Abstract
BACKGROUND
Little is known about the effects of alcohol dependence on cortical concentrations of glutamate (Glu) or gamma aminobutyric acid (GABA). We used proton magnetic resonance spectroscopy (MRS) to study cross-sectionally and longitudinally the concentrations of these Glu and GABA in alcohol dependent individuals (ALC) during early abstinence from alcohol.
Methods
Twenty ALC were studied at about one week of abstinence from alcohol (baseline) and 36 ALC at five weeks of abstinence and compared to 16 light/non-drinking controls (LD). Eleven ALC were studied twice during abstinence. Participants underwent clinical interviewing, blood work, neuropsychological testing, structural imaging and single-volume proton MRS at 4 Tesla. Absolute concentrations of Glu, GABA and those of other 1H MRS-detectable metabolites were measured in the anterior cingulate (ACC), parieto-occipital cortex (POC) and dorso-lateral prefrontal cortex (DLPFC). Relationships of metabolite levels to drinking severity and neurocognition were also assessed.
Results
ALC at baseline had lower concentrations of Glu, N-acetylaspartate (NAA), and choline- (Cho) and creatine-containing metabolites than LD in the ACC, but normal GABA and myo-inositol (mI) levels. At five weeks of abstinence, metabolite concentrations were not significantly different between groups. Between one and five weeks of abstinence, Glu, NAA and Cho levels in the ACC increased significantly. Higher cortical mI concentrations in ALC related to worse neurocognitive outcome.
Conclusion
These MRS data suggest compromised and regionally specific bioenergetics/metabolism in one-week-abstinent ALC that largely normalizes over four weeks of sustained abstinence. The correlation between mI levels and neurocognition affirms the functional relevance of this putative astrocyte marker.
Keywords: anterior cingulate cortex, glutamate, GABA, abstinence from alcohol, MR spectroscopy
1. INTRODUCTION
In-vivo proton magnetic resonance spectroscopy (1H MRS) studies have reported lower concentrations of N-acetylaspartate (NAA: a marker of neuronal viability) and choline-containing compounds (Cho: a marker of glial or cell synthesis/turnover) as well as altered myo-inositol levels (mI: a putative astrocyte marker) in the brain of individuals with alcohol use disorders (AUD), primarily in the frontal lobes, medial temporal lobe, and cerebellum (Bendszus et al., 2001; Durazzo et al., 2004; Ende et al., 2005; Fein et al., 1994; Schweinsburg et al., 2000; Seitz et al., 1999). Lower concentrations of frontal white matter NAA, cerebellar NAA and Cho as well as higher cerebellar mI have been associated with poorer neurocognition and motor functions in cross-sectional studies of individuals with AUD (see (Bendszus et al., 2001; Durazzo et al., 2004; Parks et al., 2002)). With sustained abstinence from alcohol, regional metabolite concentrations show variable recovery (e.g., Bartsch et al., 2007; Bendszus et al., 2001; Durazzo et al., 2006; Ende et al., 2005; Gazdzinski et al., 2008; Martin et al., 1995; Parks et al., 2002; Schweinsburg et al., 2000; Schweinsburg et al., 2001), and increases in some metabolite levels (e.g., NAA and Cho) have been associated with improvement on measures of learning and memory, processing speed and working memory (Bartsch et al., 2007; Bendszus et al., 2001; Durazzo et al., 2006; Gazdzinski et al., 2008; Parks et al., 2002). These findings parallel regional morphological recovery observed during abstinence from alcohol (for review see Durazzo and Meyerhoff, 2007) and suggest such adaptive neuroplastic changes are associated with improved neurocognition during periods of sustained sobriety (reviewed in Meyerhoff et al., 2011).
High field 1H MRS has enabled the quantitation of glutamate (Glu) and gamma aminobutyric acid (GABA), which are the primary excitatory and inhibitory neurotransmitters, respectively, in the human brain. Glu and glutamine are involved in the maintenance and promotion of several cell functions (Newsholm et al., 2003), and imbalances in brain Glu metabolism have been implicated in neurodegeneration in AUD. Specifically during withdrawal from alcohol, cerebral Glu levels change rapidly. Altered Glu (and primarily Ca2+), coupled with a chronic alcohol-induced up-regulation of N-methyl-D-aspartate receptors in the cortex, may promote neuronal “excitotoxicity” through cation and water disequilibrium, leading to increased oxidative stress and apoptosis (Lovinger 1993, Hughes 2009, Freund and Anderson, 1996, Tsai and Coyle, 1998). GABAergic neurons are involved in the synchronization of neuronal activity across brain regions (Clancy et al., 2010), and abnormalities in synaptic inhibition mediated by GABAergic neurons are associated with AUD and other common neuropsychiatric conditions (Johnson, 2005; Sanacora and Saricicek, 2007). Collectively, research suggests that alterations of regional Glu and GABA concentrations and of glutamatergic and GABAergic receptors (e.g., Green and Grant, 1998; Grobin et al., 1998; Kalivas et al., 2009; Spanagel, 2009), as well as alterations in Glu transmission from the anterior cingulate cortex (ACC) to the ventral striatum contribute to the development and maintenance of AUD and other substance use disorders (Kalivas and O'Brien, 2008; Kalivas, 2009; Cramer, 2011; Kalivas, 2009; Kalivas, 2009). Yet, only a few MRS studies have reported on the effects of AUD on concentrations of Glu, Glu + glutamine (Glx) or GABA.
Glu concentrations in the ACC of non-medicated ALC on the first day of abstinence from alcohol (i.e., during withdrawal) were elevated, but then decreased to normal levels over two weeks of abstinence (Frischknecht et al., 2010). In a randomized placebo-controlled study of acamprosate treatment of detoxified ALC, Umhau et al. (2010) observed decreasing levels of Glu in the ACC between the fourth and 25th day of acamprosate administration and trends to increasing Glu levels with placebo. For GABA, a preliminary study (Behar et al., 1999) reported significantly lower levels in the occipital cortex (OCC) of 1-month-abstinent ALC compared to controls, but Mason et al. (2006), in a larger follow-up study, observed no abnormalities in OCC GABA levels of ALC at either one week or one month of abstinence relative to controls. For technical reasons (a surface coil was used at relatively low magnetic field strength), these previous studies assessed only the ACC or OCC. To better understand the functional relevance of Glu and GABA and the potential role their regional metabolism plays in the development and maintenance of AUD, it is imperative to: a) measure cerebral Glu (and/or Glx) and GABA levels in multiple brain regions of the same cohort, b) investigate their associations with other common brain metabolites, neurocognitive function and drinking severity, and c) explore their concentration changes during early abstinence.
This report presents fully quantitative cross-sectional and longitudinal findings of single voxel MR spectroscopy (SVS) at high magnetic field strength (4 Tesla), focusing on absolute quantitation of Glu, Glx and GABA concentrations in the ACC, the parieto-occipital cortex (POC) and the dorso-lateral prefrontal cortex (DLPFC) of treatment-seeking ALC in early recovery. Based on the literature, we hypothesized 1) ALC near the inception of abstinence from alcohol have significantly higher Glx, Glu, and mI and lower NAA, Cho and GABA concentrations in the ACC, POC and DLPFC than light-drinking controls (LD), 2) metabolite concentrations normalize in all regions of ALC after approximately one month of abstinence from alcohol, and 3) NAA, Cr and Cho concentrations correlate positively while Glu and mI levels correlate negatively with measures of learning and memory, visuomotor scanning speed and working memory at both TPs. In secondary analyses, we explored the data for evidence of the excitotoxicity theory of Glu-induced neuronal injury in AUD (Freund and Anderson, 1996; Hughes, 2009; Lovinger, 1993; Tsai and Coyle, 1998), which would suggest an inverse relationship between Glu and NAA concentrations in ALC.
