Abstract
Lutein and zeaxanthin are xanthophylls that can be found highly concentrated in the macula of the retina. They are thought to protect the macula through their role as blue-light filters and because of their antioxidant and singlet oxygen quenching properties. Examination of metabolites unique to lutein and zeaxanthin such as 3′-dehydro-lutein, and of their stereochemistry may provide insight to the mechanism by which they are formed and by which they exert protection. To evaluate the formation of such metabolites, eleven monkeys were raised on a xanthophyll-free diet, and supplemented with pure lutein or pure zeaxanthin (2.2 mg/kg body weight/d). The period of supplementation ranged between 12 to 92 weeks. At study start and throughout the study, serum samples were taken and analyzed for xanthophylls using different HPLC systems. Xanthophyll metabolites were identified using UV/VIS and HR-MS detection. Lutein and zeaxanthin metabolites were found in detectable amounts with 3′-dehydro-lutein being a common metabolite of both. Using chiral-phase HPLC, two diastereomers, (3R,6′R)-3′-dehydro-lutein and (3R,6′S)-3′-dehydro-lutein, were identified and shown to be present in nearly equimolar amounts. A pathway for their formation from either lutein or zeaxanthin is proposed. These finding were comparable to results obtained with human plasma.
Keywords: Chiral analysis, 3′-Dehydro-lutein, Human plasma, Lutein, Rhesus monkey serum, Serum metabolites, Zeaxanthin
INTRODUCTION
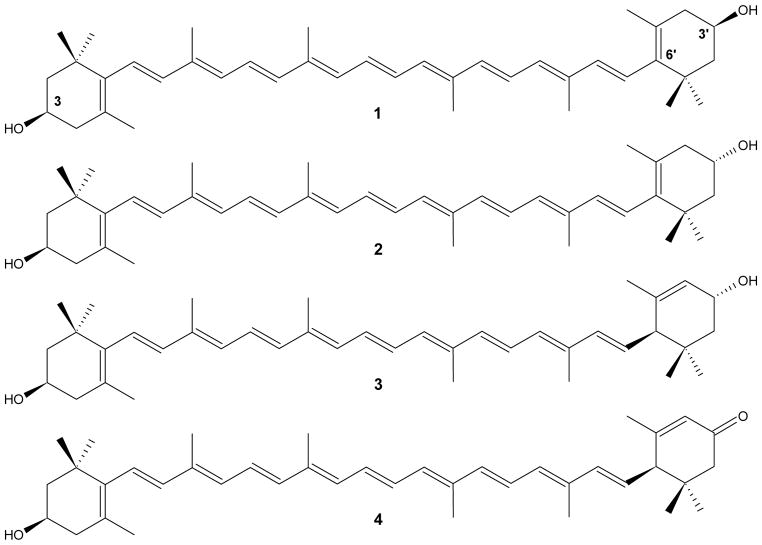
Lutein and zeaxanthin are xanthophylls (dihydroxy-carotenoids) that can be found in high concentration in the primate eye (Bone et al., 1985; Bone et al., 1993; Handelman et al., 1992; Khachik et al., 1997; Figure 1). They are especially concentrated in the macula region of the primate retina and result in its characteristic yellow colour. In the macula, lutein is present as a single stereoisomer, (3R,3′R,6′R)-β-ε-carotene-3,3′-diol, while zeaxanthin occurs primarily as a mixture of (3R,3′R)-β-β-carotene-3,3′-diol and (3R,3′S)-β-β-carotene-3,3′-diol also referred to as zeaxanthin and meso-zeaxanthin, respectively (Bone et al., 1993).
Figure 1.
Structural formula and nomenclature of (3R,3′R)-all-E-zeaxanthin 1, (RS)-meso-zeaxanthin 2, (3R,3′R,6′R)-all-E-lutein 3 and (3R,6′R)-all-E-3′-dehydro-lutein 4.
It is thought that lutein and zeaxanthin protect the macula through their role as blue-light filters and because of their antioxidant and singlet oxygen quenching properties, thereby reducing oxidative damage. In this way, lutein and zeaxanthin may reduce the risk to develop age-related macular-degeneration (AMD), the leading cause of vision loss in older adults of the Western world. This is supported by clinical and epidemiologic studies that show plasma concentrations (Eye Disease Case-Control Study Group, 1993; Gale et al. 2003) and dietary intake (Seddon et al., 1994) of these xanthophylls being inversely correlated with the risk of AMD. Furthermore, it has been shown that the content of lutein and zeaxanthin was lower in retinas of patients with AMD than in those without AMD (Bone et al., 2001). Further investigations regarding their metabolism could help to understand the mechanism by which lutein and zeaxanthin may exert their protective effects.
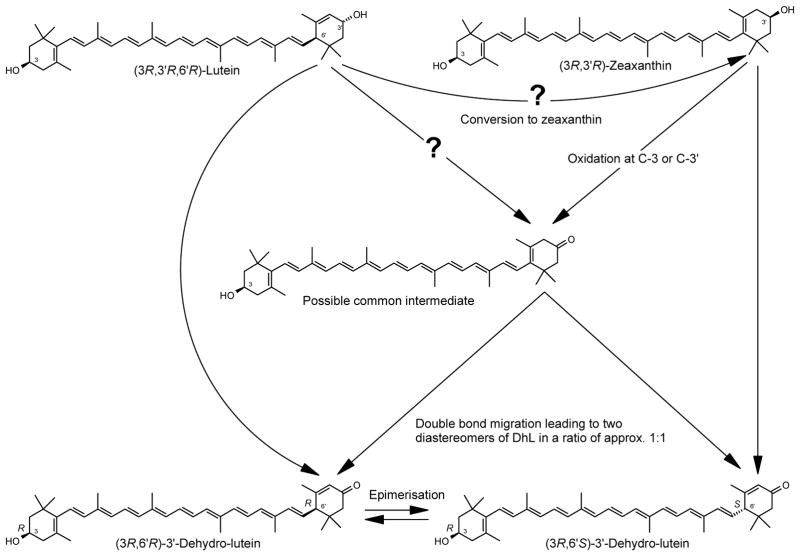
Lutein and zeaxanthin are commonly found in diet and in human serum, whereas meso-zeaxanthin is not. Meso-zeaxanthin was demonstrated to exclusively be formed in the retina of monkeys from lutein (Bone et al., 1993; Bone et al., 1997; Johnson et al., 2005). In both, the lutein and the meso-zeaxanthin molecule, the spatial orientation of the two hydroxyl groups located at the 3 and 3′ carbon atoms of the carotenoid end-groups is identical, with both hydroxyl groups pointing in opposite directions if the backbone of the molecule is regarded as projected into a plane. Thus, for the conversion of lutein into meso-zeaxanthin only the shift of one carbon-carbon double bond in the ε-ring of lutein is necessary, thereby increasing the conjugation by one double bond (Figure 1). An alternative mechanism for the formation of meso-zeaxanthin proposes that the metabolite, 3′-dehydro-lutein (# 4 in Figure 1), gives rise to meso-zeaxanthin through an enzymatic reduction pathway (Bone and Landrum, 2004). The keto-carotenoid canthaxanthin does indeed undergo reduction in the human and primate retina lending credence to this possibility (Goralzcyk et al., 1997).
