Abstract
Although the study of brain states is an old one in neuroscience, there has been growing interest in brain state specification owing to MRI studies tracing brain connectivity at rest. In this review, we summarize recent research on three relatively well-described brain states: the resting, alert, and meditation states. We explore the neural correlates of maintaining a state or switching between states, and argue that the anterior cingulate cortex and striatum play a critical role in state maintenance, whereas the insula has a major role in switching between states. Brain state may serve as a predictor of performance in a variety of perceptual, memory, and problem solving tasks. Thus, understanding brain states is critical for understanding human performance.
Brain state
The ability to maintain a brain state (see Glossary) and to switch between states is vital for self-regulation and for adapting to the varying environments that humans occupy. Brain states refer to reliable patterns of brain activity that involve the co-activation and/or connectivity of multiple large-scale brain networks. In infancy, for example, sleep, wakefulness, passive and active alertness, and crying have usually been seen as ranging along a continuum of different levels of arousal [1]. Adult brain states are multidimensional and can be identified by subjective experience (e.g., the dreaming that occurs during the brain state that involves rapid eye movement sleep – REM), changes in neuromodulation (e.g., the decrease in norepinephrine during REM sleep), or behavior (e.g. patterns of movement during REM sleep). Research has shown that brain state can serve as a predictor of performance in a variety of perceptual, memory, and problem solving tasks [2–4]. Thus understanding how brain states are acquired and maintained is a critical aspect of understanding human performance.
Recently, there has been growing interest in the specification of brain states, owing mainly to new functional magnetic resonance imaging (fMRI) studies tracing connectivity of brain networks during the resting state [5,6]. The networks active during the resting state include medial prefrontal cortex (mPFC), anterior cingulate cortex (ACC), and posterior cingulate cortex (PCC), often called the Default Mode Network (DMN), along with a number of other areas that are active and correlated at rest [7–10].
In animal and human studies sleep states have been shown to be important for learning, memory consolidation, and brain plasticity [11–14]. Although brain states such as sleep have been widely studied in both animals and humans, it has been less common to investigate brain states when awake and usually researchers focus on only one waking state [9,15]. As a consequence, the neural mechanisms of effectively achieving and maintaining appropriate brain states or switching between brain states during task performance have received little attention.
In this article, we discuss the resting state, the alert state induced by a warning signal prior to performing a task, and the state induced by meditation. For each of these states we examine biomarkers including brain activity, physiology, and behavior. These biomarkers are used to address similarities and differences between states. We then examine the literature on transitions between states: for example, how do warning signals produce a rapid transition from the resting to the alert state and how is the meditation state achieved? Finally, we explore the neural correlates of maintaining or switching brain states and highlight the significance of effective state maintenance and state switching.
The resting state
Human studies using fMRI have traditionally focused on task-evoked responses. In the past decade, however, the patterns of activity of the human brain during non-task processes (i.e., at rest) have become a focus of several studies [5,6,8–10]. In experimental settings, the resting state follows an instruction to lie quietly, relax, and not carry out any task; the eyes may be closed or open, and either fixated or not. Although the resting state does not involve an external task, it shows strong activation of a number of brain areas, some of which show reduced activation during task performance, and it consumes a greater amount of energy in comparison with task-evoked states. The resting state accounts for much of the metabolic activity of the human brain, with specific activations during tasks involving only small changes (about 5%) [8].
The default portion of the resting state seems to be common among individuals and similar areas are found active in anesthetized monkeys [16]. The intrinsic connectivity of the human brain at rest has been shown to change with development [17–20] and to differ in pathologies, such as anxiety and depression, dementia, attention deficit hyperactivity disorder (ADHD), autism, and schizophrenia [21,22].
Electrophysiological studies indicate that functional network activity during the resting state is associated with distinct oscillatory rhythms. In general, beta-band activity (12–32 Hz) indexes spontaneous mental operations and alpha-band activity (8–12 Hz) is also detected [23,24]. Coherent patterns in gamma frequency activity (33–45 Hz) have been found during wakeful rest and REM sleep [25]. These results provide electrophysiological markers associated with differences between the resting, wakeful and sleep states.
The alert state
One important approach to understanding state transitions is to address their relation to slow waves found in electrophysiological recordings. Slow waves, such as the contingent negative variation (CNV), are closely related to aspects of fMRI, since both result from synaptic activity that produces local field potentials [8,26,27]. The influence of a warning signal on brain activation patterns allows study of the rapid transition to the high levels of alertness needed for rapid and efficient performance when the target occurs. After each response in a standard reaction time task, the individual relaxes the high level of vigilance required to perform the task, allowing the brain to transition toward the resting state.
A warning signal thus induces two phases of brain activity: one prior to the target and the other following the target. We refer to these as changes in phasic alertness. The change in brain state during the period between warning and target reflects mainly a suppression of ongoing activity [28]. In the central nervous system, the CNV begins with the warning signal and may remain present until target presentation. This negative change appears to arise, at least in part, in ACC and adjacent structures [29–31]. The CNV may arise within 100 ms after the warning, in which case it is superimposed upon the event-related potential associated with the warning signal. If the target interval is predictable, the individual may not display the CNV until just prior to the occurrence of the target. The ACC is a gateway to autonomic responses, so it should be no surprise that the alert state is also indexed by widespread autonomic changes, such as slowing of heart rate and a decrease in skin conductance [32]. The state being generally inhibitory, it produces a dominance of the parasympathetic autonomic system over the sympathetic system [28,33]. The state change following the occurrence of the target generally involves sympathetic dominance, including increased heart rate [28].
