Abstract
Deficits in prefrontal cortical activity are consistent observations in a number of psychiatric diseases with two major regions consistently implicated being the mPFC and OFC, regions that carry out independent, but complementary forms of cognitive processing in changing environmental conditions. Information from the prefrontal cortex is integrated in the nucleus accumbens to guide goal-directed behavior. Anatomical studies have demonstrated that distinct prefrontal cortical regions provide an overlapping but distinct innervation of NAc subregions, however how information from these distinct regions regulates NAc output has not been conclusively demonstrated. Here we demonstrate that, while neurons receiving convergent glutamatergic inputs from the mPFC and OFC have a synergistic effect on single-spike firing, medium spiny neurons that receive a monosynaptic input from only one region are actually inhibited by activation of the complementary region. Therefore, the mPFC and OFC negatively regulate evoked activity within the lateral and medial regions of the NAc, respectively, and exist in a state of balance with respect to their influence on information processing within ventral striatal circuits.
Keywords: Medial prefrontal cortex, orbitofrontal cortex, nucleus accumbens, electrophysiology, schizophrenia
Introduction
Deficits in prefrontal cortical activity are consistent observations in a number of psychiatric diseases with two major regions consistently implicated being the mPFC and OFC. These regions carry out independent, but complementary forms of cognitive processing in changing environmental conditions. Thus, the mPFC is intimately involved in higher-order dimensional set-shifting (Birrell and Brown, 2000; Dias et al., 1996; Floresco et al., 2006; Ragozzino et al., 1999; Rogers et al., 2000; Smith et al., 2004; Stefani et al., 2003), and working memory (Abi-Dargham et al., 2002; Cohen et al., 1997; Funahashi et al., 1993; Sawaguchi and Goldman-Rakic, 1991) whereas the OFC more specifically regulates reversal learning and affective associations (Jentsch et al., 2002; McAlonan and Brown, 2003; O’Doherty et al., 2003; O’Doherty et al., 2001; Rolls, 2000; Schoenbaum et al., 1999). These inputs are integrated within NAc sub-territories to regulate motivational state and govern goal-directed behavior.
Glutamatergic inputs to the NAc arise from multiple cortical and sub-cortical regions (Groenewegen et al., 1999). These inputs are topographically arranged and provide differential innervation of functionally distinct sub-regions of the NAc (Groenewegen et al., 1999). Thus, the medial regions of the NAc are heavily innervated by afferents arising in the ventral medial regions of the prefrontal cortex, and are suggested to be associated with processing information associated with the motivational significance of reward-related cues (Berendse et al., 1992; Brog et al., 1993). In contrast, the more lateral regions of the NAc receive preferential innervation from the dorsal mPFC and orbitofrontal cortices, and are more often associated with somatomotor functions of the ventral striatum due to its considerable association with the dorsolateral ventral pallidum (Berendse et al., 1992; Brog et al., 1993). Thus, the connectivity of NAc sub-territories provides a unique foundation by which information from the cortex can be integrated to govern goal-directed behavior. Indeed, electrophysiological studies have demonstrated that activity from converging glutamatergic inputs are integrated in individual medium spiny neurons (Groenewegen et al., 1999; McGinty and Grace, 2009a; O’Donnell and Grace, 1995). More specifically, in vivo intracellular recordings from single neurons in the NAc shell have demonstrated that glutamatergic inputs can gate the cortical regulation of NAc activity (O’Donnell and Grace, 1995). Thus, coordinated afferent activity results in a transition from a hyperpolarized state (−80 mV) to a relatively depolarized state (−60 mV) (Gruber and O’Donnell, 2009; O’Donnell and Grace, 1995). This activity is postulated to gate NAc neuronal activity since action potentials are only observed during the depolarized ‘up-state’ (Gruber and O’Donnell, 2009; O’Donnell and Grace, 1995). Hence the anatomical connectivity of the NAc, combined with the ability to integrate neuronal activity, makes it ideally positioned to regulate motivational state and orchestrate goal-directed behavior. Here we examine the regulation of afferent-induced neuronal activity in the NAc by two distinct prefrontal cortical regions, the mPFC and OFC and demonstrate that these inputs exist in a state of balance with respect to their influence on information processing within ventral striatal circuits.
Materials and Methods
All experiments were performed in accordance with the guidelines outlined in the USPHS Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center.
