Abstract
Nutrient restriction (NR) prolongs longevity via enhanced mitochondrial function. To test the hypothesis that NR enhances resistance to ischemia/reperfusion (IR) arrhythmias via preserved calcium (Ca) cycling and mitochondrial function. We examined the protective effects of NR on regional IR in cultured neonatal rat ventricular myocyte monolayers. Optical mapping of intracellular Ca and mitochondrial membrane potential Δψm was performed using Rhod 2-AM and TMRE, respectively. Regional ischemia was mimicked by covering a portion of monolayer with a glass coverslip until loss of Ca propagation, and reperfusion was mimicked by removing the coverslip. NR was mimicked by culture in serum- and glucose-free medium for 24 hours.
Relative to controls, NR monolayers sustained Ca oscillations during longer periods of ischemia (19.2±1.8 min vs 10.4±1.4 min, p < 0.001); attenuated increases in Ca transient duration (CaD) and time decay Constant (Tau) during ischemia; preserved Conduction velocity (CV) during early reperfusion, leading to protection against reperfusion arrhythmias; had minimal “rebound” decreased CaD and Tau during reperfusion; and had no depolarization of Δψm during IR. NR attenuates IR arrhythmias via 1) stable calcium cycling; and 2) prevention of Δψm depolarization during IR. Enhanced mitochondrial resistance to IR arrhythmias may play a role in NR-induced longevity prolongation.
Keywords: nutrient restriction, calcium cycling, mitochondria, ischemia, reperfusion
1. Introduction
Nutrient restriction (NR), either a reduced energy intake or fasting, has been proven, in animal models, to increase longevity and reduce the incidence of age-associated diseases, including cancer, diabetes, and kidney disease.1–4 Although the molecular mechanisms by which NR exerts its protective effects on heart function remain uncertain, its beneficial impact on cardiovascular disease has been reported in multiple models of disease processes affecting the cardiovascular system. A range of indirect beneficial effects has been shown, including improving lipid profile and endothelial function, and decreasing inflammation in atherosclerosis, decreases in blood pressure and left ventricular hypertrophy, increases in heart rate variability, decreasing sympathetic outflow, (for review see Han et al5 and references therein). Direct cardioprotective effects of NR have been shown as preservation of diastolic function6 and prevention of pressure overload hypertrophy,7 and as increased tolerance to ischemia and reperfusion.8–12 However, there are little data regarding NR effects on the electrophysiological effects of IR.
Previous studies suggest multiple cellular mechanisms involved in NR-induced increase life span and protection from injury. One mechanism is related to the beneficial impact on mitochondrial function and control of oxidative stress. Studies in rodents and using cell culture models of NR demonstrate that NR causes a decrease in ROS production, attenuates the accumulation of oxidative damage, and increases mitochondrial biogenesis and bioenergetic efficiency.4, 13–19
In a previous study,20 we have shown that in vitro coverslip-induced IR exhibited similar electrophysiology changes (action potential duration shortening, Ca transient broadening, slowed conduction velocity, Ca overload) observed in acute regional IR in vivo, including IR-induced arrhythmias.
The aims of this study were: (1) to investigate the effects of NR on IR arrhythmias and calcium cycling stability during regional IR in cardiomyocyte cultures, and (2) to investigate the effects of NR on mitochondrial membrane potential during IR.
2. Methods
2.1 NR Cultured NRVM Monolayers
NRVM were isolated by standard methods and plated on 18×18-mm polyvinyl chloride coverslips. Briefly, hearts were harvested from 2- to 3-day-old neonatal Sprague-Dawley rats, and were digested with collagenase (80 U/ml, Worthington Biochemical Corp, Lakewood, NJ) and pancreatin (800 µg/mL, Sigma-Aldrich, St Louis, Mo). Myocytes were isolated with the use of a Percoll (Pharmacia Biotech AB, Uppsala, Sweden) gradient and plated at a density of 1.5×106 cells per coverslip. Monolayers were cultured in complete medium (RPMI 1640, 25mM Glucose, 5% neonatal calf serum, and 10% horse serum) for 2 to 3 days to ensure full confluence before cells were subjected to NR. NR monolayers were grown in RPMI 1640, which is glucose and serum free for 24 hrs before experimental use.
2.2 Regional IR Model
Regional IR in NRVM monolayers was performed as previously described with modifications.20 After proper staining, specimens were transferred into a recording/perfusion chamber (RC-27L, Warner Instruments, Hamden, CT) and superfused with oxygenated Tyrode’s solution at 37°C. The monolayer was stimulated at 2 Hz (bipolar stimuli at 5 V at the edge of the coverslip) with a Grass stimulator (Astro-MedInc, West Warwick, RI). Confluent specimens with homogeneous electrical propagation (i.e. propagation of Ca transients throughout the entire monolayer with even conduction velocity) were selected for experimental use.
After obtaining baseline optical recordings, we lowered a 5-mm diameter glass coverslip onto the near central area of the 18×18-mm monolayer to create a regional ischemic zone, surrounded by a non-ischemic zone. The rim of tissue under the coverslip within 1 mm of the non-ischemic zone was defined as the border zone. Optical mapping recordings were obtained as per different protocols during regional ischemia. For Ca mapping, recordings were obtained every minute until the ischemic zone became unexcitable, as determined by a complete absence of propagation (lack of Ca transients) into the area under the coverslip. We have previously validated the correlation between loss of calcium cycling and loss of excitability in this model.20 Afterwards, the coverslip was lifted to begin the reperfusion period. Ischemic loss of excitability was always reversible. For Δψm mapping, optical recordings were obtained every 2 minutes, but longer periods of (irreversible) ischemia were required to detect changes in Δψm (see below).
