Abstract
Background
Studies have shown that the incidence of melanoma in situ (MIS) is increasing significantly.
Objective
This study analyzes selected clinical and demographic characteristics of MIS cases observed in private dermatology practices in the US.
Methods
This study collected 257 MIS cases from 4 private dermatology practices in the US from January 2005 through December 2009, recording age, gender, anatomic location, lesion size, patient-reported change in lesion and concern about lesion. Case totals for invasive melanoma during the same period were recorded.
Results
The data collected showed a higher incidence of MIS in sun-exposed areas of older patients, especially males. The median age of patients at the time of MIS detection was 69. The most common site for MIS was the head-neck region. The number of MIS cases collected exceeded the number of invasive malignant melanoma (MM) cases during the study period, with an observed ratio of 1.37:1.
Limitations
For 136 patients, data were collected retrospectively for lesion size, location, gender, and age. For these patients, patient-reported change in lesion and concern about lesion were not collected. Patients often did not consent to a full body examination, therefore, it is possible that MIS lesions may have been missed in double-clothed areas.
Conclusion
Careful attention to pigmented lesions, even lesions < 4mm, on sun-exposed areas, including scalp, trunk, and feet, will facilitate earlier diagnosis of MIS. As only 30.4% of males and 50% of females had concern about these lesions, it still falls to the dermatologist to discover MIS.
Keywords: melanoma in situ, melanoma, sun-exposure, early detection, patient concern, scalp, trunk
Introduction
The incidence of melanoma in situ (MIS) is increasing faster than the incidence of invasive melanoma. 1,2,3,4,5,6 In 2005, an estimated 59,580 cases of invasive melanoma and 46,170 MIS were diagnosed.2 In contrast, an estimated 58,720 cases of invasive melanoma and 53,170 cases of MIS were diagnosed in 2009.1 The purpose of this report is to present clinical characteristics of MIS seen in a recent 5-year period in four private practice clinics in the US.
Methods
All pathology reports with a diagnosis of melanoma in situ (MIS) and invasive melanoma (MM) encountered from January, 2005 through December, 2009 in 3 clinics were included: The Dermatology Center, Rolla, MO; Columbia Dermatology, Columbia, MO; and Sheard & Drugge, Stamford, CT. At Skin & Cancer Associates, Plantation, FL, 90 only cases of MIS enrolled in the study NIH R44 CA-101639-02A2, beginning in 2007, were included in the current study. At this clinic, 4% of MIS and MM cases during the study period were not included due to time constraints. No retrospective cases were included from this clinic. For both the chart review and the NIH study, all cases with the histopathology diagnosis “lentigo maligna” or “melanoma in situ” were included in the MIS totals. Lesions lacking a definite diagnosis, e.g. those with a diagnosis of “evolving MIS,” “features of MIS,” or “consistent with MIS,” were not included. These criteria allow for greater certainty that we have not included lesions that do not fulfill histopathologic criteria for MIS. Clinical information was obtained prospectively for 121 total patients studied per the above NIH protocol, approved by the Phelps County Regional Medical Center Institutional Review Board (IRB). For these NIH study patients, a history of patient-identified lesion change and concern was tabulated. For the other 136 patients and five of the NIH study patients with incomplete data, information was collected retrospectively using diagrams in medical records including anatomic location, gender, age and size, with the modified protocol approved by the Phelps County Regional Medical Center IRB. Five patients lacked information on size of lesion. Dermoscopy was used to assess all lesions.
Dermoscopic mole monitoring was used to assess size and dermoscopic stability on nevi with an equivocal exam which were not biopsied initially.
Results
1. Patient characteristics
The study included 155 males and 102 females, ranging in age from 16 to 96, with a median age of 69 (Figure 1). 187 of 257 patients (117 males, 70 females) were 60 and older. Under the age of 60, the numbers of females and males in the study were more nearly equal (38 males, 32 females). Of the 100 patients for whom race was recorded, 99 were white and 1 was black
Figure 1.
Occurrence of melanoma in situ by age: 257 melanomas in situ, 4 private practice clinics in Plantation, Florida; Columbia and Rolla, Missouri; and Stamford, Connecticut: 2005–2009.
