Abstract
Background
Treatment of perimitral flutter (PMF) requires bidirectional mitral isthmus (MI) block, which can be difficult with radiofrequency ablation (RFA). The vein of Marshall (VOM) is located within the MI.
Objective
To test whether VOM ethanol infusion could help achieve MI block.
Methods
Perimitral conduction was studied in patients undergoing ablation of atrial fibrillation (AF). Group 1 included 50 patients with a previous AF ablation undergoing repeat ablation, 30 of which had had MI ablation. Spontaneous (8/50) or inducible PMF (21/50) was confirmed by activation mapping. Group 2 included 21 patients undergoing de novo VOM ethanol infusion. The VOM was cannulated with a quadripolar catheter for pacing and with an angioplasty balloon to deliver up to four 1mL infusions of 98% ethanol. Voltage maps were created before and after VOM ethanol. Bidirectional MI block was verified by differential pacing. RFA times required to achieve it were assessed.
Results
In Group 1, VOM ethanol infusion acutely terminated PMF in 5/29 patients. RFA needed to achieve bidirectional MI block was 2.2±1.6 min. Presence of PMF or previous MI ablation did not affect RFA times. In Group 2, RFA needed to achieve bidirectional MI block was 2.0±1.6 min (p=NS). Five patients had bidirectional MI block achieved solely by VOM ethanol without RFA. In both groups, ablation after VOM ethanol was required in the annular aspect of the MI. There were no acute complications.
Conclusion
VOM ethanol infusion is useful in the treatment of PMF and assists in reliably achieving bidirectional MI block.
Introduction
Clinical failures of catheter ablation of atrial fibrillation (AF) can be caused by atrial flutters,1-3 rather than recurrent AF. Macro-reentrant tachycardia involving the mitral annulus, -perimitral flutter (PMF)- causes to 33-60% of such flutters.1, 3-5 Catheter ablation of PMF involves, most commonly, the creation of a linear lesion from the mitral annulus to the left inferior pulmonary vein (LIPV), in the so-called mitral isthmus (MI).6, 7 Achieving a complete ablation (defined by bidirectional conduction block, across the MI ablation line) is critical to obtain enduring success, but can be technically difficult,7-9 with success rates that vary in different studies (eg, reported as 32%,10 64%,11 or 71%12), but that are consistently suboptimal. Incomplete ablation of the MI is proarrhythmogenic,13, 14 increasing the risk of recurrent flutter by up to 4 times.13 Thus, adjunctive approaches, such as radiofrequency ablation (RFA) inside the coronary sinus,15, 16 (CS, in close proximity to the circumflex coronary artery), placing the RFA lesions anteriorly,10 or occluding CS blood flow17 have been proposed.
The vein of Marshall (VOM) is located in the epicardial aspect of the MI. It has been identified as a potential obstacle for ablation of the MI, due this close anatomical relationship.18 We have developed a technique for retrograde VOM ethanol infusion and have shown its feasibility and ablative effects in left atrial tissue.19, 20 Here we hypothesized that: 1) Atrial tissue around the VOM participates in PMF; 2) VOM ethanol infusion can have therapeutic value in creating conduction block across the MI.
Methods
Patient population
PMF most commonly occurs after a previous AF ablation procedure, which may include ablation in the MI that could confound the effects of VOM ethanol infusion. To eliminate previous ablation as a possible confounding factor, we tested the effect of VOM ethanol infusion in patients undergoing MI ablation with and without a previous procedure.
Patient Group 1
We studied perimitral conduction in 50 patients with a previous AF ablation presenting for a repeat procedure due to recurrent AF or flutter. Out of 61 patients consented, the VOM was cannulated in 54 and the protocol was completed in 50.
Patient Group 2
A group of 21 patients undergoing a first ablation procedure for symptomatic, drug-refractory AF was studied. Out of 24 consented patients, the protocol was completed in 21.
Reasons for failed VOM cannulation included: absent, small, or tortuous VOM.
Procedural strategy
Patients provided informed consent and the protocol was approved by the local IRB, overseen by the FDA (IND # 105083), and an external Data Safety Monitoring Board. Under general anesthesia, a quadripolar catheter was positioned in the His bundle, and a decapolar catheter in the CS via a femoral vein. The VOM was cannulated as previously described,19, 20 using a sheath designed for left ventricular pacing lead delivery that was inserted in the CS via the right internal jugular vein (Figure 1A). A sub-selector catheter (LIMA angiographic guide) was inserted through the sheath and manipulated so that its tip faced posteriorly and superiorly. Angiographic contrast was injected and the VOM was identified as an atrial branch of the CS that arose at the level of the valve of Vieussens and that was directed posteriorly. A quadripolar catheter (1.7F Pathfinder Mini®, Cardima, or 4F IBI, St Jude Medical) was inserted in the VOM. A circular duodecapolar catheter was inserted in the left atrium and used to pace from the left atrial appendage (LAA). Heparin was administered to maintain the Activated Clotting Time (ACT) between 350 and 400 seconds throughout the procedure.
