Abstract
Background
Patients with thalassemia have low circulating levels of many nutrients, but the contribution of dietary intake has not been assessed.
Objective
Assess dietary intake in a large contemporary sample of patients with thalassemia.
Design
Prospective, longitudinal cohort study using a validated food frequency questionnaire
Participants
221 patients (19.7±11.3 yrs, 106 female) categorized into three age groups: young children (3–7.9 y), older children/adolescents (8–18.9 yr), and adult (≥ 19 yr). 78.8% β-thalassemia; 90% chronically transfused.
Setting
10 hematology outpatient clinics in the United States and Canada.
Main outcome measures
Comparison of intake with U.S. Dietary Reference Intakes, and correlation with serum 25-OH vitamin D and total body iron stores.
Statistical Analyses Performed
Intake was defined as inadequate if less than the estimated average requirement (EAR). Chi-square, Fisher’s exact and Student’s t-test were utilized to compare intake between age categories and logistic regression analysis to test the relationship between intake and outcomes, controlling for age, gender and race.
Results
Over 30% of patients consumed inadequate levels of vitamin A, D, E, K, folate, calcium, and magnesium. The only nutrients for which >90% of patients consumed adequate amounts were riboflavin, vitamin B12 and selenium. Dietary inadequacy increased with increasing age group (p<0.01) for vitamins A, C, E, B6, folate, thiamin, calcium, magnesium and zinc. Over half the sample took additional supplements of calcium and vitamin D, although circulating levels of 25-OH vitamin D remained insufficient in 61% of patients. Dietary iron intake was not related to total body iron stores.
Conclusion
Patients with thalassemia have reduced intake of many key nutrients. These preliminary findings of dietary inadequacy is concerning and supports the need for nutritional monitoring to determine which patients are at greatest risk for nutritional deficiency. Future research should focus on the effect of dietary quality and nutritional status on health outcomes in thalassemia.
Keywords: Thalassemia, dietary intake, iron, vitamin D
Introduction
Thalassemia, a term that defines a group of deficiencies in the production of the alpha or beta globin chain of hemoglobin, is one of the most common single gene disorders in humans. Nearly 60% of the total population is affected in some regions of Thailand, Laos and Cambodia (1), and although a much smaller prevalence is found in North American (0.1%) its incidence is increasing (1, 2,3). Over 200 alpha and beta globin gene mutations have been identified. In its most severe form, individuals with either alpha- (especially Hemoglobin H constant spring) or beta-thalassemia require routine red blood cell transfusions soon after birth for survival.
The most common cause of death is cardiac failure resulting from transfusional iron overload(4,5). However, as chelation therapies have improved, patients are living longer and nutritional status is becoming increasingly important. Patients with thalassemia commonly exhibit inadequate growth, poor immune function, increased oxidative stress and decreased bone mineralization all morbidities with linkages to poor nutritional status(6,7,8). Recently it has been shown that patients with thalassemia have reduced body fat and lean mass and that these alterations in body composition are related to both reduced growth and decreased bone density.(9) Additionally, Claster et al (2009) reported that over half of a sample of regularly transfused patients with thalassemia residing in Los Angeles had deficient circulating levels of vitamin A, C, D and selenium(10). However, there is a paucity of data on the contribution of dietary intake to these possible nutritional deficiencies in thalassemia.
The objective of this study was to assess the dietary intake of key nutrients in a large, contemporary sample of patients with thalassemia, identified through the Thalassemia Clinical Research Network (TCRN), and to compare their intake to the United States Dietary Reference Intakes. The specific hypotheses for this study were as follows: (a) patients with thalassemia have an inadequate dietary intake of key nutrients in comparison with age and gender specific recommendations, (b) dietary intake of vitamin D is insufficient to maintain adequate vitamin D status and (c) dietary intake of iron is unrelated to total body iron stores, particularly in chronically transfused patients.
Methods
This study was conducted as part of the TCRN Thalassemia Longitudinal Cohort study. The TCRN is an National Heart Lung and Blood Institute funded research network composed of six core centers in the United States, Canada and the United Kingdom and their 10 associated satellite centers. Patients with thalassemia who were regularly cared for at one of these centers were invited to participate in the longitudinal cohort study between May, 2007 and December, 2009. The overall goal of the longitudinal cohort study was to describe the prevalence and incidence of complications related to thalassemia. The study is a prospective, longitudinal, cohort study with baseline and annual collection of routine clinical care data through chart review, patient questionnaires and detailed genotyping characterization. Patient inclusion criteria were: diagnosis of thalassemia regardless of genotype but in general, more severe phenotypes who required a minimum of annual monitoring of co-morbidities at their local clinic, both genders and all ages. Exclusion criteria included subjects with thalassemia trait, those with alpha thalassemias and a hemoglobin >9 g/dL with no history of significant complications, those who had received a bone marrow transplant, or those subjects unwilling to be followed on an annual basis. The protocol was approved by the TCRN Data and Safety Monitoring Board and by the ethical review boards of all participating TCRN institutions. Informed written consent, and assent in the case of a minor, was obtained from all participants.
At baseline and again one year later subjects were asked to complete a validated, self administered food frequency questionnaire. The adults (19+ years) completed the 110 food item, Block 2005© (Nutrition Quest, Berkeley, CA) questionnaire (11,12), the older children and adolescents (aged 8 – 18 years) completed the 77 food item Block 2004©, and the care-givers of young children (<8 years) completed the 90 food item Block 2004© for kids (13,14,15). All questionnaires were designed to estimate usual intake from a wide array of commonly consumed foods. The food lists were developed from National Health and Nutrition Examination Survey (1999–2002) dietary recall data; the nutrient database was developed from the United States Department of Agriculture Food and Nutrient Database for Dietary Studies, version 1.0 (16,17). For most individuals the questionnaire takes 30 minutes to complete. Portion size was quantified for each food according to a series of pictures and at the end of each questionnaire, a series of "adjustment" questions improved the accuracy in assessing fat and carbohydrate intake. On the adult and adolescent questionnaires, supplement usage was also quantified. Questionnaires were sent to Nutrition Quest (Berkeley, CA) for analysis and then results forwarded to the TCRN data coordinating center (New England Research Institutes, Watertown, MA). Intake of individual nutrients were quantified and defined as inadequate if less than the estimated average requirement (EAR) for age and gender (18,19,20,21,22). By definition, the EAR is the intake level at which the reference data indicate that the needs of 50% of a healthy population will be met. It is typically used to assess the diets of groups of individuals after corrections have been made for day-to-day variation (23,24). For vitamin K where an EAR was not available, the adequate intake recommendation was used in its place. The tolerable upper intake level for a nutrient was defined as the level above which the habitual intake of a particular nutrient may result in adverse effects. Additionally individual food choices were quantified into ‘MyPyramid’ food group servings within each food frequency questionnaire. These servings were then compared to the USDA recommended servings for dairy, whole grains, fruits, vegetables and meat [http://www.mypyramid.gov]. Since the time when the analytical and statistical analyses were performed in this study, the United States Department of Agriculture recommendations have been changed from ‘MyPyramid’ food group servings to ‘MyPlate’. Unfortunately, data from the Block Food Frequency could not be reanalyzed to reflect this change.
