of availability, followed by a lower rate across the subsequent 20-min bins (Fig. 2). Naltrexone did not disrupt this general pattern of drinking behavior in either group. Instead, doses of naltrexone (1.0 and 3.2 mg/kg) significantly decreased the number of drinks during the first 20 min in both groups (Fig. 2).
Fig. 2.
Experiment 1: The effects of acute naltrexone on the pattern and number of drinks per 20-min interval of the 120-min self-administration period (C3) in the (a) Alcohol Group and (b) the Control Group. Data shown are group mean drinks (+ SEM) for each successive time bin of availability of alcohol or the non-alcoholic beverage, and * indicates p<0.05 for pairwise comparison for each naltrexone dose vs. vehicle within each time bin
Although self-administration responses were of primary interest when naltrexone was administered 5-min before the CSR, measures of seeking in C2 were also analyzed. Naltrexone did not systematically reduce the number of FI responses, FI response latency (C2-Link 1) or FR response rate (C2-Link 2) to gain access to C3 in either the Alcohol or Control Groups. Responses on inactive operanda (right lever; drinkometer) in C2 were not affected by acute naltrexone for either group.
Experiment 2: Naltrexone Effects on Seeking Under the CSR
Naltrexone significantly decreased the number of left lever responses emitted during the FI (C2-Link 1), but not FI response latency (C2-Link 1), or FR response rate (C2-Link 2), to gain access to alcohol (Table 1a). Naltrexone did not alter any of the C2 measures in the Control Group (Table 1b). For both groups, there was little or no responding on the right lever or the drinkometer during C2-Link 2 during the BL and naltrexone did not alter this low level of behavior on the inactive operanda.
Table 1.
Experiment 2: Effects of acute naltrexone (30-min pretreatment) on seeking and self-administration for alcohol and a non-alcohol beverage under the CSR.
| A. Alcohol Group | Component 2 Link 1 (C2-Link 1) (Seeking) | Component 2 Link 2 (C2-Link 2) (Seeking) | Component 3 (C3) (Self-administration) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Naltrexone (mg/kg) | Left Lev FI Resp | FI Resp Latency (s) | Left Lev FR Resp Rate | Right Lev Resp | Drink Contacts | Drink Contacts | Volume (ml) | g/kg Alc Consumed | ||||||||
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | |
| BL | 127.8 | 38.4 | 610.6 | 7.6 | 3.13 | 0.24 | 0.25 | 0.25 | 0.0 | 0.0 | 17.7 | 3.2 | 598.3 | 63.7 | 1.07 | 0.11 |
| V | 140.8 | 51.1 | 605.3 | 2.6 | 3.32 | 0.37 | 0.0 | 0.0 | 0.0 | 0.0 | 16.8 | 3.3 | 573.8 | 25.6 | 1.04 | 0.13 |
| 0.32 | 38.8 | 22.0 | 620.0 | 10.1 | 3.60 | 0.76 | 0.0 | 0.0 | 0.0 | 0.0 | 12.3 | 2.3 | 465.0 | 39.2 | 0.81 | 0.08 |
| 1.0 | 48.8 | 46.4 | 680.9 | 46.8 | 2.24 | 0.85 | 0.0 | 0.0 | 0.0 | 0.0 | 13.0 | 4.7 | 422.5 | 100.8 | 0.79 | 0.21 |
| 3.2 | 19.8 | 11.2 | 649.8 | 37.0 | 2.38 | 1.02 | 0.0 | 0.0 | 0.0 | 0.0 | 10.8 | 5.6 | 383.8 | 233.9 | 0.70 | 0.40 |
| F1 (4,12) | 3.48 | 1.8 | 0.9 | nc | nc | 0.7 | 0.7 | 0.7 | ||||||||
| B. Control Group | Component 2 Link 1 (C2-Link 1) (Seeking) | Component 2 Link 2 (C2-Link 2) (Seeking) | Component 3 (C3) (Self-administration) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Naltrexone (mg/kg) | Left Lev FI Resp | FI Resp Latency (s) | Left Lev FR Resp Rate | Right Lev Resp | Drink Contacts | Drink Contacts | Volume (ml) | N/A2 | |||||||
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | ||
| BL | 94.3 | 23.8 | 609.0 | 4.4 | 2.66 | 0.45 | 1.89 | 1.83 | 0.13 | 0.13 | 34.2 | 3.3 | 993.8 | 6.3 | |
| V | 194.5 | 61.0 | 603.6 | 3.2 | 2.19 | 0.19 | 0.08 | 0.08 | 0.25 | 0.25 | 34.5 | 1.8 | 1000 | 0.0 | |
| 0.32 | 174.0 | 84.1 | 746.2 | 134.1 | 2.82 | 0.38 | 0.0 | 0.0 | 0.25 | 0.25 | 34.3 | 5.2 | 1000 | 0.0 | |
| 1.0 | 105.0 | 51.1 | 605.4 | 5.1 | 2.28 | 0.22 | 0.0 | 0.0 | 0.0 | 0.0 | 24.5 | 7.3 | 937.5 | 62.5 | |
| 3.2 | 153.3 | 36.6 | 616.7 | 8.3 | 2.70 | 0.33 | 0.0 | 0.0 | 0.0 | 0.0 | 20.0 | 8.9 | 875.0 | 125.0 | |
| F1 (4,12) | 0.6 | 1.0 | 1.36 | nc | nc | 2.0 | 1.0 | ||||||||
Data shown are group means (±SEM) for baseline (BL), vehicle (V) and each naltrexone dose (mg/kg). FI, Fixed Interval; FR, Fixed Response; Lev, Lever; Resp, Response; Alc, Alcohol.
