Abstract
Novel therapies are urgently needed to improve clinical outcomes for patients with acute myeloid leukemia (AML). The investigational drug alisertib (MLN8237) is a novel Aurora A kinase inhibitor being studied in multiple Phase I and II studies. We investigated the preclinical efficacy and pharmacodynamics of alisertib in AML cell lines, primary AML cells, and mouse models of AML. Here we report that alisertib disrupted cell viability, diminished clonogenic survival, induced expression of the FOXO3a targets p27 and BIM, and triggered apoptosis. A link between Aurora A expression and sensitivity to ara-C was established, suggesting that Aurora A inhibition may be a promising strategy to increase the efficacy of ara-C. Accordingly, alisertib significantly potentiated the anti-leukemic activity of ara-C in both AML cell lines and primary blasts. Targeted FOXO3a knockdown significantly blunted the pro-apoptotic effects of the alisertib/ara-C combination, indicating that it is an important regulator of sensitivity to these agents. In vivo studies demonstrated that alisertib significantly augmented the efficacy of ara-C without affecting its pharmacokinetic profile and led to the induction of p27 and BIM. Our collective data indicate that targeting Aurora A with alisertib represents a novel approach to increase the efficacy of ara-C that warrants further investigation.
Keywords: Aurora kinase A, acute myeloid leukemia, apoptosis, cytarabine, FOXO
Acute myeloid leukemia (AML) accounts for 80 percent of adult acute leukemia.1 Currently utilized therapeutic agents do not achieve long-term survival for the majority of patients with this disease. Elderly patients with AML have a particularly dismal prognosis with less than 10% achieving long term survival.2 They are less able to tolerate intensive therapy with cytotoxic agents and they also have a higher prevalence of pre-existing myelodysplasia, unfavorable cytogenetics, and multidrug resistance than their younger counterparts.3 Moreover, a standard induction approach remains to be established for elderly patients with AML due, in part, to the aforementioned factors along with the poor representation of elderly patients in clinical trials. A recent study demonstrated that elderly patients with good or intermediate risk cytogenetics that received therapy with low-dose cytarabine (LDAC) had a significant survival advantage over patients that received supportive care. Despite this, no patients with unfavorable cytogenetics achieved complete responses (CRs) on this study.4 Furthermore, most elderly patients will not benefit from intensive chemotherapy and have a median survival of less than 6 months with this approach.5 These poor results underscore the need to develop less toxic targeted therapies based on our understanding of the molecular aberrations in AML in order to improve clinical outcomes.
Aurora kinase A is a serine/threonine kinase that functions as a central mitotic regulator. Aurora A activity is required for mitotic entry, mitotic spindle assembly and accurate chromosome separation.6–8 Aurora A and the related kinase Aurora B have been reported to be aberrantly expressed in a number of malignancies including leukemia’s.9 It is hypothesized that their overexpression contributes to the increased proliferative rate that is a hallmark feature of cancer cells by promoting cell cycle progression. The discovery that Aurora kinases were abnormally expressed in cancer cells prompted the development of agents that inhibit their activity.10–12 To date, the pan-Aurora kinase inhibitor MK-0457/VX-680, the Aurora B selective inhibitor AZD1152, and the multi-kinase inhibitor with anti-Aurora effects KW-2449 have shown pre-clinical activity in models of AML.9, 13–17 Aurora A selective inhibitors have not been previously evaluated in models of AML. Alisertib is an orally available adenosine triphosphate (ATP)-competitive and reversible selective inhibitor of Aurora A kinase.18 It has a benzazepine core scaffold and is approximately 200-fold more selective for Aurora A than Aurora B in cells and also has a high degree of selectivity for Aurora A when compared with a large panel of other kinases in enzymatic assays. Early preclinical studies revealed broad-spectrum anticancer activity in preclinical models. Alisertib is currently undergoing clinical evaluation in several Phase I and II clinical trials for patients with solid tumors and heme-lymphatic malignancies.
Considering the dual role of Aurora A in regulating cell cycle progression and programmed cell death and the high basal expression of Aurora A in AML cells, we hypothesized that AML cells would be particularly sensitive to alisertib.10 To test this hypothesis, we investigated the efficacy and pharmacodynamic effects of alisertib in AML cell lines, primary AML blasts, and mouse models of AML.
Materials and Methods
Cells and cell culture
MV4-11, PL-21, and MOLM-13 cells were obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ GmBH, Braunschweig, Germany). HL-60, OCI-AML2, SH2, SKM-1, NOMO-1 and KG-1 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). All cell lines were maintained as previously described.19 Normal peripheral blood mononuclear cells (PBMC) and pan T-cells from healthy donors were purchased from Stem Cell Technologies (Vancouver, Canada). T cells were cultured as previously described.20 Primary human AML cells were obtained from the bone marrow of patients at the CTRC at UTHSCSA after obtaining informed consent in accordance with an approved institutional IRB protocol and processed as previously described.21
Quantitative RT-PCR
Total RNA was isolated using the RNeasy Plus Mini Kit (Qiagen Inc., Valencia, CA) and treated with TURBO DNA-free™ Kit (Ambion Inc., Foster City, CA). First-strand cDNA synthesis was performed using the high-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). AURKA transcripts were amplified using a TaqManÆ Gene expression assay (Applied Biosystems, Foster City, CA). Relative gene expression was calculated with the 2−ΔΔCt method using GAPDH as a housekeeping gene.22
Chemicals and reagents
Reagents were obtained from: Alisertib (MLN8237, Millennium Pharmaceuticals, Cambridge, MA, USA), cytarabine (CTRC pharmacy), anti-tubulin, anti-aurora A, anti-phospho-Aurora A, anti-Aurora B, anti-phospho-FOXO3a, anti-FOXO3a, anti-BIM, and anti-p27 antibodies (Cell Signaling, Beverly, MA, USA). Anti-PCNA was purchased from DAKO (Glostrup, Denmark).
Analysis of cell cycle effects and apoptosis
Apoptosis (sub-G0/G1 DNA content) and cell cycle distribution were evaluated by PI/FACS analysis as previously described.19 Active caspase-3 staining with the Active Caspase-3 FITC Apoptosis kit (BD Biosciences, San Jose, CA, USA) followed by flow cytometry was used as a second measure of drug-induced apoptosis and carried out as previously described.21, 23
MTT assay
Cell viability was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as previously described.19
Colony assays
AML cells were treated for 24h with the indicated concentrations of alisertib and cytarabine and then washed twice in PBS, incubated in cytokine-free Methocult H4230, and scored as previously described.24, 25
Immunoblotting
AML cells were incubated with alisertib, cytarabine or the combination for 24h. Cells were then lysed and subjected to SDS-PAGE as previously described.24
RNA interference
AURKA SMARTpool, AURKB SMARTpool, or siCONTROL siRNA directed at luciferase (Dharmacon, Lafayette, CO) were transfected into AML cells as previously described using the Nucleofector II according to the manufacturer’s instructions (Amaxa Inc., Gaithersburg, MD, USA).23 Immunoblotting was utilized to assess knockdown efficiency. Transfected cells were treated with the indicated concentrations of alisertib for 48h. Drug-induced apoptosis was quantified by PI/FACS as described above. FOXO3a and control shRNA lentiviral particles were introduced into MV4-11 cells according to the manufacturer’s protocol (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Stable clones were selected following puromycin treatment.
Measuring alisertib and cytarabine plasma levels
Female naive CB-17 SCID mice were given a single dose of alisertib (20 mg/kg orally) and cytarabine (ara-C, 50 mg/kg IP) alone or in combination and sacrificed at various time points (1, 2, 4, 6, 8, 16 and 24 hours). Blood was drawn using cardiac puncture and plasma was isolated. Extractions were performed separately for the analysis of alisertib and cytarabine, and alisertib and cytarabine plasma concentrations were determined by LC/MS/MS. For alisertib, 25 µL of each sample was mixed with 50 µL of internal standard solution [13CD315N2]alisertib. The prepared samples were then extracted by solid phase extraction (Isolute C8 50 µm). The samples were washed, evaporated, and reconstituted. Ten microliters of the sample solution was loaded onto a 5 µm Sunfire C8, 2.1 mm internal diameter × 50 mm, HPLC column (Waters, Milford, MA, USA). For cytarabine, twenty-five microliters of each sample was mixed with 150 µL of internal standard solution cytarabine 13C3. The prepared samples were vortexed and then eluted from the plate. Ten microliters of the sample solution was loaded onto a 5 µm Hypersil Silica, 3.0 mm internal diameter × 50 mm, HPLC column (Thermo Fisher, Hanover Park, IL, USA). An API 4000 LC/MS/MS (MDS Sciex) was used for detection of alisertib (m/z 519.1→328.1), [13CD315N2]alisertib (m/z 526.1→329.1), cytarabine (m/z 244.2→112.1), and cytarabine 13C3(m/z 247.1→115.1). Quantification was based upon integrating peaks corresponding to elution of the drug and internal standard in the extracted product ion chromatograms. The lower limit of quantitation (LLQ) for alisertib and cytarabine was 5.00 ng/mL.
Pharmacokinetics analysis
Pharmacokinetic analysis of the plasma concentration data was performed using WinNonlinÆ Professional, Version 4.0 (Pharsight Corp., Mountain View, CA, USA). Kinetic parameters were estimated using a non-compartmental model.
In vivo evaluation of alisertib and ara-C
MOLM-13 and KG-1 cells were harvested, washed in PBS, and suspended in a mixture of HBSS and Matrigel (BD Biosciences, San Jose, CA, USA). An in vivo model of AML was generated by injection of MOLM-13/KG-1 cells into the flanks of female nude mice. After tumor growth reached 150 mm3, mice were randomly assigned to receive alisertib 20 mg/kg BID orally (n=10), cytarabine 75 mg/kg TIW Intraperitoneally (n=10), vehicle control (n=10) or both alisertib and cytarabine (n=10) for 14 days. Mice were monitored daily and tumor volumes and body weights were measured twice weekly as previously described.26 At the completion of the study, tumors were excised, formalin-fixed and paraffin-embedded for immunohistochemical analysis.