2.3 Optical Mapping
NRVM monolayers were stained by immersion into oxygenated Tyrode’s solution (in mmol/L: 136 NaCl, 5.4 KCl, 1.8 CaCl2, 0.33NaH2PO4, 1 MgCl2, 10 HEPES, and 10 glucose; pH 7.3) containing the fluorescent Ca dye Rhod-2AM (10 µmol/L for 40 minutes) plus 0.016% (wt/wt) Pluronic (Molecular Probes, Eugene, OR) or the mitochondrial membrane potential dye TMRE (100 nmol/L for 20 minutes) at 37°C. For Ca mapping, fluorescence was excited by green light from a 532 nm laser light source (B&W TEK Inc, Newark, DE) and recorded at 585 nm with charge-coupled device cameras (Cascade 128+; 128×128 pixels, Photometrics, Tucson, AZ), acquiring at 2 ms per frame. For Δψm mapping, fluorescence was excited by two halogen light sources (MHAB-150W, Moritex USA Inc., San Jose, CA) into one optical fiber and filtered at 520±35 nm. The emitted fluorescence was recorded at 585 nm with a CMOS imaging system (MiCAM ULTIMA, BrainVision Inc., Tokyo, Japan). Microscopic recording of Δψm changes was performed on an inverted microscope (Axiovert 200, Carl Zeiss, Inc, Gottingen, Germany).
2.4 Data Analysis
Optical mapping Ca data were analyzed with custom software. Ca transient amplitude was measured as the relative increase in fluorescence from onset to peak (arbitrary units). The rise time was measured from the baseline to the peak. The time decay of the Ca transient (Tau) was calculated by single exponential fitting using Origin (Microcal, Northampton, MA) software. We assessed the presence of diastolic Ca overload during ischemia by calculating the ratio of trough diastolic Ca fluorescence in the ischemic zone divided by that of the non-ischemic zone in individual monolayers. Thus, each monolayer served as its own control. TMRE were analyzed with Image J (NIH). All data were expressed as mean±SD. Differences were compared using the χ2 test, and Student’s t-test where appropriate. Results were considered statistically significant if p < 0.05.
3. Results
3.1 Effects of NR on Calcium Cycling and Arrhythmias during IR
Typical microscopic and macroscopic morphologies of cultured NRVM monolayers are shown in Figure 1. Although control and NR cultures were overall confluent, NR cultures appeared less dense. We tracked propagation of the electrical stimulus using fluorescence mapping of intracellular Ca as a surrogate. At baseline, both NR and control monolayers exhibited uniform propagation during pacing at 2 Hz, without significant conduction blocks throughout the monolayers. Figure 2 shows representative examples. Before ischemia, the conduction velocities (CV) of both groups were comparable (0.18±0.06 m/s for control vs 0.15±0.02 for NR, NS). During ischemia, the ischemic zone sustained propagations for 19.2±1.8 min (n = 6) in NR monolayers compared with 10.4±1.4 min in control (n = 6, p < 0.001). Ultimately, the ischemic zone became unexcitable during later ischemia, leading to the formation of stable macroreentry with single or double direction waves circulating around the ischemic area.20 During reperfusion, propagation recovered rapidly in both groups but with impaired CV within the ischemic region in control. The combination of recovered excitability and persistently slow CV, as well as ectopic beats arising the border zone, led to reperfusion arrhythmias, as shown previously20 (Figure 3). Arrhythmias were found in 4/6 of control monolayers, as opposed to none NR monolayers.
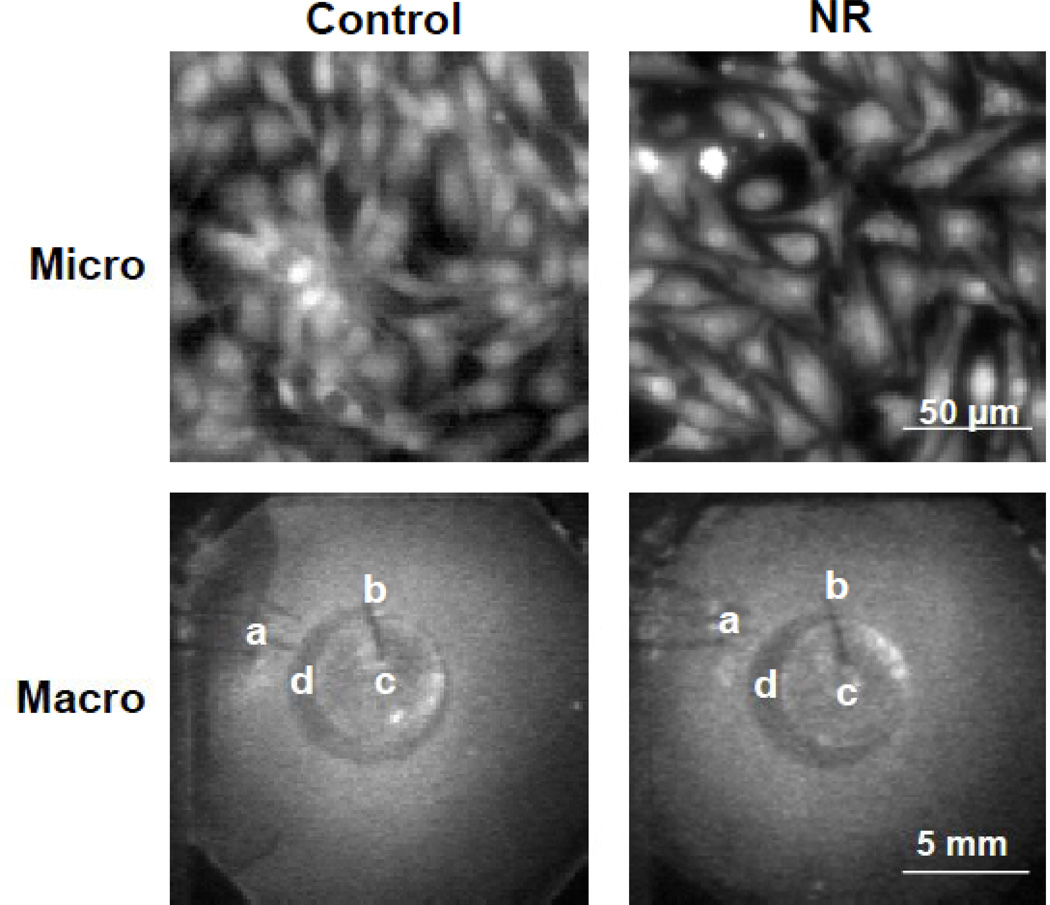
Figure 1.
