Abstract
Objectives
To determine empirically the diseases contributing most commonly and strongly to death in older adults, accounting for coexisting diseases.
Design
Longitudinal
Setting
United States
Participants
Twenty two thousand eight hundred ninety Medicare Current Beneficiary Survey participants, a national representative sample of Medicare beneficiaries, enrolled during 2002 – 2006.
Measurements
Chronic and acute diseases were ascertained from Medicare claims data. Diseases contributing to death during follow-up were identified empirically via regression models among all diseases with a frequency of ≥ 1% and hazard ratio for death of > 1. The additive contributions of these diseases, adjusting for co-existing diseases, were calculated using a longitudinal extension of average attributable fraction; 95% confidence intervals were estimated from bootstrapping.
Results
Fifteen diseases and acute events contributed significantly to death, together accounting for nearly 70% of death. Heart failure (20.0%), dementia (13.6%), chronic lower respiratory disease (12.4%), and pneumonia (5.3%) made the largest contributions to death. Cancers, including lung, colorectal, lymphoma, and head and neck, together contributed to 5.6% of death. The other disease and events included acute kidney injury, stroke, septicemia, liver disease, myocardial infarction, and unintentional injuries.
Conclusion
The extent of the contribution of some diseases such as dementia and respiratory disease to death in older adults may be underappreciated, while the contribution of other diseases may be overestimated, with methods that focus on determining a single underlying cause. Current conceptualization of a single underlying cause may not account adequately for the contribution to death of coexisting diseases experienced by older adults.
Keywords: death, coexisting diseases, multiple chronic conditions
INTRODUCTION
Most deaths in developed countries occur in persons over the age of 65 years who have multiple coexisting diseases.1–3 Accurate assessment of the contribution of coexisting diseases to death is needed to measure the burden and effect of diseases in the aging population.4–6
To determine cause of death in the United States, the National Center for Health Statistics (NCHS) and National Vital Statistics System (NVSS) use the World Health Organization (WHO) algorithmic decision rules and adjudication applied to death certificate data.7–9 For the purpose of mortality statistics, every death is attributed to one underlying condition.9 This attribution is used both at the person level to assign cause(s) of death and at the population level to determine the relative and absolute frequencies of causes.1,10–13 The condition listed on the last line of the death certificate usually is accepted as the underlying cause of death, although the automated decision rules may derive a different underlying cause.9
In addition to ascertaining the underlying cause the current system used by the Centers for Disease Control and Prevention (CDC) also produces data on multiple causes of death.9,14–15 This system, however, considers each disease separately. The proportion of deaths attributed to these co-existing diseases currently is not determined.2
As has been well chronicled, death certificates may be inaccurate or incomplete, often leading investigators to adjudicate cause of death from health record data.16–18 While expanding the clinical information used, this approach remains driven by decision rules for assigning a single cause among all the conditions present. These rules are influenced by custom and precedence. The contribution of dementia and injury, for example, have been underestimated.2,16,19,20 Conversely, decision rules and custom encourage citing other disease categories, such as cancer and cardiovascular diseases, as the underlying cause, often at the exclusion of coexisting diseases.2,9,21–23 Algorithmic decision rules, therefore, may both over- and under-estimate the contribution of some diseases. Even when fully reported, the assumption of a single underlying cause, while reasonable for individuals with a single disease or little co-morbidity, may not be appropriate for older adults with co-existing diseases.
To determine empirically the contribution of coexisting chronic and acute diseases to death among older adults, we applied a longitudinal extension of the average attributable fraction method to Medicare claims data that accounted for interrelationships among co-existing diseases and for timing of disease onset relative to death.
METHODS
Study Population
The study population included participants in the Medicare Current Beneficiary Survey (MCBS). The MCBS sample is drawn from The Center for Medicare and Medicaid Services (CMS) Medicare enrollment file and, with use of CMS-provided weights, is statistically representative of the national Medicare population.24,25 Persons over 85 years old are over-sampled. The 22,890 participants at least 65 years old who had at least one interview between 2002 and 2006 and who did not belong to a health maintenance organization (HMO) were included. The 3,923 HMO members were excluded because they lacked health claims which were used to ascertain diseases. The Access to Care and Cost and Use files were used. Baseline was enrollment in the cohort. Participants were followed until death or end of follow-up which was up to 41 months. The protocol was approved by the Yale University institutional review board.
Data
Chronic and acute diseases were ascertained from hospital, outpatient, physician, and skilled nursing facility Medicare claims data. Claims, which include International Classification of Diseases, Clinical Modification (ICD-9 CM) codes, are submitted by physicians and other health care providers to get reimbursement for their services. Up to 10 ICD-9 CM codes were available for each hospitalization.26 All disease claims were assigned to a single level Clinical Classification Software (CCS) code based on the ICD-9 CM codes.27 Only codes relating to symptoms; physical, laboratory or imaging findings; and conditions relevant to pregnancy or birth were removed. All other conditions and diseases were included. When appropriate, clinically identical or similar disease codes were combined, resulting in 97 chronic diseases and conditions and 36 acute diseases and events. To avoid counting a condition twice, chronic conditions such as COPD and heart failure, which have acute exacerbations, were only counted as chronic conditions, not as both acute and chronic conditions. Medicare claims data were available beginning nine months before enrollment. Chronic diseases reported in claims during these nine months were considered prevalent. Otherwise, chronic diseases were assigned onset coincident with the first claim after enrollment in the cohort. Deaths were ascertained from Medicare vital status files.
Statistical Analysis
MCBS yearly weights24 were averaged for each participant. Chronic diseases reported in claims data prior to the baseline were included as time-constant variables; chronic diseases first reported after baseline were time-constant after onset. Acute diseases were accounted for on a monthly basis and assigned one, three or six months duration based on empiric observation of their association with death. To avoid over-counting, at least nine months between claims were required to count as a recurrence of an acute disease.
All statistical tests, including main effects and two factor interactions, were two-sided with P < 0.05 indicative of statistical significance. Analyses were performed using SAS (version 9.2), SUDAAN (version 10), and MATLAB® (version R2009A).