2. MATERIALS AND METHODS
2.1. Participants
Forty-four (39 males, 5 females) ALC were recruited from the San Francisco VA Medical Center Substance Abuse Day Hospital and the San Francisco Kaiser Permanente Chemical Dependence Recovery Programs. All ALC participants met DSM-IV criteria for alcohol dependence at the time of study. Male ALC had consumed more than 150 alcoholic drinks (one drink contains 13.6 g of pure ethanol) per month for at least 8 years while female ALC had consumed more than 80 drinks per month for at least 6 years prior to enrollment. All ALC participants were tested daily for breath alcohol while in outpatient treatment to ensure sobriety. Inclusion and exclusion criteria are fully detailed elsewhere (Durazzo et al., 2004). In brief, participants were excluded for a history of abuse or dependence on other substances within the past five years (other than nicotine), and for neurological or psychiatric disorders that are known to affect brain neurobiology or neurocognition. Hepatitis C, type-2 diabetes, hypertension, unipolar mood disorder (major depression and/or substance-induced mood disorder) were permitted in the ALC cohort given their high prevalence in AUD (Hasin et al., 2007; Mertens et al., 2003; Mertens et al., 2005; Parekh and Klag, 2001; Stinson et al., 2005). Sixteen age-matched LD were recruited from the local community and had no history of medical or psychiatric conditions known to influence the outcome measures of this study.
2.2. Clinical Assessment
ALC participants completed the Structured Clinical Interview for DSM-IV Axis I Disorder Patient Edition, Version 2.0 (First et al., 1998) and standardized questionnaires for alcohol withdrawal (CIWA-Ar; Addiction Research Foundation Clinical Institute of Withdrawal Assessment for Alcohol; Sullivan et al., 1989), depression (Beck Depression Inventory (BDI); Beck, 1978) and anxiety symptomatology (State-Trait Anxiety Inventory, Y-2, STAI; Spielberger et al., 1977) within one day of MR study. We assessed alcohol consumption over lifetime with the lifetime drinking history (LDH; Skinner and Sheu, 1982; Sobell et al., 1988; Sobell and Sobell, 1990). From the LDH we derived the age at onset of heavy drinking (defined as >100 alcoholic drinks per month) and estimated the average number of alcoholic drinks consumed per month over 1 year, 3 years, 8 years before enrollment and over lifetime.
A brief neurocognitive battery assessed aural working memory (Wechsler Adult Intelligence Scale-III (WAIS-III) Digit Span), visuomotor scanning speed and incidental learning (WAIS-III Digit Symbol; Wechsler, 1997), auditory-verbal (Delis et al., 2000) and visuospatial learning and memory (Brief Visual memory Test-Revised; Benedict, 1997) in LD as well as in ALC at TP1 and TP2 follow-up (for details, see Durazzo et al., 2007).
To evaluate the nutritional status and alcohol-related or other hepatocellular injury in ALC, we obtained laboratory tests for serum albumin, pre-albumin, alanine aminotransferase (ALT), aspartate aminotransferase (AST) and gamma-glutamyltransferase (GGT) within 3 days of the MR scans at each time point.
Of the 44 ALC participants, 20 were scanned at time point 1 (TP1 at 9 ± 4 days of abstinence from alcohol) and 36 scanned at TP2 (34 ± 7 days of abstinence). Not all participants contributed spectra from every VOI. Eleven participants were scanned at both TPs (at approximately 9 and 34 days of abstinence) and used for longitudinal analyses. The sixteen LD were each scanned once.
Table 1 shows demographics, laboratory and alcohol consumption variables for LD and ALC studied at TP1. ALC showed significantly greater depressive and anxiety symptomatology. The demographics of ALC studied at TP2 were similar to those at TP1. Also, the demographics, laboratory and alcohol consumption variables of the smaller longitudinal sample were representative of the ALC group shown in Table 1.
Table 1.
Demographics, laboratory and alcohol consumption variables for LD and ALC at TP1
| Variable | LD | ALC | p-value |
|---|---|---|---|
| Number of participants (male, female) | 16 (14, 2) | 20 (17, 3) | - |
| Age (years) | 49.0 ± 10.1 | 53.9 ± 8.8 | NS |
| Education (years) | 15.4 ± 2.7 | 13.8 ± 2.3 | 0.048 |
| Monthly alcohol consumption: 1 year average | 19.8 ± 21.7 | 338.7 ± 168.9 | - |
| Monthly alcohol consumption: 3 years average | 19.8 ± 21.8 | 329.5 ± 164.6 | - |
| Monthly alcohol consumption: lifetime average | 18.2 ± 16.9 | 232.6 ± 130.7 | - |
| Age of onset of heavy drinking (years) | - | 22.8 ± 5.7 | - |
| Months of heavy drinking | - | 290 ± 125.6 | - |
| Smoker (%) | 35 | 65 | - |
| MCV | 93.2 ± 3.8 | 96.2± 5.0 | NS |
| Prealbumin | 28.2 ± 6.2 | 27.2 ± 9.6 | NS |
| AMNART | 115.3 ± 8.3 | 116.4 ± 6.0 | NS |
| CIWA- Ar | - | 0.63 ± 1.4 | - |
| GGT | 52.5 ± 91.6 | 55.6 ± 40.5 | NS |
| Albumin | 4.1 ± 0.3 | 4.2 ± 0.3 | NS |
| AST | 22.6 ± 7.1 | 32.6 ± 10.1 | 0.031 |
| ALT | 19.3 ± 8.1 | 30.9 ± 12.0 | 0.057 |
| WBC | 6.1 ± 1.6 | 6.1 ± 1.1 | NS |
| RBC | 4.6 ± 0.4 | 4.3 ± 0.4 | NS |
| Hemoglobin | 14.8 ± 1.1 | 14.1 ± 1.4 | NS |
| Hematocrit | 42.7 ± 3.2 | 41.0 ± 4.1 | NS |
| BDI | 5.4 ± 4.8 | 13.1 ± 7.9 | 0.004 |
| STAI-Y2 | 33.5 ± 7.4 | 45.6 ± 10.3 | 0.002 |
Note: mean ± standard deviation; AMNART, American National Adult Reading test; MCV, mean corpuscular volume; CIWA, Addiction Research Foundation Clinical Institute of Withdrawal Assessment for Alcohol; GGT, gamma-glutamyltransferase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; WBC, White Blood Cells; RBC, Red Blood Cells; BDI, Beck Depression Inventory; STAI-Y2, Trait-Anxiety-Index
2.3 Imaging and Processing Methods
MRI data were acquired on a 4 Tesla Bruker MedSpec system using an 8-channel transmit-receive head coil and a Siemens Trio console (Siemens, Erlangen, Germany). A Magnetization Prepared Rapid Gradient (TR/TE/TI = 2300/3/950 ms, 7° flip angle, 1.0 × 1.0 × 1.0 mm3 resolution) and a turbo spin-echo (TR/TE = 8400/70 ms, 150° flip angle, 0.9 × 0.9 × 3 mm3 resolution) sequences were used to acquire 3-D sagittal T1-weighted and 2D axial T2-weighted anatomical images, respectively. The T1 and T2 images were then displayed and volumes-of-interest (VOIs) for MRS placed over the ACC (35 × 25 × 20 mm3), in the POC (20 × 40 × 20 mm3) and right DLPFC (size: 40 × 20 × 20 mm3), maximizing inclusion of much gray matter (GM) as possible (Figure 1 shows the three VOIs on axial T2-weighted images). The T1 images were also used for segmentation into GM, WM and cerebrospinal fluid (CSF), using the expectation maximization segmentation method (Leemput et al., 1999), and the segmented data used to estimate tissue fraction (TF) and CSF contributions to each VOI. After 3-D shimming and water suppression of each VOI, NAA, Cr, Cho, mI and Glu signals were acquired with a Stimulated Echo Acquisition Mode (STEAM) sequence (TR/TE/TM = 2000/12/10 ms, 90° flip angle, 2000Hz spectral bandwidth, 2.5 minutes acquisition time). Immediately afterwards, a reference water signal was also collected from the same VOIs with the same STEAM sequence but without water suppression and used for scaling metabolite peak areas. Signals from GABA and Glx were acquired immediately after the water spectrum from the exact same VOIs with a modified J-editing sequence (MEGA PRESS: TR/TE = 2000/71, 90° flip angle, 2000 Hz spectral bandwidth, 12.5 minutes acquisition time; Kaiser et al., 2008). The MRS data were processed using a combination of an in-house written Matlab program and IDL (Research Systems, Inc., Boulder, CO) with SITOOLS (Soher et al., 1996). First, the matlab program was used to convert the time domain data (raw data) to frequency domain data (i.e., 1-D Fourier transformation) after DC correction and apodization with a 2 Hz Lorentz-Gaussian filter. Using a priori information of metabolite frequencies, phases and amplitudes, the metabolite peak areas (METarea) as well as the reference water peak area (H20area) were then fitted, together with baselines using SITOOLS. A correction factor (CF), which accounted for differences between the transmitter voltage amplitudes used to acquire the metabolite and unsuppressed water signal (for each participant) was calculated as , (where METtrans-amp is the transmitter voltage amplitude for the metabolite and H2Otrans-amp is that for the corresponding reference water signal). The water peak area was then adjusted with CF as H20area adjusted area = H20 *CF. The metabolite peak areas were then scaled according to the formula The METscaled values were then used to estimate metabolite concentrations ([MET]) (in mmol) while accounting for TF for each VOI according to the formula
Figure 1.