In vivo Electrophysiology
Male Sprague Dawley rats (250-350g) were anaesthetized with chloral hydrate (400 mg/kg i.p.) and placed in a stereotaxic apparatus. Anesthesia was maintained by supplemental administration of chloral hydrate as required to maintain suppression of limb compression withdrawal reflex. A core body temperature of 37°C was sustained by a thermostatically controlled heating pad (KOPF – TCAT-2LV). Bipolar concentric stimulating electrodes (KOPF/Rhodes NEX100) were implanted in the ventral mPFC (A/P +3.0, M/L +0.5, D/V −5.0 mm from bregma) and OFC (A/P +3.0, M/L +3.5, D/V −5.0 mm from bregma). Extracellular glass microelectrodes (impedance 6-14MΩ) were lowered into either medial (A/P +1.5, M/L +1.0 mm from bregma and −5.0 to −7.5mm ventral of brain surface) or lateral (A/P +1.5, M/L +2.0 mm from bregma and −5.0 to −7.5mm ventral of brain surface) regions of the NAc using a hydraulic microdrive. Alternating current pulses (0.5 Hz, 0.25 msec, 1mA) were examined and cells with latencies <30msec were included in the study. Once a stable medium spiny neuron was identified, 30 current pulses (0.5 Hz, 0.25 msec) were applied to each PFC region separately. The OFC and mPFC were then stimulated simultaneously (30 pulses) before increasing the inter-stimulus intervals (ISI’s) by 10ms at a time. A total of ten rats were used with multiple neurons recorded from each rat.
Drug administration
For acute administration of drugs into the PFC, rats were implanted with a 23 gauge cannula 2.0mm dorsal to the mPFC (A/P +3.0, M/L +0.5, D/V −3.0 mm from bregma) or OFC (A/P +3.0, M/L +3.5, D/V −3.0 mm from bregma). In addition, bipolar concentric stimulating electrodes (KOPF/Rhodes NEX100) were implanted in the alternate PFC region. Single current pulses (0.5 Hz, 0.25 msec, 1mA) were administered and cells with latencies <30msec were included in the study. Once a stable medium spiny neuron was identified, baseline spike probability was recorded for 20 mins prior to chemical activation (NMDA: 0.75μg/0.5μl in Dulbecco’s PBS) of the alternate PFC region. Drugs were infused through a 30-gauge injection cannula protruding 2.0 mm past the end of the implanted guide cannula which was left in situ for the duration of the experiments to ensure adequate diffusion of drug into the surrounding tissue. Evoked NAc neuron activity was recorded for up to 40 minutes following infusions. Neurons displaying a >25% change in spike probability, compared to baseline, were considered to be increased or decreased by NMDA activation. For control experiments, Dulbecco’s PBS (0.5μl) was injected into either the PFC or OFC. A total of twenty two rats were used and received only one injection per region.
Histology
At the cessation of the electrophysiology experiments, the recording site was marked via electrophoretic ejection of Pontamine sky blue from the tip of the recording electrode (−15μA constant current (5 sec on; 2 sec off) for 20-30 mins) and stimulating electrodes marked by passing constant current across the poles (10 sec, 0.3mA). Rats were killed by an overdose of anesthetic (chloral hydrate, additional 400 mg/kg i.p.), decapitated and their brains removed, fixed for at least 48 hours (8% w/v paraformaldehyde, 1% w/v potassium ferricyanide, in PBS), and cryoprotected (25% w/v sucrose in PBS) until saturated. Brains were sectioned (50 μm coronal sections), mounted onto gelatin-chrom alum coated slides and stained with Cresyl violet for histochemical verification of electrode and/or cannula sites. All histology was performed with reference to a stereotaxic atlas (Paxinos and Watson, 1986).
Analysis
Electrophysiological analysis of single unit neuron activity was performed using commercial computer software (LabChart Pro - ADInstruments). All data are represented as the mean ± standard error of the mean (SEM) unless otherwise stated.
Materials
Chloral hydrate, Dulbecco’s phosphate buffered saline, and NMDA were all purchased from Sigma (St Louis, MO). All other chemicals and reagents were of either analytical or laboratory grade and purchased from various suppliers.