Recent studies have reported the identification of 3′-dehydro-lutein (in some literature also referred to as 3′-oxolutein or 3′-ketolutein) in human serum after supplementation with either lutein or zeaxanthin at doses ranging from 1–20.5 mg/d for 42 days (Hartmann et al., 2004; Thurmann et al., 2005). It seemed likely that, in course of the metabolic oxidation of lutein or zeaxanthin, two diastereomers of 3′-dehydro-lutein with opposite configuration at C-6′, namely (3R,6′R)-3′-dehydro-lutein and (3R,6′S)-3′-dehydro-lutein respectively could be formed. However, chiral analysis of 3′-dehydro-lutein extracted from human serum after supplementation with either lutein or zeaxanthin (Hartmann et al., 2004; Thurmann et al., 2005) indicated that the two diastereomers (3R,6′R)-3′-dehydro-lutein and (3R,6′S)-3′-dehydro-lutein are formed independently when lutein or zeaxanthin was supplemented (Höller et al., 2005). While more 3′-dehydro-lutein was formed during supplementation of humans with zeaxanthin than with lutein, both stereoisomers always occurred in approximately equimolar concentrations. A possible mechanism involving oxidation at C-3′ and a consecutive isomerisation at C-6′ was proposed (Figure 2).
Figure 2.
Possible pathways in humans and rhesus monkeys from either lutein or zeaxanthin which may lead to formation of two diastereomers of 3′-dehydro-lutein (DhL) in a ratio of 1:1: via oxidation at C-3′ followed by epimerisation at C-6′ (dotted lines) or via formation of a common intermediate (solid lines).
Since the human plasma samples investigated were not totally free of other carotenoids, including lutein and zeaxanthin from diet, it was not possible to unambiguously deduce the origin of the (3R,6′R)-3′-dehydro-lutein and (3R,6′S)-3′-dehydro-lutein from either lutein or zeaxanthin.
The absorption and metabolism of xanthophylls varies widely among animal species which limits the use of most animal models. Therefore, the only appropriate model in this regard is the primate. 3′-dehydro-lutein is found in the retina (Khachik et al., 1997). Its absolute stereochemistry could influence its biological function in this tissue. Further, it may be an intermediate in the conversion of lutein to meso-zeaxanthin. Therefore, it is important to understand the absolute stereochemistry and the formation of this metabolite from lutein and zeaxanthin.
In the present study, we had the unique opportunity to study monkeys that had been on a xanthophyll-free diet from birth to age 7–16 years. Serum and tissue analyses showed no detectable lutein, zeaxanthin, or any xanthophyll metabolites (Johnson et al., 2005; Neuringer et al., 2004) allowing us to do supplementation and to evaluate the individual metabolites of lutein and zeaxanthin. The effect of dietary supplementation with either pure lutein or pure zeaxanthin on the formation of metabolites in serum could be followed more readily and without any interference from the other xanthophylls or their metabolites.
MATERIALS AND METHODS
Subjects
Eleven rhesus monkeys (Macca mulatta) born at the Oregon National Primate Research Center (ONPRC) were reared on one of two semi-purified diets, both of which contained adequate levels of all known essential nutrients, but no detectable xanthophylls. The semi-purified diets were fed to the mothers of the subjects throughout pregnancy and to the subjects from the day of birth until the completion of the study. As described in more detail in Neuringer et al. (2004), the two diets differed only in their fat source and, therefore, in fatty acid composition, with one containing low levels and one adequate levels of n-3 fatty acids in the form of α-linolenoic acid. The animals also received limited amounts of very low carotenoid foods such as wheat or rice cereals, white rice, sweetened drinks, gelatine, pineapple, and banana. They were fed three times per day and fresh water was available at all times.
Beginning at 7 to 16 years of age, while all of the monkeys continued on their semi-purified diets, the diets of five monkeys were supplemented with pure lutein and six with pure zeaxanthin, at 3.9 μmol/kg per day (2.2 mg/kg per day). This dose represented 7.7 times the average daily xanthophyll intake from the standard laboratory diet (described below). Further details on the study design have been published elsewhere (Johnson et al., 2005; Neuringer et al., 2004).
For xanthophyll supplementation, lutein (100 % trans) was purified to make it zeaxanthin free, and zeaxanthin (90 % trans, 10 % 13-cis) was synthesized by DSM Nutritional Products Ltd. (Kaiseraugst, Switzerland) and formulated into gelatine beadlets. Doses of beadlets were determined for each animal based on the analysis of xanthophyll content and current body weight. Supplements were provided daily for 4 to 12 months and, due to limited supply of the pure xanthophylls, four times per week thereafter until the conclusion of the study. The duration of daily supplementation varied within each group but was matched between two groups. However, because lutein had to be specially purified, its supply was more limited, resulting in a shorter duration of supplementation at four times per week. The lutein- and zeaxanthin-fed groups were balanced to the extent possible based on gender, n-3 diet group, and body weight. For this study, the duration period for which serum samples were available varied from 12–48 weeks among the lutein-fed monkeys and from 26–92 weeks for the zeaxanthin-fed monkeys.
Data from the lutein- and zeaxanthin-fed monkeys were compared with data from normal control monkeys fed a standard stock diet (Purina 5047 Monkey Chow; Ralston Purina, Richmond, IN, USA) providing a daily carotenoid intake of approximately 0.26 μmol/kg per day lutein, 0.24 μmol/kg per day zeaxanthin, and 0.035 μmol/kg per day β-carotene (means of four analyses). These animals also received supplemental fruits and vegetables (typically, one fourth to one half an apple, or one half carrot approximately three times per week), which contributed an estimated maximum additional daily average of ~ 3 nmol/kg of lutein plus zeaxanthin or <1% of the intake from the stock diet. They were housed under the same conditions as the experimental diet groups.
All procedures were approved by the Institutional Animal Care and Use Committee of the ONPRC, Oregon Health and Science University, and conformed to NIH guidelines and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Serum Xanthophyll Analyses
Normal-phase HPLC
Monkey serum was prepared for extraction as previously described (Yeum et al., 1996) with minor modifications. Briefly, 200 μL of sample was added to 3 mL CHCl3/MeOH (2:1) and 500 μL 0.85 % saline. Given that no other carotenoids were fed to these animals, canthaxanthin or cryptoxanthin in hexane was added as an internal standard (150 μL, from DSM Nutritional Products, Basel, Switzerland). However, in the zeaxanthin fed monkeys there was co-elution of the internal standard with an unknown component. In this case, the analysis was repeated without internal standard and the contribution of the unknown to the internal standard peak was calculated. The mixture was vortexed for 30 s and centrifuged at 1000 g for 10 min at 4°C.