Figure 1.
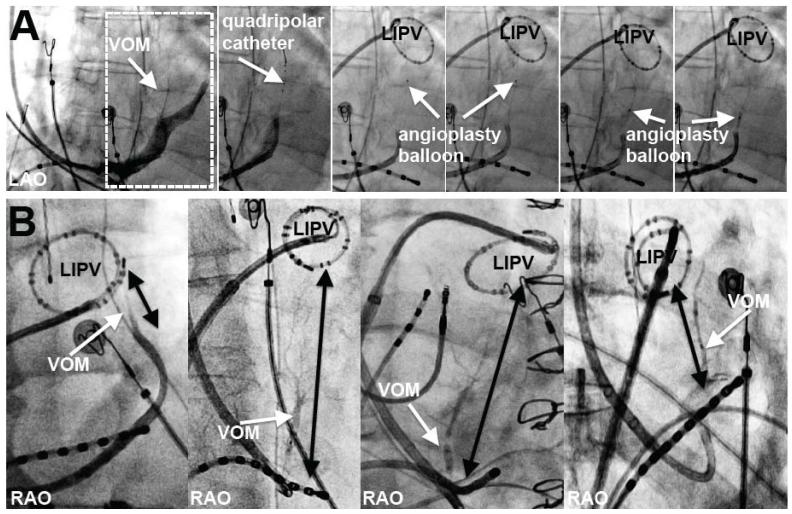
VOM cannulation technique and VOM relationship with the MI. A, Procedural steps: from left to right: initial CS venogram (dotted rectangle shows VOM and area in subsequent panels), VOM with a quadripolar catheter, and an angioplasty balloon at different levels of the VOM where ethanol is delivered. B, Anatomical variability of the MI length (black arrows from left inferior pulmonary vein, LIPV, to the CS) in selective VOM venograms. Right (RAO) or left (LAO) anterior oblique views as indicated.
If the presenting rhythm was AF, cardioversion was performed. Three-dimensional maps of the left atrial geometry and bipolar voltage amplitude were performed using the NavX system (St Jude Medical, Minneapolis, MN) in either flutter or sinus rhythm. Atrial flutter, if spontaneously present, was mapped and entrained from proximal and distal CS and VOM. PMF was diagnosed when the entire reentrant circuit (cycle length) was mapped around the mitral annulus, and entrainment (at threshold output) from the proximal and distal CS led to post-pacing intervals within 30 ms of the cycle length. If in sinus rhythm, attempts to induce flutter were performed by burst pacing down to 200 ms.
VOM ethanol infusion procedure
The VOM quadripolar catheter was removed and an angioplasty wire (BMW, Abbott, Abbott Park, Illinois) was advanced into the VOM. An angioplasty balloon (8 mm length, 2 mm diameter, Voyager OTW, Abbott or 6 mm length, 1.5 mm diameter, Medtronic, Minneapolis, MN) was advanced over the wire as distally as possible. Depending on the length of the VOM, up to four balloon occlusive injections of 98% ethanol (1 cc over 2 minutes) were delivered. Starting in the most distal VOM, the balloon was slightly retracted sequentially after each injection, so that the last injection was given from the most proximal portion of the VOM. After ethanol infusion, a repeat voltage map was performed to delineate the ethanol-induced low-voltage area. Scar was defined as bipolar voltage amplitude <0.1 mV.
Pacing from the LAA and the CS (on either side of the ethanol-induced scar) was used to assess perimitral conduction.
The procedure continued with RFA using a Thermocool® catheter (Biosense-Webster, Diamond Bar, CA) navigated with the Artisan® robotic sheath (Hansen Medical, Mountain View, CA). RFA was performed initially targeting the MI at the ethanol-induced scar, at areas of the endocardial scar where signals were still present, delivering a power of 25-35 W with 17-30 cc/min of saline irrigation, during either PMF or while performing LAA pacing. RFA times required to achieve bidirectional MI block were measured. Block was confirmed with differential pacing, and reconfirmed after a 30-minute wait. Burst pacing was performed to re-induce PMF after block was achieved. RFA was then continued as needed in each case to perform de-novo PV isolation (Group 2) or re-isolate the PVs (Group 1), ablate complex fractionated potentials, or other atrial flutters if present. Ethanol levels were measured in mixed venous blood at the end of the procedure.
Data analysis
Fluoroscopic data were analyzed off line using Osirix Software (Pixmeo, Geneva). Data are presented as mean ± standard deviation. Quantitative variables were compared using Student’s t test. Proportions were compared using χ2 or Fisher’s exact test when applicable. A p value < 0.05 was considered significant.
Results
Patient characteristics
The Table summarizes patient characteristics and procedural parameters. Group 1 patients had a greater likelihood of having persistent AF, and had a modestly lower left ventricular ejection fraction. A prior MI ablation line had been performed in the index procedure in 30/50 patients. Group 2 patients had most commonly paroxysmal AF, and none had atrial flutter clinically. In Group 1 patients, PMF was spontaneously present in 8/50 patients and was induced by burst pacing from the VOM, LAA, or distal CS in an additional 21/50 patients. Thus, a total of 29 patients with confirmed PMF were studied. In one patient, repeated flutter inductions led to clockwise and counterclockwise PMF. Overall, of 30 PMF induced, 17 were counterclockwise and 13 were clockwise. The PMF cycle length was 289±61 ms.