A warning signal triggers neural activity in the locus coeruleus, which is the source of the neuromodulator norepinephrine (NE) [34]. Warning signal effects can be blocked by drugs such as guanfacine and clonidine, which have the effect of reducing NE release [35]. Drugs that increase NE release can also enhance the warning signal effect [35]. The NE pathway includes major nodes in the frontal lobes and parietal areas in the dorsal part of the visual pathways [36].
How does the alert state influence task performance? The warning increases rapid motor responses to signals. However, it appears to accomplish this in a specific way [37]. The alert state speeds the motor response without altering signal quality. This accords with the anatomical distribution of NE, which does not appear to influence the ventral pathways involved in object recognition. If the target is one that might be missed because it is very short or masked by other input, the probability of detecting the signal is increased by a warning signal. However, if there is a more easily detectable target, the speeded reaction time is often accompanied by increased error. This suggests that, whereas the response is speeded, the quality of information to which the person responds is reduced, resulting in increased error rates. Posner [37] argued that these effects can be understood if the warning signal allows faster input to parts of the brain that mediate conscious detection of the target [37]. This allows not only for enhanced overt response, but also for the priority of the signal to consciousness.
The study of resting state and alerting provides some important clues to the neural systems involved in change of state. First, state changes can be rapid and voluntary. The warning signal has an effect within one to two hundred milliseconds, but only if the individual knows that it signals the arrival of a target. Second, the warning interval terminates the resting state and begins an adjustment that involves cortical, subcortical, and autonomic activity. Third, brain stem neuromodulators are involved in the change of state. Finally, the brain state potentiates attention to enhance the priority of target events.
The change of state in alerting appears to take place in two stages. Stage A follows the warning and is a quiet vigilant state. Stage B includes the activity following the target. These two stages involve different biomarkers: increased parasympathetic activity, including lower heart rate, pupil diameter, and skin conductance response in stage A and more sympathetic activity in stage B [28]. Stage A of the alert state involves the neurotransmitter NE derived from locus coeruleus [38,39]. In the case of visual search where a specific target is to be found among distracters, stage B has been shown to involve a sustained suppression of gamma-band oscillations within frontal and parietal areas involved in orienting and executive control [40,41].
The meditation state
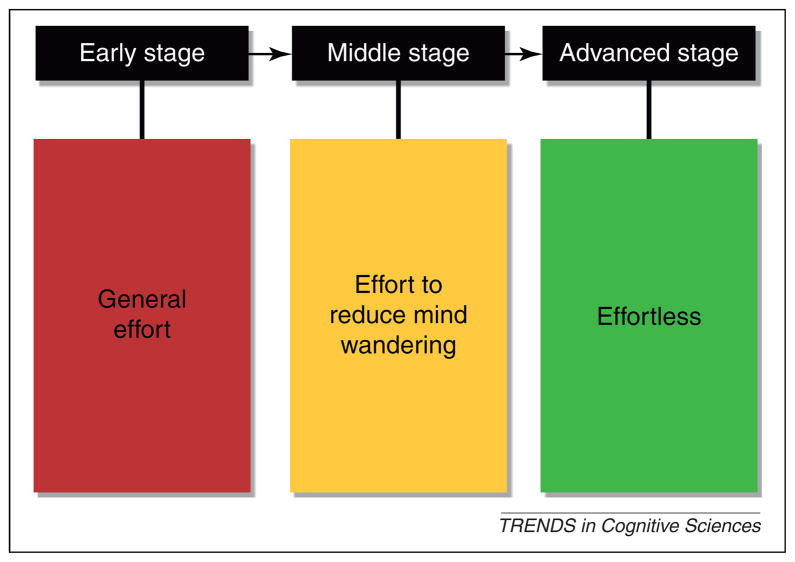
Unlike the alert state, meditation requires specific training. It is, therefore, difficult to separate the meditation state from the training that produces it. There are many forms of meditation practice, including transcendental meditation, Buddhist meditation, mindfulness meditation, and others [42–45]. Whether the state induced by these various forms of training is the same is not known, so it is difficult to describe a meditation state independent of the training needed to reach it. Faced with this issue, we have chosen to focus mainly on the training and meditation state induced by one form of mindfulness meditation called Integrative Body-Mind training (IBMT) [46–48]. It seems likely that other forms of mindfulness meditation involve the same state and perhaps other meditation practices do as well [49–52]. We also divide the state description into three stages of practice (Figure 1).
Figure 1.
Three stages of meditation. Different stages of meditation, including early, middle (intermediate) and advanced, involve different amounts of effort.
IBMT shares several key components with other forms of meditation, including relaxation, mental imagery, and mindfulness. The meditation state is facilitated through training and trainer-group dynamics, harmony, and resonance [33,46–48,51–53]. Another important reason for using IBMT as an example of meditation training is that it has been studied scientifically, using randomized trials in comparison with controls rather than depending on preexistent meditation groups [33,46–48,51–53]. With IBMT, the meditation state can be induced by as few as five 20–30 minute sessions. IBMT improves attention and self-regulation, and induces neuroplasticity through interaction between the central and the autonomic nervous systems (see Box 1 for behavioural effects of the two levels of training).