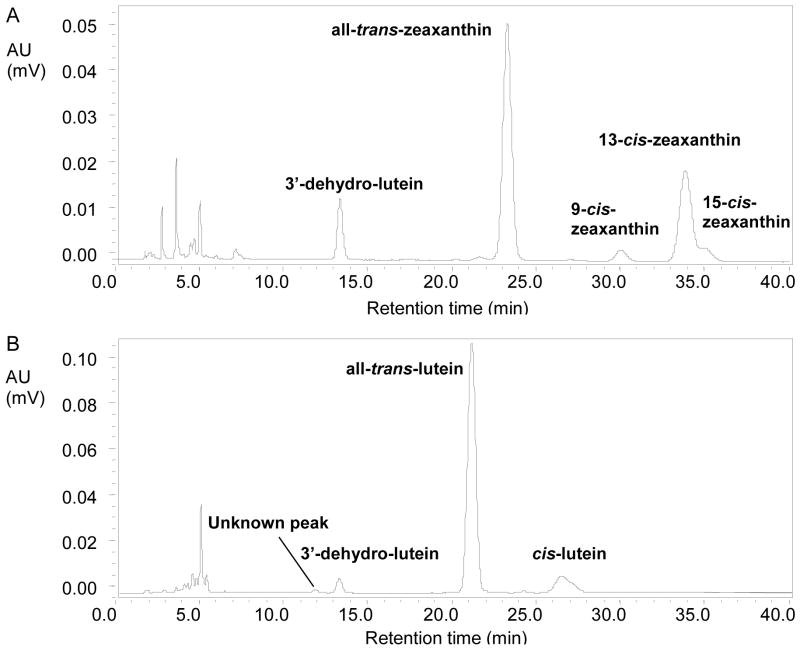
The CHCl3 layer was removed and evaporated to dryness under nitrogen. To the remaining mixture, 3 mL hexane was added, vortexed for 30 s and again centrifuged as above. The hexane layer was combined with the first extraction and evaporated to dryness under nitrogen. The residue was re-dissolved in100 μL hexane and sonicated for 30 s. A 50 μL aliquot was injected into the HPLC system equipped with an HPLC pump (Waters, 616), an autosampler (Waters, 717 plus, 13°C) and a Photodiode Array Detector (Waters, 996). Carotenoids were quantified using a normal-phase, isocratic HPLC system, as previously described (Hartmann et al., 2004) using a polar column (Lichrosorb, Si-60, 5 μm, 250 × 4 mm; Phenomenex, Torrance, CA, USA) with minor modifications. For optimal separation of lutein and zeaxanthin, the mobile phase ratio hexane (Sigma-Aldrich Chemicals, St. Louis, MO, UAS) to acetone (Sigma, 99.9 % water free) was modified to 85:15 (v/v) at a flow rate of 1 mL/min. Carotenoids were quantified at 445 nm. For identification, peaks were compared with HPLC retention times and absorption spectra of known standards of lutein and zeaxanthin (DSM Nutritional Products, Basel, Switzerland) and synthetic 3′-dehydro-lutein (Molnar et al., 2006) provided by J. Landrum. This HPLC method results in baseline separations of lutein, zeaxanthin, and 3′-dehydro-lutein (Figure 3).
Figure 3.
Normal-phase HPLC chromatograms of serum extracts: (A) zeaxanthin-fed monkey; (B) lutein-fed monkey.
Serum Xanthophyll Analysis using HPLC-MS
For HPLC-MS analyses, monkey serum (300 μL) was extracted three times using the same method described above for the normal-phase HPLC system, using 2 mL hexane instead of 3 mL. All three extractions were combined, dried down under nitrogen and re-dissolved in 140 μL of the mobile phase (hexane:acetone; 85:15, v/v) and sonicated for 30 seconds. A 60 μL aliquot was injected twice into the HPLC system and fractions were collected under red light. The fractions were dried down under nitrogen and re-dissolved in 120 μl ethanol. A 50 μl sample was injected twice into the HPLC-MS (Bruker Esquire 3000plus LCMS (n)-System) with an APCI interface and an ion trap as earlier described by Putzbach et al. (2005). The mass spectra recording was in a mass region of 400–700 m/z, the detection of the APCI in a positive ion mode at 400°C. The voltage of the corona needle was 4 kV. Nitrogen was used as drying and carrier gas at a flow rate of 5 mL/min with a nebuliser pressure of 65 psi. The ionisation chamber and dry gas temperature was 300°C. The compound stability and trap drive level was at 75%.
Chiral-phase HPLC analysis and online HR-MS analysis
Reference compounds
(3R,6′S)- and (3R,6′R)-3′-dehydro-lutein. (3R,6′R)-3′-dehydro-lutein was available from chemical synthesis. In order to obtain the (3R,6′S)-isomer, zeaxanthin was fed to laying hens, and the obtained egg yolk was extracted according to published procedures (Schiedt, 1998). The extract was purified by semi-quantitative normal-phase (Si-60) HPLC. The 3′-dehydro-lutein peak fraction contained all-trans-3′-dehydro-lutein as the only carotenoid as judged by 1H NMR spectroscopy (data not shown). This fraction was directly used for chiral-phase HPLC.
HPLC-HR-MS analysis of serum
Monkey serum was extracted and analysed as previously described with minor modifications (Höller et al., 2005; Khachik et al., 2002). Briefly, 500 μL water and 1000 μL ethanol were added to 500 μL serum sample. After vortexing 1000 μL n-hexane/chloroform (80/20, v/v) were added and the mixture was shaken for 10 min in the dark at RT. After centrifugation for 10 min at 8°C for phase separation, 800 μL of the upper organic phase was transferred into a 1.5 mL microcentrifuge vial and dried in a SpeedVac at RT and reduced pressure. The residue was dissolved in 200 μL n-hexane/2-propanol (95/5, v/v) containing 350 mg/L BHT. 50 μL were used for HPLC analysis on a Chiralpak AD, 250 × 4.6 mm column (Daicel Chemical Industries, Ltd). For elution, 5% isopropanol in n-hexane was used at a flow rate of 1.0 mL/min. Carotenoids were detected using DAD (300 – 600 nm), monitoring wavelength at 450 nm. Identification of the peaks was based on analysis of synthetic (3R,6′R)-3′-dehydro-lutein, and 3′-dehydro-lutein [1:2 mixture of (3R,6′R)- and (3R,6′S)-3′-dehydro-lutein] isolated from egg yolk as described above (Figure 4). High resolution MS data was acquired on an Orbitrap LTQ mass spectrometer (Thermo Finnigan) operating in FTMS scan mode with APCI+. A mass accuracy of 1.5 ppm or better was achieved using a common phthalate from background as lock mass.
