Abstract
Background/Objectives
Decision aids designed for use with older patients need to address heterogeneity in likelihood of benefits and harms and facilitation of clinician-patient communication. We designed a tool for nonvalvular atrial fibrillation (NVAF)to inform patients of their individual stroke and bleed risks, assist in clarifying priorities, and promote communication.
Design
Clustered randomized controlled trial.
Setting
Primary care clinics.
Participants
Patients with NVAF.
Intervention
Completion of tool prior to regularly scheduled visit.
Measurements
Primary outcomes included the 100-point Informed and Values Clarity subscales of the Decisional Conflict Scale (lower scores indicate more informed and greater clarity.) Secondary outcomes included knowledge, patient-clinician communication, and change in treatment.
Results
69 patients were enrolled in the intervention group and 66 in the control group. Following their visit, intervention participants had lower scores on the Informed [mean difference (95% CI) = −11.9 (−21.1, −2.7)] and Values Clarity subscales [mean difference (95% CI) = −14.6 (−22.6, −6.6)]. Greater proportions of intervention compared to control participants knew medications for reducing stroke risk (61% vs 31%, p<0.001) and side effects (49% vs 37%, p=0.07). Stroke and bleeding risk was discussed more frequently in the intervention than control group (71% vs 12% and 69% vs 20%, p<0.0001). Five intervention participants expressed a preference for medication that was disconcordant with their current treatment plan. There was no change in treatment plan in either group.
Conclusion
The tool was effective in improving perceived and actual knowledge and value clarity and in increasing physician-patient communication but did not change treatment. Key words: atrial fibrillation, decision making, communication
INTRODUCTION
Decision aids have been shown to improve patient knowledge, reduce decisional conflict, and in some studies to increase patient participation in the process of decision making.1 All decision aids inform patients about available treatment options, some include value clarification exercises, and many ask individuals to make a treatment choice. There are many challenges to developing decision aids including, how to present probabilistic information and uncertainty, balance risk-benefit information, and address the variability patients’ health literacy and numeracy skills. There are also a number of specific challenges that are particularly important when developing decision aids for older adults. First, because of the increased prevalence of comorbid conditions with aging, the benefits and harms of each option may vary widely.2 Therefore, decision aids need to provide individualized information regarding the probable outcomes of different treatment options based on patients’ risk factor and comorbidity profiles. Second, because older persons’ preferences are shaped by the sequelae of disease-specific outcomes on broader domains of health, most notably physical and cognitive function,3 decision aids must include these outcomes in addition to traditional disease-specific outcomes. Finally, although older persons want their opinions to inform decision making, they are, on average, more likely to want their physician to make the treatment decision.4 This preferred approach to decision making may best accommodated by a decision aid that promotes clinician-patient communication about treatment options without asking the patient to make the decision. Enhancing communication is particularly important given the evidence that physicians are less likely to have a participatory decision making style with older persons.5
Treatment of non-valvular atrial fibrillation (NVAF) to reduce stroke risk is a complex, preference-sensitive decision that depends upon an understanding of the value patients place on the tradeoffs between the decreased risk of stroke versus increased risk of bleed associated with each of the medications and the inconveniences associated with taking warfarin. We sought to build upon several existing decision aids that have been shown to help patients with NVAF make an informed choice6–8 in order to address the issues of use of these tools with older persons. The tool was designed in order to 1) inform patients of their individual incremental risks of stroke and major bleed associated with both aspirin and warfarin, and the sequelae associated with each of these risks, 2) assist patients in clarifying their priorities and appreciate how their values contribute to decision making, and 3) encourage collaborative discussions by providing both patients and physicians with prompts to facilitate communication.
METHODS
Participants
Participants were persons with NVAF receiving primary care at VA Connecticut Healthcare System who had at least two primary care visits in the last 12 months. Potential participants with NVAF were identified by searching databases containing patient diagnoses and electrocardiograph results. Participants with paroxysmal atrial fibrillation were included if they had at least two episodes of atrial fibrillation, with the most recent episode documented in the last twelve months and/or were receiving therapy with aspirin and/or warfarin. Exclusion criteria identified patients who had: 1) aphasia, cognitive, visual or hearing impairment; 2) medications managed by a clinician outside of the VA; 3) comorbid illness requiring treatment with warfarin (mechanical valve or thrombo-embolic disease); 4) contraindication to ASA or warfarin therapy (allergy to either medication, intra-cerebral hemorrhage, gastrointestinal bleed treated within last year, gastrointestinal bleed without identified source, active substance abuse); 5) regular use of non-steroidal anti-inflammatory agents or two anti-platelet agents (for whom calculators for bleeding risks with anticoagulation are not available);6) life expectancy less than 12 months based on the opinion of the primary care clinician; 7) nursing home residence. Patients were asked to do a clock-drawing task, setting the hands to 1:45. They were excluded if the numbers 1,4, and 5 were circled, they did not include hands designating the time, numbers were missing, or all the numbers were placed on one side. Patients were also excluded if they could not identify the option with the best benefit/harm profile from a set of three hypothetical medications (see Appendix 1).
