Abstract
Rationale
Varenicline is believed to work, in part, by reducing craving responses to smoking cues and by reducing general levels of craving; however, these hypotheses have never been evaluated with craving assessed in the natural environments of treatment-seeking smokers.
Objectives
Ecological momentary assessment procedures were used to assess the impact of varenicline on cue-specific and general craving in treatment-seeking smokers prior to quitting.
Methods
For 5 weeks prior to quitting, 60 smokers carried personal digital assistants that assessed their response to smoking or neutral cues. During Week 1 (Baseline), participants did not receive medication; during Weeks 2–4 (Drug Manipulation), participants were randomized to receive varenicline or placebo; during Week 5 (Standard Therapy), all participants received varenicline. Craving was assessed before each cue; cue-specific craving and attention to cue were assessed after each cue.
Results
During all phases, smoking cues elicited greater craving than neutral cues; the magnitude of this effect declined after the first week. General craving declined across each phase of the study. Relative to the placebo condition, varenicline was associated with a greater decline in general craving over the Drug Manipulation Phase. Varenicline did not significantly attenuate cue-specific craving during any phase of the study.
Conclusions
Smoking cues delivered in the natural environment elicited strong craving responses in treatment-seeking smokers, but cue-specific craving was not affected by varenicline administered prior to the quit attempt. These findings suggest that the clinical efficacy of varenicline is not mediated by changes in cue-specific craving during the pre-quit period of treatment-seeking smokers.
Keywords: smoking, cue reactivity, craving, ecological momentary assessment, varenicline
Most cigarette smokers report that they would like to quit smoking (Centers for Disease Control and Prevention 2007), but most smokers who attempt cessation fail to maintain abstinence (Hughes et al. 2008). Varenicline (Chantix) was approved by the FDA as cigarette cessation pharmacotherapy in 2006 (Rollema et al. 2007) and functions principally at the α4β2n acetylcholine receptor as both an antagonist and partial agonist, making it a unique treatment option (Jorenby et al. 2006). Initial studies indicated that varenicline produces higher short-term and long-term abstinence rates than other effective treatments (e.g., bupropion; Nides et al. 2008). Although the mechanisms responsible for varenicline’s efficacy are not clearly established, one hypothesis is that varenicline may reduce the experience of craving.
Craving is a salient experience for most smokers that is present before, during, and after attempts at quitting (Tiffany et al. 2009). General (or background) craving levels have been shown to predict post-quit relapse (e.g., Killen et al. 2006). In heavy, daily cigarette smokers, craving is augmented during abstinence and can be detected within 30 minutes of the person’s last cigarette (Schuh and Stitzer 1995), peaks within 3 to 6 hours, and is readily reversed by the administration of nicotine (Teneggi et al. 2002; Tiffany et al. 2000). Smoking related cues can also trigger craving. Cue-specific craving, which has been studied comprehensively in laboratory-based studies, appears to operate somewhat independently of abstinence-induced craving. For example, cue-specific craving is generally not augmented during abstinence (Bailey et al. 2010; Drobes and Tiffany 1997; Maude-Griffin and Tiffany 1996; Tiffany et al. 2000) and is not specifically attenuated by nicotine replacement in abstinent smokers (Tiffany et al. 2000).
In smoking treatment studies, varenicline has reduced cigarette craving relative to placebo during abstinence from smoking (Gonzales et al. 2006; Nides et al. 2006; Patterson et al. 2009; Tonstad et al. 2006). This may be due to varenicline’s nicotine agonist properties, which are theorized to attenuate abstinence-induced symptoms. Varenicline also functions as a partial nicotine antagonist (by blocking nicotine binding). Consequently, those who smoke while taking varenicline may experience a decrease in the positively reinforcing effects of nicotine, with a corresponding weakening of the association between cigarette cues and positive outcomes. This extinction may lead to a diminution in cue-specific craving (Rollema et al. 2007).
To date, two published studies have examined varenicline’s impact on cue-specific craving. In a non-treatment study, Brandon et al. (2011) found that after two weeks of drug therapy, varenicline reduced cue-specific craving relative to placebo following overnight abstinence. Importantly, this study was limited by differential attrition whereby substantially more participants in the varenicline condition relative to the placebo condition dropped out before the final assessment. In another non-treatment study, Franklin et al. (2011) found that, after drug administration, varenicline users showed a decrease in cue-specific craving from baseline but placebo users did not. However, this study always presented neutral cues before smoking cues, making it difficult to determine the extent to which increased craving was specific to cue content.
Although craving in the natural environments of smokers is of greatest interest, nearly all studies of cue-specific craving have taken place in laboratories; little is known about the impact of smoking cues on craving in the natural environment of smokers. Ecological momentary assessment (EMA) provides a method to address this limitation. Contemporary studies with EMA have participants carry personal digital assistants (PDAs), which are used to collect information about events and reactions in real time in participants’ natural environments (Shiffman 2009). Recently, EMA procedures have been used to examine cue-specific craving in the natural environment. Warthen and Tiffany (2009) developed the CREMA (cue reactivity – ecological momentary assessment) procedure in which cigarette smokers carried PDAs over an 8-day period. Photographs or imagery sentences containing either smoking-related or neutral cues were presented on the PDAs several times each day. Cue-specific craving effects in the natural environment were as large as those generated through laboratory-based assessments; Wray et al. (2011) replicated and extended these findings to in vivo cues. As CREMA distributes assessments over a broad range of contexts, situations, and times, the resulting data are likely to produce a more representative sampling of cue-specific craving effects than those generated with assessments confined to laboratory settings.