Immunohistochemistry
Paraffin-embedded tumor sections (4–6 µm thick) were mounted on slides and stained with antibodies against PCNA, BIM, and p27 as previously described.25
Statistical analyses
Statistical significance of differences observed between samples was determined using the Tukey-Kramer Comparison Test or the Student’s t test. Differences were considered significant in all experiments at p < 0.05.
Results
Targeting Aurora A with alisertib disrupts the viability of AML cell lines and primary AML cells
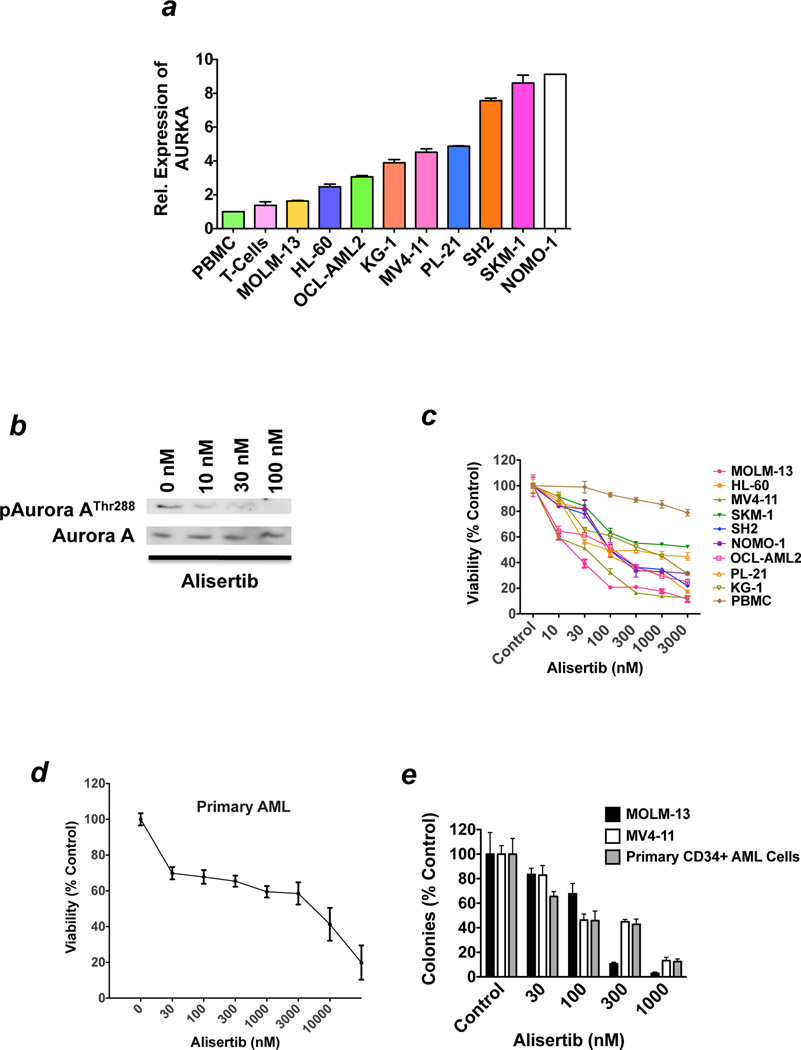
In order to investigate the therapeutic potential of targeting Aurora A activity in AML, we first assessed the relative expression of the AURKA gene in normal PBMCs and a panel of nine human AML cell lines using a quantitative RT-PCR method. AURKA expression levels were significantly higher in all 9 AML cell lines than in normal PBMC and proliferating T-cell controls (Figure 1a). Our findings are consistent with a recent study that demonstrated prevalent AURKA overexpression in AML cells from patients.27 We next treated MV4-11 cells with three concentrations of alisertib to examine the effects of this agent on Aurora A autophosphorylation as a measure of Aurora A activity. Our results showed that alisertib caused a dose-dependent reduction in the phosphorylation of Aurora A on its Thr288 auto-phosphorylation site. Our findings are consistent with other recent preclinical studies with alisertib and demonstrate that this agent effectively inhibits Aurora A activity in AML cells (Figure 1b).26, 28 We subsequently treated a panel of nine AML cell lines and normal PBMC controls with various concentrations of alisertib and quantified the consequential effects on cell viability by MTT assay. Clinically achievable concentrations of alisertib preferentially inhibited the in vitro growth and survival of AML cell lines as compared with normal PBMCs, indicating that this agent may have therapeutic selectivity (Figure 1c).29 Importantly, in vitro treatment with alisertib also diminished the viability of primary blasts obtained form patients with AML (n = 3, Figure 1d) and disrupted the clonogenic survival of AML cell lines and primary CD34+ AML cells from patients (Figure 1e).
Figure 1. Alisertib impairs the growth and survival of AML cell lines and primary blasts form patients with AML.
(a) Relative expression of AURKA in normal PBMC, T-cells, and AML cell lines. The basal expression levels of AURKA were quantified by qRT-PCR. n = 3 ± SD. (b) Alisertib treatment abrogates Aurora kinase A autophosphorylation. MV4-11 AML cells were treated with the indicated concentrations of alisertib for 24 hours. The levels of phospho-Aurora A (Thr288) and total Aurora A were assessed by immunoblotting. (c) Alisertib selectively diminishes the viability of AML cells. Normal PBMC and a panel of 9 human AML cell lines were treated with the indicated concentrations of alisertib for 72 hours. Cell viability was measured by MTT assay. n = 3 ± SD. (d) Alisertib has activity against primary AML cells. Primary cells from 3 different patients with AML were treated ex vivo with alisertib for 72 hours. Cell viability was measured by MTT assay. n = 3 ± SD. (e) Treatment with alisertib inhibits clonogenic survival. MOLM-13 and MV4-11 AML cell lines and primary CD34+ cells from AML patients (n = 3) were treated with the indicated concentrations of alisertib for 24 hours. Drug was washed away and cells were seeded in Methocult. Colonies were scored on day 14. n = 3 ± SD.
Alisertib modulates cell cycle distribution and induces apoptosis
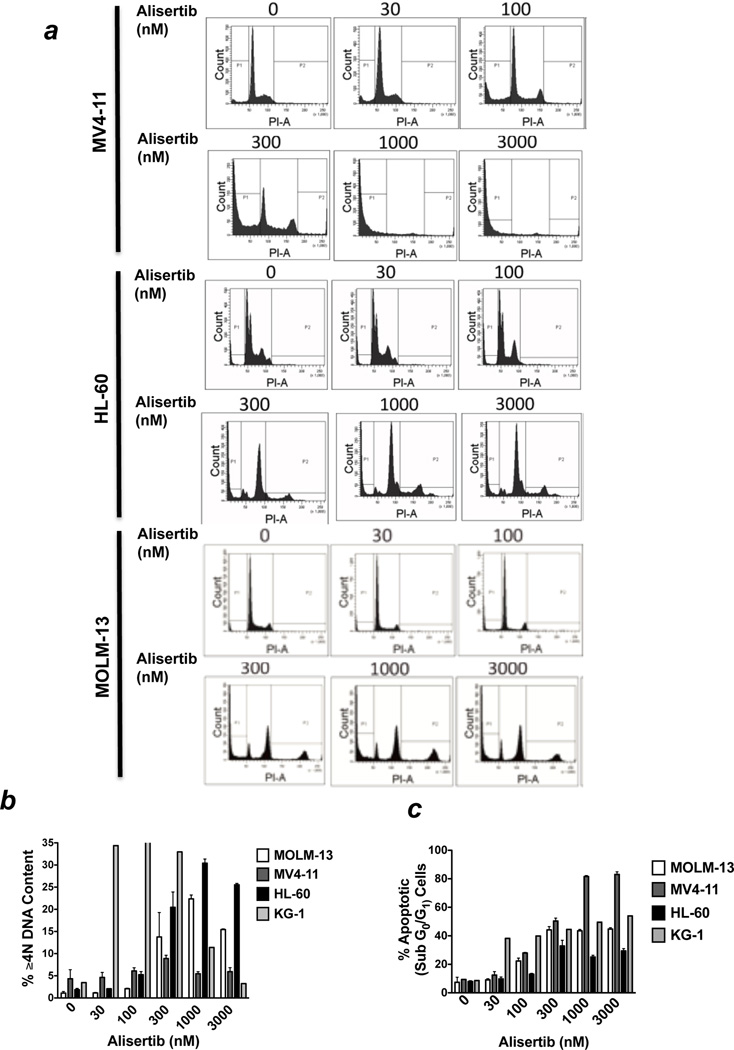
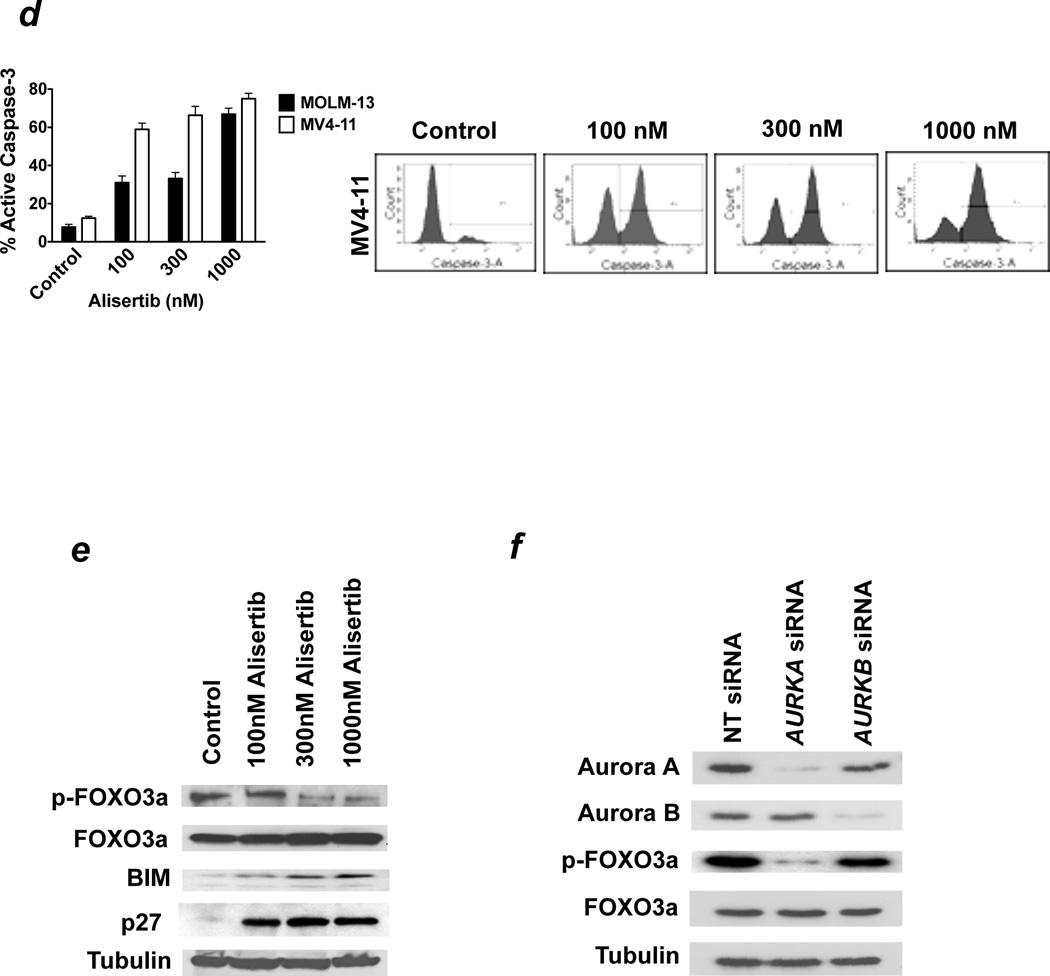
Genetically or pharmacologically antagonizing the activity of Aurora kinase A has been reported to produce mixed effects on cell cycle dynamics including polyploidy and G2/M growth arrest.7, 30–32 In order to assess the cell cycle-related effects of alisertib, we treated MV4-11, HL-60, KG-1, and MOLM-13 cells with 5 different concentrations of alisertib for 48h and quantified the effects on cell cycle distribution using PI/FACS analysis (Figure 2a). These analyses showed dose-dependent increases in the percentage of cells with ≥4N DNA content (Figure 2b) and with sub-G0/G1(apoptotic) DNA (Figure 2c). Considering that DNA fragmentation (sub-G0/G1 DNA) is a hallmark feature of apoptosis, we quantified the effects of alisertib on caspase-3 activation as a second measure of apoptosis to confirm the pro-apoptotic effects of this agent. Treatment with alisertib for 48h led to a concentration-dependent increase in the percentages of cells expressing the active form of