All participants completed written informed consent. The protocol was approved by the Human Subjects Subcommittee of VA Connecticut Healthcare System and the Yale School of Medicine. The trial was registered at Clinical Trials. gov: NCT00829478.
Randomization
Patients enrolled in the VA Connecticut Healthcare System receive primary care services through one of two groups of clinicians (firms). In order to avoid contamination, we randomized subjects at the level of the firm so that all subjects in one firm received the intervention and all subjects in the second firm were included in the control arm.
Decision Support Tool
The tool was designed to conform to the International Patient Decision Aids Standards.9 The details of the tool and its development are presented elsewhere.10 Briefly, the tool was developed as a program to run on a laptop computer, administered by a research nurse. The tool includes education about the connection between NVAF and stroke, about the different treatment options, and why treatment for NVAF involves a choice. Participants are provided with individualized information regarding their risk of stroke and bleed and the sequelae of these outcomes. An example of the presentation of these risks is provided in Appendix 2.
Risks were estimated based on comorbidities as determined by chart review conducted by the research nurse prior to meeting with the patient supplemented by self-report for history of falls. Baseline stroke risk was estimated using the CHADS2 algorithm.11 Stroke risks associated with aspirin and warfarin were estimated by multiplying baseline stroke risk by the relative risk reductions published in a meta-analysis.12 Baseline bleeding risk and bleeding risk with aspirin were taken from a systematic review,13 and bleeding risk with warfarin was estimated using the HEMORR2HAGES score.14 All person-year outcomes were converted to 5-year risks using the DEALE approach.15
Participants were asked to discuss which option they thought was best for them and why. These reasons were entered into the tool. They were provided with a print-out of their risk information and reasons for their preferred option, and were assisted in writing down questions or concerns they had for their clinician. Clinicians in the intervention arm were provided a copy of the participant’s print-out along with a card containing a prompt inviting the patient to talk about their treatment options: “I know you just learned about your treatment options for your atrial fibrillation. Can you tell me how you feel about them?” The research nurse reminded the clinician to use the prompt during the visit.
Procedures and Measures
Patients with an upcoming appointment (within 6 months) were enrolled in the trial. Enrollment occurred from October 2008 through December 2009. Baseline data were collected in a face-to-face interview prior to patients’ regularly scheduled visit with their primary care provider, and, for patients in the intervention group, this was followed by administration of the tool. Descriptive variables included sociodemographics: age, gender, ethnicity/race, marital status, education, and health literacy (measured using the REALM-SF)16; and health: comorbidities and global quality of life. Details regarding the outcome measures are provided in Table 1. The two primary outcome variables were the Informed and Values Clarity subscales of the low-literacy version of the Decisional Conflict Scale,17 obtained in an interview with a research assistant blinded to intervention assignment immediately following the visit with the primary care provider. Secondary outcomes of knowledge, anxiety, and worry were obtained during this interview, and both the primary and these secondary outcomes were re-assessed following the participant’s primary care visit by an interviewer blinded to the participant’s group assignment. The secondary outcome of rationale for preferred treatment was taken from the responses provided by participants in the intervention arm as they completed the decision tool.
Table 1.
Outcome Measures
| Primary outcome measure | Measurement approach |
|---|---|
| Feeling informed and having clear values | Informed and Values Clarity subscales of the low-literacy version of the Decisional Conflict Scale. 100-point scale with lower scores indicating feeling more informed and having greater clarification of values. |
| Secondary outcome measures | |
| Knowledge of treatment options and major adverse effects | In the absence of validated scales to measure knowledge regarding medications for NVAF, participants were asked open-ended questions about treatment options to reduce stroke risk in NVAF and about side effects of aspirin and warfarin. Responses for treatment options were considered correct if both aspirin and warfarin were named. Responses for adverse effects were considered correct if they included a mention of ulcers, bleeding, thinning the blood, or stroke. |
| Accuracy of stroke and bleeding risk | Participants were asked to estimate their own 5-year stroke and bleeding risk coded on a 0 to 100 numeric rating scale. Accuracy of estimates was calculated by comparing the participant’s estimate to the estimate provided by the algorithms used in the decision support tool. For each participant, the algorithms were selected to reflect the medication(s) being taken by the participant. For example, stroke risk for a participant taking warfarin was calculated by multiplying the CHADS2 score by the relative risk reduction provided by warfarin. We report the value obtained by taking the absolute value of the difference between the participant’s risk as estimated by the decision support tool and the estimate provided by the participant. |
| Anxiety | Spielberger State Anxiety Index.29 Ten-point scale with lower scores indicated less anxiety. |
| Worry | Two single-item measures: “How worried are you about having a stroke over the next 5 years?” and “How worried are you about having a bleed over the next 5 years?” coded on a 0 to 10 scale (lower scores = less worry). |
| Rationale for preferred treatment: | Participants in the intervention group were asked to comment aloud on how they felt about each option and to describe which option they preferred and why. These comments were transcribed verbatim by the research assistant and classified by the authors into nine groups. |
| Discussion of NVAF-related outcomes | Audiotapes of clinical encounters were reviewed by trained coders blinded to participants’ intervention status, who assessed whether the prompt and printout were used during the encounter and whether there was a discussion of bleeding and stroke risk. Two investigators independently reviewed the transcripts of participants choosing treatment that was discordant with their current medications for NVAF. |
| Change in treatment plan | Medical records were reviewed by an investigator blinded to participants’ intervention status for a change in the medication prescribed for treatment of NVAF. |
Visits were audiotaped. Trained coders indicated whether there was explicit mention of the handout and whether or not the physician used the prompt. Coders also indicated if stroke or bleed were mentioned during the visit. Of the 135 encounters, three were not audiotaped because of technical difficulties. Of the 132 audiotapes, 8 (4 from each group) could not be coded because the recordings were of poor quality, leaving a final sample of 124. In addition, two investigators independently reviewed the transcripts of participants choosing treatment that was discordant with their current medications for NVAF, and change in treatment.