To date, CREMA has produced robust cue-specific craving effects in the natural environments of non-treatment seeking smokers; this procedure has not been tested in smokers enrolled in a treatment program. The current study examined cue-specific craving using EMA with smokers enrolled in a smoking cessation trial of varenicline. Drug exposure was manipulated by varying the duration of varenicline administration prior to the target quit date (i.e., 4 weeks vs. 1 week of exposure). Cue-specific and general levels of craving were assessed in the period leading up to the target quit date. We expected that the CREMA procedure would yield a more sensitive index of varenicline’s impact on cue-specific craving than available through conventional laboratory-based assessments of cue reactivity.
Method
Study Design
Sixty daily adult smokers (25 males/35 females) were recruited for a smoking treatment study in Western New York. Eligible participants were at least 18 years of age and were seeking smoking cessation treatment. Prior to their target quit date, participants were randomized to receive either 1 or 4 weeks of varenicline and completed daily study assessments (see Hawk et al. in press for detailed treatment methodology). Participants carried PDAs that were used to collect data for 7 consecutive weeks over the course of the study and received $20–$64 weekly based on degree of adherence with study procedures.
The general treatment procedures are depicted in Table 1. At Visit 1, participants attended an orientation, completed initial health assessments, and were trained in the use of the PDA1. Data were collected from participants for 7 consecutive days during an initial Baseline assessment phase. Participants returned to the clinic for Visit 2 (Day 8) and were randomized to receive active varenicline or placebo for the next 3 weeks (Drug Manipulation Phase). Participants taking varenicline received 0.5 mg once daily for 3 days, 0.5 mg bid for 4 days, then 1.0 mg bid for the remainder of the study; those receiving placebo were provided with identical appearing medication. At Visit 4 (Day 29), all participants were given varenicline for 1 week before their quit attempt (Standard Therapy Phase). Participants whose course of varenicline began at Visit 4 followed the same dosing regimen as those who began varenicline at the second clinic visit. Visit 5 (Day 36) was on the participant’s target quit date (TQD). After this session, participants were in the Post-Target Quit Date (Post-TQD) phase for the remainder of the study. Data collected daily on PDAs included Morning Assessments, Cigarette Logs, Smoking Symptom sessions, and CREMA sessions. Participants completed a Morning Assessment each day before smoking their first cigarette. During Cigarette Logs, participants were instructed to log each cigarette smoked. Participants received an alarm that initiated a Smoking Symptom session two times per day; data from this session and Cigarette Logs are not reported in this paper. Participants also received daily alarms signaling CREMA sessions that involved cue manipulations two times per day and were paid $1.50 for each CREMA session completed. In order to remain in the study, participants were required to maintain a minimum weekly compliance rate of 3/7 morning assessments and 12/24 alarms. The data reported in this paper come from CREMA sessions administered during the Baseline, Drug Manipulation, and Standard Therapy phases. During these phases, participants were instructed to smoke as usual.
Table 1.
Overview of treatment design.
| Study Days | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 |
|---|---|---|---|---|---|
| Phase | Baseline | Drug Manipulation | Standard | ||
| Visits | #1 | #2 | #3 | #4 | |
| Varenicline group | V | V | V | V | |
| Placebo group | P | P | P | V | |
Note: TQD = Target Quit Date; V = varenicline; P = placebo;
Materials
PDAs (Palm Tungsten E2) were programmed using a modified version of the Purdue Momentary Assessment Tool software (Weiss et al. 2004). Participants did not have access to other PDA functions. This software package was used in two previous CREMA studies that were successful in generating cue-specific craving (Warthen and Tiffany 2009; Wray et al. 2011).
Procedures
Participants were consented at Visit 1; they then completed self-report questionnaires that included demographic and smoking history information. Participants were told to begin logging cigarettes smoked immediately following Visit 1. They were also told to initiate a Morning Assessment the morning after their first clinic visit and to complete one each day. CREMA and Smoking Symptom session alarms began the following day during a participant’s chosen 12-hour time period. For example, a participant could have alarms occur between 700 and 1900 hours. Although alarms were only administered during this 12-hour period, participants were instructed to log all of their cigarettes, even those smoked outside of the twelve-hour CREMA and Smoking Symptom assessment period.
The PDA alarm assessments were delivered on full days between clinic visits. Between Visits 3 and 4 as well as 5 and 6, there were 13 days each of alarm assessments. CREMA sessions and Smoking Symptom sessions were delivered until Visit 5. On 29 of the 31 days of data collection, two of the four alarms signaled CREMA sessions and two of the four alarms signaled Smoking Symptom sessions. On the first and tenth day of the 13-day assessment block (i.e., between Visits 3 and 4) one CREMA and three Smoking Symptom sessions were delivered. After Visit 5, only Smoking Symptom sessions were delivered. Table 2 shows the number of CREMA sessions and Smoking Symptom sessions administered between each visit. Study procedures were approved by the Roswell Park Cancer Institute Institutional Review Board.
Table 2.
CREMA and Smoking Symptom Sessions.
| Phase | Baseline | Drug Manipulation | Standard | ||
|---|---|---|---|---|---|
| Study Days | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 |
| CREMA Sessions | 12 | 12 | 13 | 11 | 12 |
| Smoking Symptom Sessions | 12 | 12 | 14 | 14 | 12 |
Assessments
CREMA Sessions
Each day, 2 CREMA session alarms were distributed across the 12-hour period and never occurred within 30 minutes of each other. At the beginning of each CREMA session, participants completed assessments that included the Craving Questionnaire (an assessment of craving with a rating scale from 1–5 comprised of the items “Nothing would be better than smoking a cigarette right now,” “All I want right now is a cigarette,” “I crave a cigarette right now,” and “I have an urge for a cigarette,” α=.95 {Carter and Tiffany 2001}) as well as two mood items (results from the mood items will not be reported in this paper). The Craving Questionnaire has been used previously in several studies of cue-specific craving and has produced robust cue-specific craving effects (e.g., Carter and Tiffany 2001; Warthen and Tiffany 2009; Wray et al. 2011).