VOM, the mitral isthmus and PMF
Although the origin of the VOM relative to the CS ostium was variable, the VOM ran consistently towards the left inferior PV (LIPV) in the regions considered as the MI.7 The length of the MI, as measured from the CS at the VOM ostium to the LIPV was variable (2.9±0.8 cm, range 1.1-4.8 cm). The VOM length measured 3.5±1.4cm (p=0.04, compared with the MI length). The VOM its branches spanned the entire length of the MI or beyond (VOM longer than the MI) in 40/71 patients. Figure 1B shows examples of different MI widths with the VOM visualized.
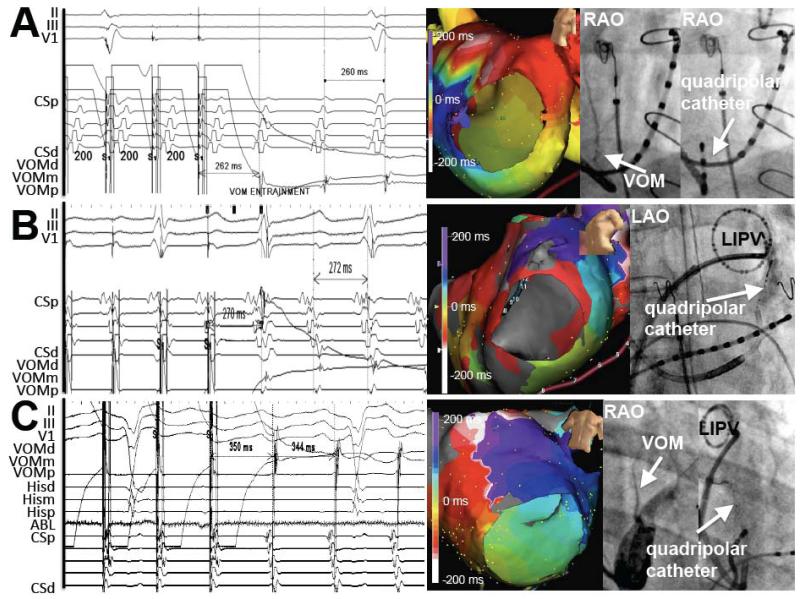
Entrainment from the VOM led to postpacing intervals within 30 ms of the tachycardia cycle length in 22 patients (in the remainder, flutter terminated or changed activation sequence). Figure 2AB shows examples. In 4 of these patients, entrainment was overt, with changes in the CS activation sequence during VOM pacing, likely reflecting local propagation in the distal CS of the paced stimulus within the reentrant circuit. Figure 2C shows an example.
Figure 2.
PMF response to entrainment from the VOM. Tracings are accompanied by 3D maps and right anterior oblique views of the VOM venogram and VOM quadripolar catheter location. Post-pacing intervals match the tachycardia cycle length. A, Concealed entrainment from the proximal VOM in a clockwise PMF. B, Concealed entrainment of counterclockwise PMF. C, Overt entrainment of counterclockwise PMF.
Previous MI line and PMF
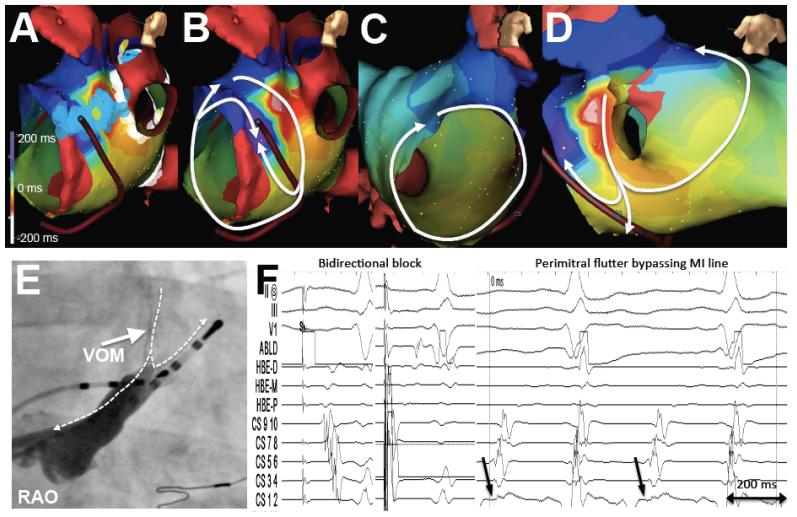
In 30/50 patients of Group 1, an MI ablation line had been performed at the index procedure. In 14 of them, bidirectional MI block had been documented. At the repeat procedure, all 30 patients had recurrent conduction across the MI, and PMF was present in 4 of these patients. In one of them, the VOM could have provided a potential mechanism as an epicardial conduction pathway bypassing the previous endocardial ablation line in the MI. Figure 3 shows the three dimensional maps of a clockwise PMF in which the earliest CS activation arises in the mid-to-proximal CS. This occurred in the setting of a previous MI ablation with apparent bidirectional MI block. The VOM joins the CS at the location of the earliest CS activation. Although a posterior gap in the MI line could have led to a conducting pathway, this would not explain the earliest activation site in the mid CS. A second reentrant loop around the left PVs is present, forming a figure-eight pattern using the using the left atrial ridge as the common limb. VOM ethanol led to bidirectional MI block and eliminated PMF inducibility.
