Abstract
Racial/ethnic minorities experience persistent health disparities due in part to their exposure to chronic SES and psychosocial risk. The hypothalamic-pituitary-adrenal axis and its hormonal end product, cortisol, are believed to mediate the associations between chronic stress and poor health. In this study, racial/ethnic differences in diurnal salivary cortisol rhythms in 179 preadolescent youths and the contributing roles of SES risk, psychosocial risk, perceived discrimination, harsh parenting, and parental monitoring were examined. The analyses revealed racial/ethnic differences in diurnal cortisol rhythms, with African Americans having significantly flatter morning-to-evening cortisol slopes than Caucasians and with Latinos having significantly lower evening cortisol levels than Caucasians. Greater psychosocial risk and less parental monitoring were associated with flatter cortisol slopes. Racial/ethnic differences on the cortisol measures persisted when controlling for SES, psychosocial risk, and parenting quality. The need to assess chronic risk across the lifespan and disentangle possible genetic from environmental contributors is discussed.
Keywords: cortisol, race/ethnicity, preadolescent youths, psychosocial risk, parental monitoring
Introduction
Health disparities among racial/ethnic minorities in the United States are well documented. In comparison to Caucasians, African Americans and Latinos experience disproportionately greater morbidity and mortality across many diseases, including cancer, cardiovascular disease, and diabetes (Centers for Disease Control and Prevention, 2011; National Center for Health Statistics, 2010; Walsemann et al., 2008). Despite recent improvements in the health of the general population (National Center for Health Statistics, 2010; Sondik et al., 2010), these improvements have not impacted all racial/ethnic groups equally; there are stable or increasing prevalence rates for chronic and life-threatening diseases among many minority populations (Orsi et al., 2010; Sondik et al., 2010). Thus, understanding the complex, multidimensional nature of health disparities and identifying the underlying mechanisms of these disparities is increasingly important (see Adler and Rehkopf, 2008). Prior research findings suggest that the hypothalamic-pituitary-adrenal (HPA) axis is a potential mediator between chronic stress and poor physical health (Chrousos, 2009; de Kloet et al., 1998; McEwen, 2008). As such, the HPA axis might play a critical role in the persistence of disparate morbidity and mortality among racial/ethnic minorities.
Racial/Ethnic Differences in Diurnal Cortisol Rhythms
Cortisol, a glucocorticoid hormone, is the end product of HPA axis activation in humans. In healthy populations, the HPA axis exhibits a diurnal pattern of activity (Schmidt-Reinwald et al., 1999; Stone et al., 2001). Cortisol levels typically peak 30-45 min after waking and decline gradually throughout the day to levels near zero in the evening. The HPA axis also responds to physical and psychological stress (Johnson et al., 1992; Sapolsky et al., 2000). Exposure to stress has been shown to have a profound effect on the functioning of the HPA system, but the direction of the effect (i.e., increased vs. decreased diurnal cortisol levels) appears to depend upon factors such as the type, chronicity, and severity of the stressor (Miller et al., 2007). For example, exposure to chronic stress (vs. acute stress) is more often associated with lower morning and higher evening cortisol levels, resulting in a flatter diurnal slope (Fries et al., 2005; Gunnar and Vazquez, 2001; Miller et al., 2007). In turn, flatter diurnal slopes have been associated with chronic disease (Kumari et al., 2011; Nijm et al., 2007).
The literature on racial/ethnic disparities in cortisol is not extensive but demonstrates divergent diurnal cortisol rhythms for African Americans and Latinos compared to Caucasians (Cohen, Schwartz, et al., 2006; DeSantis et al., 2007; Hajat et al., 2010; Suglia et al., 2010; c.f., Cohen, Doyle, et al., 2006). African Americans have been found to have flatter diurnal cortisol slopes, with lower morning levels and higher evening levels, than Caucasians across studies of adolescents (DeSantis et al., 2007), pregnant women (Suglia et al., 2010), adults (Cohen, Schwartz, et al., 2006; Hajat et al., 2010), and elderly participants (McCallum et al., 2006). Although prior researchers have shown that the diurnal cortisol rhythms for Latinos and Caucasians also differ, the nature of the differences is inconsistent across studies. For example, Hajat et al. found that, similar to African American adults, Latino adults had significantly lower morning cortisol levels compared to Caucasian adults. However, in contrast to African Americans and Caucasians, Latinos demonstrated a steeper decline in cortisol late in the day, resulting in lower evening cortisol levels. In contrast, DeSantis et al. found that Latino adolescents demonstrated flatter cortisol slopes with higher evening levels compared to Caucasians. In sum, although African Americans have consistently been shown to have lower morning and higher evening cortisol levels compared to Caucasians, the research findings with Latinos have been somewhat equivocal.
