Abstract
A comprehensive understanding of the neurobiology of alcohol cue reactivity is critical to identifying the neuropathology of alcohol use disorders (AUD) and developing treatments that may attenuate alcohol craving and reduce relapse risk. Functional neuroimaging studies have identified many brain areas in which alcohol cues elicit activation. However, extant studies have included relatively small numbers of cases, with AUD of varying severity, and have employed many different cue paradigms. We used activation likelihood estimation, a quantitative, coordinate-based meta-analytic method, to analyze the brain areas activated by alcohol-related cues across studies, and to examine whether these areas were differentially activated between cases and controls. Secondarily, we reviewed correlations between behavioral measures and cue-elicited activation, as well as treatment effects on such activation. Data analyzed were from 28 studies of 679 cases and 174 controls. Among cases, alcohol cues elicited robust activation of limbic and prefrontal regions, including ventral striatum, anterior cingulate, and ventromedial prefrontal cortex. As compared to controls, cases demonstrated greater activation of parietal and temporal regions, including posterior cingulate, precuneus, and superior temporal gyrus. Cue-elicited activation of ventral striatum was most frequently correlated with behavioral measures and most frequently reduced by treatment, but these results often derived from region-of-interest analyses that interrogated only limbic regions. These findings support long-standing theories of mesolimbic involvement in alcohol cue processing, but suggest that cue-elicited activation of other brain areas may more clearly differentiate cases from controls. Prevention and treatment for AUD should consider interventions that may reduce cue-elicited activation of these areas.
Keywords: alcoholism, addiction, activation likelihood estimation, fMRI, PET
Introduction
The study of alcohol use disorders (AUD) has devoted significant attention to alcohol cue reactivity, or the psychological and physiological responses elicited by exposure to alcohol-related stimuli. Such stimuli induce alcohol craving (i.e., cue-elicited craving) (Monti et al., 1987), a phenomenon believed to result from conditioned associations between stimuli that precede alcohol ingestion (e.g., an image of one’s favored beer; the smell or taste of liquor), the positively reinforcing, pleasurable effects of alcohol (Drummond et al., 1990, Niaura et al., 1988) and the negatively reinforcing attenuation of withdrawal symptoms that occurs after its use (Wise, 1988). Drug-related cues robustly elicit reinstatement of drug self-administration after extinction in animal models of addiction (Bouton et al., 2006), and a litany of authors has argued that craving and cue reactivity represent the core behavioral pathology of end-stage addiction, as they indicate a fundamental shift in motivated behavior (Anton, 1999, Kalivas & Volkow, 2005, Koob & Volkow, 2010, Pickens & Johanson, 1992, Robinson & Berridge, 1993, Sinha & O’Malley, 1999, Skinner & Aubin, 2010). However, subjective craving is difficult to measure among human subjects (Sayette et al., 2000) and, in alcohol research, has been inconsistently associated with relapse after abstinence (Monti et al., 2000, Niaura et al., 1988, Roberts et al., 1999). Further, while animal models have yielded a host of neurobiological substrates for craving and cue reactivity, the manner in which these phenomena are represented in the human brain is less well understood. Thus, more objective and neurobiologically informative measurements of alcohol cue reactivity and craving have been sought.
Functional neuroimaging (e.g., functional magnetic resonance imaging (fMRI), positron emission tomography (PET), and single photon emission computed tomography (SPECT)) has emerged as a powerful, noninvasive method to study alcohol cue reactivity. In the last decade, nearly 30 studies have used functional imaging to probe how heavy drinkers and individuals with AUD respond to alcohol cues. This line of research has suggested that cue-elicited brain activation represents a core component of alcohol-induced neuropathology (Heinz et al., 2009). Accordingly, intervention research has begun to target cue-elicited activation as an index of treatment efficacy (Hermann et al., 2006, Mann et al., 2009, Myrick et al., 2008, 2010, Vollstadt-Klein et al., 2011). However, different studies have included relatively small numbers of subjects with AUD of varying severity, have employed a variety of different cue reactivity paradigms, and have reported many different areas of cue-elicited activation. Hence, no consensus exists on where in the brain treatments for alcoholism should reduce such activation. While several qualitative reviews of functional imaging studies of alcohol cue reactivity exist (Buhler & Mann, 2011, Heinz et al., 2009, Sinha & Li, 2007, Yalachkov et al., 2012), none has used quantitative meta-analysis to systematically characterize the brain areas activated by alcohol cues across subject populations, cue exposure paradigms, and imaging modalities.
Given the substantial number of studies and the lack of a clear consensus regarding the brain areas activated by alcohol cues, the primary aim of this study was to use a validated quantitative meta-analytic method for functional neuroimaging studies, the activation likelihood estimation (ALE) approach (Laird et al., 2005, Turkeltaub et al., 2002), to analyze the stereotactic coordinates of cue-elicited activation reported in extant studies. Secondarily, we sought to characterize the states and traits related to this activation by systematically reviewing correlations between activation and behavioral measures, and to describe nascent efforts to reduce cue-elicited activation by reviewing 6 treatment studies that have reported such effects.
Materials and Methods
Literature search and selection
Papers were identified on PubMed (http://www.ncbi.nlm.nih.gov/pubmed) using the keywords “alcohol,” “alcoholism,” “dependence,” “abuse,” “craving,” “cue,” “fMRI,” “PET,” and “SPECT.” The reference sections of identified papers were then consulted for additional relevant citations. Papers were included if they presented an analysis of brain response to alcohol-related cues, either within heavy drinkers or individuals with AUD or between such individuals and control subjects. Secondary analyses of previously published samples (e.g., reports of genetic moderators of cue reactivity) were not included. Treatment studies were included if they reported a pre-treatment scan or if some subjects received placebos. This search yielded 28 total studies (listed in Table 1 by year of publication).
Table 1.
Summary of methodological details of functional imaging studies of alcohol cue reactivity.