Figure 4.
Chromatograms visualising the separation of lutein 3, zeaxanthin 4, and two diastereomers of 3′-dehydro-lutein [(3R,6′R)-DhL 1 and (3R,6′S)-DhL 2] on a the normal-phase (upper chromatogram) and the chiral-phase (lower chromatogram) HPLC system used in the current study. A human plasma extract derived from a subject supplemented with lutein and zeaxanthin of the LUXEA study (Thurmann et al., 2005) was used.
Statistics
These analyses occurred subsequent to the primary monkey studies (Johnson et al., 2005; Neuringer et al., 2004). Therefore, there was limited amount of serum available and not all time points could be evaluated, making absolute comparisons between groups difficult. Relative comparisons were possible by expressing xanthophyll compound as a percent of the total serum xanthophylls. The mean value among the time points measured for each animal was used for each xanthophyll compound. For the xanthophylls of interest (3′-dehydro-lutein, lutein, zeaxanthin), this varied little for each animal (<10 % CV). Data was expressed as mean ± se and differences among the groups (lutein-fed, zeaxanthin-fed, control) were assessed using one-way ANOVAs followed by Bonferri-Dunn post hoc test for comparisons among 3 groups (SigmaStat 3.1). Significant difference was set at p <0.017. Evaluation of the data using relative comparisons (rather than absolute) does not compromise the primary aim and qualitative nature of this work. That is, the identification of metabolites specific to lutein and zeaxanthin.
RESULTS
Serum Carotenoid Analysis by HPLC
At baseline, no measurable amounts of xanthophylls could be found in the serum of the monkeys raised on a xanthophyll-free diet. In our previous work, the same finding was reported for each time point investigated through-out the duration of the study (Johnson et al., 2005). After supplementation with either lutein or zeaxanthin, there were measurable amounts of xanthophylls in the serum of these animals.
Normal-phase HPLC
The normal-phase HPLC analysis revealed a more detailed separation of these xanthophylls and their metabolites than the reversed-phase HPLC.
The chromatograms of the zeaxanthin-fed monkey serum showed all-trans-zeaxanthin, 9-cis-, 13-cis- and 15-cis-zeaxanthin, and a compound that eluted at the retention time of standard 3′-dehydro-lutein (Figure 3A). In lutein-fed monkey serum, apart from all-trans-lutein and its cis-isomers, 3′-dehydro-lutein and an unknown peak, were detected (Figure 3B). Because there was only all-trans-lutein in the supplement, these metabolites are believed to be the result of in vivo conversion. For further identification, the metabolites were collected and analyzed by HPLC-MS.
The percent contributions of individual xanthophylls to the total xanthophyll serum concentration are given in Table 1. For both lutein-fed and zeaxanthin-fed monkey serum, the trans-isomer was the major isomer, followed by cis-isomers. This largely reflects the composition of the supplement along with in vivo isomerisation. In monkey serum of the control group a somewhat different pattern was observed. Here, cis-lutein was relatively more abundant than the trans isomer, although the difference between the two was not significant. The relative amount of 3′-dehydro-lutein was not different between the two supplemented groups, but in each it was significantly greater than in serum of monkeys of the control group (p <0.017).
Table 1.
Xanthophyll content (% total serum xanthophyll) in serum of xanthophyll-free monkeys supplemented with lutein (100% trans) or zeaxanthin (90% trans, 10% cis) (2.2 mg/kg body weight) and in control monkey serum (mean ± se);
| Lutein (all-trans) | Zeaxanthin (all-trans) | 3′-Dehydro-lutein | Lutein (13 cis) | Lutein (cis)* | Zeaxanthin (13 cis) | Zeaxanthin (cis)* | unknown | |
|---|---|---|---|---|---|---|---|---|
| Lutein-fed n = 5 | 72.3 ± 1.3a | n.d. | 6.3 ± 0.9a | 17.9 ± 0.7a | 2.1 ± 0.8 | n.d. | n.d. | 1.4 ± 0.3a |
| Zeaxanthin- fed n = 6 | n.d. | 55.5 ± 1.8a | 8.1 ± 0.8a | n.d. | n.d. | 33.0 ± 1.6a | 3.3 ± 0.6a | n.d. |
| Control n = 5 | 19.7 ± 10.6b | 42.5 ± 10.6a | 1.3 ± 0.1b | 30.6 ± 13.2a | n.d. | 3.0 ± 0.5b | 3.8 ± 1.0a | 0.1 ± 0.1b |
cis isomers other than 13 cis
n.d. not detected.
For each xanthophyll compound (columns), mean values having different superscripts are significantly different (p <0.017 for post hoc comparisons).
Apart from 3′-dehydro-lutein, serum of the lutein-fed monkeys did not share common carotenoids with the serum of zeaxanthin-fed monkeys. This was confirmed by combining serum extracts from each group and analyzing them by HPLC. There was no co-elution of peaks. However, nearly all identified unique and shared compounds from both groups could be detected also in serum of the control group.
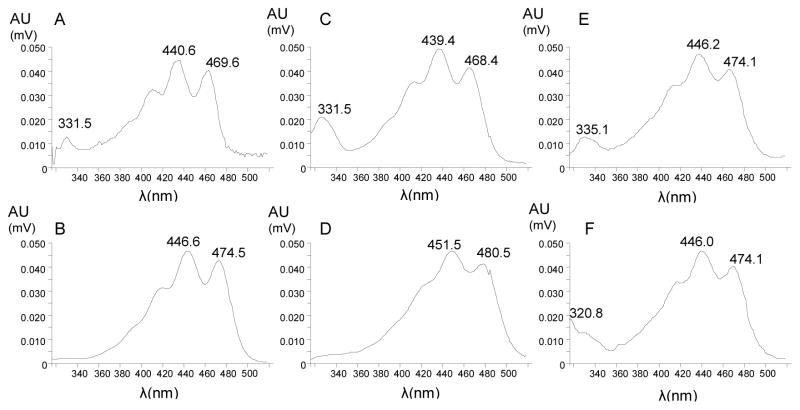
In both the reverse-phase and normal-phase HPLC systems, xanthophylls were identified by their absorption spectra (Figure 5). Both, the peak in the zeaxanthin-fed monkey serum that eluted at the retention time of standard 3′-dehydro-lutein as well as the one from lutein-fed monkey serum showed the spectra referring to this compound (Figures 5E, 5F).
Figure 5.