Circulating 25-OH vitamin D (ng/mL) and liver iron concentration, a proxy for total body iron stores, were assessed clinically and recorded if performed within the previous year. Height and weight were self-reported as part of the food frequency questionnaire. Other relevant medical history, such as, thalassemia genotype, year chronic transfusion was initiated, and serum ferritin and liver iron concentration values were obtained by review of medical records. As medical history and laboratory data were obtained primarily through chart review, not all patients have all data points, therefore sample size is included in each table when the sample is less than the total.
Statistical Analysis
Calculated Variables and Definitions
Age groups were defined as young children (3–7.9 years), older children and adolescents (8.0–18.9 year), and adults (≥ 19+ years). Subjects were categorized as transfused if they were currently receiving transfusion therapy on a routine basis, eight or more transfusions during the 12 months prior to entering the study. They were categorized as non-transfused, if they were receiving fewer than eight transfusions per year or were not currently receiving transfusion therapy. Body mass index was calculated as kilogram of body weight per height in square meters. Estimated energy requirement (EER) was calculated for each individual using their age, gender, height and weight according to the Institute of Medicine Dietary Reference Intake equations (25). Given anecdotal evidence which suggests that the majority of patients with thalassemia participate in limited physical activity outside the home (26), EER was estimated based on a sedentary lifestyle activity coefficient of 1.0. Percentage of EER was calculated as %EER= (kcal/EER) multiplied by 100.
Analyses
Continuous variables were summarized as means with standard deviations and categorical variables were summarized as percentages. General linear models were used to model the effect of iron and vitamin D intake on total body iron stores and circulating vitamin D levels after controlling for age, gender, race and transfusion status. When the influence of race was explored, racial categories were collapsed to compare Caucasian vs. non-caucasian races (asian, black, mixed, other). Subgroup analyses were also performed in the non-transfused thalassemia group alone. All inferences are based on two-tailed tests with a threshold of alpha = 0.05 for declaring significance. All analyses were conducted using Statistical Analysis Software (version 9.1.3, 2006, SAS Institute, Cary, NC).
Results
Subject Characteristics
A total of 302 subjects with thalassemia from sites which submitted food frequency questionnaire surveys were originally recruited and consented to participate in the larger trial, of which 221 completed the baseline nutritional assessments (73.2%). There were no significant differences between those who completed or did not complete the food frequency questionnaire in terms of gender, ethnicity or transfusion status. However, in the adult cohort, subjects who completed the food frequency questionnaire were younger compared to the non-completers, 29.4 vs. 33.8 years, p=0.02; and in the child cohort, the subjects who completed the questionnaire were 2 years older (5.9 vs. 3.7 years, p<0.001) compared to those who did not. Of the 221 subjects included in this study, 60 were from Canada (14 younger children, 32 older children/adolescents, 14 adults).
The majority of the subjects were receiving chronic transfusion therapy (Table 1), as this type of subject commonly received routine clinical monitoring and was therefore more likely to be included in the Thalassemia Longitudinal Cohort Study selection process. Iron overload, reflected in elevated serum ferritin and liver iron concentration, was significantly higher in the adult group of subjects who were on transfusion therapy for a longer duration (10.3 yrs vs. 9.3 to 9.4 yrs in the younger subjects, p<0.001). There was a non-significant correlation between liver iron concentration and years of chronic transfusion therapy for the group as a whole (r=0.09, p=0.26). A slightly different distribution of thalassemia genotype and race were exhibited by age group (Table 1), with a larger percentage of Asians in the youngest cohort. There were, however, no differences in gender distribution by age category. Serum 25OH vitamin D was insufficient (<30 ng/dL) in 63% of the subject population [mean: 27.2 ± 13.1 ng/dL].
Table 1.
Baseline characteristics of subjects with thalassemia, including anthropometrics, genotype, transfusion history and total body iron stores
| Total | Young Children | Older Children & Adolescents |
Adults | p-value2 | |
|---|---|---|---|---|---|
| Number of Subjects | 221 | 30 | 83 | 108 | - |
| Age, years 1 | 19.7±11.3 | 5.4 ±1.4 | 12.6±3.3 | 29.0 ±8.3 | - |
| Gender, % male | 48.0% | 40.0% | 54.2% | 45.4% | 0.31 |
| Race, % | Asian, 51.6% Caucasian, 43.4% Other, 5.0% | Asian, 80.0% Caucasian, 13.3% Other, 6.7% | Asian, 53.0% Caucasian, 38.6% Other, 8.4 % | Asian, 42.6% Caucasian, 55.6% Other, 1.9% | <0.0001 |
| Body Mass Index, kg/m2 | 20.3±4.5 | 15.9±2.7 | 18.3±4.1 | 22.9 ±3.3 | - |
| Thalassemia Genotype,3 % of sample | β-thalassemia, 78.8% E- β Thal, 13.8% Hb H/CS, 4.6% Other, 2.8% | β-thalassemia, 73.3% E- β Thal, 10.0% Hb H/CS, 13.3% Other, 3.3% | β-thalassemia, 80.5% E- β Thal, 8.5% Hb H/CS, 6.0% Other, 5.0% | β-thalassemia, 79.0% E- β Thal, 19.0% Hb H/CS, 1.0% Other, 1.0% | 0.01 |
| Chronically transfused, % of sample | 90.0% | 96.0% | 94.5% | 85.4% | 0.09 |
| Years on chronic transfusion therapy | 16.5±11.0 | 3.8±1.7 | 10.5±4.0 | 24.7±9.6 | <0.0001 |
| Serum ferritin, ng/mL (range) 4,5 | 1236 (85 to 14,835) {n=216} | 921 (89 to 3218) {n=29} | 1136 (85 to 9480) {n=82} | 1441 (129 to 14,835) {n=105} | 0.004 |
| Liver Iron Concentration, 6 mg/kg (range) | 9.6 (1.0 – 42.9) {n=195} | 9.4 (3.0 – 21.3) {n=23} | 9.3 (1.1 – 42.9) {n=75} | 10.0 (1.0 – 40.0) {n=97} | <0.0001 |
Values are presented as Mean±SD (range)
For continuous variables, p-values are from ANOVA, for categorical variables, p-values are from Fisher’s Exact test.