| First author | Year | Cases, N | Age ± SD | % Male | Status | Controls, N | Modality | Cue type | Contrasted cue(s) | Analysis | WB threshold |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Modell | 1995 | 9 AD | 44 | 78 | Outpt | None | SPECT | Tastea | Water | ROI | n/a |
| Braus | 2001 | 4 AD | 39 ± 6 | 50 | Detox | None | 1.5T fMRI | Visual | Resting baseline | WB | NR |
| George | 2001 | 10 AD | 30 ± 10 | 80 | NTS | None | 1.5T fMRI | Visual, taste | Neutral beverage | WB c | p < .01, cluster p < .05 |
| Schneider | 2001 | 10 AD | 41 ± 8 | 100 | Detox | 10 A, G | 1.5T fMRI | Olfactory | Resting baseline | WB | p ≤.05, k ≥ 64 |
| Wrase | 2002 | 6 AD | 44 ± 9 | NR | Detox | None | 1.5T fMRI | Visual | Abstract | WB, ROI | p < .05, k ≥ 10e |
| Tapert | 2003 | 15 AUD | 17 ± 1 | 60 | NTS | 15 A, G | 1.5T fMRI | Visuala | Neutral beverage | WB | p < .05, k ≥ 515 |
| Grüsser | 2004 | 10 AD | 41 ± 8 | 50 | Detox | 10 A, G | 1.5T fMRI | Visual | Neutral/abstract | ROI | n/a |
| Heinz | 2004 | 9 M | 45 ± 7 | 100 | Detox | 12 A | 1.5T fMRI | Visual | Neutral/abstract | ROI | n/a |
| Kareken | 2004 | 10 FHP HD | 23 ± 3 | NR | NTS | 5 FHN SD | 1.5T fMRI | Olfactory | Neutral | ROI | n/a |
| Myrick | 2004 | 10 AD | 34 ± 12 | 80 | NTS | 10 SD | 1.5T fMRI | Visual, taste | Neutral beverage | WB | p ≤ .05, cluster p < .05 |
| Tapert | 2004 | 8 AUD | 20 ± 1 | 0 | NTS | 9 A, G, SD | 1.5T fMRI | Visual (words) | Neutral | WB | p < .05, k ≥ 243 |
| Hermann | 2006 | 10 AD | 40 ± 7 | 100 | Detox | 10 A | 1.5T fMRI | Visual | Neutral/abstract | WB | p < .001, k ≥ 236 |
| Lingford-Hughes | 2006 | 6 AD | 41 ± 8 | 100 | Outpt | 6 G, SD | PET | Visual | Neutral beverage | WB, ROI | Cluster p < .05f |
| Olbrich | 2006 | 21AD | 41 ± 7 | 100 | Detox | None | PET | Visual, olfactory a, b | Water | WB, ROI | p < .001 |
| Heinz | 2007 | 12 AD | 39 ± 7 | 50 | Detox | 12 A, G | 1.5T fMRI | Visuala | Neutral | WB | p < .001, k ≥ 236 |
| Park | 2007 | 9 AUD | 23 ± 2 | 89 | NTS | 9 A | 3T fMRI | Visual, taste | Neutral beverage | WB | Cluster p < .05f |
| Wrase | 2007 | 13 AD | 42 ± 8 | 100 | Detox | 16 A | 1.5T fMRI | Visual | Neutral | WB | p < .001, k ≥ 64 |
| Bragulat | 2008 | 9 HD | 23 ± 3 | 60 | NTS | None | 1.5T fMRI | Olfactory a,b | Neutral, appetitive | WB, ROI | p < .001 |
| Filbey | 2008 | 37 HD | 23 ± 2 | 68 | NTS | None | 3T fMRI | Tastea | Appetitive juice | WB, ROI | p < .05 FDR |
| Myrick | 2008 | 24 AD | 25 ± 6 | 75 | NTS, PLA | 17 SD | 1.5T fMRI | Visual, taste | Neutral beverage | WB, ROI | p < .001, k ≥ 405 |
| Kareken | 2010 | 14 FHP HD | 24 ± 2 | 50 | NTS | 12 FHN HD | 3T fMRI | Olfactory a,b | Appetitive odor | ROI | n/a |
| Myrick | 2010 | 16 AD | 31 ± 8 | 81 | NTS, PLA | None | 3T fMRI | Visual, taste | Neutral beverage | WB, ROI | p < .005, k ≥ 405 |
| Ray | 2010 | 10 HD | 19–21 | 50 | Mandated | None | 3T fMRI | Visual | Neutral | WB | p < .05 |
| Vollstädt-Klein | 2010 | 21 HD | 49 ± 11 | 57 | NTS | 10 SD d | 3T fMRI | Visual | Neutral | WB, ROI | p < .001, k ≥ 270 |
| Claus | 2011 | 326 HD | 32 ± 6 | 69 | NTS & TS | None | 3T fMRI | Tastea | Appetitive juice | WB, ROI | p < 7 × 10−16 |
| Ihssen | 2011 | 11 HD | 27 ± 6 | 91 | NTS | 11 SD | 3T fMRI | Visual | Neutral | WB | p < .05, k ≥ 837 |
| Schacht | 2011 | 9 AD | 35 ± 12 | 67 | NTS | None | 3T fMRI | Visual, taste | Neutral beverage | ROI | n/a |
| Vollstädt-Klein | 2011 | 30 AD | 47 ± 9 | 63 | Detox | None | 3T fMRI | Visual | Neutral | WB, ROI | p < .001, k ≥ 270 |
Cues were individualized to each participant.
Participants also received intravenous ethanol infusion during alcohol cue exposure.
Only the anterior third of the brain was imaged.
Controls were included with cases for some within-participants contrasts.
Voxel size was not specified, so k is in number of voxels.
No height threshold reported.
Abbreviations: AD = Alcohol Dependence; HD = heavy drinkers; SD = social drinkers; FHP/FHN = family history positive/negative for alcoholism; NR = not reported; Outpt = current outpatient treatment; Detox = recent inpatient detoxification; NTS = non-treatment-seeking; TS = treatment-seeking; PLA = placebo-treated; Mandated = university-mandated referral; A = age-matched; G = gender-matched; ROI = region of interest; WB = whole brain; FDR = false discovery rate.
Subject characteristics
The 28 studies identified comprised 679 cases and 174 controls, the disparity owing to the fact that many studies did not include controls. As expected, cases encompassed a broad range of severity, from teen and young adult heavy drinkers to recently detoxified or treatment-seeking adult alcoholics. Most cases were adult male heavy drinkers (range of drinking reported: 5.0–7.3 drinks per drinking day and 2.8–4.2 drinking days per week, although studies did not all report the same drinking parameters).Table 1 notes exceptions (i.e., subjects who met Diagnostic and Statistical Manual of Mental Disorders, revised 4th edition (DSM-IV) (American Psychiatric Association, 2000) diagnostic criteria for Alcohol Dependence (AD); those with AUD, which includes both Alcohol Abuse and Dependence; and those who were family-history-positive (FHP) for alcoholism), as well as these subjects’ treatment status at the time of scanning. AD subjects drank more heavily than heavy drinkers (range: 5.7–24.8 drinks per drinking day and 4.9–5.1 drinking days per week). Controls, when included, were most often age- and/or gender-matched psychiatrically healthy individuals. Some studies used social drinkers (i.e., individuals who reported drinking fewer than 14 drinks per week) or individuals who were family-history-negative (FHN) for alcoholism as controls. No studies reported including subjects with any psychiatric or neurological comorbidities (except nicotine dependence) nor those with current psychotropic medication use, though some studies did not report this information.
Selection of cues and contrasts
Of the studies identified, most (21 of 28) used visual cues, chosen primarily from standardized image sets (e.g., the Normative Appetitive Picture System (Stritzke et al., 2004)) and alcohol print advertisements. These cues were occasionally combined with taste or olfactory cues, and were sometimes individualized, such that subjects were exposed to images (or the taste or smell) of their favorite alcoholic beverage. All studies except one (Heinz et al., 2007) presented cues with block designs, in which blocks of the alcohol cues were interspersed with blocks of other stimuli and periods of resting baseline. In most studies, a “neutral” stimulus of the same sensory modality as the alcohol cue was used to control for the sensory experience of interest (e.g., vision, olfaction). Some studies used a stronger “appetitive” neutral cue, intended to elicit, and thereby control for, activation related to non-addictive motivated or acquisitive behavior (for visual stimuli, a picture of a non-alcoholic beverage; for olfactory stimuli, an appetizing odor). Two studies that employed alcohol taste cues used a sweet, novel juice as an appetitive control (Claus et al., 2011, Filbey et al., 2008). To avoid biasing meta-analysis results in favor of studies that reported multiple contrasts, if studies reported contrasts against both resting baseline and a neutral or appetitive cue, only the latter, more conservative contrast was included. We considered conducting separate analyses for different sensory stimuli (e.g., visual, olfactory, taste), but there were not enough foci of activation from different studies for each type of cue to support this approach.
For studies that conducted a whole-brain analysis, Table 1 also lists each study’s threshold for inference of statistical significance (height thresholds are uncorrected p values unless voxel- or cluster-wise correction is noted; extent thresholds (k) are expressed in mm3). Most identified studies used uncorrected voxel-wise height thresholds, often paired with cluster extent thresholds. Though common, this approach may not adequately control false positive results (Bennett et al., 2009). Only two studies used corrected voxel-wise thresholds (i.e., family-wise error or false-discovery rate (FDR) correction) or their uncorrected equivalents (Claus et al., 2011, Filbey et al., 2008). Regrettably, the ALE approach does not allow results from these studies to be given greater weight in the meta-analysis. Nonetheless, this approach, given a sufficient number of studies and subjects, is intended to eliminate non-specific “noise” activations regardless of their original thresholding.