UV/VIS Spectra of carotenoids from monkey serum: (A) unknown peak from lutein-fed monkey serum; (B) all-trans-lutein from lutein-fed monkey serum; (C) 13-cis- lutein from lutein-fed monkey serum; (D) all-trans-zeaxanthin from zeaxanthin-fed monkey serum; (E) 3′-dehydro-lutein from zeaxanthin-fed monkey serum (F) 3′-dehydro-lutein from lutein-fed monkey serum.
Serum Carotenoid Analyses by HPLC-MS
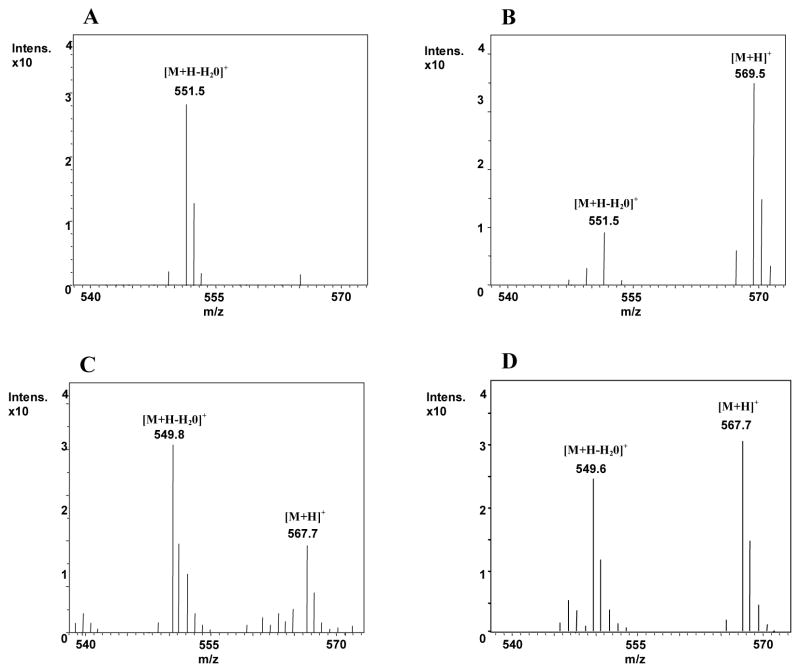
HPLC-MS data confirmed our identification of all-trans-lutein and all-trans-zeaxanthin in monkey serum (Figures 6A and 6B). For the lutein-fed monkey serum, the peak which eluted at the retention time of standard 3′-dehydro-lutein had the mass unit pattern as standard 3′-dehydro-lutein (Figure 6C). Based on the absorption spectrum and HPLC-MS, the identity of the unknown peak (RT ~13.5 min) in these sera could not be defined and needs further analysis. In the zeaxanthin-fed monkey serum, the peak which eluted at the retention time of standard 3′-dehydro-lutein had an APCI-MS mass unit pattern of 3′-dehydro-lutein (Figure 6D).
Figure 6.
HPLC-MS spectra: (A) lutein standard, the quasimolecular ion [M+H]+ is not stable under the experimental conditions and is therefore not observed; (B) zeaxanthin standard (C) 3′-dehydro-lutein from lutein-fed monkey serum; (D) 3′-dehydro-lutein from zeaxanthin-fed monkey serum.
Chiral-phase HPLC analysis and HR-MS detection
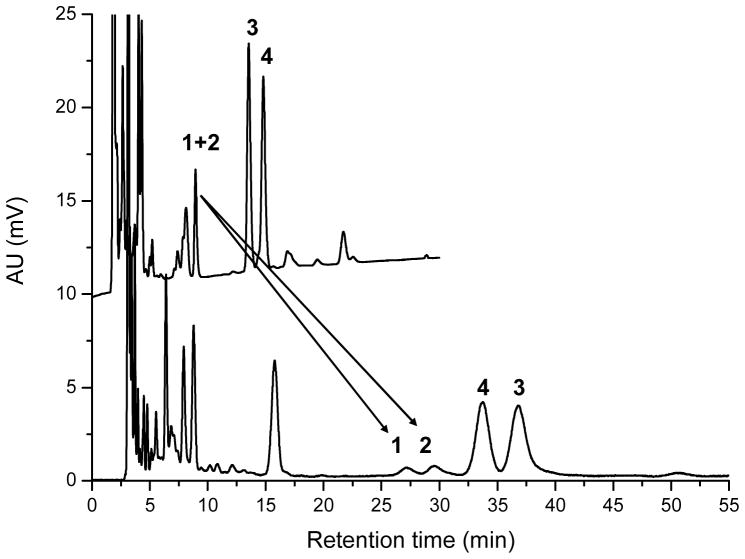
Chiral-phase HPLC analysis is an ideal tool to further investigate the identity and absolute stereochemistry of 3′-dehydro-lutein. The separation of (3R,6′S)- and (3R,6′R)-3′-dehydro-lutein, lutein and zeaxanthin on chiral-phase HPLC in comparison to normal-phase (Si-60) HPLC is shown in Figure 4. We first applied this chiral-phase separation successfully to the analysis of 3′-dehydro-lutein in human plasma (Höller et al., 2005) out of the LUXEA-study Hartmann et al., 2004; Thurmann et al., 2005). In the recent work, we coupled the chiral separation to HR-MS detection, which enabled us to establish the exact molecular masses of the carotenoids in the chromatograms investigated. For the two diastereomers (3R,6′S)- and (3R,6′R)-3′-dehydro-lutein in the egg yolk extract (Höller et al., 2005) a molecular formula of C40H55O2 was determined for the quasimolecular ion [M+H]+, which confirmed the assignment of the peaks. The (3R,6′S)-isomer was expected because of the zeaxanthin supplementation, while we expected the (3R,6′R)-isomer to arise from the natural lutein content of the feed (Schiedt, 1998).
This chiral-phase HPLC system is also suitable for the separation of (3R,3′R)-zeaxanthin and meso-zeaxanthin. If present, the latter would elute between (3R,6′S)-3′-dehydro-lutein and (3R,3′R)-zeaxanthin at around 32 min (Figures 4 and 7). However, in both human and monkey samples investigated we did not detect meso-zeaxanthin.
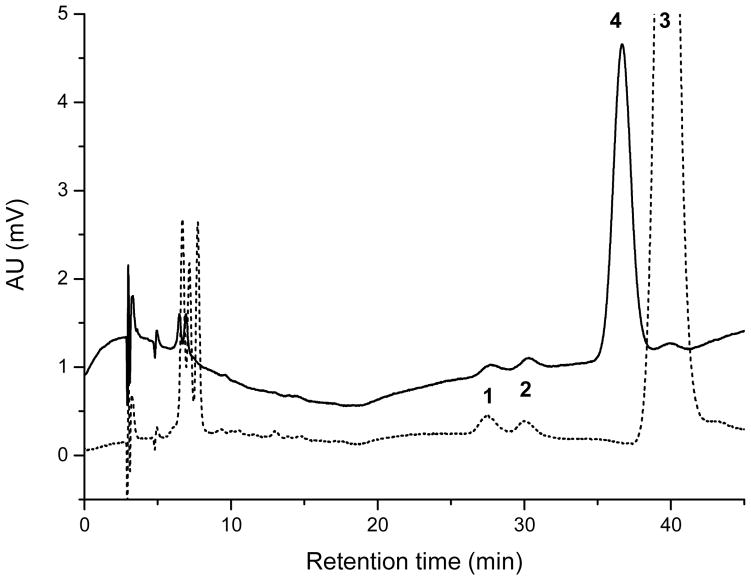
Figure 7.