The cue manipulation was delivered following baseline questions. Cues were delivered through two modes of presentation: photographs and in vivo. Each of these modes consisted of two types of stimulus content: smoking related and neutral. We used a total of 60 photographs (30 smoking related and 30 neutral) selected from our previous CREMA research (48 photos; Warthen and Tiffany 2009; Wray et al. 2011) as well as 12 new photographs chosen for this study. The new photos were taken from the same source as the original photo set (http://www.sxc.hu/). Participants were shown a photograph depicting either smoking (e.g., cigarettes, lighters) or neutral content (e.g., pencils, flowers), or they were instructed to take out and hold a single cigarette or a non-smoking related neutral object (e.g., keys). Participants were instructed to look at the cue until an alarm sounded 10 seconds after the onset of the trial. At this point during photographic trials, the photograph was removed from the screen. During in vivo trials, participants were instructed to return the cigarette to the pack and hide the pack out of sight or put the neutral object out of sight. Participants then responded to post-cue assessments, including the Craving Questionnaire and mood items, and were asked how carefully they looked at the photograph/object, their level of distraction during each trial, and, on in vivo trials, whether or not they held an object/cigarette. A second cue trial of the same cue type and mode was then administered using the same procedures and assessments described for the first cue presentation. When the second trial was completed, participants were informed that they had earned $1.50 for completing the CREMA session.
Cues presented during CREMA sessions were counterbalanced and randomized across participants, with each photographic cue utilized only one time per participant. Each cue category (photographic neutral, photographic smoking, in vivo neutral, and in vivo smoking) was presented the same number of times across the study; additionally, each cue category was presented no more than once every 2 consecutive days. Each session consisted of the presentation of two successive cue-trials of the same type and mode of presentation. Participants had the opportunity to complete a total of 60 CREMA sessions.
Results
Data Reduction
For each of the 5 weeks of CREMA data collection, responses to the four items of the Craving Questionnaire before stimulus presentation were collapsed to produce an average general (pre-cue) rating. These ratings were then averaged across all sessions within each week for each participant. Responses to the Craving Questionnaire given after each of the two stimulus presentations within each session were averaged to generate post-cue ratings for each session within each week and then averaged across each of the four stimulus categories. Ratings of focus and distraction following each cue were similarly collapsed to form a single scale - attention to cue. Therefore, each participant who completed the study had 5 weeks of pre-cue and post-cue data (1 week of Baseline, 3 weeks of Drug Manipulation, and 1 week of Standard Therapy). Following the methods of Carter and Tiffany’s meta-analysis (1999), cue-specific craving was indexed as the difference between craving rated post-smoking cue and craving rated post-neutral cue. Mixed-design ANOVAs were conducted to analyze the effect of drug (placebo and varenicline), sex (male and female), week (1, 2, 3, 4, and 5), stimulus type (smoking and neutral), and mode of presentation (photo and in vivo) on post-cue craving and attention to cue. For pre-cue craving, mixed-design ANOVAs were conducted to analyze the effect of drug, sex, and week.
Participant Characteristics
Of the 60 participants enrolled at the Visit 1, 59 remained in the study through week 5. One participant dropped out between weeks 4 and 5. Three participants were missing entire weeks of data due to lost or malfunctioning PDAs. Participants missing a full week of data were excluded from analyses for the Phase in which they missed a week. Thirty-two participants (14 male) were randomized to receive varenicline and 28 participants (11 male) were randomized to receive placebo during the Drug Manipulation Phase of the study. On average, participants were 47.7 (SD=9.3) years old, had been smoking 27 years (SD=11.6), and had made 5.5 lifetime quit attempts. Their average Fagerström Test of Nicotine Dependence score was 5.2 (SD=2.7). During the baseline week, participants smoked an average of 17.7 cigarettes per day (SD=5.6). None of these characteristics varied significantly between the conditions.
CREMA Sessions
Participants completed an average of 51.25 (89.4%) CREMA sessions. Of the total sessions, 3 (0.4%) photographic neutral, 6 (0.8%) photographic smoking, 10 (1.4%) in vivo neutral, and 37 (4.8%) in vivo smoking were excluded because participants indicated that they had not viewed a photo or handled an object or cigarette. On average, participants indicated that they had last smoked a cigarette 37 minutes prior to the start of each CREMA session.
Pre-Cue Results
Pre-Cue Craving Ratings
Week 1
As we anticipated, an ANOVA on craving rated before cue presentations collected from the Baseline Phase (week 1) revealed no baseline differences between the two drug conditions, F (1, 56) = 0.23, p = 0.64, ηp2 = 0.00. There was no significant main effect for sex nor was there a significant sex by drug interaction.
Weeks 2–4
Pre-cue craving from the Drug Manipulation Phase (weeks 2–4) was analyzed in a similar manner, with the additional within-subjects factor of week (2, 3, and 4) included. We did not find a significant main effect across the varenicline or placebo conditions, F (1, 54) = 0.22, p = 0.64, ηp2 = 0.00, or a significant sex effect. A significant effect of week was found, F (2, 108) = 7.03, p < .01, ηp2= 0.12. Contrast testing revealed that craving was significantly higher during week 2 than during week 4, F (1, 54) = 11.98, p < .001, ηp2= 0.18. Craving levels were not significantly different between weeks 2 and 3, or weeks 3 and 4 (p > .05). The interaction between drug condition and week approached significance, F (2, 108) = 3.15, p = 0.052, ηp2 = 0.12. Follow-up analyses indicated that there was a significant main effect of week for those on varenicline, F (2, 58) = 9.15, p < .001, ηp2 = 0.24, but not for those on placebo (p > .05). Contrast testing revealed that craving was significantly higher during Week 2 than Week 3, F (1, 28) = 8.83, p = <.001, ηp2 = 0.24, but was not significantly different between Week 3 and Week 4.