The role of contextual risk factors
Low SES and psychosocial risk have often been associated with dysregulated diurnal cortisol levels in the general population (Gustafsson et al., 2010; Miller et al., 2007; c.f., Dowd et al., 2009). These risk factors might similarly affect the relationship between minority status and dysregulated diurnal cortisol rhythms. Racial/ethnic minorities are disproportionately more likely to live in poverty and to be at greater risk for psychosocial stressors such as racism and discrimination, interparental violence, parental depression and stress, and multiple caregiver transitions (Fomby and Cherlin, 2007; Hatch and Dohrenwned, 2007; Holman et al., 2000; Raphael et al., 2010; Spence et al., 2011). Moreover, exposure to adversity without sufficient access to financial resources appears to be especially detrimental (Matthews and Gallo, 2011; Thoits, 2010); racial/ethnic minorities are less likely to have access to such resources (Thoits, 2010). However, racial/ethnic differences in diurnal cortisol rhythms have been found even when SES, psychosocial, and health-related risk factors are controlled (Cohen, Schwartz, et al., 2006; DeSantis et al., 2007; Hajat et al., 2010; Suglia et al., 2010). Furthermore, although minorities living in poverty appear to be the most disadvantaged in terms of health outcomes (Williams and Jackson, 2005), the interaction between SES and race/ethnicity has not been supported when examining differences in biological indicators of stress (Cohen, Schwartz, et al., 2006; Juster et al., 2010). Thus, additional factors should be considered when examining racial/ethnic differences in diurnal cortisol rhythms. Potential contributors that have yet to be examined include parenting qualities such as harsh parenting and parental monitoring.
The role of harsh parenting and parental monitoring
Within the last decade, a consensus has emerged that early life experiences have a substantial influence on physical and mental health (Shonkoff et al., 2009). Exposure to family adversity (e.g., harsh parenting, abuse, and neglect) has been consistently linked with indicators of poor health in childhood (Flaherty et al., 2009), adolescence (Miller and Chen, 2010), and adulthood (Dube et al., 2009). Previous research findings also suggest that there are racial/ethnic differences in family adversity. For example, differential exposure to harsh parenting has been found between racial/ethnic groups, with African American and Latino youths being more likely to experience harsh discipline and injurious spanking compared to Caucasian youths (Cardona et al., 2000; Hawkins et al., 2010; MacKenzie et al., 2011; Pinderhughes et al., 2000). Racial/ethnic differences have also been found in terms of parental monitoring, with African American parents monitoring their youths less than Caucasian or Latino parents (Tolma et al., 2011). In turn, these parenting qualities have been linked to dysregulated diurnal cortisol rhythms in children (Gunnar, 1998; Gunnar et al., 1996; Gunnar and Donzella, 2002; Lucas-Thompson and Goldberg, 2011). For example, low maternal involvement and warmth has been linked to flatter diurnal cortisol slopes in children (Flinn and England, 1997; Pendry and Adam, 2007). Notably, responsive and supportive caregiving has been found to attenuate the negative effects of being raised in stressful environments (Evans et al., 2007). Similarly, a parenting intervention that increases parental responsiveness and monitoring has been shown to impact diurnal cortisol rhythms in foster children, with cortisol slopes becoming more akin to those of non-foster children (Fisher and Stoolmiller, 2008; Fisher et al., 2007). Although these parenting qualities have been associated with dysregulated diurnal cortisol rhythms, little research has been conducted to examine this relationship in racially/ethnically diverse samples.
Objectives and Hypotheses of the Current Study
We examined racial/ethnic differences in diurnal cortisol rhythms in a diverse sample of preadolescents, expanding upon prior research by examining the roles of contextual risk factors (i.e., SES risk, psychosocial risk, and perceived discrimination) and parenting (i.e., harsh parenting and parental monitoring). In line with previous findings (Cohen, Schwartz, et al., 2006, DeSantis et al., 2007; Hajat et al., 2010), we hypothesized that African American youths would have significantly flatter diurnal cortisol slopes compared to Caucasian youths, exhibiting lower morning and higher evening levels. Due to the inconsistent research findings on cortisol in Latinos, we did not make any a priori hypotheses regarding the direction of the effect for this group. We further predicted that racial/ethnic differences would persist after controlling for contextual risk factors. However, considering the racial/ethnic differences in harsh parenting and parental monitoring and the link between these parenting qualities and dysregulated cortisol, we hypothesized that the racial/ethnic differences in cortisol would be accounted for by these parenting variables.