Box 1. Training effects of IBMT.
One week of training (2–3 h)
Increased efficiency in executive attention
Improved mood
Reduced stress hormone following a meditation session
Improved immunoreacivity following a meditation session
Improved parasympathetic activity
Improved brain activity and connectivity in self-control networks
One month of training (10–11 h)
Further improvements of executive attention
Improved alertness
Improved mood
Reduced stress hormone at baseline
Improved immunoreactivity at baseline
Induced white matter changes in self-control networks
Stages of meditation training
IBMT, like many meditation traditions, emphasizes the need to learn to use attention regulation early in the practice. However, different meditation techniques apply different strategies of attention control and self-regulation in achieving a similar or the same state, which may involve different mental processes and brain networks [43,44,47,54–56]. Here, we use IBMT as an example and identify three stages of meditation.
There are several partially overlapping stages of IBMT. It should be noted that most studies average measures from different stages of meditation together to determine mechanisms or effects, or compare them without identifying the stage involved [44]. In this review, we differentiate early and middle stages that involve effortful control from an advanced, more effortless stage of meditation. The stages clearly overlap, and intermediate stages, in which effort is needed for maintenance of the state, can also be observed.
Stage 1: early stage
The early stage of achieving the meditative state appears to involve the use of attentional control. To accomplish this, one reduces or even completely eliminates attention to external sensory inputs, including visual and auditory stimuli. This state requires conscious control and mental effort. The state differs from the vigilant state in that there is no orientation toward an external signal. However, it involves attention control networks, often including lateral prefrontal cortex (PFC) and parietal (PC) areas [43,50,52,55,56].
The concept of effort involves the subjective experience of strain in connection with striving for a goal. In experimental psychology, effort has been defined as the amount of energy needed to accomplish a task. As such, effort is one of a cluster of motivational concepts that carry the idea of underlying energizing systems involved in task performance [52,57].
Varying effort during state transitions often involves different brain networks [58–61]. If one exerts high effort on a task (voluntarily control), the attention control networks that include the lateral PFC and parietal cortex are involved [50,56,62]. If the practitioner uses voluntary control to achieve the meditative state, as many novices do, these attentional networks are generally active [33,55,60]. If less effort is involved, there is little activity in attentional control networks. Instead, the stage mainly involves the ACC [33,47,50].
Stage 2: intermediate stage
In the intermediate (middle) stage of meditation, the participant exerts the appropriate effort to deal with distractions and the wandering mind. A major feature of the intermediate meditation state involves rapid switches in focus as the mind wanders to various contents, which would be expected to involve attentional control networks. In one recent fMRI study [63], fourteen meditation practitioners from multiple traditions with varying practice time performed meditation during fMRI scanning. When participants realized that their mind was wandering, they pressed a button and returned their focus to the breath. Four intervals of mind wandering were constructed from the button press: (i) prior to awareness of mind wandering; (ii) awareness of mind wandering; (iii) shifting of attention; and (iv) sustained attention. The results indicated that mind wandering prior to awareness was associated with the default network of the resting state as measured with MRI, whereas cognitive processes during awareness of mind wandering, shifting of attention, and sustained attention engaged parts of the attentional networks that include the lateral PFC and parietal cortex [63]. In another study, fifteen Vipassana meditators (mean practice: 7.9 years, 2 h daily) showed stronger activations in the dorsal medial prefrontal cortex and the ACC bilaterally, compared to controls [64]. Unfortunately, these studies did not measure the effort level in achieving the meditation state.
Stage 3: advanced stage
In the advanced stage of training, usually thought to be obtained with little or no effort, meditation is maintained by activity in the ACC, left insula, and striatum. There is also a reduction of activity in the lateral PFC and parietal cortex [33,50–52] (Figure 1). The experienced practitioner achieves a state of high parasympathetic dominance with strong activation in the ACC and left insula [50,64–67]. Connections between the ACC, striatum and autonomic nervous systems are also enhanced [33,50,64]. One study tested and reported a laterality effect, whereby the left insula is responsible for parasympathetic effects, whereas the right insula involves sympathetic activity [48]. These findings are also consistent with previous evidence [66,67]. The strong parasympathetic activity is similar to the period between a warning signal and a subsequent target described above. However, unlike the alert state, in the meditation state, the person is not strongly oriented to the location of an external target, but instead concentrates or has an open focus on an internal cue, such as the breath, and on achieving a state that is awake, relaxed, in the moment, and free from a sense of evaluation [33,43,44]. Although meditation is associated with relaxation and high parasympathetic activity, there are some exceptions that show an increase in sympathetic activity [93]. Whether these practices require clear effort during the tasks and induce sympathetic activity warrants further investigation.
Using IBMT to achieve the meditation state, Tang et al. showed that five days of training differed from relaxation training chiefly in greater activity in the ventral ACC, left insula, and striatum [33]. Unlike relaxation and the other tasks used in cognitive switching studies [68] and the early stage of meditation, the more advanced stages show reduced activity in the lateral PFC and parietal cortex [52,56]. This difference may underlie the low subjective difficulty or effort reported by participants in obtaining the meditation state, in comparison with effortful control or reappraisal processing during detection of mind wandering and/or refocusing the target during the meditation, which often induces brain activation in the attention networks [43,50–52,55,56,62]. IBMT involves less effort to control thoughts, but instead induces a state of restful alertness. The process nevertheless becomes even more effortless when the practitioner develops expertise from short-term to long-term training [46,51]. It should be noted that both short-term training, such as IBMT, and long-term meditation showe activation of ventral or/and dorsal ACC [33,43,49,50,69,70]. Whether different effort, training strategies or meditation styles (such as concentration-based meditation) engage different neural networks warrants further investigation.