Activation likelihood estimation
Meta-analysis was conducted with the revised version (Eickhoff et al., 2009) of the ALE approach (Laird et al., 2005, Turkeltaub et al., 2002), using the GingerALE (v2.0.4) software package (available at http://brainmap.org/ale). ALE assesses the overlap between coordinates of activation reported in different studies by modeling them as spatial probability (Gaussian) distributions centered at the respective coordinates. This approach captures the inherent uncertainty associated with each activation focus. To determine whether these spatial distributions converge, voxel-wise activation probabilities are tested against a null distribution that assumes random spatial association between the results obtained in different studies. The revised version of the approach weights the distributions applied to each activation focus by the number of subjects in the study that reported the activation, resulting in tighter distributions around activation foci from studies with more subjects. Further, by assuming the spatial relationship between foci from a given study is fixed, and testing the distributions of these foci against randomly distributed between-study foci, it allows random-effects inference to the entire population of studies analyzed.
ALE analysis requires coordinates in the same stereotactic space. To enable localization of results to Brodmann areas (BAs), which are only defined in Talairach space, coordinates from studies reported in Montreal Neurological Institute (MNI) space were transformed to Talairach space (Talairach & Tournoux, 1988) using the icbm2tal algorithm (Lancaster et al., 2007), which provides an improved fit over the mni2tal transformation (Brett et al., 2002). MNI coordinates that had been previously transformed into Talairach space with mni2tal were transformed back into MNI space and then re-transformed into Talairach space with the icbm2tal algorithm.
To restrict meta-analytic results to gray matter, ALE analyses were constrained by a gray matter mask (i.e., a mask containing voxels in which the likelihood of gray matter was > 10%, based on the International Consortium on Brain Mapping (ICBM) tissue probability maps (Evans et al., 1994)), and were thresholded at a voxel-wise p < .05 (FDR corrected for multiple comparisons), with clusters > 200 mm3. Peaks of the resulting clusters were labeled by reference to the Talairach Daemon atlas (Lancaster et al., 1997, Lancaster et al., 2000), and all clusters were displayed on a single-subject Talairach template (colin27) (Kochunov et al., 2002).
Two primary ALE analyses were conducted: one of the coordinates reported in studies that conducted a within-subjects whole-brain (WB) analysis, which comprised 163 foci from 16 studies (560 cases), and one of the coordinates reported in studies that conducted a between-subjects (cases vs. controls) WB analysis, which comprised 63 foci from 9 studies (109 cases and 102 controls). The studies included in each analysis are listed in Supplementary Tables 1 and 2. Within- and between-subjects studies were not mutually exclusive; some studies reported both kinds of analyses. Because more than half of the cases in the within-subjects analysis came from one study (Claus et al., 2011), this analysis was repeated with subjects from this study excluded. The primary ALE analyses excluded 51 foci from 11 within-subjects region-of-interest (ROI) analyses and 14 foci from 4 between-subjects ROI analyses, as well as one within-subjects study (Modell & Mountz, 1995) and one between-subjects study (Kareken et al., 2010) that reported parameter estimates for activation averaged across ROIs, rather than stereotactic coordinates for these activations. Analyses with the ROI coordinates included and after excluding the Claus study are in the Supplementary Materials.
Systematic review of behavioral correlations and treatment effects
Since there were not enough studies that reported correlations with any single behavioral measure to perform an ALE analysis, we conducted a systematic review of the 13 studies that reported at least one correlation between a behavioral measure and brain activation (see Table 4). Correlations with a variety of measures were reported, including psychometrically validated measures of AUD severity (the Alcohol Dependence Scale (ADS; Skinner & Allen, 1982); Alcohol Use Disorders Identification Test (AUDIT; Saunders et al., 1993); and Obsessive-Compulsive Drinking Scale (OCDS; Anton et al., 1996)), craving (the Alcohol Urge Questionnaire (AUQ; Bohn et al., 1995); and Desires for Alcohol Questionnaire (DAQ; Love et al., 1998)), and loss of control (the Failed Control subscale of the Impaired Control Scale (ICSFC; Heather et al., 1993)) and various summary statistics of quantity and frequency of alcohol consumption. In vivo craving, assessed with visual analog scales while subjects were in the scanner, was also analyzed; in these studies, the mean craving rating across all alcohol cue trials was the variable used for correlation. Correlations were calculated with a variety of activation measures, including whole-brain activation, masks of activation main effects, and ROIs. Author labels for areas of activations were used.
Table 4.
Behavioral correlates of alcohol cue-elicited brain activation
| Behavioral measure | First author, year | N | Midbrain/limbic | Prefrontal | Motor | Par | Temp | Occ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VTA | Thal | DS | VS | Amg | Ins | ACC | PCC | OFC | vmPFC | latPFC | |||||||
| ADS | Claus, 2011 | 326 | L, R | R | L | L | L | L | L | L | |||||||
| AUDIT | Filbey, 2008 | 37 | L, R | L, R | L, R | M | L, R | M | |||||||||
| Claus, 2011 | 326 | L | R | R | L | L | |||||||||||
| AUQ | Filbey, 2008 | 37 | R | M | |||||||||||||
| DAQ | |||||||||||||||||
| -Mild desires to drink | Tapert, 2003 | 15 | La | L | R | R | L, R | ||||||||||
| -Strong desires and intentions to drink | Tapert, 2003 | 15 | R | ||||||||||||||
| Tapert, 2004 | 8 | L | |||||||||||||||
| -Reinforcing effects | Tapert, 2003 | 15 | Ra | Ra | |||||||||||||
| Tapert, 2004 | 8 | L | |||||||||||||||
| ICSFC | Claus, 2011 | 326 | L | L, R | R | L | L, R | L, R | |||||||||
| OCDS | Wrase, 2007 | 13 | R | ||||||||||||||
| Vollstädt-Klein, 2010 | 31 | R | Lb | ||||||||||||||
| In-scanner craving | Modell, 1995 | 9 | R | ||||||||||||||
| Myrick, 2004 | 10 | L | L | L | |||||||||||||
| Park, 2007 | 9 | L, R | L | L, R | L, R | L, R | |||||||||||
| Filbey, 2008 | 37 | R | |||||||||||||||
| Myrick, 2008 | 107 | R | |||||||||||||||
| Drinking | |||||||||||||||||
| -Drinks per month | Tapert, 2003 | 15 | R | L | L, R | ||||||||||||
| -Alcohol intake after scan | Grüsser, 2004 | 10 | M | ||||||||||||||
| -Heavy drinking days | Bragulat, 2008 | 9 | L | ||||||||||||||
| -While on 15mg APZ | Myrick, 2010 | 14 | R | ||||||||||||||
| -Years regular drinking | Claus, 2011 | 326 | L | L, R | L, R | ||||||||||||
Results from ROI analyses are bolded.
Negative correlation;
Negative correlation among heavy drinkers (N = 21) only;
Among male participants (N = 20) only.
Abbreviations: VTA = ventral tegmental area; Thal = thalamus; DS = dorsal striatum; VS = ventral striatum; Amg = amygdala; Ins = insula; ACC = anterior cingulate; PCC = posterior cingulate; OFC = orbitofrontal cortex; vmPFC = ventromedial prefrontal; latPFC = lateral prefrontal; Motor = pre-, post-central gyri, cerebellum; Par = parietal; Temp = temporal; Occ = occipital; APZ = aripiprazole.
For treatment effects, ALE analysis was not feasible because of the diversity of treatments used and because more than half the subjects came from one study (Myrick et al., 2008). Systematic review identified 6 studies that reported a treatment effect on cue-elicited activation (see Table 5). These studies comprised 119 subjects who received active treatments and 55 who received placebos. A broad range of treatments were employed, including monitored abstinence after medical detoxification (Braus et al., 2001), acute (Hermann et al., 2006) or short-term (Myrick et al., 2008, 2010, Schneider et al., 2001) pharmacotherapies (e.g., doxepin (DXP), amisulpride (AMS), naltrexone (NTX), ondansetron (OND), aripiprazole (APZ)), cognitive behavioral therapy (CBT), and behavioral cue-exposure therapy (Vollstadt-Klein et al., 2011). Methods were also variable; some studies scanned subjects both before and after treatment, while others scanned only after treatment, but compared subjects who received active treatments to those who received placebos. Activation differences were calculated both across the whole brain and in ROIs. Author labels for activations were again used.