T2-weighted images showing ACC (left), right DLPFC (middle) and POC (right) volumes-of-interest
2.4 Data Analyses
We conducted three main statistical analyses using SPSS, v18. In the first analysis, multivariate analysis of covariance (MANCOVA) was used for cross-sectional group comparisons at TP1 and TP2 to test for differences in NAA, Cr, Cho, mI and Glu concentrations between ALC and LD in each VOI. GABA and Glx were also analyzed similarly, but separately from NAA, Cr, Cho, mI and Glu, because the number of spectra from the J-editing sequence (i.e., 15 for POC of ALC at TP1) with acceptable quality was less than those from the STEAM sequence (i.e., 20 POC of for ALC at TP1). Although age was not significantly different between the groups, the age range of the participants was large (28 – 68), and age is associated with metabolite concentrations (Schuff et al., 2001); therefore, we used it as a covariate in the analyses. Also, because cigarette smoking is associated with brain metabolite concentrations in individuals with AUD (Durazzo et al., 2004; Durazzo et al., 2006; Durazzo et al., 2011), we covaried for smoking status in all cross-sectional analyses. We evaluated for group differences in total brain tissue, GM, WM, CSF contributions to the VOIs, but none was significantly different between the groups. Table 2 shows the percentage of GM, WM and CSF contributions to each VOI for ALC at TP1 and LD, depicting similar contributions of each tissue type in both groups.
Table 2.
GM, WM and CSF contributions to ACC, POC and DLPFC in 16 LD and 20 ALC at TP1
| Tissue type (%) | ACC | POC | DLPFC |
|---|---|---|---|
|
GM in LD GM ALC |
46.8 ± 4.1 45.4 ± 2.9 |
60.4 ± 4.0 60.8 ± 4.5 |
40.3 ± 5.3 39.0 ± 4.8 |
|
WM in LD WM in ALC |
33.5 ± 4.4 34.3 ± 5.8 |
29.2 ± 4.8 29.8 ± 4.8 |
52.8 ± 6.8 53.7 ± 7.3 |
|
CSF in LD CSF in ALC |
18.8 ± 4.7 19.4 ± 4.4 |
9.5 ± 5.8 8.6 ± 3.0 |
6.1 ± 2.6 6.4 ± 3.4 |
Note: mean ± standard deviation
The second analysis assessed metabolite level changes between TP1 and TP2 using paired t-tests on metabolite-to-water ratios (i.e., ratio of metabolite peak areas to corresponding reference water peak areas) of the longitudinal sample (11 ALC). In the third analyses, relationships (Spearman’s Rho) between metabolite concentrations within each VOI, as well as correlations between metabolite concentrations and measures of auditory-verbal, visuomotor scanning speed, visuospatial learning and memory, delayed visual memory and working memory were assessed in ALC and LD separately. Correlations of metabolite concentrations with drinking severity, as well as associations between changes in metabolite levels and changes in the aforementioned neurocognitive measures were also assessed in ALC only. A p-value of ≤ 0.05 was considered statistically significant in all the analyses, except analyses that assessed inter-correlations between Glu (or Glx) and Cr, Cho or mI, which required correction for multiple comparisons. The conservative Bonferroni method was used to correct for the effect of multiple comparisons on these correlations.
3. RESULTS
3.1. Participant Characterization
ALC and LD participants were not significantly different on age, but LD had significantly more years of education than ALC (p = 0.048). ALC participants had significantly higher aspartate aminotransferase levels than LD participants at TP1 (p = 0.031). All other laboratory measures as well as total brain tissue, GM, WM and CSF contributions to the VOIs did not differ significantly between ALC and LD groups.
Two ALC participants (10%) received benzodiazepine treatment at TP1; all of their metabolite concentrations were within the range of ALC who did not receive benzodiazepine treatment, but these participants did not provide data at TP2. Also, three ALC participants were positive for hepatitis C at TP1 and four at TP2, but again these participants were not part of the longitudinal sample as they did not have data at both TPs. Nine ALC participants had medically controlled hypertension at either TP; two of these participants had data at both TP1 and TP2 and were part of the longitudinal sample. However, there were no significant differences between participants with and without hepatitis C or controlled hypertension on any of our outcome measures, suggesting these conditions did not likely influence group findings. Comparison of the participants of the longitudinal sample with the rest of the ALC group in terms of age, drinking severity, neurocognitive measures, laboratory variables and medical/ psychiatry conditions showed no significant group differences on any of these measures. Therefore our longitudinal sample can be considered representative of the entire sample in this report.
3.2. Cross-sectional Group Comparisons
TP1 (9 ± 4 days of abstinence)
In the ACC, MANOVA, with age and cigarette smoking as covariates, indicated significant differences between LD and ALC across metabolite levels (F = 3.11, p = 0.028) for the STEAM sequence data. Follow-up t-tests showed significantly lower concentrations of Glu (p = 0.012), NAA (p = 0.001), Cr (p = 0.014), and a trend for lower Cho (p = 0.085) compared to LD. For the J-editing sequence data (GABA and Glx), MANCOVA gave statistically similar metabolite levels in LD and ALC; however, the follow-up t-tests showed a trend for lower Glx (p = 0.066) in ALC compared to LD. GABA and mI concentrations did not differ significantly between groups. See Table 3 for group means, standard deviations and effect sizes.
Table 3.
ACC metabolite concentrations at 9±4 days (TP1) and 34±7 days of abstinence (TP2) (cross-sectional samples)
| Metabolite | LD (12) | ALC (18): TP1 |
ALC (31): TP2 |
ES, p1 | ES, p2 |
|---|---|---|---|---|---|
| Glu | 4.08 ± 0.54 | 3.46 ± 0.79 | 3.87 ± 0.98 | 0.92, 0.012 | 0.26, NS |
| Glx | 2.23 ± 0.71 | 1.79 ± 0.59 | 2.18 ± 1.12 | 0.67, 0.066 | 0.05, NS |
| GABA | 1.31 ± 0.25 | 1.26 ± 0.39 | 1.44 ± 0.63 | 0.15, NS | 0.27, NS |
| NAA | 5.52 ± 0.90 | 4.46 ± 0.76 | 5.15 ± 1.30 | 1.27, 0.001 | 0.33, NS |
| Cr | 4.63 ± 0.77 | 3.89 ± 0.90 | 4.48 ± 1.08 | 0.88, 0.014 | 0.15, NS |
| Cho | 1.37 ± 0.21 | 1.25 ± 0.25 | 1.30 ± 0.36 | 0.52, 0.085 | 0.24, NS |
| mI | 4.28 ± 1.19 | 4.19 ± 0.94 | 4.41 ± 1.08 | 0.08, NS | 0.011, NS |
Note: mean ± standard deviation; ES stands for effect size, p1 is the p-value for LD measures versus TP1-ALC measures; while p2 is the p-value for LD measures versus TP2-ALC measures.
In the POC and DLPFC, similar MANCOVA analysis showed no significant group differences of metabolite concentrations. Predicted follow-up comparisons showed no significant differences between the groups in any of the metabolite levels. See Tables 4 and 5.
Table 4.
POC metabolite concentrations at TP1 and TP2 (cross-sectional samples).
| Metabolite | LD (16) | ALC (20): TP1 |
ALC (31): TP2 |
ES, p1 | ES, p2 |
|---|---|---|---|---|---|
| Glu | 4.18 ± 0.62 | 4.03 ± 0.48 | 4.05 ± 0.81 | 0.27, NS | 0.18, NS |
| Glx | 2.06 ± 0.46 | 1.89 ± 0.50 | 1.93 ± 0.41 | 0.35, NS | 0.30, NS |
| GABA | 1.50 ± 0.36 | 1.50 ± 0.30 | 1.53 ± 0.46 | 0.00, NS | 0.07, NS |
| NAA | 5.56 ± 1.09 | 5.27 ± 0.74 | 5.49 ± 1.0 | 0.31, NS | 0.07, NS |
| Cr | 4.56 ± 0.95 | 4.48 ± 0.61 | 4.54 ± .85 | 0.10, NS | 0.02, NS |
| Cho | 0.89 ± 0.42 | 0.83 ± 0.31 | 0.87 ± 0.25 | 0.16, NS | 0.11, NS |
| mI | 3.49 ± 0.84 | 3.41 ± 0.70 | 3.28 ± 0.82 | 0.10, NS | 0.25, NS |
Abbreviations as in Table 3.