An underlined F ratio indicates a significant (p<0.05) ANOVA, nc=not calculated
g/kg alcohol consumed does not apply (“NA”) to the Tang group
Bolded numbers indicate a significant (p<0.05) Dunnett’s post-hoc test comparison to vehicle.
While there was some evidence of a decrease in self-administration and consumption as a function of naltrexone dose under the longer pretreatment interval, this effect was not statistically significant for either group. Naltrexone produced decreases in one (Control Group) or two (Alcohol Group) baboons, and no consistent change in the remaining baboons.
Experiment 3: Effects of Acute Naltrexone on Within-Session Extinction
At BL, the Alcohol Group showed greater resistance to extinction as evidenced by higher numbers of seeking responses under extinction and longer times to reach extinction criterion, when compared to the control group (Table 2). Naltrexone significantly reduced total number of C2-Link 2 left lever seeking responses during extinction in the Alcohol Group, with 3.2 mg/kg significantly decreased compared to vehicle (Table 2). Responses on inactive operanda (right lever and drinkometer), which were previously low under BL conditions (see Table 1), occurred at higher rates during extinction in the Alcohol Group (Table 2), but not in a dose-related manner. In the Control Group, total number of C2-Link 2 left lever responses varied across baboons. For those baboons that did not show an effect, responding was maintained near vehicle levels regardless of dose, and in one case, was even increased at the highest dose. Similarly, the time to meet the extinction criteria (Table 2) was significantly reduced by 3.2 mg/kg naltrexone in the Alcohol but not Control group. Intervening BPs determined using the between-session PR procedure (which did not include naltrexone pretreatment) were comparable in the Alcohol (1086.5) and Control (1072) groups.
Table 2.
Experiment 3: Effects of acute naltrexone (30-min pretreatment) on seeking responses for alcohol or a non-alcoholic beverage during within-session extinction tests.
| A. Alcohol Group | Component 2 Link 1 (C2-Link 1) | Component 2 Link 2 (C2-Link 2) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Naltrexone (mg/kg) | Left Lever FI Resp | FI Resp Latency (s) | Left Lever FR Resp | Right Lever Resp | Drink Contacts | Time to EXT Criterion (s) | ||||||
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | |
| V | 160.0 | 74.5 | 618.4 | 16.0 | 2109.6 | 919.2 | 139.9 | 77.5 | 3.5 | 1.0 | 108.7 | 21.8 |
| 0.32 | 145.8 | 87.1 | 606.6 | 2.4 | 2040.5 | 753.2 | 150.5 | 118.8 | 6.5 | 4.7 | 87.7 | 24.1 |
| 1.0 | 20.0 | 13.8 | 810.7 | 107.6 | 1161.5 | 433.9 | 753.3 | 659.3 | 0.5 | 0.2 | 100.5 | 17.2 |
| 3.2 | 16.0 | 14.7 | 921.1 | 206.9 | 432.5 | 143.2 | 19.5 | 10.3 | 0.1 | 0.1 | 47.8 | 14.2 |
| F (3,9) | 1.4 | 1.5 | 3.9 | 0.9 | 1.7 | 7.5 | ||||||
| B. Control Group | Component 2 Link 1 (C2-Link 1) | Component 2 Link 2 (C2-Link 1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Naltrexone (mg/kg) | Left Lever FI Resp | FI Resp Latency (s) | Left Lever FR Resp | Right Lever Resp | Drink Contacts | Time to EXT Criterion (s) | ||||||
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | |
| V | 155.8 | 55.0 | 610.9 | 7.6 | 294.3 | 89.2 | 1.8 | 1.4 | 0.3 | 0.3 | 67.6 | 12.4 |
| 0.32 | 36.5 | 32.6 | 788.5 | 139.0 | 530.8 | 333.5 | 8.8 | 8.1 | 1.0 | 1.0 | 65.3 | 65.3 |
| 1.0 | 196.5 | 87.0 | 623.6 | 19.4 | 711.5 | 161.5 | 12.0 | 7.9 | 0.8 | 0.8 | 82.8 | 82.8 |
| 3.2 | 59.3 | 22.7 | 656.1 | 27.9 | 362.8 | 218.3 | 21.8 | 17.9 | 0.8 | 0.8 | 69.7 | 69.7 |
| F (3,9) | 2.5 | 1.3 | 1.1 | 1.2 | 0.3 | 0.4 | ||||||
Data shown are group means (±SEM) for vehicle (V) and each naltrexone dose (mg/kg). FI, Fixed Interval; FR, Fixed Response; Lev, Lever; Resp, Response; EXT, extinction.
An underlined F ratio indicates a significant (p<0.05) ANOVA; Bolded numbers indicate a significant (p<0.05) Dunnett’s post-hoc test comparison to vehicle.