It is unclear when the highest stage of meditation is obtained. One study of sustained attention-based meditation showed expert meditators (EMs) with 19,000 hours of practice had more overall brain activation than novices, but EMs with 44,000 hours had less activation [60]. However, another study found that the amount of time participants spent meditating each day, rather than the total number of hours of meditative practice over their lifetime, was correlated with attention performance [71].
The meditation state clearly differs from the resting state, particularly in the early stages, in that it involves effort. However, it is not yet clear whether the resting state changes as a result of meditation training. Studies indicate that meditation training may change the resting state, but different directions of change have been reported: two studies found increased resting state activity [72,73], whereas two others showed reduced resting state activity [74,75]. Plausible explanations for these conflicting results include the following: (i) the differences are due to the form of meditation used in the studies, (ii) differences between studies arise because the participants are in different stages of meditation involving varying amounts of effort, or (iii) differences may be due to varying numbers of persons achieving the meditation state during testing following the training. More research will be needed to resolve these inconsistencies.
Neural correlates of brain state control
Following earlier proposals [76,77], we hypothesize that the control of brain states includes the two components of switching and maintenance [76,77]. These two components are also present in the transition from rest to alertness and may be general characteristics of state control induced by instruction. The initial response to a warning signal involves an active voluntary response orchestrated by frontal areas including the ACC [30], whereas continued maintenance involves a direct current shift that remains present until the target occurs over the hemisphere opposite the expected target [78,79]. The transition to the alert state following a warning and most of the tasks described above requires only instruction to perform at a reasonably high level. However, establishing the meditation state not only requires instruction, but also involves practice and experience in achieving the state [31,36].
Switching between states
In an fMRI study of three different switching situations involving auditory or visual tasks in comparison with a task-free resting state, significant activation of the brain’s executive attention network was observed, along with activation of a third network comprising the right fronto-insular cortex (rFIC) and ACC, when participants perceived salient events [68]. These results indicate that the rFIC is likely to play a major role in switching between distinct brain networks across task paradigms and stimulus modalities [68]. The anterior insula and ACC have a close functional relationship and are co-activated at rest, suggesting that this connection is of fundamental importance [80,81]. Leber et al. found that in several brain regions, including basal ganglia, ACC, lateral PFC and PCC, neural activity preceding each trial predicted subsequent cognitive flexibility in switching between tasks [82].
Maintenance of states
Maintenance of some brain states can be difficult. For example, maintaining vigilance over long periods of time leads to a decline in detection performance [83], called the vigilance decrement, and to participant complaints about task difficulty. Similarly, in the early stages of meditation training, effort is required to maintain the state. As discussed above, mind wandering is frequent. Effort in this stage is indicated by increases in frontal and parietal activity [33,43,55,59]. However, it is still possible to use different training strategies, such as IBMT or open-minded practice, to maintain the state with less effort [33,43, 51,52,56].
In general, like other skill learning, long-term meditation is thought to enhance the ability to maintain the meditative state. As discussed previously, the early phase of meditation involves effortful control to enter the state [52,55]. Tang et al. [33] found similar frontal and parietal activation during relaxation training, during which the practitioner uses voluntary control to achieve a relaxed and calm state. The practitioner often experiences effort in preventing the mind from wandering and thus the executive attention network is engaged [43,51].
Training improves the ability to achieve and maintain the meditation state even if it is not practiced regularly [46]. The reduced difficulty in maintenance of the meditative state may be due to involvement of the autonomic nervous system in avoiding switching out of the state. The involvement of autonomic control in maintaining the meditation state may be compared to the task-based cognitive control exerted by the executive attention network [62]. In expert task performance, moment-to-moment control via a frontal-parietal system is suppressed, because the expert relies on fast automatic responses that are ballistic and need no control; however, the higher-level strategic control via the cingulo-opercular system may remain [62].
Relationships between states
The alert state and the meditation state
The three brain states are compared in Table 1. The meditation state differs from the alert state induced by a warning signal in several crucial ways. First, the alert state can be induced by the simple instruction to expect a target, without requiring any practice, whereas the meditation state requires specific instruction and practice. Second, the alert state requires an external target, whereas the meditation state may not involve a target event. Third, the alert state involves primarily the neuromodulator NE, whereas dopamine (DA) has often been shown to be important to the meditation state [51,84]. Finally, the alert state involves a reduction in ACC activity, likely in order to keep the mind clear to perceive and respond quickly to the target. The meditation state, however, shows increased ACC activity that serves to regulate mind wandering.
Table 1.
