Abstract
Rationale
Once dependent on alcohol or opioids, negative affect may accompany withdrawal. Dependent individuals are hypothesized to “self-medicate” in order to cope with withdrawal, which promotes escalated drug or alcohol use.
Objectives
The current study aimed to develop a reliable animal model to assess symptoms that occur during spontaneous alcohol and opioid withdrawal.
Methods
Dependence was induced using intermittent alcohol exposure or pulsatile heroin delivery and assessed for the presence of withdrawal symptoms during acute withdrawal by measuring somatic signs, behavior in the forced swim test (FST) and air-puff induced 22-kHz ultrasonic vocalizations (USVs). Additional animals subjected to eight weeks of alcohol vapor exposure were evaluated for altered somatic signs, operant alcohol self-administration and 22-kHz USV production, as well as performance in the elevated plus-maze (EPM).
Results
During spontaneous withdrawal from pulsatile heroin or intermittent alcohol vapor, animals displayed increased somatic withdrawal signs, FST immobility and 22-kHz USV production, but did not show any behavioral change in the EPM unless the duration of exposure was extended to four weeks. Following eight weeks of alcohol vapor exposure, animals displayed somatic withdrawal signs, escalated alcohol self-administration and increased 22-kHz USVs.
Conclusions
These paradigms provide consistent methods to evaluate the behavioral ramifications, and neurobiological substrates, of alcohol and opioid dependence during spontaneous withdrawal. As immobility in the FST and percent open-arm time in the EPM were dissociable, with 22-kHz USVs paralleling immobility in the FST, assessment of air-puff induced 22-kHz USVs could provide an ethologically-valid alternative to the FST.
Keywords: alcohol, anxiety, comorbidity, dependence, depression, diacetylmorphine, elevated plus-maze, ethanol, forced swim test, heroin, immobility, opiate, operant, opioid, osmotic minipumps, negative affect, self-administration, ultrasonic vocalizations, vapor exposure, withdrawal
Introduction
Approximately 7.9% of the population 18 years could be diagnosed with an alcohol use disorder (AUD) within the past year, with 3.4% diagnosable as alcohol-dependent (Substance Abuse and Mental Health Services Administration 2005). Furthermore, there have been progressive increases in licit and illicit opioid abuse such that approximately 2.2 million people are currently dependent on opioids (Substance Abuse and Mental Health Services Administration 2010). Moreover, the societal cost of alcohol and opioid abuse in the United States has been estimated to range from $184.6 to $234.8 and $55 billion per year, respectively (Birnbaum et al. 2011; Harwood et al. 1998; Rehm et al. 2009). Chronic exposure to alcohol or opioids can induce dependence and result in the production of negative affective states which are predominantly evident during withdrawal (Koob and Le Moal 1997; Markou et al. 1998) and are hypothesized to be the basis of negative reinforcement learning (for review, see Walker 2012; Smith et al. 2011). The negative affective behaviors associated with alcohol and opioid dependence have different etiologies (i.e., pre-existing or exposure-induced, respectively) and there is evidence for both perspectives (Hasin and Grant 2002; Schuckit et al. 1997). However, since the comorbidity between alcohol or opioid dependence and negative affective states is high (Grant and Harford 1995; Nunes et al. 1994), it is important to develop treatments for these symptoms that arise from these conditions.
Therefore, the creation of reliable animal models of dependence-induced negative affect is critical in order to provide a valid method for pharmacotherapeutic development efforts. Chronic exposure to alcohol vapor is a well-established method to induce alcohol dependence (Nealey et al. 2011; Rogers et al. 1979), and allows the experimenter to titrate the animal's blood alcohol levels (BALs) and to maintain a high degree of control over dependence induction. Refinement of the model has resulted in animals being intermittently exposed to alcohol vapor for four weeks (as opposed to chronic exposure which has low face validity), which reliably produces symptoms of alcohol withdrawal characterized by an escalated response pattern according to both fixed- and progressive ratio schedules of reinforcement (O'Dell et al. 2004; Walker and Koob 2007). Underlying these behavioral changes is an altered brain circuitry that not only results in motivational changes, but also produces negative affective states during withdrawal (Koob 2003; 2009; Koob and Le Moal 2001; Walker et al. 2012). Rats experiencing acute and protracted alcohol withdrawal show increased intracranial self-stimulation thresholds (Schulteis et al. 1995), decreased latencies to immobility, as well as increased total immobility time in the modified forced swim test (Getachew et al. 2010; Walker et al. 2010), both of which could be considered indicative of depressive-like behaviors or attenuated motivational states. Therefore, alcohol vapor exposure can induce dependence and the resulting withdrawal state is comprised of an array of symptoms that include altered somatic, motivational and negative affective behaviors.
However, parallel models of opioid dependence and withdrawal need to be further developed with the goals of enhancing face, predictive, and construct validity. Experimentally, opioid dependence is typically induced via the implantation of subcutaneous morphine pellets (Cochin et al. 1979; Harris and Aston-Jones 2003; Walker et al. 2003) or osmotic minipumps (Hay et al. 2010; Kalinichev and Holtzman 2003). Under ordinary circumstances, these models are less than ideal as the experimenter must remove the morphine pellet or osmotic minipump (which can agitate the organism) to induce spontaneous withdrawal, or administer an opioid receptor antagonist, such as naloxone, to precipitate withdrawal. As such, these paradigms do not parallel clinical populations that cycle through periods of drug use and withdrawal (Baker et al. 2004; Kaplan 1992). However, these models do produce similar motivational and affective alterations that are seen during withdrawal in alcohol-dependent organisms. For example, one study showed that morphine-dependent rats in withdrawal, both spontaneous and precipitated, spent less time in the open arms of the elevated plus maze, findings that indicate opioid withdrawal has anxiogenic effects (Schulteis et al. 1998) comparable to those observed during alcohol withdrawal (Valdez et al. 2002; Zhang et al. 2007).
Given that the face validity of precipitated opioid withdrawal does not reflect clinical opioid abuse patterns (Contarino and Papaleo 2005; Kaplan 1992), it is unclear whether the predictive validity of precipitated withdrawal models would be applicable under all conditions. However, there are very few studies that have characterized spontaneous opioid withdrawal in animal models. One study used osmotic minipumps to chronically administer heroin and assess withdrawal (Azar et al. 2004) in a manner that allowed for control of the timing and duration of spontaneous withdrawal. However, that study solely measured body weight during spontaneous withdrawal, while the present experiment attempts to characterize spontaneous withdrawal by evaluating a constellation of symptoms, both somatic and those indicative of negative affect.
Several animal models have been proposed to assess negative affective states resembling depression or anxiety. Due to a high degree of predictive validity for antidepressant efficacy, immobility in the forced swim test (FST) has been posited as a measure of depressive-like behavior (Cryan et al. 2002; Duman 2010). Likewise, the percentage of open-arm time in the elevated plus maze (EPM) has been proposed as a measure of anxiety-like behavior in rodents as it also has high predictive validity for both anxiolytic and anxiogenic drugs (Pellow et al. 1985). However, other measures may represent negative affective states in a more ethologically-valid manner. 22-kHz ultrasonic vocalizations (USVs) have been shown to signify aversive states in rats (Knutson et al. 2002), but determining whether 22-kHz USVs might be representative of anxiety- or depressive-like states (or both) has been difficult. If 22-kHz USVs could be demonstrated to better approximate negative affective states resembling either depression or anxiety, their utility might increase due to enhanced specificity and could offer a reasonable alternative to the FST or EPM. Furthermore, given that 22-kHz USVs are active measures of negative affective states (as opposed to passive measures like the FST and EPM), their measurement and interpretation is not confounded by nonspecific locomotor effects of drugs in the same way passive measures are (e.g., Kliethermes 2005).
The current experiments were designed to test three main hypotheses: 1) pulsatile heroin delivery would induce dependence and allow for consistent behavioral testing during spontaneous withdrawal, 2) induced 22-kHz USVs would increase during spontaneous withdrawal from heroin and alcohol concomitant with motivational and somatic withdrawal indices and 3) acute withdrawal-induced 22-kHz USVs could be distinguished from negative affective behavior resembling anxiety as indexed by percent open-arm time in the EPM. To this end, we utilized modified osmotic minipumps in a manner that allowed pulsatile delivery of heroin, rather than a continuous infusion regimen. This method affords precise control over the timing of infusions to achieve identical exposure timing parameters (14 hours on / 10 hours off) as the alcohol vapor exposure paradigm used in the studies; which better models human opioid abuse patterns (Kaplan 1992). Subsequently, following alcohol or heroin dependence induction, somatic, motivational and affective-like behaviors were assessed during acute withdrawal.
Methods
Animals
One hundred and thirty-six male Wistar rats (~70 days old and bred from Charles River Laboratory (Hollister, CA) breeding pairs) were used in the present studies. All animals were group-housed (2–3 rats per cage) in a temperature controlled vivarium (21±2º C) on a 12-hr reverse light cycle with ad libitum food and water. All animals were handled for one week prior to experimentation and animal care adhered to the National Research Council’s Guide for the Care and Use of Laboratory Animals (1996) and Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (2003), with all procedures approved by the Washington State University Institutional Animal Care and Use Committee. Every effort was made to use the least amount of animals, minimize animal suffering, and utilize alternatives to in vivo techniques.