Thalassemia Genotype: E-B Thal: E-beta thalassemia, HbH/CS: Hemoglobin H/constant spring
Where numbers of subjects are different than the group as a whole, sample size is included in {}.
Due to skewness of the data, serum ferritin and liver iron concentration are presented as Median (range) and all analyses are performed in log scale
Liver iron concentration: determined from either magnetic resonance imaging, liver biopsy or Ferritometer.
When data were analyzed for the adult sub-group who completed the food frequency questionnaire at both time points (n=46), there were no differences between the average intakes at baseline and those at year 1 in any of the macro- or micronutrients assessed (data not shown), that is the variability within the nutrient studied was greater than the change observed between the baseline and year 1 visits. Given the stability of the dietary intake data, for simplicity, only the baseline data are presented herein.
The average servings of many of the major food groups were lower than that recommended for healthy adolescents and adults (Table 2; 27). In particular, servings of dairy products (milk, yogurt, cheese), and whole grains (cereals, grains, bread) were significantly lower for both the older children/adolescents and adult groups (all p<0.01). Adult patients with thalassemia consumed an adequate number of servings per day of vegetables, particularly dark green vegetables, and meat (meat, poultry, legumes).
Table 2.
Food Group Servings: Comparison between Subjects with Thalassemia and 2006 USDA Recommendations
| Older Children & Adolescents | Adults | |||
|---|---|---|---|---|
| Food Group | USDA Recommendation* |
Thalassemia N=83 |
USDA Recommendation |
Thalassemia N=108 |
| Whole Grains, oz | 3.0 – 3.5 | 0.9 ± 1.1 | 3.0 – 4.0 | 0.8 ± 0.7 |
| Fruits, cups | 1.5 – 2.0 | 1.3 ± 1.2 | 1.5 – 2.0 | 1.0 ± 0.8 |
| Vegetables, cups | 2.0 – 3.0 | 1.7 ± 1.4 | 2.5 – 3.0 | 2.9 ± 2.6 |
| Dark Green Vegetables, cups | 0.29 – 0.43 | 0.3 ± 0.3 | 0.43 | 0.4 ± 0.5 |
| Orange Vegetables, cups | 0.21 – 0.29 | 0.1 ± 0.1 | 0.29 | 0.1 ± 0.2 |
| Dairy, cups | 3.0 | 1.3 ± 1.0 | 3.0 | 1.3 ± 1.2 |
| Meat, oz | 5.0 – 6.0 | 4.4 ± 4.0 | 5.0 – 6.5 | 5.1 ± 4.7 |
Food Groups: collection of foods that share similar nutritional properties.
The Food Guide Pyramid was designed to educate consumers about daily servings of each food group which comprise a healthy diet based on the 2005 Dietary Guidelines for Americans [27].
USDA Recommendation- ‘My Pyramid’ food group recommendations are the minimum number of servings for an individual who participates in less than 30 minutes of activity per day (www.mypyramid.gov). Ranges (in shaded columns) are provided for gender differences. My food group servings were not calculated in the Block kids FFQ; therefore, are not available for the “young child” group.
Macronutrient Intake
Body mass index was in the normal range (18–25) for 74% of adult thalassemia subjects studied, and weight (Δ=1.2±4.6 kg) and body mass index (Δ=0.4±2.1kg/m2) did not change significantly between the baseline and year 1 assessment for the adults in this study. Estimated average kilocalorie intake was between 107 to 163% of estimated caloric requirement based on age, weight, height and an assumed sedentary lifestyle. The difference between intake and expenditure was much greater in younger children compared to adults (p=0.021), but not different between the adolescents and adults (Table 3). Older children and adolescents with thalassemia met the acceptable macronutrient distribution range for fat intake (Table 3: 25–35% of kcal as fat), carbohydrate (45–65% of kcal) and protein (10–30% of kcal). However, adults were consuming more kilocalories as fat on average than is considered acceptable for healthy adult individuals (~38% vs. acceptable range: 20–35%). Average dietary fiber intake was far less than recommendations (25 to 38 grams), regardless of age group considered.