caspase-3, indicating the induction of apoptosis significantly contributes to the activity of this agent (Figure 2d). Notably, MOLM-13 cells displayed significantly greater sensitivity to alisertib in MTT and colony formation assays than in assays that directly measured apoptosis. This suggests that the unique genetic background of individual cell types may be important in determining the cellular response to Aurora A inhibition (growth inhibition, apoptosis, or both) and that suppression of cell proliferation and stimulation of apoptosis both underlie the anticancer activity of alisertib. The combined effects of growth inhibition/cell cycle disruption and apoptosis that we observed in our FACS analyses were supported by immunoblotting analyses, which revealed that alisertib treatment led to a significant rise in the levels of the cyclin-dependent kinase inhibitor, p27, and of the microtubule-associated BH3-only apoptotic regulator, BIM in MV4-11 cells. The increased levels of p27 and BIM were correlated with a significant reduction in the transcriptionally inactive, cytosolically localized (phosphorylated) form of FOXO3a, which is an important transcriptional regulator of both p27 and BIM expression (Figure 2e).33, 34
Figure 2.
(a–c) Alisertib disrupts cell cycle dynamics and induces apoptosis. MV4-11, MOLM-13, and HL-60 cells were treated with the indicated concentrations of alisertib for 48 hours. PI/FACS was utilized to assess drug-related effects on cell cycle distribution and apoptosis. Representative histograms are shown in panel. The percentages of cells with ≥4N and sub-G0/G1 DNA content are quantified in panels (b) and (c), respectively. n = 3 ± SD. (d) Alisertib activates caspase-3. MOLM-13 and MV4-11 cells were treated with the indicated concentrations of alisertib for 48 hours. The percentages of cells expressing active caspase-3 were quantified using a FACS-based method as detailed in the Materials and methods. Representative histograms from experiments conducted with MV4-11 cells are shown. n = 3 ± SD. (e) Alisertib induces the expression of the FOXO3a targets p27 and BIM. MV4-11 cells were treated with the indicated concentrations of alisertib for 24 hours. Protein lysates were subjected to SDS-PAGE and membranes were probed with antibodies as described in the Materials and methods. Tubulin documented equal protein loading. (f) Aurora A preferentially regulates FOXO3a phosphorylation levels. MV4-11 cells were transfected with non-targeted (NT) control siRNA, AURKA siRNA, or AURKB siRNA using the Nucleofector II. The effects of targeted Aurora A and Aurora B knockdown on the levels of phospho-FOXO3a were assessed by immunoblotting. Tubulin documented equal protein loading.
In order to investigate whether Aurora A and Aurora B inhibition have similar effects on FOXO3a phosphorylation status, we utilized siRNA to knockdown AURKA and AURKB expression, respectively. Our results showed that AURKA siRNA led to a significantly greater reduction in phospho-FOXO3a levels than AURKB siRNA (Figure 2f). This suggests that Aurora A may play a more prominent role in the regulation of FOXO3a phosphorylation status than Aurora B.
ara-C induces the expression of Aurora A
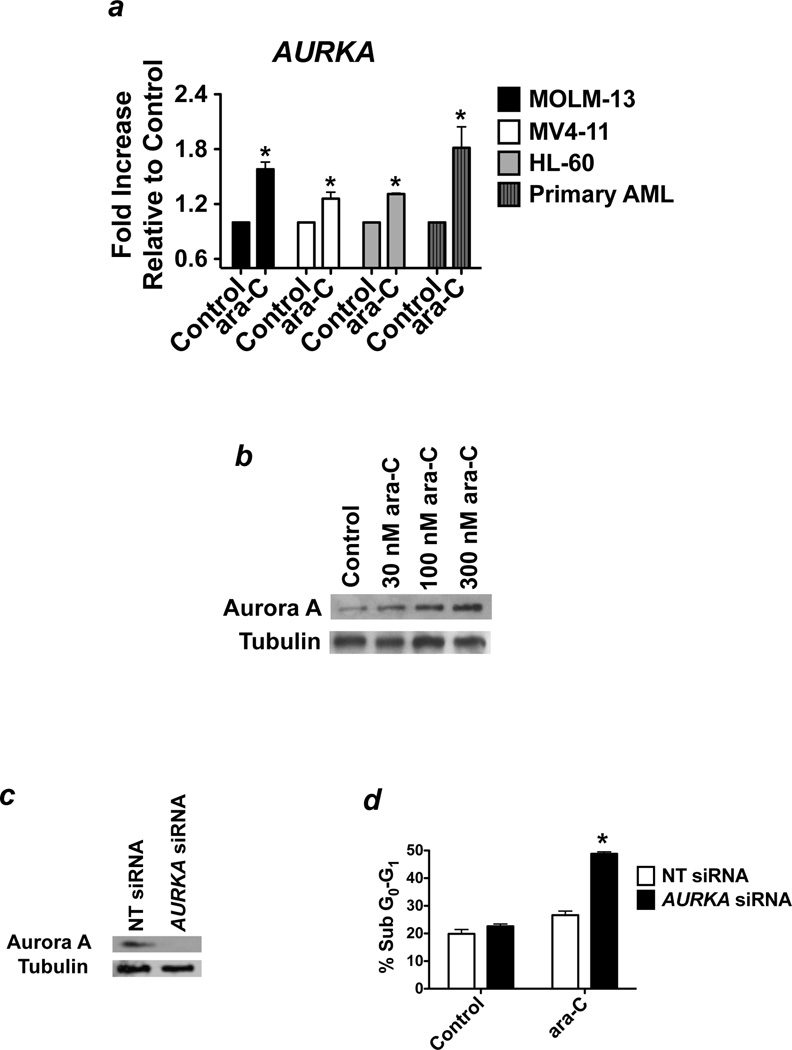
A number of studies have reported a link between Aurora kinase expression and resistance/reduced sensitivity to anticancer agents.35–40 However, the mechanistic basis for this correlation has not been well investigated. We hypothesized that the stress response induced by cytotoxic drugs such as cytarabine (ara-C), a frontline agent in AML therapy, could result in increased expression of Aurora A. In order to explore this possibility, we treated AML cell lines (HL-60, MV4-11, MOLM-13) and primary blasts from patients with AML (n = 3) with ara-C for 24h and quantified the effects on the expression of Aurora A by quantitative RT-PCR and immunoblotting (Figure 3a–b). Our results showed that acute exposure to ara-C was sufficient to trigger a significant increase in Aurora A expression in both established cell lines and primary AML cells. We next utilized siRNA to knockdown Aurora A expression in MV4-11 cells to assess whether expression of Aurora A significantly impacted cellular sensitivity to ara-C (Figure 3c). Direct comparison of the pro-apoptotic effects of ara-C in cells transfected with non-targeted control siRNA and Aurora A-targeted siRNA revealed that ara-C was nearly twice as effective at inducing apoptosis when Aurora A expression was diminished, suggesting that targeting Aurora A activity may be an effective strategy to increase the therapeutic efficacy of ara-C (Figure 3d).