Pre-Dependence Training
Acquisition of Operant Alcohol Self-administration
Animals were trained to self-administer a 10% alcohol solution with a sweetener-fade technique during 30 minute operant self-administration sessions (Samson 1986). Operant training took place in standard operant conditioning chambers (Med Associates, St. Albans, VT) with custom drinking wells (Behavioral Pharma, La Jolla, CA). Operant alcohol self-administration training has been detailed in previous publications (Walker and Koob 2007). Using a continuous schedule of reinforcement (fixed-ratio 1; FR-1), acquisition of the operant response occurred using sweetened fluid as the reinforcer (0.125% saccharin and 3% glucose), which is preferred to sucrose (Valenstein et al. 1967) and does not require food or water deprivation. 10% alcohol (w/v) was added to the sweetened fluid, and over three weeks, sweetener was slowly removed until only 10% alcohol (w/v) was left in the solution. After responding for alcohol stabilized (defined as three sessions with <10% deviation), the animals were split into two groups matched for operant alcohol self-administration (g/kg) and assigned to either air- or alcohol vapor-exposed conditions (n = 10 / grp). All animals were subjected to a four week exposure period prior to reinitiating self-administration.
Dependence Induction Procedures
Intermittent Alcohol Vapor Exposure
Intermittent exposure to alcohol vapor (14 hrs on / 10 hrs off; seven days per week) induces increased motivational and negative affective states indicative of alcohol dependence (Nealey et al. 2011; Valdez et al. 2002; Walker et al. 2008; Walker et al. 2010; Walker and Koob 2007; Walker and Koob 2008). The apparatus (La Jolla Alcohol Research, Inc., La Jolla, CA) allows blood alcohol levels (BALs) to be titrated by the experimenter by adjusting the rate of 95% alcohol that is vaporized and introduced into the air flow supplying the sealed environmental chambers that the animals were housed in. This allowed the experimenter to keep the animal’s BALs within a desired level (175–225 mg%), which was determined semiweekly by collecting blood from the tails (~100 μl). After centrifugation, plasma samples were assayed for alcohol content using the Analox AM1 (Analox Instruments Ltd., Lunenburg, MA).
Pulsatile Heroin Exposure
Four doses of daily pulsatile heroin exposure (14hrs on, 10hrs off) were selected for use in the osmotic minipumps representing vehicle, 0.75 mg, 1.5 mg and 3 mg heroin /14 hrs. Although osmotic minipumps are generally used for continuous infusion experimental designs, the present approach (see Fig. 1) utilized a modification that provided the means to deliver heroin in a pulsatile manner for up to 30 days. By filling the pumps with saline and attaching polyethylene (PE60) tubing to the pump, based on the tubing inner diameter and pump flow rate characteristics, the volume needed for different infusion periods (e.g., 14 or 10 hour periods) could be determined. Prior to filling, the tubing was prepared using the Lynch coil technique (Lynch et al. 1980) which consisted of coiling the PE tubing around a cylinder with a similar circumference as that of the pump, dipping the coil into heated water for one minute to soften the thermoformable PE tubing and then immersing the coil into ice water to harden the PE and fix it in that particular coiled shape. Once formed, the tubing was filled with alternating heroin solution and mineral oil according to the desired time course for a 30-day period using a miniature perfusion manifold (Harvard Apparatus, MA) that provided the means to accurately fill the coil with two alternating solutions using microinfusion pumps for precise volume delivery. Priming of the osmotic minipump occurred overnight in 37°C saline to confirm appropriate flow rate and functioning prior to surgical implantation.
Fig. 1.
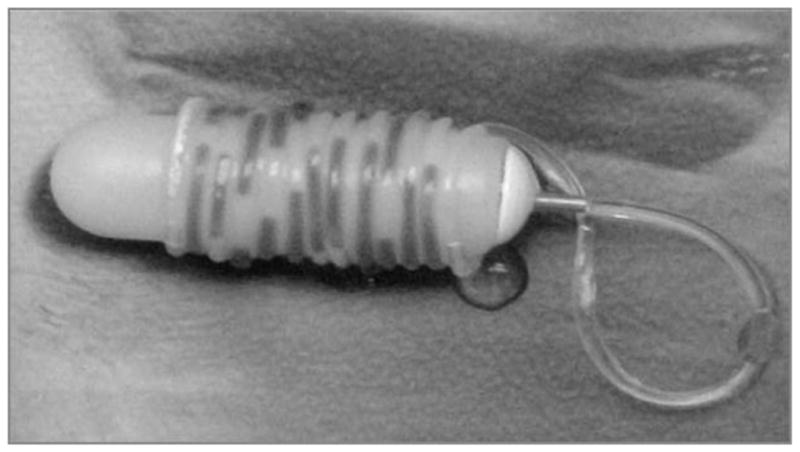
Modified osmotic minipump that allows for pulsatile heroin delivery according to the desired experimental schedule. Preparation of the thermoformable polyethylene tubing so that it will remain tightly wrapped and untangled occurs via the Lynch coil method (Lynch et al. 1980). 50 ul of food coloring distinguished the drug solution and can be seen in this figure as the darker portions in the tubing.
Animals were anesthetized with isoflurane gas (4% for induction and ~2% for maintenance) and the minipumps were implanted subcutaneously in the posterior region of the back after shaving, sterilizing and making an incision with a scalpel. Once the osmotic minipump was appropriately positioned, the incision was closed with surgical staples, which were removed after three days. Following surgery, animals were allowed to recover for four additional days and received daily antibiotic injections of Baytril (5 mg/kg, SC) during that time period.
The doses of heroin used in the present study were determined based on previous experiments which showed that rats demonstrating escalated self-administration patterns had comparable levels of heroin intake if allowed to self-administer heroin for 12 or 23 hours (Chen et al. 2006; Greenwell et al. 2009). In those studies, the amount self-administered was approximately 3 mg per day, which was consistent with our lab’s intravenous heroin self-administration data for one-hour sessions multiplied by 14 hours (unpublished data). The minipump infused at a rate of ~2.33 μl per hour (Alzet model 2ML4 by Durect) and therefore, in conjunction with the known volume of the tubing used, one could calculate the precise amount that would be needed so that the desired daily heroin dose was achieved within a 14 hour timeframe and was then followed by mineral oil at the same rate for 10 hours.
Post-Dependence Behavioral Measures
Acute Withdrawal Operant Alcohol Self-Administration
After the initial four-week alcohol vapor exposure period, both the air- and vapor-exposed animals were tested in 30 minute operant alcohol self-administration sessions twice a week, at a time point corresponding to six hours into withdrawal for the dependent animals. After each session, animals were returned to their air- or vapor-exposed chambers. Animals were allowed to engage in operant self-administration sessions twice weekly until they reached stable responding (< 10% deviation over three days), at which point their lever presses (providing an index of the volume of 10% alcohol (w/v) that was consumed) and weights were used to calculate the g/kg data used in the statistical analyses.
Elevated Plus-Maze
Air- or alcohol- exposed groups were tested in the EPM at a time point corresponding to 6–10 hours into withdrawal for the dependent animals. The maze consists of a raised Plexiglas platform (50 cm high) with two open arms and two closed arms of equal length (47 cm × 10 cm each) and a 10 × 10 cm center platform. The floors of the EPM were black, but the Plexiglas walls of the closed arms were clear (40 cm high). Each animal was placed in the center of the platform facing the same direction and allowed to explore the maze for 5 minutes. Illumination in all arms was approximately 50 lux. Each animal was recorded by video and AnyMaze video tracking software (Stoelting Co; Wood Dale, IL) was used to score the amount of time spent in the open arm, closed arm, and center platform. The maze was cleaned with Quatricide® and dried between each animal.
Forced Swim Test
The heroin- and alcohol-exposed groups were tested in the FST at a time point corresponding to 6–10 hours into withdrawal for the dependent animals. The FST apparatus was a custom-built clear Plexiglas® cylinder (diameter = 34 cm, height = 79.5 cm). The cylinder was filled to 53 cm with water temperature at 24±2°C. Illumination at the surface of the water was approximately 25 lux. For the heroin-exposed animals, on day one the rats were placed in the apparatus for 15 minutes and behavior was not recorded. On day two, behavior in the apparatus was recorded by video during a 5-min test session. Alternatively, the alcohol vapor-exposed animals were subjected to an initial 15-min test session that was recorded by videotape, but did not undergo a second day of testing. Elimination of the second day test was based on the demonstration that alcohol vapor-induced immobility appeared to be sensitive to evaluation on the first day (Getachew et al. 2008; Getachew et al. 2010) rather than the second day of FST testing during acute withdrawal (Walker et al. 2010). Test sessions from both exposure conditions were analyzed with AnyMaze video tracking software (Stoelting Co, Wood Dale, IL). Immobility was defined as a lack of active swimming with animals floating to maintain the head above water with only minor paw movement. Swimming was defined as active movement of the animals with all four paws and climbing was defined by active attempts to climb the walls of the apparatus to escape.