Representative morphologies of neonatal rat myocyte monolayers. Cells were stained with rhod-2 AM and visualized under an inverted microscope while propagation was mapped macroscopically. Con, control; NR, nutrient restriction. Slightly less dense cultures were obtained under NR conditions. a, Pacing electrodes. b, Handling needle glued to the center of the coverslip for manipulation. c, Five mm-diameter glass coverslip for ischemia induction. d, Transmitted image of the water immersion objective of the microscope underneath the monolayer.
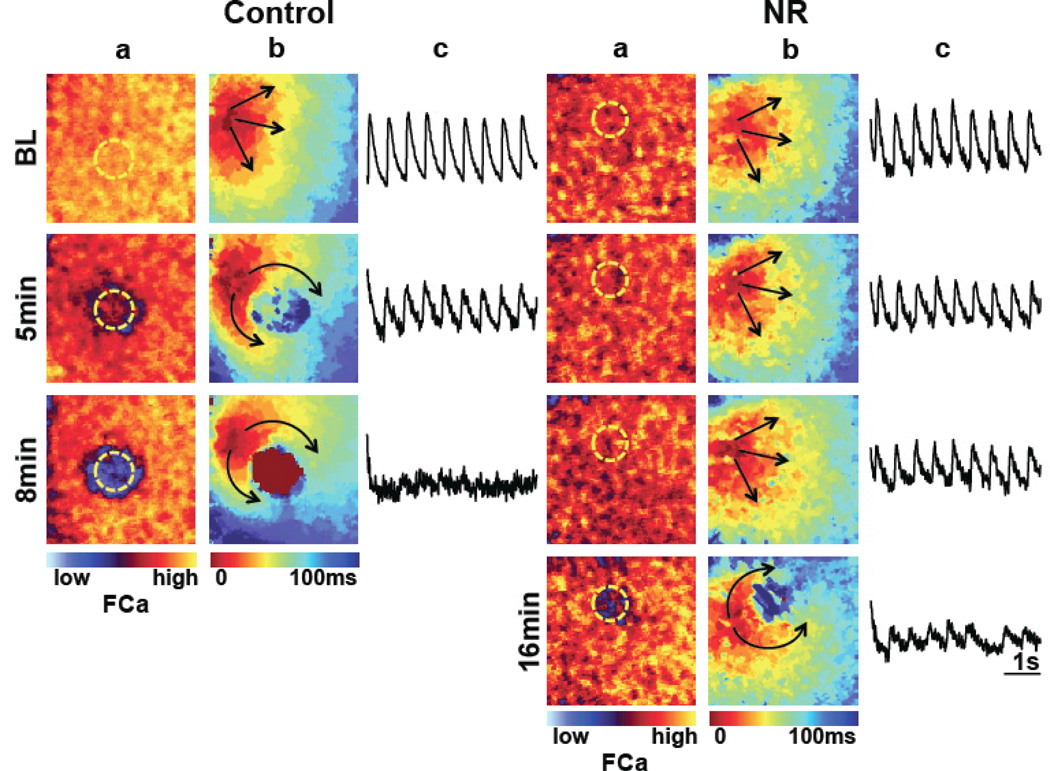
Figure 2.
Impulse propagation during regional ischemia in control (left) and NR (right) monolayers, at baseline (BL) and during continuing ischemia (duration in minutes). All monolayers were paced at 2 Hz from the left border. a, Snapshots of peak systolic Ca fluorescence (FCa). With prolonged ischemic times, a decrease in peak systolic Ca is seen in the ischemic zone (marked in yellow dashed circle). In this examples, Ca transients were absent after 8 min of ischemia in the control specimens, and significantly blunted after 16 minutes in NR monolayers; b, Isochronal activation maps. Regional slowing of propagation is seen in the ischemic zone at 5 minutes in controls, but required 16 minutes to develop in NR monolayers. Upon loss of excitability, the propagation spread around the ischemic zone (which appeared smaller in NR monolayers). c, Ca signal tracings from the ischemic region.