Table 5.
Treatment effects on alcohol cue-elicited brain activation
| First author, year | N | Type and length of treatment | Placebo N | Scans | Brain areas in which treatment reduced activation | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thal | DS | VS | ACC | Ins | Amg | OFC | vmPFC | latPFC | Par | Occ | CBL | |||||
| Braus, 2001 | 4 | Monitored abstinence × 21–112 d | n/a | Pre/post | * L, R | |||||||||||
| Schneider, 2001 | 10 | 150 mg DXP, CBT × 21 d | n/a | Pre/post | * R | * L | ||||||||||
| Hermann, 2006 | 10 | 400 mg AMS (one dose) | n/a | Pre/post | R | |||||||||||
| Myrick, 2008 | 23 | 50 mg NTX × 7 d | 24 | Post | R | L, R | R | R | ||||||||
| 23 | 0.5 mg OND × 7 d | 24 | Post | L | R | R | R | |||||||||
| 20 | 50 mg NTX, 0.5 mg OND × 7 d | 24 | Post | R | R | R | ||||||||||
| Myrick, 2010 | 14 | 15 mg APZ × 14 d | 16 | Post | R | |||||||||||
| Vollstädt-Klein, 2011 | 15 | Cue-exposure therapy × 21 d | 15 | Pre/post | L | L | L, R | L | L | L, R | ||||||
Results from ROI analyses are bolded.
Not statistically tested.
Abbreviations: d = days; CBL = cerebellum. See Table 4 for other brain area abbreviations.
Results
ALE meta-analysis of within-subjects cue-elicited activation
Table 2 and Figure 1 display areas in which alcohol cues elicited greater activation than contrasted cues among cases only. ALE values were greatest in a large cluster that encompassed ventral striatum (VS) (i.e., caudate head) and ventral anterior cingulate cortex (ACC). Other supra-threshold clusters were primarily left-sided, with peaks in ventromedial prefrontal cortex (vmPFC), posterior cingulate cortex (PCC), claustrum/insula, precuneus, thalamus, temporal cortex (middle temporal and parahippocampal gyri), and primary and secondary visual processing areas (inferior occipital and fusiform gyri). The analysis that excluded the large Claus study (Claus et al., 2011) yielded similar results to the analysis that included this study (see Supplementary Table 3). The analysis conducted with coordinates from the ROI studies included yielded high ALE values in similar areas, though the cluster encompassing ventral striatum and ventral ACC was larger, and additional clusters with peaks in right insula, left VS, left cerebellum, left midbrain (substantia nigra), and bilateral inferior frontal gyrus were also significant (see Supplementary Table 4).
Table 2.
Regions of activation in which alcohol cues elicited greater activation than contrasted cues among cases only
| Anatomical region | BA | x | y | z | ALE (corrected p)* | Volume (mm3) |
|---|---|---|---|---|---|---|
| R caudate head | - | 8 | 4 | −2 | 0.017 | 2176 |
| R caudate body | - | 6 | 14 | 6 | 0.014 | |
| R lateral globus pallidus | - | 14 | 0 | 4 | 0.012 | |
| L anterior cingulate | 25 | −2 | 8 | −10 | 0.012 | |
| L medial frontal gyrus | 10 | −2 | 52 | 6 | 0.014 | 872 |
| L medial frontal gyrus | 32 | 0 | 40 | 6 | 0.013 | |
| L posterior cingulate | 30 | −4 | −50 | 18 | 0.014 | 864 |
| L posterior cingulate | 29 | −10 | −48 | 12 | 0.013 | |
| L cingulate gyrus | 31 | −4 | −38 | 30 | 0.018 | 600 |
| R claustrum | - | 32 | 10 | −6 | 0.017 | 464 |
| L middle temporal gyrus | 21 | −58 | −30 | −4 | 0.016 | 368 |
| L precuneus | 7 | −2 | -62 | 60 | 0.018 | 352 |
| L thalamus | - | −20 | −22 | 12 | 0.013 | 320 |
| L fusiform gyrus | 37 | −50 | −42 | −12 | 0.014 | 312 |
| L anterior cingulate | 24 | −2 | 24 | 16 | 0.013 | 312 |
| R inferior occipital gyrus | 18 | 28 | −88 | −10 | 0.014 | 288 |
| R inferior occipital gyrus | 18 | 28 | −88 | −8 | 0.014 | |
| L thalamus | - | −2 | −6 | 10 | 0.013 | 288 |
| L thalamus | - | −12 | −8 | 12 | 0.010 | |
| L parahippocampal gyrus | 34 | −20 | −12 | −16 | 0.012 | 216 |
| L middle frontal gyrus | 6 | −20 | 22 | 58 | 0.016 | 208 |
p < .05, corrected, voxelwise, for the false discovery rate. BA = Brodmann area. ALE = activation likelihood estimate. Coordinates are in Talairach space. For clusters with sub-peaks, primary peak is bolded and sub-peaks are italicized.
Figure 1.
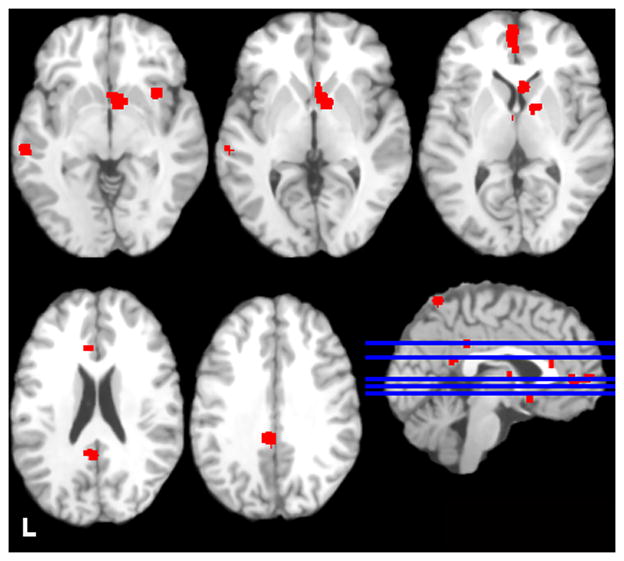
Regions in which activation was greater to alcohol cues than contrasted cues among cases only. Images are neurologically oriented and thresholded at a voxel-wise p < .05 (FDR corrected for multiple comparisons), with clusters > 200 mm3. Talairach z coordinates of displayed slices are, from top left to bottom right, −5, 0, 5, 20, and 30. Blue lines display location of these slices.
ALE meta-analysis of between-subjects cue-elicited activation
Table 3 and Figure 2 display areas in which alcohol cues elicited greater activation in cases than controls. ALE values were greatest in a cluster with its peak in the left PCC. Other supra-threshold clusters encompassed left superior temporal gyrus and bilateral precuneus. The analysis conducted with coordinates from the ROI studies included yielded identical ALE values in the same clusters (see Supplementary Table 5).
Table 3.
Regions of activation in which cases had greater alcohol cue-elicited activation than controls
| Anatomical region | BA | x | y | z | ALE (corrected p)* | Volume (mm3) |
|---|---|---|---|---|---|---|
| L posterior cingulate | 23 | −6 | −36 | 26 | 0.017 | 720 |
| L superior temporal gyrus | 38 | −52 | 10 | −14 | 0.016 | 320 |
| R precuneus | 19 | 34 | −74 | 36 | 0.012 | 320 |
| L precuneus | 31 | −12 | −58 | 26 | 0.011 | 240 |
p < .05, corrected, voxelwise, for the false discovery rate. BA = Brodmann area. ALE = activation likelihood estimate. Coordinates are in Talairach space.
Figure 2.