Somatic Withdrawal Signs
Somatic withdrawal signs during acute spontaneous withdrawal in heroin-treated rats were assessed using a modified Gellert and Holtzman (1978) scale. Based on this scale, both checked (teeth chatter, abnormal posture, irritability, diarrhea, swallowing, profuse salivation, erection/ejaculation, chromodacryorrhea) and graded (escape attempts, abdominal restrictions, and wet shakes) signs were assessed. Checked withdrawal signs were scored as either present or absent, while graded withdrawal signs were tallied based on the frequency of the behavior. Assessment of withdrawal signs occurred every other day following the surgical recovery period until 23 days after minipump implantation. Starting on day 19, the last three observation sessions (i.e., 19, 21 and 23) were recorded and averaged for analysis. Each observation lasted 5 minutes and occurred 6–10 hours into the withdrawal period.
Somatic withdrawal signs indicative of alcohol dependence were also assessed 6–10 hours into withdrawal using the methods described in Schulteis et al. (1995). Four behaviors were assessed and scored on a scale of 0–2: hyperirritability upon touch, presence of the ventromedial distal flexion response (measured by gently grasping the rat by the scruff of the neck and checking the retraction of the limbs towards the body), tail stiffness / rigidity, and abnormal posture or gait. Animals were observed for three minutes and given a score that ranged from 0–8.
Ultrasonic Vocalizations
Alcohol- or heroin-dependent animals were tested during spontaneous acute withdrawal to evaluate behaviors indicative of negative affective-like states induced by dependence. Production of 22-kHz USVs was assessed by administering a puff of air (~60 psi) to the nape of the animal’s neck from a distance of approximately 10 cm. Animals were tested 6–10 hours into withdrawal and each session consisted of three trials of 15 air-puffs each. The three trials were separated by one minute and each air-puff was separated by 15 seconds. The air-puffs for each trial continued until the animal vocalized. Once a vocalization occurred during the trial, vocalizations were then recorded until they ceased for one minute, or until the animals vocalized for 10 minutes total and the animal advanced to the next trial. The approach used to induce USVs was based on the work of others (Knapp et al. 1998; Knapp and Pohorecky 1995). Vocalizations were recorded with a P48 Electret Ultrasound Microphone (Avisoft Bioacoustics, Germany), E-MU Systems Audio/MIDI Interface (Scotts Valley, Ca) and SeaPro software (created by AEST, Italy). All vocalizations were counted with Avisoft Bioacoustics software (Berlin, Germany). The number and duration of vocalizations from the second trial was used for all data analyses.
Timeline: Opioid-Exposed Animals
All heroin-exposed (diacetylmorphine; provided by the NIDA Drug Supply program and dissolved in 0.9% saline) animals were implanted with osmotic minipumps (considered experimental day 1) corresponding to four different doses of heroin (n = 6 / dose) and allowed to recover until the fifth experimental day. Somatic withdrawal sign evaluations occurred every other day until stability (≤ 20% deviation for three days) was achieved. Once stability was achieved, the average of the final three sessions (experiment days 19, 21 and 23) were used as the dose-response data for somatic withdrawal signs, with measurement of 22-kHz USVs and immobility in the FST occurring on subsequent days (experimental days 24 and 25–26, respectively).
Timeline: Alcohol-Exposed Animals
Animals were assigned to air- or vapor-exposure conditions and following two weeks of intermittent alcohol vapor exposure, independent measurement of somatic withdrawal signs (n = 10 / exposure condition), FST behaviors (e.g., latency to immobility, immobility time, swim time and climbing time; n = 6 / exposure condition), percent open-arm time in the EPM (n = 9 / exposure condition) or induced 22-kHz USVs (n = 6 / exposure condition) were assessed. Based on the results of the two-week vapor exposure experiment, two additional groups of animals (n = 9 / exposure condition) were subjected to intermittent alcohol vapor for a four week period prior to EPM evaluation.
To assess the relationship between escalated self-administration and 22-kHz USVs, animals were trained to self-administer 10% alcohol (w/v) prior to one-month of air- or vapor-exposure (n = 10 / exposure condition). Subsequent acute withdrawal self-administration sessions occurred twice weekly until stable responding was achieved and then the animals were assessed for induced 22-kHz USV production. The total time to completion and length of vapor exposure for the animals previously trained to self-administer alcohol was eight weeks. A final group of animals was evaluated for somatic withdrawal signs following eight weeks of alcohol vapor exposure (n = 6 / exposure condition).
Statistics
In the Laboratory of Alcoholism and Addictions Neuroscience at Washington State University we strive to identify strategies that minimize the unnecessary use of animals and protect against the commission of Type I and Type II errors. Therefore, instead of solely focusing on the p-values ≤ α when conducting statistical analyses, power (1-β) was calculated for all analyses with α= 0.05 and β = 0.2, such that the p-value(s) ≤ 0.05 must be accompanied by power ≥ 0.8 for the data to be acceptable. Cohorts of animals were equally added to each group within each experiment in a random manner with statistical analyses being conducted for each experiment once n=5 in every group. If the criteria for α and β were satisfied, the addition of animals to that component of the experiment ceased. In those cases that sample size was approximating commonly accepted sample sizes in preclinical research settings (e.g., n = 10) and the criteria for α and β had not been satisfied, the empirically-determined effect size was used to conduct an a priori power analysis to determine the projected sample size given α = 0.05 and β = 0.2. If the projected sample size exceeded 20 animals per group, that experiment was concluded and the null hypothesis accepted.
Statistics: Heroin-treated animals
Somatic withdrawal scores, 22-kHz USVs (number and duration) and immobility time in the FST were independently analyzed using a univariate analysis of variance (ANOVA) with heroin dose as the between-group factor. If a main effect of dose was determined, post-hoc Dunnett tests were used to compare the vehicle condition to each of the three heroin doses. A two-way mixed-model ANOVA assessed the time course for somatic withdrawal scores was evaluated using a heroin dose as the between-group and test session as the within-subject factors. If a main effect or interaction was discovered, post-hoc comparisons were independently made for each dose during each experimental day. Lastly, Pearson correlations evaluated the relationship between somatic withdrawal scores and 22-kHz USVs.
Statistics: Alcohol-treated animals
Because the somatic withdrawal scores following two or eight weeks of alcohol vapor exposure were calculated using what many consider to be ordinal / categorical data, non-parametric independent-sample Mann-Whitney U tests were used for the analysis with exposure level as the between-groups factor. Operant self-administration of alcohol (g/kg) following eight weeks of vapor exposure, 22-kHz USVs (number and duration) following two or eight weeks of vapor exposure were separately analyzed using a univariate ANOVA with exposure condition as the between-groups variable. Behavior in the FST (latency to immobility, immobility time, swim time and climbing time) following two weeks of vapor exposure was analyzed using a multivariate ANOVA and behaviors showing no effect of independent variable manipulation were loaded as a covariates and the analysis repeated. Percent open-arm time in the EPM following two or four weeks of air or vapor exposure was analyzed with a two-way ANOVA with exposure condition and exposure duration as the between-groups variables. Appropriate post-hoc comparisons were conducted if a main effect or interaction were found. Pearson correlations between alcohol self-administration (g/kg) and 22-kHz USVs (number and duration) following eight weeks of exposure were calculated for the air- and vapor-exposed conditions.
Results
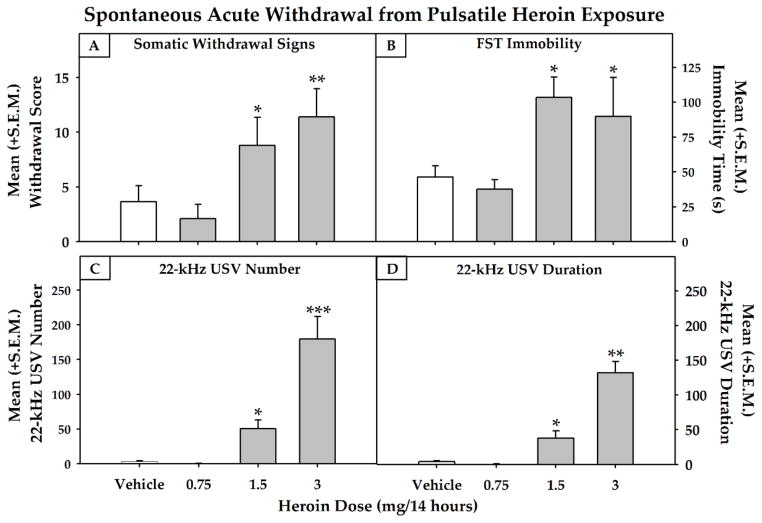
As a result of post-surgical complications, two animals in the lowest heroin dose group were removed from the study during the time period between recovery and actual testing. Additionally, four heroin-treated animals (one from the vehicle, one from the 1.5 mg/kg and two from the 3.0 mg/kg heroin groups) did not complete the entire FST test process due to agitation at the well-healed incision location. Therefore, of the 136 animals that began the study, 130 were included in the data analysis. The impact of different doses of pulsatile heroin on withdrawal scores (Fig. 2, panel A), immobility in the FST (Fig. 2, panel B) and the number and duration of 22-kHz USVs (Fig. 2, panels C and D) were assessed using a univariate ANOVA. A main effect of Dose was found for withdrawal scores (F (3, 18) = 6.336, p < 0.01, power = 0.921), immobility in the FST (F (3, 17) = 6.169, p < 0.01, power = 0.886) and for the number (F (3, 18) = 19.962, p < 0.001, power = 1.0) and duration (F (3, 18) = 33.845, p < 0.001, power = 1.0) of 22-kHz USVs. Dunnett tests identified that the 1.5 and 3.0 mg/day heroin increased withdrawal scores (p < 0.05 and p < 0.01, respectively), immobility in the FST (ps < 0.05) and the number (p < 0.05 and p < 0.001, respectively) and duration (p < 0.05 and p < 0.01, respectively) of 22-kHz USVs.