Comparison of three brain states
| Resting State | Alert State | Meditation State | |
|---|---|---|---|
| Brain Networks | DMN including mPFC, ACC, PCC, and others | Right PFC, PC, and others | Stage 1, Lateral PFC, PC Stage 3, ACC, insula, striatum |
| EEG | Alpha dominance | Desynchronized EEG signal | Mixed bands including alpha, theta, gamma |
| ANS | Sympathetic dominance | Stage A, parasympathetic dominance; Stage B, sympathetic dominance | Parasympathetic dominance |
| Neuromodulator | Norepinepherine (NE) | Dopamine (DA) |
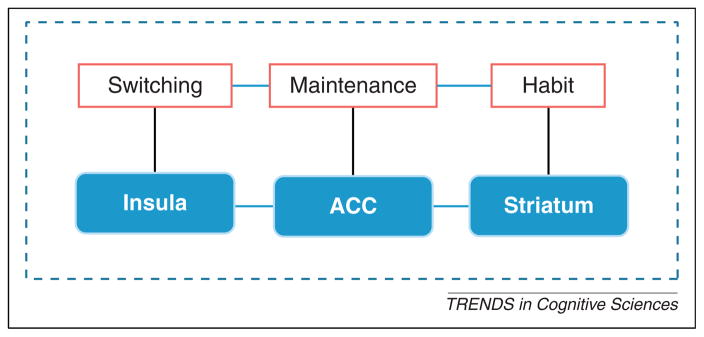
As mentioned previously, five days of IBMT increase brain activity in the ACC, insula, and striatum [33]. One month of IBMT improves white matter connectivity between the ACC, striatum, and other regions [47,53]. Based on these results and related work, we propose the insula, ACC, and stiatum (IAS) as key neural correlates of changing brain states (Figure 2). Because of its role in attention and self-regulation [32,47,50,65], we hypothesize that the ACC is involved in maintaining a state by reducing conflict with other states; the insula serves a primary role in switching between states [50,68,85], and the striatum is linked to the reward experience and formation of habits [46,47,51,52] needed to make maintenance easier. The insula and ACC work together to support the role of the autonomic nervous system in maintaining the meditation state [32,46,47,52,80].
Figure 2.
The IAS hypothesis. Key neural correlates of changing brain states include the insula, ACC, and striatum (IAS). The ACC is involved in maintaining a state by reducing conflict with other states; the insula serves a primary role in switching between states; the striatum is linked to the reward experience and formation of habits required to make state maintenance easier.
Future directions and applications
In this review, we have examined three brain states: the resting, alert and meditative states. We now consider the significance of understanding these states and suggest directions for future research (Box 2).
Box 2. Directions for future research.
Individual differences in switching and maintaining brain states
Developmental differences in brain states
Critical or sensitive periods for training brain state control
Relationship between PFC, ACC, insula, and striatum during different stages of meditative practice
Dynamics of change in brain states
Self-regulation and control of brain states
Educational success through control of brain states
Health benefits from control of brain states
Resting state data have been widely applied to the study of normal development [17–20] and to a variety of neuropsychiatric disorders, including schizophrenia, anxiety, and autism [21,22]. Making use of the ability to image the connectivity of the brain’s resting state will continue to increase our understanding of development and its disorders.
Sleep deprivation and drugs have also been repeatedly shown to influence the alert state. Tasks that require maintaining alertness over time have been shown to deteriorate following being deprived of sleep. Difficulties in obtaining the alert state during task performance have also been shown in normal aging and have been described as an important cause of ADHD [86]. Future research is needed on individual and developmental differences in the alert state and in the means of achieving the alert state.
Much of this review was devoted to the description of states observed in meditation training. Like all skills, achieving the meditative state differs among individuals. These differences may be due to genetic variations known to influence attention [87]. Future studies should be directed toward understanding the reasons for these individual differences. The brain mechanisms of expertise are not fully known [88]. As one form of expertise it will be useful to understand differences between novices and experts in reaching and maintaining the meditation state and the benefits they might gain from doing so.
In the early stage of training, the PFC is often involved in achieving both the meditation and the alert state. The interaction between the PFC, ACC, and striatum, which is more prominent in the advanced stages, would also benefit from continuing research.
Although IBMT has been taught with benefit to children, young adults, and the elderly [24,33,46–48,51,53,89,90], it is still unknown whether the ability to achieve the meditation state and its benefits is constant at different ages. Now that meditation studies can be done with relatively brief periods of training, it should be possible for future studies to explore whether sensitive periods exist and what benefits occur at different ages.
As we understand more about brain states and means for achieving and maintaining them, applications can also be more fully directed toward achieving benefits. These include application to clinical populations, for example, those suffering from ADHD, stress, mood disorders, drug abuse or other syndromes thought to benefit from meditation training. Meditation and training of alerting may also be applied to a wider population in the educational system to improve the development of attention and self-regulation, now widely thought to be crucial to success in school [91,92].
Acknowledgments
We would like to thank Rongxiang Tang for assistance with artwork on all figures. This work was supported by 973 Program 2012CB518200, the Office of Naval Research, and NIH grants HD 060563 and R21DA030066 (to Y.Y.T. and M.I.P.).