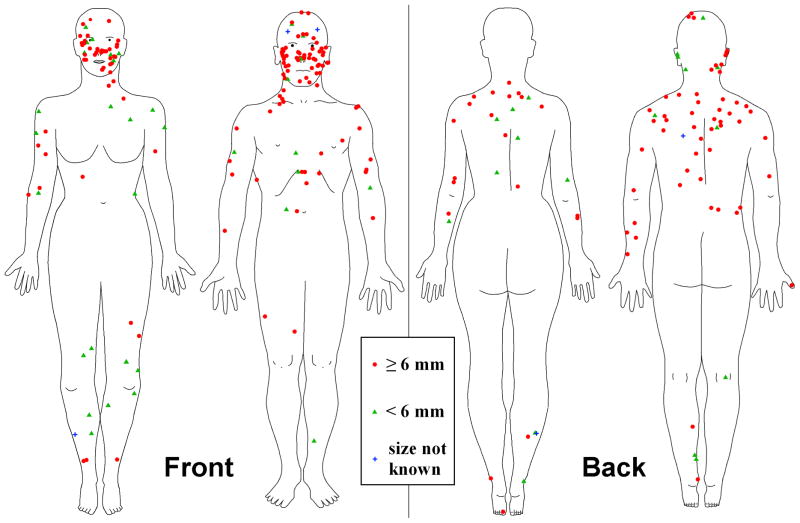
2. Anatomic location and gender
Anatomic location of all 257 lesions is shown in Figure 2. No MIS were detected in the region between the navel and the upper thigh for either gender or on female scalp/ears. The most concentrated MIS location was the head-neck, which accounted for 119 of 257 (46.3%) of lesions. Of the 119 head-neck lesions, 43 (36.1%) were female and 76 (63.9%) were male. Of this head-neck subset of lesions, 39 (90.7%) of the females and 72 (94.7%) of the males were at least 50 years of age. Females had more MIS on the legs than males: 20 vs. 8. Males had more MIS on the left arm than the right arm (12 left, 8 right), while female MIS were nearly equal (7 left, 8 right). Males had more MIS on the back, 33 vs. 15; chest, 6 vs. 3; and abdomen, 8 vs. 2.
Figure 2.
Anatomic location: 257 melanomas in situ, 4 private practice clinics in Plantation, Florida; Columbia and Rolla, Missouri; and Stamford, Connecticut: 2005–2009.
3. Lesion size
Size of the lesion, defined as the greatest diameter of the lesion, ranged from 1–120 mm, with a median of 9mm for both genders. 53 lesions (20.6%) were < 6mm, and 13 lesions (5.1%) < 4mm. For the 53 lesions < 6mm, 32 (60.4%) were in females.
4. Concern/change
Only the minority of patients scored for concern/change had concern about the lesion (47 of 121 or 38.8%) or had noticed change (57 of 121 or 47.1%). Of those with lesions < 6mm, a similar percentage had concern (36.7%) or noticed change (56.7%). Overall, 50.0% of females had concern and only 30.4% of males had concern.
5. Ratio of MIS/melanoma cases
The MIS to invasive melanoma case ratio (MIS:MM) over the five-year period was 260/190 (1.37:1). Each clinic participating in the study diagnosed more MIS than invasive melanoma (MIS:MM > 1.0 for all four clinics). This excess of MIS cases differs from the national ratio of MIS:MM reported by the Center for Disease Control and Prevention (CDC) over the same time period: 250,820/302,910 (0.83:1) 1,2,4,5,6
Comment
Our study provides support for correlation of MIS with the amount of incident ultraviolet light exposure. Although this association is also found for invasive melanoma, large series show 2–3% of melanomas occurring between navel and upper thigh7, locations that did not exhibit MIS in our study. We encouraged all patients to have full body examination, but many patients declined genital and breast examinations. MIS occurred more frequently on the head-neck (119 out of 257) than the trunk (67 out of 257), a reversal of invasive melanoma distribution.7,8
It was recently reported that MIS has left-sided predominance.9 We observed no overall left-sided predominance (124 right, 125 left, 8 midline) or in head-neck (58 right, 57 left, 4 midline) or back (22 right, 24 left) areas. However, a tendency towards gender differences did exist on arms, with left-sided predominance observed in males (12 left, 8 right) and females (7 left, 8 right). One possible explanation is that for the older age group, males were the driver, and before automobiles were air conditioned, windows were often rolled down.