Week 5
During the Standard Therapy Phase (week 5), we did not find a significant main effect of drug condition, F (1, 55) = 1.64, p = 0.21, ηp2= 0.03, or sex, nor any significant interactive effect.
Change in Craving Over Weeks
Inspection of the means (Table 3) suggested that pre-cue craving decreased systematically across phases of the study. Data from weeks 2–4 were collapsed to produce an average Drug Manipulation Phase craving rating, which was then compared to the Baseline and Standard Therapy Phases. Analyses revealed a significant effect of phase, F (2, 110) = 24.10, p < .001, ηp2= 0.30. Contrast tests revealed a significant decline in pre-cue craving from Phase 1 to Phase 2, F (1, 55) = 8.91, p < .01, ηp2= 0.14, and from Phase 2 to Phase 3, F (1, 55) = 20.59, p < .001, ηp2= 0.27.
Table 3.
Means and (standard deviations) of pre-cue ratings
| Baseline | Drug Manipulation | Standard Therapy | |||
|---|---|---|---|---|---|
|
| |||||
| Rating | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 |
| Craving | |||||
| Placebo | 2.27 (0.65) | 2.16 (0.62) | 2.15 (0.68) | 2.02 (0.60) | 1.84 (0.55) |
| Varenicline | 2.31 (0.69) | 2.24 (0.78) | 1.97 (0.67) | 1.91 (0.70) | 1.65 (0.63) |
Relationship between craving and smoking levels
Although participants were not instructed to restrict their smoking level, analyses from this study (Hawk et al. in press) suggest that women receiving active varenicline reduced their smoking levels compared women in the placebo group. As such, we tested whether smoking level moderated the effect of treatment condition on craving during this critical Drug Manipulation Phase of the study. Using regression analyses to control for the effects of Baseline Phase craving and cigarettes smoked, smoking level was not significantly related to craving in the varenicline group (β = 0.18, t{31} = 0.96, p > .05) nor the placebo group (β = 0.04, t{27} = 0.21, p > .05) during the Varenicline v. Placebo Phase of the study, suggesting that the relationship between drug condition and craving was not moderated by differential levels of smoking in our sample.
Cue-Specific Craving Results
Post-Cue Craving Ratings
Week 1
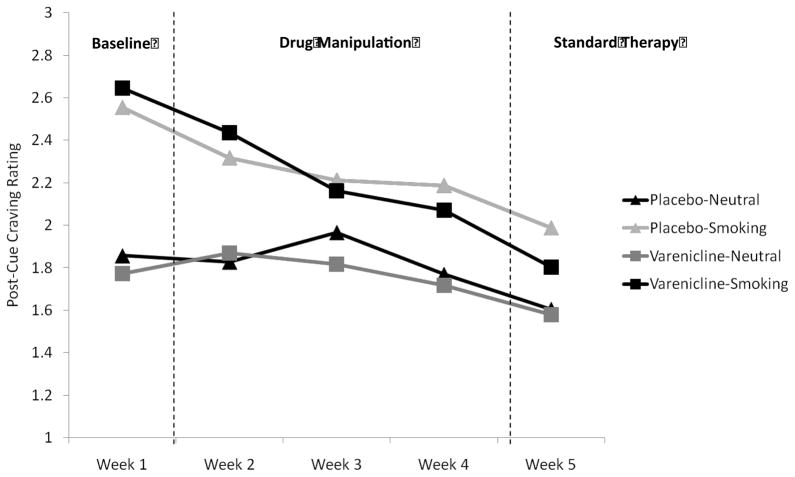
During the Baseline Phase, participants reported significantly greater craving after smoking cues than after neutral cues, F (1, 54) = 85.66, p < .001, ηp2= 0.61, indicating that cue-specific craving was robust during this portion of the study (see Figure 1). There was not a significant main effect of drug condition, F (1, 54) = 0.00, p = 0.96, ηp2=0.00, nor was there a significant drug condition by cue type interaction, F (1, 54) = 1.46, p = 0.23, ηp2= 0.03, signifying that no drug group differences existed at baseline. There were no other significant main or interactive effects of drug condition, mode, or sex (ps > .05).
Figure 1.
Average weekly craving reported after viewing neutral or smoking cues as a function of drug condition.
Weeks 2–4
During the Drug Manipulation Phase, smoking cues continued to elicit significantly greater craving than neutral cues, F (1, 46) = 27.74, p < .001, ηp2= 0.38. There was also a significant effect of week, F (2, 92) = 4.89, p < .05, ηp2 = 0.10. Examination of post-cue craving across those weeks indicated that post-cue craving during week 2 was significantly higher than post-cue craving during week 4, F (1, 46) = 7.71, p < .01, ηp2= 0.14. Craving was also significantly higher during week 3 than during week 4, F (1, 46) = 5.64, p < .05, ηp2= 0.11. There was no significant main effect of drug condition, F (1, 46) = 0.01, p = 0.93, ηp2= 0.00, nor was there a significant drug condition by cue type interaction, F (1, 54) = 0.61, p = 0.44, ηp2= 0.01. Further, there were no significant main or interactive effects of drug condition, mode, or sex on post-cue craving (all ps>.05).
Week 5
During week 5 (Standard Therapy), participants continued to report significantly greater craving after smoking cues relative to neutral cues, F (1, 54) = 15.37, p < .001, ηp2= 0.24. There was not a significant main effect of drug condition, F (1, 54) = 0.65, p = 0.42, ηp2 = 0.01 nor was there a significant drug condition by cue type interaction, F (1, 54) = 1.16, p = 0.29, ηp2= 0.02. There were no other significant main or interactive effects of drug condition, mode, or sex (ps > .05).