Table 5.
DLPFC metabolite concentrations at TP1 and TP2 (cross-sectional samples).
| Metabolite | LD (12) | ALC (12): TP1 |
ALC (25): TP2 |
ES, p1 | ES, p2 |
|---|---|---|---|---|---|
| Glu | 3.13 ± 0.67 | 3.21 ± 0.57 | 3.08 ± 0.87 | 0.13, NS | 0.06, NS |
| Glx | 2.21 ± 0.94 | 2.18 ± 0.76 | 2.15 ± 0.86 | 0.06, NS | 0.07, NS |
| GABA | 1.54 ± 0.49 | 1.56 ± 0.47 | 1.71 ± 0.78 | 0.04, NS | 0.26, NS |
| NAA | 5.35 ± 0.97 | 5.48 ± .96 | 5.35 ± 1.01 | 0.13, NS | 0.00, NS |
| Cr | 4.43 ± 0.74 | 4.72 ± 0.66 | 4.48 ± .81 | 0.35, NS | 0.06, NS |
| Cho | 1.08 ± 0.13 | 1.09 ± 0.19 | 1.08 ± 0.21 | 0.06, NS | 0.00, NS |
| mI | 3.60 ± 1.00 | 3.80 ± 0.79 | 3.76 ± 0.84 | 0.02, NS | 0.17, NS |
Abbreviations as in Table 3
TP2 (34 ± 7 days of abstinence)
The MANCOVA indicated no significant metabolite level differences between LD and ALC at TP2 in any VOI. See Tables 3 – 5.
3.3. Longitudinal Change of Metabolite Ratios in 11 ALC
In the ACC between TP1 and TP2, ALC demonstrated significant increases in ratios of Glu (p = 0.008), NAA (p = 0.034), and Cho (p = 0.037) to water. Figure 4 depicts the increase of ACC Glu-to-water ratios in most ALC participants over time. A plot of ACC Glu concentrations (combining cross-sectional TP1 and TP2 data) against days of abstinence showed a moderate positive correlation (r = 0.31, p = 0.034) between Glu and time (see supplementary Figure 11), further supporting Glu increases in early abstinence. There were no significant longitudinal changes in ratios of Cr, mI, and GABA to water in the ACC.
Figure 4.
Plots of ACC Glu/H20 in 11 ALC at TP1 and TP2. The gray region indicates mean ± standard deviation of Glu/H20 in LD. Glu increases in the 11 ALC were significant (p = 0.008), even when removing the individual with the greatest change.
In the POC, the Glu-to-water ratio increased significantly between TP1 and TP2 (p = 0.049). Ratios of NAA (p = 0.092), Cr (p = 0.053) and Cho (p = 0.065) to water tended to increase over the same interval.
In the DLPFC, there were no significant longitudinal changes in any of the metabolite ratios of this small sample.
3.4. Cross-sectional Correlations among Main Outcome Measures in ALC
TP1
Glu and NAA concentrations correlated positively in the ACC (r = 0.73, p = 0.001) (see Figure 5a), POC (r = 0. 58, p = 0.007; supplementary Figure 22), and DLPFC (r = 0.64, p = 0.018). mI concentrations of the ACC and POC related negatively to visuomotor scanning speed, delayed visual memory and verbal learning; POC mI also correlated negatively with visuospatial memory and auditory-verbal learning (all r < −0.48, p < 0.047, see Table 6). In the DLPFC, Glx correlated negatively with verbal learning and visuospatial learning (both r < −0.51, p < 0.014). Average number of drinks per month over one year prior to study was positively related to mI in the ACC (r – 0.64, p = 0.04); no other associations between alcohol consumption and metabolite concentrations were observed in the other VOIs. See Table 6 for statistically significant associations within the ALC group.
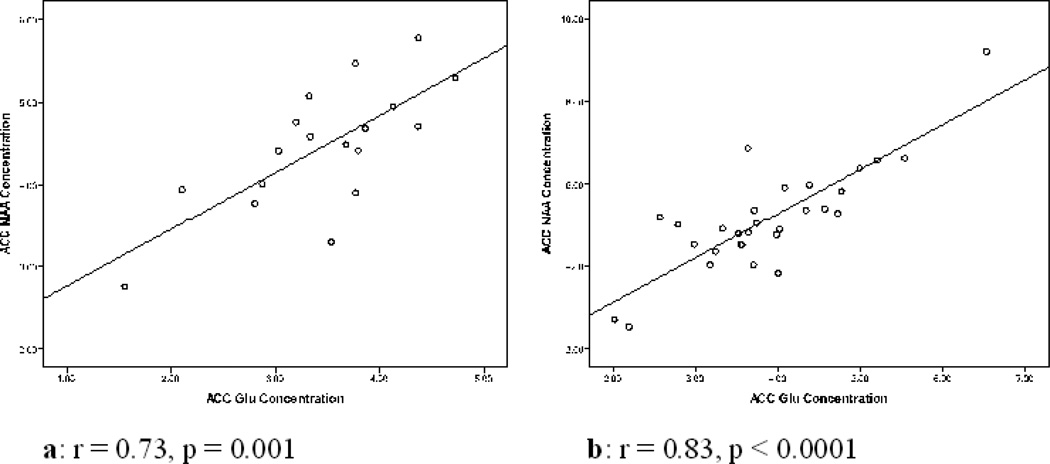
Figure 5.
Plots of ACC NAA concentration against ACC Glu concentration. a: plot for TP1, b: plot for TP2.
Table 6.
Spearman correlations among TP1 and TP2 main outcome measures for ALC.
| VOI | Correlations | TP1 correlation coefficient |
TP1 p value |
TP2 correlation coefficient |
TP2 p value |
|---|---|---|---|---|---|
| ACC | Glu vs. NAA | 0.73 | 0.001 | 0.83 | 0.0001 |
| Glu vs. Cr | 0.83 | 0.004 | 0.63 | 0.004 | |
| Glu vs. Cho | 0.74 | 0.004 | 0.78 | 0.002 | |
| Glx vs. NAA | - | NS | NS | ||
| mI vs. monthly alcohol consumption: 1 year average | 0.64 | 0.004 | - | NS | |
| mI vs. verbal learning | −0.52 | 0.040 | - | NS | |
| mI vs. delayed visual memory | −0.49 | 0.047 | - | NS | |
| mI vs. visuomotor scanning speed | −0.53 | 0.027 | - | NS | |
| POC | Glu vs. NAA | 0.58 | 0.007 | 0.92 | 0.0001 |
| Glu vs. Cr | 0.68 | 0.004 | 0.92 | 0.001* | |
| Glu vs. Cho | 0.78 | 0.001 | 0.78 | 0.001* | |
| mI vs. verbal learning | −0.51 | 0.039 | - | NS | |
| mI vs. visuomotor scanning speed | −0.48 | 0.040 | - | NS | |
| mI vs. visuospatial memory | −0.67 | 0.002 | 0.43 | 0.024 | |
| mI vs. auditory-verbal learning | −0.51 | 0.039 | −0.35 | 0.020 | |
| DLPFC | Glu vs. NAA | 0.64 | 0.018 | 0.62 | 0.001 |
| Glu vs. Cr | 0.57 | 0.040 | 0.52 | 0.008 | |
| Glu vs. mI | 0.69 | 0.009 | - | NS | |
| Glx vs verbal learning | - | NS | −0.58 | 0.011 | |
| Glx vs. visuospatial learning | - | NS | −0.51 | 0.014 | |
| Cho vs. working memory | - | NS | 0.41 | 0.0–43 | |
| mI vs. BDI | - | NS | −0.55 | 0.005 | |
| Glu vs. BDI | - | NS | −0.43 | 0.035 |
p value corrected for 3 comparisons
TP2
Similar to the observation at TP1, Glu and NAA concentrations at TP2 correlated positively in the ACC (r = 0.83, p < 0.0001) (see Figure 5b), POC (r = 0.92, p < 0.0001) (supplementary Figure 33), and DLPFC (r = 0.62, p = 0.001). However, in contrast to TP1, the ACC Glx concentration also related positively to the corresponding NAA concentration (r = 0.60, p = 0.004). Auditory-verbal learning related negatively to POC mI concentrations (r = −0.36, p = 0.020). Working memory related positively to DLPFC Cho concentration (r = 0.44, p = 0.008) and negatively to DLPFC mI concentration (r = −0.40, p = 0.025). BDI was negatively correlated to mI (r = −0.55, p = 0.005) and Glu (r = −0.43, p = 0.035) in the DLPFC.