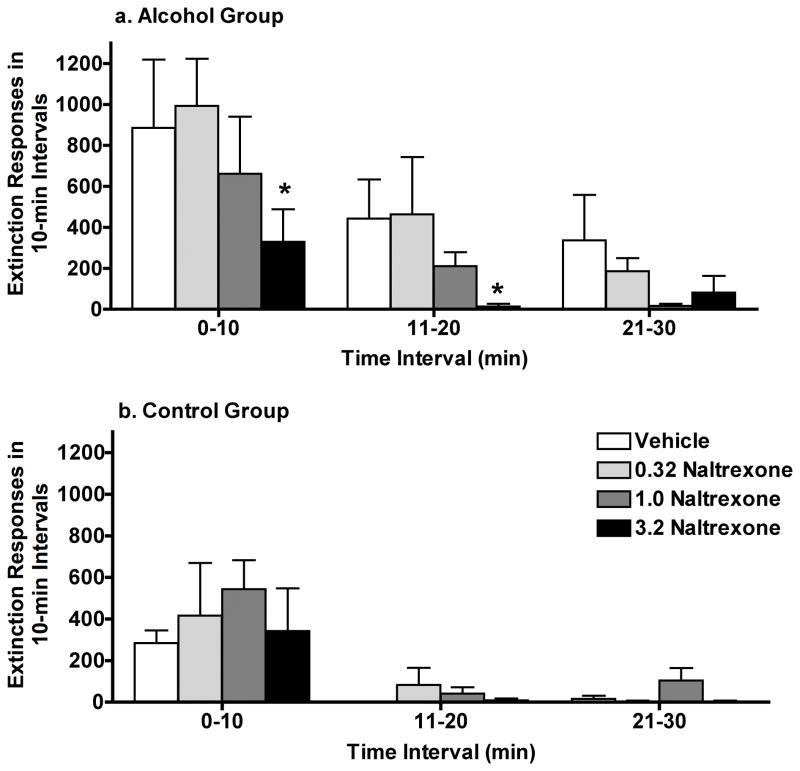
The number of responses was greatest in the first 10 min of extinction and progressively decreased over successive 10-min bins until the extinction criterion was met (Fig 3). Compared to vehicle, naltrexone (3.2 mg/kg) decreased the number of seeking responses across successive bins of the extinction period in the Alcohol Group and reached significance during the early bins when responses were highest (0–10 and 11–20 bins) (Fig. 3a). Naltrexone did not reduce the magnitude of responses in early bins of the extinction period in the Control Group (Fig. 3b).
Fig 3.
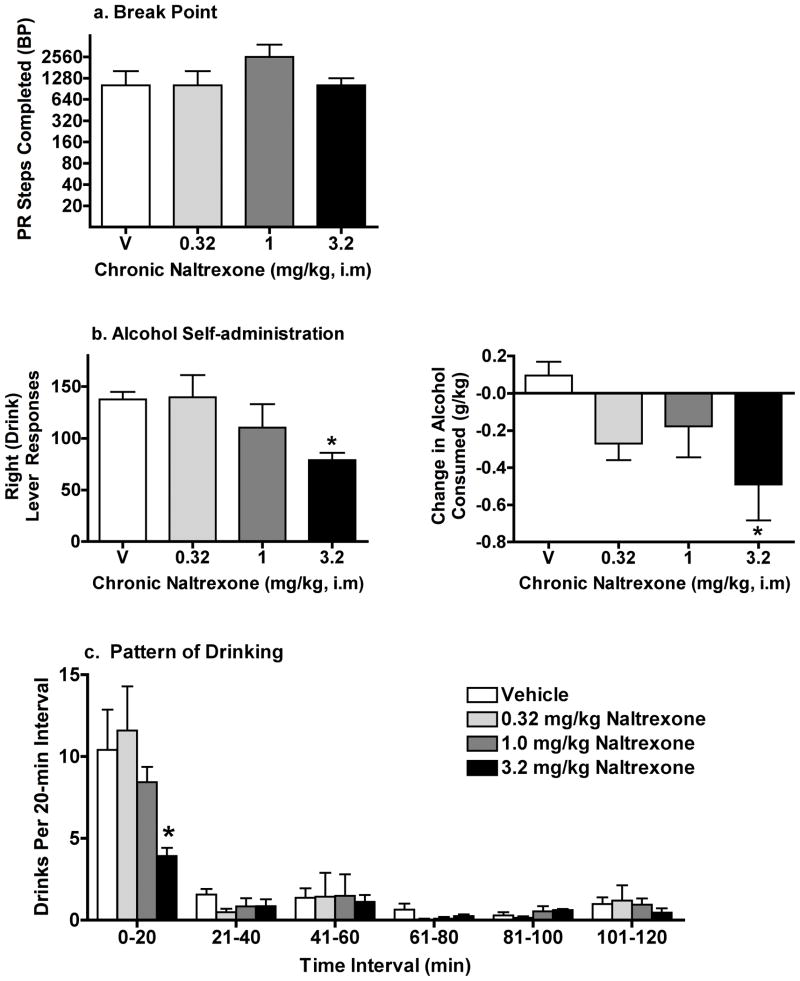
Experiment 3: The effects of chronic naltrexone on motivation to obtain and self-administer alcohol in the Alcohol Group. Data shown for a) are the mean (± SEM) breaking point for each chronically administered naltrexone dose and vehicle (V). Data shown for b) are the mean (± SEM) of the first 5 days of chronic naltrexone dosing on right lever (drink) responses and change in g/kg alcohol self-administered. Data shown for c) are the mean (± SEM) of the first 5 days of chronic naltrexone dosing on number of drinks per 20-min interval of the 120-min alcohol self-administration period (C3). Other details as in Fig. 1 and 2
Experiment 4: Effects of Chronic Naltrexone on Seeking Under the Between-Session PR Procedure
When naltrexone was administered chronically under the between session PR procedure, BP did not vary as a function of chronic naltrexone dose (Fig. 4a); baboons continued to reach maximal BPs, comparable to those obtained under vehicle, across all doses.