Material and Methods
Participants
The participants (N = 242) were recruited as a long-term follow-up subsample from the Healthy Families America (HFA) San Diego clinical trial (Landsverk et al., 2002). The HFA intervention is a widely implemented home visitation program for high-risk families with newborns aimed at improving parenting, promoting healthy child development, and preventing maltreatment. The original study recruited 488 families at birth based on being at risk for child maltreatment, and the children were followed for 36 months. The follow-up study was conducted after a 6-year hiatus, when the children were 9-12 years old. No significant intervention effects on reducing child maltreatment have been found to date in the HFA San Diego clinical trial (Landsverk et al., 2002). Nonetheless, we examined these potential group effects.
Salivary cortisol samples were collected for 196 of the youths. Youths were excluded if they were taking medications containing corticosteroids (n = 5), did not provide two of the three cortisol samples for at least 2 of the 3 sampling days (n = 1), ate full meals 30 min prior to each cortisol collection (n = 1), or did not provide questionnaire data (n = 10). The final analytic sample consisted of 179 youths, aged 9-12 years (M = 10.97 years, SD = 0.68 years; 53% female), and their primary parent. The youths were racially/ethnically diverse based on parent reports: 50% (n = 90) Latino or Hispanic descent, 16% (n = 29) multiracial, 15% (n = 27) African American, 15% (n = 27) Caucasian, and 4% (n = 6) Asian American or Pacific Islander. The parents were primarily female (92%) and were biological parents (89%) or biological relatives (8%). Additional caregivers included adoptive parents, foster parents, and stepparents. The youths in the final analytic sample did not differ significantly from the excluded youths in terms of age, gender, race/ethnicity, intervention status, SES risk, psychosocial risk, perceived discrimination, harsh parenting, or parental monitoring.
Procedures
All study procedures were approved by the IRBs for San Diego State University, Children’s Hospital of San Diego, and Oregon Social Learning Center. Parent consent/permission and child assent were obtained prior to participation. Assessments were completed in the family’s homes (n = 140) or over the phone for families who had moved from the area (n = 39). Assessments were conducted in English (n = 158) or Spanish (n = 21) based on family preference. The youths and parents separately completed assessments that each lasted approximately 2.5 hr. At the end of the assessments, the assessors demonstrated the salivary cortisol collection procedures.
Measures
Translation and cultural equivalence of measures
The study measures were translated into Spanish and then back-translated into English and compared to the original English versions. Modifications were made in consultation with the back-translator. The final versions of the translated measures were reviewed by native Spanish-speaking translators. Disagreements among translators were discussed and resolved by consensus to ensure that the translations corresponded with the Spanish dialect spoken locally. The reading level of the measures was purposefully kept low due to the low literacy levels of many of the monolingual Spanish-speaking families. To ensure that the translated measures were equivalent, we compared the internal reliabilities for the translated Spanish versions and the original English versions. Reliabilities for the Spanish and English versions were similar across measures: .67-.95. The lowest coefficient alpha (.67) was found for the English version of the Parent-Child Conflict Tactics Scale. Although there is no absolute cutoff number designating an alpha as adequate or inadequate, an alpha of .70 tends to be the standard cutoff (Schmitt, 1996). However, because the alpha was .71 when examining the English and Spanish versions together, this measure was retained for the current study. Nevertheless, we discuss the implications of the low alpha in our limitations section: correlations with variables that have low coefficient alphas can be attenuated (Schmitt, 1996).
SES and psychosocial risk
Composite risk scores were constructed for SES and psychosocial risk. The SES risk score was calculated by standardizing and averaging family income and parental education scores. Annual, after-tax family income was assessed via parent report. The parents were provided with a table depicting 11 possible income categories: “less than $4,900” to “$50,000 or more” in increments of $5,000. Parental education was categorized as 0 (no GED or high school diploma), 1 (GED or high school diploma), 2 (some college), 3 (associate’s degree), or 4 (bachelor’s or graduate degree). For two-parent families, the parental education scores were averaged. SES risk scores were recoded so that higher scores were indicative of greater risk. Two parents were uncertain of their family income, and their risk scores were based on parental education only. There were no missing data for parental education.