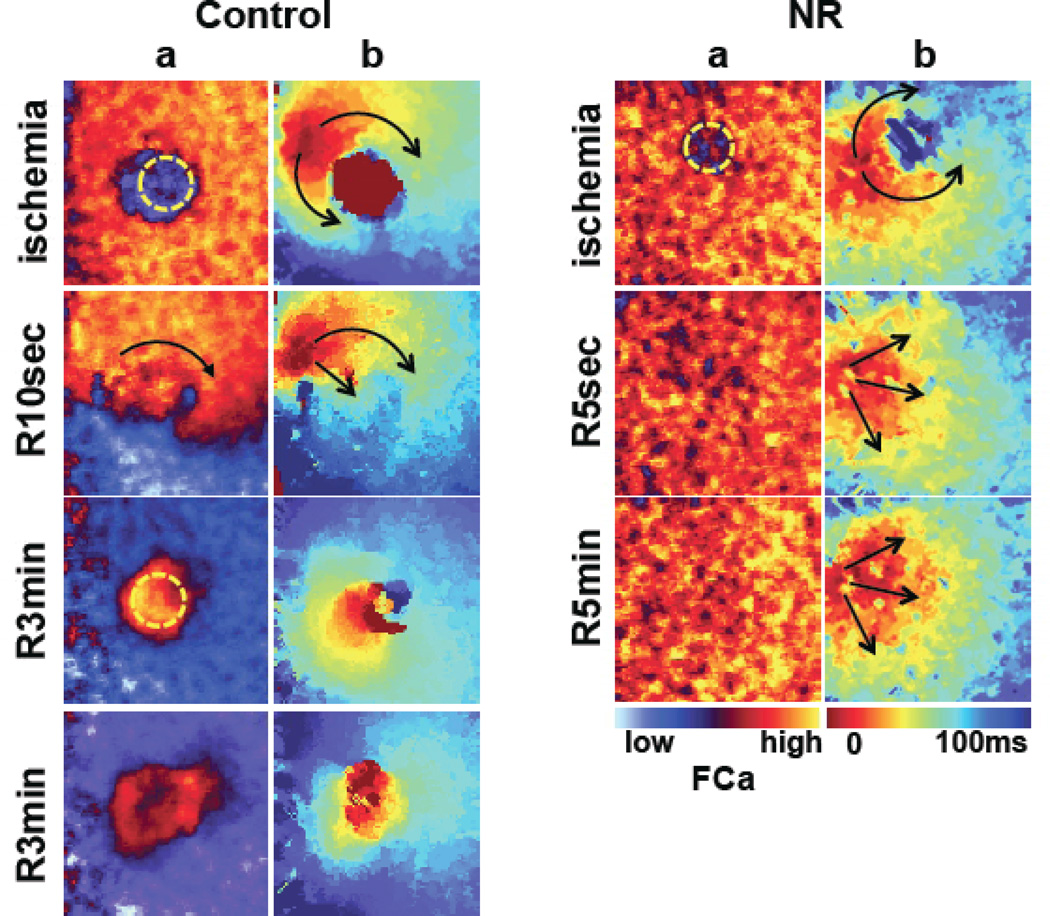
Figure 3.
Impulse propagation during reperfusion in control (left) and nutrient restriction (right) monolayers. a, Snapshot of systolic Ca fluorescence; b, Isochronal activation maps. At the end of ischemia, both control and NR monolayers had decreased systolic Ca in the ischemic zone (consistent with absent Ca transient), and propagation surrounded the ischemic zone without activating it. Upon early (10 seconds) reperfusion (R), the reperfused zone in controls (R10 sec) remained with slow conduction and propagation went still around it, whereas in NR monolayers, propagation had normalized as early as 5 seconds into reperfusion. Over the ensuing minutes into reperfusion, ectopic beats arose from the reperfused area border zone in control monolayers, but not in NR monolayers.
3.2 Effects of NR on Ca Cycling during IR
Intracellular Ca2+ cycling is profoundly altered during IR, with decreased Ca2+ uptake in to the sarcoplasmic reticulum, and prolonged diastolic Ca2+ decay (longer time decay constant, Tau) and diastolic Ca overload.20 This, combined with shortened action potential duration, is a major cellular mechanism of arrhythmogenesis.20 During ischemia, progressive increases in Ca transient duration (CaD) and Tau were prominent in control but attenuated in NR. During early reperfusion, decreased CaD and Tau were prominent during reperfusion in controls, but minimal in NR. Figure 4 shows representative examples of Ca transients during IR in control and in NR monolayers, along with their divergent progression over time.
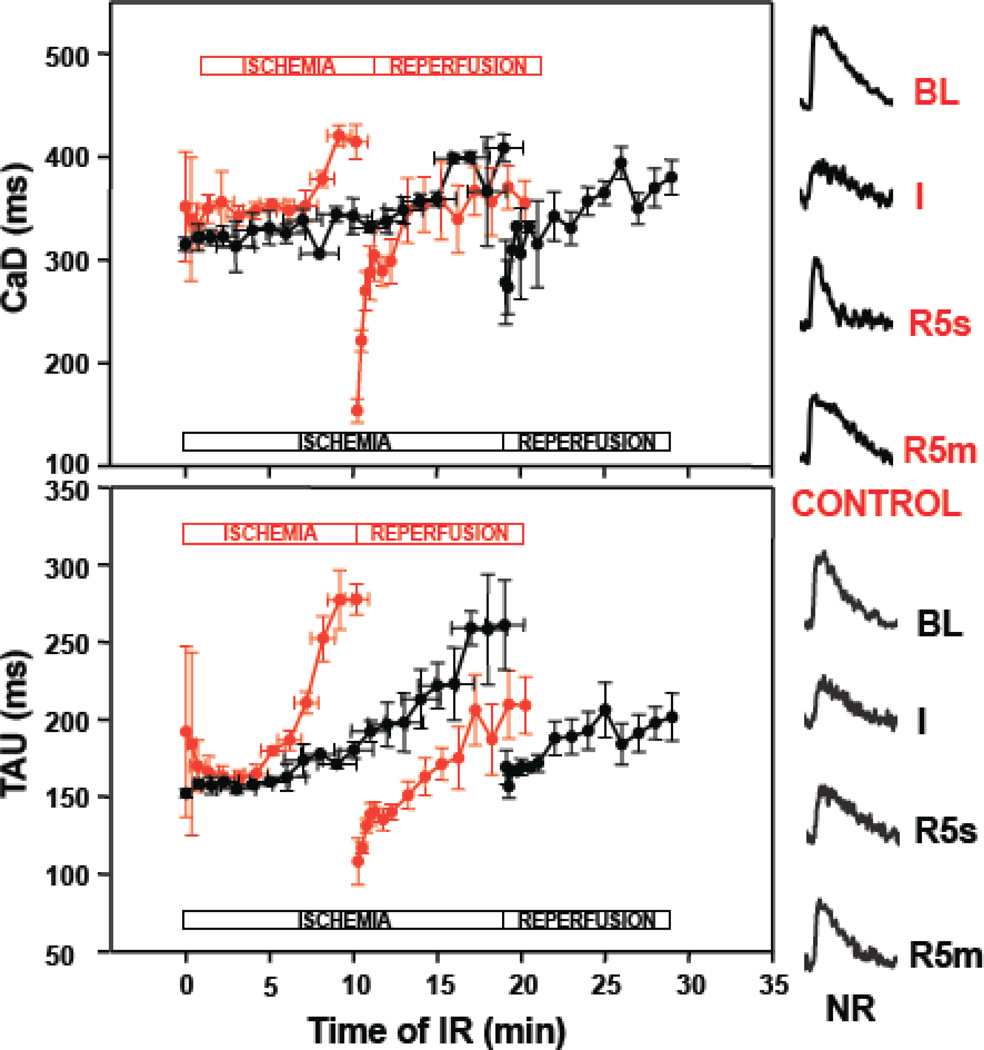
Figure 4.