Fig 4.
Experiment 4: Effects of acute naltrexone on extinction of seeking in the within-session extinction tests in C2-Link 2 for the (a) Alcohol Group and (b) the Control Group. Data shown are group mean (+ SEM) number of left lever responses per 10 min interval for the first 60 min of the within-session extinction test session and * indicates p<0.05 for pairwise comparison for each dose vs. vehicle within each interval
Examination of PR sessions in which the ratio requirement was completed showed that under vehicle and under no pretreatment conditions, right lever responses, contacts, and volume of alcohol consumed in C3 remained stable regardless of the ratio value in C2-Link 2, a result that has also been previously reported (Kaminski et al. 2008). Because at least five consecutive sessions were conducted with each dose, C3 self-administration measures were analyzed as the mean of the first 5 days of dosing, without consideration of the FR (X) value in C2. Fig. 4b shows that both the number of right lever (drink) responses (F(3,12) = 4.2) and g/kg alcohol consumed (F(3,15) = 4.9) decreased as a function of chronic naltrexone dose; pairwise comparisons confirmed the decrease relative to BL at 3.2 mg/kg for both measures. Similar to acute administration conditions, chronically administered naltrexone did not disrupt the general pattern of drinking behavior. Chronic administration of 3.2 mg/kg naltrexone significantly decreased the number of drinks during the first 20 min (Fig. 4c).
DISCUSSION
The primary findings of the present study were 1) naltrexone produced modest, but dose-dependent, decreases in alcohol drinking, under both acute and chronic dosing conditions in baboons with long-term self-administration experience under a CSR, 2) naltrexone’s effects on alcohol intake were primarily by decreasing the magnitude of the first drinking bout, an effect also observed for intake of the non-alcoholic beverage, 3) in the context of alcohol access and ongoing drinking, the motivation to obtain alcohol in the CSR was largely unaffected by naltrexone, and 4) alcohol seeking responses, which were highly resistant to extinction, were dose-dependently and selectively decreased by naltrexone during within-session extinction tests. Each of these findings is discussed in detail below.
Throughout the current experiments, the non-alcoholic beverage maintained a higher rate of self-administration than alcohol. However, BPs determined prior to naltrexone testing for the Control Group matched BPs of the Alcohol Group. Similarly, BPs obtained during Experiment 3 (which did not include naltrexone pretreatment) were also similar for the two reinforcers, demonstrating the two fluids functioned as comparable reinforcers.
The FDA recommended dose of naltrexone for treatment of alcohol dependence is 50 mg, although higher doses (100–150 mg/kg) are used successfully in clinical trials (Anton et al 2003; Yoon et al 2011). Using an interspecies dose conversion formula (Dews 1976; Mordenti and Chappell 1989), the FDA recommended dose of 50 mg naltrexone would be comparable to a dose of 0.92 mg/kg in baboons. Thus, doses of 1 mg/kg or higher would be expected to reduce alcohol drinking in baboons.
To evaluate effects on self-administration responses, in Experiment 1, 0.32–3.2 mg/kg naltrexone was administered 50–60 min before alcohol access. Acute naltrexone dose-dependently reduced alcohol self-administration responses and total amount of alcohol consumed. Naltrexone administered for five consecutive days (Experiment 4) reduced alcohol self-administration responses and consumption in ways similar to that observed under acute administration conditions (Experiment 1). Individual differences were seen in the Control Group, with effects on the non-alcoholic beverage that were similar to those on alcohol in two of the four baboons (Experiment 1).
The concentration of alcohol (4% w/v) used in the present experiment has previously been shown to maintain stable intake. While baboons will drink high concentrations of alcohol (e.g., 16% w/v) during induction of alcohol drinking (Henningfield et al. 1981), lower concentrations of alcohol are generally preferred and maintain higher rates of operant self-administration (Ator and Griffiths 1992). Baboons will titrate volume of higher concentrations to achieve g/kg intake within a similar intoxicating range. Although the effects of naltrexone were not evaluated across a range of concentrations, previous research in nonhuman primates (Williams et al. 1997) demonstrated that naltrexone (0.1 mg/kg, administered 30 min prior to the session) decreased alcohol-reinforced responding similarly across a range of concentrations (0.25% w/v – 4% w/v), that maintained both low intake (0.25% w/v) and high intake (4% w/v), shifting the ethanol-concentration curve downward in an unsurmontable manner.
Studies in laboratory animals have consistently reported that naltrexone administration reduces alcohol self-administration and intake, and many studies have reported this effect was not selective for alcohol (Egli 2005). For example, in nonhuman primates, doses of 0.1–3.0 mg/kg naltrexone, administered 30 min before self-administration, reduced self-administration of alcohol and Tang (Shelton and Grant 2001), saccharin (Rodefer et al. 1999), water (Williams and Woods 1999), and sucrose (Williams et al. 1998). Although it has been argued that the reduction of intake of alcohol and other orally consumed substances by naltrexone may, in part, be related to changes in palatability (Ferraro et al 2002; Hill et al. 1997), studies demonstrating decreased intravenous self-administration of alcohol in monkeys (Altshuler et al. 1980; Williams et al. 1998) provide evidence for opioid mediation of reinforcement, which is unrelated to any buccal or gustatory effects (Olszewski et al. 2011).