Changes in Cue-Specific craving over weeks
Examination of data across all weeks (Table 4) suggested that cue-specific craving effects declined systematically across each phase of the study. Exploratory analyses of post-cue-craving from the three phases revealed a significant Cue Type by Phase interaction, F (2, 110) = 15.86, p < .001, ηp2= 0.22. Follow-up analyses showed that cue-specific craving was higher in the Baseline Phase than during both the Drug Manipulation Phase, F (1, 55) = 20.46, p < .001, ηp2= 0.27, and the Standard Therapy Phase, F (1, 55) = 19.93, p < .001, ηp2= 0.26. The Drug Manipulation Phase was not significantly different than the Standard Therapy Phase (p >.05).
Table 4.
Means and (standard deviations) of post-cue ratings as divided by cue type (smoking v. neutral) and drug condition (placebo v. varenicline)
| Baseline | Drug Manipulation | Standard Therapy | |||
|---|---|---|---|---|---|
|
| |||||
| Rating | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 |
| Craving | |||||
| Placebo | |||||
| Neutral | 1.86 (0.75) | 1.83 (0.76) | 1.97 (0.77) | 1.77 (0.66) | 1.60 (0.59) |
| Smoking | 2.55 (0.93) | 2.32 (1.00) | 2.21 (0.91) | 2.19 (0.87) | 1.99 (0.95) |
| Varenicline | |||||
| Neutral | 1.77 (0.77) | 1.87 (0.83) | 1.82 (0.72) | 1.72 (0.71) | 1.58 (0.60) |
| Smoking | 2.65 (0.99) | 2.44 (1.01) | 2.16 (0.99) | 2.07 (0.97) | 1.80 (0.85) |
| Attention | |||||
| Placebo | |||||
| Neutral | 4.54 (0.52) | 4.75 (0.42) | 4.76 (0.39) | 4.81 (0.39) | 4.75 (0.48) |
| Smoking | 4.57 (0.47) | 4.69 (0.46) | 4.79 (0.42) | 4.82 (0.44) | 4.77 (0.45) |
| Varenicline | |||||
| Neutral | 4.53 (0.65) | 4.67 (0.60) | 4.70 (0.49) | 4.66 (0.68) | 4.62 (0.78) |
| Smoking | 4.61 (0.55) | 4.61 (0.61) | 4.69 (0.65) | 4.72 (0.56) | 4.66 (0.72) |
Cue-Specific Craving controlling for Pre-Cue Craving
In some cue-reactivity research, cue-specific craving has been indexed as the difference between pre-cue to post-cue craving. To examine cue-specificity defined in this fashion, mixed-design ANOVAs were conducted on change in craving (from pre to post-cue craving). In these analyses, we continued to see strong cue-specific craving effects across the five weeks (Fs ranging from 14.18 to 62.02, ps < .001), but, as in the previous analyses, there were no significant effects of varenicline on cue-specific craving (Fs ranging from 0.16–2.14, ps > .05).
Attention to Cue
Across all weeks, attention to cue was rated significantly higher during photographic cue trials than during in vivo cue trials, F (1, 43) = 18.39, p< .001, ηp2= 0.30. A significant cue mode by drug condition interaction revealed that photographic cues elicited greater attention in the placebo group (ηp2= 0.33) than in the varenicline group (ηp2=0.27). There was also a significant effect of week on attention, F (4, 172) = 9.84, p< .001, ηp2= 0.19; attention to cue increased from week 1 to week 2, F (1, 43) = 18.35, p< .001, ηp2= 0.29, and significantly increased again from week 2 to week 3, F (1, 43) = 4.12, p< .05, ηp2= 0.09. Attention did not increase significantly after week 3. There were no other significant main or interactive effects.
Discussion
This study evaluated the impact of varenicline on general and cue-specific craving in the natural environment of treatment-seeking smokers over the period leading up to their target quit date. We found no significant differences between varenicline and placebo on general (pre-cue) levels of craving; however, a probe of the nearly significant week by drug interaction during the Drug Manipulation Phase revealed that craving showed a significant early decline when participants began taking active doses of varenicline (this decline was not observed in the placebo condition). This is consistent with several previous studies that have found varenicline to significantly reduce general levels of craving relative to placebo (Brandon et al. 2011; Gonzales et al. 2006; Jorenby et al. 2006, Patterson et al. 2009; Tonstad et al. 2006; West et al. 2008). Results of the current study extend the findings of earlier treatment studies by including multiple assessments during the pre-quit period; other varenicline studies have focused more extensively on craving rated after the quit date or after abstinence and no study had multiple pre-quit craving assessments in the natural environment. Furthermore, the current study assessed craving using EMA at random time points, suggesting that pre-quit exposure to varenicline has an impact on craving in the natural environment of treatment-seeking smokers.
During each of the five weeks of assessment, smoking cues elicited significantly greater craving than neutral cues. However, we found no evidence that varenicline significantly affected cue-specific craving during any phase of the study. Previous studies of cue-specific craving and varenicline have yielded mixed results. Brandon et al. (2011) found a significant difference in cue-specific craving between participants receiving varenicline versus placebo. Limitations in that study as well as methodological differences from the current investigation may account for the discrepant results. In Brandon et al. (2011), participants were not treatment-seeking smokers, data was not collected in the smokers’ natural environment, and, participants were overnight-abstinent at the time of cue presentation and assessment. Participants in our study were assessed at random times in their natural environment, and, consequently, their recent exposure to cigarettes varied.