Figure 3. Aurora A blunts the efficacy of ara-C.
(a) Effects of ara-C treatment on AURKA expression. Human AML cell lines (MOLM-13, HL-60, and MV4-11) and primary AML cells were treated with the indicated concentrations of ara-C for 24 hours. qRT-PCR was utilized to quantify the impact of drug treatment on the relative expression of AURKA. n = 3 ± SD, (*p ≤ 0.05, ara-C treatment vs. control). (b) ara-C causes a dose-dependent increase in Aurora A levels. MV4-11 cells were treated with the indicated concentrations of ara-C for 24 hours. Protein lysates were subjected to SDS-PAGE, blotted, and probed with an Aurora A specific antibody. Tubulin documented equal protein loading. (c–d) Targeting Aurora A increases the pro-apoptotic effects of ara-C. MV4-11 cells were transfected with non-target control or Aurora A-targeted siRNA as described in the Materials and Methods. Immunoblotting was utilized to assess knockdown efficiency (panel c). Cells transfected with non-target control and Aurora A siRNA were treated with ara-C for 48 hours. Drug-induced apoptosis was quantified by PI/FACS (panel d). n = 3 ± SD, (*p ≤ 0.05, AURKA siRNA vs. non-targeted siRNA).
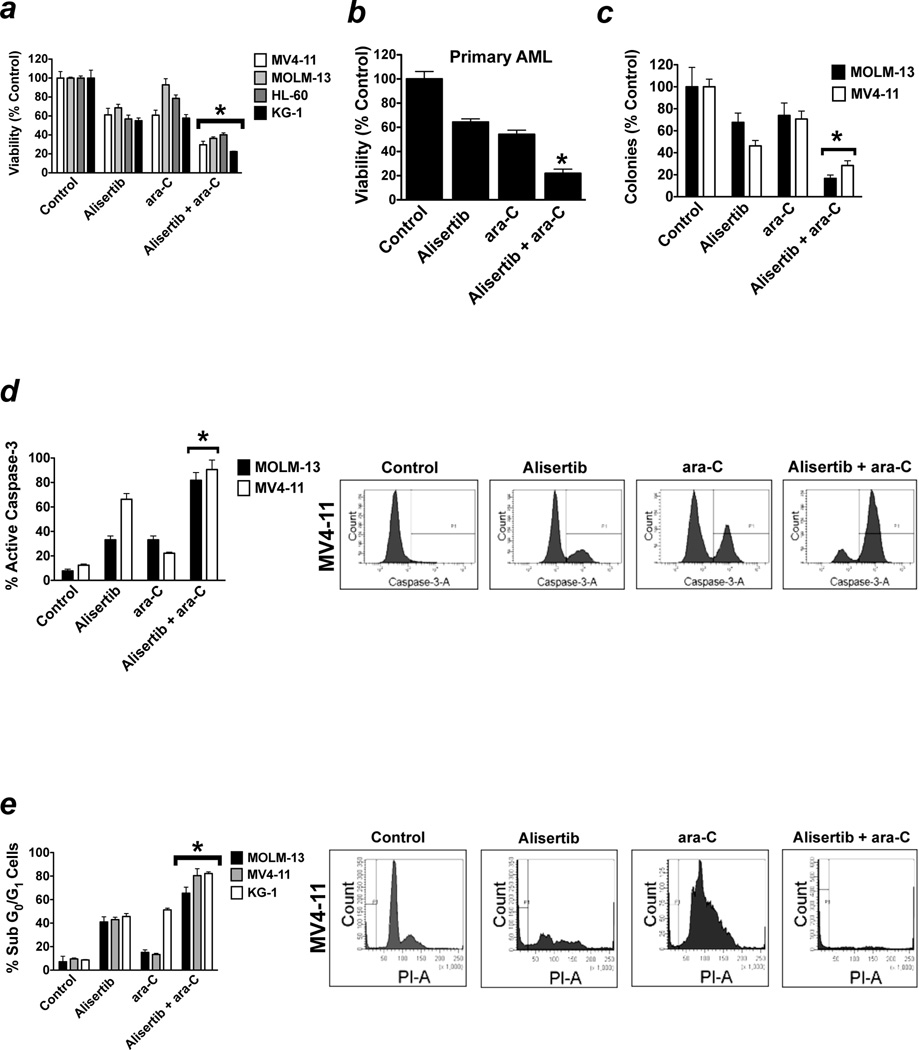
Alisertib significantly augments the in vitro activity of ara-C
Based on our data demonstrating that targeted knockdown of Aurora A significantly increased the sensitivity of MV4-11 cells to ara-C, we hypothesized that inhibition of Aurora A activity with alisertib would potentiate the anticancer effects of ara-C. We tested our hypothesis by treating AML cell lines (HL-60, KG-1, MV4-11, and MOLM-13) with alisertib, ara-C, or both drugs for 72h. Quantification of cell viability by MTT assay showed that alisertib cooperated with ara-C to reduce AML cell viability (Figure 4a). Investigation of the effects of these agents in primary AML blasts confirmed that alisertib significantly increased the ability of ara-C to disrupt AML cell viability (Figure 4b). Colony formation assays demonstrated that inhibition of Aurora A activity with alisertib significantly enhanced the ability of ara-C to suppress the clonogenicity of AML cells (Figure 4c). Considering that earlier investigations have shown that Aurora A can function to inhibit apoptosis, we next tested whether antagonizing Aurora A activity with alisertib could potentiate the pro-apoptotic effects of ara-C. Quantification of the percentages of cells with active caspase-3 (Figure 4d) and with sub-G0/G1 DNA content (Figure 4e) following 48h treatment with alisertib, ara-C, or both drugs revealed that the level of apoptosis induction was significantly greater in cells treated with alisertib + ara-C than with either drug alone. These data indicate that targeting Aurora A with alisertib may be an effective strategy to increase the anti-leukemic activity of ara-C.
Figure 4. Alisertib significantly increases the efficacy of ara-C.
(a–b) MLN8237 potentiates the anti-leukemic effects of ara-C. Human AML cell lines (MV4-11, MOLM-13, Hl-60, and KG-1, panel a) and primary AML cells (panel b) were treated with 100 nM alisertib, 100 nM ara-C, or the combination for 72 hours. Percentages of viable cells were determined by MTT assay. n = 3 ± SD, (*p ≤ 0.05, combination drug treatment vs. single agent drug treatments). (c) The combination of alisertib and ara-C cooperate to disrupt clonogenic survival. MOLM-13 and MV4-11 cells were treated 100 nM alisertib, 100 nM ara-C, or both for 24 hours. Drugs were washed away and cells were plated in Methocult. Colonies were scored on day 14. n = 3 ± SD, (*p ≤ 0.05, combination drug treatment vs. single agent drug treatments). (d–e) Alisertib augments ara-C-mediated apoptosis. Cells were treated with 100 nM alisertib, 100 nM ara-C, or both drugs for 48 hours. Drug-induced apoptosis was quantified by active caspase-3 staining (d) and PI/FACS (e). Representative histograms generated from experiments conducted with MV4-11 cells are shown. n = 3 ± SD, (*p ≤ 0.05, combination drug treatment vs. single agent drug treatments).
FOXO3a is a critical regulator of cellular sensitivity to alisertib and ara-C
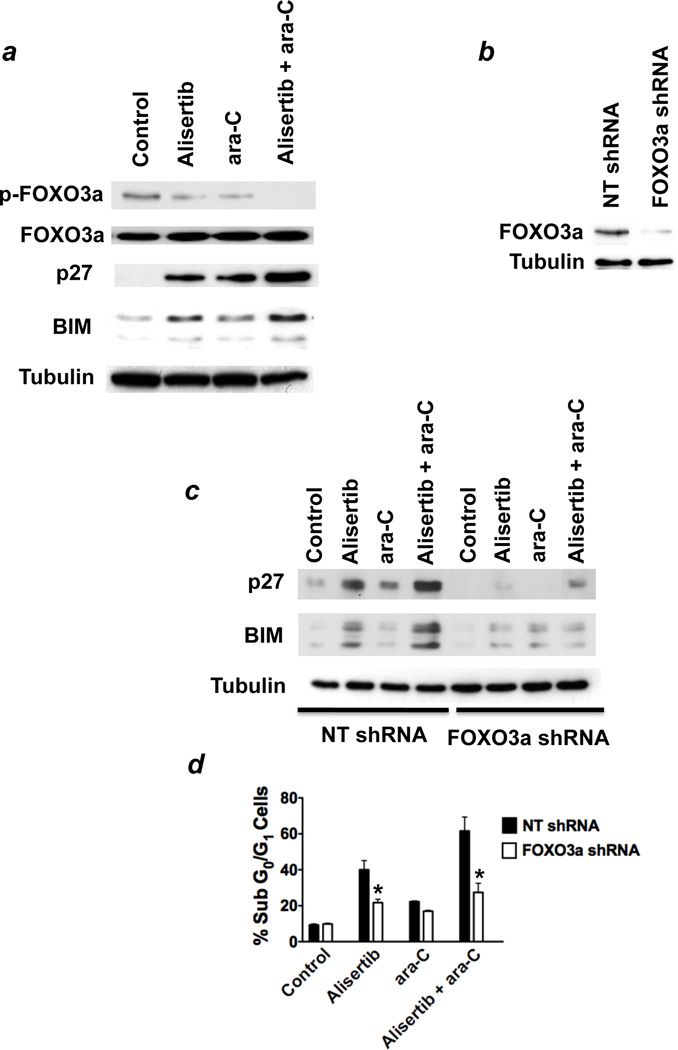
FOXO3a is a member of the forkhead family of transcription factors and regulates the expression of a large number of target genes with critical roles in processes that are essential for oncogenic transformation and malignant pathogenesis. Phosphorylation of FOXO3a sequesters it in the cytosol and consequentially inhibits its transcriptional activity. Upon its dephosphorylation, FOXO3a translocates to the nucleus where it can initiate the transcription of its target genes.33, 34 Given that treatment with alisertib led to a significant elevation in the levels of dephosphorylated (transcriptionally active) FOXO3a and the induction of the FOXO3a targets p27 and BIM (Figure 2e), we hypothesized that these downstream effects of Aurora A inhibition may contribute to the ability of this agent to increase the efficacy of ara-C. In order to investigate this possibility, we first conducted immunoblot analyses of the levels of total and phosphorylated FOXO3a, p27, and BIM following treatment with alisertib, ara-C, or the combination of these drugs. Our results showed that the levels of phospho-FOXO3a and its targets p27 and BIM were more profoundly changed by treatment with both alisertib and ara-C as compared with the effects produced by either single agent treatment (Figure 5a). This suggested that the combined effects of these drugs on FOXO3a play an important role in regulating sensitivity to these agents.