Table 3.
Average Dietary Intake of Energy, Macro-Nutrients, and Micro-Nutrients in Subjects with Thalassemia Separated by Age Group 1
| Total | Young Children | Older Children & Adolescents |
Adults | |
|---|---|---|---|---|
| Number of Subjects | 221 | 30 | 83 | 108 |
| Energy Intake & Requirements | ||||
| Total kilo-calories | 1813 ± 908 | 1852 ± 853 | 1752 ± 813 | 1850 ± 996 |
| Estimated Energy Requirement (kcal/d) | 1749 ± 439 | 1203 ± 110 | 1580 ± 346 | 2008 ± 360 |
| %Estimated Energy Requirement 2 | 116.9 ± 91.7% | 163.4 ± 79.1% | 114.8 ± 52.9% | 106.9 ± 111.9% |
| Macro-Nutrients | ||||
| Protein, grams | 70.7 ± 38.6 | 66 ± 30.3 | 66.7 ± 33.6 | 75.2 ± 43.8 |
| Protein, % of kcal | 15.7 ± 3.4 | 14.4 ± 2.4 | 15.2 ± 3.0 | 16.5 ± 3.7 |
| Carbohydrate, % of kcal | 49.1 ± 8.4 | 52.9 ± 5.2 | 51.9 ± 6.7 | 45.8 ± 9.1 |
| Dietary fiber, grams | 13.8 ± 7.9 | 13.4 ± 6.8 | 13.7 ± 7.7 | 14 ± 8.3 |
| Fat, % of kcal | 35.9 ± 6.3 | 34.4 ± 4.7 | 34.0 ± 5.4 | 37.8 ± 6.8 |
| Saturated Fat, grams | 23.6 ± 13.1 | 25.3 ± 11.7 | 22.1 ± 11.0 | 24.3 ± 14.8 |
| Cholesterol, mg | 256.1 ± 161.6 | 240.3 ± 115.1 | 242.9 ± 168.7 | 270.9 ± 167.1 |
| Fat Soluble Vitamins | ||||
| Vitamin A, RAE | 724.6 ± 554.3 | 1250.1 ± 766.9 | 539.3 ± 306.1 | 720.9 ± 544.3 |
| Vitamin D, IU | 149.1 ± 103.7 | 194.7 ± 96.2 | 153.1 ± 101 | 133 ± 104.7 |
| Vitamin E, mg | 6.9 ± 3.9 | 7.2 ± 3 | 6.0 ± 3.7 | 7.5 ± 4.1 |
| Vitamin K, ug | 143.1 ± 151.7 | 57.7 ± 39.3 | 85.8 ± 84.8 | 189.6 ± 176.4 |
| Water soluble vitamins | ||||
| Vitamin C, mg | 121.0 ± 83.3 | 122.6 ± 92.2 | 135.2 ± 86.6 | 109.5 ± 76.8 |
| Folate, mcg | 292.5 ± 162.1 | 352 ± 192.1 | 315.8 ± 154.9 | 257.5 ± 151.5 |
| Thiamin, mg | 1.4 ± 0.7 | 1.6 ± 0.8 | 1.4 ± 0.7 | 1.4 ± 0.8 |
| Niacin, mg | 19.0 ± 10.7 | 18.0 ± 9.1 | 17.5 ± 9 | 20.5 ± 12.1 |
| Riboflavin, mg | 1.8 ± 0.9 | 2 ± 0.9 | 1.7 ± 0.8 | 1.8 ± 0.9 |
| Minerals | ||||
| Calcium, mg | 792.1 ± 417 | 858.7 ± 354.6 | 785.6 ± 404.3 | 778.4 ± 444.1 |
| Magnesium, mg | 245.6 ± 126 | 234.9 ± 110.4 | 227.3 ± 111.6 | 263 ± 138.8 |
| Selenium, mcg | 93.9 ± 52.5 | 89.1 ± 41.1 | 87.0 ± 45.0 | 100.7 ± 59.8 |
| Phosphorus, mg | 1158.1 ± 577.1 | 1210.3 ± 528.3 | 1124.4 ± 535.9 | 1169.8 ± 623.2 |
| Copper, mg | 1.2 ± 0.7 | 1.1 ± 0.5 | 1.1 ± 0.6 | 1.3 ± 0.8 |
| Iron, mg | 12.5 ± 6.7 | 13.1 ± 7.5 | 12.1 ± 6.1 | 12.5 ± 7 |
| Zinc, mg | 10.2 ± 6.3 | 8.6 ± 4.2 | 9.7 ± 5.0 | 11.0 ± 7.6 |
Data expressed as Mean ± SD. All data in this table are total nutrient intakes from diet alone, supplementation not included.
% Estimated Energy Requirement (EER): Kilocalorie intake/EER, where EER is calculated from individual patient age, weight, height, and physical activity coefficient of 1.0 = sedentary physical activity.
Vitamins and Mineral Intake from Dietary Sources
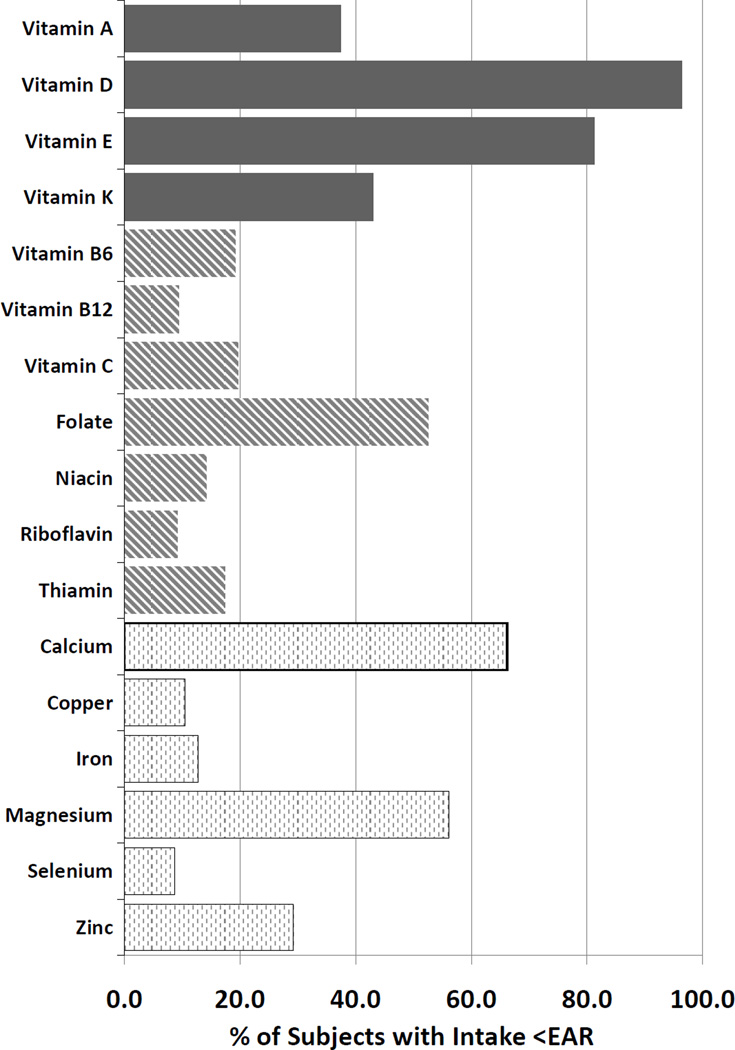
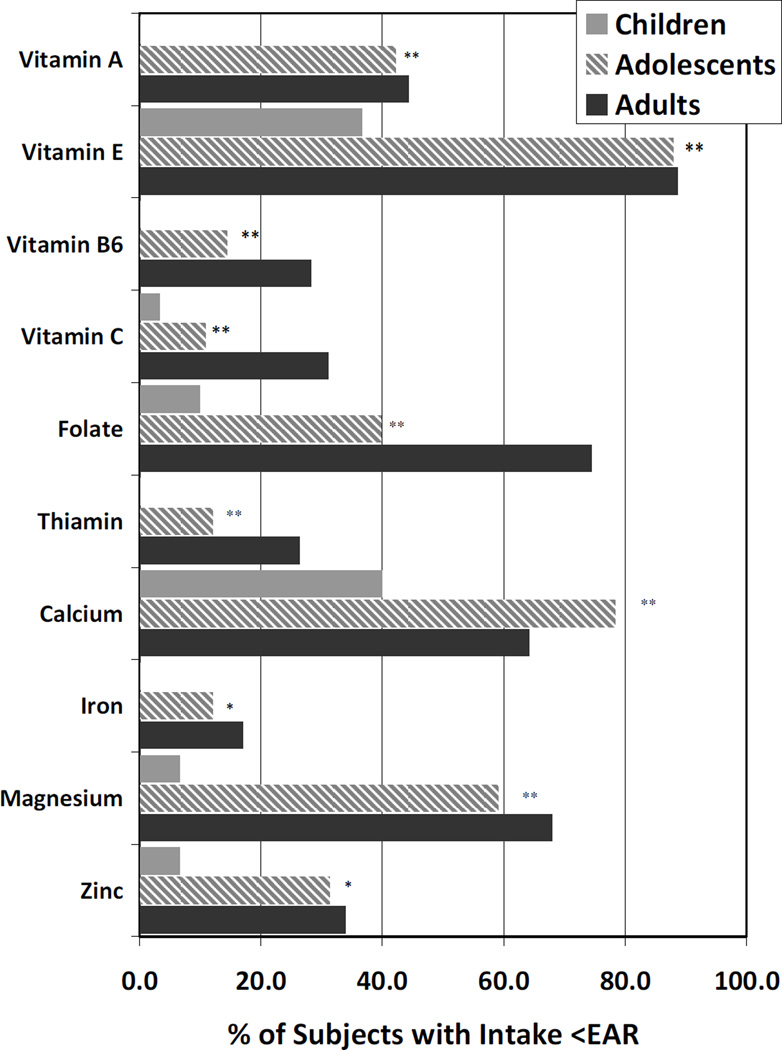
Over 30% of patients consumed less than the estimated average requirement (EAR) of vitamin A, D, E, folate, calcium and magnesium (Figure 1). The only nutrients for which >90% of patients consumed at least the EAR were vitamin B12, riboflavin and selenium. No subjects consumed greater than the upper limit for vitamin D or E. Two adult subjects consumed more than 2500 mg/d of calcium, the upper limit for adults, and 68% of young children consumed more than the upper limit of vitamin A (900 ug/day), although it is unclear what proportion of these are pro-vitamin A sources. Adults were more likely than children to consume less than the EAR of most every nutrient studied (Figure 2). These differences by age group were statistically significant with the exception of vitamin D, K, B12, niacin, riboflavin, copper and selenium.