Results
Distinct subregions of the prefrontal cortex are known to provide a topographic input to the medium spiny neurons of the nucleus accumbens (Groenewegen et al., 1999). Consistent with anatomical data, we report that single-pulse activation of the ventral mPFC preferentially activated neurons located in the medial NAc, whereas activation of the OFC primarily excited neurons in the NAc core. It should be noted that there was some overlap of these inputs with a population of neurons in both the core and shell responding to activation of both afferents. Although neurons receiving input from only one prefrontal pathway were specifically targeted, neurons that responded to both afferents were encountered and in these neurons the simultaneous activation of both the mPFC and OFC resulted in an increase in spike probability, and a decrease in the variance of spike latency (Figure 2), consistent with previous examinations of convergent glutamatergic inputs to NAc neurons (McGinty and Grace, 2009b). The majority of neurons recorded responded to only one PFC input and in these neurons the simultaneous activation of the mPFC and OFC did not significantly alter evoked spike probability (Figures 3 & 4). To further investigate any potential interactions between these inputs, we examined the effect of temporally separating the activation of PFC inputs. Interestingly, this unmasked an inhibitory effect of cortical stimulation on afferent-evoked responses in the majority of NAc neurons (25 out of 41 neurons - 61%). Specifically, in accumbens neurons responding only to mPFC activation, the pre-stimulation of the OFC produced a pronounced inhibition (in 13 out of 22 neurons – 59%) of mPFC-evoked activity with a maximum response (change in spike probability: −59.2 ± 7.9 %) observed with a 20ms ISI (Figures 3 & 4). This response was time dependent, occurring only when the stimulus was preceded by 10-30 ms (p<0.05 1-way ANOVA: Figure 4). A similar response was observed in the NAc core. Thus, neurons activated by the OFC were inhibited (in 12 out of 19 neurons – 63%) by activation of the mPFC from 10-40ms prior (p<0.05 1-way ANOVA: Figure 4). Thus, it appears that the mPFC and OFC negatively regulate firing of NAc neurons driven by the other cortical input.
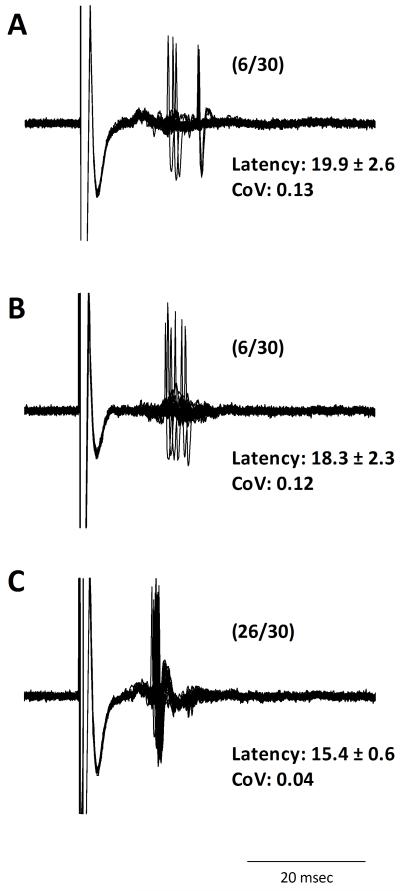
Figure 2.
A subset of medium spiny neurons in the NAc receive converging inputs from both the mPFC and OFC. In medium spiny neurons that responded to electrical stimulation of both the OFC (A) and mPFC (B), the simultaneous activation of both PFC regions induced an augmented response than that observed with each input alone (C). Data in parentheses reflects percent spike probability.
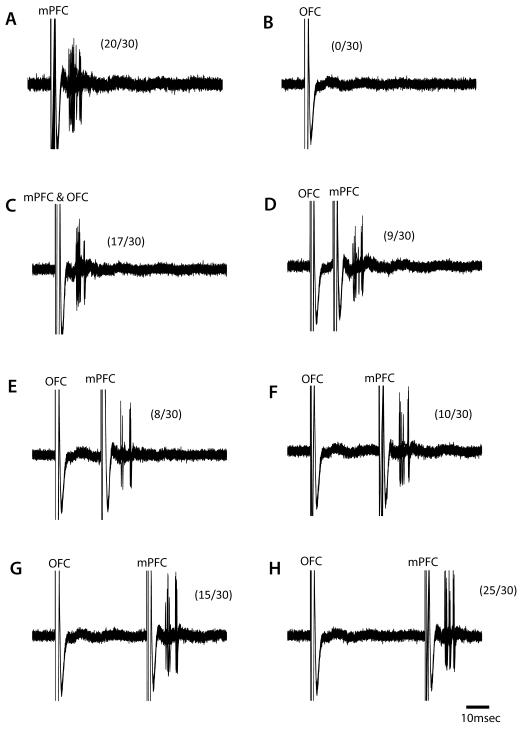
Figure 3.