Fig. 2.
Mean (+S.E.M.) somatic withdrawal scores (panel A, n=4–6), immobility time (panel B, n=4–6) and 22-kHz USV number (panel C, n=4–6) and duration (panel D, n=4–6) were dose-dependently increased during acute withdrawal using pulsatile exposure to induce heroin dependence (* = p<0.05, **p<0.01 and ***p<0.001 compared to the vehicle-treated condition).
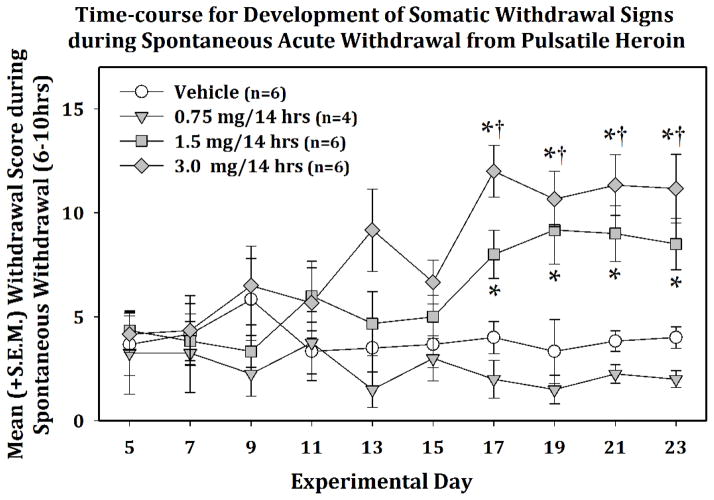
The two-way mixed-model ANOVA conducted on the time-course for somatic withdrawal scores (see Fig. 3) indicated a main effect of Dose (F (3, 18) = 8.938, p ≤ 0.001, power = .983), a main effect of Session (F (9, 162) = 2.382, p < 0.05, power = .909) and a Dose × Session interaction (F (27, 162) = 1.918, p < 0.01, power = .993). Post-hoc comparisons were performed on the withdrawal scores for each dose during the experimental time-course and indicated that, on Experimental Days 17 – 23, the 1.5 and 3.0 mg dose significantly differed (p<0.05) from the vehicle-treated animals during those sessions and the 3.0 mg dose of heroin significantly increased (p<0.05) somatic scores produced during spontaneous withdrawal when compared to the first day of score measurement (i.e., Experimental Day 5).
Fig. 3.
Mean (+/−S.E.M.) somatic scores during spontaneous acute withdrawal (6–10 hrs) from pulsatile heroin. Following the second week of spontaneous withdrawal, the 1.5 and 3.0 mg/14hr doses produced a significant increase in somatic withdrawal scores that stabilized by day 23 (* = p<0.05 when compared to vehicle-treated controls and † = p<0.05 when compared to baseline data collected on Experimental Day 5).
The relationship between somatic withdrawal signs and 22-kHz USVs (n=22) was assessed during acute withdrawal (6–10 hrs) using Pearson correlation tests. Significant positive correlations between somatic scores and the number (r = .730, p<0.01; trendline: y = 12.053x - 20.289) and duration (r = .784, p<0.01; trendline: y = 8.9899x - 15.199) of 22-kHz USVs were identified.
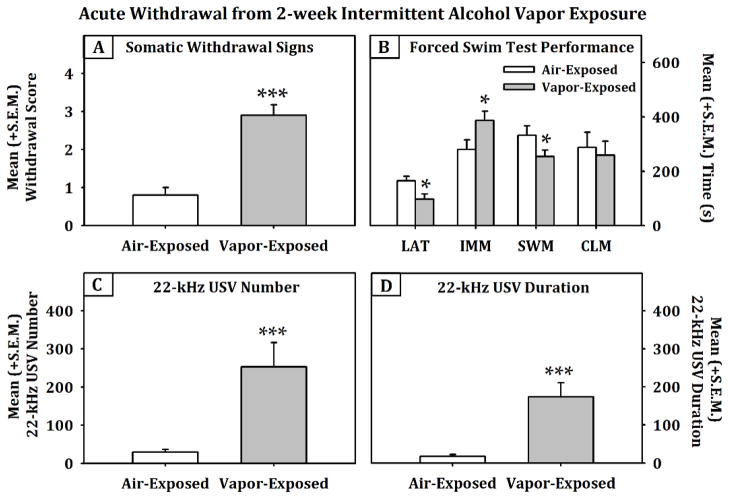
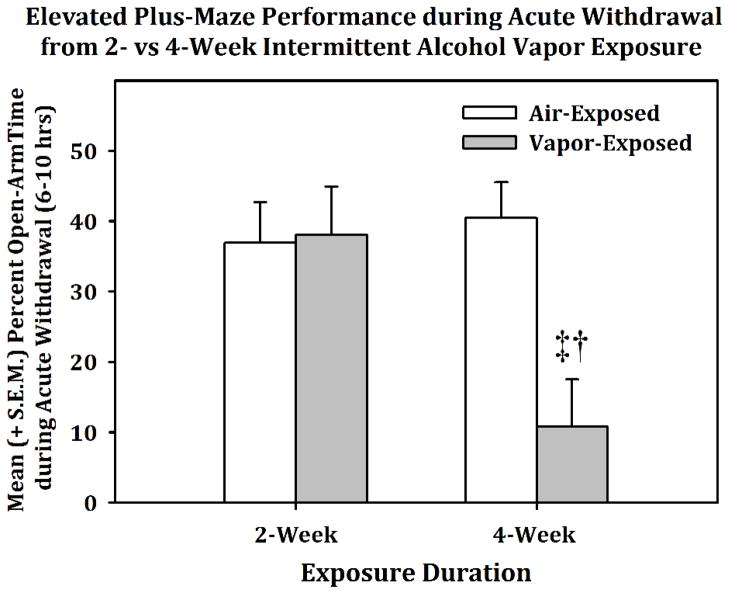
Figure 4 displays the effects of a two-week air or intermittent alcohol vapor exposure period on somatic withdrawal scores (panel A), behavior in the FST (panel B), as well as the number (panel C) and duration (panel D) of 22-kHZ USVs during acute withdrawal (6–10 hrs). An independent-samples Mann-Whitney U Test indentified a significant effect of vapor exposure on somatic withdrawal scores (p<0.001). The multivariate ANOVA conducted on behaviors in the FST indicated a main effect of vapor exposure on latency to immobility (F (2, 9) = 10.705, p < 0.05, power = 0.8), total immobility time (F (2, 9) = 10.705, p < 0.05, power = 0.811) and swim time (F (2, 9) = 10.705, p < 0.05, power = 0.81) when using climbing time as a covariate. Similarly, univariate ANOVAs showed that vapor-exposed animals had a significantly increased number and duration of 22-kHz USVs (F (1, 10) = 12.291, p < 0.01, power = 0.884; F (1, 10) = 17.687, p < 0.01, power = 0.965, respectively) during acute withdrawal when compared to the air-exposed controls. Conversely, when the EPM data (see Fig. 5) for rats in acute withdrawal (6–10 hrs) following exposure to intermittent alcohol vapor for two (n = 9 / exposure condition) or four weeks (n = 9 / exposure condition) was analyzed, the two-way ANOVA indicated a main effect of Condition (F (1, 32) = 7.347, p < 0.05), a main effect of Duration (F (1, 32) = 5.11, p < 0.05) and Condition × Duration interaction (F (1, 32) = 8.589, p < 0.01, power = .859). Post-hoc comparisons confirmed that the four-week vapor exposed animals spent a significantly lower percentage of time in the open arms when compared to the four-week air-exposed animals (F (1, 16) = 10.601, p < 0.01, power = .863) and when compared to the two-week vapor-exposed animals (F (1, 16) = 9.858, p < 0.01, power = .838). Therefore, the developmental pattern of 22-kHz USVs corresponded to immobility behavior in the FST, but not behavior in the EPM.
Fig. 4.
Mean (+S.E.M.) somatic withdrawal scores (panel A, n=10/grp), forced swim test behavior (panel B, n=6/grp) and 22-kHz USVs (panels C and D, n=6/grp) following two weeks of air or intermittent alcohol vapor exposure (*=p<0.05 and ***=p<0.001/ when comparing air and vapor exposure conditions), LAT = Latency to immobility, IMM = immobility, SWM = swim time and CLM = climbing time.
Fig. 5.
Mean (+S.E.M.) percent time in the open-arms of the elevated plus-maze (EPM) following 2-weeks (n=9/grp) or 4-weeks (n=9/grp) of intermittent alcohol vapor exposure. Open-arm time in the EPM during acute withdrawal was not impacted by 2-weeks, but significantly decreased by 4-weeks of vapor exposure (‡ = p<0.05 when compared to the 4- week air-exposed group and † = p<0.05 when compared to the 2-week vapor-exposed group.