Figure 1.
Percentage of Subjects with Dietary Intake less than the Estimated Average Intake (EAR) for Specific Nutrients (All subjects, n=221)
Footnote: Fat soluble vitamins (black bars), water soluble vitamins (striped bars), minerals (light bars)
Figure 2.
Dietary Inadequacy Increases with Advancing Age for Some Essential Nutrients
Footnote: **denotes significance between age groups in individual nutrients by p<0.001, and * by p<0.05. Only nutrients for which there was a significant difference by age group are included herein (omitted Vitamins B12, D, K, niacin, riboflavin, copper and selenium) For some nutrients (Vitamins A & B6, thiamin and iron), none of the young children were consuming less than the EAR, therefore, the % in this figure is zero. Adult subjects are represented by the black bars, older children & adolescents by the striped bars, and younger children by the light grey bars.
Supplementation
Many subjects (31–57%) took multivitamin and mineral supplements in addition to their usual diet. The most common supplements consumed were calcium and vitamin D (Table 4). Multivitamin/mineral supplements were also commonly consumed by nearly half of all adolescents and adults. Whether or not the multivitamin/mineral supplements most commonly chosen contained iron, were data that were unfortunately not available in the from the Block food frequency questionnaire.
Table 4.
Percentage of Subjects Taking Micronutrient Supplements by Age Group 1
| All N=191 |
Adolescents N=83 |
Adults N=108 |
||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Multivitamin/Mineral supplements 2 | ||||||
| 76 | 44.0 | 32 | 44.0 | 44 | 44.0 | |
| Single Nutrient Supplements | ||||||
| Fat Soluble Vitamins | ||||||
| Vitamin A | 74 | 39.2 | 31 | 37.3 | 43 | 40.6 |
| Vitamin D | 107 | 56.6 | 34 | 41.0 | 73 | 68.9 |
| Vitamin E | 79 | 41.8 | 31 | 37.3 | 48 | 45.3 |
| Water Soluble Vitamins | ||||||
| Vitamin C | 94 | 49.7 | 32 | 38.6 | 62 | 58.5 |
| Folate | 92 | 48.7 | 32 | 38.6 | 60 | 56.6 |
| Thiamin | 75 | 39.7 | 31 | 37.3 | 44 | 41.5 |
| Riboflavin | 75 | 39.7 | 31 | 37.3 | 44 | 41.5 |
| Niacin | 75 | 39.7 | 31 | 37.3 | 44 | 41.5 |
| Vitamin B-6 | 75 | 39.7 | 31 | 37.3 | 44 | 41.5 |
| Vitamin B-12 | 75 | 39.7 | 31 | 37.3 | 44 | 41.5 |
| Minerals | ||||||
| Calcium | 100 | 52.9 | 32 | 38.6 | 68 | 64.2 |
| Copper | 59 | 31.2 | 31 | 37.3 | 28 | 26.4 |
| Magnesium | 71 | 37.6 | 31 | 37.3 | 40 | 37.7 |
| Selenium | 60 | 31.7 | 31 | 37.3 | 29 | 27.4 |
| Zinc | 65 | 34.4 | 31 | 37.3 | 34 | 32.1 |
Supplementation usage information only obtained within the food frequency questionnaire for the adolescent and adult subjects who answered this section of the questionnaire.
Multivitamin/mineral supplementation usage assumed to be without iron for most subjects due to their iron overload co-morbidity. However, multivitamins with or without iron were not options on the Block FFQ, and therefore the actual number of subjects taking multivitamin supplements without iron was not obtained.
Relationship of intake to body iron or circulating vitamin D levels
General linear models were used to explore the relationship between dietary iron intake and liver iron concentration, a proxy for total body iron. Liver iron concentration was assessed on average within 4.4 ± 3.4 months of the food frequency evaluation by either magnetic resonance imaging (72.1% of patients), liver biopsy (4.1%) or Ferritometer™ (23.8%). There was no significant relationship between dietary iron intake and liver iron concentration in the group as a whole (p=0.13) or after controlling for age, transfusion status, gender and race (p=0.11). Similar results were observed when total body iron was estimated from serum ferritin; no relationship was observed between dietary iron intake and ferritin after controlling for age, gender, race and transfusion status (p=0.3). There was also no significant relationship between iron intake and total body iron when the same analysis was limited to only transfused subjects (n=105) [p=0.09 for liver iron concentration and p=0.4 for ferritin]. There was also no relationship between dietary vitamin D intake or supplemental vitamin D and serum levels of 25OH vitamin D after controlling for age, transfusion status, gender, and race (both p=NS).
Discussion
This is the first study of dietary intake patterns in patients with thalassemia, a population identified previously as having a multitude of risk factors for altered nutritional status(6,7,10). What is clear from these data is that many patients with thalassemia residing in the United States and Canada consume inadequate intakes of key food groups and essential nutrients, and that intake appears to worsen with increasing age. Nutrients of particular concern are vitamin A, D, E, calcium and magnesium. The level of inadequacy is particularly relevant given previous reports of reduced circulating levels of essential nutrients (7,10,28,29,34) which suggest that nutrient requirements for patients with thalassemia may actually be higher than recommendations for the U.S. population.