Figure 3.
PMF occurring after apparent bidirectional MI block. A, Lesion location in the MI obtained by RFA. B and C, Activation maps of flutter showing a clockwise rotation around the mitral annulus that, as it reaches the location of the MI ablation lesions, blocks in the anterior aspect of the MI. Conduction proceeds in the posterior aspect of the MI, but does not propagate anteriorly; instead, it continues posteriorly to reach the mitral annulus (CS) far past the MI ablation line, at a location that coincides with the VOM origin. D, Bystander loop around the left pulmonary veins. E, CS venogram showing the VOM insertion close to the earliest atrial activation in the CS catheter. Dashed arrows mark the activation sequence. F, Intracardiac tracings. Pacing from the ablation catheter (ABLD) above the RFA line (left panel) shows earliest activation in the CS ostium. Pacing from below the RFA line (mid panel) shows delay reaching the ABLD catheter, proving bidirectional MI block. Right panel shows the activation sequence during PMF, with the mid-CS (arrows, site of VOM insertion) activating prior to the distal CS, suggesting that the VOM could act as a conduction pathway bypassing the RFA line.
VOM Ethanol infusion: MI Scar, and effect on perimitral conduction and PMF
Ethanol led to a low-voltage area in the LA, which was localized within the MI. Depending on VOM location (i.e. distance from the CS ostium to the VOM origin), size and branching patterns, the scar encompassed variable portions of the MI. Scar area measured 8.8±4.5 cm2 in Group 1 patients vs 7.7±3.2 cm2 in Group 2 patients (p=0.43). Figures 4 and 5 show examples.
Figure 4.
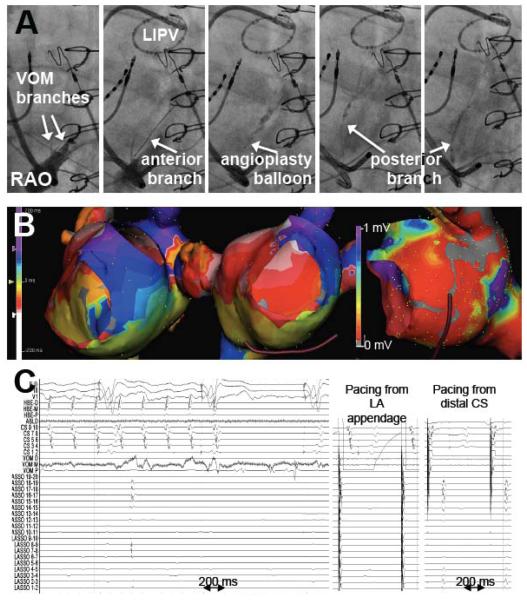
Left inferior pulmonary vein isolation (LIPV), PMF termination and bidirectional MI block with VOM ethanol. Top row, VOM venograms showing successive cannulation of anterior and posterior branches with the angioplasty balloon, and the location of the LIPV as marked by the circular catheter. Mid row, isochronal maps of the left atrium showing counterclockwise PMF (left panel), and voltage amplitude map showing the low-voltage area created by ethanol infusion. Bottom row, electrograms during ethanol infusion showing LIPV isolation with a dissociated beat and flutter termination (left panel). Mid and right panels show differential pacing across the MI, proving bidirectional block.
Figure 5.
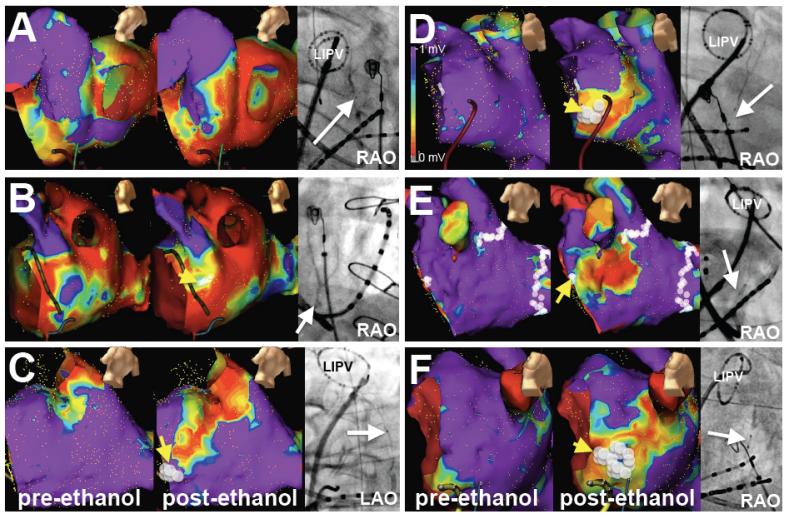
VOM anatomy and low-voltage scar creation in the MI. A-C, Bipolar voltage maps in patients with previous AF catheter ablation (Group 1). Left panels show voltage maps at baseline, prior to ethanol injection. Mid panels show maps after ethanol. Right panels show RAO projections of selective VOM venograms with a circular duodecapolar catheter in the left inferior pulmonary vein (LIPV). A and B had a previous ablation in the MI, C did not. D-F, Voltage maps and VOM venograms in patients without any prior ablation (Group 2). White dots in the MI indicate the areas that required RFA to achieve isthmus block (yellow arrows, not required in A). Bipolar voltage scale as in D.