Ca transient duration (CaD, top) and time decay constant (TAU, bottom) during IR in control (red) versus nutrient restriction (NR, black) monolayers. In control monolayers, during ischemia there were progressive prolongation of the Ca transient and Tau, that culminated in loss of excitability at 10.4±1.4 minutes (horizontal error bars show variability in times to reach loss of excitability). Upon reperfusion, a “rebound” shortening of both CaD and Tau were seen. Although similar prolongations in CaD and Tau were seen in NR monolayers at the end of the ischemic period (p=NS) the time to reach loss of excitability was longer (19.2±1.8 min, p<0.001), and there was no “rebound” shortening of CaD or Tau upon early reperfusion. Examples of control and NR Ca transients during IR are shown in the right: BL, baseline; I, ischemia; R5s, early reperfusion; R5m, late reperfusion.
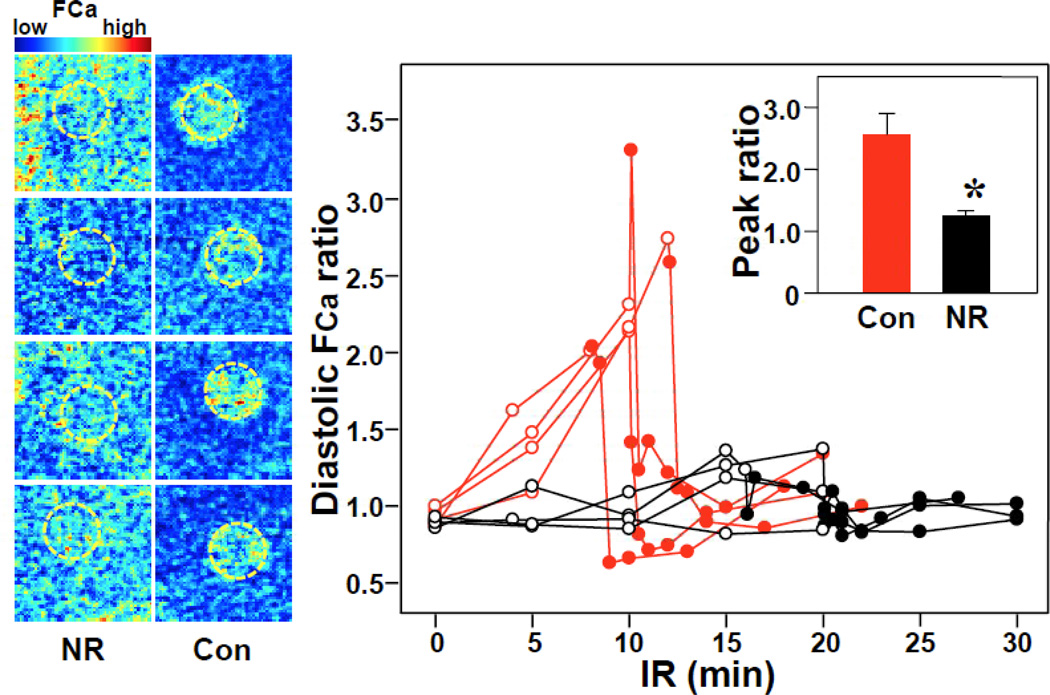
It is also well known that ischemia leads to calcium overload. Relative to the non-ischemic zone, the ischemic zone consistently showed increased diastolic Ca fluorescence at the time of loss of excitability and early reperfusion in both control and NR monolayers. However, the peak ratios of ischemic/nonischemic diastolic Ca were consistently higher in controls, indicating a mitigated calcium overload in NR monolayers. Figure 5 shows examples and the temporal trends of such ratios.
Figure 5.
Diastolic Ca overload during IR. Left, Snapshots of end-diastolic Ca fluorescence (FCa) at the end of ischemia (upon loss of excitability) in 4 representative examples of control (Con) and nutrient restriction (NR) monolayers. There was significant variation of Ca fluorescence between specimens, therefore fluorescence in the ischemic zone was normalized to that of the non-ischemic zone in each monolayer (diastolic FCa ratio). In control monolayers, higher diastolic Ca fluorescence outlines the ischemic zone (dashed yellow circumference), which does not occur in NR monolayers. Right, Diastolic FCa ratio over time during ischemia and reperfusion. Red: Control, Black, NR. Open circle: during ischemia, Closed circle: during reperfusion. Right inset, Peak ratio during IR.*P<0.05 vs control by t-test.