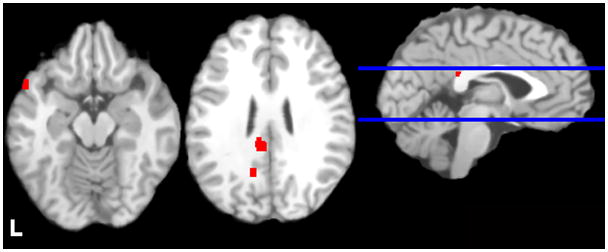
Regions of alcohol cue-elicited activation that were greater in cases than controls. Images are neurologically oriented and thresholded at a voxel-wise p < .05 (FDR corrected for multiple comparisons), with clusters > 200 mm3. Talairach z coordinates of displayed slices are, from left to right, −15 and 25. Bl ue lines display location of these slices.
Behavioral correlates of cue-elicited activation
Table 4 lists correlations between cue-elicited activation and behavioral measures. Correlations were reported across a wide variety of brain areas. Across all measures, the area most frequently positively correlated with severity of dependence, amount of drinking, impaired control, and magnitude of craving was VS (10 positive correlations across 6 different measures from 7 studies), though 8 of these correlations derived from ROI analyses that interrogated only or primarily limbic regions. VS activation was particularly frequently correlated with in vivo craving (3 of 5 studies that reported such a correlation), though one study (Vollstadt-Klein et al., 2010) also reported a negative correlation between VS activation and OCDS score among heavy drinkers. Parietal (7 positive correlations across 5 measures from 3 studies) and lateral PFC (8 positive correlations across 5 measures from 4 studies) activations were also frequently positively correlated; half of the lateral PFC correlations and all of the parietal correlations derived from WB analyses.
Treatment effects on cue-elicited activation
Table 5 displays brain areas in which cue-elicited activation was reduced after treatment. Treatment most frequently attenuated VS activation; 4 of 6 studies reported this effect. However, of these studies, one (Braus et al., 2001) did not statistically test the effect (i.e., they reported above-threshold activation before treatment and below-threshold activation after treatment, but did not test the significance of the difference between scans). This is an example of the “imager’s fallacy” (Henson, 2005). Further, one study reported the difference between the active and placebo groups at an uncorrected voxel-wise WB threshold of p < .05 (Myrick et al., 2008), and the other two (Myrick et al., 2010, Vollstadt-Klein et al., 2011) found it with ROI analyses that only tested the striatum. Two of 6 studies reported treatment-attenuated activation in dorsal striatum, ACC, lateral PFC, and parietal cortex.
Discussion
Taken together, the results of this coordinate-based meta-analysis indicate that alcohol-related cues, which induce subjective craving and are believed to contribute to relapse among abstinent alcoholics, elicit activation of a variety of brain areas, including the ventral striatum (VS), ventral anterior cingulate cortex (ACC), and ventromedial PFC (vmPFC), among heavy drinkers and individuals with AUD. However, activation in these areas may not differentiate these individuals from control subjects. Instead, cue-elicited activation of parietal and temporal regions, including posterior cingulate (PCC), precuneus, and superior temporal gyrus, may be selectively enhanced among heavy drinkers and individuals with AUD. A secondary review of behavioral correlations and treatment effects on cue-elicited activations indicated that, while behavioral measures related to AUD have demonstrated some consistency in their association with cue-elicited VS activation and treatments have most often reported reduced VS activation, most of the reported associations have derived from ROI analyses, which have often interrogated only limbic and prefrontal ROIs, and all of the treatment effects have derived from ROI analysis or inadequate statistical analysis.
Across all within-subjects, whole-brain analyses, the likelihood of cue-elicited activation was greatest in a cluster with its peak in the ventromedial part of head of the right caudate nucleus, which encompasses the nucleus accumbens (NAcc). This area is a primary target of the mesolimbic dopamine (DA) pathway, which animal models of addiction suggest is a critical substrate for reward processing (e.g., Wise, 2002). There was also a high likelihood of cue-elicited activation in ventral ACC and adjacent vmPFC (i.e., BAs 10, 25, and 32), which are projection targets of the mesocortical DA pathway and reciprocally project to NAcc. DA release in NAcc is both an acute pharmacological effect of alcohol (Imperato & Di Chiara, 1986, Yoshimoto et al., 1992) and a necessary precursor for animals to learn to self-administer alcohol (Weiss & Porrino, 2002). When an alcohol-related stimulus is presented, DA release in NAcc may signal the salience and reward associated with the stimulus (Schultz et al., 1997), and may subserve “wanting” (i.e., craving) it (Robinson & Berridge, 1993). Ventromedial PFC and ventral ACC may contribute to these processes by encoding the reward value of the stimulus, particularly as it is influenced by an individual’s current emotional state (Kennerley & Walton, 2011). Thus, activation of these areas within cases suggests that alcohol-related cues engage neural circuits associated with reward processing among heavy drinkers and individuals with AUD.
However, VS, ACC, and vmPFC activation were not different between cases and controls. While this might be seen as surprising, in fact, only one WB study (Ihssen et al., 2011) has reported greater VS activation in cases than controls, and studies that have reported greater ACC and vmPFC activation in cases have not consistently identified the same sub-regions of these relatively large and functionally heterogeneous areas. In the Ihssen study, cases were described as heavy drinkers (more than 21 drinks/week), but alcohol-related psychopathology (i.e., AUD) was not reported. In two additional small studies of heavy drinkers, Kareken and colleagues reported greater ROI-based VS and vmPFC activation among those who were FHP relative to social drinkers (Kareken et al., 2004) and FHN heavy drinkers (Kareken et al., 2010). No studies have reported greater VS activation among individuals with AUD, rather than heavy drinkers. Of the 12 total studies that contributed coordinates to the between-subjects analysis, 9 used subjects with AUD, and 6 of these used treatment-seeking subjects. Thus, two possible explanations for our finding are that 1) heavy drinkers, but not individuals with AUD, demonstrate greater VS, vmPFC, and ACC activation; and/or 2) controls also perceive alcohol cues as novel and rewarding, and the cue-elicited salience signal putatively generated in these areas is also present among controls. Support for the first explanation may come from recent work that suggests that alterations in neural circuits besides the mesocorticolimbic DA pathways underlie the progression from early- to end-stage addiction (Kalivas & O’Brien, 2008); thus, cue-elicited activation of dopaminergic areas may be enhanced among heavy drinkers, but not treatment-seeking alcoholics. With respect to the second explanation, two recent studies have reported that light social drinkers (mean = 2 drinks/week) display robust alcohol cue-elicited activation of VS, vmPFC, ACC, and other reward-related areas (Seo et al., 2011), and that social drinkers demonstrate greater VS and vmPFC activation than heavy drinkers (Vollstadt-Klein et al., 2010).
Alternatively, the relatively small number of studies that included controls (relative to those that included cases) may indicate that the cases vs. controls effect size for VS, vmPFC, and ACC was simply too small to be statistically significant. Given the smaller numbers of studies and subjects, the ALE analysis of between-subjects studies clearly had less power to detect differences than the within-subjects analysis. Most studies that did include controls did not report activation separately for them, so it was not possible to conduct a separate ALE analysis of cue-elicited activation within this group. Further, VS is a relatively small and functionally coherent region, and as such may be more suitable for ROI analysis than whole-brain analyses (e.g., Poldrack, 2007), which contributed the majority of the between-subjects coordinates. Nonetheless, our findings suggest that alcohol cues may not activate VS, vmPFC, and ACC differently between cases and controls.