To estimate the contribution to death of diseases accounting for the effects of coexisting diseases, we followed a two step approach. The first step fit a multivariable model with main effects and significant two factor interactions. The second step used that model to calculate conditional probabilities in order to provide an overall average effect of each disease that accounted for all its longitudinal combinations with other diseases. Candidate diseases were all diseases with occurrence (present at baseline plus onset after baseline) of ≥1% and significant bivariate association with death in a Cox model. For pairs of diseases with significant association with death and correlations >.20, the disease with the stronger association with death was selected.28 All diseases selected were then entered concurrently into a multivariable model and diseases maintaining significance in the multivariable models were retained. All two factor interactions between diseases showing significance in the multivariable model were tested and those retaining significance in a forward selection Cox model were kept. The coefficients from a pooled logistic regression of retained diseases and significant interactions then were used to calculate average attributable fraction (AAF).29 The pooled logistic model, which can be calculated from a simpler data structure, is equivalent to a Cox model when each unit of time is short (i.e., one month) and the probability of the outcome (i.e., death) within each unit is small.
The AAF was extended for longitudinal data to estimate the additive and unordered contribution of each disease to the occurrence of death.30–32 Precision of the longitudinal AAFs was estimated by generating bootstrap samples. Longitudinal AAFs can be interpreted as the average, longitudinal proportion of deaths attributed to the disease. Although additive, accounting for interrelationships among diseases ensures that AAFs do not sum to over 100 percent. To estimate the diseases contributing to death according to age and gender, we employed the same techniques described for the entire cohort for the subgroups <80 vs. 80+years and men vs. women.
RESULTS
The 22,890 MCBS cohort members were an average of 76.4 (±·06) years old (range 65 –111 years); 57.4% were female. Participants were followed for a median of 15 months (range, 1 to 41; interquartile range, 4 to 28). The cohort included 12.8% individuals who were nonwhite; 6.1% were of Hispanic ethnicity. A total of 2,445 participants (11%) died. Of these deaths, 748 occurred in individuals 65 to 79 years and 1697 in those ≥ 80 years old; 1,407 of the deaths were in females and 1,038 were in males.
As displayed in Table 1, 39 chronic diseases had a prevalence of ≥1% and a significant bivariate hazard ratio for death of >1. The chronic diseases with the strongest bivariate association with death included cancers of the liver, pancreas, lung, and bone; heart failure; dementia; psychotic disorders; and chronic lower respiratory disease, all with hazard ratios of ≥ 3·5.
Table 1.
Frequency of Chronic Diseases and Bivariate Hazard Ratios for Death in the MCBS Cohort (N = 22890)a
| Diseaseb | Present at Baseline |
Onset after Baseline |
Hazard Ratio c |
|---|---|---|---|
| Chronic Diseases | n (%) | n (%) | (95%CI) |
| Malignancy/Cancer | |||
| Lung | 238 (1.0) | 172 (0.8) | 7.5 (6.2–9.0) |
| Liver, pancreas | 68 (0.3) | 60 (0.3) | 7.2 (5.5–9.5) |
| Bone, connective tissue | 75 (0.3) | 64 (0.3) | 4.3 (3.0–6.1) |
| Head, neck | 160 (0.7) | 85 (0.3) | 3.0 (2.3–3.8) |
| Lymphoma | 164 (0.7) | 69 (0.3) | 2.9 (2.2–3.8) |
| Leukemia | 111 (0.5) | 55 (0.2) | 2.4 (1.7–3.5) |
| Colorectal | 393 (1.6) | 174 (0.7) | 2.2 (1.8–2.6) |
| Renal | 84 (0.4) | 39 (0.2) | 2.2 (1.4–3.4) |
| Bladder | 226 (0.9) | 75 (0.3) | 1.9 (1.4–2.6) |
| Prostate | 993 (4.2) | 248 (1.1) | 1.5 (1.3–1.7) |
| Breast | 581 (2.6) | 161 (0.7) | 1.3 (1.0–1.6) |
| Circulatory/Cardiovascular | |||
| Heart failure | 3416 (13.2) | 1596 (6.4) | 5.1 (4.7–5.5) |
| Peri-, endo-, myocarditis | 964 (4.0) | 615 (2.6) | 3.0 (2.6–3.3) |
| Pulmonary heart disease | 565 (2.3) | 475 (2.0) | 3.0 (2.7–3.4) |
| Cardiac dysrhythmias | 5246 (21.4) | 2227 (9.6) | 2.9 (2.7–3.2) |
| Peripheral, visceral | 3912 (15.7) | 2005 (8.6) | 2.3 (2.1–2.6) |
| Coronary atherosclerosis | 6262 (25.9) | 1805 (7.8) | 2.1 (1.9–2.2) |
| Cerebrovascular | 2989 (12.0) | 1712 (7.2) | 2.1 (1.9–2.3) |
| Heart valve disorders | 3114 (13.0) | 1817 (7.8) | 2.0 (1.8–2.2) |
| Other heart disease | 1335 (5.5) | 807 (3.4) | 1.9 (1·7–2·2) |
| Conduction disorders | 928 (3.7) | 664 (2.7) | 2.0 (1.7–2.2) |
| Hypertension | 14369 (61.0) | 2190 (9.7) | 1.3 (1.2–1.4) |
| Respiratory/Lung | |||
| Interstitial, other lung disease | 1835 (7.6) | 1400 (5.9) | 3.6 (3.3–3.9) |
| COPD | 3688 (15.3) | 1496 (6.3) | 2.9 (2.7–3.1) |
| Asthma | 1381 (6.1) | 625 (2.8) | 1.3 (1.1–1.5) |
| Digestive/Gastrointestinal | |||
| Hepatitis, liver | 1415 (6.3) | 1016 (4.6) | 2.1 (1.8–2.3) |
| Biliary tract | 640 (2.7) | 557 (2.4) | 1.9 (1.6–2.2) |