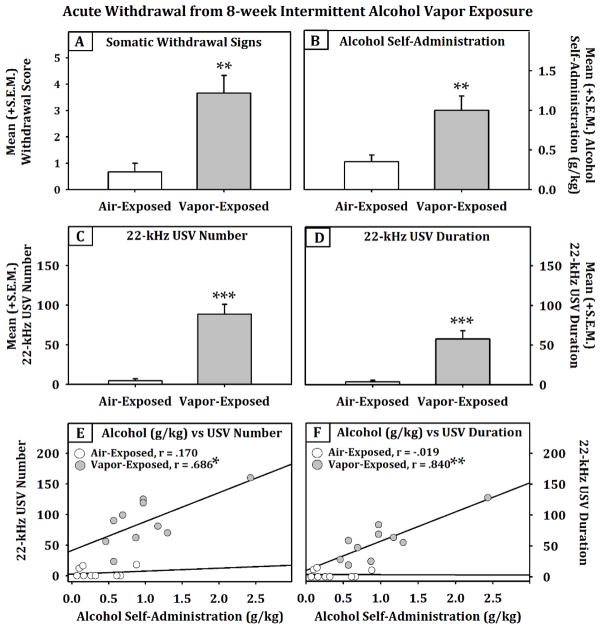
The animals that were exposed to intermittent alcohol vapor for eight weeks displayed a significant increase (see Fig. 6) in somatic withdrawal signs (Independent-Samples Mann-Whitney U test, p = 0.004) and operant self-administration of 10% (w/v) alcohol (g/kg; F (1, 18) = 10.464, p < 0.01, power = .864) when compared to the air-exposed animals. In the alcohol self-administering animals that were in withdrawal, both the number (F (1, 18) = 43.852, p < 0.001, power = 1.0) and duration (F (1, 18) = 27.126, p < 0.001, power = .998) of 22-kHz USVs were significantly elevated when compared to the air-exposed group. The relationship between alcohol self-administration and 22-kHz USVs (number and duration; n=10 / exposure condition) was assessed in the air- and vapor-exposed animals during acute withdrawal (6–10 hrs) using Pearson correlation tests (see Fig. 6). In the air-exposed animals, there was no evidence of any correlations between alcohol self-administration and 22-kHz USV production. However, it was interesting to identify that in the vapor-exposed animals that were dependent, significant positive correlations were determined for both the number (r = .686, p < 0.05; trendline: y = 12.053x - 20.289) and duration (r = .840, p < 0.01; trendline: y = 8.9899x - 15.199) of 22-kHz USVs and self-administration of alcohol (g/kg).
Fig. 6.
Mean (+S.E.M.) somatic withdrawal scores (panel A, n=6/grp), operant alcohol self-administration (panel B, n=10/grp) and 22-kHz USVs (panels C and D, n=10/grp) after 8-weeks of air or intermittent alcohol vapor exposure (**=p<0.01 and ***=p<0.001, when comparing air and vapor exposure conditions). In vapor-exposed animals, alcohol self-administration and 22-kHz USVs were shown to be predictive of each other (panels E and F;* =p<0.05 and ** =p<0.01).
Discussion
In accordance with the hypothesis that pulsatile delivery of heroin via osmotic mini-pumps would induce dependence and exhibit spontaneous withdrawal-induced behavioral change, the results showed that animals who received pulsatile heroin exposure dose-dependently increased their somatic withdrawal signs over the course of three weeks. This time frame is consistent with evidence showing that opiate dependence occurs approximately 7 - 20 days after the initiation of chronic opiate treatment (Blasig et al. 1973; Fernandes et al. 1977; Marshall and Weinstock 1971), although the present data demonstrated that, using a pulsatile delivery method that resulted in repeated cycles of exposure and spontaneous withdrawal on a daily basis, the development of a dependence-like phenotype (as indexed by somatic withdrawal signs) took approximately two weeks. Likewise, animals that were exposed to intermittent alcohol vapor for two weeks showed increased somatic withdrawal signs during acute withdrawal.
Supportive of our second hypothesis, animals exposed to pulsatile heroin showed dose-dependent increases in 22-kHz USVs during spontaneous withdrawal, just as those exposed to intermittent alcohol vapor did. Therefore, pulsatile heroin and intermittent alcohol vapor exposure increase the number and duration of 22-kHz USVs which supports the use of these approaches to reliably assess dependence-induced negative affective-like states. Under a comparable exposure timeframe, alcohol- and heroin-exposed animals in acute withdrawal showed a significant increase in immobility time in the FST when compared to control animals. It must be noted that there is a slight disconnect in the timing of withdrawal-induced immobility in the FST between these two drug-classes (i.e. heroin-exposed animals show sensitivity on the second day, whereas alcohol-exposed animals are most sensitive on the first day of testing). However, the predictive validity of the model does not seem to be compromised as other studies have shown that increased immobility on the first day of the FST during withdrawal is sensitive to anti-depressant treatment (Getachew et al. 2008; Getachew et al. 2010) or correlates with other behaviors indicative of anhedonic-like behavior (Chartoff et al. 2012), such as increased thresholds for intracranial self-stimulation.
Following eight weeks of intermittent vapor exposure, somatic withdrawal signs were still present and animals that displayed escalated operant alcohol self-administration also had increased production of 22-kHz USVs. Indeed, those animals also showed a significant correlation between the number and duration of 22-kHz USVs and levels of self-administration, whereas the air-exposed animals displayed no such correlation. It follows that observation of either behavior in alcohol-dependent animals would be predictive of the occurrence of the other behavior. The escalated self-administration observed in dependent rats has been extensively characterized and shown to be a learned plasticity-dependent response hypothesized to remove the negative affective state that is present during withdrawal (for review, see Walker 2012; Smith et al. 2011). Considerable interest in genetically selected lines of rats such as the alcohol Preferring (P) and Non-preferring (NP) rats has occurred due to similarities in P rat alcohol consumption, as well as other behaviors, to those diagnosed with AUDs (for review, see Bell et al. 2006). One factor considered as a putative contributor to the excessive alcohol consumption exhibited by P rats was enhanced ‘emotionality’, a state that could drive a P rat to engage in excessive alcohol consumption in order to remove that state. Because of the possibility for enhanced ‘emotionality’, P have been tested for air-puff induced 22-kHz USVs, in which USVs were shown to be negatively correlated with alcohol intake (Knapp et al. 1997), a pattern opposite of that observed in the present experiment. While those data generated with P rats did not support the concept of negative reinforcement as a basis for excessive alcohol consumption in P rats, the present results showing that 22-kHz USVs are positively correlated with alcohol self-administration in dependent animals does. Consequently, the co-occurrence of these behaviors supports the need for further investigations into the neurobiological basis of negative reinforcement learning.
The use of osmotic minipumps to induce drug dependence for the study of affect during withdrawal has seldom been tested. For example, one study used subcutaneous osmotic minipumps to continuously deliver amphetamine to rats and removed the minipumps to induce withdrawal (Paterson et al. 2000). However, the current models used intermittent drug exposure to induce dependence and there are several benefits to intermittent exposure. In alcohol dependence, intermittent alcohol vapor exposure produces escalation in operant responding in the first period of withdrawal, escalation that is not seen in animals exposed to continuous alcohol vapor (Lopez and Becker 2005; O'Dell et al. 2004). Likewise, seizures are a common physiological symptoms of alcohol withdrawal and are increased with intermittent schedules of alcohol exposure (Matsumoto et al. 2001); although in the present study, target BALs were such that withdrawal-induced seizures were non-existent. Periods of drug exposure and withdrawal, therefore, appear important for development of the neurophysiological changes that characterize alcohol dependence (O'Dell et al. 2004). Multiple withdrawal cycles via intermittent exposure also increase negative affective-like behavior. Alcohol dependent animals exposed to multiple withdrawal cycles spend less time in social interactions and show increased anxiety-like behaviors on the EPM (Breese et al. 2005; Overstreet et al. 2004). Likewise, repeated alcohol withdrawal sensitizes withdrawal-induced anxiety; therefore stress caused by withdrawal increasingly induces negative affective behaviors during protracted abstinence (Breese et al. 2005; Walker et al. 2010). Therefore, intermittent alcohol exposure could putatively result in a more rapid escalation in operant responding, as well as increased somatic and affective withdrawal signs, and the current method of pulsatile delivery of heroin was designed in consideration of such factors.