The between-subjects ALE analysis indicated several areas in which cases did display greater activation than controls, including two parietal regions: PCC and the adjacent precuneus. A cluster encompassing PCC (BAs 23, 29, 30 and 31) and precuneus (BAs 7 and 31) was also among the largest within-subjects activations. These areas, which have reciprocal connectivity with adjoining parietal regions, thalamus, hippocampus, and PFC (Cavanna & Trimble, 2006, Vann et al., 2009), have classically been associated with memory (Cabeza & Nyberg, 2000), perception of emotional salience (Maddock, 1999), and processing self-relevant information (Cavanna & Trimble, 2006), but accumulating evidence suggests that they may also underlie risky decision making (Hayden et al., 2008, McCoy & Platt, 2005) and subjective valuation of potential rewards (Kable & Glimcher, 2007). To this end, single-cell recordings from primate PCC indicate that neuronal activity in this area is correlated with the amount of risk associated with a behavioral option and an animal’s preference for a risky option (McCoy & Platt, 2005). Thus, the fact that cases demonstrated greater cue-elicited activation of these areas than controls might be interpreted as evidence that these individuals perceive alcohol cues as a risky but preferable option. Consistent with these interpretations, a very large within-subjects study (Claus et al., 2011) compared cue-elicited activation between heavy drinkers who were seeking treatment (perhaps those who had made more risky decisions related to their alcohol use) and those who were not, and reported a large area of greater activation among treatment-seekers in PCC and precuneus. Further, this study reported correlations between precuneus activation and a variety of measures of AUD severity, including AUDIT score and years of heavy drinking. Thus, there are several indications that cue-elicited parietal activation may be greater among cases, and may scale with AUD severity.
There may be other explanations for the differences in PCC and precuneus activation between cases and controls. Greater cue-elicited activation in these areas could reflect resistance to craving among heavy drinkers and individuals with AUD. Nicotine-dependent subjects instructed to either resist or allow craving while viewing cigarette-related and neutral-content videos display greater cue-elicited activation in PCC and precuneus when they resist craving relative to allowing it (Brody et al., 2007). Although nicotine craving is not precisely analogous to alcohol craving, both phenomena elicit similar psychophysiological effects (Carter & Tiffany, 1999). Alternatively, given the proximity of PCC and precuneus to primary visual cortex and the fact that most studies used visual cues, activation differences in these areas could reflect differences in secondary visual processing between cases and controls.
These findings complicate interpretation of the behavioral correlations and treatment effects reviewed here, many of which implicated VS but derived from ROI analyses. Given the relationship between NAcc DA release, the pharmacological effects of alcohol, and the acquisition of alcohol self-administration, it is logical that studies of cue reactivity and craving have used ROI analysis to focus on this region. However, it is possible that alcohol craving is correlated with cue-elicited VS activation, but that this relationship holds among controls as well as heavy drinkers and individuals with AUD (though the extent to which controls “crave” alcohol is debatable). In support of this hypothesis, the same study that reported cue-elicited VS activation among social drinkers found that this activation correlated with alcohol craving among male social drinkers (Seo et al., 2011). In contrast, WB analyses, which are more theoretically agnostic, may reveal unpredicted substrates for behavior. For a behavioral measure to demonstrate correlation with activation in a WB analysis, the magnitude of the relationship must be quite high to surpass multiple comparisons correction. Nonetheless, studies that have conducted such analyses have often found correlations between AUD-related behaviors and parietal activation, including PCC and precuneus, and treatment effects in these regions have been reported (Vollstadt-Klein et al., 2011). The only study to use ROI analysis of a parietal region reported a correlation between PCC cue-elicited activation and heavy drinking days (Bragulat et al., 2008). Given the meta-analytic findings reported here, greater attention to these regions, particularly in future treatment studies, may be warranted.
Limitations and future directions
This study suffered from several limitations. First, the ALE approach to meta-analysis is inherently limited by its reliance on stereotactic coordinates that represent clusters of varying spatial extent and statistical significance. It has been improved from earlier implementations by the inclusion of probability distributions around coordinates weighted by the number of subjects in the studies that produced them, but it cannot differentiate between coordinates that are the peaks of very large or very small areas of activation if they derive from studies of equal size. The ALE approach is also limited by the fact that functional imaging studies, unlike other behavioral science studies, traditionally do not report results that did not survive statistical thresholding, precluding the inclusion of sub-threshold but consistent activations across studies (see Lieberman & Cunningham, 2009, for further discussion of this issue). In the present study, two studies included in the within-subjects analyses administered intravenous ethanol during alcohol cue exposure (Bragulat et al., 2008, Olbrich et al., 2006), raising the possibility that the meta-analytic results conflated the pharmacological and cue-elicited effects of alcohol. Additionally, while there is no indication that placebo medications affect alcohol cue reactivity (e.g., Hutchison et al., 2006), two treatment studies in which subjects received placebos (Myrick et al., 2008, Myrick et al., 2010) were included in the within-subjects analyses. However, within-subjects results were unchanged when all four of these studies were excluded.
Future studies of alcohol cue reactivity should consider differences between subgroups of heavy drinkers and individuals with AUD (e.g., treatment-seekers; individuals with comorbid psychopathology or drug use), as well as differences between males and females. With one exception (Tapert et al., 2004), all studies analyzed included primarily male participants. While this distribution is representative of the population of individuals with AUD, some evidence suggests that men and women may react differently to alcohol cues (e.g., Seo et al., 2011). Future studies should also address differences in functional and effective connectivity between cases and controls. One possibility for the failure to find differences in the areas of greatest within-subjects cue-elicited activation between cases and controls is that connectivity between these regions and other parts of the brain, rather than merely their individual activation, is differentially affected in AUD. Reduced functional connectivity, particularly between frontal regions and striatum, parietal cortex, and cerebellum, has been reported among both individuals with AUD (Park et al., 2010, Rogers et al., 2012) and FHP adolescents (Herting et al., 2011, Wetherill et al., 2012), but these studies did not use cue reactivity tasks. Given our findings of differential cue-elicited activation between cases and controls in secondary visual processing areas, analysis of connectivity between these areas and regions associated with emotional and salience processing (e.g., amygdala, ACC, OFC) that are known to modulate visual activation (e.g., Wendt et al., 2011) might be particularly informative.