Our demographic data confirm that for MIS, as for invasive melanoma, older males are at increased risk. Frequent and thorough surveillance of this demographic group is therefore warranted. Only 37 of 257 patients were less than 50 years old. These findings support previous findings of increased incidence of MIS in older patients .10 Our findings show a greater number of MIS in males for most age groups, confirming results from other studies.11,12 Females predominate in the 20–40 yrs age group, a cohort for which tanning bed and other ultraviolet light exposure is greater.13 Only in the > 90 yrs age group does greater female life expectancy allow this ratio to change, again showing female predominance.
Our findings differ from those of a recent study by Rosina in terms of lesion distribution and role of the dermatologist in lesion discovery.11 Rosina found most MIS on the trunk, while our study located most MIS in the head-neck region. The Rosina study, in a hospital setting, is not strictly comparable. The Rosina study excluded all patients with lentigo maligna (LM), usually located on the head and neck from the series.14 Excluding LM from MIS data is a departure from the current standards of many pathologists, who consider LM a subtype of MIS.14,15
Our data show a greater number of MIS than invasive melanoma detected in each of four private practice dermatology clinics in the US, which is a reversal of estimated MIS:MM reported from the CDC for the same time period. The reason for this disparity between data collected by dermatologists in private dermatology clinics and data gathered by the CDC from various specialties across the US is not known. This disparity might be attributed to the use of dermoscopy in our four clinics, which is believed to reduce the benign to malignant ratio for biopsied lesions.17,18 Data from the Argenziano study obtained from members of the International Dermoscopy Society serve to support this hypothesis. The MIS:MM ratio for non-pigmented lesion clinics during the 3 years when our study overlaps their study, 2005–2007, give an MIS:MM ratio of 0.980 for 1616 cases,17 higher than the 0.83 ratio from the US as a whole. Although the article lacked information on use of dermoscopy, the clinics involved in the study were all recruited by board members of the International Dermoscopy Society. The low MIS:MM case ratio of 0.430 for 2525 cases in pigmented lesion clinics could have been due to the much higher fraction of invasive melanomas seen in the pigmented lesion clinics.17
Our data show almost exclusive location of MIS in body areas not double-clothed. Therefore, MIS can be detected even in patients who refuse full body skin examination. It is our experience that most patients declining full body skin examination will consent to this examination when asked a second time and/or when they are offered a gown.
Caveat
One weakness in our study is that MIS in patients declining total body examination may have been missed in areas that remained covered. Another weakness of this study is the incomplete tabulation of change and concern, as these data were obtained only for the 121 NIH study patients. The NIH study patients did not differ from the other patients in terms of age (median: 69 yrs - both groups), but did show a lower male:female ratio (1.33 vs. 1.72 for chart review patients). Since a higher percentage of females report both change and concern in comparison with males, the finding that only the minority of patients had concern about their MIS or noted change would likely still be supported if concern and change statistics had been taken from the entire group. The four clinics, two in Missouri, one in Connecticut, and one in Florida, provide only a small sample of private practice patients in the US. Therefore, data may not be generalizable to MIS patients in private practices elsewhere in the US, in university settings, or in other countries.
However, the clinics are located in states with invasive melanoma incidence rates that represent both low (Missouri (11.94–20.32 per 100,000 population)), and medium high rates (Connecticut and Florida (20.33–24.73 per 100,000 population)). 19 The clinics as a group are reasonably representative of clinics with varied ultraviolet light exposure as measured by average daily total global solar radiation (AVGLO): Florida, high; Connecticut, low; and Missouri (2 clinics): median.20 Socioeconomic status has been shown to affect early-stage melanoma incidence.21 Median per-capita income for counties where these clinics are located is slightly below the national average; yet average income in these counties is above the national average: Boone County, Missouri (8% below national average), Broward County, Florida (2% below the national average), Phelps County, Missouri (24% below national average), and Fairfield County, Connecticut (79% above the national average).22
The Florida clinic provided no cases during 2005–2006. That omission allowed for a better balance of the 257 total MIS found in the four clinics: Columbia MO, 68; Plantation FL, 77; Rolla MO, 50; Stamford CT, 62.