| UGI disorders | 4498 (18.7) | 2669 (11.4) | 2.6 (2.4–2.9) |
| Abdominal hernia | 1385 (5.9) | 980 (4.3) | 1.3 (1.1–1.4) |
| Genitourinary/Endocrine | |||
| Nephritis, chronic renal failure | 974 (3.8) | 658 (2.6) | 3.3 (2.9–3.6) |
| Diabetes | 5680 (24.3) | 1192 (5.2) | 1.4 (1.3–1.5) |
| Hematologic | |||
| Deficiency, other anemias | 5101 (20.5) | 2132 (9.0) | 3.0 (2.7–3.4) |
| Coagulation, hemorrhagic disorders | 1015 (4.2) | 695 (3.0) | 2.8 (2.4–3.2) |
| Connective tissue | |||
| Other CTDs | 7458 (31.3) | 3009 (13.2) | 1.4 (1.3–1.6) |
| RA, Lupus | 907 (4.0) | 356 (1.6) | 1.1 (0.9–1.3) |
| Neurologic | |||
| Epilepsy | 605 (2·.) | 304 (1.3) | 3.0 (2.6–3.5) |
| Parkinson disease | 446 (1.7) | 143 (0.5) | 2.4 (2.0–3.0) |
| MS, degenerative CNS disorders | 1187 (4.8) | 846 (3.5) | 2.3 (2.0–2.6) |
| Other nervous system disorders | 3852 (16.0) | 2062 (8.8) | 1.8 (1.7–2.0) |
| Psychiatric/Cognitive | |||
| Dementia | 1907 (6.4) | 579 (2.3) | 4.1 (3.7–4.6) |
| Schizophrenia, other psychotic disorders | 816 (3.0) | 481 (1.8) | 4.1 (3.6–4.7) |
| Alcohol, substance abuse | 239 (1.0) | 153 (0.7) | 2.4 (1.9–3.1) |
| Mood disorders, depression | 2359 (9.5) | 950 (4.0) | 2.1 (2.0–2.4) |
| Anxiety disorders | 1332 (5.6) | 751 (3.3) | 1.5 (1.4–1.8) |
Abbreviations: MCBS, Medicare Current Beneficiary Survey; HR, hazard ratio; CI, confidence intervals; CTD, connective tissue diseases; CNS, central nervous system; COPD, chronic obstructive pulmonary disease; GI, gastrointestinal; MS, multiple sclerosis; RA, rheumatoid arthritis
Analyses included participants from 2002–2006. Numbers are unweighted; the percentages and incidence rates are weighted. Chronic diseases with a weighted prevalence (present at baseline) plus cumulative incidence (onset after baseline) of ≥ 1% for which the hazard ratio was >1 and the 95% CI excluded 1 are included in the Table.
Other chronic diseases with hazard ratio >1 but prevalence of less than 1% included UGI cancer, brain cancer, and multiple myeloma.
Hazard Ratio is for the overall occurrence of the disease (i.e. present at baseline plus onset after baseline).
Twenty-four acute diseases and events were significantly associated with death in bivariate analysis (Table 2). Septicemia, acute kidney injury (AKI), myocardial infarction (MI), pneumonia, acute intestinal events, gangrene, stroke, hip fracture, bacterial infections, hemorrhage, syncope, traumatic head injury, and other unintentional injury had incident hazard ratios ≥ 3·5.
Table 2.
Frequency of Diseases and Bivariate Hazard Ratios for Death in the MCBS Cohort (N = 22890)a
| Acute Diseases and Eventsb | No. Events (No. persons) |
Incidence rate (per 1000 person years) |
Hazard ratio (95%CI) |
|---|---|---|---|
| Infections | |||
| Septicemia | 1083 (859) | 30.1 | 13.8 (12.2–15.6) |
| Pneumonia, Influenza | 3690 (2711) | 105.7 | 8.1 (7.3–9.1) |
| Bacterial infection | 1343 (1009) | 38.7 | 4.8 (3.9–5.9) |
| STI | 67 (65) | 2.2 | 3.1 (0.8–11.9) |
| Other infection | 331 (246) | 9.8 | 2.8 (1.7–4.5) |
| Meningitis, encephalitis, CNS infection | 118 (93) | 3.7 | 2.6 (1.1–6.4) |
| Infective arthritis, osteomyelitis | 383 (272) | 12.2 | 2.4 (1.6–3.8) |
| Acute hemorrhage | 762 (590) | 23.0 | 4.0 (3.0–5.3) |
| Circulatory / Cardiovascular | |||
| Myocardial infarction | 1161 (901) | 34.2 | 8.3 (7.1–9.8) |
| Gangrene | 221 (167) | 6.6 | 6.1 (4.1–9.0) |
| Acute stroke | 3066 (2084) | 87.4 | 5.4 (4.8–6.1) |
| Syncope | 1798 (1682) | 53.0 | 3.6 (2.8–4.8) |
| Thromboembolism | 1243 (908) | 37.4 | 3.2 (2.5–4.1) |
| Digestive/Gastrointestinal | |||
| Intestinal infection, Gastroenteritis | 1549 (1182) | 47.0 | 2.4 (1.8–3.0) |
| Intestinal obstruction, abscess, peritonitis | 936 (741) | 28.1 | 6.9 (5.6–8.4) |
| G.I. hemorrhage | 2752 (2054) | 84.4 | 3.2 (2.7–3.7) |
| Genitourinary | |||
| Acute kidney injury | 1778 (1275) | 51.2 | 9.4 (8.2–10.7) |
| Urinary tract infections | 7472 (4853) | 223.0 | 2.6 (2.3–2.9) |
| Unintentional injuries | |||
| Hip fracture | 707 (517) | 19.2 | 5.3 (4.2–6.6) |
| Traumatic head injury | 512 (391) | 14.8 | 5.2 (3.8–7.2) |
| Other injury | 6097 (4276) | 180.6 | 3.6 (3.1–4.0) |
| Fall | 1911 (1439) | 54.2 | 3.1 (2.5–3.8) |
| Complications of medical or surgical care | 4215 (2924) | 131.7 | 3.0 (2.6–3.5) |
| MVA | 132 (107) | 4.3 | 2.3 (1.0–5.2) |
| Fractures other than hip | 2428 (1788) | 71.1 | 2.2 (1.8–2.7) |
Abbreviations: MCBS, Medicare Current Beneficiary Survey; HR, hazard ratio; CI, confidence intervals; MVA, Motor vehicle accident; STI, sexually transmitted infection; UGI, upper gastrointestinal
Analyses included participants from 2002–2006. Numbers are unweighted; the percentages and incidence rates are weighted. Acute diseases and events are only counted once regardless of number of claims until there has been at least 9 months between claims at which time another episode of the acute disease is recorded (see Methods). All acute diseases and events for which the hazard ratio was >1 and the 95% CI excluded 1 are included in the Table.