A subset of medium spiny neurons in the NAc are inhibited by prefrontal cortical activation. A representative neuron in the medial NAc that is excited by electrical activation the mPFC (A), but not OFC (B). The simultaneous activation of both afferents (C) did not appear to alter spike probability compared to mPFC activation alone. Each trace from D-H represents an increasing interstimulus interval of 10ms. Interestingly, stimulation of the OFC prior to the mPFC significantly attenuated the response to mPFC stimulation when separated by 10-30ms. Data in parentheses reflects percent spike probability.
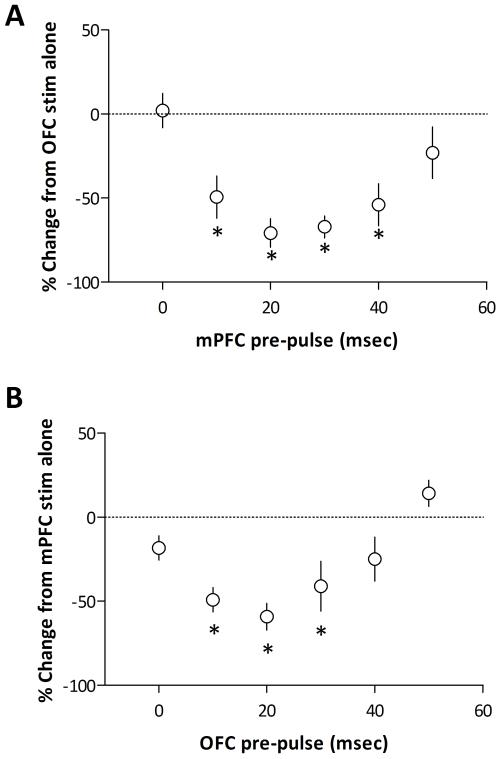
Figure 4.
Group data demonstrating the negative regulation of evoked NAc activity by PFC subregions. Neurons recorded in the lateral NAc (A) were primarily activated by the OFC and in these neurons a preceding input from the mPFC significantly attenuates OFC-evoked responses (* represents significant difference from OFC activation alone: p<0.05, 1-way ANOVA; n = 12). Similarly, in medial NAc neurons activated by mPFC stimulation (B), the preceding activation of OFC afferents significantly inhibited evoked activity (* represents significant difference from mPFC activation alone: p<0.05, 1-way ANOVA; n = 13).
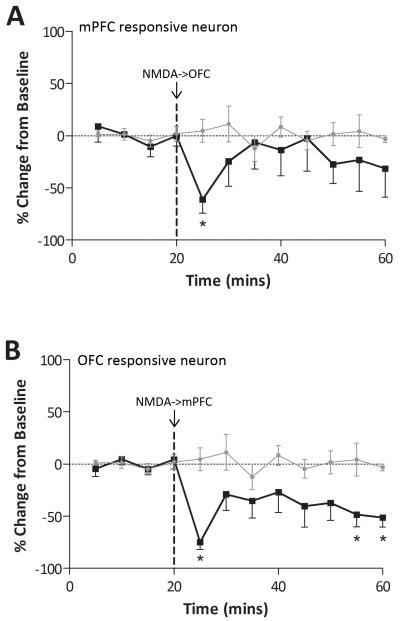
To further examine the relationship between the mPFC and OFC regulation of NAc activity, we investigated how tonic PFC activation altered evoked activity. Consistent with the responses observed with paired-pulse stimulation (Table 1), intra-mPFC NMDA (0.75μg/0.5μl) significantly attenuated OFC-evoked responses (change in spike probability at 5 mins −74.9 ± 6.9 %: p<0.05 c.f. vehicle infusion, 2-way ANOVA: Figure 5) whereas intra-OFC NMDA inhibited the response to mPFC stimulation (change in spike probability at 5 mins −61.1 ± 13.1 %: p<0.05 c.f vehicle infusion, 2-way ANOVA: Figure 5). It should be noted that a subset of neurons displayed an increase in evoked-activity that likely reflects neurons receiving convergent inputs as detailed above (Table 1).