In this observational study, the dietary intake patterns may be linked to some major health concerns that occur frequently in thalassemia. Most convincingly, the limited intake of dairy products and the key nutrients found in these foods, vitamin D, calcium and magnesium may play a role in the development of osteoporosis. Vogiatzi et al (2009) has shown that nearly half of all transfused patients with thalassemia have low bone mass and are at a significant increased risk for fracture.(8) Low bone mass was strongly predicted by reduced serum vitamin D after controlling for age, weight and hypogonadism. In the present study, over half the sample consumed less than the EAR for calcium and magnesium, and nearly all subjects, regardless of age were consuming less than the EAR for vitamin D. Furthermore, serum vitamin D remained deficient in over 60% of the sample despite routine supplementation with both calcium and vitamin D. This is similar to what has been observed in other cross-sectional samples of patients with thalassemia (7,10). Recently, it has been found that supplementation with vitamin D must be at a much higher dose, over 2000 IU/day, in order to maintain sufficiency (29). Additionally, given that many patients revert back to deficient levels when supplementation is stopped, it is recommended that vitamin D levels be monitored every 6 months in this population (29). Clearly, focus on improving intake of dairy foods and/or the adequacy of bone forming nutrients through higher dose supplementation is crucial and may have lasting effects on preventing one of the most common ailments in these patients.
It must also be noted that osteoporosis is a multi-factorial disease, particularly in patients with thalassemia. Hypogonadism is common and also strongly linked to the development of low bone mass at a young age (8). Additionally, given the nature of the bone deficits observed with reduced bone geometries and cortical bone deficits (30), the role of limited physical activity and increased sedentary behaviors cannot be dismissed.
Cardiomyopathy due to transfusional iron overload is the most common cause of death in thalassemia (4,5,31). Iron overload can be successfully managed with chelation; however, vitamin C deficiency diminishes its effectiveness (32,33). Others have observed significantly reduced levels of ascorbate in transfused subjects (10,34). Additionally, iron overload, leads to an increase in non-transferrin bound iron and a sequential reduction in circulating antioxidants (35,36). In this study, adolescent and adult subjects consumed few orange vegetables, foods known to be rich in antioxidants. Furthermore, the inadequacy of dietary intake of key antioxidants (vitamin E, C and zinc) increased with advancing age. Some subjects, perhaps recognizing their diets were inadequate, or who were prescribed them by their physicians, supplemented with either a multivitamin or vitamin C or E alone. However, the supplemental amount required to reduce oxidative stress in this iron-toxic population remains an unanswered question.
Dietary iron reduction has for decades been the focus of nutritional intervention in patients with thalassemia given that iron overload is a cause of significant morbidity in both transfused and non-transfused subjects (37). However, there is a paucity of data which link dietary iron intake with total body iron stores in thalassemia. In this study, there was not a significant relationship between dietary iron intake and total body iron stores estimated by either recent liver iron concentration or average serum ferritin measurement after correction for age, gender, race and transfusion status. Clearly, for transfused subjects, the transfusional load of iron [200 mg iron/unit × 2 units every 3 weeks= 19 mg/day] far outweighs the estimated absorption of iron from the diet [average iron intake in this study: 12.5 mg/day × 10% absorption= 1.25 mg/day]. However, classic studies have shown that with very low hemoglobin levels (<9 g/dL) iron absorption increases dramatically and can approach 20% (38). Therefore, the relationship between dietary iron intake and iron stores is likely to be quite different in the non-transfused patient with thalassemia, and could not be explored thoroughly in this study due to the limited number of non-chronically transfused subjects.
Given these preliminary dietary data in adequately transfused patients with thalassemia, it is suggested that dietitians shift the focus of the nutritional message away from avoiding iron-rich diets towards concentrating on a more well-balanced diet rich in antioxidants and minerals (6). When iron is avoided in the diet, frequently zinc intake is reduced; an essential nutrient which as been shown to be particularly beneficial to immune status, bone health and growth in thalassemia (39,40). As noted, intake of dairy foods is also low, which may in part be related to lactose intolerance. Therefore strategies for increasing dietary calcium and magnesium should emphasize non-dairy foods. Finally, shifting the focus towards more fruits, vegetables and whole grains will not only enhance antioxidant intake, but also fiber and folate, critically important to red cell metabolism.
Whole grain and dairy intake was surprisingly low in this group of subjects. However, when compared to the general population, intake of many of these food groups was not that different. In a recent publication from the National Cancer Institute, over 90% of adult females from the National Health and Nutrition Examination Survey database do not meet the minimum recommendations for whole grains, dairy, fruit or orange and dark green leafy vegetables (41). The majority of adult males and adolescents also did not meet the dietary guidelines for many nutrient rich food groups; though an overconsumption of fats and added sugars was ubiquitous. The United States Department of Agriculture recently shifted its recommendations away from the “pyramid” structure to the “my plate” approach (http://www.choosemyplate.gov/). Perhaps this approach will aid all Americans, including patients with thalassemia, a simpler way to plan their meals and meet their overall dietary guidelines.
In the present study, many subjects with thalassemia reported taking additional dietary supplements. The most common single nutrient supplements were calcium and vitamin D, presumably because so many patients are known to have low bone mass. Nearly half of all subjects also reported taking multivitamin mineral supplements, presumably without iron. Supplemental intake is much higher than what is typically observed in another prevalent hemoglobinopathy, sickle cell disease (42). Dietary and supplemental intake are presented separately in this manuscript, however, if multivitamins are adhered to in this population, they may serve to significantly augment the daily dietary intake of many patients with thalassemia.
Capturing the true dietary intake of groups of individuals is challenging, and every assessment technique has its flaws. Though the tools used in this study have been extensively validated and compared with other tools (11–15, 43), food frequency questionnaires in general have been shown to both underestimate (44) and overestimate intake in individuals (45). The benefit to the use of food frequency questionnaires is that they tend to capture intake patterns over time compared to 24 hour recalls or records that may alter intake and are less representative of overall intake patterns. Additionally, the food frequency tool has been shown recently to be suitable for testing many diet-disease associations (46). The aim of this study was to compare the usual intake of a population (thalassemia) with the current United States dietary recommendations, which by definition are not intended for daily consideration. If subjects in this study tended to overestimate intake, then a very conservative estimate of the prevalence of deficiency would have been made in patients with thalassemia. One way to estimate validity of intake data are to compare to energy expenditure estimates. Adults in this cohort were relatively weight stable during the period of observation, baseline to year one. Given this information, the adults estimated kilocalorie intake should be similar to their estimated energy requirement. For these adult subjects, %EER was close to 100% (Table 3); therefore, the assumption was made that these subjects lived a relatively sedentary lifestyle. In other words, the actual caloric intake and the percentage of estimated intake required for sedentary individuals was rather similar.