Figure 5. FOXO3a regulates sensitivity to the alisertib/ara-C combination.
(a) Alisertib and ara-C cooperate to induce expression of the FOXO3a targets p27 and BIM. MV4-11 cells were treated for 24 hours with 100 nM alisertib, 100 nM ara-C, or both agents. Immunoblotting was utilized to assess the effects of drug treatment on the levels of phospho-FOXO3a, total FOXO3a, p27, and BIM. Tubulin documented equal protein loading. (b) Generation of MV4-11 cells with stable FOXO3a knockdown. Cells were infected with non-targeted control or FOXO3a-targeted shRNA using a lentiviral approach. Stable cell lines were selected with puromycin treatment. Immunoblotting was utilized to assess knockdown efficiency. (c) FOXO3a is required for maximal induction of p27 and BIM by alisertib/ara-C. MV4-11 cells expressing control or FOXO3a-targeted shRNA were treated with 100 nM alisertib, 100 nM ara-C, or both drugs for 24 hours. Protein lysates were subjected to SDS-PAGE and the levels of p27 and BIM were evaluated by immunoblotting. Tubulin documented equal protein loading. (d) Targeted knockdown of FOXO3a blunts the pro-apoptotic effects of the alisertib/ara-C combination. MV4-11 cells expressing control or FOXO3a-targeted shRNA were treated with 100 nM alisertib, 100 nM ara-C, or both drugs for 48 hours. Drug-induced apoptosis was quantified by PI/FACS. n = 3 ± SD, (*p ≤ 0.05, FOXO3a shRNA vs. non-targeted control shRNA).
To determine whether FOXO3a is required for the induction of p27 and BIM expression in response to treatment with alisertib and ara-C, we used a lentiviral approach to generate MV4-11 cells with stable shRNA-mediated knockdown of FOXO3a (Figure 5b). We next treated cells expressing non-targeted control shRNA and FOXO3a shRNA with alisertib, ara-C, and the combination of these drugs and assessed the consequential effects on the expression of p27 and BIM by immunoblotting. Treatment with either single agent or the combination of both agents led to significant increases in the expression of p27 and BIM. In contrast, cells expressing FOXO3a-targeted shRNA displayed only minor changes in the levels of p27 and BIM following single agent and combination treatments (Figure 5c). Our results demonstrate that FOXO3a is required for maximal induction of p27 and BIM by alisertib and ara-C. We next evaluated the potential role of FOXO3a as a regulator of the efficacy of the alisertib/ara-C combination. MV4-11 cells stably expressing non-targeted or FOXO3a shRNA were treated with alisertib, ara-C, or both drugs for 48h and drug-induced apoptosis was quantified by PI/FACS. Targeted knockdown of FOXO3a blunted the pro-apoptotic effects of alisertib/ara-C by more than 50% of what was observed on cells expressing control shRNA (Figure 5d). Collectively, these data support a role for FOXO3a as a critical mediator of the therapeutic efficacy of this combination.
Alisertib potentiates the in vivo efficacy of ara-C
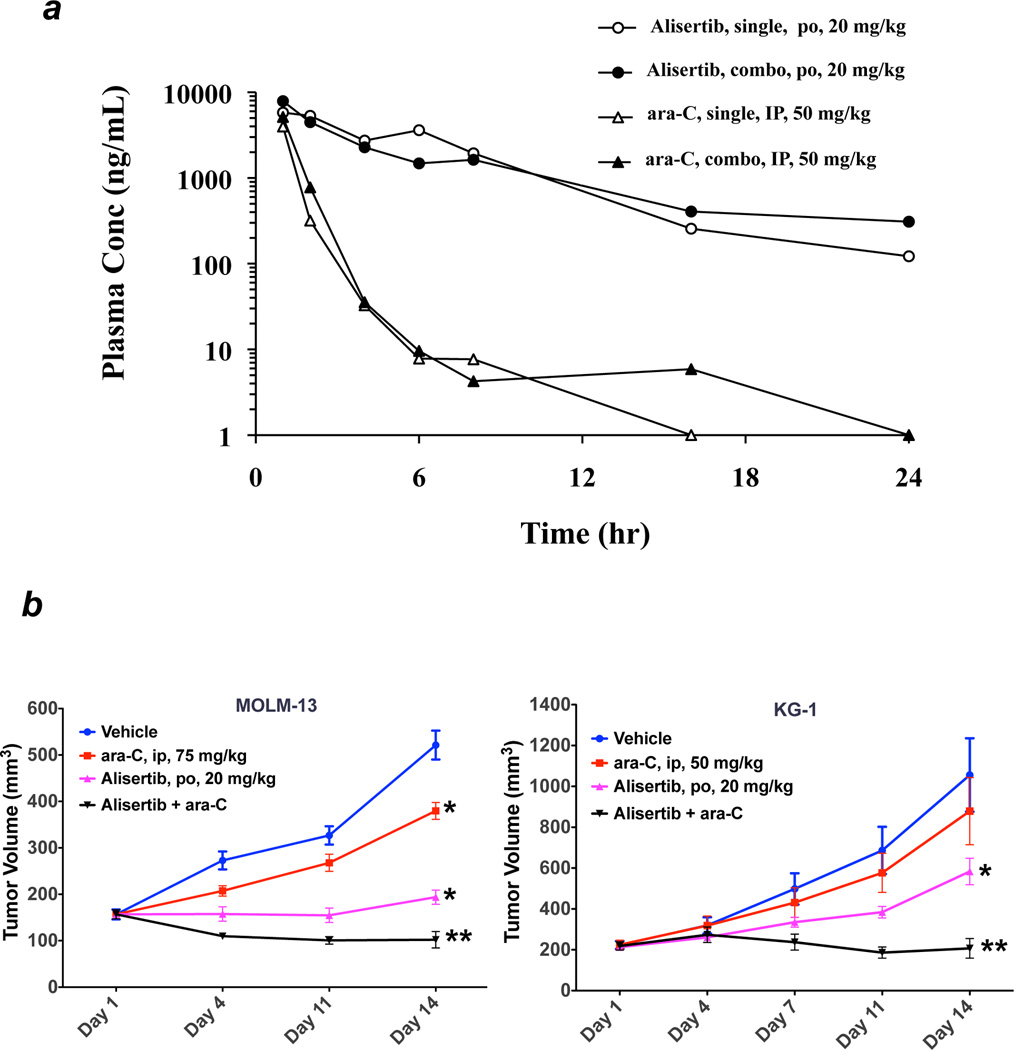
Our in vitro data demonstrated that targeting Aurora A with alisertib enhanced the efficacy of ara-C in AML cell lines and primary blasts form patients with AML. In order to further investigate the therapeutic potential of this novel strategy, we first conducted a pharmacokinetic (PK) experiment to ascertain any possible effects that co-administration of alisertib may have on the PK profile of ara-C. Mice were given a single dose of alisertib (20 mg/kg orally) and ara-C (50 mg/kg IP) alone or in combination and sacrificed at various time points. Plasma concentrations of ara-C and alisertib were determined using an LC/MS/MS method. Pharmacokinetic parameters were estimated using a non-compartmental model. Our results demonstrate that the addition of either agent did not significantly affect the pharmacokinetics of the other (Figure 6a). We next conducted xenograft studies to investigate the in vivo therapeutic potential of the combination of alisertib and ara-C. MOLM-13 and KG-1 cells were injected subcutaneously into the flanks of immunodeficient nude mice. Vehicle, alisertib, ara-C or the combination of alisertib and ara-C were administered to mice for 14 days. In both KG-1 and MOLM-13 AML models, single agent treatment with alisertib or ara-C had substantial effects on tumor burden. The combination of both agents was well tolerated without a significant impact on animal body weight and resulted in significantly greater tumor growth inhibition than what was achieved by either agent alone (Figure 6b). These findings indicate that inhibition of Aurora A activity with alisertib may be an attractive strategy to heighten the anti-leukemic activity of ara-C.
Figure 6. In vivo efficacy of alisertib and ara-C.