Table 1. Drug Induced Changes in Excitability.
| Excitation | Inhibition | No Change | |
|---|---|---|---|
| NMDA-> OFC | 25.0% | 62.5% (59%) | 12.5% |
| NMDA-> mPFC | 16.7% | 83.3% (63%) | - |
Proportion of accumbens neurons responding to NMDA activation (0.75μg/0.5μl) of the OFC or mPFC (n=6-8 neurons). Numbers in parenthesis represent percentage of neurons inhibited by electrical stimulation (n=19-22 neurons).
Figure 5.
Chemical activation of PFC subregions negatively regulates evoked activity in NAc subregions. NMDA activation of the OFC significantly attenuated mPFC-evoked activity in the medial NAc (A: * represents significant difference from vehicle: p<0.05, 2-way ANOVA; n = 5). Similarly, NMDA activation of the mPFC significantly inhibited the OFC-evoked spike firing in lateral NAc neurons (B: * represents significant difference from vehicle: p<0.05, 2-way ANOVA; n = 5).
Discussion
Here we demonstrate that although the mPFC and OFC innervate somewhat anatomically distinct sub-regions of the NAc (Groenewegen et al., 1999), activity within these cortical regions is integrated to determine the output of individual NAc neurons. As mentioned above, the prefrontal cortex provides a relatively dense innervation of the NAc (Groenewegen et al., 1999). Furthermore, this innervation is topographic with distinct cortical regions innervating unique sub-territories of the NAc (Groenewegen et al., 1999). Thus the ventral medial subregions of the PFC primarily innervate the NAc shell, whereas the OFC provides a more dense innervation of the NAc core (Groenewegen et al., 1999). Given the complementary roles of the mPFC and OFC in the regulation of executive function, it would seem likely that the NAc is a site of convergence where these prefrontal cortical regions interact to guide behavior. Our current data suggest that although the mPFC and OFC innervate somewhat anatomically distinct sub-regions of the NAc (Groenewegen et al., 1999), activity within these cortical regions is integrated to determine the output of individual NAc neurons. Thus, the excitatory response to OFC stimulation in neurons of the NAc can be attenuated by the pre-stimulation of the mPFC and vice versa. Given the relatively little overlap between the OFC and mPFC inputs to the NAc (Groenewegen et al., 1999), this negative regulation likely involves activation of feed-forward and feedback GABAergic pathways. Indeed, anatomical studies have demonstrated evidence for direct connections between the core and shell (van Dongen et al., 2005) while recent data from intracellular recordings suggest that cortical activation can inhibit NAc neurons via the local release of GABA (Gruber et al., 2009). Thus, it appears that the prefrontal cortex can differentially regulate NAc activity, and moreover, that the inputs from the mPFC and OFC interact dynamically to determine NAc output. Such a regulation provides a temporal and anatomically distinct mechanism by which the NAc can integrate behaviorally relevant inputs to guide goal-directed behavior. Moreover, this system provides the neurophysiological framework whereby a dysfunction within cortical inputs may result in the aberrant integration of motivational and sensory input and therefore contribute to the pathophysiology of neuropsychiatric diseases.
There is a significant literature from both clinical and preclinical investigations demonstrating a role for these regions in neuropsychiatric disorders including schizophrenia (Abi-Dargham et al., 2002; Chakirova et al., 2010; Homayoun and Moghaddam, 2008; Manoach et al., 2000; Nishimura et al., 2011; Schobel et al., 2009a; Weinberger et al., 1986). The data presented here suggest that the mPFC and OFC negatively regulate evoked activity within the NAc and therefore exist in a state of balance with respect to their influence on information processing within ventral striatal circuits. Furthermore, we posit that a pathological disruption in PFC activity would produce dramatic effects on this equilibrium resulting in aberrant goal-directed behavior. Indeed, there is considerable evidence that these PFC regions play a significant role in the cognitive deficits associated with schizophrenia. In addition, human imaging studies in schizophrenia patients have demonstrated that the OFC and mPFC are differentially affected in the disease. Specifically, an increase in regional cerebral blood volume (rCBV) is present in the orbitofrontal cortex of schizophrenia patients, whereas a decreased rCBV is reported in the dorsolateral prefrontal cortex (Schobel et al., 2009b). A similar finding has been reported in a rodent model of schizophrenia in that the inhibition of mPFC neuronal activity following amphetamine is amplified following repeated psychostimulant administration and the opposite response is observed in OFC neurons, i.e. an augmented excitatory response to amphetamine following repeated treatment (Homayoun and Moghaddam, 2006). Thus, it appears that schizophrenia is associated with distinct alterations in mPFC and OFC structure and function that, when combined with enduring alterations in sub-cortical dopamine transmission, likely result in an enhanced OFC input and attenuated mPFC input to the NAc. Given the results of the current study, this would likely result in an augmented output of the medial core of the nucleus accumbens as well as a decreased activity throughout the more lateral regions.