The only acute event with hazard ratio >1 but prevalence of less than 1% was spinal cord injury.
The contributions to death of the 15 chronic and acute diseases according to multivariate analyses are listed in Table 3. Together these diseases contributed to 67.6% of deaths. Accounting for the other diseases singly and in combination, heart failure (20.0%), dementia (13.6%), and chronic lower respiratory disease (12.4%) contributed the greatest proportions to death.
Table 3.
Diseases Contributing to Death in the MCBS Cohort by the Empiric Methoda
| Disease / Condition | Proportional Contribution Percent (95% CI)b |
|---|---|
| Heart failure | 20.0 (17.9–22.2) |
| Dementia | 13.6 (12.1–15.2) |
| Chronic lower respiratory disease c | 12.4 (10.4–14.9) |
| Pneumonia, influenza | 5.3 (4.5–6.3) |
| Lung cancer | 4.1 (3.3–5.2) |
| Acute kidney injury | 3.3 (2.7–4.1) |
| Stroke | 2.4 (2.0–2.9) |
| Septicemia | 1.4 (1.1–1·9) |
| Liver disease, hepatitis | 1.2 (0.5–2.5) |
| Myocardial infarction | 1.1 (0.7–1.4) |
| Colorectal cancer | 0.6 (0.2–1.1) |
| Unintentional injuries d | 0.6 (0.3–0.9) |
| Acute intestinal events (obstruction, peritonitis, abscess) | 0.6 (0.3–0.9) |
| Lymphoma | 0.5 (0.2–0.7) |
| Head and neck cancer | 0.4 (0.1–0.7) |
| Total | 67.6 (66.0–69.4) |
Medicare Current Beneficiary Survey participants 2002–2006. Diseases identified by claims data. Process for selecting these diseases described under Statistical Analysis.
Proportional contribution determined using longitudinal extension of the average attributable fraction; 95% confidence intervals were estimated by generating 100 bootstrap samples. The proportions reported here include the direct effect of each disease plus the effect of significant interactions between pairs of diseases, e.g. between lung cancer and heart failure or between acute cardiovascular disease and dementia. In no case did the combination of two diseases account for a higher proportion of deaths than the effects of the individual diseases added together (i.e. no interactions were synergistic).
Chronic lower respiratory disease included chronic obstructive pulmonary disease, interstitial lung disease, and other chronic lower respiratory disease.
Unintentional injuries included only hip fracture and head trauma.
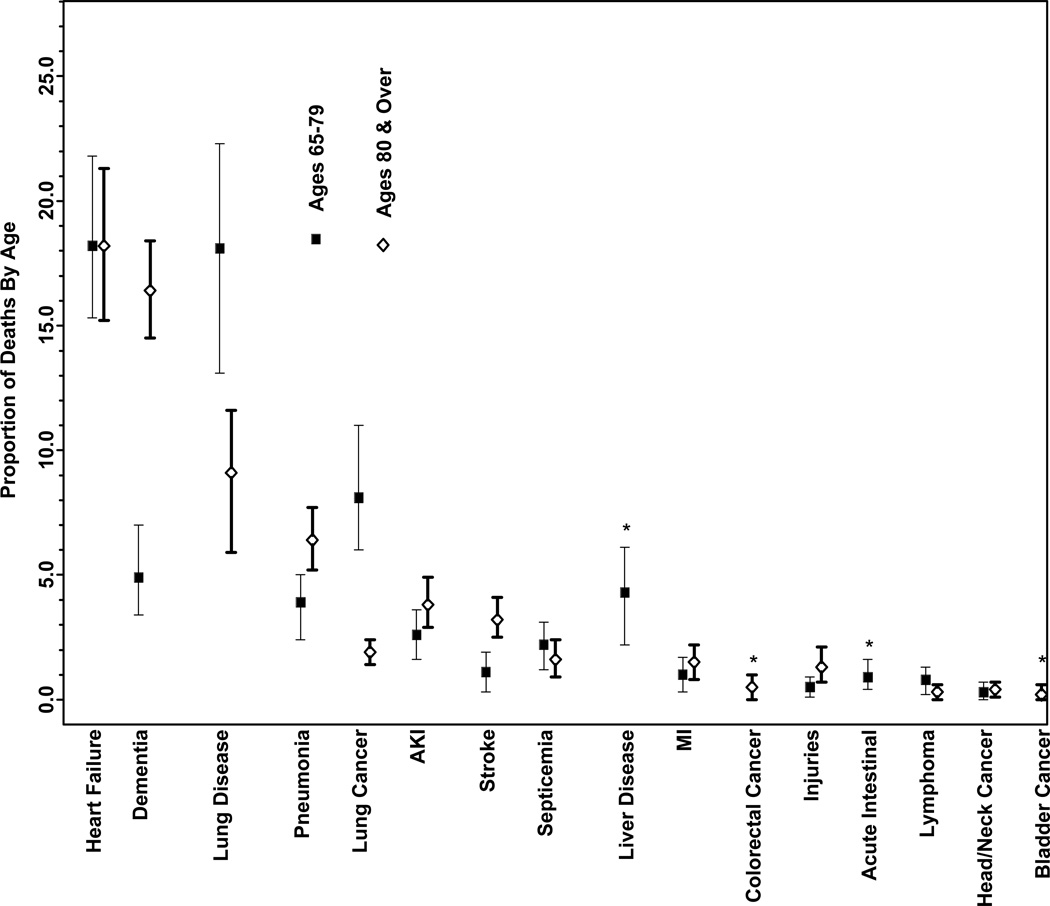
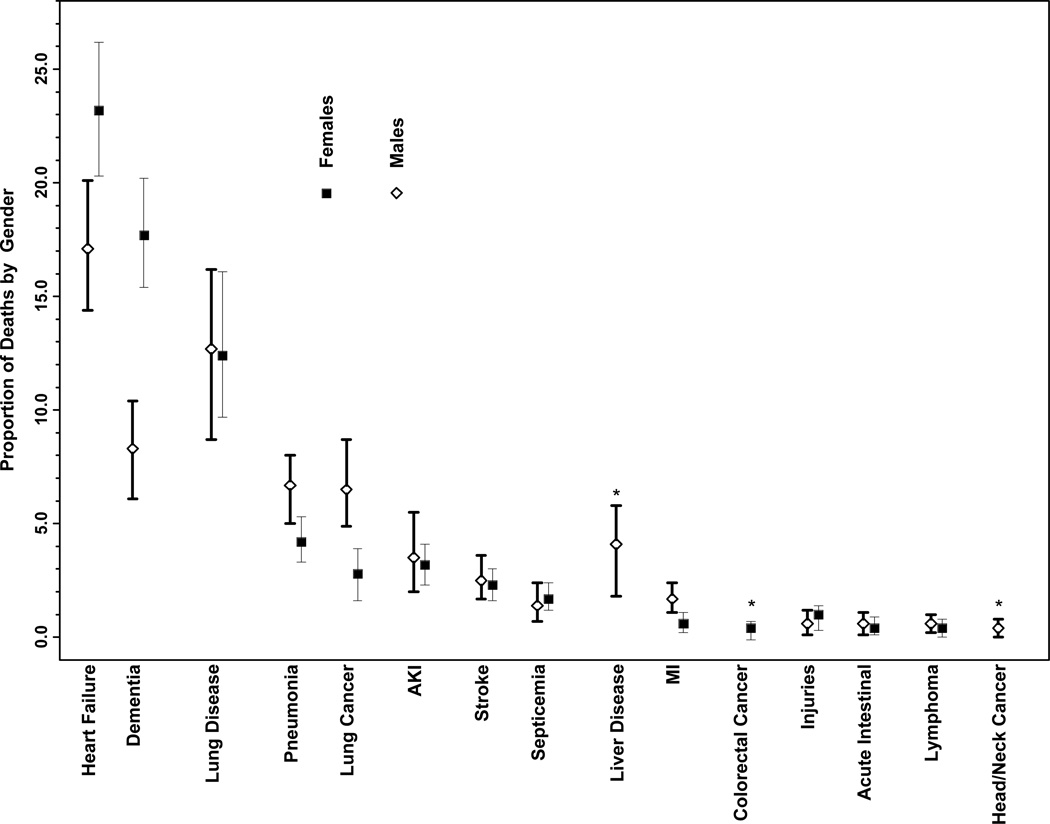
The diseases contributing to death according to age and gender are displayed in the Figure. Bladder cancer emerged as a significant contributor (0.2%) only among those ≥80 years as did liver disease (4.3%) and acute intestinal events (0.9%) only among those 65–79 years old. Dementia contributed a greater proportion to deaths among those ≥ 80 years old (16.4%) than those 65–79 years old (4.9%). Chronic lower respiratory disease was a greater contributor among those 65–79 years old (18.1%) than among those ≥80 years old (9.1%) as was lung cancer (8.1% among those 65–79 years old and 1.9% among those ≥80 years old). The other diseases were similar between age strata. The diseases contributing significantly to death were similar among men and women, with a few exceptions. The proportion of death attributed to dementia was higher among females (17.7%) than males (8.3%). Heart failure also contributed to a slightly higher proportion of death among females (23.2%) than males (17.1%) while lung cancer contributed to a higher proportion of death among men (6.5%) than women (2.8%).