Conclusions
In summary, this meta-analysis demonstrated that across studies, alcohol-related cues elicited activation of ventral striatum, anterior cingulate, and ventromedial PFC among heavy drinkers and individuals with AUD. Measures of AUD severity, craving, loss of control, and drinking quantity and frequency have most frequently been correlated with cue-elicited VS activation, but many of the reported associations have derived from ROI analyses, as have reported treatment-induced reductions in VS activation. In contrast, cue-elicited activation of other regions, including two parietal regions (posterior cingulate and precuneus), differentiated individuals with AUD from healthy controls. Though somewhat less frequent, behavioral correlations and treatment effects in parietal regions have also been reported. To adequately understand the neurobiological substrates of alcohol cue reactivity and craving and the manner in which AUD treatments may ameliorate neuropathology related to these phenomena, greater attention to brain regions besides VS, ACC, and vmPFC may be warranted.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institute of Alcohol Abuse and Alcoholism (NIAAA). Dr. Schacht was supported by T32 AA007474, Dr. Anton by K05 AA017435, and Dr. Myrick by the Charleston Alcohol Research Center (P50 AA010761). Portions of this work were presented at the 2011 NIAAA Training Directors Meeting, Providence, RI.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 2000. text rev. [Google Scholar]
- Anton RF, Moak DH, Latham PK. The obsessive compulsive drinking scale: A new method of assessing outcome in alcoholism treatment studies. Arch Gen Psychiatry. 1996;53:225–31. doi: 10.1001/archpsyc.1996.01830030047008. [DOI] [PubMed] [Google Scholar]
- Anton RF. What is craving? Models and implications for treatment. Alcohol Res Health. 1999;23:165–73. [PMC free article] [PubMed] [Google Scholar]
- Bennett CM, Wolford GL, Miller MB. The principled control of false positives in neuroimaging. Soc Cogn Affect Neurosci. 2009;4:417–22. doi: 10.1093/scan/nsp053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res. 1995;19:600–6. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry. 2006;60:352–60. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Bragulat V, Dzemidzic M, Talavage T, Davidson D, O’Connor SJ, Kareken DA. Alcohol sensitizes cerebral responses to the odors of alcoholic drinks: an fMRI study. Alcohol Clin Exp Res. 2008;32:1124–34. doi: 10.1111/j.1530-0277.2008.00693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braus DF, Wrase J, Grüsser S, Hermann D, Ruf M, Flor H, Mann K, Heinz A. Alcohol-associated stimuli activate the ventral striatum in abstinent alcoholics. J Neural Transm. 2001;108:887–94. doi: 10.1007/s007020170038. [DOI] [PubMed] [Google Scholar]
- Brett M, Johnsrude IS, Owen AM. The problem of functional localization in the human brain. Nat Rev Neurosci. 2002;3:243–9. doi: 10.1038/nrn756. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Jou J, Tiongson E, Allen V, Scheibal D, London ED, Monterosso JR, Tiffany ST, Korb A, Gan JJ, Cohen MS. Neural substrates of resisting craving during cigarette cue exposure. Biol Psychiatry. 2007;62:642–51. doi: 10.1016/j.biopsych.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhler M, Mann K. Alcohol and the human brain: a systematic review of different neuroimaging methods. Alcohol Clin Exp Res. 2011;35:1771–93. doi: 10.1111/j.1530-0277.2011.01540.x. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Neural bases of learning and memory: functional neuroimaging evidence. Curr Opin Neurol. 2000;13:415–21. doi: 10.1097/00019052-200008000-00008. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–40. [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–83. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Claus ED, Ewing SW, Filbey FM, Sabbineni A, Hutchison KE. Identifying neurobiological phenotypes associated with alcohol use disorder severity. Neuropsychopharmacology. 2011;36:2086–96. doi: 10.1038/npp.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond DC, Cooper T, Glautier SP. Conditioned learning in alcohol dependence: implications for cue exposure treatment. Br J Addict. 1990;85:725–43. doi: 10.1111/j.1360-0443.1990.tb01685.x. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30:2907–26. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AC, Collins DL, Neelin P, MacDonald D, Kamber M, Marrett TS. Three-dimensional correlative imaging: Applications in human brain mapping. In: Thatcher RW, Hallett M, Zeffiro T, John ER, Huerta M, editors. Functional Neuroimaging: Technical Foundations. New York: Academic Press; 1994. pp. 145–62. [Google Scholar]
- Filbey FM, Claus E, Audette AR, Niculescu M, Banich MT, Tanabe J, Du YP, Hutchison KE. Exposure to the taste of alcohol elicits activation of the mesocorticolimbic neurocircuitry. Neuropsychopharmacology. 2008;33:1391–401. doi: 10.1038/sj.npp.1301513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden BY, Nair AC, McCoy AN, Platt ML. Posterior cingulate cortex mediates outcome-contingent allocation of behavior. Neuron. 2008;60:19–25. doi: 10.1016/j.neuron.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heather N, Tebbutt JS, Mattick RP, Zamir R. Development of a scale for measuring impaired control over alcohol consumption: a preliminary report. J Stud Alcohol. 1993;54:700–9. doi: 10.15288/jsa.1993.54.700. [DOI] [PubMed] [Google Scholar]
- Heinz A, Wrase J, Kahnt T, Beck A, Bromand Z, Grüsser SM, Kienast T, Smolka MN, Flor H, Mann K. Brain activation elicited by affectively positive stimuli is associated with a lower risk of relapse in detoxified alcoholic subjects. Alcohol Clin Exp Res. 2007;31:1138–47. doi: 10.1111/j.1530-0277.2007.00406.x. [DOI] [PubMed] [Google Scholar]
- Heinz A, Beck A, Grüsser SM, Grace AA, Wrase J. Identifying the neural circuitry of alcohol craving and relapse vulnerability. Addict Biol. 2009;14:108–18. doi: 10.1111/j.1369-1600.2008.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson R. What can functional neuroimaging tell the experimental psychologist? Q J Exp Psychol A. 2005;58:193–233. doi: 10.1080/02724980443000502. [DOI] [PubMed] [Google Scholar]
- Hermann D, Smolka MN, Wrase J, Klein S, Nikitopoulos J, Georgi A, Braus DF, Flor H, Mann K, Heinz A. Blockade of cue-induced brain activation of abstinent alcoholics by a single administration of amisulpride as measured with fMRI. Alcohol Clin Exp Res. 2006;30:1349–54. doi: 10.1111/j.1530-0277.2006.00174.x. [DOI] [PubMed] [Google Scholar]
- Herting MM, Fair D, Nagel BJ. Altered fronto-cerebellar connectivity in alcohol-naive youth with a family history of alcoholism. NeuroImage. 2011;54:2582–9. doi: 10.1016/j.neuroimage.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison KE, Ray L, Sandman E, Rutter M-C, Peters A, Davidson D, Swift R. The effect of olanzapine on craving and alcohol consumption. Neuropsychopharmacology. 2006;31:1310–7. doi: 10.1038/sj.npp.1300917. [DOI] [PubMed] [Google Scholar]
- Ihssen N, Cox WM, Wiggett A, Fadardi JS, Linden DE. Differentiating heavy from light drinkers by neural responses to visual alcohol cues and other motivational stimuli. Cereb Cortex. 2011;21:1408–15. doi: 10.1093/cercor/bhq220. [DOI] [PubMed] [Google Scholar]
- Imperato A, Di Chiara G. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J Pharmacol Exp Ther. 1986;239:219–28. [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat Neurosci. 2007;10:1625–33. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–13. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33:166–80. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Kareken DA, Claus ED, Sabri M, Dzemidzic M, Kosobud AEK, Radnovich AJ, Hector D, Ramchandani VA, O’Connor SJ, Lowe M, Li T-K. Alcohol-related olfactory cues activate the nucleus accumbens and ventral tegmental area in high-risk drinkers: preliminary findings. Alcohol Clin Exp Res. 2004;28:550–7. doi: 10.1097/01.alc.0000122764.60626.af. [DOI] [PubMed] [Google Scholar]
- Kareken DA, Bragulat V, Dzemidzic M, Cox C, Talavage T, Davidson D, O’Connor SJ. Family history of alcoholism mediates the frontal response to alcoholic drink odors and alcohol in at-risk drinkers. NeuroImage. 2010;50:267–76. doi: 10.1016/j.neuroimage.2009.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerley SW, Walton ME. Decision making and reward in frontal cortex: complementary evidence from neurophysiological and neuropsychological studies. Behav Neurosci. 2011;125:297–317. doi: 10.1037/a0023575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Lancaster J, Thompson P, Toga AW, Brewer P, Hardies J, Fox P. An optimized individual target brain in the Talairach coordinate system. NeuroImage. 2002;17:922–7. [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–38. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp. 2005;25:155–64. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Rainey LH, Summerlin JL, Freitas CS, Fox PT, Evans AC, Toga AW, Mazziotta JC. Automated labeling of the human brain: a preliminary report on the development and evaluation of a forward-transform method. Hum Brain Mapp. 1997;5:238–42. doi: 10.1002/(SICI)1097-0193(1997)5:4<238::AID-HBM6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–31. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp. 2007;28:1194–205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Soc Cogn Affect Neurosci. 2009;4:423–8. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love A, James D, Willner P. A comparison of two alcohol craving questionnaires. Addiction. 1998;93:1091–102. doi: 10.1046/j.1360-0443.1998.937109113.x. [DOI] [PubMed] [Google Scholar]