That varenicline appears to operate on general levels of craving but not on cue-specific craving suggests that different psychological processes may govern these different manifestations of craving. Human and animal studies on learning have found that goal-directed learning (outcome-response) and stimulus-directed learning (stimulus-outcome) can activate different behavioral profiles (Hogarth et al. 2010; Ostlund and Balleine 2008). Addiction researchers have proposed a dual-process model that includes both stimulus-outcome and outcome-response contingencies as processes that operate independently (Rescorla and Solomon 1967; Tiffany 1990). Considering these theories, one could conceptualize general craving as activating a goal-directed pathway--that is, general craving is influenced by a smoker’s current satiety and the current incentive value of the cigarette outcome. Cue-specific craving, on the other hand, has been shown to be robust to manipulations of satiety (Bailey et al. 2010; Drobes and Tiffany 1997; Maude-Griffin and Tiffany 1996; Tiffany et al. 2000). In our study, varenicline reduced levels of general craving compared to placebo; suggesting that varenicline may operate in part by reducing the incentive value of a cigarette by diminution of the outcome representation. Conversely, cue-specific craving was not altered by varenicline administration, supporting the hypothesis that cue-specific craving is a dissociable entity from general craving.
To date, no studies have directly compared the effects of varenicline before and after the target quit date. Varenicline may function differently in smokers who are preparing for cessation than in those who have already quit. Smokers who have quit are more likely to experience abstinence-based craving, which varies as a function of time since last cigarette (Schuh and Stitzer 1995), and thus may be more sensitive to the effects of varenicline. Laboratory-based cue-reactivity studies of varenicline have typically required some period of abstinence prior to the cue assessment (e.g., a 12-hour period; e.g., Brandon et al. 2011); even studies that do not require a period of abstinence interposed an extended interval between the last cigarette smoked and time that craving is rated (>60 minutes; Franklin et al. 2011). In such studies, this interval may be long enough to generate some degree of abstinence-induced craving. In the current study, the average interval reported between the last cigarette smoked and the subsequent cue-reactivity assessment was 37 minutes; as such, participants were not likely to experience much abstinence-induced craving.
Participants reported paying greater attention to photographic cues than in vivo cues in this study, which was perhaps due to the novelty of these cues. This difference was observed regardless of smoking or neutral cue content. We found that this effect was more pronounced for those in the placebo condition. This unanticipated finding may suggest that varenicline suppresses attentional processing of both smoking and non-smoking stimuli. However, the fact that there was no difference in craving between photographic and in vivo cues suggests that these attentional effects had no impact on craving.
Analyses of the smoking data from this study (Hawk et al. in press) indicate that varenicline had an impact on smoking rates such that women who received varenicline reported smoking significantly fewer cigarettes than women receiving placebo during both the Drug Manipulation Phase and the Standard Therapy Phase. Among men, varenicline had no impact on smoking rates during any pre-quit phase of the study. In the current study, those on varenicline reported a small decrease in general craving during the Drug Manipulation Phase relative to those on placebo. Changes in smoking levels complicate interpretations of changes in craving to the extent that a decrease in smoking could be associated with either increases or decreases in craving. That is, lower levels of craving may result in reductions in smoking, or lower smoking levels might trigger increased craving. In the latter case, an overall impact of varenicline on craving might be overshadowed by a simultaneous increase in craving generated by reductions in smoking. While a direct test of these possibilities was not possible, craving ratings and smoking levels were not significantly associated, suggesting that they operated independently of one another. A better test of these relationships could be produced through procedures in which smoking level is held constant or a substantially larger sample could be used to tease apart the complex of associations across craving and smoking levels.
This was the first study to assess reactivity to smoking cues presented in smokers’ natural environments in a treatment-seeking sample. Previous research with this procedure has been restricted to cigarette smokers not attempting to quit (Warthen and Tiffany 2009; Wray et al. 2011). Our results indicate that even smokers enrolled in a treatment program showed increased craving in response to smoking cues compared to neutral cues, an effect that prevailed across multiple weeks. Further, we found that cue-specific craving was stronger during the Baseline Phase than either the Drug Manipulation Phase or the Standard Therapy Phase. Our previous CREMA research has not found that cue-specific craving declines over time (Warthen and Tiffany 2009; Wray et al. 2011), but the current study differed from that research in several important ways. Our sample consisted of treatment-seeking smokers who were preparing to quit smoking while the previous studies assessed non-treatment seekers. Additionally, the current study assessed cue-specific craving over 5 weeks, while the previous studies examined only collected reactivity data for 8 days.
There are several processes that might be responsible for the observed change in magnitude of cue-specific craving over the course of the study. Because the decrease occurred between Phase 1 (Baseline) and Phase 2 (Drug Manipulation), participants may have felt that, after the Baseline week, “treatment had begun,” and, consequently, may have worked to actively manage their responses to smoking related cues. Notably, participants received brief cognitive-behavioral therapy with the onset of Phase 2, which may have provided tools and skills to manage craving. It is also possible that participants habituated to the cues over multiple presentations. Research on cue-exposure treatments provides evidence that repeated exposure to smoking cues may lead to a reduction in craving over repeated trials during laboratory sessions (Lee et al. 2004). Research has also suggested that reductions in craving following repeated exposures of cigarette cues may mediate the link between the cue and smoking behavior (O’Connell et al. 2011); however, investigations of cue-exposure therapy have yielded only limited results when considering smoking behavior and relapse rates (Conklin and Tiffany 2002).
General craving decreased systematically across each phase of the study as participants approached their target quit date. In contrast, the decrease for cue-specific craving, was only observed between Baseline and Drug Manipulation. Because the decrease in craving was found across both drug conditions, it may be attributed to any number of factors, including the brief therapy that participants received or their participation in a quitting program. Two nicotine patch studies also found that craving decreased in the pre-quit period; the decrease was larger for those wearing the patch than those not (Rezaishiraz et al. 2007; Rose et al. 2006). The decrease in cue-specific craving was not likely attributable to lack of attention paid to the cue; attention actually increased during the early weeks of the study.