VOM ethanol infusion alone acutely terminated PMF in 5 patients (out of the 29 patients with documented PMF, ethanol was administered during PMF in 19). Figure 4 shows an example. Large, multi-branched VOMs were present in these patients, in whom large scar surface areas were mapped after VOM. VOM ethanol infusion led to PMF termination by itself only in cases with previous MI ablation. In others, ethanol did not terminate PMF, and did not significantly alter its cycle length.
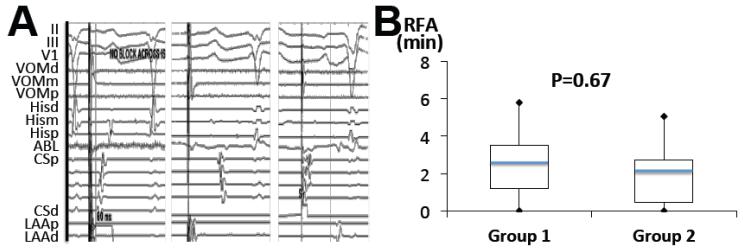
In 4/50 patients in Group 1, and 1/21 patients in Group 2, bidirectional MI block was achieved solely by VOM ethanol infusion. These were 5 patients with large ethanol-induced scar areas, and in 4/5 a previous MI line had been performed at the index procedure (Figure 4 and 5A). Most commonly, however, a viable portion of the MI remained at its most annular portion. Thus, RFA was usually required in order to achieve bidirectional MI block. We directed RFA applications to the remaining viable areas of the MI. Most of RFA needed was at the most anterior, annular aspect of the MI (anterior to the low-voltage area created by ethanol). Minimal additional endocardial RFA was needed to achieve bidirectional MI block block in both patient groups (2.2±1.6 min in Group 1 vs 2.1±1.6 in Group 2, p=0.67). Figure 5 shows examples of the RFA ablation sites required to achieve bidirectional MI block. Figure 6 shows an example of perimitral conduction after ethanol and after RFA, and the RFA times required to obtain bidirectional MI block in both groups. The differences in RFA time required to achieve block in patients with and without a previous MI ablation were not significant (2.0±1.5 min in patients without a previous MI line vs. 2.5±1.7 min in patients with a previous MI ablation). RFA was not needed in the CS except in 1 patient in Group 1. At the end of the procedure, bidirectional MI block was achieved in all 71 patients (100%) and PMF was not inducible in any of them.
Figure 6.
MI conduction after VOM ethanol infusion and block after ablation, and RFA times required to achieve bidirectional MI block. A, In most cases, pacing from LAA after VOM ethanol had intact conduction through the MI (left panel, propagation first in the distal CS, CSd). After supplemental RFA, propagation is first in the proximal CS (CSp, mid panel). Bidirectional MI block is proven by pacing from CSd with a long delay to reach the LAA (right panel). B, Box plots (minimum, quartiles, maximum, -blue line is mean) of RFA times, in patients with (Group 1) and without previous ablation (Group 2).
Patient follow-up
There were no complications directly attributable to VOM instrumentation or ethanol infusion. Ethanol levels measured in mixed venous blood at the end of the procedure were undetectable in all patients. Two patients presented with subacute hemopericardium 2 and 4 weeks after the procedure, successfully drained without sequelae. At a follow-up of 16.2±6.2 months (range 2-36), in Group 1, 7 patients had recurrent AF and 7 patients had recurrent flutter or atrial tachycardia. One of them had recurrent PMF due to recurrent conduction across the MI at the anterior site ablated with RFA. Repeat RFA (21 seconds) restored block. Other tachycardias were roof-dependent flutter (2), LAA atrial tachycardia (2), and right atrial flutter (2). In Group 2, 6/21 patients have had recurrent atrial arrhythmias (all recurrent AF, due to reconnected PV demonstrated on repeat procedures), all of which had persistent bidirectional MI block.