Analysis
All analyses were conducted according to original intervention assignments and were performed at the level of the participant. The primary outcome measures were analyzed with multiple linear regression models, as were secondary outcomes measured using scales (knowledge of treatment stroke and bleeding risk, anxiety, worry). Baseline values of the outcomes were included as covariates. The binary outcomes for knowledge of medications and side effects were analyzed with multiple logistic regression models, again controlling for baseline values of the outcomes. Adding a random effect for physician clusters did not contribute to better fitting regression models, so this variable was not retained.
Statistical analyses were conducted with SAS software (version 9.22). P-values less than 0.05 for two-sided significance tests are reported as statistically significant. A sample size was calculated for a two-sided test with Type 1 error of 0.05 and power of 0.80 assuming amoderate effect size (Cohen’s d) of 0.5.18 Using these assumptions 64 study participants were required for each of the intervention and control groups; increasing these figures by 5% for possible missing values yielded an overall study sample of 135 study participants.
RESULTS
Baseline characteristics
135 participants were assigned to the intervention or the control group (see Figure 1 and Appendix 3). Nearly all participants were male and white; a majority was at least 75 years old and had greater than a high school education. Fewer than 10% of participants were receiving ASA alone. At baseline, knowledge of treatment options for stroke risk reduction in NVAF and their adverse effects was poor. Mean level of worry regarding the risk of future stroke and bleed was low (Table 2). The prevalence of comorbid conditions was high, with substantial variability in participants’ stroke and bleeding risk, as reflected in the CHADS2 and HEMORR2HAGES scores (Table 3).
Figure 1.
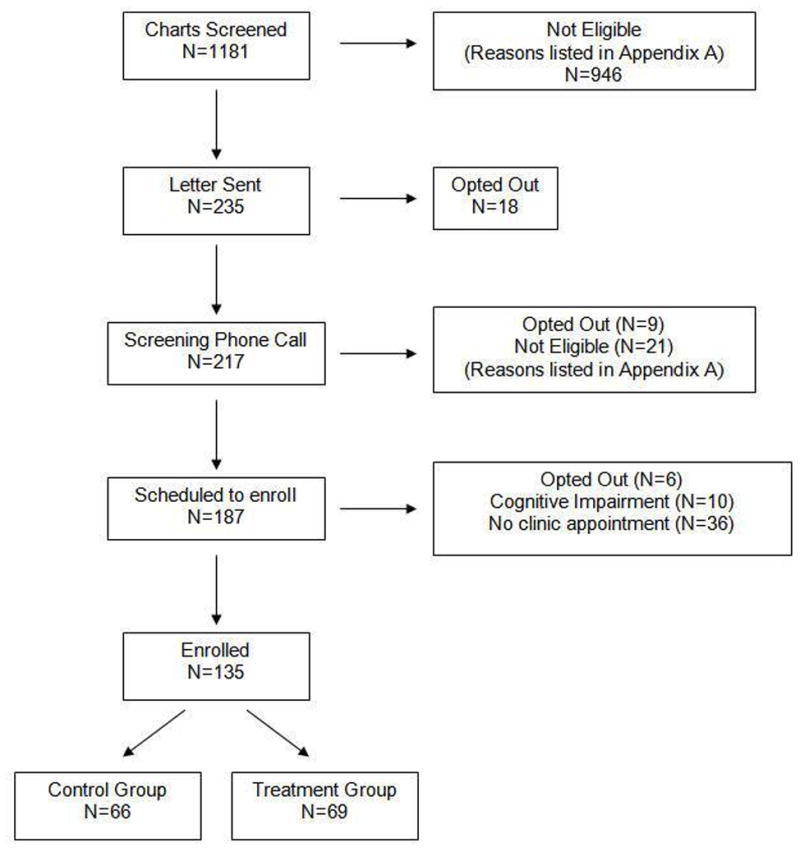
Flowchart of screening and enrollment.