Figure 1. The Proportional Contribution of Diseases to Death by an Empiric Method Among Older Adults According to Age and Gender.
The proportional contribution of diseases to death among women and men and among individuals <80 years and 80+ years was determined empirically from claims data for the Medicare Current Beneficiary Survey 2002–2006 cohort using longitudinal extension of the average attributable fraction; 95% confidence intervals were estimated by generating 100 bootstrap samples.
Abbreviations: AKI, acute kidney injury; MI, myocardial infarction
* The disease contributed significantly to death only in one age group or gender.
DISCUSSION
We applied an empiric approach to delineating the contribution of diseases to death among older adults that accounted for the individual and interacting effects of coexisting diseases. Chronic diseases together accounted for 52%, and acute diseases for 15%, of deaths, supporting the predominant importance of chronic disease in older adults. The diseases contributing to death were similar between men and women and among older adults of all ages, with a few exceptions. As expected, a higher proportion of deaths were attributed to dementia among older, than younger, individuals and among women than men.18,33 Chronic lower respiratory disease and lung cancer contributed more among younger, than older, persons; the latter disease also contributed more among men than women. The slightly higher proportion of deaths attributed to heart failure in women than men corroborates recent reports.34
While we did not have “single underlying cause” data for participants who died, the causes should be quite similar to those reported by the CDC for this age group because MCBS is a nationally representative cohort. Direct comparison is not possible because the categories of diseases are not always identical, as noted below for cardiovascular disease and cancer. Nevertheless, many of the diseases and their proportions are similar to CDC reports of the 10 most common causes of death in persons 65 years and older, although the proportions are discrepant for some diseases. Using the algorithmic rules and adjudication of death certificate data, the CDC determined that the leading causes of death in persons 65 years and older in the U.S. in 2004 were cardiovascular (30.4%), cancer (22%), cerebrovascular/stroke (7.4%), chronic lower respiratory disease (6%), Alzheimer/dementia (3.7%), diabetes (3.1%), pneumonia/influenza (3.0%), kidney (2.0%), unintentional injury (2%), and septicemia (1.5%).15 Diabetes is the only disease included in the CDC report that was not included in our list while liver disease and acute intestinal events were included in our method but are not leading causes by CDC methods.