Both somatic and affective withdrawal signs were assessed for heroin-exposed animals during spontaneous withdrawal. In opioid dependence models, withdrawal is generally precipitated by an opioid-receptor antagonist such as naloxone or naltrexone (Barr et al. 1998; Gellert and Holtzman 1978; Goeldner et al. 2011; Koob and Le Moal 1997). One study did assess somatic withdrawal signs with comparable methodology to the present study, however, the complete spectrum of somatic signs were evaluated under conditions of precipitated withdrawal and only the animal’s weight (which decreased) was measured under conditions of spontaneous withdrawal from chronic heroin exposure (Azar et al. 2004). In contrast, the current model allows for assessment of both somatic withdrawal signs and negative affective-like states in opioid dependence, as well as for repeated testing in the multiple periods of withdrawal. The present model suggests enhanced predictive and construct validity in that somatic and psychological withdrawal signs were dose-dependently increased in heroin-exposed animals. Importantly, daily drug exposure and spontaneous withdrawal has further face validity than models of precipitated opioid withdrawal because human opioid abusers experience spontaneous withdrawal and then take more drugs to relieve the withdrawal symptoms (i.e., negative reinforcement / self-medication), on a daily basis (Baker et al. 2004; Kaplan 1992). Of additional note is the fact that somatic withdrawal scores were correlated with 22-kHz USV production; an association that should be clinically relevant, in that, humans experiencing physiological withdrawal symptoms are likely to have dependence-induced depression that persists much longer than those that do not experience withdrawal (Brown and Schuckit 1988)
In the pulsatile heroin-exposure model, animals are passively receiving heroin versus actively self-administering heroin under conditions of long-access, and methodological difference could influence dependence and withdrawal signs. In cocaine self-administration binge models, yoked-control animals passively received cocaine based on operant responses made by a self-administering animal and emitted more 22-kHz USVs after the last cocaine infusion than the self-administering animals (Mutschler and Miczek 1998). This difference indicates that there may be an aversive quality to passively receiving cocaine. Conversely, passive chronic morphine treatment produces escalated heroin self-administration (Walker et al. 2003) as do long-access heroin self-administration models (Ahmed et al. 2000; Vendruscolo et al. 2011; Walker et al. 2000), and these effects indicate that passive or active opioid exposure are both sufficient to produce escalated responding for heroin as long as the daily duration of exposure is of sufficient length, otherwise the rapidity of escalation suffers (Vendruscolo et al. 2011). Additional support comes from ventral tegmental area (VTA) electrophysiological evidence showing highly correlated cellular responses to non-contingent or contingent heroin administration (Steffensen et al. 2006).
Historically, there have been attempts to differentiate between anxiety- and depressive-like behaviors when assessing negative affect. There appear to be different biological underpinnings for depression and anxiety (Krishnan and Nestler 2008; Nutt and Stein 2006), and it is important to separate these conditions. In the present experiment, immobility in the FST was considered a putative measure of negative affective symptoms resembling depression (Cryan et al. 2002; Cryan et al. 2005a) during withdrawal. There is extensive evidence that 22-kHz USVs are indicative of negative affective states in rats (Burgdorf et al. 2001; Knutson et al. 2002; Portfors 2007), and air-puffs have been validated as a non-painful method for inducing these vocalizations (Knapp and Pohorecky 1995). The current results of escalated USVs during alcohol- and opioid-withdrawal are consistent with the published literature. Namely, animals exposed to alcohol liquid diet showed an increase in number and duration of USVs during withdrawal (Moy et al. 1997; Moy et al. 2000) and adult rats in morphine withdrawal also produce significantly more low USVs than saline controls (Vivian and Miczek 1991). In agreement, increased immobility in the FST is observed during alcohol- and opioid-withdrawal (see present results and Molina et al. 1994; Walker et al. 2010).
Of considerable interest, and consistent with our hypothesis, is the fact that after two weeks of alcohol vapor exposure, immobility in the FST and 22-kHz USV production were significantly increased during acute withdrawal, but open-arm time in the EPM was unaltered. Furthermore, it was not that animals could not display an anxiety-like phenotype using the vapor model because after four weeks of vapor exposure, the percentage of time spent in the open-arms of the EPM was significantly decreased during acute withdrawal. Taken together, these results suggest that a depressive-like phenotype is produced during withdrawal after two weeks of intermittent vapor exposure, but an anxiety-like phenotype is not produced under the same conditions. Ultimately, a transition from the FST to the use of 22-kHz USVs as a measure of negative affect resembling depression might be warranted given that the temporal characteristics of 22-kHz USV production in alcohol-dependent animals are consistent with immobility in the FST, but not open-arm time in the EPM. However, such a statement cannot be made lightly in the face of numerous studies that have been conducted to evaluate anxiety-like behavior in the EPM following chronic alcohol exposure. An assessment of 26 journal articles (using the Pubmed database) utilizing the EPM to assess anxiety-like behavior in alcohol exposed rats identified very few that used as short of an exposure period as that used in the present study and all used a continuous-available, rather than intermittently-available, alcohol liquid diet to induce dependence. Of those, one systematically examined the developmental time course for maximal anxiety-like behavior and indentified that 24-hrs into withdrawal was optimal time following 15 days of continuously-available access to an alcohol liquid diet (Pandey et al. 1999). Others evaluated EPM performance at a withdrawal time point more consistent with that in the present study (i.e., 6–8 hrs into withdrawal), but still had methodology that deviated considerably from ours that included the use of Sprague-Dawley rats, rather than Wistar rats, and a continuously-available liquid diet approach (e.g., Devaud et al. 1999; Moy et al. 1997; Moy et al. 2000). Thus, the ability to measure an anxiety-like phenotype in those studies could be attributable to many methodological factors, with continuous availability posited as the best explanation. Additional vapor exposure EPM studies have been conducted, but the shortest duration of exposure was three weeks, with testing conducted 12 hrs into withdrawal (Valdez et al. 2002). In the present study, two weeks of vapor exposure was insufficient, whereas four weeks of exposure induced a decrease in percent open-arm time. It follows that the measurable anxiety-like levels must have occurred somewhere between 2 – 4 weeks, although the exact timing is unknown. Therefore, our data is consistent with the literature, in as much as there is not a good comparison for the two week intermittent vapor exposure regimen prior to testing in the EPM six hrs into withdrawal. It should be noted that when assessing motivational-like indices of dependence and withdrawal, it was shown that a 2-week intermittent vapor exposure schedule produced a superior dependence-like behavioral profile of escalated alcohol self-administration during acute withdrawal than continuous exposure to alcohol vapor (O'Dell et al. 2004).
Because 22-kHz USVs paralleled immobility in the FST, a behavior posited to be indicative of a depressive-like state (Cryan et al. 2002; Cryan et al. 2005b), measurement of 22-kHz USVs could be an ethologically-valid approach for the assessment of an aspect of “negative affect” that is distinct from behavioral change in the EPM under shorter alcohol vapor exposure conditions at the time point of withdrawal utilized in the present study. One benefit of utilizing 22-kHz USVs to assess negative affect is the well defined neurocircuitry underlying their production (Wright and Panksepp 2011) that can aid in future neurobiological investigations. However, it remains that 22-kHz USVs have been interpreted by some as anxiety-like responses, but a systematic assessment of the conditions under which 22-kHz USVs are produced suggests that 22-kHz USVs are produced in anxiety models that have an inherent depressive-like component as well (Vivian and Miczek 1993). Further research will have to be conducted in order to address whether 22-kHz USVs are indicative of negative affect resembling depression or anxiety in a manner satisfactory to all.
In summary, intermittent alcohol vapor exposure and pulsatile heroin delivery lead to dependence as determined by multiple indices of withdrawal in rats. In addition to somatic symptoms, increases in FST immobility and 22-kHz ultrasonic vocalizations were shown to accompany withdrawal following two weeks of intermittent alcohol vapor exposure. Given that 22-kHz USVs parallel immobility in the FST suggests that they can be used as a measure of negative affect in the rat that is distinct from behavioral change in the EPM under certain conditions. Furthermore, in animals exposed to eight weeks of alcohol vapor exposure, escalation of alcohol self-administration was observed and significantly increased 22-kHz USVs. Consequently, these methods should provide a basis to evaluate the neurobiological substrates of negative affective behavior in alcohol- and opioid-dependent rats and assist in pharmacotherapeutic development efforts to treat opioid and alcohol dependence.
Acknowledgments
Support for this research was provided in part by R01AA020394-01 from the National Institute on Alcohol Abuse and Alcoholism, RGA 11-014 from the Hope for Depression Research Foundation, research grants from the WSU Alcohol and Drug Abuse Research Program awarded to BMW and DJR according to the State of Washington Initiative Measure No. 171 and WSU Department of Psychology research grants awarded to ASP and DJR. The authors would like to thank the NIDA Drug Supply Program for their assistance with the study. The authors are particularly appreciative of the assistance provided by Dr. Jaak Panksepp and Paolo Iacobucci with the technicalities related to USV measurement and lively discussions related to affective states. None of the authors have any financial, personal or organizational conflicts of interest to report in relation to this manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism, the National Institutes of Health or the State of Washington.