Taken as a whole we demonstrate that the mPFC and OFC negatively regulate evoked activity within the lateral and medial regions of the NAc, respectively, and therefore exist in a state of balance with respect to their influence on information processing within ventral striatal circuits.
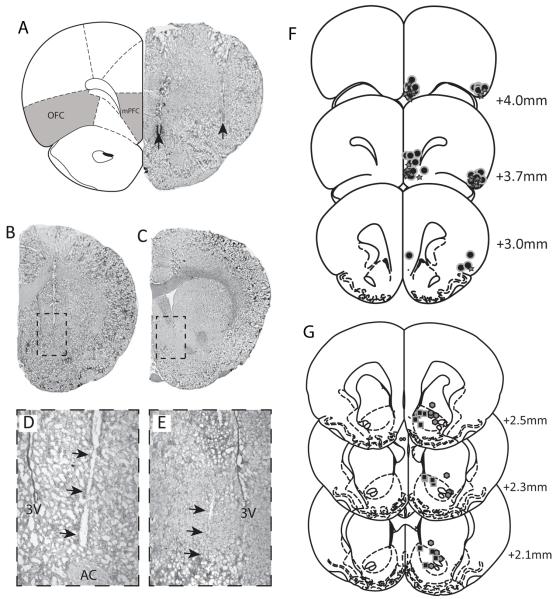
Figure 1.
Representative brain sections demonstrating the localization of stimulating electrode placements (arrows) within the mPFC and OFC (A) and recording electrode locations within the lateral (B expanded in D) and medial (C expanded in E) NAc. The locations of each identified recording electrode (f: lateral octagons - medial squares), stimulating electrode (G: star) or infusion site (g: circle) are depicted with reference to a stereotaxic atlas adapted from Paxinos and Watson (Paxinos and Watson, 1986).
Acknowledgements
This work was supported by the National Institutes of Health (R01 MH090067 to D.J.L.) and a NARSAD award from the Maltz Family Foundation (D.J.L).
Footnotes
Statement of Interest
None
References
- Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, et al. Prefrontal Dopamine D1 Receptors and Working Memory in Schizophrenia. Journal of Neuroscience. 2002;22(9):3708–3719. doi: 10.1523/JNEUROSCI.22-09-03708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendse HW, Galis-de Graaf Y, Groenewegen HJ. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. Journal of Comparative Neurology. 1992;316(3):314–347. doi: 10.1002/cne.903160305. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. Journal of Neuroscience. 2000;20(11):4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. Journal of Comparative Neurology. 1993;338(2):255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- Chakirova G, Welch KA, Moorhead TWJ, Stanfield AC, et al. Orbitofrontal morphology in people at high risk of developing schizophrenia. European Psychiatry. 2010;25(6):366–372. doi: 10.1016/j.eurpsy.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, et al. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386(6625):604–611. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Primate analogue of the Wisconsin Card Sorting Test: Effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behavioral Neuroscience. 1996;110(5):872–886. doi: 10.1037//0735-7044.110.5.872. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Magyar O, Ghods-Sharifi S, Vexelman C, et al. Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting. Neuropsychopharmacology. 2006;31(2):297–309. doi: 10.1038/sj.npp.1300825. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Dorsolateral prefrontal lesions and oculomotor delayed-response performance: Evidence for mnemonic ‘scotomas. Journal of Neuroscience. 1993;13(4):1479–1497. doi: 10.1523/JNEUROSCI.13-04-01479.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AVJ, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Annals New York Academy Science. 1999;877:49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- Gruber AJ, O’Donnell P. Bursting activation of prefrontal cortex drives sustained up states in nucleus accumbens spiny neurons in vivo. Synapse. 2009;63(3):173–180. doi: 10.1002/syn.20593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber AJ, Powell EM, O’Donnell P. Cortically Activated Interneurons Shape Spatial Aspects of Cortico-Accumbens Processing. Journal of Neurophysiology. 2009;101(4):1876–1882. doi: 10.1152/jn.91002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. Progression of cellular adaptations in medial prefrontal and orbitofrontal cortex in response to repeated amphetamine. Journal of Neuroscience. 2006;26(31):8025–8039. doi: 10.1523/JNEUROSCI.0842-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. Orbitofrontal cortex neurons as a common target for classic and glutamatergic antipsychotic drugs. Procedings National Academy Science USA. 2008;105(46):18041–18046. doi: 10.1073/pnas.0806669105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Olausson P, Garza Ii R, Taylor JR. Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacology. 2002;26(2):183–190. doi: 10.1016/S0893-133X(01)00355-4. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Gollub RL, Benson ES, Searl MM, et al. Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biological Psychiatry. 2000;48(2):99–109. doi: 10.1016/s0006-3223(00)00227-4. [DOI] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behavioral Brain Research. 2003;146(1-2):97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- McGinty VB, Grace AA. Activity-dependent depression of medial prefrontal cortex inputs to accumbens neurons by the basolateral amygdala. Neuroscience. 2009a;162(4):1429–1436. doi: 10.1016/j.neuroscience.2009.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinty VB, Grace AA. Timing-dependent regulation of evoked spiking in nucleus accumbens neurons by integration of limbic and prefrontal cortical inputs. Journal of Neurophysiology. 2009b;101(4):1823–1835. doi: 10.1152/jn.91162.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y, Takizawa R, Muroi M, Marumo K, et al. Prefrontal cortex activity during response inhibition associated with excitement symptoms in schizophrenia. Brain Research Reviews. 2011;1370(C):194–203. doi: 10.1016/j.brainres.2010.11.003. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Critchley H, Deichmann R, Dolan RJ. Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. Journal of Neuroscience. 2003;23(21):7931–7939. doi: 10.1523/JNEUROSCI.23-21-07931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, et al. Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience. 2001;4(1):95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- O’Donnell P, Grace AA. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. Journal of Neuroscience. 1995;15(5 Pt 1):3622–3639. doi: 10.1523/JNEUROSCI.15-05-03622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press Australia; Sydney: 1986. [Google Scholar]
- Ragozzino ME, Detrick S, Kesner RP. Involvement of the prelimbic-infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. Journal of Neuroscience. 1999;19(11):4585–4594. doi: 10.1523/JNEUROSCI.19-11-04585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Andrews TC, Grasby PM, Brooks DJ, et al. Contrasting cortical and subcortical activations produced by attentional-set shifting and reversal learning in humans. Journal of Cognitive Neuroscience. 2000;12(1):142–162. doi: 10.1162/089892900561931. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The orbitofrontal cortex and reward. Cerebral Cortex. 2000;10(3):284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: Involvement in working memory. Science. 1991;251(4996):947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- Schobel SA, Kelly MA, Corcoran CM, Van Heertum K, et al. Anterior hippocampal and orbitofrontal cortical structural brain abnormalities in association with cognitive deficits in schizophrenia. Schizophrenia Research. 2009a;114(1-3):110–118. doi: 10.1016/j.schres.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobel SA, Lewandowski NM, Corcoran CM, Moore H, et al. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Archives General Psychiatry. 2009b;66(9):938–946. doi: 10.1001/archgenpsychiatry.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Neural encoding in orbitofrontal cortex and basolateral amygdala during olfactory discrimination learning. Journal of Neuroscience. 1999;19(5):1876–1884. doi: 10.1523/JNEUROSCI.19-05-01876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AB, Taylor E, Brammer M, Rubia K. Neural Correlates of Switching Set as Measured in Fast, Event-Related Functional Magnetic Resonance Imaging. Human Brain Mapping. 2004;21(4):247–256. doi: 10.1002/hbm.20007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani MR, Groth K, Moghaddam B. Glutamate receptors in the rat medial prefrontal cortex regulate set-shifting ability. Behavioral Neuroscience. 2003;117(4):728–737. doi: 10.1037/0735-7044.117.4.728. [DOI] [PubMed] [Google Scholar]
- van Dongen YC, Deniau JM, Pennartz CMA, Galis-de Graaf Y, et al. Anatomical evidence for direct connections between the shell and core subregions of the rat nucleus accumbens. Neuroscience. 2005;136(4):1049–1071. doi: 10.1016/j.neuroscience.2005.08.050. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Archives General Psychiatry. 1986;43(2):114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]