Similar to CDC reports,15 cardiovascular disease was the leading cause of death in the current analysis, although the proportional contribution was lower. Heart failure was the predominant cardiovascular contributor to death in our analysis. Direct comparison with CDC results for cardiovascular diseases is not possible because CDC focus is on presumed etiological classifications. For example, heart failure would not be reported as a cause of death but would likely be classified by presumed underlying causes such as ischemic heart disease or hypertensive heart disease by the CDC. The accuracy of these presumed etiologies are unclear, particularly in older adults with multiple possible etiological causes. Of note, only one percent of death was attributed to acute myocardial infarction (MI), supporting recent reports of reduced MI fatality rates.35
Cancers, including lung, colorectal, lymphoma, and head and neck, together contributed to 5.6% of death, lower than the 22% of deaths reported by the CDC.15 We included only cancers with an increased risk of death in our initial analytical steps and with a frequency of at least 1%, perhaps partially explaining the discrepancy with CDC results. However, because we included all but the most uncommon cancers with increased risk of death, this is unlikely the entire explanation. Previous investigations have revealed a higher rate of coexisting conditions in older adults with, than without, cancer and have shown that mortality in persons with cancer is partially related to coexisting diseases.36,37 In the current analyses, the bivariate association of cancer with death was reduced by inclusion of coexisting diseases in the multivariable model. LE-AAF determines an overall average effect for each disease that reflects adjustment for all the existing combinations of other diseases. The lower proportion of death attributed to cancers by the empiric method supports observations of preferential reporting of cancer as the single underlying cause of death in the algorithmic method that does not account for the effect of coexisting conditions.22,23 An accurate determination of cancer to death in older adults requires further investigation.
Conversely, our empiric approach suggested that the contribution of chronic lower respiratory disease may be underestimated. While recognized by the CDC as the fourth leading cause of death,15 the 12.4% attributed to chronic lower respiratory diseases by our empiric method was twice the CDC estimate.
Dementia had the second highest contribution to death, accounting for 13·6% of deaths in the cohort, a proportion similar to the 10.6% reported by Ives who used clinical data beyond that included in the death certificate.16 The contribution of dementia to death is likely still underestimated by current cause of death methods.16,19,21 From the current analysis we cannot determine whether dementia directly led to death, accelerated death from other diseases, or resulted in less aggressive treatment of coexisting conditions. Regardless of the reasons, dementia was a potent contributor to death.
Diabetes, the sixth leading cause of death in the CDC report15 was not a contributor in the empiric method. The lack of contribution was partially explained by expected high correlation between diabetes and other diseases, most notably cardiovascular and renal diseases. Correlation with other diseases, however, was unlikely the total explanation. In bivariate analysis, the hazard ratio for diabetes was 1.4, lower than for many other diseases. Diabetics who survive to older ages may have less lethal disease than younger persons. Due to changing definitions and better surveillance,39 some persons who carry the diagnosis may be less ill than earlier cohorts of diabetics. These findings in an older population do not negate concerns about the adverse effects of the obesity-related increase in diabetes among younger populations.
Pneumonia, septicemia, renal disease, and unintentional injuries are all included in CDC leading causes of death for the older population as well as in our empiric approach.15 The estimates of the proportion of deaths contributed by these acute diseases and events were similar with the two methods except for unintentional injury which accounted for 2% of deaths according to the CDC and only 0.6% by our method.
This study has strengths and well as limitations. The MCBS is nationally representative of the fee-for-service Medicare population. We cannot determine whether results generalize to Medicare HMO members who lacked claims data.
Claims data provide a more complete ascertainment of diseases than those included on death certificates and thorough reporting of coexisting conditions is necessary to empirically determine contributors to death. Claims data, however do have limitations.40–42 Diagnosis codes in claims are assigned to obtain reimbursement; conditions that provide more lucrative reimbursement receive priority.40,41 Studies comparing claims and medical records generally show good agreement although comorbid conditions are underreported in claims databases suggesting we may have underestimated the contribution of some diseases.41,42
Our empiric method for determining the contribution of diseases to death has both advantages and disadvantages. Tracking diseases over time in the entire population of individuals who survived or died avoided the bias inherent in ascertaining diseases only in those who died. Our method accounted to some extent for the effect of disease duration. We had only nine months of claims prior to the baseline, however, so that we could not account for prior duration. Our analytical method addressed the critical limitation of attributable fractions of not accounting for interrelationships among diseases, thus allowing them to sum to over 100%.30–32 The AAF has a distinctive ability to accommodate a high number of coexisting diseases, crucial to determining the contribution of diseases to deaths in older adults, most of whom have multiple coexisting diseases. Because highly correlated diseases could not be included together, we may have missed the contribution of highly correlated diseases. On the other hand, ascribing death to several correlated diseases may overestimate their contributions. Indeed, the high correlations across supposedly separate diseases raise questions about current classification of diseases.
The empiric method “explained” 68% of death in the older population, a similar amount attributed to the 10 leading causes of with the method used by the CDC.15 Clearly the 15 diseases in Table 3 do not explain all deaths. Diseases with a prevalence of less than 1% likely contributed to death, but were not considered. Whereas both stages of our modeling exhibited good fit, statistical models never explain all the variation in an outcome.
This empiric method applies only to the population level; results do not address diseases contributing to death in individuals. Furthermore, this method identifies associations of diseases with death but cannot establish causality.
We lacked data on the official cause of death for cohort members so can not compare these causes with our findings. Determining exact cause of death can be difficult and misleading even with a wealth of clinical data, including autopsy, particularly for frail and elderly individuals.22,44 Comparison of a careful review of all available data with the officially assigned cause of death in a group of older adults showed many discrepancies. For example, “ill-defined” was the cause in 22.7% of decedents according the clinical review only 4% of whom had this listed as the official cause.44 Cardiovascular was the most frequently assigned official cause in these individuals, a cause not well supported by the clinical data in these individuals.