This study was limited with respect to the size of the cohort of non-transfused subjects. The majority of the subjects (90%) were chronically transfused, therefore, the generalizability of these results to non-transfused subjects with thalassemia is limited. Additionally, due to the present study design, circulating levels of many nutrients were not measured and therefore the relationship between dietary intake and other co-morbidities in thalassemia could not be explored further. This was beyond the scope of this study; however, it is the obvious next step in understanding the full effects of nutritional status on overall health in patients with thalassemia.
Conclusions
Patients with thalassemia have reduced intake of many key nutrients (vitamin A, D, E, K, folate, calcium and magnesium). Furthermore, intake of some essential nutrients appears to worsen with age. The level of dietary inadequacy is concerning particularly when these data are combined with previous reports of decreased circulating essential nutrients and the prevalence of many co-morbidities with nutritional linkages. These preliminary findings support the need for nutritional monitoring to determine which patients are at greatest risk for nutritional deficiency. Optimizing dietary intake through nutrient dense foods and appropriate use of supplementation where necessary may improve overall health in these patients. Given the limitations of this study, future research should focus more directly on the effect of dietary quality and nutritional status on health outcomes in thalassemia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vichinsky EP. Changing patterns of thalassemia worldwide. Ann NY Acad Sci. 2005;1054:18–24. doi: 10.1196/annals.1345.003. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham MJ, Macklin EA, Neufeld EJ, Cohen AR. Complications of B-thalassemia major in North America. Blood. 2004;104(1):34–39. doi: 10.1182/blood-2003-09-3167. [DOI] [PubMed] [Google Scholar]
- 3.Vichinsky EP, Macklin EA, Waye JS, Lorey F, Olivieri NF. Changes in the epidemiology of thalassemia in North America: a new minority disease. Pediatrics. 2005;116(6):e818–e825. doi: 10.1542/peds.2005-0843. [DOI] [PubMed] [Google Scholar]
- 4.Zurlo MG, De Stefano P, Borgna-Pignatti C, et al. Survival and causes of death in thalassaemia major. Lancet. 1989;2(8653):27–30. doi: 10.1016/s0140-6736(89)90264-x. [DOI] [PubMed] [Google Scholar]
- 5.Fung EB, Harmatz P, Milet M, et al. Morbidity and mortality in chronically transfused subjects with thalassemia or sickle cell disease: a report from the multicenter study of iron overload. Am J Hematol. 2007;82(4):255–265. doi: 10.1002/ajh.20809. [DOI] [PubMed] [Google Scholar]
- 6.Fung EB. Nutritional deficiencies in patients with thalassemia. Ann NY Acad Sci. 2010;1202:188–196. doi: 10.1111/j.1749-6632.2010.05578.x. [DOI] [PubMed] [Google Scholar]
- 7.Vogiatzi MG, Macklin EA, Trachtenberg FL, et al. Differences in the prevalence of growth, endocrine and vitamin D abnormalities among the various thalassemia syndromes in North America. Br J Haematol. 2009;146(5):546–556. doi: 10.1111/j.1365-2141.2009.07793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vogiatzi M, Macklin EA, Fung EB, et al. Bone disease in thalassemia: a frequent and still unresolved problem. J Bone Miner Res. 2009;24(3):543–557. doi: 10.1359/jbmr.080505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fung EB, Xu Y, Kwiatkowski J, et al. Relationship between chronic transfusion therapy and body composition in subjects with thalassemia. J Pediatr. 2010;157(4):641–647. doi: 10.1016/j.jpeds.2010.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Claster S, Wood JC, Noetzli L, et al. Nutritional deficiencies in iron overloaded patients with hemoglobinopathies. Am J Hematol. 2009;84(6):344–348. doi: 10.1002/ajh.21416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mares-Perlman JA, Klein BEK, Klein R, Ritter LL, Fisher MR, Freudenheim JL. A diet history questionnaire ranks nutrient intakes in middle-aged and older men and women similarly to multiple food records. J Nutr. 1993;123(3):489–501. doi: 10.1093/jn/123.3.489. [DOI] [PubMed] [Google Scholar]
- 12.Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43(12):1327–1335. doi: 10.1016/0895-4356(90)90099-b. [DOI] [PubMed] [Google Scholar]
- 13.Cullen KW, Watson K, Azkievi I. Relative reliability and validity of the Block kids questionnaire among youth aged 10 to 17 years. J Am Diet Assoc. 2008;108(5):862–866. doi: 10.1016/j.jada.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Smith C, Fila S. Comparison of the kid's Block food frequency questionnaire to the 24-hour recall in urban Native American youth. Am J Hum Biol. 2006;18(5):706–709. doi: 10.1002/ajhb.20475. [DOI] [PubMed] [Google Scholar]
- 15.Marshall TA, Eichenberger Gilmore JM, Broffitt B, Stumbo PJ, Levy SM. Relative validity of the Iowa fluoride study targeted nutrient semi-quantitative questionnaire and the Block kids' food questionnaire for estimating beverage, calcium, and vitamin D intakes by children. J Am Diet Assoc. 2008;108(3):465–472. doi: 10.1016/j.jada.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 16.United States Department of Agriculture. Nutrition Monitoring Division. Washington, DC: U.S. GPO; Human Nutrition Information Service. Composition of foods: raw, processed, prepared (Revised Handbooks # 8-1, Dairy and Egg Products-1976 to # 8-21, Fast Foods - 1988) pp. 1976–1988. [Google Scholar]
- 17.NHANES III. National Health and Nutrition Examination Survey. Maryland, Hyattsville: National Center for Health Statistics; pp. 1999–2002. [Google Scholar]
- 18.Institute of Medicine, Food and Nutrition Board. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin and choline. Washington, DC: National Academy Press; 1998. pp. 1–16. [PubMed] [Google Scholar]
- 19.Institute of Medicine, Food and Nutrition Board. Dietary reference intakes for vitamin C, vitamin E, selenium and carotenoids. Washington, DC: National Academy Press; 2000. pp. 1–20. [PubMed] [Google Scholar]