The psychosocial risk score was constructed with four indicators of risk: parental depression, parenting stress, caregiver transitions, and interparental violence. Each has been associated with dysregulated cortisol patterns (Davies et al., 2008; Dougherty et al., 2011; Flinn and England, 1997; Koch et al., 2010; Luecken et al., 2009; Pendry and Adam, 2007). Parental depression was assessed via the Center for Epidemiologic Studies Depression Scale (Radloff, 1977). The parents were asked to rate the frequency with which they experienced each item during the past week: 0 (rarely or none of the time) to 3 (most or all of the time). This scale has been shown to have good construct validity and reliability (Knight et al., 1997). For the current study, the coefficient alpha for the scale was .91. Parenting stress was measured via the Parent Daily Report (Chamberlain and Reid, 1987), a 40-item checklist of daily child problem behaviors and the parental stress associated with these problem behaviors. Responses were dichotomized as 0 (not stressful) or 1 (stressful). An average daily parenting stress score was calculated across the 3 salivary cortisol sampling days. This measure has been shown to have acceptable reliability and validity (Weinrott et al., 1979). Caregiver transitions were measured via a parent interview to assess any instance in which a person moved in or out of the child’s home or the child’s primary parent changed since birth. Interparental violence was measured using the Revised Conflict Tactics Scales (Straus et al., 1996), which assesses the frequency of various methods for handling conflict with a romantic partner during the past year: 0 (never) to 6 (more than 20 times). This measure has been shown to have good construct and discriminant validity and internal consistency (Straus et al., 1996). For this study, an interparental violence composite was constructed by averaging responses from the Psychological Aggression and Physical Assault subscales (8 and 12 items, respectively). The coefficient alpha was .80 for psychological aggression and .81 for physical assault. Data from the psychosocial risk measures were missing for some participants: parental depression (n = 1), parenting stress (n = 11), and interparental violence (n = 6). No participant was missing data for more than one measure. As with the SES risk score, scores on the psychosocial risk measures were standardized and averaged to create a composite score. The psychosocial risk scores were transformed using a square-root transformation to normalize its positively skewed distribution.
Perceived discrimination
Perceived racial/ethnic discrimination was assessed using a 12-item, self-report measure for youth adapted by Martinez et al. (2002) from a similar measure for adults (Kessler et al., 1999). The youths were asked to endorse negative events that occurred “because of your race, ethnicity, skin color, language, or nationality” during the past 3 months. For endorsed items, each youth was asked to indicate how stressful each event was using a 5-point Likert-type scale. A coefficient alpha for the current sample was .78. A logarithmic transformation was used to correct for the positively skewed distribution.
Harsh parenting
The Parent-Child Conflict Tactics Scales (Straus et al., 1998), a parent-report questionnaire, was used to assess the frequency of harsh parenting practices during the past year: 0 (never) to 6 (more than 20 times). The measure has been shown to have adequate test-retest reliability, internal consistency, and construct validity (Straus et al., 1998). For the current study, scores from the Psychological Aggression and Weekly Discipline subscales were summed to construct an 8-item index of harsh parenting, with a coefficient alpha of .71. A square-root transformation was used to normalize the positively skewed distribution.
Parental monitoring
Parental monitoring was assessed via the Monitor and Parent-Child Relationship Questionnaire (Capaldi and Wilson, 1998). The parents were asked to rate the frequency of six parental monitoring experiences over the past 6 months (e.g., “How often has your child played out of adult eyesight and hearing by themselves”): 1 (never) to 5 (very often). Scores were recoded so that higher scores indicate higher levels of parental monitoring. The coefficient alpha was .72. A square-root transformation was used to normalize the negatively skewed distribution.
Salivary cortisol
Salivary cortisol was collected three times per day for 3 days (i.e., nine samples) in the child’s home. Saliva collection occurred 30 min after waking (morning), between 4:00 and 5:00 p.m. (afternoon), and 30 min prior to bedtime (evening). Prior to each collection, the youth was instructed not to eat, drink, or brush his/her teeth; deviations from these guidelines were recorded. The parent also recorded the youth’s general health, medication use, wake and bed times, and saliva collection times on a brief questionnaire each day. To stimulate salivation at each sampling time, the youth chewed Trident® Original Flavor sugarless gum for 1 min and used a straw to expel saliva into a prelabeled vial. The vials were then labeled with the collection date and time by the parent, refrigerated, and mailed to the laboratory after all of the samples had been collected. In the laboratory, the samples were stored at −20° C until they were mailed to the Biochemical Laboratory at the University of Trier for analysis. The samples were assayed in duplicate using a competitive solid phase time-resolved fluorescence immunoassay with fluoromeric end point detection (Dressendörfer et al., 1992). The lower sensitivity limit of this assay is 0.006 μg/dl. The samples from each youth were included in the same assay batch to minimize within-subject variability. Duplicates varying by more than 15% were reassayed. The intraassay coefficients of variance ranged 4.44-5.00%, and the interassay coefficients of variance ranged 6.61-8.31%.