All operant responses were recorded in real time allowing the examination of the within-session patterning of alcohol self-administration. Similar to previous reports with rats (Samson et al. 2000), baboons (Weerts et al. 2006) and other primates (Boyle et al. 1998; Grant et al. 2009; Macenski and Meisch 1992; Rodefer et al. 1999; Vivian et al. 2001; Williams et al. 1998), baboons in the current study engaged in “loading;” the greatest magnitude of drinking occurred early in the alcohol self-administration period followed by lower rates of drinking for the remainder of the period. Naltrexone administered acutely (Experiment 1) or chronically (Experiment 4) did not delay onset of drinking or alter the general within-session patterning of drinking. Instead, the magnitude of the initial drinking bout was significantly reduced for both alcohol and the nonalcoholic reinforcer, further suggesting that naltrexone reduced reinforcing effects that were directly related to consumption. These data are consistent with human laboratory alcohol administration studies showing that naltrexone significantly decreased self-reported ratings of alcohol liking and best effects (King et al. 1997; McCaul et al. 2000; O’Malley et al. 2010).
Naltrexone did not decrease most alcohol seeking measures in C2 of the CSR. In Experiment 2, acute doses of naltrexone tended to decrease the number of responses directed towards obtaining the daily supply of alcohol, but did not prolong latency to obtain alcohol (FI response latency), and did not alter any seeking measure in the Control Group. In Experiment 4, chronically administered naltrexone also did not reduce BPs to obtain alcohol. This finding is consistent with a recent controlled laboratory study in social drinkers, which also found naltrexone did not alter alcohol seeking BPs (Setiawan et al. 2011). The present data are consistent with previous research showing that alcohol seeking responses, which provide a measure of the motivation to drink in the CSR, are largely under the control of stimuli/cues which have acquired conditioned reinforcing and/or eliciting properties, and are highly resistant to change (Kaminski et al. 2008). Previous research has shown that environmental or contextual stimuli associated with drug use are an important mitigating factor in relapse to drug use (Childress et al. 1992; Collins and Brandon 2002; Crombag and Shaham 2002).
It is noteworthy then, that in Experiment 3, acute doses of naltrexone selectively facilitated extinction of seeking responses in the Alcohol Group. Naltrexone did not decrease extinction responding in a clear dose-related manner in any baboon in the Control Group (e.g., a decrease at 0.32 mg/kg was seen in one baboon, no change in two, and an increase in one). This finding is consistent with rodent studies showing naltrexone (doses ranging from 0.1–3.0 mg/kg) selectively facilitated extinction of responses previously maintained by alcohol (Bienkowski et al. 1999; Czachowski and DeLory 2010) and attenuated alcohol- and cue-induced reinstatement of alcohol self-administration (Bienkowski et al. 1999; Lê et al. 1999; Liu and Weiss 2002). The current study further determined that it selectively decreased resistance to extinction by suppressing seeking responses in the early phase of extinction and decreasing the length of time responding persisted. This finding is noteworthy given that under vehicle conditions alcohol showed greater resistance to extinction when compared to the non-alcoholic reinforcer.
The baboons in the current study had long-term self-administration experience under the CSR with either alcohol or the non-alcoholic beverage. In the Alcohol Group, baboons drank 1 g/kg/day of alcohol, which was maintained at stable levels 7 days/week. Mean blood alcohol level (BAL) of 88.2 mg/dl (>0.08%) were previously determined in these same baboons after comparable alcohol intake (mean 0.93 g/kg) (Kaminski et al. 2008). This level of drinking is important, as relatively few models using outbred animals generate patterns of volitional alcohol intake that exceed a threshold associated with risk of harm (Leeman et al. 2010). Problematic or “at risk” drinking in man includes patterns of drinking to intoxication (e.g., 0.8 to 1 g/kg, BAL > 0.08%) within a single drinking period (binge) as well as regular drinking at this level across days (heavy drinking). Thus, the level and pattern of alcohol intake by baboons under the CSR is pharmacologically relevant based on NIAAA definitions, particularly in the context of their long-term drinking history and exposure to alcohol-related cues in the drinking environment.
The observed decreases in seeking responses under extinction indicate that naltrexone facilitates extinction of persistent behaviors associated with alcohol-related cues. When alcohol was available, naltrexone produced modest decreases in alcohol drinking, but did not eliminate it. The results, then, support those studies showing that naltrexone may work, in part, by preventing drinking episodes from becoming a full-fledge relapse into heavy drinking (Anton et al. 1999, 2004; O’Malley and Froehlich 2003, Pettinati et al. 2006). The consistency of the present results with available clinical data and observations suggests that the CSR procedure, in which seeking responding and consumption can be evaluated in the same session, is a valid model for evaluation of novel treatment medications.
Footnotes
DISCLOSURE/CONFLICT OF INTEREST
This research was supported by NIH/NIAAA R01AA15971. Dr. Weerts was a co-investigator on a contract in human subjects (A Phase 2 Study of LY2196044 Compared with Naltrexone and Placebo in the Treatment of Alcohol Dependence) funded by Lilly Research Laboratories. Drs. Duke and Kaminski have no conflicts to disclose.