The current approach used by the WHO and the CDC works well with individuals with a single or few diseases in whom the cause of death is reliably determined and clinical judgment and the decision rules and algorithms are very likely to coincide. Decision rules and algorithms, and even clinical judgment, are inherently more subjective, however, when trying to allocate death to one of several co-existing conditions. The CDC recognizes the importance of reporting multiple causes of death but does not yet assess their relative contribution.15 Methods that determine the contribution of a disease while accounting for the contribution of coexisting diseases would better align CDC and WHO methods with current clinical reality. Such methods could be used at the population-level, as in the current study, and, once developed, at the person-level. Assuming acute and chronic diseases are entered accurately and completely, the use of an empiric method with electronic health record (EHR) data might allow real time determination of the contribution of co-existing diseases of death.
Other alternatives to assigning an underlying disease as a cause of death in older adults have been proposed. For example, investigators have shown that most deaths among older adults have been shown to follow one of four trajectories, namely sudden death, terminal illness such as from cancer, organ failure, and frailty.43 Empirically determining the contribution of multiple diseases complements these other approaches.
Death in older adults results from the accumulated effect of coexisting diseases, a fact not accurately reflected by current methods. The contribution of some diseases may be overestimated, and others underestimated, with approaches that focus on identifying a single underlying cause. Given the difficulty in disentangling the effect of multiple conditions experienced by older adults, the concept of conditions contributing to death may be more appropriate than the current concept of single underlying cause of death. An accurate, empiric method for determining the proportional contribution of multiple diseases is needed to measure the burden of disease and establish public health, clinical, and research priorities for the aging population.
ACKNOWLEDGMENTS
This study was supported by RO1 AG030109 and the Yale Pepper Center (P30 AG021342) from the National Institute on Aging. Results were calculated using the Yale University Biomedical High Performance Computing Center, whose instrumentation was funded by RR19895 from the National Institutes of Health.
We thank Nicholas Carriero, Ph.D. for providing guidance with MATLAB® coding and the development of batch scripts
Sponsor’s Role: None.
Funding: This study was supported by RO1 AG030109 and the Yale Pepper Center (P30 AG021342) from the National Institute on Aging. Results were calculated using the Yale University Biomedical High Performance Computing Center, whose instrumentation was funded by RR19895 from the National Institutes of Health.
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Authors’ Contributions:
All authors contributed to (1) the conception and design, or acquisition of data or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, and (3) final approval of the version to be published.
Mary E. Tinetti, M.D. 1, 2, 3
Terrence E. Murphy, Ph.D. 1, 2, 3
Cary P. Gross, M.D. 2, 3
Haiqun Lin, M.D. 1, 2, 3
Heather G. Allore, Ph.D. 1, 2, 3
REFERENCES
- 1.Hoyert DL, Kung HC, Smith BL. Deaths: preliminary data for 2003. Natl Vital Stat Rep. 2005;53:1–48. [PubMed] [Google Scholar]
- 2.Wall MM, Huang J, Oswald J, et al. Factors associated with reporting multiple causes of death. BMC Med Res Methodol. 2005;5:4. doi: 10.1186/1471-2288-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Israel RA, Rosenberg HM, Curtin LR. Analytical potential for multiple cause-of-death data. Am J Epidemiol. 1986;124:161–179. doi: 10.1093/oxfordjournals.aje.a114375. [DOI] [PubMed] [Google Scholar]
- 4.Gross CP, Anderson GF, Powe NR. The relation between funding by the National Institutes of Health and the burden of disease. N Engl J Med. 1999:340, 1881–1887. doi: 10.1056/NEJM199906173402406. [DOI] [PubMed] [Google Scholar]
- 5.Lopez AD, AbouZahr C, Shibuya K, et al. Keeping count: births, deaths, and causes of death. Lancet. 2007;370:1744–1746. doi: 10.1016/S0140-6736(07)61419-6. [DOI] [PubMed] [Google Scholar]
- 6.Ezzati M, Lopez AD, Rodgers A, et al. the Comparative Risk Assessment Collaborating Group. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–1360. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- 7.Manual for coding cause of death. [Accessed September 10, 2010]; at www.cdc.gov/nchs/data/dvs/2a2005a.pdf.
- 8.World Health Organization. 10th ed. Geneva: World Health Organization; 1992. International statistical classification of disease and related health problems. [Google Scholar]
- 9.Gorina Y, Lentzer H. Multiple causes of death in old age. Aging Trends. 2008;9:1–9. [PubMed] [Google Scholar]
- 10.Mortality data from the National Vital Statistics System. [Accessed September 10, 2010]; at http://cdc.gov/nchs/deaths.htm. [PubMed]
- 11.Janssen F, Kunst AE. ICD coding changes and discontinuities in trends in cause-specific mortality in six European countries, 1950–99. Bull World Health Org. 2004;82:904–913. [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson RN. Deaths: Leading causes for 2000. Natl Vital Stat Rep. 2002;50:1–85. [PubMed] [Google Scholar]
- 13.Jemal A, Ward E, Hao Y, et al. Trends in the leading causes of death in the United States, 1970–2002. JAMA. 2005;294:1255–1259. doi: 10.1001/jama.294.10.1255. [DOI] [PubMed] [Google Scholar]
- 14.National Center for Health Statistics. Mortality data, multiple-cause-of-death public-use data files. [Accessed September 10, 2010]; at http://www.cdc.gov/nchs/products/elec_prods/subject/mortmcd.htm.