Of the 1611 possible saliva samples from our 179 participants, 34 were missing because they were not collected or returned, 2 were excluded because of out-of-range cortisol values (> 2.0 μg/dl), 35 were excluded for being collected outside the specified sampling window, and 14 were excluded for being collected after an illness or injury. Only participants who provided two of the three cortisol samples for at least 2 of the 3 sampling days were included in our analysis (N = 179). For day 1, 88% had three useable samples, 10% had two, and 2% had one or none. For day 2, 78% had three useable samples, 15% had two, and 7% had one or none. For day 3, 81% had three useable samples, 13% had two, and 6% had one or none.
Morning, afternoon, and evening cortisol values were significantly correlated at p < .001 across the 3 sampling days (r = .36-.46, .33-.55, and .36-.38, respectively) and were averaged across days to create more reliable measures. The average cortisol values for each sampling time were then transformed using a logarithmic transformation to correct for positive skew. In addition, three outliers (i.e., values greater than 2.5 SD above or below the mean) for the morning, five for the afternoon, and five for the evening were replaced with less extreme values (i.e., the next highest or lowest value) to further normalize the distribution for values at each sampling time. Cortisol slope coefficients were calculated by regressing the untransformed average cortisol values on the average sampling times for each participant. Higher cortisol slopes indicated a flatter slope (i.e., a smaller decline across the day). Two cortisol slope coefficients were more than 2.5 SD below the mean and were replaced with the next steepest slope coefficient. All cortisol variables were standardized so that each unit of change represented 1 SD.
Covariates
The covariates included age, gender, nonsteroid medication usage, average hours of sleep, average wake time, and average latency to morning sampling (i.e., the time between wake time and the morning sampling time). Potential covariates that were not used in the analyses because they were not associated with race/ethnicity or cortisol slope include intervention status, level of pubertal development, menstruation status, average expressed negative emotion, and depressive symptoms.
Statistical Analyses
Bivariate Pearson correlation coefficients were computed to examine the relationships between the covariates, contextual risk variables, parenting variables, race/ethnicity, and cortisol slope coefficients. Covariates that were significantly correlated with race/ethnicity or cortisol slope were retained and incorporated into a hierarchical multiple regression analysis predicting cortisol slope. Step 1 of the regression model included covariates and racial/ethnic group variables dummy coded to examine potential differences between youths in each minority group and the Caucasian youths. (Because only 6 youths were identified as Asian or Pacific Islander, these youths were included with the multiracial group in our analyses.) The contextual risk variables were added in Step 2, and the parenting variables were added in Step 3. To aid in the interpretation of these findings, similar hierarchical multiple regression analyses were conducted for each sampling time. Partial correlations from the hierarchical multiple regression models are reported for significant findings to indicate effect size.
Results
Preliminary Analyses
The average cortisol slope coefficients and average untransformed values for each sampling time (measured in μg/dl) are shown in Table 1. As expected, the majority of the slope coefficients (96%) were negative, indicating decreasing cortisol levels across the day. Bivariate correlations indicated significant associations between race/ethnicity, cortisol slope, and a number of the covariates, contextual risk variables, and parenting variables (see Table 2). That is, race/ethnicity was related to age, nonsteroid medication usage, SES risk, psychosocial risk, perceived discrimination, and parental monitoring; and cortisol slope was related to gender, wake time, psychosocial risk, and parental monitoring. Likewise, we found significant associations when examining the bivariate correlations between race/ethnicity and cortisol slope.