3.3 Effects of NR on Δψm during IR
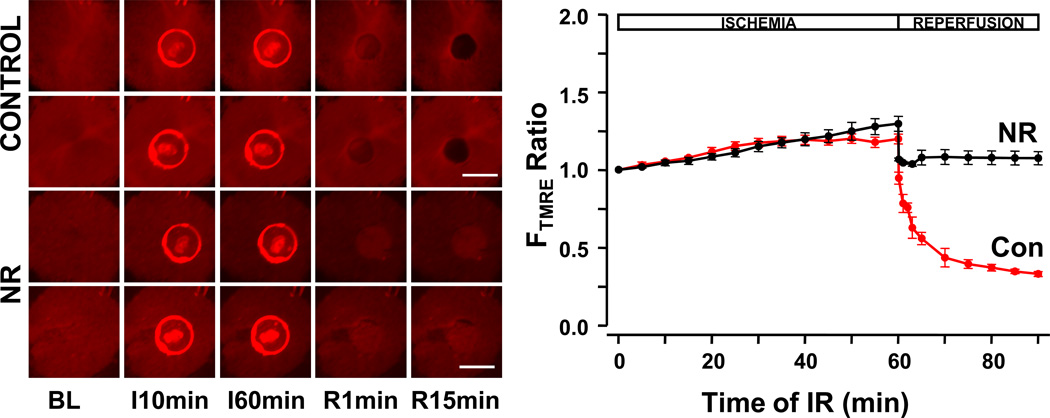
Mitochondria play an important role during IR arrhythmias.21 To find the effects of NR on mitochondrial function, we monitored the fluorescence intensity changes of TMRE during IR. During reversible ischemia –i.e. during the ischemic times required to lose Ca cycling, as used when measuring Ca transients-, neither NR and control monolayers developed mitochondrial depolarizations as shown by persistence of TMRE fluorescence (preservation of Δψm, data not shown), which illustrates that loss excitability and cessation of contraction develop much faster during ischemia than alterations in mitochondrial potential, which tend to precede cell death. This required induction of irreversible ischemia, which we created by prolonging the ischemia time to 60 minutes. With prolonged ischemia, we observed that Δψm depolarized at the end of ischemia without recovery during reperfusion in controls, but NR monolayers had no Δψm depolarization even after 60 min of ischemia. Figure 6 shows representative examples and the temporal trend of TMRE fluorescence in the IR region (relative to the perfused region) in controls vs NR monolayers.
Figure 6.
Mitochondrial depolarization during IR. Monolayers were stained with TMRE. Left, two representative examples are shown for each group. I, ischemia; R, reperfusion. Bar=5mm, field size 13mm × 13mm. During irreversible (60 min) ischemia a glass coverslip artifact is visible over the ischemic zone. Upon reperfusion, the reperfused area shows a dramatic decrease in fluorescence in controls, but not in NR monolayers. Right, average of TMRE fluorescence ratio (FTMRE, ischemic zone -pixels without artifact- vs nonischemic zone). Red curves represent control (Con), black curves represent NR (n=5). A dramatic decrease in fluorescence was consistently seen in controls, consistent with mitochondrial depolarization upon reperfusion, which was not present in NR monolayers.
4. Discussion
The main findings of our work are that NR promoted resistance to IR and its arrhythmogenic consequences via several mechanisms: 1) NR promotes stability of Ca cycling during regional IR; 2) NR decreases reperfusion arrhythmias; 3) NR preserves mitochondrial polarization during prolonged IR.
4.1 NR Preserves Calcium Cycling And Protects Against Arrhythmias During IR
In our study, NR promoted a reduction in the cell density during culture but it did not change the propagation at baseline. The reduction in the number of live cells may be the result of attenuated cell growth and division induced by serum and glucose deprivation. During ischemia, NR monolayers remained excitable longer than normal cultured cells, with a prolongation of more than 90% of the ischemic time required to lose excitability. The implications of this finding, if confirmed in larger animals in vivo, are significant: Sudden cardiac death is the most common initial presentation of heart disease, and most commonly occurs in the setting of acute coronary syndromes leading to ischemia. Besides the longevity-prolonging effects of NR, which seem to preserve overall health and retard aging, our findings support a protective value of NR against the acute insult of IR and its electrophysiological consequences.
Arguably, Ca cycling derangements are central to the electrophysiological destabilization and ensuing arrhythmias during IR. The combination of prolonged Ca transients, intracellular Ca overload, shortened action potential duration, and intercellular uncoupling (due to closure of gap junctions) all conspire to create an arrhythmogenic milieu during IR.20 Our experiments showed that NR attenuated the progressive changes of Ca transient duration and Tau during IR. During reperfusion, CV fully recovered in NR monolayers which prevented the development of arrhythmias.
The resistance to IR observed in the NR monolayers is likely to be multifactorial. Proximate mediators could be linked to intracellular Ca cycling and perhaps stability of the action potential (not tested here). Both can be ultimately linked to preserved ATP generation in NR monolayers. Decreasing ATP during ischemia would decrease the function of the sarcoplasmic reticulum Ca ATPase pump (SERCA) and lead to prolonged Ca transient decay and duration, along with diastolic cytoplasmic Ca overload. Additionally, decreasing function of the Na/K ATPase pump would lead to Na accumulation22 and sarcolemmal Na-Ca exchanger operating in reverse mode, in turn, leads to additional Ca overload, which can worsen cell injury by disrupting mitochondrial function.23 In control, we observed that the ischemic area developed diastolic Ca overload compared to non-ischemic area, which was mitigated in NR monolayers (Figure 5). This suggested that NR may protect myocytes against IR injury by preserving mitochondrial function and providing ATP to maintain stability of Ca cycling and prevent arrhythmogenesis.