Glossary
- Alert state
the brain state that follows a warning related to a target event requiring a rapid response
- Brain state
the reliable patterns of brain activity that involve the activation and/or connectivity of multiple large-scale brain networks
- Contingent Negative Variation (CNV)
a negative direct current shift in electrophysiological recordings that occurs when a warning signal leads one to prepare for an upcoming target
- Default Mode Network (DMN)
a brain network that includes the medial prefrontal cortex (mPFC), anterior cingulate cortex (ACC), and posterior cingulate cortex (PCC); all these regions are active in the resting state
- Integrative Body-Mind Training (IBMT)
this mindfulness-based meditation technique originates from ancient eastern contemplative traditions, including traditional Chinese medicine, Zen, etc. IBMT stresses no effort or less effort to control thoughts, and the achievement of a state of restful alertness that allows a high degree of awareness and balance of the body, mind, and environment. The meditation state is facilitated through training and trainer-group dynamics, harmony, and resonance. A number of randomized clinical trials indicate that IBMT improves attention and self-regulation and induces neuroplasticity through interaction between the central and the autonomic nervous systems
- Local Field Potential (LFP)
electric potential generated in a volume of neural tissue by a local population of neurons
- Resting state
brain activity following the instruction to close or keep eyes open, relax, and not perform any task or think about anything specifically
- Resting state connectivity
the set of brain areas that show correlation during the resting state, including the default mode network and several other brain networks
References
- 1.Thoman EB. Sleeping and waking states in infants: a functional perspective. Neurosci Biobehav Rev. 1990;14:93–107. doi: 10.1016/s0149-7634(05)80165-4. [DOI] [PubMed] [Google Scholar]
- 2.Baldassarre A, et al. Individual variability in functional connectivity predicts performance of a perceptual task. Proc Natl Acad Sci USA. 2012;109:3516–3521. doi: 10.1073/pnas.1113148109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kounios J, Jung-Beeman M. Aha! The cognitive neuroscience of insight. Curr Dir Psychol Sci. 2009;18:210–216. [Google Scholar]
- 4.Yoo JJ, et al. When the brain is prepared to learn: enhancing human learning using real-time fMRI. Neuroimage. 2012;59:846–852. doi: 10.1016/j.neuroimage.2011.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deco G, et al. Emerging concepts for the dynamical organization of resting-state activity in the brain. Nat Rev Neurosci. 2011;12:43–56. doi: 10.1038/nrn2961. [DOI] [PubMed] [Google Scholar]
- 6.Kelly C, et al. Characterizing variation in the functional connectome: promise and pitfalls. Trends Cogn Sci. 2012;16:181–188. doi: 10.1016/j.tics.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–689. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- 8.Raichle ME. Two views of brain function. Trends Cogn Sci. 2010;14:180–190. doi: 10.1016/j.tics.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Raichle ME, et al. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeo BT, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272–1278. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- 12.Walker MP, Stickgold R. Sleep, memory, and plasticity. Annu Rev Psychol. 2006;57:139–166. doi: 10.1146/annurev.psych.56.091103.070307. [DOI] [PubMed] [Google Scholar]
- 13.Walker MP, Stickgold R. Sleep-dependent learning and memory consolidation. Neuron. 2004;44:121–133. doi: 10.1016/j.neuron.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 14.Wamsley EJ, et al. Dreaming of a learning task is associated with enhanced sleep-dependent memory consolidation. Curr Biol. 2010;20:850–855. doi: 10.1016/j.cub.2010.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris KD, Thiele A. Cortical state and attention. Nat Rev Neurosci. 2011;12:509–523. doi: 10.1038/nrn3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vincent JL, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- 17.Fair DA, et al. The maturing architecture of the brain’s default network. Proc Natl Acad Sci USA. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao W, et al. Evidence on the emergence of the brain’s default network from 2-week-old to 2-year-old healthy pediatric subjects. Proc Natl Acad Sci USA. 2009;106:6790–6795. doi: 10.1073/pnas.0811221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Power JD, et al. The development of human functional brain networks. Neuron. 2010;67:735–748. doi: 10.1016/j.neuron.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrews-Hanna JR, et al. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Broyd SJ, et al. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33:279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Zhang D, Raichle ME. Disease and the brain’s dark energy. Nat Rev Neurol. 2010;6:15–28. doi: 10.1038/nrneurol.2009.198. [DOI] [PubMed] [Google Scholar]
- 23.Laufs H, et al. Electroencephalographic signatures of attentional and cognitive default modes in spontaneous brain activity fluctuations at rest. Proc Natl Acad Sci USA. 2003;100:11053–11058. doi: 10.1073/pnas.1831638100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mantini D, et al. Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci USA. 2007;104:13170–13175. doi: 10.1073/pnas.0700668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He BJ, et al. Electrophysiological correlates of the brain’s intrinsic large-scale functional architecture. Proc Natl Acad Sci USA. 2008;105:16039–16044. doi: 10.1073/pnas.0807010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JH, et al. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature. 2010;465:788–792. doi: 10.1038/nature09108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Logothetis NK, et al. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 28.Kahneman D. Attention and Effort. Prentice-Hall; 1973. [Google Scholar]