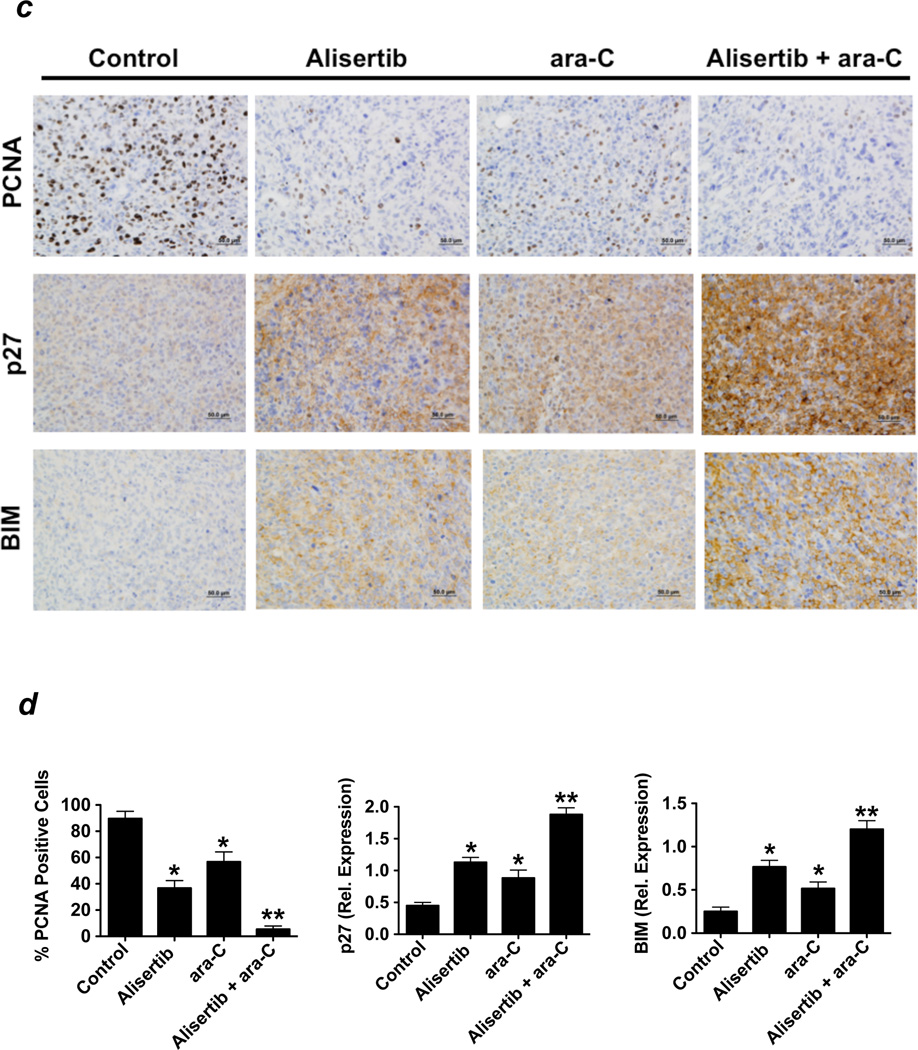
(a) Alisertib does not significantly impact the pharmacokinetic profile of ara-C. Mice were administered vehicle, alisertib, ara-C, or both as indicated. The plasma levels of each drug were quantified as detailed in the Materials and Methods. (b) Alisertib augments the in vivo activity of ara-C. MOLM-13 and KG-1 cells were injected into the flanks of nude mice. Vehicle, alisertib (20 mg/kg po BID), ara-C (75 mg/kg ip TIW for MOLM-13, 50 mg/kg ip QDx5) or both drugs were administered for 14 days. Tumor volume was measured biweekly. n = 10 ± SD, *p≤ 0.05 (controls vs. single agents, **p≤ 0.05 (single agents vs. combination). (c–d) The alisertib/ara-C combination induces the expression of p27 and BIM in vivo. Immunohistochemistry was carried out on tumor specimens from mice in each treatment group to quantify the effects of drug treatment on the expression of the FOXO3a targets p27 and BIM. PCNA was used as a general measure of tumor cell proliferation. Representative images are shown from each treatment group (c). Positive cells were scored manually under 20X magnification (d). Mean ± SD, n = 5, *p≤ 0.05 (controls vs. single agents, **p≤ 0.05 (single agents vs. combination).
Our earlier in vitro assays demonstrated that FOXO3a is an important regulator of therapeutic activity of the alisertib/ara-C combination. We utilized an immunohistochemical approach to quantify the impact of in vivo administration of these agents on the expression of the FOXO3a targets p27 and BIM and on proliferating cell nuclear antigen (PCNA) as a general measure of proliferative activity. Consistent with our in vitro observations, the alisertib/ara-C combination was significantly more effective at inducing the expression of p27 and BIM and also at globally diminishing tumor cell proliferation than either alisertib or ara-C alone (Figure 6c–d). Our collective findings indicate that inhibition of Aurora A activity with alisertib represents a novel approach to increase the growth inhibitory and pro-apoptotic effects of treatment with ara-C.
Discussion
Given its intrinsic overexpression in cancer, essential functions in the regulation of mitosis, and potential roles in promoting drug resistance and disease progression, Aurora kinase A is an attractive target for cancer therapy. As a result, a number of Aurora kinase inhibitors that have varying degrees of activity against Aurora A are currently in development.12 Alisertib can be distinguished from the majority of other small molecule inhibitors of Aurora kinase activity in development due to its selectivity for Aurora A versus Aurora B. Although earlier preclinical studies with alisertib have demonstrated significant anticancer activity in a range of tumor types, its potential efficacy has not been previously investigated in models of AML.31, 32, 41–43 We hypothesized that the prevalent overexpression of Aurora A in AML and highly proliferative nature of this malignancy would render it particularly sensitive to alisertib. We conducted a series of preclinical experiments with the aim to ascertain the antileukemic activity and pharmacodynamic effects of targeting Aurora A with alisertib in AML cell lines, primary AML blasts, and mouse xenograft models of AML.
Our in vitro assays demonstrated that alisertib has pleiotropic effects in AML cells. Exposure to alisertib diminished cell viability and clonogenic survival, disrupted cell cycle dynamics, and induced apoptosis. Interestingly, our data demonstrated that cellular sensitivity to alisertib was not directly correlated with Aurora A expression levels in AML cells. These findings are consistent with those from other preclinical and early phase clinical trials with alisertib, which have also failed to show a direct relationship between Aurora A expression levels and sensitivity to alisertib.44 The reason for this phenomenon is unknown. It is possible that alisertib may achieve similar degrees of Aurora A inhibition in cells with relatively low, intermediate, and high basal Aurora A expression. If this were the case, sensitivity to alisertib would not appear to be directly linked to Aurora A levels. Pharmacodynamic studies from ongoing and planned Phase II and Phase III clinical trials with alisertib may help to clarify this issue.
Phosphorylation of the transcription factor FOXO3a sequesters it in the cytosol where it is transcriptionally inactive. Upon its dephosphorylation, FOXO3a translocates to the nucleus where it can initiate transcription of its target genes. Interestingly, the growth inhibitory and pro-apoptotic effects we observed in AML cells treated with alisertib were associated with a significant reduction in the levels of phospho-FOXO3a and significantly increased expression of the FOXO3a transcriptional targets p27 and BIM. Given that p27 is a critical component of the G2/M cell cycle transition and that BIM is a microtubule-associated pro-apoptotic factor, the observed induction of p27 and BIM expression is a predictable consequence of Aurora A inhibition. These findings are consistent with those of earlier investigations conducted in other cancer models that also demonstrated elevated p27 and BIM levels following treatment with Aurora kinase inhibitors.31, 45
Several prior studies have established a link between Aurora A overexpression and resistance to therapeutic agents, supporting a role for Aurora A in the regulation of chemosensitivity.35–40 However, it is not completely clear at this time whether this is an intrinsic or acquired relationship. We postulated that treatment with conventional cytotoxic agents such as ara-C could promote elevated levels of Aurora A during the genotoxic stress response. Indeed, our data demonstrate that in vitro treatment with ara-C leads to increased expression of Aurora A in AML cell lines and primary blasts from patients. In order to investigate the potential therapeutic implications of ara-C-mediated induction of Aurora A expression, we first utilized siRNA as a proof of principle to demonstrate that targeted knockdown of Aurora A significantly increased the pro-apoptotic effects of ara-C. This suggested that Aurora A may play a chemoresistance role with respect to ara-C and that targeting its activity could be of therapeutic benefit.
We further investigated the potential impact of targeting Aurora A activity with alisertib on the efficacy of ara-C in a series of in vitro experiments in AML cell lines and primary AML cells. These assays demonstrated that alisertib significantly increased both the growth inhibitory and pro-apoptotic effects of ara-C. Interestingly, the combination of both drugs led to enhanced induction of the FOXO3a targets p27 and BIM compared to either single agent treatment. Our targeted knockdown assays demonstrated that FOXO3a expression was required for maximal alisertib/ara-C mediated induction of these specific targets and consequently, for the combination to most effectively trigger apoptosis. To our knowledge, this is the first report demonstrating a link between Aurora A kinase inhibition and FOXO3a activity. Our subsequent pharmacokinetic and mouse xenograft studies validated the potential therapeutic benefit of combining alisertib and ara-C for AML therapy. Additionally, immunohistochemical assays conducted with specimens obtained form mice treated with alisertib/ara-C established p27 and BIM as pharmacodynamic effectors of these agents.
The FOXO3a-related effects that occur downstream of Aurora A inhibition by alisertib are very interesting. The exact mechanism by which alisertib induces the expression of FOXO3a transcriptional targets remains to be fully elucidated. We were unable to co-immunoprecipitate Aurora A and FOXO3a, which indicates that FOXO3a is unlikely to be a direct Aurora A phospho-substrate. A previous study conducted in models of multiple myeloma demonstrated that alisertib treatment leads to activation of protein phosphatase 2A (PP2A).32 Additional studies are required to clarify this relationship mechanistically. However, considering that FOXO3a is a critical regulator of cell death the ability of alisertib to increase FOXO3a activity may allow it to potentiate the efficacy of a broad range of anticancer agents.