3.5. Correlations among Longitudinal Changes of Main Outcome Measures in ALC
In the ACC between TP1 and TP2, the change of the Glu-to-water ratio related positively to changes in the corresponding NAA (r = 0.92, p < 0.001), Cr (r = 0.72, p = 0.013) and Cho (r = 0.92, p < 0.001) ratios.
Similarly, in the POC, the Glu-to-water ratio change correlated positively to the corresponding changes in ratios of NAA (r = 0.62, p = 0.044), Cr (r = 0.68, p = 0.020) and Cho (r = 0.76, p = 0.007) to water.
In the DLPFC, similar to a lack of cross-sectional associations, there were no significant correlations between changes in any of the metabolite-to-water ratios.
Between TP1 and TP2, measures of delayed visual memory, processing speed and visuomotor scanning speed (all p < 0.001) as well as working memory (p = 0.029) improved. However, these neurocognitive improvements did not correlate significantly with any of the metabolite-to-water ratio changes in any of the VOIs.
3.6. Cross-sectional Correlations among Main Outcome Measures in LD
Glu and NAA concentrations related positively in the POC (r = 0.91, p < 0.001) and DLPFC (r = 0.74, p = 0.006), but not in the ACC (r = 0.29, p = 0.34). Working memory was positively related to Cho concentrations in the ACC (r = 0.74, p = 0.015) and POC (r = 0.81, p = 0.029), but not in the DLPFC, where a corresponding relationship was observed in ALC at TP2. Delayed visual memory related positively to POC NAA concentration (r = 0.78, p = 0.039). Neurocognitive measures did not correlate with mI in any of the VOIs, as was frequently observed in the ALC group.
4. DISCUSSION
In this 4 Tesla MRS study, we observed lower concentrations of Glu and Glx in the ACC, but not in the POC or DLPFC, of treatment-seeking ALC at approximately nine days of abstinence (TP1), which normalized over four weeks of sustained abstinence from alcohol. We also observed lower concentrations of NAA and Cho in the ACC of ALC at TP1, which is consistent with previous findings in treatment seeking ALC (Bendszus et al., 2001; Durazzo et al., 2004; Ende et al., 2005; Fein et al., 1994; Schweinsburg et al., 2000; Seitz et al., 1999). In contrast to previous reports, ACC Cr levels were significantly lower in ALC at TP1 compared to LD. All effect sizes for the significant metabolite group differences in the ACC were at least 0.88, indicating large magnitude of differences and/or tight variance between ALC and LD on these measures. Importantly, these cohorts did not differ significantly on GABA concentrations in any VOI at TP1 or TP2. Longitudinal analyses indicated that significant metabolite changes over four weeks of abstinence were primarily apparent in the ACC for Glu, NAA, Cr, and Cho. Although none of the POC metabolite concentrations in ALC at TP1 (n=20) were significantly different from LD, POC Glu-to-water ratios increased significantly between TP1 and TP2 (n = 11), while the POC ratios of NAA, Cr and Cho to water tended to increase over the same time. Thus, at TP2, there were no significant metabolite concentration differences between ALC and LD in any VOI. The overall findings suggest that ALC, at approximately nine days of abstinence demonstrated abnormally low concentration of the general metabolic pool of Glu as well as compromised neuronal integrity (i.e., decreased NAA concentration) and cellular bioenergetics (i.e., decreased Cr concentration) in the ACC, which largely normalized within one month of sustained abstinence from alcohol. Glu, NAA and Cr levels are closely tied to cellular oxidative phosphorylation (Baslow and Guilfoyle, 2007; Newsholme et al., 2003; Pan and Takahashi, 2005; Ross and Bluml, 2001); therefore the significantly reduced concentrations of these metabolites in the ACC may indicate a general compromise of bioenergetics/metabolism of tissue in that region after acute detoxification, which recovers to normal over approximately one month of sustained abstinence from alcohol. We posit that the strong recovery of biomarkers of neuronal integrity and cellular bioenergetics in the ACC of ALC during short-term abstinence is functionally relevant, given the role of the ACC in self-monitoring as well as in regulation of emotional and affective tone and behavior (Bush et al., 2000; 2002).
Recently, Umhau et al. reported decreasing MRS-measured ACC Glu concentrations in acamprosate-treated abstinent ALC between the fourth and twenty-fifth day of medication and, similar to our findings, a trend to increasing Glu concentrations in placebo-treated abstinent ALC within the same time interval (Umhau et al., 2010). However, as they did not study a light-drinking control group, they were unable to make a statement about the relative Glu level at the beginning of treatment. Our cross-sectional data showed that ACC Glu concentration in ALC at TP1 (9 ± 4 days) were significantly lower than in LD; four weeks later at TP2, the Glu concentrations in ALC had increased to concentrations statistically similar to LD. Since acamprosate suppresses Glu concentrations, it suggests that levels of this metabolite in the acamprosate treated patients of Umhau et al. may have been further suppressed below normal concentrations during the three weeks of pharmacotherapy. In this context, it is worth noting that Glu imbalance has been implicated in addictive disorders by limiting a person’s ability to adapt to new information, such as changing substance use behavior despite adverse consequences (Kalivas, 2009). When this imbalance is ameliorated in preliminary clinical trials with a regimen of agents that indirectly increase Glu, craving for and desire to use cocaine and to smoke cigarettes both decrease (for reviews see Justin et al., 2008; Kalivas and Volkow, 2011; Olive et al., 2011). Thus, ACC Glu increases within the first few weeks of pharmacotherapy-free abstinence from alcohol may parallel these observations, underlying successful abstinence from alcohol.
Elevated levels of MRS-measured (i.e., mostly intracellular) Glx or Glu concentrations have been reported in humans and in animals during withdrawal from alcohol. Frischknecht and colleagues reported high Glx concentration in ALC at the first day of withdrawal from alcohol (Frischknecht et al., 2010). Similarly, high extracellular Glu concentrations in the striatum of alcohol-dependent rats between 12 and 24 hours of withdrawal from alcohol were also observed using microdialysis (Rossetti and Carboni, 1995). However, after 36 hours of withdrawal, the Glu concentrations returned to normal levels. Furthermore, after 24 weeks of vaporized alcohol exposure of rats, Zahr and colleagues found elevated MRS-measured concentrations of Glx and Glu (in addition to Cho) in the basal ganglia (Zahr et al., 2009). However, they did not measure metabolite concentrations during withdrawal. Since our TP1 data were acquired at about nine days from the initiation of abstinence, it is possible we missed the brief critical period occurring immediately after cessation of alcohol consumption where Glu may be elevated. Together, our new findings and those of the literature suggest dynamic Glu levels during early abstinence: elevated levels of Glu (or Glx) at withdrawal appear to drop rapidly to below normal levels (as we observed at approximately nine days of abstinence) and then increase again towards normal concentrations within three to four weeks of abstinence from alcohol.
As opposed to ACC Glu concentrations, all cross-sectional regional GABA concentrations in this ALC sample during early abstinence were not significantly different from those in controls in any of the VOIs. Furthermore, regional cortical GABA concentrations did not change appreciably over time. A previous small study found that GABA levels were significantly lower in the occipital cortex (OCC) of five ALC at one month of abstinence compared to nine controls (Behar et al., 1999). However, in a follow-up study with five non-smoking and seven smoking ALC, the same group observed elevated OCC GABA in the non-smoking ALC at one week of abstinence from alcohol (relative to controls), which normalized after four weeks of abstinence. However, neither the entire ALC nor the smoking ALC group demonstrated lower OCC GABA compared to controls. GABA levels that were lower in the smoking ALC than in the non-smoking ALC did not change during abstinence (Mason et al., 2006). Our analyses in a larger cohort showed no significant GABA concentration differences in any VOI (including the POC) between smoking and non-smoking ALC at either TP. We also observed no significant main effect of smoking when analyzing the ALC and LD groups combined; nevertheless, smoking was used as a covariate in our analyses since it has been shown to affect brain metabolite concentrations in individuals with AUD (Durazzo et al., 2004; 2006; 2011). However, it should be noted that the voxel size in the two previous studies was smaller and placed at a different region of the occipital cortex compared to the POC voxel size and placement in this study. Taken together, these studies suggest higher or unchanged GABA levels at one week of abstinence and likely normal GABA levels after four to five weeks of sustained abstinence.