Chiral-phase HPLC chromatograms of serum samples from lutein supplemented (dotted line) and zeaxanthin supplemented monkey serum (solid line). Peak identification: 1 (3R,6′R)-3′-dehydro-lutein, 2 (3R,6′S)-3′-dehydro-lutein, 3 lutein, 4 zeaxanthin. Unidentified carotenoids eluting around 6–8 min could include anhydrolutein.
Analysis of serum from rhesus monkeys
Our previous investigation of human plasma had indicated that (3R,6′S)- and (3R,6′R)-3′-dehydro-lutein are formed from either lutein or zeaxanthin in an approx. equimolar ratio (Höller et al., 2005). The rhesus monkey sera from animals raised on a carotenoid-free diet now offered the unique opportunity to further elaborate this finding without interference from other carotenoids. Due to the relatively poor sensitivity of LC-MS detection for many carotenoids, and their low content in the sera investigated, a relatively large volume (500 μL) of serum was needed for the analyses. Therefore, only 13 selected monkey serum samples could be analysed for 3′-dehydro-lutein using chiral-phase HPLC with DAD and HR-MS detection. Four samples were from animals before supplementation, four from animals which had received lutein, and five from animals which had received zeaxanthin (Table 2). In plasma samples taken from animals prior to supplementation no carotenoids were detected. In five serum samples from animals that received lutein (two animals) or zeaxanthin (one animal) both (3R,6′S)- and (3R,6′R)-3′-dehydro-lutein were detected in approx. equimolar concentrations, qualitatively confirming our earlier findings from human plasma samples. In three serum samples from two different animals no 3′-dehydro-lutein could be detected using the chiral-phase analysis, although its presence had been established using the normal-phase HPLC-system. This finding can be explained by the lower sensitivity of the chiral-phase analysis. Identity of the two diastereomers was established by co-elution of the reference compounds from synthesis and egg yolk, DAD and HR-MS data. Both normal- (Figure 4) and chiral-phase (Figure 7) HPLC revealed the presence of additional carotenoids with retention times shorter than 10 min. Their pattern differed in the chromatograms of serum after lutein supplementation in contrast to supplementation with zeaxanthin and mostly showed spectra in the visible range similar to lutein. The short retention times pointed to relatively lipophilic compounds. Possible candidates could include anhydro-lutein, lutein-esters or 3,3′-di-dehydro-lutein. Unfortunately, no unambiguous HR-MS data could be obtained because of the limited sample material available, leaving the identification of these compounds unsolved.
Table 2.
Relative content of (3R,6′R)- and (3R,6′S)- 3′-dehydro-lutein in selected monkey serum samples as determined by chiral-phase HPLC with VIS detection. Amounts [%] are given relative to the concentration in the serum of the carotenoid that had been supplemented, zeaxanthin or lutein.
| Sample ID# | Weeks of supplementation | Zeaxanthin [%]a | Lutein [%]a | (3R,6′R)-3′-Dehydro- lutein [%] | (3R,6′S)-3′-Dehydro- lutein [%] |
|---|---|---|---|---|---|
| 12756 | 0 (baseline) | n.d. | n.d. | n.d. | n.d. |
| 13980 | 0 (baseline) | n.d. | n.d. | n.d. | n.d. |
| 13980 | 12 | 100 | n.d. | 19 | 19 |
| 13980 | 32 | 100 | n.d. | 22 | 22 |
| 14114 | 0 (baseline) | n.d. | n.d. | n.d. | n.d. |
| 14362 | 12 | n.d. | 100 | 7 | 7 |
| 14362 | 16 | n.d. | 100 | 11 | 11 |
| 15866 | 0 (baseline) | n.d. | n.d. | n.d. | n.d. |
| 15866 | 12 | 100 | n.d. | n.d. | n.d. |
| 15866 | 32 | 100 | n.d. | n.d. | n.d. |
| 16361 | 12 | n.d. | 100 | 7 | 7 |
| 16361 | 24 | n.d. | 100 | n.d. | n.d. |
100 indicates supplementation with the respective xanthophyll. Remaining samples were from animals before supplementation.
n.d. not detected.
DISCUSSION
Evidence from animal, clinical and epidemiologic studies suggests a protective role for xanthophylls in AMD prevention (Landrum et al., 1997; Krinsky et al., 2003). Therefore, further characterisation of their metabolisms may provide insights to the mechanisms by which xanthophylls exert their protective effects.
This study allowed us to investigate the individual metabolites of lutein and zeaxanthin. The xanthophyll-free monkeys of the present study were fed with pure lutein (100% trans) or pure zeaxanthin (90% trans, 10% cis). Therefore, clear insight into in vivo conversion was possible. Retention time data, spectral analysis and chiral-phase HPLC with HR-MS detection were employed to identify metabolites specific to lutein and zeaxanthin, focussing on 3′-dehydro-lutein.
One important finding of this study was the presence of 3′-dehydro-lutein in both lutein-fed and zeaxanthin-fed monkey serum. The conversion of 3′-dehydro-lutein from lutein was ~6%. Thurman et al. have also shown this to occur in humans (Thurmann et al., 2005). However, our study is unique in that this conversion was measured without the interference of 3′-dehydro-lutein possibly formed from zeaxanthin (Hartmann et al., 2004).
3′-Dehydro-lutein has been reported to be present in plasma and tissue of different species, including fish (Matsuno et al., 1986b), iguanas Costantini et al., 2005), birds (Matsuno et al., 1986a; McGraw and Schuetz, 2004), monkeys (Khachik et al., 1997) and humans (Khachik et al., 1992). Despite its possible importance for metabolomic investigations, it seems that the absolute stereochemistry of this metabolite has received thus far only little attention. Only in few cases the stereochemistry was directly analysed, e.g., in egg yolk. Here, 3′-dehydro-lutein was shown to be either present as a scalemic mixture (Matsuno et al., 1986a) or as a single stereoisomer (Schiedt, 1998), depending on whether the feed of the animals contained both lutein and zeaxanthin or only zeaxanthin. Related investigations in human or monkey plasma seemingly have never been conducted. In most related literature either no information on the absolute stereochemistry is given, or an R, R-configuration is claimed which, however, appears to be merely deduced by analogy from theoretically possible precursor carotenoids. One reason for this lack of data might be the difficulty to obtain (3R,6′S)-3′-dehydro-lutein as reference compound. In contrast to the (3R,6′R)-isomer which is well characterised and can easily be obtained by oxidation of lutein (Molnar et al., 2006), the synthesis of this diastereomer is difficult. Therefore, we choose it to be formed by biological transformation of zeaxanthin by laying hens (Höller et al., 2005; Schiedt, 1998). Another practical problem is the separation of the two isomers. Although the two compounds are diastereomers, their separation requires chiral-phase HPLC which can hardly be applied for routine analysis of carotenoids in plasma samples due to its complexity and relatively low sensitivity. Having both diastereomers as reference material and a chiral-phase HPLC made current investigations possible.