4.2 NR Improves on Mitochondrial function during IR
Mitochondria play many important roles in the function of cells, including the production of ATP,24 the initiation of the intrinsic pathway of apoptosis,25 and the modulation of calcium homeostasis.26 Mitochondria play a central role in the pathophysiological response to IR. There is compromise of oxidative phosphorylation, reducing ATP generation and shifting it toward glycolysis. Reactive oxygen species are also released from compromised mitochondria.27 Mitochondrial depolarization leads to action potential shortening and loss of excitability.21 Opening of mitochondrial permeability transition pore (MPTP) determines an immediate collapse of ΔΨm that is followed by ATP depletion.28, 29 Such mitochondrial derangements have been linked to IR arrhythmias.21 We observed that NR protected mitochondria against depolarization during severe IR. NR has been linked to induction of mitochondrial biogenesis and improvement of bioenergentic efficiency.16 More recently, NR has been shown to attenuate myocardial oxidative damage after ischemia/reperfusion by suppressing mitochondrial ROS production during early reperfusion.30 The mechanisms seem to be related to enhanced activity of sirtuins and increased deacetylation (and thus improved function) of key proteins of the electron-transport chain.30 To our knowledge, we show for the first time that NR also confers resistance to mitochondrial depolarization during IR and link this protection to the preservation of calcium cycling and eletrophysiological stability in this setting.
4.3 Limitations
We did not perform voltage recordings in monolayers, thus propagation maps were based on Ca recordings. However, we have shown good concordance of voltage and Ca maps in previous work.20 The dye Rhod-2 we used in this study is a non-ratiometric (single wavelength) indictor, which only provides relative, non-quantified Ca measurements and is subject to loading and bleaching artifacts. In order to avoid these, each monolayer served as its own control. Relative Ca measurements are adequate to assess the stability of Ca cycling in the ischemic zone and non-ischemic zone in each individual monolayers. Simultaneous mapping of both Ca and Δψm would have strengthened the study, but we were limited by the similar excitation and emission wavelengths of the dyes used (similar for TMRE and Rhod-2). Rhod-2 has been suggested to have selective accumulation in mitochondria and was thus used to monitor mitochondrial calcium in a variety of cells 31–34. However, in the loading protocol used, it is an effective indicator of cytosolic Ca.35–37 NR by glucose and serum deprivation may not be comparable to other protocols of nutrient- or calorie- restriction. An in vivo model of NR and of regional myocardial ischemia may be best to evaluate the degree of IR injury and the effect of NR. However, myocyte monolayers allowed study of cardiac NR effects in isolation, excluding extracardiac and metabolic confounding factors. The dramatic impact of NR as tested on monolayers suggests a significant physiologic adaptation even in this reductionistic model and warrants further testing in other, more physiological ones.
5. Conclusions
Our study lend further support to the mitochondria as central mediator of the beneficial effects of NR, and expands the previous knowledge to include protective effects in IR arrhythmias and mediated by stable Ca cycling.
Acknowledgments
Funding sources: NIH/NHLBI 1R21HL097305 and 1R41HL104819 (MV), and The Methodist Hospital Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
References
- 1.Colman RJ, Anderson RM, Johnson SC, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider NL. Effects of intermittent feeding upon growth and life span in rats. Gerontology. 1982;28:233–241. doi: 10.1159/000212538. [DOI] [PubMed] [Google Scholar]
- 3.Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider NL. Effects of intermittent feeding upon growth, activity, and lifespan in rats allowed voluntary exercise. Exp.Aging Res. 1983;9:203–209. doi: 10.1080/03610738308258453. [DOI] [PubMed] [Google Scholar]
- 4.Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han X, Ren J. Caloric restriction and heart function: is there a sensible link? Acta Pharmacol Sin. 2010;31:1111–1117. doi: 10.1038/aps.2010.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shinmura K, Tamaki K, Sano M, et al. Impact of long-term caloric restriction on cardiac senescence: caloric restriction ameliorates cardiac diastolic dysfunction associated with aging. J Mol Cell Cardiol. 2011;50:117–127. doi: 10.1016/j.yjmcc.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 7.Seymour EM, Parikh RV, Singer AA, Bolling SF. Moderate calorie restriction improves cardiac remodeling and diastolic dysfunction in the Dahl-SS rat. J.Mol.Cell Cardiol. 2006;41:661–668. doi: 10.1016/j.yjmcc.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 8.Chandrasekar B, Nelson JF, Colston JT, Freeman GL. Calorie restriction attenuates inflammatory responses to myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2001;280:H2094–H2102. doi: 10.1152/ajpheart.2001.280.5.H2094. [DOI] [PubMed] [Google Scholar]
- 9.Shinmura K, Tamaki K, Bolli R. Short-term caloric restriction improves ischemic tolerance independent of opening of ATP-sensitive K+ channels in both young and aged hearts. J Mol.Cell Cardiol. 2005;39:285–296. doi: 10.1016/j.yjmcc.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Ahmet I, Wan R, Mattson MP, Lakatta EG, Talan M. Cardioprotection by intermittent fasting in rats. Circulation. 2005;112:3115–3121. doi: 10.1161/CIRCULATIONAHA.105.563817. [DOI] [PubMed] [Google Scholar]
- 11.Shinmura K, Tamaki K, Bolli R. Impact of 6-mo caloric restriction on myocardial ischemic tolerance: possible involvement of nitric oxide-dependent increase in nuclear Sirt1. Am J Physiol Heart Circ Physiol. 2008;295:H2348–H2355. doi: 10.1152/ajpheart.00602.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shinmura K, Tamaki K, Saito K, Nakano Y, Tobe T, Bolli R. Cardioprotective effects of short-term caloric restriction are mediated by adiponectin via activation of AMP-activated protein kinase. Circulation. 2007;116:2809–2817. doi: 10.1161/CIRCULATIONAHA.107.725697. [DOI] [PubMed] [Google Scholar]