The pattern of our findings across VOIs and TPs does not support the glutamate-related excitotoxicity theory of neuronal injury in ALC during early abstinence. Specifically, in ALC, Glu was not elevated relative to LD in any VOI at TP1 or TP2, and Glu concentrations correlated positively with NAA, Cr and Cho at both TP1 and TP2 in ALC in almost all the VOIs, similar to what we observed in our LD. Furthermore, increases in Glu-to-water ratios during abstinence were also positively related to increases in the ratios of NAA, Cr and Cho to water. Given that NAA and Cr levels, like Glu levels, are closely linked to the general viability of cell bioenergetics, their positive correlations with Glu in the ALC group suggest these metabolites reflect the metabolic integrity of glutamatergic neurons (predominately interneurons) in the VOIs investigated.
Though we did not observe significant mI differences between ALC (at either TP) and LD, mI consistently showed highly robust correlations with multiple neurocognitive measures at both TPs. At TP1, ACC and POC mI concentrations were negatively related to visuomotor scanning speed with POC mI also relating negatively to visuospatial learning and memory as well as auditory-verbal learning. At TP2, POC mI concentrations related negatively to auditory-verbal learning, while working memory related negatively to mI and positively to Cho in the DLPFC. The negative association of mI concentrations with the neurocognitive measures in the three VOIs of this study is consistent with our previous findings, where we observed strong negative correlations between regional mI and neurocognition in a largely independent sample (Durazzo et al., 2004; 2006). Higher regional mI levels are associated with poorer cognition in Alzheimer Disease and HIV-positive individuals (Chang et al., 2002; Chantal et al., 2002; Parnetti et al., 2007; Salvan et al., 1998) and increased mI was shown to be associated with inflammatory processes in the central nervous system, where it may reflect reactive astrogliosis (Bitsch et al, 1999; Ross et al., 1998). However, the extent to which mI is preferentially localized in glial versus neuronal tissue is unclear (Fisher et al., 2002). Although we did not detect elevated mI in samples of different abstinent ALC in our previous studies (Durazzo et al., 2004; 2006; 2010), one group reported higher than normal mI levels in the ACC of recently detoxified ALC (Schweinsberg et al. 2000).
This study has several limitations that may influence the generalizability of our findings. First, the TP1 and longitudinal samples were modest in size and comprised of predominantly male treatment-seeking alcohol dependent individuals; as such sex effects could not be evaluated. The large VOI sizes (necessitated by our goal to quantitate GABA concentrations) and the skull curvature led to inclusion of MR signal from non-targeted regions (i.e., inclusion of white matter, as can be seen in Table 2), thereby limiting tissue specificity. Also, our J-editing sequence was originally optimized for quantitation of GABA only; as a result, the signal of the Glx resonance was generally noisier than that of Glu in the STEAM spectra. This can best be appreciated from the larger standard deviations of the Glx than the Glu means shown in the tables. The poorer J-edited signal quality likely attributed to the lack of correlations of Glx and GABA levels with other metabolite levels as well as with the neurocognitive measures. Furthermore, we did not screen participants for DSM-IV Axis II disorders, such as antisocial personality disorders (Grant et al., 2004; Pridmore et al., 2005) or measure potential group differences in nutrition, exercise and genetic predispositions; all of these conditions may contribute to altered brain neurobiology.
In conclusion, we found lower Glu and Cr concentrations in addition to lower NAA and Cho in the ACC of ALC at about one week of abstinence from alcohol compared to LD. No significant group differences in any of the metabolite levels were apparent in the POC and the DLPFC, suggesting specific frontal effects in this abstinent alcohol dependent cohort. The lower ACC concentrations suggest a general compromised bioenergetics/metabolism in medial prefrontal brain, because the affected metabolites are all tied to cellular oxidative phosphorylation. The metabolite concentrations largely normalize over four weeks of sustained abstinence. Our observation of positive correlations between Glu and NAA concentrations in all three brain regions of ALC at TP1 and TP2, together with the positive correlations between longitudinal Glu and NAA changes between the time points do not seem to support the excitotoxicity theory of Glu-induced neuronal injury in ALC; rather, these correlations suggest some compromised integrity of glutamatergic neurons in the ACC of ALC in early abstinence. Finally, although we found no significant mI concentration differences in any of our group comparisons, the moderately strong correlations between mI levels and neurocognition affirm the functional relevance of this putative astrocyte marker in ALC.
Supplementary Material
Figure 2.
STEAM sequence spectrum (TR/TE/TM = 2000/12/20 ms) with main resonances for NAA, Glu, Cr, Cho and mI
Figure 3.
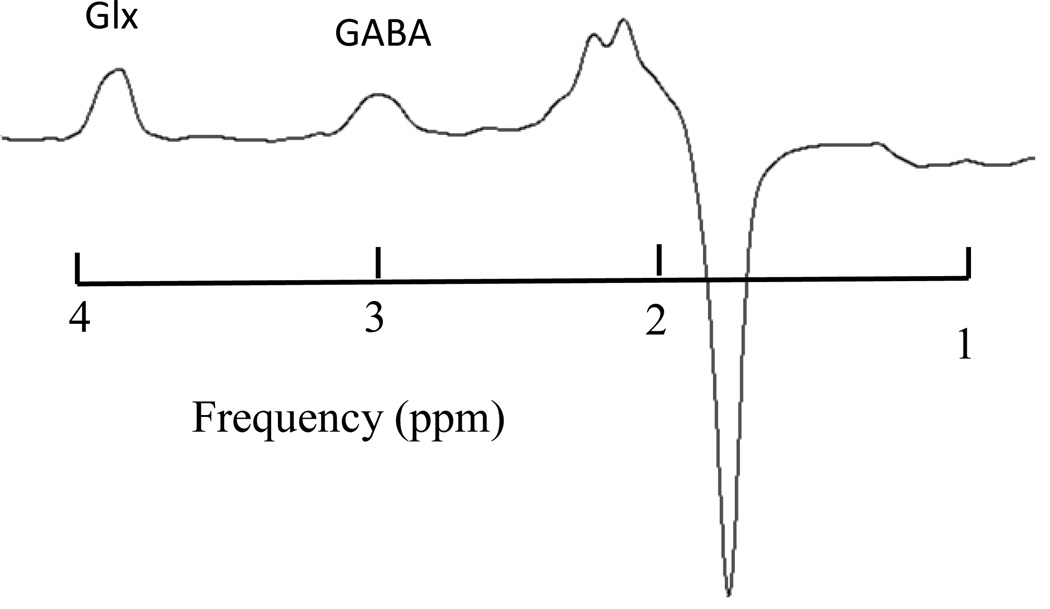
J-edited spectrum of GABA and Glx (TR/TE = 2000/71 ms)
Acknowledgement
This research was supported by the Radiology Research Service of the Veteran's Administration Medical Center. The authors wish to thank Drs. Susanna Fryer and David Pennington for their intellectual contributions to the manuscript
Role of Funding Sources
This research was funded by NIAAA and NIDA through grants AA10788 (DJM) and DA24136 (TCD), respectively. Apart from the funding, NIAAA and NIDA did not play any role in the study design, collection, analysis and interpretation of the data, or in the writing and submission of this report for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Contributors
Drs. Dieter Meyerhoff and Timothy Durazzo designed the research and wrote the study protocol. Dr. Anderson Mon was responsible for data acquisition, processing, literature review of related work and writing of the first draft of the manuscript. Drs. Timothy Durazzo and Dr. Anderson Mon undertook the data analysis. All authors have approved the submission of the manuscript.