Early diagnosis of melanoma at the MIS stage is critical because, unlike invasive melanoma, MIS does not affect life expectancy. 23 In addition, early detection allows for less invasive therapy. Toren and Parlette discusses various options for the management of MIS, including radiation, topical imiquimod, and cryotherapy.16 In our study, all MIS were excised, either with conservative excision or in a minority of cases, employing Mohs surgery with frozen sections and immunostains. Clinical margins of 5mm were obtained on all lesions when allowed by anatomic constraints. For lesions which were entirely scoop shaved, margins were taken from the biopsy scar. For larger lesions which had been sampled, margins were determined at the time of excision by dermoscopy, 24 and Woods lamp in some cases. Closure for some large lesions was delayed until pathology margins were determined, corresponding to “slow Mohs.”16 Imiquimod was used adjunctively in 2% of patients, but was never used as a monotherapy. No recurrences were observed during the duration of the study, and no second MIS occurred during the study, but two recurrences were observed later. Follow-up time was too short to determine accurate rates for recurrence or incidence of 2nd primary MIS. We conclude that careful attention to pigmented lesions, even ones smaller than 4mm, on sun-exposed areas, including scalp, trunk, and feet, will facilitate earlier diagnosis of MIS. We agree with Lucas et al.; some cases of early MIS may not demonstrate abnormal features on clinical and dermoscopic examination alone.25 These early MIS may be measured, photographed clinically and dermoscopically, and followed for change. In all four clinics, some MIS were biopsied only after dermoscopic change was documented. Only a minority of patients had any concern or noticed change in their lesions before biopsy. For the majority of MIS, it still falls to the dermatologist to discover the lesion and make the diagnosis.
Capsule Summary.
The incidence of melanoma in situ is increasing.
This article provides an anatomic distribution of melanoma in situ with analysis of age, gender, lesion size, patient-observed change and concern, and calculation of the melanoma in situ to invasive melanoma case ratio
This information emphasizes the need for careful attention to pigmented lesions, even 1–2mm, on sun-exposed areas to facilitate earlier diagnosis of MIS. Only a minority of patients had concern about these lesions, thus the role of clinicians in detecting these lesions is paramount.
Acknowledgments
The authors wish to thank Dr. Elizabeth Drugge and Kevin Iwasczuk who acquired many images and data for the study.
Role of the Sponsors: The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of NIH, the sponsor. The sponsor had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; or in the preparation, review, or approval of the manuscript.
Funding/Support: This publication was made possible by Grant Number SBIR R44 CA-101639-02A2 of the National Institutes of Health (NIH).
Footnotes
Author Contributions: Dr. William V. Stoecker and Ms. Sherea M. Stricklin had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Financial Disclosure of the Authors: No financial disclosures or conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sherea M. Stricklin, University of Missouri - School of Medicine, Columbia, MO.
William V. Stoecker, Stoecker & Associates, Rolla, MO and University of Missouri - School of Medicine, Department of Dermatology, Columbia, MO.
Joseph M. Malters, The Dermatology Center, Rolla, MO.
Rhett Drugge, Sheard & Drugge PC, Stamford, CT.
Margaret Oliviero, Skin and Cancer Associates, Plantation, FL.
Harold S. Rabinovitz, Skin and Cancer Associates, Plantation, FL.
Lindall Perry, Columbia Dermatology & Mohs Skin Cancer Surgery, Columbia, MO
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009 Jul-Aug;59(4):225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Murray T, Ward E, Samuels A, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005 Jan-Feb;55(1):10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 3.Criscione VD, Weinstock MA. Melanoma thickness trends in the United States, 1988–2006. J Invest Dermatol. 2010;130(3):793–7. doi: 10.1038/jid.2009.328. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Seigel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006 Mar-Apr;(2):106–30. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 5.Jemal A, Seigel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 6.Jemal A, Seigel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 7.Habif TF. Clinical Dermatology. 5. St. Louis, MO: Mosby; 2009. p. 863. [Google Scholar]