Reference List
- 1.Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000;22:413–421. doi: 10.1016/S0893-133X(99)00133-5. [DOI] [PubMed] [Google Scholar]
- 2.Azar MR, Ahmed SH, Lintz R, Gutierrez T, Stinus L, Koob GF. A non-invasive gating device for continuous drug delivery that allows control over the timing and duration of spontaneous opiate withdrawal. J Neurosci Methods. 2004;135:129–135. doi: 10.1016/j.jneumeth.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- 4.Barr GA, Zmitrovich A, Hamowy AS, Liu PY, Wang S, Hutchings DE. Neonatal withdrawal following pre- and postnatal exposure to methadone in the rat. Pharmacol Biochem Behav. 1998;60:97–104. doi: 10.1016/s0091-3057(97)00596-0. [DOI] [PubMed] [Google Scholar]
- 5.Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict Biol. 2006;11:270–288. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- 6.Birnbaum HG, White AG, Schiller M, Waldman T, Cleveland JM, Roland CL. Societal costs of prescription opioid abuse, dependence, and misuse in the United States. Pain Med. 2011;12:657–667. doi: 10.1111/j.1526-4637.2011.01075.x. [DOI] [PubMed] [Google Scholar]
- 7.Blasig J, Herz A, Reinhold K, Zieglgansberger S. Development of physical dependence on morphine in respect to time and dosage and quantification of the precipitated withdrawal syndrome in rats. Psychopharmacologia. 1973;33:19–38. doi: 10.1007/BF00428791. [DOI] [PubMed] [Google Scholar]
- 8.Breese GR, Overstreet DH, Knapp DJ, Navarro M. Prior multiple ethanol withdrawals enhance stress-induced anxiety-like behavior: inhibition by CRF1- and benzodiazepine-receptor antagonists and a 5-HT1a-receptor agonist. Neuropsychopharmacology. 2005;30:1662–1669. doi: 10.1038/sj.npp.1300706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown SA, Schuckit MA. Changes in depression among abstinent alcoholics. J Stud Alcohol. 1988;49:412–417. doi: 10.15288/jsa.1988.49.412. [DOI] [PubMed] [Google Scholar]
- 10.Burgdorf J, Knutson B, Panksepp J, Shippenberg TS. Evaluation of rat ultrasonic vocalizations as predictors of the conditioned aversive effects of drugs. Psychopharmacology (Berl) 2001;155:35–42. doi: 10.1007/s002130100685. [DOI] [PubMed] [Google Scholar]
- 11.Chartoff E, Sawyer A, Rachlin A, Potter D, Pliakas A, Carlezon WA. Blockade of kappa opioid receptors attenuates the development of depressive-like behaviors induced by cocaine withdrawal in rats. Neuropharmacology. 2012;62:167–176. doi: 10.1016/j.neuropharm.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen SA, O'Dell LE, Hoefer ME, Greenwell TN, Zorrilla EP, Koob GF. Unlimited access to heroin self-administration: independent motivational markers of opiate dependence. Neuropsychopharmacology. 2006;31:2692–2707. doi: 10.1038/sj.npp.1301008. [DOI] [PubMed] [Google Scholar]
- 13.Cochin J, Miller JM, Rosow CE, Grell R, Poulsen JL. The influence of the mode of morphine administration on tolerance and dependence. NIDA Res Monogr. 1979;27:36–47. [PubMed] [Google Scholar]
- 14.Contarino A, Papaleo F. The corticotropin-releasing factor receptor-1 pathway mediates the negative affective states of opiate withdrawal. Proc Natl Acad Sci U S A. 2005;102:18649–18654. doi: 10.1073/pnas.0506999102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- 16.Cryan JF, Page ME, Lucki I. Differential behavioral effects of the antidepressants reboxetine, fluoxetine, and moclobemide in a modified forced swim test following chronic treatment. Psychopharmacology (Berl) 2005a;182:335–344. doi: 10.1007/s00213-005-0093-5. [DOI] [PubMed] [Google Scholar]
- 17.Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev. 2005b;29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Devaud LL, Matthews DB, Morrow AL. Gender impacts behavioral and neurochemical adaptations in ethanol-dependent rats. Pharmacol Biochem Behav. 1999;64:841–849. doi: 10.1016/s0091-3057(99)00164-1. [DOI] [PubMed] [Google Scholar]
- 19.Duman CH. Models of depression. Vitam Horm. 2010;82:1–21. doi: 10.1016/S0083-6729(10)82001-1. [DOI] [PubMed] [Google Scholar]
- 20.Fernandes M, Kluwe S, Coper H. Quantitative assessment of tolerance to and dependence on morphine in mice. Naunyn Schmiedebergs Arch Pharmacol. 1977;297:53–60. doi: 10.1007/BF00508810. [DOI] [PubMed] [Google Scholar]
- 21.Gellert VF, Holtzman SG. Development and maintenance of morphine tolerance and dependence in the rat by scheduled access to morphine drinking solutions. J Pharmacol Exp Ther. 1978;205:536–546. [PubMed] [Google Scholar]
- 22.Getachew B, Hauser SR, Taylor RE, Tizabi Y. Desipramine blocks alcohol-induced anxiety- and depressive-like behaviors in two rat strains. Pharmacol Biochem Behav. 2008;91:97–103. doi: 10.1016/j.pbb.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Getachew B, Hauser SR, Taylor RE, Tizabi Y. Alcohol-induced depressive-like behavior is associated with cortical norepinephrine reduction. Pharmacol Biochem Behav. 2010;96:395–401. doi: 10.1016/j.pbb.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goeldner C, Lutz PE, Darcq E, Halter T, Clesse D, Ouagazzal AM, Kieffer BL. Impaired emotional-like behavior and serotonergic function during protracted abstinence from chronic morphine. Biol Psychiatry. 2011;69:236–244. doi: 10.1016/j.biopsych.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grant BF, Harford TC. Comorbidity between DSM-IV alcohol use disorders and major depression: results of a national survey. Drug Alcohol Depend. 1995;39:197–206. doi: 10.1016/0376-8716(95)01160-4. [DOI] [PubMed] [Google Scholar]
- 26.Greenwell TN, Funk CK, Cottone P, Richardson HN, Chen SA, Rice KC, Zorrilla EP, Koob GF. Corticotropin-releasing factor-1 receptor antagonists decrease heroin self-administration in long- but not short-access rats. Addict Biol. 2009;14:130–143. doi: 10.1111/j.1369-1600.2008.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris GC, Aston-Jones G. Enhanced morphine preference following prolonged abstinence: association with increased Fos expression in the extended amygdala. Neuropsychopharmacology. 2003;28:292–299. doi: 10.1038/sj.npp.1300037. [DOI] [PubMed] [Google Scholar]
- 28.Harwood HJ, Fountain D, Livermore G. Economic costs of alcohol abuse and alcoholism. Recent Dev Alcohol. 1998:307–330. doi: 10.1007/0-306-47148-5_14. [DOI] [PubMed] [Google Scholar]
- 29.Hasin DS, Grant BF. Major depression in 6050 former drinkers: association with past alcohol dependence. Arch Gen Psychiatry. 2002;59:794–800. doi: 10.1001/archpsyc.59.9.794. [DOI] [PubMed] [Google Scholar]
- 30.Hay JL, Kaboutari J, White JM, Salem A, Irvine R. Model of methadone-induced hyperalgesia in rats and effect of memantine. Eur J Pharmacol. 2010;626:229–233. doi: 10.1016/j.ejphar.2009.09.056. [DOI] [PubMed] [Google Scholar]
- 31.Kalinichev M, Holtzman SG. Changes in urination/defecation, auditory startle response, and startle-induced ultrasonic vocalizations in rats undergoing morphine withdrawal: similarities and differences between acute and chronic dependence. J Pharmacol Exp Ther. 2003;304:603–609. doi: 10.1124/jpet.102.044206. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan CD. In: Drug craving and drug use in the daily life of heroin addicts. de Vries MW, editor. Cambridge University Press; New York: 1992. pp. 193–218. [Google Scholar]
- 33.Kliethermes CL. Anxiety-like behaviors following chronic ethanol exposure. Neurosci Biobehav Rev. 2005;28:837–850. doi: 10.1016/j.neubiorev.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Knapp DJ, Duncan GE, Crews FT, Breese GR. Induction of Fos-like proteins and ultrasonic vocalizations during ethanol withdrawal: further evidence for withdrawal-induced anxiety. Alcohol Clin Exp Res. 1998;22:481–493. [PubMed] [Google Scholar]
- 35.Knapp DJ, Kampov-Polevoy AB, Overstreet DH, Breese GR, Rezvani AH. Ultrasonic vocalization behavior differs between lines of ethanol-preferring and nonpreferring rats. Alcohol Clin Exp Res. 1997;21:1232–1240. [PubMed] [Google Scholar]
- 36.Knapp DJ, Pohorecky LA. An air-puff stimulus method for elicitation of ultrasonic vocalizations in rats. J Neurosci Methods. 1995;62:1–5. doi: 10.1016/0165-0270(95)00044-5. [DOI] [PubMed] [Google Scholar]
- 37.Knutson B, Burgdorf J, Panksepp J. Ultrasonic vocalizations as indices of affective states in rats. Psychol Bull. 2002;128:961–977. doi: 10.1037/0033-2909.128.6.961. [DOI] [PubMed] [Google Scholar]
- 38.Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- 39.Koob GF. Dynamics of neuronal circuits in addiction: reward, antireward, and emotional memory. Pharmacopsychiatry. 2009;42(Suppl 1):S32–S41. doi: 10.1055/s-0029-1216356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- 41.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 42.Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lopez MF, Becker HC. Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology (Berl) 2005;181:688–696. doi: 10.1007/s00213-005-0026-3. [DOI] [PubMed] [Google Scholar]
- 44.Lynch HJ, Rivest RW, Wurtman RJ. Artificial induction of melatonin rhythms by programmed microinfusion. Neuroendocrinology. 1980;31:106–111. doi: 10.1159/000123059. [DOI] [PubMed] [Google Scholar]