Alisertib has been evaluated in several Phase I and Phase II studies in solid tumors and hematological malignancies.46–48 It is generally very well tolerated and adverse events such as myelosuppresion and gastrointestinal mucosal damage are reversible and consistent with the inhibition of Aurora A activity of alisertib with predominant effects on proliferative tissues such as the bone marrow and gastrointestinal tract. Promising antitumor activity has been observed particularly in hem-lymphatic malignancies.46 Notably, alisertib has also been evaluated as a single agent in a Phase II study of 57 patients with advanced acute myeloid leukemia or myelodysplastic syndrome.47 While objective responses (13% of the population) including one complete response were observed in this study, it is likely that combination therapy with cytotoxic agents such as cytarabine would allow greater initial disease control facilitating sustained Aurora kinase A inhibition. Our collective data demonstrate that the combination of alisertib and ara-C is effective and well tolerated in preclinical models of AML. Based on this promising preclinical data, a Phase I/II study is warranted to investigate the safety and activity of the alisertib/ara-C combination in patients with AML who would not be expected to benefit from conventional therapy.
Novelty and Impact.
Very few patients with acute myeloid leukemia (AML) achieve long-term survival with conventional therapy. Novel approaches are urgently required to improve clinical outcomes for patients with this disease. Aurora kinase A is a central mitotic regulator that is overexpressed in AML and many other malignancies. Our data demonstrate that targeting Aurora A with alisertib represents a new strategy to disrupt AML cell survival and augment the efficacy of standard ara-C therapy that warrants further investigation.
Acknowledgements
This work was supported by grants from LeukemiaTexas (J.S.C) and the National Cancer Institute Cancer Center Core Support Grant# CA054174. Mengkun Zhang, Johnny J. Yang, and Jeffrey Ecsedy are employees of Millennium Pharmaceuticals, Inc.
References
- 1.Yamamoto JF, Goodman MT. Patterns of leukemia incidence in the United States by subtype and demographic characteristics, 1997–2002. Cancer Causes Control. 2008;19:379–390. doi: 10.1007/s10552-007-9097-2. [DOI] [PubMed] [Google Scholar]
- 2.Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE, Anderson JE, Petersdorf SH. Age and acute myeloid leukemia. Blood. 2006;107:3481–3485. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stone RM. The difficult problem of acute myeloid leukemia in the older adult. CA Cancer J Clin. 2002;52:363–371. doi: 10.3322/canjclin.52.6.363. [DOI] [PubMed] [Google Scholar]
- 4.Burnett AK, Milligan D, Prentice AG, Goldstone AH, McMullin MF, Hills RK, Wheatley K. A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer. 2007;109:1114–1124. doi: 10.1002/cncr.22496. [DOI] [PubMed] [Google Scholar]
- 5.Kantarjian H, Ravandi F, O'Brien S, Cortes J, Faderl S, Garcia-Manero G, Jabbour E, Wierda W, Kadia T, Pierce S, Shan J, Keating M, et al. Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood. 2010;116:4422–4429. doi: 10.1182/blood-2010-03-276485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fu J, Bian M, Jiang Q, Zhang C. Roles of Aurora kinases in mitosis and tumorigenesis. Mol Cancer Res. 2007;5:1–10. doi: 10.1158/1541-7786.MCR-06-0208. [DOI] [PubMed] [Google Scholar]
- 7.Marumoto T, Honda S, Hara T, Nitta M, Hirota T, Kohmura E, Saya H. Aurora-A kinase maintains the fidelity of early and late mitotic events in HeLa cells. J Biol Chem. 2003;278:51786–51795. doi: 10.1074/jbc.M306275200. [DOI] [PubMed] [Google Scholar]
- 8.Hoar K, Chakravarty A, Rabino C, Wysong D, Bowman D, Roy N, Ecsedy JA. MLN8054, a small-molecule inhibitor of Aurora A, causes spindle pole and chromosome congression defects leading to aneuploidy. Mol Cell Biol. 2007;27:4513–4525. doi: 10.1128/MCB.02364-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang XF, Luo SK, Xu J, Li J, Xu DR, Wang LH, Yan M, Wang XR, Wan XB, Zheng FM, Zeng YX, Liu Q. Aurora kinase inhibitory VX-680 increases Bax/Bcl-2 ratio and induces apoptosis in Aurora-A-high acute myeloid leukemia. Blood. 2008;111:2854–2865. doi: 10.1182/blood-2007-07-099325. [DOI] [PubMed] [Google Scholar]
- 10.Ikezoe T, Yang J, Nishioka C, Tasaka T, Taniguchi A, Kuwayama Y, Komatsu N, Bandobashi K, Togitani K, Koeffler HP, Taguchi H. A novel treatment strategy targeting Aurora kinases in acute myelogenous leukemia. Mol Cancer Ther. 2007;6:1851–1857. doi: 10.1158/1535-7163.MCT-07-0067. [DOI] [PubMed] [Google Scholar]
- 11.Harrington EA, Bebbington D, Moore J, Rasmussen RK, Ajose-Adeogun AO, Nakayama T, Graham JA, Demur C, Hercend T, Diu-Hercend A, Su M, Golec JM, et al. VX-680, a potent and selective small-molecule inhibitor of the Aurora kinases, suppresses tumor growth in vivo. Nat Med. 2004;10:262–267. doi: 10.1038/nm1003. [DOI] [PubMed] [Google Scholar]
- 12.Kelly KR, Ecsedy J, Mahalingam D, Nawrocki ST, Padmanabhan S, Giles FJ, Carew JS. Targeting Aurora Kinases in Cancer Treatment. Curr Drug Targets. 2011 doi: 10.2174/138945011798829410. [DOI] [PubMed] [Google Scholar]
- 13.Yang J, Ikezoe T, Nishioka C, Tasaka T, Taniguchi A, Kuwayama Y, Komatsu N, Bandobashi K, Togitani K, Koeffler HP, Taguchi H, Yokoyama A. AZD1152, a novel and selective aurora B kinase inhibitor, induces growth arrest, apoptosis, and sensitization for tubulin depolymerizing agent or topoisomerase II inhibitor in human acute leukemia cells in vitro and in vivo. Blood. 2007;110:2034–2040. doi: 10.1182/blood-2007-02-073700. [DOI] [PubMed] [Google Scholar]
- 14.Kojima K, Konopleva M, Tsao T, Nakakuma H, Andreeff M. Concomitant inhibition of Mdm2-p53 interaction and Aurora kinases activates the p53-dependent postmitotic checkpoints and synergistically induces p53-mediated mitochondrial apoptosis along with reduced endoreduplication in acute myelogenous leukemia. Blood. 2008;112:2886–2895. doi: 10.1182/blood-2008-01-128611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiskus W, Wang Y, Joshi R, Rao R, Yang Y, Chen J, Kolhe R, Balusu R, Eaton K, Lee P, Ustun C, Jillella A, et al. Cotreatment with vorinostat enhances activity of MK-0457 (VX-680) against acute and chronic myelogenous leukemia cells. Clin Cancer Res. 2008;14:6106–6115. doi: 10.1158/1078-0432.CCR-08-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oke A, Pearce D, Wilkinson RW, Crafter C, Odedra R, Cavenagh J, Fitzgibbon J, Lister AT, Joel S, Bonnet D. AZD1152 rapidly and negatively affects the growth and survival of human acute myeloid leukemia cells in vitro and in vivo. Cancer Res. 2009;69:4150–4158. doi: 10.1158/0008-5472.CAN-08-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shiotsu Y, Kiyoi H, Ishikawa Y, Tanizaki R, Shimizu M, Umehara H, Ishii K, Mori Y, Ozeki K, Minami Y, Abe A, Maeda H, et al. KW-2449, a novel multikinase inhibitor, suppresses the growth of leukemia cells with FLT3 mutations or T315I-mutated BCR/ABL translocation. Blood. 2009;114:1607–1617. doi: 10.1182/blood-2009-01-199307. [DOI] [PubMed] [Google Scholar]
- 18.Sells T, Ecsedy J, Stroud S, Janowick D, Hoar K, LeRoy P, Wysong D, Zhang M, Huck J, Silverman L, Chen W, Bembenek M, et al. MLN8237: an orally active small molecule inhibitor of Aurora A kinase in phase I clinical trials. AACR Meeting Abstracts. 2008;2008 237-. [Google Scholar]
- 19.Carew JS, Nawrocki ST, Krupnik YV, Dunner K, Jr, McConkey DJ, Keating MJ, Huang P. Targeting endoplasmic reticulum protein transport: a novel strategy to kill malignant B cells and overcome fludarabine resistance in CLL. Blood. 2006;107:222–231. doi: 10.1182/blood-2005-05-1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee CC, Lin SJ, Cheng PJ, Kuo ML. The regulatory function of umbilical cord blood CD4(+) CD25(+) T cells stimulated with anti-CD3/anti-CD28 and exogenous interleukin (IL)-2 or IL-15. Pediatr Allergy Immunol. 2009;20:624–632. doi: 10.1111/j.1399-3038.2008.00843.x. [DOI] [PubMed] [Google Scholar]
- 21.Swords RT, Kelly KR, Smith PG, Garnsey JJ, Mahalingam D, Medina E, Oberheu K, Padmanabhan S, O'Dwyer M, Nawrocki ST, Giles FJ, Carew JS. Inhibition of NEDD8-activating enzyme: a novel approach for the treatment of acute myeloid leukemia. Blood. 2010;115:3796–3800. doi: 10.1182/blood-2009-11-254862. [DOI] [PubMed] [Google Scholar]