- 8.Pruthi DK, Guilfoyle R, Nugent Z, Wiseman MC, Demers AA. Incidence and anatomic presentation of cutaneous malignant melanoma in central Canada during a 50-year period: 1956 to 2005. J Am Acad Dermatol. 2009;61(1):44–50. doi: 10.1016/j.jaad.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 9.Butler ST, Fosko SW. Increased prevalence of left-sided skin cancers. J Am Acad Dermatol. 2010 Mar 10; doi: 10.1016/j.jaad.2009.11.032. [DOI] [PubMed] [Google Scholar]
- 10.Crocetti E, Caldarella A, Chiarugi A, Nardini P, Zappa M. Does in situ melanoma really come before invasive melanoma? Descriptive epidemiology questions this relationship. Tumori. 2011 Mar-Apr;97(2):257. doi: 10.1700/667.7797. [DOI] [PubMed] [Google Scholar]
- 11.Rosina P, Tessari G, Giodano M, Girolomoni G. Clinical and diagnostic features of in situ melanoma and superficial spreading melanoma: a hospital based study. J Eur Acad Dermatol Venereol. 2011 Mar; doi: 10.1111/j.1468–3083.2011.04015.x. [DOI] [PubMed] [Google Scholar]
- 12.Merrill RM. Risk-adjusted melanoma skin cancer incidence rates in Whites (United States) Melanoma Res. 2011 Dec;21(6):535–40. doi: 10.1097/CMR.0b013e328349420f. [DOI] [PubMed] [Google Scholar]
- 13.Ting W, Schultz K, Cac NN, Peterson M, Walling HW. Tanning bed exposure increases the risk of malignant melanoma. Int J Dermatol. 2007 Dec;46(12):1253–7. doi: 10.1111/j.1365-4632.2007.03408.x. [DOI] [PubMed] [Google Scholar]
- 14.Smallberger GJ, Siegel DM, Khachemoune A. Lentigo maligna. 2008 Nov-Dec;21(6):439–46. doi: 10.1111/j.1529-8019.2008.00244.x. [DOI] [PubMed] [Google Scholar]
- 15.Swetter SM, Boldrick JC, Jung SY, Egbert BM, Harvell JD. Increasing incidence of lentigo maligna melanoma subtypes: northern California and national trends 1990–2000. J Invest Dermatol. 2005 Oct;125(4):685–91. doi: 10.1111/j.0022-202X.2005.23852.x. [DOI] [PubMed] [Google Scholar]
- 16.Toren KL, Parlette EC. Managing Melanoma In Situ. Semin Cutan Med Surg. 2010 Dec;29(4):258–63. doi: 10.1016/j.sder.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Argenziano G, Cerroni L, Zalaudek I, Staibano S, Hofmann-Wellenhof R, et al. Accuracy in melanoma detection: A 10-year multicenter survey. J Am Acad Dermatol. doi: 10.1016/j.jaad.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 18.Carli P, De Giorgi V, Crocetti E, Mannone F, Massi D, Chiarugi A, et al. Improvement of malignant/benign ratio in excised melanocytic lesions in the ‘dermoscopy era’: a retrospective study 1997–2001. Br J Dermatol. 2004;150:687–92. doi: 10.1111/j.0007-0963.2004.05860.x. [DOI] [PubMed] [Google Scholar]
- 19.Watson M, Johnson CJ, Chen VW, Thomas CC, Weir HK, Sherman R, et al. Melanoma surveillance in the United States: Overview of methods. J Am Acad Dermatol. 2011 Nov;65(5 Suppl 1):S6–S16. doi: 10.1016/j.jaad.2011.04.037. [DOI] [PubMed] [Google Scholar]
- 20.Richards TB, Johnson CJ, Tatalovich Z, Cockburn M, Eide MJ, Henry KA, et al. Association between cutaneous melanoma incidence rates among white US residents and county-level estimates of solar ultraviolet exposure. J Am Acad Dermatol. 2011 Nov;65(5 Suppl 1):S50–7. doi: 10.1016/j.jaad.2011.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ajani UA, Johnson CJ, Roland KB, Eide M, Jemal A, Negoita S, Bayakly RA, Ekwueme DU. Association of cutaneous melanoma incidence with area-based socioeconomic indicators-United States, 2004–2006. J Am Acad Dermatol. 2011 Nov;65(5 Suppl 1):S58–68. doi: 10.1016/j.jaad.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 22.U.S. Census Bureau. [Accessed 19 November 2011]; < http://quickfacts.census.gov/qfd/index.html>.
- 23.Mocellin S, Nitti D. Cutaneous melanoma in situ: translational evidence from a large population-based study. Oncologist. 2011;16(6):896–903. doi: 10.1634/theoncologist.2010-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robinson JK. Use of digital epiluminescence microscopy to help define the edge of lentigo maligna. Arch Dermatol. 2004 Sep;140(9):1095–1100. doi: 10.1001/archderm.140.9.1095. [DOI] [PubMed] [Google Scholar]
- 25.Lucas CR, Sanders LL, Murray JC, Myers SA, Hall RP, Grichnik JM. Early melanoma detection: nonuniform dermoscopic features and growth. J Am Acad Dermatol. 2003 May;48(5):663–71. doi: 10.1067/mjd.2003.283. [DOI] [PubMed] [Google Scholar]