- 13.Gredilla R, Sanz A, Lopez-Torres M, Barja G. Caloric restriction decreases mitochondrial free radical generation at complex I and lowers oxidative damage to mitochondrial DNA in the rat heart. FASEB J. 2001;15:1589–1591. doi: 10.1096/fj.00-0764fje. [DOI] [PubMed] [Google Scholar]
- 14.Sohal RS, Ku HH, Agarwal S, Forster MJ, Lal H. Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech.Ageing Dev. 1994;74:121–133. doi: 10.1016/0047-6374(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 15.Hepple RT, Baker DJ, McConkey M, Murynka T, Norris R. Caloric restriction protects mitochondrial function with aging in skeletal and cardiac muscles. Rejuvenation.Res. 2006;9:219–222. doi: 10.1089/rej.2006.9.219. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Lluch G, Hunt N, Jones B, et al. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc Natl Acad Sci U S A. 2006;103:1768–1773. doi: 10.1073/pnas.0510452103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colom B, Oliver J, Roca P, Garcia-Palmer FJ. Caloric restriction and gender modulate cardiac muscle mitochondrial H2O2 production and oxidative damage. Cardiovasc Res. 2007;74:456–465. doi: 10.1016/j.cardiores.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Minamiyama Y, Bito Y, Takemura S, et al. Calorie restriction improves cardiovascular risk factors via reduction of mitochondrial reactive oxygen species in type II diabetic rats. J Pharmacol Exp.Ther. 2007;320:535–543. doi: 10.1124/jpet.106.110460. [DOI] [PubMed] [Google Scholar]
- 19.Broderick TL, Belke T, Driedzic WR. Effects of chronic caloric restriction on mitochondrial respiration in the ischemic reperfused rat heart. Mol.Cell Biochem. 2002;233:119–125. doi: 10.1023/a:1015506327849. [DOI] [PubMed] [Google Scholar]
- 20.de DC, Pai RK, Chen F, et al. Electrophysiological consequences of acute regional ischemia/reperfusion in neonatal rat ventricular myocyte monolayers. Circulation. 2008;118:2330–2337. doi: 10.1161/CIRCULATIONAHA.108.789149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akar FG, Aon MA, Tomaselli GF, O'Rourke B. The mitochondrial origin of postischemic arrhythmias. J Clin Invest. 2005;115:3527–3535. doi: 10.1172/JCI25371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shivkumar K, Deutsch NA, Lamp ST, Khuu K, Goldhaber JI, Weiss JN. Mechanism of hypoxic K loss in rabbit ventricle. J Clin Invest. 1997;100:1782–1788. doi: 10.1172/JCI119705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang S, Radhakrishnan J, Ayoub IM, Kolarova JD, Taglieri DM, Gazmuri RJ. Limiting sarcolemmal Na+ entry during resuscitation from ventricular fibrillation prevents excess mitochondrial Ca2+ accumulation and attenuates myocardial injury. J Appl Physiol. 2007;103:55–65. doi: 10.1152/japplphysiol.01167.2006. [DOI] [PubMed] [Google Scholar]
- 24.Saraste M. Oxidative phosphorylation at the fin de siecle. Science. 1999;283:1488–1493. doi: 10.1126/science.283.5407.1488. [DOI] [PubMed] [Google Scholar]
- 25.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 26.Rizzuto R, Simpson AW, Brini M, Pozzan T. Rapid changes of mitochondrial Ca2+ revealed by specifically targeted recombinant aequorin. Nature. 1992;358:325–327. doi: 10.1038/358325a0. [DOI] [PubMed] [Google Scholar]
- 27.Aon MA, Cortassa S, O'Rourke B. Percolation and criticality in a mitochondrial network. Proc Natl Acad Sci U S A. 2004;101:4447–4452. doi: 10.1073/pnas.0307156101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev. 1999;79:1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- 29.Di LF, Bernardi P. Mitochondria and ischemia-reperfusion injury of the heart: fixing a hole. Cardiovasc Res. 2006;70:191–199. doi: 10.1016/j.cardiores.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 30.Shinmura K, Tamaki K, Sano M, et al. Caloric restriction primes mitochondria for ischemic stress by deacetylating specific mitochondrial proteins of the electron transport chain. Circ Res. 2011;109:396–406. doi: 10.1161/CIRCRESAHA.111.243097. [DOI] [PubMed] [Google Scholar]
- 31.Babcock DF, Herrington J, Goodwin PC, Park YB, Hille B. Mitochondrial participation in the intracellular Ca2+ network. J Cell Biol. 1997;136:833–844. doi: 10.1083/jcb.136.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hajnoczky G, Robb-Gaspers LD, Seitz MB, Thomas AP. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995;82:415–424. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- 33.Jou MJ, Peng TI, Sheu SS. Histamine induces oscillations of mitochondrial free Ca2+ concentration in single cultured rat brain astrocytes. J Physiol. 1996;497(Pt 2):299–308. doi: 10.1113/jphysiol.1996.sp021769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rutter GA, Burnett P, Rizzuto R, et al. Subcellular imaging of intramitochondrial Ca2+ with recombinant targeted aequorin: significance for the regulation of pyruvate dehydrogenase activity. Proc Natl Acad Sci U S A. 1996;93:5489–5494. doi: 10.1073/pnas.93.11.5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Del Nido PJ, Glynn P, Buenaventura P, Salama G, Koretsky AP. Fluorescence measurement of calcium transients in perfused rabbit heart using rhod 2. Am J Physiol. 1998;274:H728–H741. doi: 10.1152/ajpheart.1998.274.2.H728. [DOI] [PubMed] [Google Scholar]
- 36.de Diego C, Pai RK, Chen F, et al. Electrophysiological consequences of acute regional ischemia/reperfusion in neonatal rat ventricular myocyte monolayers. Circulation. 2008;118:2330–2337. doi: 10.1161/CIRCULATIONAHA.108.789149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang S, Chen J, Chen MT, Vernier PT, Gundersen MA, Valderrabano M. Cardiac myocyte excitation by ultrashort high-field pulses. Biophys J. 2009;96:1640–1648. doi: 10.1016/j.bpj.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]