Discussion
This study investigated the role of VOM ethanol infusion during MI ablation. The main findings of our study are: 1) The VOM and neighboring tissues are within the PMF circuit; 2) VOM ethanol infusion assists in achieving bidirectional MI block consistently (100% when VOM cannulation is technically feasible) and with minimal RFA times, and it does so in patients with and without prior left atrial ablation. In prior studies, success rates in achieving bidirectional MI block with RFA have been reported to be between 32-92%, and reported ablation times required in these studies varies around 15±8 minutes, with ablation within the CS needed in 54-91%.7, 9, 11, 15, 18, 21-24 Strategies to optimize thermal RFA-induced injury in the MI, specifically to overcome the heat loss due to CS blood flow have been proposed, such as intra-CS RFA or balloon occlusion of the distal CS.25 Chemical ablation from the VOM effectively targets MI myocardium from the epicardial aspect. Our acute success rate (100%), total ablation time, and minimal (1 in 71 patients) requirement of CS ablation to achieve bidirectional MI block compare very favorably to these previously reported results.
Anatomical features such as myocardial thickness and presence of pouches,26, 27 isthmus length and relationship to the circumflex artery,11 and high takeoff of the LIPV23 have been described as possible obstacles to MI ablation. Other factors such as local coronary flow28 and acute edema in response to RFA are also associated with failure to achieve MI block. Lastly, an epicardial CS connection providing an epicardial bridge29 (localized in ligament of Marshall area30) could circumvent the MI and lead to recurrent PMF or failure to achieve MI block with endocardial ablation. Figure 3 shows an example compatible with this hypothesis. All of these obstacles can be overcome by VOM ethanol infusion.
Although the VOM is consistently located in the MI, most of the time ethanol-induced scar did not span the entire MI and thus did not lead to bidirectional MI block by itself. The location of the scar helps understand the need for RFA in the most anterior aspect of the MI (Figure 5). Since the VOM arises from the posterior aspect of the CS, and 6-8 mm of angioplasty balloon are inserted in the VOM for selective injection, it is clear that a substantial portion of the MI lies anterior to the ethanol injection site.
Chemical ablation via arterial ethanol infusion has been used to ablate cardiac arrhythmias, specifically for ventricular tachycardia,31, 32 and for the AV junction.33 Our prior experience with venous chemical ablation shows that VOM ethanol infusion is feasible and safe in humans. We have shown it decreases RFA time in the left inferior PV, and may have a role as an adjunct to PVAI.20 Our data support its clinical utility for PMF ablation.
Limitations
Our study is non-randomized. Patients with PMF had had a previous ablation, including MI ablation in 30/50, which could have decreased the need for RFA. The inclusion of patients without any RFA (group 2) should correct this limitation.
Conclusions
VOM ethanol infusion creates a scar across the MI that minimizes RFA needed to achieve bidirectional MI block with consistent success.
Table.
Clinical characteristics and procedural parameters.
| Group 1 (n=50) |
Group 2 (n=21) |
pValue | |
|---|---|---|---|
| Age | 64±9 | 63±8 | 0.78 |
| Female Gender (n,%) | 13 (26%) | 7 (33%) | 0.77 |
| Paroxysmal AF (n,%) | 22 (44%) | 18 (86%) | <0.001 |
| Left ventricular EF (%) | 56±11 | 64±8 | <0.01 |
| Left atrial volume by MRI or CT (cc) | 110±42 | 108±29 | 0.83 |
| Previous MI line (n,%) | 30 (60%) | 0 (0%) | <0.001 |
| PMF present (n,%) | 8 (16%) | 0 (0%) | 0.09 |
| PMF induced (n,%) | 21 (42%) | 0 (0%) | <0.001 |
| Ethanol-induced scar (cm2) | 8.8±4.5 | 7.7±3.2 | 0.43 |
| RF time (min) | 2.2±1.7 | 2.0±1.6 | 0.67 |
| Bidirectional block (n,%) | 50/50 | w 21/21 | 1 |
| VOM procedure time (min) | 56±17 | 58±14 | 0.65 |
| VOM fluoroscopy time (min) | 8.5±4.6 | 8.6±4.5 | 0.9 |
| Total procedure time (min) | 217±70 | 203±52 | 0.43 |
| Total fluoroscopy time (min) | 26±13.1 | 17.5±5.5 | 0.01 |
| Recurrent AF (n,%) | 7 (14%) | 5 (23%) | <0.001 |
| Recurrent flutter (n,%) | 7 (14%) | 0 (0%) | <0.001 |
Acknowledgments
Financial Support: NIH/NHLBI 1R21HL097305, The Methodist Hospital Research Institute
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COI Disclosure: None relevant
REFERENCES