Limitations
Some limitations in the current study should be considered. First, the sample size (60 participants) was relatively small for a treatment study and may have precluded the detection of significant drug effects. In contrast, however, repeated-measures analyses of certain variables helped us to observe important significant outcomes of cue type, mode, and week. Second, in exchange for analyses in the natural environment, the CREMA methodology provides less experimental control than cue-reactivity studies conducted in the laboratory. Participant compliance with study procedures was known to us only through self-report rather than direct observation—for example, participants were expected to view an in vivo object or cigarette, and, unless they indicated that they had not done so, we had to assume compliance. Notably, however, participants were told during training that no penalty would be given if a participant did not actually hold an object or cigarette. Finally, generalizability of study findings may also be considered a limitation, as the demands of the CREMA procedure may have reduced the number of smokers willing to participate. However, study dropout was low, which suggests that the CREMA procedure was not excessively burdensome to those who enrolled.
Summary
In this study, varenicline did not significantly reduce cue-specific craving prior to the quit attempt when assessed in the natural environment. Varenicline did appear to have an early impact on general craving among those who received an extended course of active medication. Irrespective of drug condition, craving decreased over time; general craving continued to decline across all phases of the study and cue-specific craving decreased significantly in the early weeks of the study. In the first study ever study that utilized cue reactivity and ecological momentary assessment in a clinical drug trial, we found robust cue-specific craving that continued up through the participants’ target quit date. Overall, we found strong cue-specific craving assessed in the natural environment of quitting smokers - these findings demonstrate the continued utility of the CREMA procedure for examining cue-specific craving. Though the CREMA procedure was highly sensitive for the detection of cue-specific craving, the study produced no evidence that varenicline had any impact on cue-specific craving raving among smokers preparing to quit.
Acknowledgments
This research was funded in part by a 2008 Global Research Award for Nicotine Dependence (GRAND), an independent investigator initiated research program, sponsored by Pfizer, Inc., awarded to Martin C. Mahoney, MD, Ph.D. and a grant from the National Institute on Drug Abuse (R21 DA019653) to Stephen T. Tiffany, Ph.D. The authors have no financial relationship with the organizations that sponsored the research.
Footnotes
Training protocol available from Stephen Tiffany
References
- Bailey SR, Goedeker KC, Tiffany ST. The impact of cigarette deprivation and cigarette availability on cue reactivity in smokers. Addict. 2010;105:364–372. doi: 10.1111/j.1360-0443.2009.02760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon TH, Drobes DJ, Unrod M, Heckman BW, Oliver JA, Roetzheim RC, Karver SB, Small BJ. Varenicline effects on craving, cue reactivity, and smoking reward. Psychopharmacol. 2011 doi: 10.1007/s00213-011-2327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addict. 1999;94:327–340. [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. Cue Exposure: Time for change. Addict. 2002;97:1219–1223. doi: 10.1046/j.1360-0443.2002.00205.x. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Cigarette smoking among adults—United States, 2006. Morb and Mort Wkly Rep. 2007;56:1157–1161. [PubMed] [Google Scholar]
- Drobes DJ, Tiffany ST. Induction of smoking urge through imaginal and in vivo procedures: Physiological and self-report manifestations. J of Abnorm Psychol. 1997;106:15–25. doi: 10.1037//0021-843x.106.1.15. [DOI] [PubMed] [Google Scholar]
- Franklin T, Wang ZW, Suh JJ, Hazan R, Cruz J, Li Y, Goldman M, Detre JA, Childress AR, et al. Effects of varenicline on smoking cue-triggered neural and craving responses. Arch of Gen Psychiatr. 2011 doi: 10.1001/archgenpsychiatry.2010.190. Advanced online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Reeves KR, et al. Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs sustained release bupropion and placebo for smoking cessation. JAMA. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Hawk LW, Ashare RL, Lohnes SF, Schlienz NJ, Rhodes JD, Tiffany ST, Gass JC, Cummings KM, Mahoney MC. The effects of extended pre-quit varenicline treatment on smoking behavior and short-term abstinence: A randomized clinical trial. Clin Pharmacol and Ther. doi: 10.1038/clpt.2011.317. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hogarth L, Dickinson A, Duka T. The associative basis of cue elicited drug taking in humans. Psychopharmacol. 2010;208:337–351. doi: 10.1007/s00213-009-1735-9. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Peters EN, Naud S. Relapse to smoking after 1 year of abstinence: A meta-analysis. Addict Behav. 2008;33:1516–1520. doi: 10.1016/j.addbeh.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR, et al. Efficacy of varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs. placebo or sustained-release bupropion for smoking cessation: A randomized controlled trial. JAMA. 2006;296:56–64. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP, Murphy GM, Hayward C, Arredondo C, Cromp D, Schatzberg AF. Extended treatment with bupropion SR for cigarette smoking cessation. J of Consult and Clin Psych. 2006;74:286–294. doi: 10.1037/0022-006X.74.2.286. [DOI] [PubMed] [Google Scholar]