- 22.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carew JS, Nawrocki ST, Kahue CN, Zhang H, Yang C, Chung L, Houghton JA, Huang P, Giles FJ, Cleveland JL. Targeting autophagy augments the anticancer activity of the histone deacetylase inhibitor SAHA to overcome Bcr-Abl-mediated drug resistance. Blood. 2007;110:313–322. doi: 10.1182/blood-2006-10-050260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nawrocki ST, Carew JS, Maclean KH, Courage JF, Huang P, Houghton JA, Cleveland JL, Giles FJ, McConkey DJ. Myc regulates aggresome formation, the induction of Noxa, and apoptosis in response to the combination of bortezomib and SAHA. Blood. 2008;112:2917–2926. doi: 10.1182/blood-2007-12-130823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carew JS, Nawrocki ST, Reddy VK, Bush D, Rehg JE, Goodwin A, Houghton JA, Casero RA, Jr, Marton LJ, Cleveland JL. The novel polyamine analogue CGC-11093 enhances the antimyeloma activity of bortezomib. Cancer Res. 2008;68:4783–4790. doi: 10.1158/0008-5472.CAN-07-6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly KR, Ecsedy J, Medina E, Mahalingam D, Padmanabhan S, Nawrocki ST, Giles FJ, Carew JS. The novel Aurora A kinase inhibitor MLN8237 is active in resistant chronic myeloid leukemia and significantly increases the efficacy of nilotinib. J Cell Mol Med. 2011;15:2057–2070. doi: 10.1111/j.1582-4934.2010.01218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lucena-Araujo AR, de Oliveira FM, Leite-Cueva SD, dos Santos GA, Falcao RP, Rego EM. High expression of AURKA and AURKB is associated with unfavorable cytogenetic abnormalities and high white blood cell count in patients with acute myeloid leukemia. Leuk Res. 2011;35:260–264. doi: 10.1016/j.leukres.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 28.Sehdev V, Peng D, Soutto M, Washington MK, Revetta F, Ecsedy JA, Zaika AI, Rau T, Schneider-Stock R, Belkhiri A, El-Rifai W. The Aurora kinase A inhibitor MLN8237 Enhances Cisplatin-induced Cell Death in Esophageal Adenocarcinoma Cells. Mol Cancer Ther. 2012 doi: 10.1158/1535-7163.MCT-11-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cervantes-Ruiperez A, Burris HA, Cohen RB, Dees EC, Infante JR, Fingert H, Shinde V, Venkatakrishnan K, Chakravarty A, Tabernero J. Pharmacokinetic (PK) and pharmacodynamic (PD) results from two phase I studies of the investigational selective Aurora A kinase (AAK) inhibitor MLN8237: Exposure-dependent AAK inhibition in human tumors. ASCO Annual Meeting. 2010;28:15. [Google Scholar]
- 30.Marumoto T, Hirota T, Morisaki T, Kunitoku N, Zhang D, Ichikawa Y, Sasayama T, Kuninaka S, Mimori T, Tamaki N, Kimura M, Okano Y, et al. Roles of aurora-A kinase in mitotic entry and G2 checkpoint in mammalian cells. Genes Cells. 2002;7:1173–1182. doi: 10.1046/j.1365-2443.2002.00592.x. [DOI] [PubMed] [Google Scholar]
- 31.Kelly KR, Ecsedy J, Medina E, Mahalingam D, Padmanabhan S, Nawrocki ST, Giles FJ, Carew JS. The novel Aurora A kinase inhibitor MLN8237 is active in resistant chronic myeloid leukemia and significantly increases the efficacy of nilotinib. J Cell Mol Med. 2010 doi: 10.1111/j.1582-4934.2010.01218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gorgun G, Calabrese E, Hideshima T, Ecsedy J, Perrone G, Mani M, Ikeda H, Bianchi G, Hu Y, Cirstea D, Santo L, Tai YT, et al. A novel Aurora-A kinase inhibitor MLN8237 induces cytotoxicity and cell-cycle arrest in multiple myeloma. Blood. 2010;115:5202–5213. doi: 10.1182/blood-2009-12-259523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maiese K, Chong ZZ, Shang YC. OutFOXOing disease and disability: the therapeutic potential of targeting FoxO proteins. Trends Mol Med. 2008;14:219–227. doi: 10.1016/j.molmed.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang JY, Hung MC. A new fork for clinical application: targeting forkhead transcription factors in cancer. Clin Cancer Res. 2009;15:752–757. doi: 10.1158/1078-0432.CCR-08-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anand S, Penrhyn-Lowe S, Venkitaraman AR. AURORA-A amplification overrides the mitotic spindle assembly checkpoint, inducing resistance to Taxol. Cancer cell. 2003;3:51–62. doi: 10.1016/s1535-6108(02)00235-0. [DOI] [PubMed] [Google Scholar]
- 36.Yang H, He L, Kruk P, Nicosia SV, Cheng JQ. Aurora-A induces cell survival and chemoresistance by activation of Akt through a p53-dependent manner in ovarian cancer cells. Int J Cancer. 2006;119:2304–2312. doi: 10.1002/ijc.22154. [DOI] [PubMed] [Google Scholar]
- 37.Dutta-Simmons J, Zhang Y, Gorgun G, Gatt M, Mani M, Hideshima T, Takada K, Carlson NE, Carrasco DE, Tai YT, Raje N, Letai AG, et al. Aurora kinase A is a target of Wnt/beta-catenin involved in multiple myeloma disease progression. Blood. 2009;114:2699–2708. doi: 10.1182/blood-2008-12-194290. [DOI] [PubMed] [Google Scholar]
- 38.Sumi K, Tago K, Kasahara T, Funakoshi-Tago M. Aurora kinase A critically contributes to the resistance to anti-cancer drug cisplatin in JAK2 V617F mutant-induced transformed cells. FEBS Lett. 2011;585:1884–1890. doi: 10.1016/j.febslet.2011.04.068. [DOI] [PubMed] [Google Scholar]
- 39.Wu CC, Yu CT, Chang GC, Lai JM, Hsu SL. Aurora-A promotes gefitinib resistance via a NF-kappaB signaling pathway in p53 knockdown lung cancer cells. Biochem Biophys Res Commun. 2011;405:168–172. doi: 10.1016/j.bbrc.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Cammareri P, Scopelliti A, Todaro M, Eterno V, Francescangeli F, Moyer MP, Agrusa A, Dieli F, Zeuner A, Stassi G. Aurora-a is essential for the tumorigenic capacity and chemoresistance of colorectal cancer stem cells. Cancer Res. 2010;70:4655–4665. doi: 10.1158/0008-5472.CAN-09-3953. [DOI] [PubMed] [Google Scholar]
- 41.Maris JM, Morton CL, Gorlick R, Kolb EA, Lock R, Carol H, Keir ST, Reynolds CP, Kang MH, Wu J, Smith MA, Houghton PJ. Initial testing of the aurora kinase A inhibitor MLN8237 by the Pediatric Preclinical Testing Program (PPTP) Pediatr Blood Cancer. 2010;55:26–34. doi: 10.1002/pbc.22430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qi W, Cooke LS, Liu X, Rimsza L, Roe DJ, Manziolli A, Persky DO, Miller TP, Mahadevan D. Aurora inhibitor MLN8237 in combination with docetaxel enhances apoptosis and anti-tumor activity in mantle cell lymphoma. Biochem Pharmacol. 2011;81:881–890. doi: 10.1016/j.bcp.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carol H, Boehm I, Reynolds CP, Kang MH, Maris JM, Morton CL, Gorlick R, Kolb EA, Keir ST, Wu J, Wozniak AE, Yang Y, et al. Efficacy and pharmacokinetic/pharmacodynamic evaluation of the Aurora kinase A inhibitor MLN8237 against preclinical models of pediatric cancer. Cancer Chemother Pharmacol. 2011 doi: 10.1007/s00280-011-1618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hook KE, Garza SJ, Lira ME, Ching KA, Lee NV, Cao J, Yuan J, Ye J, Ozeck M, Shi ST, Zheng X, Rejto PA, et al. An integrated genomic approach to identify predictive biomarkers of response to the Aurora kinase inhibitor PF-03814735. Mol Cancer Ther. 2012 doi: 10.1158/1535-7163.MCT-11-0184. [DOI] [PubMed] [Google Scholar]
- 45.Dai Y, Chen S, Venditti CA, Pei XY, Nguyen TK, Dent P, Grant S. Vorinostat synergistically potentiates MK-0457 lethality in chronic myelogenous leukemia cells sensitive and resistant to imatinib mesylate. Blood. 2008;112:793–804. doi: 10.1182/blood-2007-10-116376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Swaminathan Padmanabhan TCS, Julie M. Vose, Craig B. Reeder, Jesus G Berdeja, Kevin T. McDonagh, Andre Goy, Kevin R Kelly, Xiaofei Zhou, Hua Liu. Howard Fingert and Nathan Fowler: Phase I Study of An Investigational Aurora A Kinase Inhibitor MLN8237 In Patients with Advanced Hematologic Malignancies. Annual Meeting of the American Society of Hematology. 2010 [Google Scholar]
- 47.Stuart L, Goldberg PF, Michael D Craig, Emmanuel Gyan, John Lister, Jeannine Kassis, Arnaud Pigneux MD, Gary J Schiller, JungAh Jung E. Jane Leonard, Howard Fingert, and Peter Westervelt: Phase 2 Study of MLN8237, An Investigational Aurora A Kinase (AAK) Inhibitor In Patients with Acute Myelogenous Leukemia (AML) or Myelodysplastic Syndromes (MDS) Annual Meeting of the American Society of Hematology. 2010 [Google Scholar]
- 48.Dees EC, Infante JR, Burris HA, Astsaturov IA, Stinchcombe T, Liu H, Galvin K, Venkatakrishnan K, Fingert H, Cohen RB. Phase I study of the investigational drug MLN8237, an Aurora A kinase (AAK) inhibitor, in patients (pts) with solid tumors. ASCO Annual Meeting. 2010;28:15s. [Google Scholar]