References
- Altshuler, Phillips PE, Feinhandler DA. Alterations of ethanol self-administration by naltrexone. Life Sci. 1980;26:679–84. doi: 10.1016/0024-3205(80)90257-x. [DOI] [PubMed] [Google Scholar]
- Anton RF, Moak DH, Waid LR, Latham PK, Malcolm RJ, Dias JK. Naltrexone and cognitive behavioral therapy for the treatment of outpatient alcoholics: results of a placebo-controlled trial. Am J Psychiatry. 1999;156:1758–1764. doi: 10.1176/ajp.156.11.1758. [DOI] [PubMed] [Google Scholar]
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–17. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Ator NA, Griffiths RR. Oral self-administration of triazolam, diazepam and ethanol in the baboon: drug reinforcement and benzodiazepine physical dependence. Psychopharmacology (Berl) 1992;108:301–12. doi: 10.1007/BF02245116. [DOI] [PubMed] [Google Scholar]
- Bienkowski P, Kostowski W, Koros E. Ethanol-reinforced behaviour in the rat: effects of naltrexone. Eur J Pharmacol. 1999;374:321–327. doi: 10.1016/s0014-2999(99)00245-9. [DOI] [PubMed] [Google Scholar]
- Bouza C, Angeles M, Muno A, Amate JM. Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: a systematic review. Addiction. 2004;99:811–828. doi: 10.1111/j.1360-0443.2004.00763.x. [DOI] [PubMed] [Google Scholar]
- Boyle AE, Stewart RB, Macenski MJ, Spiga R, Johnson BA, Meisch RA. Effects of acute and chronic doses of naltrexone on ethanol self-administration in rhesus monkeys. Alcohol Clin Exp Res. 1998;22:359–366. [PubMed] [Google Scholar]
- Bullock DH. Repeated conditioning-extinction sessions as a function of the reinforcement schedule. J Exp Analysis Beh. 1960;3:241–243. doi: 10.1901/jeab.1960.3-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock DH, Smith WC. An effect of repeated conditioning-extinction upon operant strength. J Exp Psych. 1953;46:349–352. doi: 10.1037/h0054544. [DOI] [PubMed] [Google Scholar]
- Childress A, Ehrman R, Rohsenow D, Robbins S, O’Brien C. Classically conditioned factors in drug dependence. In: Lowinson P, Luiz P, Millman RB, Langard G, editors. Substance Abuse: A Comprehensive Textbook. Williams and Wilkins; Baltimore: 1992. pp. 56–69. [Google Scholar]
- Ciccocioppo R, Martin-Fardon R, Weiss F. Effect of selective blockade of μ1 δ opioid receptors on reinstatement of alcohol-seeking behavior by drug-associated stimuli in rats. Neuropsychopharmacology. 2002;27:391–399. doi: 10.1016/S0893-133X(02)00302-0. [DOI] [PubMed] [Google Scholar]
- Clark GC, Taylor BW. Effects of repeated extinction of an operant on characteristics of extinction curves. Psych Rep. 1960;6:226. [Google Scholar]
- Cohen C, Perrault G, Griebel G, Soubrie P. Nicotine-associated cues maintain nicotine-seeking behavior in rats several weeks after nicotine withdrawal: reversal by the cannabinoid (CB1) receptor antagonist, rimonabant (SR141716) Neuropsychopharmology. 2005;30:145–55. doi: 10.1038/sj.npp.1300541. [DOI] [PubMed] [Google Scholar]
- Collins B, Brandon T. Effects of extinction context and retrieval cues on alcohol cue reactivity among nonalcoholic drinkers. J Consult Clin Psychol. 2002;70:390–397. [PubMed] [Google Scholar]
- Crombag H, Shaham Y. Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behav Neurosci. 2002;116:169–173. doi: 10.1037//0735-7044.116.1.169. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, DeLory MJ. Acamprosate and naltrexone treatment effects of ethanol and sucrose seeking and intake in ethanol-dependent and nondependent rats. Psychopharmacology. 2010;204:335–348. doi: 10.1007/s00213-009-1465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson D, Palfai T, Bird C, Swift R. Effects of naltrexone on alcohol self-administration in heavy drinkers. Alcohol Clin Exp Res. 1999;23:195–203. [PubMed] [Google Scholar]
- Dews PB. Interspecies differences in drug effects: behavioral. In: Usdin E, Forrest IS, editors. Psychotherapeutic Drugs, Part I. Marcel Dekker; New York: 1976. pp. 175–214. [Google Scholar]
- Egli M. Can experimental paradigms and animal models be used to discover clinically effective medications for alcoholism? Addict Biol. 2005;10:309–319. doi: 10.1080/13556210500314550. [DOI] [PubMed] [Google Scholar]
- Ferraro FM, Hill KG, Kaczmarek HJ, Coonfield DL, Kiefer SW. Naltrexone modifies the palatability of basic tastes and alcohol in outbred male rats. Alcohol. 2002;27:107–114. doi: 10.1016/s0741-8329(02)00220-3. [DOI] [PubMed] [Google Scholar]
- Gracy KN, Dankiewicz LA, Weiss F, Koob GF. Heroin-specific stimuli reinstate operant heroin-seeking behavior in rats after prolonged extinction. Pharmacol Biochem Behav. 2000;65:489–94. doi: 10.1016/s0091-3057(99)00234-8. [DOI] [PubMed] [Google Scholar]