- Lee J, Lim Y, Graham SJ, Kim G, Wiederhold BK, Wiederhold MD, Kim IY, Kim SI. Nicotine craving and cue exposure by using virtual environments. CyberPsychol & Behav. 2005;7:705–713. doi: 10.1089/cpb.2004.7.705. [DOI] [PubMed] [Google Scholar]
- Maude Griffin PM, Tiffany ST. Production of smoking urges through imagery: The impact of affect and smoking abstinence. Exp and Clin Psychopharmacol. 1996;4:198–208. [Google Scholar]
- Nides M, Oncken C, Gonzales D, Rennard S, Watsky EJ, Anziano R, Reeves KR. Smoking cessation with varenicline, a selective α4β2 nicotinic partial agonist. Arch Intern Med. 2006;166:1561–1568. doi: 10.1001/archinte.166.15.1561. [DOI] [PubMed] [Google Scholar]
- Nides M, Elbert D, Reus VI, Christen AG, Make BJ, Billing CB, Williams KE. Varenicline versus bupropion SR or placebo for smoking cessation: A pooled analysis. American J of Health Beh. 2008;32:664–675. doi: 10.5555/ajhb.2008.32.6.664. [DOI] [PubMed] [Google Scholar]
- O’Connell KA, Shiffman S, DeCarlo LT. Does extinction of responses to cigarette cues occur during smoking cessation? Addict. 2011;106:410–417. doi: 10.1111/j.1360-0443.2010.03172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. The disunity of Pavlovian and instrumental values. Behav Brain Sci. 2008;31:456–457. [Google Scholar]
- Patterson C, Jepson A, Strasser J, Loughead K, Perkins R, Gur J, Frey S, Siegel C, Lerman Varenicline improves mood and cognition during smoking abstinence. Bio Psychiatr. 2009;65:144–149. doi: 10.1016/j.biopsych.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA, Solomon RL. Two-process learning theory: relationships between Pavlovian conditioning and instrumental training. Psychol Rev. 1967;74:151–183. doi: 10.1037/h0024475. [DOI] [PubMed] [Google Scholar]
- Rezaishiraz H, Hyland A, Mahoney MC, O’Connor RJ, Cummings KM. Treating smokers before the quit date: Can nicotine patches and denicotinized cigarettes reduce cravings? Nic and Tob R. 2007;9:1139–1146. doi: 10.1080/14622200701684172. [DOI] [PubMed] [Google Scholar]
- Rollema H, Shrikhande A, Ward KM, Tingley FD, III, Coe JW, O’Neill BT, Tseng E, Bertrand D, et al. Pre-clinical properties of the α4β2 nicotinic acetylcholine receptor partial agonists varenicline, cytosine and dianicline translate to clinical efficacy for nicotine dependence. Bri J of Pharmacol. 2007;160:334–345. doi: 10.1111/j.1476-5381.2010.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Kukovich P. Precessation treatment with nicotine skin patch facilitates smoking cessation. Nic and Tob R. 2006;8:89–101. doi: 10.1080/14622200500431866. [DOI] [PubMed] [Google Scholar]
- Schuh KJ, Stitzer ML. Desire to smoke during spaced smoking intervals. Psychopharmacol. 1995;120:289–295. doi: 10.1007/BF02311176. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Ecologicial momentary assessment (EMA) in studies of substance use. Psych Assess. 2009;21:486–497. doi: 10.1037/a0017074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teneggi V, Tiffany ST, Squassante L, Milleri S, Zivani L, Bye A. Smokers deprived of cigarettes for 72 h: Effect of nicotine patches on craving and withdrawal. Psychopharmacol. 2002;164:177–187. doi: 10.1007/s00213-002-1176-1. [DOI] [PubMed] [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug use behavior: Role of automatic and nonautomatic processes. Psychol Rev. 1990;97:47–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Tiffany ST. Drug craving and affect. In: Kassel J, editor. Substance abuse and emotion. Washington: District of Columbia; 2010. pp. 83–108. [Google Scholar]
- Tiffany ST, Cox LS, Elash CA. Effects of transdermal nicotine patches on abstinence-induced and cue-elicited craving in cigarette smokers. J of Clin and Consult Psychol. 2000;68:233–240. doi: 10.1037//0022-006x.68.2.233. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urgs. Br J Addict. 1991;86:1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Warthen MW, Goedeker KC. The functional significance of craving in nicotine dependence. In: Bevins R, editor. The motivational impact of nicotine and its role in tobacco use. New York, New York: 2009. pp. 171–197. [DOI] [PubMed] [Google Scholar]
- Tonstad S, Tonnesen P, Hajek P, Williams KE, Billing CB, Reeves KR. Effect of maintenance therapy with varenicline on smoking cessation: A randomized controlled trial. JAMA. 2006;296:64–71. doi: 10.1001/jama.296.1.64. [DOI] [PubMed] [Google Scholar]
- Warthen MW, Tiffany ST. Evaluation of cue reactivity in the natural environment of smokers using ecological momentary assessment. Exp and Clin Pychopharmacol. 2009;17:70–77. doi: 10.1037/a0015617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Cue-provoked craving and nicotine replacement therapy in smoking cessation. J of Clin and Consult Psychol. 2004;72:1136–1143. doi: 10.1037/0022-006X.72.6.1136. [DOI] [PubMed] [Google Scholar]
- Weiss HM, Beal DJ, Lucy SL, MacDermid SM. [Accessed 29 February 2012];Constructing EMA studies with PMAT: The Purdue Momentary Assessment Tool user’s manual. 2004 Available via http://www.ruf.rice.edu/~dbeal/pmatusermanual.pdf.
- West R, Baker CL, Cappelleri JC, Bushmakin AG. Effect of varenicline and bupropion SR on craving, nicotine withdrawal symptoms, and reward effects of smoking during a quit attempt. Psychopharmacol. 2008;197:371–377. doi: 10.1007/s00213-007-1041-3. [DOI] [PubMed] [Google Scholar]
- Wray J, Godleski S, Tiffany ST. Cue-reactivity in the natural environment of cigarette smokers: A comparison of photographic and in vivo smoking stimuli. Psychol of Addict Behav. 2011 doi: 10.1037/a0023687. [DOI] [PMC free article] [PubMed] [Google Scholar]