- 20.Institute of Medicine, Food and Nutrition Board. Dietary reference intakes for vitamin A, vitamin D, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium and zinc. Washington, DC: National Academy Press; 2001. pp. 1–28. [PubMed] [Google Scholar]
- 21.Institute of Medicine, Food and Nutrition Board. Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D and flouride. Washington, DC: National Academy Press; 1997. pp. 1–20. [PubMed] [Google Scholar]
- 22.Institute of Medicine, Food and Nutrition Board. Dietary reference intakes for calcium and vitamin D. Washington, DC: National Academy Press; 2011. pp. S1–S12. [Google Scholar]
- 23.Otten JJ, Hellwig JD, Meyers LD, editors. Dietary reference intakes: the essential guide to nutrient requirements, Institute of Medicine. Washington, DC: National Academy Press; 2006. pp. 19–68. [Google Scholar]
- 24.American Dietetic Association. Practice Paper of the ADA: Using the dietary reference intakes. J Am Diet Assoc. 2011;111(5):762–770. doi: 10.1016/j.jada.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 25.Institute of Medicine, Food and Nutrition Board. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. Washington, DC: National Academy Press; 2002. pp. 5–114. [DOI] [PubMed] [Google Scholar]
- 26.Gariépy C, Lal A, Fung EB. Reduced physical activity in adult and pediatric patients with thalassemia. Blood. 2010 abstract online only. [Google Scholar]
- 27.Dietary Guidelines for Americans, 2005. 6th Edition. Washington, DC: U.S. Government Printing Office; 2005. Jan, U.S. Department of Health and Human Services and U.S. Department of Agriculture. [Google Scholar]
- 28.Livrea MA, Tesoriere L, Pintaudi AM, et al. Oxidative stress and antioxidant status in beta-thalassmia major: iron overload and depletion of lipid-soluble antioxidants. Blood. 1996;88(9):3608–3614. [PubMed] [Google Scholar]
- 29.Fung EB, Aguilar C, Micaily I, Foote D, Lal A. Treatment of vitamin D deficiency in transfusion-dependent thalassemia. Am J Hematol. 2011;86(10):871–873. doi: 10.1002/ajh.22117. [DOI] [PubMed] [Google Scholar]
- 30.Fung EB, Vichinsky EP, Kwiatkowski JK, et al. Characterization of low bone mass in young patients with thalassemia by DXA, pQCT and markers of bone turnover. Bone. 2011;48(6):1305–1312. doi: 10.1016/j.bone.2011.03.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aessopos A, Kati M, Farmakis D. Heart disease in thalassemia intermedia: a review of the underlying pathophysiology. Haematologica. 2007;92(5):658–665. doi: 10.3324/haematol.10915. [DOI] [PubMed] [Google Scholar]
- 32.O’Brien RT. Ascorbic acid enhancement of desferrioxamine induced urinary excretion in thalassemia major. Ann NY Acad Sci. 1974;232:221–225. doi: 10.1111/j.1749-6632.1974.tb20588.x. [DOI] [PubMed] [Google Scholar]
- 33.Nienhuis AW. Vitamin C and iron. N Engl J Med. 1981;304(3):170–171. doi: 10.1056/NEJM198101153040311. [DOI] [PubMed] [Google Scholar]
- 34.Dissayabutra T, Tosukhowong P, Seksan P. The benefits of vitamin C and vitamin E in children with beta-thalassemia with high oxidative stress. J Med Assoc Thai. 2005;88(Suppl 4):S317–S321. [PubMed] [Google Scholar]
- 35.Walter PB, Fung EB, Killilea DW, et al. Oxidative stress and inflammation in iron-overloaded patients with β-Thalassaemia or Sickle Cell Disease. Br J Haematol. 2006;135(2):254–263. doi: 10.1111/j.1365-2141.2006.06277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reller K, Dresow B, Collell M, et al. Iron overload and antioxidant status in patients with thalassemia major. Ann NY Acad Sci. 1998;850:463–465. doi: 10.1111/j.1749-6632.1998.tb10522.x. [DOI] [PubMed] [Google Scholar]
- 37.Mariani R, Trombini P, Pozzi M, Piperno A. Iron metabolism in thalassemia and sickle cell disease. Mediterr J Hematol Infect Dis. 2009;1(1):e2009006. doi: 10.4084/MJHID.2009.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Alarcon PA, Donovan ME, Forbes GB, Landaw SA, Stockman JA. Iron absorption in the thalassemia syndromes and its inhibition by tea. N Engl J Med. 1979;300(1):5–8. doi: 10.1056/NEJM197901043000102. [DOI] [PubMed] [Google Scholar]
- 39.Shamshirsaz AA, Bekheirnia MR, Kamgar M, et al. Bone mineral density in Iranian adolescents and young adults with beta-thalassemia major. Pediatr Hematol Oncol. 2007;24(7):469–479. doi: 10.1080/08880010701533702. [DOI] [PubMed] [Google Scholar]
- 40.Fikry SI, Saleh SA, Sarkis NN, Mangoud H. Study of serum zinc in relation to nutritional status among thalassemia patients in Damanhour Medical National Institute. J Egypt Public Health Assoc. 2003;78(1–2):73–93. [PubMed] [Google Scholar]
- 41.Krebs-Smith SM, Guenther PM, Subar AF, Kirkpatrick SI, Dodd KW. Americans do not meet federal dietary recommendations. J Nutr. 2010;140(10):1832–1838. doi: 10.3945/jn.110.124826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawchak DA, Schall JI, Zemel BS, Ohene-Frempong K, Stallings VA. Adequacy of dietary intake declines with age in children with sickle cell disease. J Am Diet Assoc. 2007;107(5):843–848. doi: 10.1016/j.jada.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 43.Boucher B, Cotterchio M, Krieger N, Nadalin V, Block T, Block G. Validity and reliability of the Block 98 Food frequency in a sample of Canadian women. Public Health Nutr. 2006;9(1):84–93. doi: 10.1079/phn2005763. [DOI] [PubMed] [Google Scholar]
- 44.Subar AF, Kipnis V, Troiano RP, et al. Using intake biomarkers to evaluate the extent of dietary misreporting in a large sample of adults: the OPEN study. Am J Epidemiol. 2003;158(1):1–13. doi: 10.1093/aje/kwg092. [DOI] [PubMed] [Google Scholar]
- 45.Jackson MD, Walker SP, Younger NM, Bennett FI. Use of a food frequency questionnaire to assess diets of Jamaican adults: validation and correlation with biomarkers. Nutr J. 2011;10(28):1–11. doi: 10.1186/1475-2891-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Midthune D, Schatzkin A, Subar AF, et al. Validating an FFQ for intake of episodically consumed foods: application to the National Institutes of Health-AARP diet and health Study. Public Health Nutr. 2011 Apr 13;:1–10. doi: 10.1017/S1368980011000632. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]