Table 1. Descriptive Statistics for Study Variables.
| Variable | N | M / % | SD | Min | Max |
|---|---|---|---|---|---|
| Covariates | |||||
| Age (years) | 179 | 10.97 | 0.68 | 9.56 | 12.63 |
| Gender (female) | 179 | 53% | |||
| Nonsteroid medication usage | 179 | 17% | |||
| Sleep (hours) | 179 | 9.13 | 0.88 | 6.67 | 12.23 |
| Wake time (A.M., hours) | 179 | 7:03 | 1.01 | 5:10 | 10:08 |
| Latency to morning sampling (minutes) | 179 | 21.29 | 15.25 | 0.00 | 88.33 |
| Race/ethnicity | |||||
| African American | 27 | 15% | |||
| Caucasian | 27 | 15% | |||
| Latino/Hispanic | 90 | 50% | |||
| Multiracial | 35 | 20% | |||
| Contextual variables | |||||
| SES risk | |||||
| Annual incomea | 177 | 6.88 | 3.00 | 1.00 | 11.00 |
| Parental educationb | 179 | 1.46 | 1.00 | 0.00 | 4.00 |
| Psychosocial risk | |||||
| Parental depression | 178 | 8.19 | 9.03 | 0.00 | 42.00 |
| Parenting stress | 168 | 4.46 | 5.55 | 0.00 | 38.00 |
| Caregiver transitions | 179 | 1.97 | 1.99 | 0.00 | 13.00 |
| Interparental violence | 173 | 5.52 | 8.77 | 0.00 | 40.50 |
| Perceived discrimination | 179 | 2.56 | 5.71 | 0.00 | 30.00 |
| Parenting variables | |||||
| Harsh parenting | 179 | 23.09 | 21.80 | 0.00 | 104.00 |
| Parental monitoring | 179 | 26.54 | 3.69 | 16.00 | 30.00 |
| Cortisol variables (μg/dl) | |||||
| Slope coefficients | 179 | −0.02 | 0.02 | −0.09 | 0.02 |
| Morning levels | 179 | 0.40 | 0.20 | 0.06 | 1.29 |
| Afternoon levels | 175 | 0.13 | 0.11 | 0.01 | 1.02 |
| Evening levels | 179 | 0.08 | 0.11 | 0.00 | 1.04 |
Note. All values are raw, untransformed values.
Annual income was categorized with 11 possible income categories, in increments of $5,000 starting from 1 (less than $4,900) to 11 ($50,000 or more).
Parental education was categorized as 0 (no GED or high school diploma), 1 (GED or high school diploma), 2 (some college), 3 (associate’s degree), and 4 (bachelor’s or graduate degree).
Table 2. Correlations Between Study Variables.
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Caucasian | — | ||||||||||||||
| 2. African American | −0.18* | — | |||||||||||||
| 3. Latino/Hispanic | −0.42*** | −0.42*** | — | ||||||||||||
| 4. Multiracial | −0.21** | −0.21** | −0.50*** | — | |||||||||||
| 5. Age | 0.15* | −0.06 | −0.20** | 0.07 | — | ||||||||||
| 6. Gender | −0.01 | −0.04 | 0.01 | 0.04 | −0.03 | — | |||||||||
| 7. Nonsteroid medication usage | 0.06 | 0.18* | −0.19** | 0.04 | 0.25** | −0.01 | — | ||||||||
| 8. Sleep | −0.06 | −0.10 | 0.10 | 0.02 | −0.13† | −0.04 | −0.04 | — | |||||||
| 9. Wake time | 0.02 | −0.06 | −0.04 | 0.08 | 0.03 | −0.11 | −0.01 | 0.41*** | — | ||||||
| 10. Latency to morning sampling | −0.09 | 0.00 | 0.12 | −0.06 | −0.01 | 0.02 | −0.20** | −0.02 | 0.01 | — | |||||
| 11. SES risk | −0.14† | −0.12 | 0.26*** | −0.09 | −0.12 | 0.11 | −0.16* | 0.12 | 0.17* | 0.13 | — | ||||
| 12. Psychosocial risk | −0.07 | 0.13† | −0.17* | 0.16* | 0.11 | 0.02 | 0.14† | −0.05 | 0.09 | −0.05 | −0.02 | — | |||
| 13. Perceived discrimination | −0.15* | 0.18* | 0.06 | −0.10 | −0.02 | −0.01 | 0.06 | −0.05 | 0.07 | 0.05 | 0.11 | 0.23** | — | ||
| 14. Harsh parenting | 0.04 | 0.11 | −0.13† | 0.03 | 0.07 | −0.02 | 0.09 | −0.03 | 0.09 | 0.00 | −0.05 | 0.42*** | 0.15* | — | |
| 15. Parental monitoring | −0.31*** | −0.18* | 0.48*** | −0.17* | −0.25** | 0.19* | −0.23** | 0.17* | −0.10 | 0.15* | 0.25** | −0.35** | −0.05 | −0.22** | — |
| 16. Cortisol slope coefficients | −0.05 | 0.25** | −0.18* | 0.05 | −0.03 | −0.15* | −0.02 | 0.04 | 0.26*** | 0.01 | −0.01 | 0.25** | 0.08 | 0.08 | −0.27*** |
| 17. Cortisol morning values | 0.08 | −0.24** | 0.10 | 0.02 | 0.09 | 0.23** | 0.06 | −0.10 | −0.33*** | −0.04 | 0.00 | −0.14† | −0.07 | −0.02 | 0.22** |
| 18. Cortisol afternoon values | 0.02 | 0.08 | −0.11 | 0.06 | −0.02 | 0.06 | −0.07 | 0.05 | −0.01 | 0.06 | 0.10 | 0.04 | 0.06 | 0.05 | −0.03 |
| 19. Cortisol evening values | 0.10 | 0.08 | −0.23** | 0.13 | 0.00 | 0.13 | −0.03 | 0.03 | 0.00 | 0.07 | −0.07 | 0.13 | 0.09 | 0.02 | −0.10 |
Note. Gender: 0 (male), 1 (female).