Table 2.
Baseline Characteristics
| Intervention | Control | |
|---|---|---|
|
| ||
| N=69 | N=66 | |
| Male, n (%) | 68 (99) | 65 (98) |
| Age, n (%) | ||
| < 65 | 17 (25) | 13 (20) |
| 65–74 | 12 (17) | 17 (26) |
| 75–84 | 32 (46) | 24 (36) |
| ≥85 | 8 (12) | 12 (18) |
| Education, n (%) | ||
| < 9th grade | 4 (6) | 1 (2) |
| 9–12th grade | 28 (40) | 32 (48) |
| > High school | 37 (54) | 33 (50) |
| Hispanic, n (%) | 3 (4) | 2 (3) |
| Non-white, n (%) | 2 (3) | 6 (9) |
| Lives alone, n (%) | 20 (29) | 15 (23) |
| Married, n (%) | 40 (58) | 41 (62) |
| Quality of life: fair/poor, n (%) | 16 (23) | 12 (18) |
| Health literacy< 9th grade, n (%) | 13 (19) | 15 (23) |
| Comorbidities, n (%) | ||
| Hypertension | 61 (88) | 56 (85) |
| Heart failure | 20 (29) | 15 (23) |
| Prior stroke/transient ischemic attack | 9 (13) | 2 (3) |
| Diabetes | 16 (23) | 22 (33) |
| Prior hospitalization for bleeding | 1 (1) | 0 |
| CHADS2 score, n (%) | ||
| 0 | 2 (3) | 3 (5) |
| 1 | 15 (22) | 18 (27) |
| 2 | 29 (42) | 27 (41) |
| 3 | 11 (16) | 14 (21) |
| 4 | 11 (16) | 3 (5) |
| 5 | 1 (1) | 1 (1) |
| HEMORR2HAGES score, n (%) | ||
| 0 | 14 (20) | 17 (26) |
| 1 | 26 (38) | 28 (42) |
| 2 | 19 (27) | 15 (23) |
| 3 | 8 (12) | 5 (8) |
| 4 | 2 (3) | 1 (1) |
| Current treatment, n (%) | ||
| Warfarin | 51 (74) | 47 (71) |
| Aspirin | 6 (9) | 5 (8) |
| Both | 12 (17) | 14 (21) |
| Decisional conflict, mean (SD)† | ||
| Informed subscale | 37.6 (31.2) | 34.1 (30.9) |
| Values Clarity subscale | 34.4 (34.9) | 25.8 (31.3) |
| Knows treatment options, n (%) | 3 (4) | 4 (6) |
| Knows major adverse effects, n (%) | 16 (23) | 20 (30) |
| Accuracy of stroke risk, mean (SD)* | 17.6 (14.3) | 15.7 (14.5) |
| Accuracy of bleeding risk, mean (SD)* | 12.8 (11.6) | 12.9 (12.6) |
| Worried about stroke, mean (SD)†† | 1.4 (2.0) | 1.7 (2.1) |
Absolute value of the difference between the participant’s stroke/bleed risk as calculated by the decision support tool based on the participant’s current medications minus the participant’s own estimate of his/her stroke/bleeding risk.
100-point scale; lower scores = feeling more informed/having greater clarity
10-point scale; lower scores = less worry/anxiety
Table 3.
Differences in Decisional Conflict Subscales, Knowledge, Worry, and Anxiety
| Intervention Group Mean * | Control Group Mean * | Difference (95% CI) * | Standard Error | R2 | P-Value | |
|---|---|---|---|---|---|---|
| Primary Outcomes: DCS† | ||||||
| Informed subscale | 13.0 | 24.8 | −11.9 (−21.1, −2.7) | 4.7 | 0.24 | .011 |
| Values clarity subscale | 6.4 | 21.0 | −14.6 (−22.6, −6.6) | 4.1 | 0.14 | <.001 |
| Secondary Outcomes | ||||||
| Accuracy of stroke risk‡ | 9.1 | 14.2 | −5.2 (−8.4, −1.9) | 1.6 | 0.37 | .002 |
| Accuracy of bleeding risk‡ | 8.7 | 13.1 | −4.4 (−7.5, −1.4) | 1.5 | 0.34 | .004 |
| Anxiety§ | 13.0 | 13.4 | −0.38 (−1.4, .67) | 0.5 | 0.43 | .477 |
| Worry about stroke§ | 1.8 | 1.6 | 0.18 (−0.31, .66) | 0.2 | 0.57 | .471 |
| Worry about bleed§ | 1.5 | 1.9 | −0.43 (−1.1, .29) | 0.4 | 0.25 | .243 |
| n/N (%)¶ | n/N (%)¶ | Odds Ratio (95% Confidence Interval) | c statistic | |||
| Knows medications | 40/66 (61) | 19/62 (31) | 3.5 (1.6, 7.7) | # | - | .001 |
| Knows side effects | 26/53 (49) | 17/46 (37) | 1.9 (0.9, 4.0) | 0.66 | - | .075 |
DCS: Decisional conflict scale
Least squares means adjusted for baseline values
100-point scale; lower scores = feeling more informed/having greater clarity
Absolute value of the difference between the participant’s stroke/bleed risk as calculated by the decision support tool based on the participant’s current medications minus the participant’s own estimate of his/her stroke/bleeding risk.