- Maddock RJ. The retrosplenial cortex and emotion: new insights from functional neuroimaging of the human brain. Trends Neurosci. 1999;22:310–6. doi: 10.1016/s0166-2236(98)01374-5. [DOI] [PubMed] [Google Scholar]
- Mann K, Kiefer F, Smolka M, Gann H, Wellek S, Heinz A. Searching for responders to acamprosate and naltrexone in alcoholism treatment: rationale and design of the PREDICT study. Alcohol Clin Exp Res. 2009;33:674–83. doi: 10.1111/j.1530-0277.2008.00884.x. [DOI] [PubMed] [Google Scholar]
- McCoy AN, Platt ML. Expectations and outcomes: decision-making in the primate brain. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2005;191:201–11. doi: 10.1007/s00359-004-0565-9. [DOI] [PubMed] [Google Scholar]
- Modell JG, Mountz JM. Focal cerebral blood flow change during craving for alcohol measured by SPECT. J Neuropsychiatry Clin Neurosci. 1995;7:15–22. doi: 10.1176/jnp.7.1.15. [DOI] [PubMed] [Google Scholar]
- Monti PM, Binkoff JA, Abrams DB, Zwick WR, Nirenberg TD, Liepman MR. Reactivity of alcoholics and nonalcoholics to drinking cues. J Abnorm Psychol. 1987;96:122–6. doi: 10.1037//0021-843x.96.2.122. [DOI] [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Hutchison KE. Toward bridging the gap between biological, psychobiological and psychosocial models of alcohol craving. Addiction. 2000;95(Suppl 2):S229–36. doi: 10.1080/09652140050111799. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Randall PK, Voronin K. Effect of naltrexone and ondansetron on alcohol cue-induced activation of the ventral striatum in alcohol-dependent people. Arch Gen Psychiatry. 2008;65:466–75. doi: 10.1001/archpsyc.65.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrick H, Li X, Randall PK, Henderson S, Voronin K, Anton RF. The effect of aripiprazole on cue-induced brain activation and drinking parameters in alcoholics. J Clin Psychopharmacol. 2010;30:365–72. doi: 10.1097/JCP.0b013e3181e75cff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niaura RS, Rohsenow DJ, Binkoff JA, Monti PM, Pedraza M, Abrams DB. Relevance of cue reactivity to understanding alcohol and smoking relapse. J Abnorm Psychol. 1988;97:133–52. doi: 10.1037//0021-843x.97.2.133. [DOI] [PubMed] [Google Scholar]
- Olbrich HM, Valerius G, Paris C, Hagenbuch F, Ebert D, Juengling FD. Brain activation during craving for alcohol measured by positron emission tomography. Aust N Z J Psychiatry. 2006;40:171–8. doi: 10.1080/j.1440-1614.2006.01765.x. [DOI] [PubMed] [Google Scholar]
- Park SQ, Kahnt T, Beck A, Cohen MX, Dolan RJ, Wrase J, Heinz A. Prefrontal cortex fails to learn from reward prediction errors in alcohol dependence. J Neurosci. 2010;30:7749–53. doi: 10.1523/JNEUROSCI.5587-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens RW, Johanson CE. Craving: consensus of status and agenda for future research. Drug Alcohol Depend. 1992;30:127–31. doi: 10.1016/0376-8716(92)90017-7. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Region of interest analysis for fMRI. Social Cognitive and Affective Neuroscience. 2007;2:67–70. doi: 10.1093/scan/nsm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JS, Anton RF, Latham PK, Moak DH. Factor structure and predictive validity of the Obsessive Compulsive Drinking Scale. Alcohol Clin Exp Res. 1999;23:1484–91. [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rogers BP, Parks MH, Nickel MK, Katwal SB, Martin PR. Reduced Fronto-Cerebellar Functional Connectivity in Chronic Alcoholic Patients. Alcohol Clin Exp Res. 2012;36:294–301. doi: 10.1111/j.1530-0277.2011.01614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Shiffman S, Tiffany ST, Niaura RS, Martin CS, Shadel WG. The measurement of drug craving. Addiction. 2000;95(Suppl 2):S189–210. doi: 10.1080/09652140050111762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider F, Habel U, Wagner M, Franke P, Salloum JB, Shah NJ, Toni I, Sulzbach C, Hönig K, Maier W, Gaebel W, Zilles K. Subcortical correlates of craving in recently abstinent alcoholic patients. Am J Psychiatry. 2001;158:1075–83. doi: 10.1176/appi.ajp.158.7.1075. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–9. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Seo D, Jia Z, Lacadie CM, Tsou KA, Bergquist K, Sinha R. Sex differences in neural responses to stress and alcohol context cues. Hum Brain Mapp. 2011;32:1998–2013. doi: 10.1002/hbm.21165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, O’Malley SS. Craving for alcohol: findings from the clinic and the laboratory. Alcohol Alcohol. 1999;34:223–30. doi: 10.1093/alcalc/34.2.223. [DOI] [PubMed] [Google Scholar]
- Sinha R, Li CSR. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev. 2007;26:25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- Skinner HA, Allen BA. Alcohol dependence syndrome: measurement and validation. J Abnorm Psychol. 1982;91:199–209. doi: 10.1037//0021-843x.91.3.199. [DOI] [PubMed] [Google Scholar]
- Skinner MD, Aubin HJ. Craving’s place in addiction theory: contributions of the major models. Neurosci Biobehav Rev. 2010;34:606–23. doi: 10.1016/j.neubiorev.2009.11.024. [DOI] [PubMed] [Google Scholar]
- Stritzke WG, Breiner MJ, Curtin JJ, Lang AR. Assessment of substance cue reactivity: advances in reliability, specificity, and validity. Psychol Addict Behav. 2004;18:148–59. doi: 10.1037/0893-164X.18.2.148. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- Tapert SF, Brown GG, Baratta MV, Brown SA. fMRI BOLD response to alcohol stimuli in alcohol dependent young women. Addict Behav. 2004;29:33–50. doi: 10.1016/j.addbeh.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. NeuroImage. 2002;16:765–80. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP, Maguire EA. What does the retrosplenial cortex do? Nat Rev Neurosci. 2009;10:792–802. doi: 10.1038/nrn2733. [DOI] [PubMed] [Google Scholar]
- Vollstadt-Klein S, Wichert S, Rabinstein J, Buhler M, Klein O, Ende G, Hermann D, Mann K. Initial, habitual and compulsive alcohol use is characterized by a shift of cue processing from ventral to dorsal striatum. Addiction. 2010;105:1741–9. doi: 10.1111/j.1360-0443.2010.03022.x. [DOI] [PubMed] [Google Scholar]
- Vollstadt-Klein S, Loeber S, Kirsch M, Bach P, Richter A, Buhler M, von der Goltz C, Hermann D, Mann K, Kiefer F. Effects of cue-exposure treatment on neural cue reactivity in alcohol dependence: a randomized trial. Biol Psychiatry. 2011;69:1060–6. doi: 10.1016/j.biopsych.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Weiss F, Porrino LJ. Behavioral neurobiology of alcohol addiction: recent advances and challenges. J Neurosci. 2002;22:3332–7. doi: 10.1523/JNEUROSCI.22-09-03332.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt J, Weike AI, Lotze M, Hamm AO. The functional connectivity between amygdala and extrastriate visual cortex activity during emotional picture processing depends on stimulus novelty. Biol Psychol. 2011;86:203–9. doi: 10.1016/j.biopsycho.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Wetherill RR, Bava S, Thompson WK, Boucquey V, Pulido C, Yang TT, Tapert SF. Frontoparietal connectivity in substance-naive youth with and without a family history of alcoholism. Brain Res. 2012;1432:66–73. doi: 10.1016/j.brainres.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. The neurobiology of craving: implications for the understanding and treatment of addiction. J Abnorm Psychol. 1988;97:118–32. doi: 10.1037//0021-843x.97.2.118. [DOI] [PubMed] [Google Scholar]
- Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36:229–40. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- Yalachkov Y, Kaiser J, Naumer MJ. Functional neuroimaging studies in addiction: multisensory drug stimuli and neural cue reactivity. Neurosci Biobehav Rev. 2012;36:825–35. doi: 10.1016/j.neubiorev.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Yoshimoto K, McBride WJ, Lumeng L, Li TK. Ethanol enhances the release of dopamine and serotonin in the nucleus accumbens of HAD and LAD lines of rats. Alcohol Clin Exp Res. 1992;16:781–5. doi: 10.1111/j.1530-0277.1992.tb00678.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