p < .10.
p < .05.
p < .01.
p < .001.
Race/Ethnicity and Cortisol Slope
Step 1 of the hierarchical linear regression, which included the covariates and racial/ethnic group variables, accounted for a significant amount of the variance in cortisol slope, R2 = .17, F(9, 160) = 3.79, p < .001 (see Table 3). Significant predictors included wake time and being African American. Later wake time was related to flatter cortisol slopes: each additional hour was associated with an increase of .28 SD (partial r = .26). The African American youths were also more likely than Caucasian youths to have flatter slopes with an average increase of .26 SD in cortisol slope for these youths (partial r = .22). No other covariates or racial/ethnic group variables significantly contributed to cortisol slope.
Table 3. Multiple Regression Analysis Predicting Cortisol Slope Coefficients (N = 179).
| Step 1 | Step 2 | Step 3 | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| B | SE | B | SE | B | SE | |
| Covariates | ||||||
| Age | −0.056 | 0.075 | −0.071 | 0.074 | −0.086 | 0.073 |
| Gender | −0.120† | 0.071 | −0.127† | 0.070 | −0.095 | 0.071 |
| Nonsteroid medication usage | −0.068 | 0.075 | −0.085 | 0.075 | −0.097 | 0.074 |
| Sleep | −0.056 | 0.078 | −0.045 | 0.078 | −0.018 | 0.078 |
| Wake time | 0.277*** | 0.078 | 0.259** | 0.079 | 0.240** | 0.079 |
| Latency to morning sampling | −0.003 | 0.072 | −0.010 | 0.072 | 0.024 | 0.071 |
| Race/ethnicity | ||||||
| African American | 0.264** | 0.093 | 0.235* | 0.094 | 0.263** | 0.094 |
| Latino/Hispanic | −0.045 | 0.106 | −0.047 | 0.107 | 0.042 | 0.115 |
| Multiracial | 0.069 | 0.096 | 0.028 | 0.100 | 0.056 | 0.096 |
| Contextual variables | ||||||
| SES risk | −0.011 | 0.075 | 0.008 | 0.075 | ||
| Psychosocial risk | 0.210** | 0.074 | 0.188* | 0.082 | ||
| Perceived discrimination | −0.024 | 0.074 | −0.025 | 0.073 | ||
| Parenting variables | ||||||
| Harsh parenting | −0.072 | 0.076 | ||||
| Parental monitoring | −0.191* | 0.090 | ||||
| R 2 | 0.168 | 0.207 | 0.232 | |||
Note. The reference category for gender is female. The reference category for race/ethnicity is Caucasian.
p < .10.
p < .05.
p < .01.
p < .001.
Step 2, which included the contextual risk variables, contributed to a significant change in the variance in cortisol slope explained, ΔR2 = .04, F(3, 166) = 2.73, p = .045. Increases in psychosocial risk were related to flatter cortisol slopes, where each unit increase in psychosocial risk was associated with an increase of .21 SD in cortisol slope (partial r = .22). Wake time and being African American remained significant predictors: each additional hour was associated with an increase of .26 SD (partial r = .25), and identifying as African American compared to Caucasian was associated with an increase of .24 SD (partial r = .19).