10-point scale; lower scores = less anxiety/worry
Percentages of outcomes among those without knowledge at baseline.
A logistic regression model using maximum likelihood estimation did not fit properly because of quasi-complete separation between two model variables; so an exact logistic regression model was fit instead. A c statistic is not obtainable from results generated by exact logistic regression models
Changes in decisional conflict, knowledge, anxiety and worry
Following their primary care visit, participants in the intervention group had significantly lower scores on the Informed and Values Clarity subscales of the Decisional Conflict Scale (effect sizes = 0.3 and 0.5, respectively), indicating that these participants were more likely to report having adequate information about their treatment choices and knowing what was most important to them in making a treatment decision (Table 3). Participants in the intervention group were significantly more likely to be able to name the medications for reducing stroke risk in NVAF and were more likely to know their adverse effects, although the latter did not reach statistical significance. Participants in the intervention group were also significantly more likely to provide accurate estimates for their risk of stroke and bleed (effect size = 0.4 for both). The intervention did not affect participants’ anxiety or worry about stroke and bleed (Table 3).
Rationale for preferred treatment
All but six participants were able to give one or more reasons for their choice that could be categorized (Table 4). The remaining six provided ambiguous statements that did not clearly relate to their stated preference. A priority to prevent stroke, regardless of the risk of bleed, was by far the most commonly cited explanation. Many noted that the recovery from bleed was usually better than from stroke. Familiarity with stroke or bleed also influenced participants’ choice. While some subjects were bothered by frequent blood tests with warfarin, others believed it provided control over their risk of bleeding.
Table 4.
Description of 65 Intervention Participants’ Rationale for Preferred Choice
| Rationale | Examples | Frequency |
|---|---|---|
| Priority to prevent stroke | I am more concerned about having a stroke then a major bleed. I choose coumadin, because the risk of stroke is more debilitating then a major bleed. I would stick with the coumadin. The last thing I want is a stroke. |
47 |
| Worried about bleed | The risk of stroke is less on the coumadin, but I am a carpenter and fall a lot so I am at a higher risk of having a major bleed. I see that I am at a higher risk of having a stroke on ASA. I would go with the ASA because I spend a lot of time in the woods and am at risk of bleeding. |
5 |
| Recovery from bleed better than from stroke | Most people will recover better from the bleed then a stroke. The side effect of bleeding can be corrected. There is a better recovery from bleed than stroke. |
15 |
| Familiarity with sequelae | My mother died of a stroke at the age of 38. I know about strokes because my mother had one and never regained the use of her right leg. I have a brother who had a stroke and he still has serious problems from it. I knew a lot of people who took aspirin and they are now gone because they had a lot of stomach problems with aspirin. |
8 |
| Familiarity with frequency | I know more people that have had strokes then major bleeds. You don’t hear about major bleeding being a problem as much as you hear about strokes. |
3 |
| Control over risk | I am not as concerned about a major bleed because I am very careful so I don’t bleed. I am not as concerned about the chance of a major bleed because I have more control over that, I can use an electric shaver. I choose the coumadin because I can control how much I take with the blood tests I choose coumadin, because my blood gets tested so I know how thin my blood is. If I took aspirin I wouldn’t know how thin my blood is. |
9 |
| Bothered by inconveniences | I don’t like having to stay away from the greens. Because I have been on coumadin so long and I have had to miss out on my green vegetables and you would like to eat them. I don’t want the blood work. |
7 |
| Perceived risk influenced by personal experience | I don’t have a problem with bleeding so I am not as worried about that. I feel that I have a less chance of bleeding then the average person because I have fewer problems in the past with bleeding. |
2 |
| Influence of physician’s opinion | My heart doctor strongly feels I should take coumadin. I talked to my doctor about going off coumadin so I could take medications for my arthritis but he feels that coumadin is my best option. The doctor thinks this is the better medication for me. |
5 |
Discussion of NVAF-related outcomes
The physician prompt was used in 83% and the participant print-out was discussed in 48% of the encounters in the intervention group. The risk of stroke was discussed more frequently in the intervention than control group (71% versus 12%, p<0.0001), as was the risk of a major bleed (69% versus 20%, p<0.0001).