Conflict of Interest
None of the authors has any conflict of interest in this study
REFERENCES
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, D.C: American Psychiatric Association; 1994. [Google Scholar]
- Bartsch AJ, Homola G, Biller A, Smith SM, Weijers HG, Wiesbeck GA, Jenkinson M, De Stefano N, Solymosi L, Bendszus M. Manifestations of early brain recovery associated with abstinence from alcoholism. Brain. 2007;130:36–47. doi: 10.1093/brain/awl303. [DOI] [PubMed] [Google Scholar]
- Baslow MH, Guilfoyle DN. Using proton magnetic resonance imaging and spectroscopy to understand brain "activation". Brain Lang. 2007;102:153–164. doi: 10.1016/j.bandl.2006.06.119. [DOI] [PubMed] [Google Scholar]
- Beck AT. Depression Inventory, Vol. Philadelphia: Center for Cognitive Therapy; 1978. [Google Scholar]
- Behar KL, Rothman DL, Petersen KF, Hooten M, Delaney R, Petroff OA, Shulman GI, Navarro V, Petrakis IL, Charney DS, Krystal JH. Preliminary evidence of low cortical GABA levels in localized 1H-MR spectra of alcohol-dependent and hepatic encephalopathy patients. Am. J. Psychiatry. 1999;156:952–954. doi: 10.1176/ajp.156.6.952. [DOI] [PubMed] [Google Scholar]
- Bendszus M, Weijers HG, Wiesbeck G, Warmuth-Metz M, Bartsch AJ, Engels S, Boning J, Solymosi L. Sequential MR imaging and proton MR spectroscopy in patients who underwent recent detoxification for chronic alcoholism: correlation with clinical and neuropsychological data. Am. J. Neuroradiol. 2001;22:1926–1932. [PMC free article] [PubMed] [Google Scholar]
- Benedict R. Brief Visuospatial Memory Test - Revised: Professional Manual, Vol. Odessa, FL: Psychological Assessment Resources, Inc.; 1997. [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc. Natl. Acad. Sci. U S A. 2002;99:523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B, Defelipe J, Espinosa A, Fairen A, Jinno S, Kanold P, Luhmann HJ, Rockland KS, Tamamaki N, Yan XX. Cortical GABAergic neurons: stretching it remarks, main conclusions and discussion. Front. Neuroanat. 2010;4:7. doi: 10.3389/neuro.05.007.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer SC, Sur M, Dobkin BH, O'Brien C, Sanger TD, Trojanowski JQ, Rumsey JM, Hicks R, Cameron J, Chen D, Chen WG, Cohen LG, deCharms C, Duffy CJ, Eden GF, Fetz EE, Filart R, Freund M, Grant SJ, Haber S, Kalivas PW, Kolb B, Kramer AF, Lynch M, Mayberg HS, McQuillen PS, Nitkin R, Pascual-Leone A, Reuter-Lorenz P, Schiff N, Sharma A, Shekim L, Stryker M, Sullivan EV, Vinogradov S. Harnessing neuroplasticity for clinical applications. Brain. 2011;134:1591–1609. doi: 10.1093/brain/awr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test, 2nd Edition, Vol. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- Durazzo TC, Gazdzinski S, Banys P, Meyerhoff DJ. Cigarette smoking exacerbates chronic alcohol-induced brain damage: a preliminary metabolite imaging study. Alcohol. Clin. Exp. Res. 2004;28:1849–1860. doi: 10.1097/01.alc.0000148112.92525.ac. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Banys P, Meyerhoff DJ. Brain metabolite concentrations and neurocognition during short-term recovery from alcohol dependence: preliminary evidence of the effects of concurrent chronic cigarette smoking. Alcohol. Clin. Exp. Research. 2006;30:539–551. doi: 10.1111/j.1530-0277.2006.00060.x. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ. Neurobiological and neurocognitive effects of chronic cigarette smoking and alcoholism. Front. Biosci. 2007;12:4079–4100. doi: 10.2741/2373. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Rothlind JC, Gazdzinski S, Banys P, Meyerhoff DJ. Chronic smoking is associated with differential neurocognitive recovery in abstinent alcoholic patients: a preliminary investigation. Alcohol. Clin. Exp. Res. 2007;31:1114–1127. doi: 10.1111/j.1530-0277.2007.00398.x. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Pathak V, Gazdzinski S, Mon A, Meyerhoff DJ. Metabolite levels in the brain reward pathway discriminate those who remain abstinent from those who resume hazardous alcohol consumption after treatment for alcohol dependence. J. Stud. Alcohol Drugs. 2010;71:278–289. doi: 10.15288/jsad.2010.71.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Mon A, Gasdzinski S, Meyerhoff DJ. Chronic cigarette smoking in alcohol dependence: associations with cortical thickness and N-acetylaspartate levels in the extended brain reward system. Addict. Biol. 2011 doi: 10.1111/j.1369-1600.2011.00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ende G, Welzel H, Walter S, Weber-Fahr W, Diehl A, Hermann D, Heinz A, Mann K. Monitoring the effects of chronic alcohol consumption and abstinence on brain metabolism: a longitudinal proton magnetic resonance spectroscopy study. Biol. Psychiatry. 2005;58:974–980. doi: 10.1016/j.biopsych.2005.05.038. [DOI] [PubMed] [Google Scholar]
- Fein G, Meyerhoff DJ, Di Sclafani V, Ezekiel F, Poole N, MacKay S, Dillon WP, Constans J-M, Weiner MW. 1H magnetic resonance spectroscopic imaging separates neuronal from Glial changes in alcohol-related brain atrophy. NIAAA Res. Mon. No. 27/Alcohol and Glial Cells. 1994:227–241. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition (SCID-I/P, Version 2.0, 8/98 revision), Vol. New York, NY: Biometrics Research Department; 1998. [Google Scholar]
- Freund G, Anderson KJ. Glutamate receptors in the frontal cortex of alcoholics. Alcohol. Clin. Exp. Res. 1996;20:1165–1172. doi: 10.1111/j.1530-0277.1996.tb01106.x. [DOI] [PubMed] [Google Scholar]
- Frischknecht U, Hermann D, Hoerst N, Tunc-Skarka N, Kiefer F, Ende G, Mann KF. Increased ACC glutamate levels in patients during alcohol withdrawal return to normal after two weeks of abstinence. Alcohol. Clin. Exp. Research. 2010;34:132A. [Google Scholar]
- Gazdzinski S, Durazzo TC, Yeh PH, Hardin D, Banys P, Meyerhoff DJ. Chronic cigarette smoking modulates injury and short-term recovery of the medial temporal lobe in alcoholics. Psychiatry Res. 2008;162:133–145. doi: 10.1016/j.pscychresns.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant B, Stinson F, Dawson D, P C, Dufour M, Compton W, Pickering RP, Kaplan K. Prevalence and co-occurance of substance use disorders and independent mood and anxiety disorders. Arch. Gen. Psychiatry. 2004;61:807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- Green KL, Grant KA. Evidence for overshadowing by components of the heterogeneous discriminative stimulus effects of ethanol. Drug Alcohol Depend. 1998;52:149–159. doi: 10.1016/s0376-8716(98)00086-6. [DOI] [PubMed] [Google Scholar]
- Grobin AC, Matthews DB, Devaud LL, Morrow AL. The role of GABA(A) receptors in the acute and chronic effects of ethanol. Psychopharmacology (Berl.) 1998;139:2–19. doi: 10.1007/s002130050685. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch. Gen. Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Alcohol withdrawal seizures. Epilepsy Behav. 2009;15:92–97. doi: 10.1016/j.yebeh.2009.02.037. [DOI] [PubMed] [Google Scholar]
- Johnson BA. Recent advances in the development of treatments for alcohol and cocaine dependence: focus on topiramate and other modulators of GABA or glutamate function. CNS Drugs. 2005;19:873–896. doi: 10.2165/00023210-200519100-00005. [DOI] [PubMed] [Google Scholar]
- Justin T, Gass M, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem. Pharmacol. 2008;75:218–265. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser LG, Young K, Meyerhoff DJ, Mueller SG, Matson GB. A detailed analysis of localized J-difference GABA editing: theoretical and experimental study at 4 T. NMR Biomed. 2008;21:22–32. doi: 10.1002/nbm.1150. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, O'Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat. Rev. Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Lalumiere RT, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology. 2009;56(Suppl. 1):169–173. doi: 10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. New medications for drug addiction hidding in glutamatergic neuroplasticity. Mol. Pshychiatry. 2011;16:974–986. doi: 10.1038/mp.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM. Excitotoxicity and alcohol-related brain damage. Alcohol. Clin. Exp. Res. 1993;17:19–27. doi: 10.1111/j.1530-0277.1993.tb00720.x. [DOI] [PubMed] [Google Scholar]