- 29.Walter WG. Slow potential waves in the human brain associated with expectancy, attention and decision. Arch Psychiatr Nervenkr. 1964;206:309–322. [PubMed] [Google Scholar]
- 30.Nagai Y, et al. Brain activity relating to the contingent negative variation: an fMRI investigation. Neuroimage. 2004;21:1232–1241. doi: 10.1016/j.neuroimage.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 31.Raichle ME. A paradigm shift in functional brain imaging. J Neurosci. 2009;29:12729–12734. doi: 10.1523/JNEUROSCI.4366-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Critchley HD, et al. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003;126:2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- 33.Tang YY, et al. Central and autonomic nervous system interaction is altered by short-term meditation. Proc Natl Acad Sci USA. 2009;106:8865–8870. doi: 10.1073/pnas.0904031106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- 35.Marrocco RT, et al. Arousal systems. Curr Opin Neurobiol. 1994;4:166–170. doi: 10.1016/0959-4388(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 36.Morrison JH, Foote SL. Noradrenergic and serotoninergic innervation of cortical, thalamic tectaland visual structures in old and new world monkeys. J Comp Neurol. 1986;243:117–128. doi: 10.1002/cne.902430110. [DOI] [PubMed] [Google Scholar]
- 37.Posner MI. Chronometric Explorations of Mind. Erlbaum; 1978. [Google Scholar]
- 38.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 39.Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- 40.Ossandón T, et al. Transient suppression of broadband gamma power in the default-mode network is correlated with task complexity and subject performance. J Neurosci. 2011;31:14521–14523. doi: 10.1523/JNEUROSCI.2483-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ossandón T, et al. Efficient ‘pop-out’ visual search elicits sustained broadband γ activity in the dorsal attention network. J Neurosci. 2012;32:3414–3421. doi: 10.1523/JNEUROSCI.6048-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams JG, Jon KZ. Mindfulness: diverse perspectives on its meaning, origins, and multiple applications at the intersection of science and dharma. Contemp Buddhism. 2011;12:1–18. [Google Scholar]
- 43.Lutz A, et al. Attention regulation and monitoring in meditation. Trends Cgn Sci. 2008;12:163–169. doi: 10.1016/j.tics.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Travis F, Shear J. Focused attention, open monitoring and automatic self-transcending: Categories to organize meditations from Vedic, Buddhist and Chinese traditions. Conscious Cogn. 2010;19:1110–1118. doi: 10.1016/j.concog.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 45.Smith H, Novak P. Buddhism: A Concise Introduction. HarperCollins Press; 2003. [Google Scholar]
- 46.Tang YY. Exploring the Brain, Optimizing the Life. Science Press; 2009. [Google Scholar]
- 47.Tang YY. Mechanism of integrative body-mind training. Neurosci Bull. 2011;27:383–388. doi: 10.1007/s12264-011-1141-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang YY, et al. Short-term meditation training improves attention and self-regulation. Proc Natl Acad Sci USA. 2007;104:17152–17156. doi: 10.1073/pnas.0707678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cahn BR, Polich J. Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychol Bull. 2006;132:180–211. doi: 10.1037/0033-2909.132.2.180. [DOI] [PubMed] [Google Scholar]
- 50.Hölzel BK, et al. How does mindfulness meditation work? Proposing mechanisms of action from a conceptual and neural perspective. Persp Psychol Sci. 2011;6:537–559. doi: 10.1177/1745691611419671. [DOI] [PubMed] [Google Scholar]
- 51.Tang YY, Posner MI. Attention training and attention state training. Trends Cogn Sci. 2009;13:222–227. doi: 10.1016/j.tics.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 52.Posner MI, et al. Training effortless attention. In: Bruya B, editor. Effortless Attention: A New Perspective in the Cognitive Science of Attention and Action. The MIT Press; 2010. pp. 410–424. [Google Scholar]
- 53.Tang YY, et al. Short-term meditation induces white matter changes in the anterior cingulate. Proc Natl Acad Sci USA. 2010;107:15649–15652. doi: 10.1073/pnas.1011043107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chiesa A, Serretti A. A systematic review of neurobiological and clinical features of mindfulness meditations. Psychol Med. 2010;40:1239–1252. doi: 10.1017/S0033291709991747. [DOI] [PubMed] [Google Scholar]
- 55.Farb NA, et al. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Soc Cogn Affect Neurosci. 2007;2:313–322. doi: 10.1093/scan/nsm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Siegel DJ. The Mindful Brain: Reflection and Attunement in the Cultivation of Well-being. Norton Press; 2007. [DOI] [PubMed] [Google Scholar]
- 57.Hockey GRJ, et al. Energetics and Human Information Processing. Matinus Nijhoff; 1986. [Google Scholar]
- 58.Petersen SE, et al. The effects of practice on the functional anatomy of task performance. Proc Natl Acad Sci USA. 1998;1995:853–860. doi: 10.1073/pnas.95.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jensen CG, et al. Mindfulness training affects attention – or is it attentional effort? J Exp Psychol Gen. 2012;141:106–123. doi: 10.1037/a0024931. [DOI] [PubMed] [Google Scholar]