Change in treatment
Among participants in the intervention group, five were taking warfarin but indicated that ASA might be a better choice. In four of the five cases, the physician had a strong preference for warfarin and communicated to the patient that aspirin would not be a suitable option. In two of these cases, the patient explicitly stated that he wanted to do whatever the doctor believed to be best. The fifth case represented a patient being seen by a resident who did not feel he/she had sufficient knowledge to support a change to ASA. During the 30 days following the primary visit, there were no changes in prescriptions for NVAF in either the intervention or control group.
DISCUSSION
In this study we examined the outcomes associated with the use of a decision aid for anticoagulation in NVAF that was designed to address several of the challenges to improving decision making for older persons. As is provided by many tools, the aid educates patients and emphasizes the availability of multiple treatment options and the role of patient preference in the decision-making process. In addition, to address the heterogeneity in outcomes among older persons, it uses calculators for both stroke and bleeding risk based on a wide range of comorbidities and risk factors to provide patients with highly individualized risk information. It also provides a description of the sequelae of these outcomes in terms of survival and function. In order to enhance clinician-patient communication, it prepares patients to discuss their values and opinions with their clinicians. The outcomes were selected to reflect the process of high-quality decision making, defined as a patient and physician participating together to arrive at an informed choice reflecting patients’ values; namely, improving patient knowledge, helping patients to clarify their values, and promoting clinician-patient communication.9,19
After viewing the tool, patients in the intervention group were able to express a preference and a reasonable rationale for their choice. Compared to patients receiving usual care, patients using the tool had greater improvements in both primary outcomes: perceived knowledge and values clarity. The effect size associated with improvement in perceived knowledge was small (0.3). Despite the small change in perceived knowledge, patients using the tool were also better able to estimate their risks of stroke and bleed compared to patients receiving usual care. It is possible that patients with prevalent NVAF may perceive that they are better informed than their objective knowledge scores indicated, as a result of having a condition that is currently being treated. The effect size associated with improved value clarity was 0.5 indicating a moderate improvement. The larger improvement noted in values clarity indicates that this may be a more responsive measure in patients with prevalent conditions, and that the tool had a meaningful benefit in helping patients think about what matters to them the most.
Our study adds to the literature demonstrating that decision aids for NVAF improve patient knowledge and decrease decisional conflict, outcomes that have been the traditional focus for evaluating the effectiveness of decision support tools. Man-Son-Hing et al6 created a decision aid consisting of a booklet, audiotape, and a worksheet to enable patients to clarify their values. This tool improved patient knowledge and their ability to make a choice.6 Thompson et al developed a decision aid using the standard gamble approach to elicit patient utilities, but found that the standard gamble task was too difficult for patients to use. However, a modified version of the tool presenting risk-benefit estimates derived from a previously developed decision analytic model,20 decreased decisional conflict to a greater extent than did provision of guideline recommendations.8
The decision tool was designed with the goal of not only achieving these outcomes but also of increasing patient participation in decision making and promoting patient-clinician communication. Although these outcomes have been much less well studied, they are increasingly recognized as key objectives for decision aids.1,21 They are particularly important among older persons, to whom clinicians may be less likely to provide information,22 despite the nearly universal preference of patients for knowledge about treatment options and discussion of their opinions.4 Nonetheless, the promotion of communication must also respect patients’ desires not to make the treatment decision, which is a commonly preferred decision-making style among older persons.4 Rather than focusing on the patient as a decision maker, the tool instead encouraged participants to articulate their opinions in preparation for engaging in further discussion with their clinician. Such an approach helps to ensure that patients’ values inform the process of decision making while accommodating different approaches to making the final treatment decision (e.g. the clinician alone, the patient alone, or the clinician and patient together). This approach is based on the finding that, when patients are more active communicators, their physicians provide more information.22 The tool used techniques with evidence for effectiveness in improving patients’ communication skills, including having the patients practice what they would say, by asking questions of the research assistant, and writing questions down.23 The results demonstrate that the tool was successful in promoting communication in the intervention group regarding stroke and bleeding risks. However, it did not affect treatment plans. Of note, however, only a small proportion of patients had a preference that was discordant with the treatment they were currently receiving.
The primary objective for the large majority of participants in the study was to decrease their risk of stroke, and most subjects had preferences that were concordant with their currently prescribed medications. However, five participants receiving warfarin stated that they preferred aspirin after completing the decision tool and all five offered rational explanations for their choice. Warfarin was not discontinued in any of these cases. The audiotapes revealed that in four of these cases, the treating clinicians had a strong preference for warfarin and convinced these patients to continue on warfarin. These cases highlight unresolved issues related to the use of decision support tools to inform clinical practice. The audiotapes demonstrated that the primary care doctors vocalized their discomfort in not doing everything possible to prevent a stroke. They anticipated greater regret associated with patients developing a stroke off of warfarin, than a bleed on warfarin. These findings suggest that some clinicians have difficulty accepting patient preferences in situations where they have a strong belief that there is a single right way to proceed. This belief that patients do not truly have a choice about treatment options represents a fundamental barrier to the implementation of decision aids into clinical care. Improving patient knowledge and preparing patients to discuss their opinions with their clinician may not be sufficient to ensure that patients’ values inform the decision-making process. This is particularly pertinent for older persons. Because of long-standing relationships with their physicians and/or lack of empowerment to question or challenge their physicians’ beliefs,24 they may, like several of the participants in the current study, agree to the physician’s recommendation even if it does not reflect their preferences. These considerations suggest that, in order for decision aids to have an effect on treatment decision making, clinicians, in addition to patients, need to be a target of intervention.