- 45.Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology. 1998;18:135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- 46.Marshall I, Weinstock M. Quantitative method for assessing one symptom of the withdrawal syndrome in mice after chronic morphine administration. Nature. 1971;234:223–224. doi: 10.1038/234223a0. [DOI] [PubMed] [Google Scholar]
- 47.Matsumoto I, Burke L, Inoue Y, Wilce PA. Two models of ethanol withdrawal kindling. Nihon Arukoru Yakubutsu Igakkai Zasshi. 2001;36:53–64. [PubMed] [Google Scholar]
- 48.Molina VA, Heyser CJ, Spear LP. Chronic variable stress or chronic morphine facilitates immobility in a forced swim test: reversal by naloxone. Psychopharmacology (Berl) 1994;114:433–440. doi: 10.1007/BF02249333. [DOI] [PubMed] [Google Scholar]
- 49.Moy SS, Knapp DJ, Criswell HE, Breese GR. Flumazenil blockade of anxiety following ethanol withdrawal in rats. Psychopharmacology (Berl) 1997;131:354–360. doi: 10.1007/s002130050303. [DOI] [PubMed] [Google Scholar]
- 50.Moy SS, Knapp DJ, Duncan GE, Breese GR. Enhanced ultrasonic vocalization and Fos protein expression following ethanol withdrawal: effects of flumazenil. Psychopharmacology (Berl) 2000;152:208–215. doi: 10.1007/s002130000507. [DOI] [PubMed] [Google Scholar]
- 51.Mutschler NH, Miczek KA. Withdrawal from a self-administered or non-contingent cocaine binge: differences in ultrasonic distress vocalizations in rats. Psychopharmacology (Berl) 1998;136:402–408. doi: 10.1007/s002130050584. [DOI] [PubMed] [Google Scholar]
- 52.National Research Council. Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington, D.C: 1996. [Google Scholar]
- 53.National Research Council (US) Committee on Guidelines. Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research. National Academy Press; Washington, D.C: 2003. [Google Scholar]
- 54.Nealey KA, Smith AW, Davis SM, Smith DG, Walker BM. kappa-opioid receptors are implicated in the increased potency of intra-accumbens nalmefene in ethanol-dependent rats. Neuropharmacology. 2011;61:35–42. doi: 10.1016/j.neuropharm.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nestler EJ, Carlezon WA. The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 56.Nunes E, Quitkin F, Brady R, Post-Koenig T. Antidepressant treatment in methadone maintenance patients. J Addict Dis. 1994;13:13–24. doi: 10.1300/j069v13n03_02. [DOI] [PubMed] [Google Scholar]
- 57.Nutt DJ, Stein DJ. Understanding the neurobiology of comorbidity in anxiety disorders. CNS Spectr. 2006;11:13–20. doi: 10.1017/s1092852900025803. [DOI] [PubMed] [Google Scholar]
- 58.O'Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- 59.Overstreet DH, Knapp DJ, Breese GR. Similar anxiety-like responses in male and female rats exposed to repeated withdrawals from ethanol. Pharmacol Biochem Behav. 2004;78:459–464. doi: 10.1016/j.pbb.2004.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pandey SC, Zhang D, Mittal N, Nayyar D. Potential role of the gene transcription factor cyclic AMP-responsive element binding protein in ethanol withdrawal-related anxiety. J Pharmacol Exp Ther. 1999;288:866–878. [PubMed] [Google Scholar]
- 61.Paterson NE, Myers C, Markou A. Effects of repeated withdrawal from continuous amphetamine administration on brain reward function in rats. Psychopharmacology (Berl) 2000;152:440–446. doi: 10.1007/s002130000559. [DOI] [PubMed] [Google Scholar]
- 62.Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 63.Portfors CV. Types and functions of ultrasonic vocalizations in laboratory rats and mice. J Am Assoc Lab Anim Sci. 2007;46:28–34. [PubMed] [Google Scholar]
- 64.Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223–2233. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- 65.Rogers J, Wiener SG, Bloom FE. Long-term ethanol administration methods for rats: advantages of inhalation over intubation or liquid diets. Behav Neural Biol. 1979;27:466–486. doi: 10.1016/s0163-1047(79)92061-2. [DOI] [PubMed] [Google Scholar]
- 66.Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- 67.Schuckit MA, Tipp JE, Bergman M, Reich W, Hesselbrock VM, Smith TL. Comparison of induced and independent major depressive disorders in 2,945 alcoholics. Am J Psychiatry. 1997;154:948–957. doi: 10.1176/ajp.154.7.948. [DOI] [PubMed] [Google Scholar]
- 68.Schulteis G, Markou A, Cole M, Koob GF. Decreased brain reward produced by ethanol withdrawal. Proc Natl Acad Sci U S A. 1995;92:5880–5884. doi: 10.1073/pnas.92.13.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schulteis G, Yackey M, Risbrough V, Koob GF. Anxiogenic-like effects of spontaneous and naloxone-precipitated opiate withdrawal in the elevated plus-maze. Pharmacol Biochem Behav. 1998;60:727–731. doi: 10.1016/s0091-3057(98)00034-3. [DOI] [PubMed] [Google Scholar]
- 70.Smith AW, Nealey KA, Wright JW, Walker BM. Plasticity associated with escalated operant ethanol self-administration during acute withdrawal in ethanol-dependent rats requires intact matrix metalloproteinase systems. Neurobiol Learn Mem. 2011 doi: 10.1016/j.nlm.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Steffensen SC, Stobbs SH, Colago EE, Lee RS, Koob GF, Gallegos RA, Henriksen SJ. Contingent and non-contingent effects of heroin on mu-opioid receptor-containing ventral tegmental area GABA neurons. Exp Neurol. 2006;202:139–151. doi: 10.1016/j.expneurol.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 72.Substance Abuse and Mental Health Services Administration. 2005 State Estimates of Substance Use & Mental Health: Substance Dependence, Abuse, and Treatment Need. 2005. [Google Scholar]
- 73.Substance Abuse and Mental Health Services Administration; Office of Applied Studies. Results from the 2009 National Survey on Drug Use and Health. DHHS; Rockville, MD: 2010. [Google Scholar]
- 74.Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- 75.Valenstein ES, Cox VC, Kakolewski JW. Polydipsia elicited by the synergistic action of a saccharin and glucose solution. Science. 1967;157:552–554. doi: 10.1126/science.157.3788.552. [DOI] [PubMed] [Google Scholar]
- 76.Vendruscolo LF, Schlosburg JE, Misra KK, Chen SA, Greenwell TN, Koob GF. Escalation patterns of varying periods of heroin access. Pharmacol Biochem Behav. 2011;98:570–574. doi: 10.1016/j.pbb.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vivian JA, Miczek KA. Ultrasounds during morphine withdrawal in rats. Psychopharmacology (Berl) 1991;104:187–193. doi: 10.1007/BF02244177. [DOI] [PubMed] [Google Scholar]
- 78.Vivian JA, Miczek KA. Diazepam and gepirone selectively attenuate either 20–32 or 32–64 kHz ultrasonic vocalizations during aggressive encounters. Psychopharmacology (Berl) 1993;112:66–73. doi: 10.1007/BF02247364. [DOI] [PubMed] [Google Scholar]
- 79.Walker BM. Conceptualizing Withdrawal-Induced Escalation of Alcohol Self- Administration as a Learned, Plasticity-Dependent Process. Alcohol. 2012 doi: 10.1016/j.alcohol.2012.01.001. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Walker BM, Drimmer DA, Walker JL, Liu T, Mathe AA, Ehlers CL. Effects of prolonged ethanol vapor exposure on forced swim behavior, and neuropeptide Y and corticotropin-releasing factor levels in rat brains. Alcohol. 2010;44:487–493. doi: 10.1016/j.alcohol.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Walker BM, Koob GF. The gamma-aminobutyric acid-B receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcohol Clin Exp Res. 2007;31:11–18. doi: 10.1111/j.1530-0277.2006.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Walker BM, Koob GF. Pharmacological Evidence for a Motivational Role of kappa- Opioid Systems in Ethanol Dependence. Neuropsychopharmacology. 2008;33:643–652. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Walker BM, Rasmussen DD, Raskind MA, Koob GF. alpha1-noradrenergic receptor antagonism blocks dependence-induced increases in responding for ethanol. Alcohol. 2008;42:91–97. doi: 10.1016/j.alcohol.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Walker BM, Valdez GR, McLaughlin JP, Bakalkin G. Targeting Dynorphin/Kappa Opioid Receptor Systems to Treat Alcohol Abuse and Dependence. Alcohol. 2012 doi: 10.1016/j.alcohol.2011.10.006. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Walker JR, Ahmed SH, Gracy KN, Koob GF. Microinjections of an opiate receptor antagonist into the bed nucleus of the stria terminalis suppress heroin self-administration in dependent rats. Brain Res. 2000;854:85–92. doi: 10.1016/s0006-8993(99)02288-x. [DOI] [PubMed] [Google Scholar]
- 86.Walker JR, Chen SA, Moffitt H, Inturrisi CE, Koob GF. Chronic opioid exposure produces increased heroin self-administration in rats. Pharmacol Biochem Behav. 2003;75:349–354. doi: 10.1016/s0091-3057(03)00094-7. [DOI] [PubMed] [Google Scholar]
- 87.Wright JS, Panksepp J. Toward affective circuit-based preclinical models of depression: sensitizing dorsal PAG arousal leads to sustained suppression of positive affect in rats. Neurosci Biobehav Rev. 2011;35:1902–1915. doi: 10.1016/j.neubiorev.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 88.Zhang Z, Morse AC, Koob GF, Schulteis G. Dose- and time-dependent expression of anxiety-like behavior in the elevated plus-maze during withdrawal from acute and repeated intermittent ethanol intoxication in rats. Alcohol Clin Exp Res. 2007;31:1811–1819. doi: 10.1111/j.1530-0277.2007.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]