- Grant KA, Bennett AJ. Advances in nonhuman primate alcohol abuse and alcoholism research. Pharmacol Ther. 2003;100:235–255. doi: 10.1016/j.pharmthera.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Ator NA, Griffiths RR. Establishment and maintenance of oral ethanol self-administration in the baboon. Drug Alcohol Depend. 1981;7:113–24. doi: 10.1016/0376-8716(81)90025-9. [DOI] [PubMed] [Google Scholar]
- Hill KG, Kiefer SW. Naltrexone treatment increases the aversiveness of alcohol for outbred rats. Alcohol Clin Exp Res. 1997;21:637–641. [PubMed] [Google Scholar]
- Jimenez-Gomez C, Shahan TA. Resistance to change of alcohol self-administration: effects of alcohol-delivery rate on disruption by extinction and naltrexone. Behav Pharmacol. 2007;18:161–169. doi: 10.1097/FBP.0b013e3280f2756f. [DOI] [PubMed] [Google Scholar]
- Johnson BA. Update on neuropharmacological treatment for alcoholism: scientific basis and clinical findings. Biochem Pharmacol. 2008;75:34–56. doi: 10.1016/j.bcp.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA. Medication treatment of different types of alcoholism. Am J Psychiatry. 2010;167:630–9. doi: 10.1176/appi.ajp.2010.08101500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Swift RM, Ait-Daoud N, DiClemente CC, Javors MA, Malcolm RJ., Jr Development of novel pharmacotherapies for the treatment of alcohol dependence: focus on antiepileptics. Alcohol Clin Exp Res. 2004;28:295–301. doi: 10.1097/01.alc.0000113409.47937.6c. [DOI] [PubMed] [Google Scholar]
- Kaminski BJ, Goodwin AK, Wand G, Weerts EM. Dissociation of alcohol-seeking and consumption under a chained schedule of oral alcohol reinforcement. Alcohol Clin Exp Res. 2008;32:1–9. doi: 10.1111/j.1530-0277.2008.00662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katner SN, Magalong JG, Weiss F. Reinstatement of alcohol-seeking behavior by drug-associated discriminative stimuli after prolonged extinction in the rat. Neuropsychopharmacology. 1999;20:471–479. doi: 10.1016/S0893-133X(98)00084-0. [DOI] [PubMed] [Google Scholar]
- Killeen TK, Brady KT, Gold PB, Simpson KN, Faldowski RA, Tyson C, Anton RF. Effectiveness of naltrexone in a community treatment program. Alcohol Clin Exp Res. 2004;28:1710–1717. doi: 10.1097/01.alc.0000145688.30448.2c. [DOI] [PubMed] [Google Scholar]
- King AC, Volpicelli JR, Frazer A, O’Brien CP. Effect of naltrexone on subjective alcohol response in subjects at high and low risk for future alcohol dependence. Psychopharmacology (Berl) 1997;129:15–22. doi: 10.1007/s002130050156. [DOI] [PubMed] [Google Scholar]
- Krank MD. Pavlovian conditioning with ethanol: sign-tracking (autoshaping), conditioned incentive, and ethanol self-administration. Alcohol Clin Exp Res. 2003;27:1592–1598. doi: 10.1097/01.ALC.0000092060.09228.DE. [DOI] [PubMed] [Google Scholar]
- Lê AD, Poulos CX, Quan B, Chow S. The effects of selective blockade of delta and mu opiate receptors on ethanol consumption by C57BL/6 mice in a restricted access paradigm. Brain Res. 1993;630:330–332. doi: 10.1016/0006-8993(93)90672-a. [DOI] [PubMed] [Google Scholar]
- Leeman RF, Heilig M, Cunningham CL, Stephens DN, Duka T, O’Malley SS. Ethanol consumption: how should we measure it? Achieving consilience between human and animal phenotypes. Addict Biol. 2010;15:109–24. doi: 10.1111/j.1369-1600.2009.00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macenski MJ, Meisch RA. Ethanol-reinforced responding of naïve rhesus monkeys: Acquisition without induction procedures. Alcohol. 1992;9:547–554. doi: 10.1016/0741-8329(92)90095-r. [DOI] [PubMed] [Google Scholar]
- Markou A, Weiss F, Gold LH, Caine SB, Schulteis G, Koob GF. Animal models of drug craving. Psychopharmacology (Berl) 1993;112:163–82. doi: 10.1007/BF02244907. [DOI] [PubMed] [Google Scholar]
- McCaul ME, Wand GS, Eissenberg T, Rohde CA, Cheskin LJ. Naltrexone alters subjective and psychomotor responses to alcohol in heavy drinking subjects. Neuropsychopharmacology. 2000;22:480–92. doi: 10.1016/S0893-133X(99)00147-5. [DOI] [PubMed] [Google Scholar]
- Monterosso JR, Flannery BA, Pettinati HM, Oslin DW, Rukstalis M, O’Brien CP, Volpicelli JR. Predicting treatment response to naltrexone: the influence of craving and family history. Am J Addict. 2001;10:258–68. doi: 10.1080/105504901750532148. [DOI] [PubMed] [Google Scholar]
- Mordenti J, Chappell W. The use of interspecies scaling in toxicokenetics. In: Yacobi A, Kelly J, Batra V, editors. Toxicokenetics and New Drug Development. Pergamon Press; New York: 1989. pp. 42–96. [Google Scholar]
- Olszewski PK, Alsio J, Schioth HB, Levine AS. Opioids as facilitators of feeding: can any food be rewarding? Physiol Behav. 2011;104:105–10. doi: 10.1016/j.physbeh.2011.04.033. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Jaffe AJ, Rode S, Rounsaville BJ. Experience of a “slip” among alcoholics treated with naltrexone or placebo. Am J Psychiatry. 1996;153:281–3. doi: 10.1176/ajp.153.2.281. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Froehlich JC. Advances in the use of naltrexone: an integration of preclinical and clinical findings. Recent Dev Alcohol. 2003;16:217–245. [PubMed] [Google Scholar]