There are several limitations to this study. We designed the trial to address decision making in patients with prevalent disease only because recruitment of patients with incident NVAF into a research study is highly challenging. The tool was therefore not administered to patients at the time of an actual treatment decision. Studies have found that the risk of major bleed may decrease over time. While we provided patients with individualized risk information based on the best available evidence, there are no validated methods for adjusting this risk in patients with prevalent NVAF. In addition, the study was conducted at a single site, in a select population of primarily white and highly-educated patients, almost all of whom were already on anticoagulation, thus limiting the generalizability of our results. Moreover, a very small proportion of subjects screened were eligible to participate in the trial. The tool used risk calculators available at the time the study was designed, but could be easily updated to include newer calculators such as the recently validated CHA2DS2VASc.25 The advent of newer agents to treat NVAF may provide treatment alternatives with lower risk of harms and fewer burdens than warfarin.26,27 However, the wide range in benefit and harm associated with aspirin and warfarin according to patients’ comorbidities in this study suggests that the currently available randomized controlled trial data for the outcomes associated with these new agents may not be generalizable to older and sicker patients. In addition, the lack of ability to reverse the effects of these agents in case of emergency may introduce new potential harms for consideration in treatment decision making.28
In summary, a multicomponent decision support tool for NVAF addressed the challenges of improving decision making among older persons and persons with multiple medical conditions by presenting patients with widely varying individualized risk estimates and providing support to encourage patient-physician communication. The tool improved patient knowledge, perception of being informed and having clear values, and discussion with clinicians. It resulted in identification of a small proportion of patients who did not share the dominant goal of reducing stroke risk. The lack of change in treatment plan for these patients highlights the complexities of incorporating patient preferences into the decision-making process.
Supplementary Material
Appendix 1: Choice task
Appendix 2: Screen shot of stroke and bleeding risk as presented to participants
Appendix 3: Reasons for exclusion
Acknowledgments
We would like to acknowledge the efforts of Karen Wu, R.N. (research assistant).
Funding sources: Dr. Fried is supported by K24 AG28443. Dr. Street is supported in part by the Houston Health Services Research and Development Center of Excellence (HFP90-020) at the Michael E. DeBakey VA Medical Center. The project described was supported by the Donaghue Foundation Practical Benefit Initiative DF #06-205 and by the Claude D. Pepper Older Americans Independence Center at Yale University School of Medicine (#P30AG21342 NIH/NIA).
Sponsor’s Role: The sponsors had no role in the design, methods, subject recruitment, data collections, or analysis and preparation of paper.
Footnotes
Author Contributions
LF: Study concept and design, acquisition of data, analysis and interpretation of data, preparation of manuscript
RLS: Study concept and design, analysis and interpretation of data, preparation of manuscript
VT: Analysis and interpretation of data, preparation of manuscript
JRO: Acquisition of data, analysis and interpretation of data, preparation of manuscript
LI: Acquisition of data, preparation of manuscript
PHVN: Analysis and interpretation of data, preparation of manuscript
TRF: Study concept and design, acquisition of data, analysis and interpretation of data, preparation of manuscript
References
- 1.O’Connor AM, Bennett CL, Stacey D, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database System Rev. 2009:CD001431. doi: 10.1002/14651858.CD001431.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Fraenkel L, Fried TR. Individualized medical decision making: Necessary, achievable, but not yet attainable. Arch Intern Med. 2010;170:566–569. doi: 10.1001/archinternmed.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fried TR, McGraw S, Agostini JV, et al. Views of older persons with multiple morbidities on competing outcomes and clinical decision-making. J Am Geriatr Soc. 2008;56:1839–1844. doi: 10.1111/j.1532-5415.2008.01923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levinson W, Kao A, Kuby A, et al. Not all patients want to participate in decision making. A national study of public preferences. J Gen Intern Med. 2005;20:531–535. doi: 10.1111/j.1525-1497.2005.04101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaplan SH, Gandek B, Greenfield S, et al. Patient and visit characteristics related to physicians’ participatory decision-making style: Results from the Medical Outcomes Study. Med Care. 1995;33:1176–1187. doi: 10.1097/00005650-199512000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Man-Son-Hing M, Laupacis A, O’Connor AM, et al. A patient decision aid regarding antithrombotic therapy for stroke prevention in atrial fibrillation: A randomized controlled trial. JAMA. 1999;282:737–743. doi: 10.1001/jama.282.8.737. [DOI] [PubMed] [Google Scholar]