The ratio of the diastereomers in the rhesus monkey plasma was found to be approx. 1:1 (Table 2). Although only a low number of samples could be investigated, our results suggest that this is independent on whether lutein or zeaxanthin is supplemented. This outcome was not expected, but is qualitatively comparable to results reported from analysis of human plasma samples which where obtained after supplementation with lutein, zeaxanthin or a combination of both (Höller et al., 2005). In analogy to the metabolism in chicken, a stereospecific conversion would have been expected to occur in rhesus monkeys and humans. Formation of 3′-dehydro-lutein from e.g. lutein was believed to occur by simple oxidation at C-3′, thereby retaining the configuration at C-6′. Possible pathways in agreement with our experimental results are depicted in Figure 2. The two 3′-dehydro-lutein diastereomers with opposite configuration at C-6′ could be formed via a common precursor derived from lutein or zeaxanthin (straight lines). However, this postulated common precursor (3-dehydro-zeaxanthin) has thus far not been described in literature. It might not be stable enough to be observed in plasma, and might be directly transformed into 3′-dehydro-lutein. In general, carotenoids with a β-3-one end group are very rare. While this mechanism seems reasonable for zeaxanthin, the corresponding conversion from lutein would require the additional migration of a double bond. Therefore, we propose the following pathway: an oxidation of lutein and zeaxanthin occurs at C-3′ and is followed by a double bond migration and epimerisation at C-6′ (dotted lines). Such an epimerisation is an uncommon reaction for carotenoids, and further work is necessary to clarify these pathways. Supplementation studies with synthetic (3R,6′R)-3′-dehydro-lutein could possibly demonstrate this epimerisation in vivo whereas the use of carbon-13 labelled lutein and zeaxanthin would be helpful to confirm the complete pathway.
In lutein- and zeaxanthin-fed monkeys, high amounts of cis-isomers were found in serum (20 % in lutein-fed, 36 % in zeaxanthin-fed) compared to the isomer ratio in the supplements. Given that there were no cis-isomers present in the lutein supplement and only 10 % in the zeaxanthin, this gives evidence for in vivo conversion. The high amount of cis-isomers in monkey serum can partly result from storage and processing. Samples were stored at −70°C and protected under nitrogen, but light-induced isomerisation cannot totally be excluded. However, the extraction procedure was performed under red light. Recent unpublished data showed that neither UV-light nor moderate heat induced isomerisation could significantly affect the isomer ratio in lutein, extracted from Zea maize which supports the idea of in vivo conversion instead of preparative artefacts. These results are similar to findings in two other monkey species where the 13-cis-isomers of lutein and zeaxanthin were found to be enriched relatively to the all-trans isomer comparing the ratios in plasma versus the ratios in the diet (Snodderly et al., 1990).
The major role of lutein and zeaxanthin in the eye is the protection of the macular against blue light induced oxidative damage. In the retina, all-trans-isomers are prominent, whereas cis-isomers are present in minute amounts only. The latter are unable to span the bilipid membrane because of their nonlinear geometry (Subczynski et al., 1991) and therefore they can only exert a limited protective function, if any.
In conclusion, we show that 3′-dehydro-lutein is a common metabolite of both, lutein and zeaxanthin in serum of rhesus monkeys. Additionally, our results suggest that two of its diastereomers, (3R,6′S)- and (3R,6′R)-3′-dehydro-lutein, are present in nearly equimolar concentrations.
Supplementary Material
Acknowledgments
The authors thank Noelle Landauer for care of experimental animals, preparation and feeding of supplements and assistance with blood sampling; Guangwen Tang, for assistance with mass spectrometry; Norman Krinsky for discussions regarding xanthophyll metabolism; John Landrum for supplying synthetic (3R,6′R)-3′-dehydro-lutein.
Supported by USDA 581950-9-001, NIH grants DK29930 and RR00163, The Foundation Fighting Blindness, and DSM Nutrition, Ltd. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the USDA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bone RA, Landrum JT, Friedes L, Gomez CM, Kilburn MD, Menendez E, Vidal I, Wang W. Distribution of lutein and zeaxanthin steroisomers in the human retina. Exp Eye Res. 1997;64:211–218. doi: 10.1006/exer.1996.0210. [DOI] [PubMed] [Google Scholar]
- Bone RA, Landrum JT, Hime GW, Cains A, Zamor J. Stereochemistry of the human macular carotenoids. Invest Ophthalmol Vis Sci. 1993;34:2033–2040. [PubMed] [Google Scholar]
- Bone RA, Landrum JT, Mayne ST, Gomez CM, Tibor SE, Twaroska EE. Macular pigment in donor eyes with and without AMD: a case-control study. Invest Ophthalmol Vis Sci. 2001;42:235–240. [PubMed] [Google Scholar]
- Bone RA, Landrum JT, Tarsis SE. Preliminary identification of the human macular pigment. Vision Res. 1985;25:1531–1535. doi: 10.1016/0042-6989(85)90123-3. [DOI] [PubMed] [Google Scholar]
- Bone RA, Landrum JT. Heterochromatic flicker photometry. Arch Biochem Biophys. 2004;430:137–142. doi: 10.1016/j.abb.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Costantini D, Dell’Omo G, Casagrande S, Fabiana A, Carosi M, Bertacche V, Marquez C, Snell H, Snell H, Tapia W, Gentile G. Inter-population variation of carotenoids in Galapagos land iguanas (Conolophus subcristatus) Comp Biochem Physiol B. 2005;142:239–244. doi: 10.1016/j.cbpb.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Eye Disease Case-Control Study Group (EDCCSG) Antioxidant status and neovascular age-related macular degeneration. Arch Ophthalmol. 1993;111:104–109. doi: 10.1001/archopht.1993.01090010108035. [DOI] [PubMed] [Google Scholar]