- 15.Heron MP. Deaths: Leading causes for 2004. Natl Vital Stat Rep. 2007;56 [PubMed] [Google Scholar]
- 16.Ives DG, Samuel P, Psaty BM, et al. Agreement between nosologist and Cardiovascular Health Study review of deaths: Implications of coding differences. JAMA. 2009;57:133–139. doi: 10.1111/j.1532-5415.2008.02056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cappola AR, Fried LP, Arnold AM, et al. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA. 2006;295:1033–1041. doi: 10.1001/jama.295.9.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newman AB, Sachs MC, Arnold AM, et al. Total and cause-specific mortality in the cardiovascular health study. J Gerontol A Biol Sci Med Sci. 2009;64:1251–1261. doi: 10.1093/gerona/glp127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ostbye T, Hill G, Steenhuis R. Mortality in elderly Canadians with and without dementia: A 5-year follow-up. Neurology. 1999;53:521–526. doi: 10.1212/wnl.53.3.521. [DOI] [PubMed] [Google Scholar]
- 20.Mann NC, Knight S, Olson LM, et al. Underestimating injury mortality using statewide databases. J Trauma. 2005;58:162–167. doi: 10.1097/01.ta.0000114067.37731.da. [DOI] [PubMed] [Google Scholar]
- 21.Lakkireddy DR, Gowda MS, Murray CW, et al. Death certificate completion: How well are physicians trained and are cardiovascular causes overstated? Am J Med. 2004;117:492–498. doi: 10.1016/j.amjmed.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 22.Nashelsky MB, Lawrence CH. Accuracy of cause of death determination without forensic autopsy examination. Am J Forensic Med Pathol. 2003;24:313–319. doi: 10.1097/01.paf.0000097857.50734.c3. [DOI] [PubMed] [Google Scholar]
- 23.Mathers CD, Ma Fat D, Inoue M, et al. Counting the dead and what they died of: an assessment of the global status of cause of death data. Bull World Health Org. 2005;83:171–177. [PMC free article] [PubMed] [Google Scholar]
- 24.Medicare Current Beneficiary Survey. [Accessed September 10, 2010]; at www.cms.hhs.gov/apps/mcbs/overview.asp.
- 25.Adler G. A Profile of the Medicare Current Beneficiary Survey. Health Care Financ Rev Summer. 1994;15:153–163. [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention (CDC) International Classification of Diseases 9th Revision, Clinical Modification (ICD-9-CM) [Accessed October 12, 2009]; at http://www.cdc.gov/nchs/icd/icd9cm.htm.
- 27.Clinical Classification Software (CCS) for ICD-9-CM. [Accessed October 10, 2010]; at www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.
- 28.Spiegelman D, Hertzmark E, Wand HC. Point and interval estimates of partial population attributable risks in cohort studies: Examples and software. Cancer Causes Control. 2007;18:571–579. doi: 10.1007/s10552-006-0090-y. [DOI] [PubMed] [Google Scholar]
- 29.D’Agostino RB, Lee ML, Belanger AJ, et al. Relation of pooled logistic regression to time dependent Cox regression analysis: The Framingham Heart Study. Stat Med. 1990;9:1501–1515. doi: 10.1002/sim.4780091214. [DOI] [PubMed] [Google Scholar]
- 30.Eide GE, Gefeller O. Sequential and average attributable fractions as aids in the selection of preventive strategies. J Clin Epidemiol. 1995;48:645–655. doi: 10.1016/0895-4356(94)00161-i. [DOI] [PubMed] [Google Scholar]
- 31.Gefeller O, Land M, Eide GE. Averaging attributable fractions in the multifactorial situation: Assumptions and interpretation. J Clin Epidemiol. 1998;51:437–441. doi: 10.1016/s0895-4356(98)00002-x. [DOI] [PubMed] [Google Scholar]
- 32.Lin H, McAvay G, Allore HG, et al. The additive contribution of multiple time-varying coexisting diseases to the occurrence of adverse health outcomes. Am J Publ Health. (in press). [Google Scholar]
- 33.Steenland K, MacNeil J, Vega I, et al. Recent trends in Alzheimer disease mortality in the United States, 1999 to 2004. Alzheimer Dis Assoc Disord. 2009;23:165–170. doi: 10.1097/wad.0b013e3181902c3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 35.Yeh RW, Sidney S, Chandra M, et al. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362:2155–2165. doi: 10.1056/NEJMoa0908610. [DOI] [PubMed] [Google Scholar]
- 36.Smith AW, Reeve BB, Bellizzi KM, et al. Cancer, comorbidities, and health-related quality of life of older adults. Health Care Financ Rev. 2008;29:41–56. [PMC free article] [PubMed] [Google Scholar]
- 37.Gross CP, McAvay GJ, Krumholz HM, et al. The Effect of Age and Chronic Illness on Life Expectancy after Diagnosis of Colorectal Cancer: Implications for Screening. Ann Intern Med. 2006;145:646–653. doi: 10.7326/0003-4819-145-9-200611070-00006. [DOI] [PubMed] [Google Scholar]
- 38.Taylor DH, Jr, Fillenbaum GG, Ezell ME. The accuracy of Medicare claims data in identifying Alzheimer's disease. J Clin Epidemiol. 2002;55:929–937. doi: 10.1016/s0895-4356(02)00452-3. [DOI] [PubMed] [Google Scholar]
- 39.Percentage of civilian, non institutionalized population with diagnosed diabetes, by age, United States, 1980–2007. [Accessed August 13, 2010]; at www.cdc.gov/diabetes/statistics/prev/national/figbyage.htm.
- 40.Klabunde CN, Harlan LC, Warren JL. Data sources for measuring comorbidity. A comparison of hospital records and Medicare claims data for cancer patients. Med Care. 2006;44:921–928. doi: 10.1097/01.mlr.0000223480.52713.b9. [DOI] [PubMed] [Google Scholar]
- 41.Kieszak SM, Flanders WD, Kosinski AS, et al. A comparison of the Charlson comorbidity index derived from medical record data and administrative billing data. J Clin Epidemiol. 1999;52:137–142. doi: 10.1016/s0895-4356(98)00154-1. [DOI] [PubMed] [Google Scholar]
- 42.Fowles JB, Lawthers AG, Weiner JP, et al. Agreement between physicians’ office records and Medicare Part B claims data. Health Care Financ Rev. 1995;16:189–199. [PMC free article] [PubMed] [Google Scholar]
- 43.Lunney JR, Lynn J, Hogan C. Profiles of older Medicare decedents. J Am Geriatr Soc. 2002;50:1108–1112. doi: 10.1046/j.1532-5415.2002.50268.x. [DOI] [PubMed] [Google Scholar]
- 44.Alpe´rovitch A, Bertrand M, Jougla E, et al. Do we really know the cause of death of the very old? Comparison between official mortality statistics and cohort study classification. Eur J Epidemiol. 2009;24:669–675. doi: 10.1007/s10654-009-9383-2. [DOI] [PubMed] [Google Scholar]