- Pettinati HM, O’Brien CP, Rabinowitz AR, Wortman SP, Oslin DW, Kampman KM, Dackis CA. The status of naltrexone in the treatment of alcohol dependence: specific effects on heavy drinking. J Clin Psychopharmacol. 2006;26:610–625. doi: 10.1097/01.jcp.0000245566.52401.20. [DOI] [PubMed] [Google Scholar]
- Ray LA, Krull JL, Leggio L. The effects of naltrexone among alcohol non-abstainers: results from the COMBINE Study. Front Psychiatry. 2010;1:1–6. doi: 10.3389/fpsyt.2010.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson K, Baillie A, Reid S, Morley K, Teesson M, Sannibale C, Weltman M, Haber P. Do acamprosate or naltrexone have an effect on daily drinking by reducing craving for alcohol? Addiction. 2008;103:953–9. doi: 10.1111/j.1360-0443.2008.02215.x. [DOI] [PubMed] [Google Scholar]
- Rodefer JS, Campbell UC, Cosgrove KP, Carroll ME. Naltrexone pretreatment decreases the reinforcing effectiveness of ethanol and saccharin but not PCP or food under concurrent progressive-ratio schedules in rhesus monkeys. Psychopharmacology. 1999;141:436–446. doi: 10.1007/s002130050854. [DOI] [PubMed] [Google Scholar]
- Rosner S, Hackl-Herrwerth A, Leucht S, Vecchi S, Srisurapanont M, Soyka M. Opioid antagonists for alcohol dependence. Cochrane Database of Systematic Reviews. 2010:12. doi: 10.1002/14651858.CD001867.pub3. [DOI] [PubMed] [Google Scholar]
- Samson HH, Czachowski CL, Slawecki CJ. A new assessment of the ability of oral ethanol to function as a reinforcing stimulus. Alcohol Clin Exp Res. 2000;24:766–73. [PubMed] [Google Scholar]
- Setiawan E, Pihl RO, Cox SML, Gianoulakis C, Palmour RM, Benkelfat C, Leyton M. The effect of naltrexone on alcohol’s stimulant properties and self-administration behavior in social drinkers: Influence of gender and genotype. Alcohol Clin Exp Res. 2011;35:1134–1141. doi: 10.1111/j.1530-0277.2011.01446.x. [DOI] [PubMed] [Google Scholar]
- Shahan TA, Burke KA. Ethanol-maintained responding of rats is more resistant to change in a context with alternative non-drug reinforcement. Behav Pharmacol. 2004;15:279–285. doi: 10.1097/01.fbp.0000135706.93950.1a. [DOI] [PubMed] [Google Scholar]
- Shelton KL, Grant KA. Effects of naltrexone and Ro15–4513 on a multiple schedule of ethanol and Tang self-administration. Alcohol Clin Exp Res. 2001;25:1576–1585. [PubMed] [Google Scholar]
- Stafford D, LeSage MG, Glowa JR. Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: a review. Psychopharmacology(Berl) 1998;139:169–184. doi: 10.1007/s002130050702. [DOI] [PubMed] [Google Scholar]
- Ulm RR, Volpicelli JR, Volpicelli LA. Opiates and alcohol self-administration in animals. J Clin Psychiatry. 1995;56 (Supple7):5–14. [PubMed] [Google Scholar]
- Unterwald EM. Naltrexone in the treatment of alcohol dependence. J Addict Med. 2008;2:121–7. doi: 10.1097/ADM.0b013e318182b20f. [DOI] [PubMed] [Google Scholar]
- Vivian JA, Green HL, Young JE, et al. Induction and maintenance of ethanol self-administration in cynomolgus monkeys (Macaca fascicularis): Long-term characterization of sex and individual differences. Alcohol Clin Exp Res. 2001;25:1087–1097. [PubMed] [Google Scholar]
- Weerts EM, Goodwin AK, Kaminski BJ, Hienz RD. Environmental cues, alcohol seeking, and consumption in baboons: effects of response requirement and duration of alcohol abstinence. Alcohol Clin Exp Res. 2006;30:2026–2036. doi: 10.1111/j.1530-0277.2006.00249.x. [DOI] [PubMed] [Google Scholar]
- Weiss F. Advances in animal models of relapse for addiction research. In: Kuhn CM, Koob GF, editors. Advances in the Neuroscience of Addiction. 2. CRC Press; Boca Rotan (FL): 2010. [PubMed] [Google Scholar]
- Williams KL, Winger G, Pakarinen ED, Woods JH. Naltrexone reduces ethanol- and sucrose-reinforced responding in rhesus monkeys. Psychopharmacology. 1998;139:53–61. doi: 10.1007/s002130050689. [DOI] [PubMed] [Google Scholar]
- Williams KL, Woods JH. Naltrexone reduces ethanol- and/or water-reinforced responding in rhesus monkeys: effect depends upon ethanol concentration. Alcohol Clin Exp Res. 1999;23:1462–1467. [PubMed] [Google Scholar]
- Yoon G, Kim SW, Thuras P, Westermeyer J. Safety, tolerability, and feasibility of high-dose naltrexone in alcohol dependence: an open-label study. Hum Psychopharmacol. 1022;26:125–32. doi: 10.1002/hup.1183. [DOI] [PubMed] [Google Scholar]
- Zarcone TJ, Ator NA. Drug discrimination: Stimulus control during repeated testing in extinction. J Exp Analysis Beh. 2000;74:283–294. doi: 10.1901/jeab.2000.74-283. [DOI] [PMC free article] [PubMed] [Google Scholar]