- Gale CR, Hall NF, Phillips DIW, Martyn CN. Lutein and zeaxanthin status and risk of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2003;44:2461–2465. doi: 10.1167/iovs.02-0929. [DOI] [PubMed] [Google Scholar]
- Goralczyk R, Buser S, Bausch J, Bee W, Zuhlke U, Barker FM. Occurrence of birefringent retinal inclusions in cynomolgus monkeys after high doses of canthaxanthin. Invest Ophthalmol Vis Sci. 1997;38:741–752. [PubMed] [Google Scholar]
- Handelman GJ, Snodderly DM, Adler AJ, Russett MD, Dratz EA. Measurement of carotenoids in human and monkey retinas. Meths Enzymol. 1992;213:220–230. doi: 10.1016/0076-6879(92)13123-f. [DOI] [PubMed] [Google Scholar]
- Hartmann D, Thurmann PA, Spitzer V, Schalch W, Manner B, Cohn W. Plasma kinetics of zeaxanthin and 3′dehydro-lutein after multiple oral doses of synthetic zeaxanthin. Am J Clin Nutr. 2004;79:410–417. doi: 10.1093/ajcn/79.3.410. [DOI] [PubMed] [Google Scholar]
- Höller U, Schierle J, Schalch W. Stereochemical investigations of 3′-dehydrolutein in human plasma samples obtained during supplementation with lutein and zeaxanthin for up to six months during the LUXEA-study. Poster presented at 14th International Carotenoid Sympsoium; Edinburgh. 2005. [Google Scholar]
- Johnson EJ, Neuringer M, Russell RM, Schalch W, Snodderly DM. Nutritional manipulation of primate retinas. III Effects of lutein or zeaxanthin supplementation on adipose and retina of xanthophylls-free monkeys. Invest Ophthalmol Vis Sci. 2005;46:692–702. doi: 10.1167/iovs.02-1192. [DOI] [PubMed] [Google Scholar]
- Khachik F, Beecher GR, Goli MB. Separation and identification of carotenoids and their oxidation products in the extracts of human plasma. Anal Chem. 1992;64:2111–2122. doi: 10.1021/ac00042a016. [DOI] [PubMed] [Google Scholar]
- Khachik F, Bernstein PS, Garland DL. Identification of lutein and zeaxanthin oxidation products in human and monkey retinas. Invest Ophthalmol Vis Sci. 1997;38:1802–1811. [PubMed] [Google Scholar]
- Khachik F, de Moura FF, Zhao DY, Aebischer CP, Bernstein PS. Transformations of selected carotenoids in plasma, liver, and ocular tissues of humans and in nonprimate animal models. Invest Ophthalmol Vis Sci. 2002;43:3383–3392. [PubMed] [Google Scholar]
- Krinsky NI, Landrum JT, Bone RA. Biologic mechanism of the protective role of lutein and zeaxanthin in the eye. Ann Rev Nutr. 2003;23:171–201. doi: 10.1146/annurev.nutr.23.011702.073307. [DOI] [PubMed] [Google Scholar]
- Landrum JT, Bone RA, Kilburn MD. The macular pigment: A possible role in protection from age-related macular degeneration. Adv, Pharmacol. 1997;38:537–556. doi: 10.1016/s1054-3589(08)60998-9. [DOI] [PubMed] [Google Scholar]
- Matsuno T, Hirono T, Ikuno Y, Maoka T, Shimizu M, Komori T. Isolation of three new carotenoids and proposed metabolic pathways of carotenoids in hen’s egg yolk. Comp Biochem Physiol B. 1986a;84:477–481. doi: 10.1016/0305-0491(86)90224-5. [DOI] [PubMed] [Google Scholar]
- Matsuno T, Maoka T, Ikuno Y. Comparative biochemical studies of carotenoids in fish-XXVII. Carotenoids in the eggs of three species of Cyprinidae. Comp Biochem Physiol B. 1986b;83:335–337. doi: 10.1016/0305-0491(86)90224-5. [DOI] [PubMed] [Google Scholar]
- McGraw KJ, Schuetz JG. The evolution of carotenoid coloration in estrilid finches: a biochemical analysis. Comp Biochem Physiol B. 2004;139:45–51. doi: 10.1016/j.cbpc.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Molnar P, Deli J, Osz E, Toth G, Zsila F, Herrero C, Landrum JT. Preparation and spectroscopic characterisation of 3′-oxolutein. Lett Org Chem. 2006;3:723–734. [Google Scholar]
- Neuringer M, Sandstrom MM, Johnson EJ, Snodderly DM. Nutritional manipulation of primate retinas: I. Effects of lutein or zeaxanthin supplements of serum and macuarlar pigment of xanthophyll-free monkeys. Invest Ophthalmol Vis Sci. 2004;45:3234–3243. doi: 10.1167/iovs.02-1243. [DOI] [PubMed] [Google Scholar]
- Putzbach K, Krucker M, Albert K, Grusak MA, Tang G, Dolnikowski GG. Structure determination of partially deuterated carotenoids from intrinsically labeled vegetables by HPLC-MS and 1H NMR. J Agric Food Chem. 2005;53:671–677. doi: 10.1021/jf0487506. [DOI] [PubMed] [Google Scholar]
- Schiedt K. In: Carotenoids, vol. 3: Biosynthesis and Metabolism. Britton G, Liaaen-Jensen S, Pfander H, editors. Birkhäuser; Basel: 1998. pp. 285–358. [Google Scholar]
- Seddon JM, Ajani UA, Sperduto RD, Hiller R, Blair N, Burton TC, Farber MD, Gragoudas ES, Haller J, et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye disease case-control study group. JAMA. 1994;272:1413–1420. [PubMed] [Google Scholar]
- Snodderly DM, Russett MD, Land RI, Krinsky NI. Plasma carotenoids of monkeys (Macaca fascicularis and Saimiri sciureus) fed a nonpurified diet. J Nutr. 1990;120:1663–1671. doi: 10.1093/jn/120.12.1663. [DOI] [PubMed] [Google Scholar]
- Subczynski WK, Markowska E, Sielewiesiuk J. Effect of polar carotenoids on the oxygen diffusion-concentration product in lipid bilayers. An EPR spin label study. Biochim Biophys Acta Biomem. 1991;1068:68–72. doi: 10.1016/0005-2736(91)90061-c. [DOI] [PubMed] [Google Scholar]
- Thurmann PA, Schalch W, Aebischer JC, Tenter U, Cohn W. Plasma kinetics of lutein, zeaxanthin, and 3′dehydro-lutein after multiple oral doses of a lutein supplement. Am J Clin Nutr. 2005;82:88–97. doi: 10.1093/ajcn.82.1.88. [DOI] [PubMed] [Google Scholar]
- Yeum KJ, Booth SL, Sadowski JA, Liu C, Tang G, Krinsky NI, Russell RM. Human plasma carotenoid response to the ingestion of controlled diets high in fruits and vegetables. Am J Clin Nutr. 1996;64:594–602. doi: 10.1093/ajcn/64.4.594. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.