- 1.Deisenhofer I, Estner H, Zrenner B, et al. Left atrial tachycardia after circumferential pulmonary vein ablation for atrial fibrillation: incidence, electrophysiological characteristics, and results of radiofrequency ablation. Europace. 2006;8:573–582. doi: 10.1093/europace/eul077. [DOI] [PubMed] [Google Scholar]
- 2.Haissaguerre M, Hocini M, Sanders P, et al. Catheter ablation of long-lasting persistent atrial fibrillation: clinical outcome and mechanisms of subsequent arrhythmias. J Cardiovasc Electrophysiol. 2005;16:1138–1147. doi: 10.1111/j.1540-8167.2005.00308.x. [DOI] [PubMed] [Google Scholar]
- 3.Chugh A, Oral H, Lemola K, et al. Prevalence, mechanisms, and clinical significance of macroreentrant atrial tachycardia during and following left atrial ablation for atrial fibrillation. Heart Rhythm. 2005;2:464–471. doi: 10.1016/j.hrthm.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 4.Ammar S, Hessling G, Reents T, et al. Arrhythmia type after persistent atrial Fibrillation Ablation Predicts Success of the Repeat Procedure. Circ Arrhythm Electrophysiol. 2011 doi: 10.1161/CIRCEP.111.963256. [DOI] [PubMed] [Google Scholar]
- 5.Chae S, Oral H, Good E, et al. Atrial tachycardia after circumferential pulmonary vein ablation of atrial fibrillation: mechanistic insights, results of catheter ablation, and risk factors for recurrence. J Am Coll Cardiol. 2007;50:1781–1787. doi: 10.1016/j.jacc.2007.07.044. [DOI] [PubMed] [Google Scholar]
- 6.Pappone C, Manguso F, Vicedomini G, et al. Prevention of iatrogenic atrial tachycardia after ablation of atrial fibrillation: a prospective randomized study comparing circumferential pulmonary vein ablation with a modified approach. Circulation. 2004;110:3036–3042. doi: 10.1161/01.CIR.0000147186.83715.95. [DOI] [PubMed] [Google Scholar]
- 7.Jais P, Hocini M, Hsu LF, et al. Technique and results of linear ablation at the mitral isthmus. Circulation. 2004;110:2996–3002. doi: 10.1161/01.CIR.0000146917.75041.58. [DOI] [PubMed] [Google Scholar]
- 8.Knecht S, Hocini M, Wright M, et al. Left atrial linear lesions are required for successful treatment of persistent atrial fibrillation. Eur Heart J. 2008;29:2359–2366. doi: 10.1093/eurheartj/ehn302. [DOI] [PubMed] [Google Scholar]
- 9.Knecht S, Wright M, Sacher F, et al. Relationship between perimitral and peritricuspid conduction times. Heart Rhythm. 2008;5:400–405. doi: 10.1016/j.hrthm.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 10.Pak HN, Oh YS, Lim HE, Kim YH, Hwang C. Comparison of voltage map-guided left atrial anterior wall ablation versus left lateral mitral isthmus ablation in patients with persistent atrial fibrillation. Heart Rhythm. 2011;8:199–206. doi: 10.1016/j.hrthm.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 11.Yokokawa M, Sundaram B, Garg A, et al. Impact of mitral isthmus anatomy on the likelihood of achieving linear block in patients undergoing catheter ablation of persistent atrial fibrillation. Heart Rhythm. 2011;8:1404–1410. doi: 10.1016/j.hrthm.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 12.Mountantonakis S, Frankel DS, Hutchinson MD, et al. Feasibility of catheter ablation of mitral annular flutter in patients with prior mitral valve surgery. Heart Rhythm. 2011;8:809–814. doi: 10.1016/j.hrthm.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 13.Anousheh R, Sawhney NS, Panutich M, Tate C, Chen WC, Feld GK. Effect of mitral isthmus block on development of atrial tachycardia following ablation for atrial fibrillation. Pacing Clin Electrophysiol. 2011;33:460–468. doi: 10.1111/j.1540-8159.2009.02625.x. [DOI] [PubMed] [Google Scholar]
- 14.Matsuo S, Yamane T, Hioki M, et al. Recurrent atrial arrhythmia in patients with atrial fibrillation following pulmonary vein isolation. J Cardiovasc Electrophysiol. 2011;22:1080–1082. doi: 10.1111/j.1540-8167.2011.02027.x. [DOI] [PubMed] [Google Scholar]
- 15.Chugh A, Oral H, Good E, et al. Catheter ablation of atypical atrial flutter and atrial tachycardia within the coronary sinus after left atrial ablation for atrial fibrillation. J Am Coll Cardiol. 2005;46:83–91. doi: 10.1016/j.jacc.2005.03.053. [DOI] [PubMed] [Google Scholar]
- 16.Bogun F, Good E, Han J, et al. Mechanical interruption of postinfarction ventricular tachycardia as a guide for catheter ablation. Heart Rhythm. 2005;2:687–691. doi: 10.1016/j.hrthm.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 17.D’Avila A, Thiagalingam A, Foley L, Fox M, Ruskin JN, Reddy VY. Temporary occlusion of the great cardiac vein and coronary sinus to facilitate radiofrequency catheter ablation of the mitral isthmus. J Cardiovasc Electrophysiol. 2008;19:645–650. doi: 10.1111/j.1540-8167.2008.01185.x. [DOI] [PubMed] [Google Scholar]