- Martin PR, Gibbs SJ, Nimmerrichter AA, Riddle WR, Welch LW, Willcott MR. Brain proton magnetic resonance spectroscopy studies in recently abstinent alcoholics. Alcohol. Clin. Exp. Res. 1995;19:1078–1082. doi: 10.1111/j.1530-0277.1995.tb00992.x. [DOI] [PubMed] [Google Scholar]
- Mason GF, Petrakis IL, de Graaf RA, Gueorguieva R, Guidone E, Coric V, Epperson CN, Rothman DL, Krystal JH. Cortical gamma-aminobutyric acid levels and the recovery from ethanol dependence: preliminary evidence of modification by cigarette smoking. Biol. Psychiatry. 2006;59:85–93. doi: 10.1016/j.biopsych.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Mertens JR, Lu YW, Parthasarathy S, Moore C, Weisner CM. Medical and psychiatric conditions of alcohol and drug treatment patients in an HMO: comparison with matched controls. Arch. Intern. Med. 2003;163:2511–2517. doi: 10.1001/archinte.163.20.2511. [DOI] [PubMed] [Google Scholar]
- Mertens JR, Weisner C, Ray GT, Fireman B, Walsh K. Hazardous drinkers and drug users in HMO primary care: prevalence, medical conditions, and costs. Alcohol. Clin. Exp. Res. 2005;29:989–998. doi: 10.1097/01.alc.0000167958.68586.3d. [DOI] [PubMed] [Google Scholar]
- Meyerhoff DJ, Durazzo TC, Ende G. Chronic alcohol consumption, abstinence and relapse: brain proton magnetic resonance spectroscopy studies in animals and humans. Curr. Top. Behav. Neurosci. 2011 doi: 10.1007/7854_2011_131. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Newsholme P, Procopio J, Lima MM, Pithon-Curi TC, Curi R. Glutamine and glutamate--their central role in cell metabolism and function. Cell Biochem. Funct. 2003;21:1–9. doi: 10.1002/cbf.1003. [DOI] [PubMed] [Google Scholar]
- Olive MF, Cleva RM, Kalivas PW, Malcoln RJ. Glutamatergic medications for the treatment of drug and behavioral addictions. Pharmacol. Biochem. Behav. 2012;100:801–810. doi: 10.1016/j.pbb.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan JW, Takahashi K. Interdependence of N-acetyl aspartate and high-energy phosphates in healthy human brain. Ann. Neurol. 2005;57:92–97. doi: 10.1002/ana.20317. [DOI] [PubMed] [Google Scholar]
- Parekh RS, Klag MJ. Alcohol: role in the development of hypertension and end-stage renal disease. Curr. Opin. Nephrol. Hypertens. 2001;10:385–390. doi: 10.1097/00041552-200105000-00014. [DOI] [PubMed] [Google Scholar]
- Parks MH, Dawant BM, Riddle WR, Hartmann SL, Dietrich MS, Nickel MK, Price RR, Martin PR. Longitudinal brain metabolic characterization of chronic alcoholics with proton magnetic resonance spectroscopy. Alcohol. Clin. Exp. Res. 2002;26:1368–1380. doi: 10.1097/01.ALC.0000029598.07833.2D. [DOI] [PubMed] [Google Scholar]
- Pridmore S, Chambers A, McArthur M. Neuroimaging in psychopathy. Aust. N. Z. J. Psychiatry. 2005;39:856–865. doi: 10.1080/j.1440-1614.2005.01679.x. [DOI] [PubMed] [Google Scholar]
- Ross B, Bluml S. Magnetic resonance spectroscopy of the human brain. Anat. Rec. 2001;265:54–84. doi: 10.1002/ar.1058. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Carboni S. Ethanol withdrawal is associated with increased extracellular glutamate in the rat striatum. Eur. J. Pharmacol. 1995;283:177–183. doi: 10.1016/0014-2999(95)00344-k. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Saricicek A. GABAergic contributions to the pathophysiology of depression and the mechanism of antidepressant action. CNS Neurol. Disord. Drug Targets. 2007;6:127–140. doi: 10.2174/187152707780363294. [DOI] [PubMed] [Google Scholar]
- Schuff N, Ezekiel F, Gamst AC, Amend DL, Capizzano AA, Maudsley AA, Weiner MW. Region and tissue differences of metabolites in normally aged brain using multislice 1H magnetic resonance spectroscopic imaging. Magn. Reson. Med. 2001;45:899–907. doi: 10.1002/mrm.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg BC, Taylor MJ, Videen JS, Alhassoon OM, Patterson TL, Grant I. Elevated myo-inositol in gray matter of recently detoxified but not long-term alcoholics: a preliminary MR spectroscopy study. Alcohol. Clin. Exp. Res. 2000;24:699–770. [PubMed] [Google Scholar]
- Schweinsburg BC, Taylor MJ, Alhassoon OM, Videen JS, Brown GG, Patterson TL, Berger F, Grant I. Chemical pathology in brain white matter of recently detoxified alcoholics: a 1H magnetic resonance spectroscopy investigation of alcohol-associated frontal lobe injury. Alcohol. Clin. Exp. Res. 2001;25:924–934. [PubMed] [Google Scholar]
- Seitz D, Widmann U, Seeger U, Nagele T, Klose U, Mann K, Grodd W. Localized proton magnetic resonance spectroscopy of the cerebellum in detoxifing alcoholics. Alcohol. Clin. Exp. Res. 1999;23:158–163. [PubMed] [Google Scholar]
- Skinner HA, Sheu WJ. Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. J. Stud. Alcohol. 1982;43:1157–1170. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Riley DM, Schuller R, Pavan DS, Cancilla A, Klajner F, Leo GI. The reliability of alcohol abusers' self-reports of drinking and life events that occurred in the distant past. J. Stud. Alcohol. 1988;49:225–232. doi: 10.15288/jsa.1988.49.225. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Self-reports issues in alcohol abuse: state of the art and future directions. Behav. Assess. 1990;12:77–90. [Google Scholar]
- Soher BJ, van Zijl PC, Duyn JH, Barker PB. Quantitative proton MR spectroscopic imaging of the human brain. Magn. Reson. Med. 1996;35:356–363. doi: 10.1002/mrm.1910350313. [DOI] [PubMed] [Google Scholar]
- Spanagel R. Alcoholism: a systems approach from molecular physiology to addictive behavior. Physiol. Rev. 2009;89:649–705. doi: 10.1152/physrev.00013.2008. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Self-Evaluation Questionaire. Palo Alto, CA: Consulting Psychologists Press; 1977. [Google Scholar]
- Stinson FS, Grant BF, Dawson DA, Ruan WJ, Huang B, Saha T. Comorbidity between DSM-IV alcohol and specific drug use disorders in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2005;80:105–116. doi: 10.1016/j.drugalcdep.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Sullivan J, Sykora K, Schneiderman J, Naranjo C, Sellers E. Assesment of alcohol withdrawal: the revised clinical institute withdrawl assesment for alcohol scale. Br. J. Addict. 1989;84:1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Tsai G, Coyle JT. The role of glutamatergic neurotransmission in the pathophysiology of alcoholism. Annu. Rev. Med. 1998;49:173–184. doi: 10.1146/annurev.med.49.1.173. [DOI] [PubMed] [Google Scholar]
- Umhau JC, Momenan R, Schwandt ML, Singley E, Lifshitz M, Doty L, Adams LJ, Vengeliene V, Spanagel R, Zhang Y, Shen J, George DT, Hommer D, Heilig M. Effect of acamprosate on magnetic resonance spectroscopy measures of central glutamate in detoxified alcohol-dependent individuals: a randomized controlled experimental medicine study. Arch. Gen. Psychiatry. 2010;67:1069–1077. doi: 10.1001/archgenpsychiatry.2010.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale - III (WMS - III), Vol. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Zahr NM, Mayer D, Vinco S, Orduna J, Luong R, Sullivan EV, Pfefferbaum A. In vivo evidence for alcohol-induced neurochemical changes in rat brain without protracted withdrawal, pronounced thiamine deficiency, or severe liver damage. Neuropsychopharmacology. 2009;34:1427–1442. doi: 10.1038/npp.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.