- 7.McAlister FA, Man-Son-Hing M, Straus SE, et al. Impact of a patient decision aid on care among patients with nonvalvular atrial fibrillation: A cluster randomized trial. CMAJ. 2005;173:496–501. doi: 10.1503/cmaj.050091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomson RG, Eccles MP, Steen IN, et al. A patient decision aid to support shared decision-making on anti-thrombotic treatment of patients with atrial fibrillation: Randomised controlled trial. Qual Saf Health Care. 2007;16:216–223. doi: 10.1136/qshc.2006.018481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elwyn G, O’Connor A, Stacey D, et al. Developing a quality criteria framework for patient decision aids: Online international Delphi consensus process. BMJ. 2006;333:417. doi: 10.1136/bmj.38926.629329.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fraenkel L, Street R, Fried T. Development of a tool to improve the quality of decision making in atrial fibrillation. BMC Med Inf Decis Mak. 2011;11:59. doi: 10.1186/1472-6947-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: Results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 12.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: Antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 13.Hernandez-Diaz S, Rodriguez LAG. Incidence of serious upper gastrointestinal bleeding/perforation in the general population: Review of epidemiologic studies. J Clin Epidemiol. 2002;55:157–163. doi: 10.1016/s0895-4356(01)00461-9. [DOI] [PubMed] [Google Scholar]
- 14.Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: Results from the National Registry of Atrial Fibrillation (NRAF) Am Heart J. 2006;151:713–719. doi: 10.1016/j.ahj.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 15.Beck JR, Pauker SG, Gottlieb JE, et al. A convenient approximation of life expectancy (the “DEALE”): II. Use in medical decision-making. Am J Med. 1982;73:889–697. doi: 10.1016/0002-9343(82)90787-2. [DOI] [PubMed] [Google Scholar]
- 16.Arozullah AM, Yarnold PR, Bennett CL, et al. Development and validation of a short-form, rapid estimate of adult literacy in medicine. Med Care. 2007;45:1026–1033. doi: 10.1097/MLR.0b013e3180616c1b. [DOI] [PubMed] [Google Scholar]
- 17.O’Connor AM. Validation of a decisional conflict scale. Med Decis Making. 1995;15:25–30. doi: 10.1177/0272989X9501500105. [DOI] [PubMed] [Google Scholar]
- 18.Rosenthal R. Effect size and tests of significance. In: Cooper HM, Hedges LV, editors. The handbook of research synthesis. New York: Russel Sage; 1994. pp. 232–236. [Google Scholar]
- 19.Sepucha KR, Fowler FJ, Jr, Mulley AG., Jr Policy support for patient-centered care: The need for measurable improvements in decision quality. Health Aff. 2004:VAR54–62. doi: 10.1377/hlthaff.var.54. Suppl Variation. [DOI] [PubMed] [Google Scholar]
- 20.Thomson R, Parkin D, Eccles M, et al. Decision analysis and guidelines for anticoagulant therapy to prevent stroke in patients with atrial fibrillation. Lancet. 2000;355:956–962. doi: 10.1016/S0140-6736(00)90012-6. [DOI] [PubMed] [Google Scholar]
- 21.Street RL., Jr Aiding medical decision making: A communication perspective. Med Decis Making. 2007;27:550–553. doi: 10.1177/0272989X07307581. [DOI] [PubMed] [Google Scholar]
- 22.Street RL. Information-giving in medical consultations: The influence of patients’ communicative styles and personal characteristics. Soc Sci Med. 1991;32:541. doi: 10.1016/0277-9536(91)90288-n. [DOI] [PubMed] [Google Scholar]
- 23.Cegala DJ. Patient communication skills training: A review with implications for cancer patients. Patient Educ Couns. 2003;50:91–94. doi: 10.1016/s0738-3991(03)00087-9. [DOI] [PubMed] [Google Scholar]
- 24.Beisecker AE. Aging and the desire for information and input in medical decisions: patient consumerism in medical encounters. Gerontol. 1988;28:330–335. doi: 10.1093/geront/28.3.330. [DOI] [PubMed] [Google Scholar]
- 25.Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The Euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 26.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 27.Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 28.del Zoppo GJ, Eliasziw M. New options in anticoagulation for atrial fibrillation. N Engl J Med. 2011;365:952–953. doi: 10.1056/NEJMe1107516. [DOI] [PubMed] [Google Scholar]
- 29.Kvaal K, Ulstein I, Nordhus IH, Engedal K. The Spielberger State-Trait Anxiety Inventory (STAI): The state scale in detecting mental disorders in geriatric patients. Int J Geriatr Psychiatry. 2005;20:629–634. doi: 10.1002/gps.1330. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1: Choice task
Appendix 2: Screen shot of stroke and bleeding risk as presented to participants
Appendix 3: Reasons for exclusion