- 18.Choi JI, Pak HN, Park JH, et al. Clinical significance of complete conduction block of the left lateral isthmus and its relationship with anatomical variation of the vein of Marshall in patients with nonparoxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2009;20:616–622. doi: 10.1111/j.1540-8167.2008.01408.x. [DOI] [PubMed] [Google Scholar]
- 19.Valderrabano M, Chen HR, Sidhu J, Rao L, Ling Y, Khoury DS. Retrograde ethanol infusion in the vein of Marshall: regional left atrial ablation, vagal denervation and feasibility in humans. Circ Arrhythm Electrophysiol. 2009;2:50–56. doi: 10.1161/CIRCEP.108.818427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valderrabano M, Liu X, Sasaridis C, Sidhu J, Little S, Khoury DS. Ethanol infusion in the vein of Marshall: Adjunctive effects during ablation of atrial fibrillation. Heart Rhythm. 2009;6:1552–1558. doi: 10.1016/j.hrthm.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fassini G, Riva S, Chiodelli R, et al. Left mitral isthmus ablation associated with PV Isolation: long-term results of a prospective randomized study. J Cardiovasc Electrophysiol. 2005;16:1150–1156. doi: 10.1111/j.1540-8167.2005.50192.x. [DOI] [PubMed] [Google Scholar]
- 22.Matsuo S, Yamane T, Date T, et al. Completion of Mitral Isthmus Ablation Using a Steerable Sheath: Prospective Randomized Comparison With a Nonsteerable Sheath. J Cardiovasc Electrophysiol. 2011 doi: 10.1111/j.1540-8167.2011.02112.x. [DOI] [PubMed] [Google Scholar]
- 24.Anousheh R, Sawhney NS, Panutich M, Tate C, Chen WC, Feld GK. Effect of mitral isthmus block on development of atrial tachycardia following ablation for atrial fibrillation. Pacing Clin Electrophysiol. 2010;33:460–468. doi: 10.1111/j.1540-8159.2009.02625.x. [DOI] [PubMed] [Google Scholar]
- 23.Takatsuki S, Extramiana F, Hayashi M, et al. High take-off left inferior pulmonary vein as an obstacle in creating a conduction block at the lateral mitral isthmus. Europace. 2009;11:910–916. doi: 10.1093/europace/eup151. [DOI] [PubMed] [Google Scholar]
- 25.Wong KC, Jones M, Qureshi N, et al. Balloon occlusion of the distal coronary sinus facilitates mitral isthmus ablation. Heart Rhythm. 2011;8:833–839. doi: 10.1016/j.hrthm.2011.01.042. [DOI] [PubMed] [Google Scholar]
- 26.Becker AE. Left atrial isthmus: anatomic aspects relevant for linear catheter ablation procedures in humans. J Cardiovasc Electrophysiol. 2004;15:809–812. doi: 10.1046/j.1540-8167.2004.03651.x. [DOI] [PubMed] [Google Scholar]
- 27.West JJ, Norton PT, Kramer CM, et al. Characterization of the mitral isthmus for atrial fibrillation ablation using intracardiac ultrasound from within the coronary sinus. Heart Rhythm. 2008;5:19–27. doi: 10.1016/j.hrthm.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 28.Kurotobi T, Shimada Y, Kino N, et al. Local coronary flow is associated with an unsuccessful complete block line at the mitral isthmus in patients with atrial fibrillation. Circ Arrhythm Electrophysiol. 2011;4:838–843. doi: 10.1161/CIRCEP.111.964478. [DOI] [PubMed] [Google Scholar]
- 29.Miyazaki S, Shah AJ, Haissaguerre M. Recurrent perimitral tachycardia using epicardial coronary sinus connection to bypass endocardial conduction block at the mitral isthmus. Circ Arrhythm Electrophysiol. 2011;4:e39–41. doi: 10.1161/CIRCEP.111.963157. [DOI] [PubMed] [Google Scholar]
- 30.Han S, Joung B, Scanavacca M, Sosa E, Chen PS, Hwang C. Electrophysiological characteristics of the Marshall bundle in humans. Heart Rhythm. 2010;7:786–793. doi: 10.1016/j.hrthm.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brugada P, de Swart H, Smeets JL, Wellens HJ. Transcoronary chemical ablation of ventricular tachycardia. Circulation. 1989;79:475–482. doi: 10.1161/01.cir.79.3.475. [DOI] [PubMed] [Google Scholar]
- 32.Okishige K, Andrews TC, Friedman PL. Suppression of incessant polymorphic ventricular tachycardia by selective intracoronary ethanol infusion. Pacing Clin Electrophysiol. 1991;14:188–195. doi: 10.1111/j.1540-8159.1991.tb05089.x. [DOI] [PubMed] [Google Scholar]
- 33.Kay GN, Bubien RS, Dailey SM, Epstein AE, Plumb VJ. A prospective evaluation of intracoronary ethanol ablation of the atrioventricular conduction system. J Am Coll Cardiol. 1991;17:1634–1640. doi: 10.1016/0735-1097(91)90659-w. [DOI] [PubMed] [Google Scholar]