- 60.Brefczynski-Lewis JA, et al. Neural correlates of attentional expertise in long-term meditation practitioners. Proc Natl Acad Sci USA. 2007;104:11483–11488. doi: 10.1073/pnas.0606552104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roy M, et al. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn Sci. 2012;16:147–156. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Annu Rev Neurosci. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hasenkamp W, et al. Mind wandering and attention during focused meditation: a fine-grained temporal analysis of fluctuating cognitive states. Neuroimage. 2012;59:750–760. doi: 10.1016/j.neuroimage.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 64.Hölzel BK, et al. Differential engagement of anterior cingulated and adjacent medial frontal cortex in adept meditators and nonmeditators. Neurosci Lett. 2007;421:16–21. doi: 10.1016/j.neulet.2007.04.074. [DOI] [PubMed] [Google Scholar]
- 65.Posner MI, et al. The anterior cingulate gyrus and the mechanisms of self regulation. Cogn Affect Behav Neurosci. 2007;7:391–395. doi: 10.3758/cabn.7.4.391. [DOI] [PubMed] [Google Scholar]
- 66.Oppenheimer SM, et al. Cardiovascular effects of human insular cortex stimulation. Neurology. 1992;42:1727–1732. doi: 10.1212/wnl.42.9.1727. [DOI] [PubMed] [Google Scholar]
- 67.Oppenheimer SM, et al. Left-insular cortex lesions perturb cardiac autonomic tone in humans. Clin Auton Res. 1996;6:131–140. doi: 10.1007/BF02281899. [DOI] [PubMed] [Google Scholar]
- 68.Sridharan D, et al. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zeidan F, et al. Mindfulness meditation improves cognition: evidence of brief mental training. Conscious Cogn. 2010;19:597–605. doi: 10.1016/j.concog.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 70.Leiberg S, et al. Short-term compassion training increases prosocial behavior in a newly developed prosocial game. PLoS ONE. 2011;6:e17798. doi: 10.1371/journal.pone.0017798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chan D, Woollacott M. Effects of level of meditation experience on attentional focus: is the efficiency of executive or orientation networks improved? J Altern Complement Med. 2007;13:651–657. doi: 10.1089/acm.2007.7022. [DOI] [PubMed] [Google Scholar]
- 72.Jang JH, et al. Increased default mode network connectivity associated with meditation. Neurosci Lett. 2011;487:358–362. doi: 10.1016/j.neulet.2010.10.056. [DOI] [PubMed] [Google Scholar]
- 73.Travis F, et al. A self-referential default brain state: patterns of coherence, power, and eLORETA sources during eyes-closed rest and Transcendental Meditation practice. Cogn Process. 2010;11:21–30. doi: 10.1007/s10339-009-0343-2. [DOI] [PubMed] [Google Scholar]
- 74.Brewer JA, et al. Meditation experience is associated with differences in default mode network activity and connectivity. Proc Natl Acad Sci USA. 2011;108:20254–20259. doi: 10.1073/pnas.1112029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Berkovich-Ohana A, et al. Mindfulness-induced changes in gamma band activity - Implications for the default mode network, self-reference and attention. Clin Neurophysiol. 2011;123:700–710. doi: 10.1016/j.clinph.2011.07.048. [DOI] [PubMed] [Google Scholar]
- 76.Dosenbach NUF, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci. 2010;14:277–290. doi: 10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 78.Harter MR, Guido W. Attention to pattern orientation-negative cortical potentials, reaction-time, and the selection process. Electroencephalogr Clin Neurophysiol. 1980;49:461–475. doi: 10.1016/0013-4694(80)90389-2. [DOI] [PubMed] [Google Scholar]
- 79.Rosler F, et al. Slow negative brain potentials as reflections of specific modular resources of cognition. Biol Psychiatry. 1997;45:109–141. doi: 10.1016/s0301-0511(96)05225-8. [DOI] [PubMed] [Google Scholar]
- 80.Medford N, Critchley HD. Conjoint activity of anterior insular and anterior cingulate cortex: awareness and response. Brain Struct Funct. 2010;214:535–549. doi: 10.1007/s00429-010-0265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bush G, et al. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 82.Leber AB, et al. Neural predictors of moment-to-moment fluctuations in cognitive flexibility. Proc Natl Acad Sci USA. 2008;105:13592–13597. doi: 10.1073/pnas.0805423105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grier RA, et al. The vigilance decrement reflects limitations in effortful attention, not mindlessness. Hum Factors. 2003;45:349–359. doi: 10.1518/hfes.45.3.349.27253. [DOI] [PubMed] [Google Scholar]
- 84.Lou HC, et al. A 15O-H2O PET study of meditation and the resting state of normal consciousness. Hum Brain Mapp. 1999;7:98–105. doi: 10.1002/(SICI)1097-0193(1999)7:2<98::AID-HBM3>3.0.CO;2-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Craig AD. How do you feel – now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 86.Halperin JM, Healey DM. The influences of environmental enrichment, cognitive enhancement, and physical exercise on brain development: can we alter the developmental trajectory of ADHD? Neurosci Biobehav Rev. 2011;35:621–634. doi: 10.1016/j.neubiorev.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Posner MI, et al. Attention genes. Dev Sci. 2007;10:24–29. doi: 10.1111/j.1467-7687.2007.00559.x. [DOI] [PubMed] [Google Scholar]
- 88.Tanaka JW, Curran T. A neural basis for expert object recognition. Psychol Sci. 2001;12:43–47. doi: 10.1111/1467-9280.00308. [DOI] [PubMed] [Google Scholar]
- 89.Tang YY. Multi-intelligence and Unfolding the Full Potentials of Brain. Dalian University of Technology Press; 2007. [Google Scholar]
- 90.Tang YY, et al. Improving executive function and its neurobiological mechanisms through a mindfulness-based intervention: advances within the field of developmental neuroscience. Child Dev Persp. 2012 doi: 10.1111/j.1750-8606.2012.00250.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Posner MI. Attention in the Social World. Oxford University Press; 2012. [Google Scholar]
- 92.Posner MI, Rothbart MK. Educating the Human Brain. APA Books; 2007. [Google Scholar]
- 93.Lutz A, et al. BOLD signal in insula is differentially related to cardiac function during compassion meditation in experts vs. novices. Neuroimage. 2009;47:1038–1046. doi: 10.1016/j.neuroimage.2009.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]