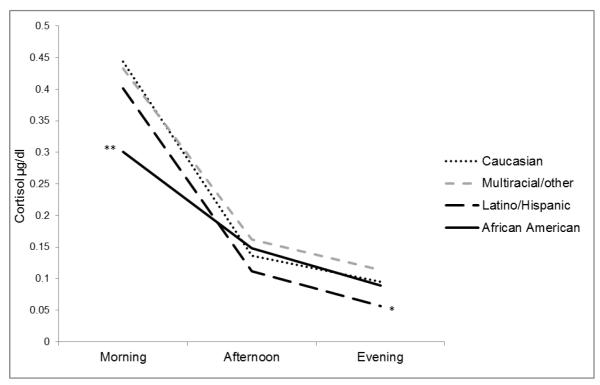
Although Step 3, which included the parenting variables, did not significantly increase the variance explained in the model, ΔR2 = .03, F(2, 164) = 2.64, p = .074, the full model accounted for a significant amount of the variance in cortisol slope, R2 = .23, F(14, 164) = 3.54, p < .001. Later wake time, being African American, having greater psychosocial risk, and having lower parental monitoring were significantly related to having flatter cortisol slopes: each additional hour was associated with an increase of .24 SD (partial r = .23), identifying as African American compared to Caucasian was associated with an increase of .26 SD (partial r = .21), each unit increase in psychosocial risk was associated with an increase of .19 SD (partial r = .18), and each unit increase in parental monitoring was associated with a decrease of .19 SD (partial r = −.16). Being African American was still associated with cortisol once the parenting variables were added to the model. Thus, differences in parenting did not account for the racial/ethnic differences in diurnal cortisol slopes. Diurnal cortisol slopes by race/ethnicity are shown in Figure 1.
Figure 1.
Racial/ethnic differences in cortisol patterns. Note. Adjusted means, after controlling for the covariates, contextual factors, and parenting variables, are for the raw, untransformed cortisol values. *Caucasian > Latino/Hispanic, p < .05. **Caucasian > African American, p < .01.
To aid in the interpretation of these results, a hierarchical linear regression was conducted for each sampling time. The results of these analyses suggest that the flatter cortisol slopes for the African American youths resulted from significantly lower morning cortisol values, even in the fully adjusted model, t(164) = −3.27, p = .001, partial r = −.25. The African American youths did not differ from the Caucasian youths in afternoon or evening values. Indeed, there were no significant racial/ethnic differences for the afternoon cortisol values. The Latino youths had significantly lower evening values compared to the Caucasian youths, even in the fully adjusted model, t(164) = −2.33, p = .021, partial r = −.18.
Discussion and Conclusions
We examined racial/ethnic differences in diurnal cortisol rhythms in a diverse sample of preadolescents. Consistent with the results of similar studies with adults (e.g., Cohen, Schwartz, et al., 2006), the magnitude of the racial/ethnic differences in diurnal cortisol slope observed in the current study were small. However, these differences persisted even after controlling for SES risk, psychosocial risk, perceived discrimination, harsh parenting, and parental monitoring. Specifically, we found that the African American youths had flatter cortisol profiles (and lower morning levels) than the Caucasian youths. Although there were no other racial/ethnic differences in diurnal cortisol slope, the Latino youths had lower evening cortisol levels than the Caucasian youths. Thus, our findings provide additional evidence for racial/ethnic differences in diurnal cortisol levels (e.g., Cohen, Schwartz, et al., 2006; Hajat et al., 2010) and suggest that these differences can be observed as early as preadolescence.
In addition to racial/ethnic differences, we found that higher levels of psychosocial risk factors (i.e., parental depression, parenting stress, multiple caregiver transitions, and interparental violence) and lower levels of parental monitoring were associated with flatter cortisol slopes in this sample of preadolescent youth. However, these factors did not account for racial/ethnic differences in cortisol slope. These results further support indications that parental psychosocial risk impacts children’s HPA system functioning (Davies et al., 2008; Dougherty et al., 2011; Flinn and England, 1997; Koch et al., 2010; Luecken et al., 2009; Pendry and Adam, 2007). Parental monitoring also influenced diurnal cortisol rhythms: youths with less parental monitoring showed flatter cortisol slopes than youths with greater parental monitoring. These results are consistent with studies demonstrating that less responsive and involved parenting is associated with flatter diurnal cortisol rhythms (Evans et al., 2007; Pendry and Adam, 2007). Moreover, our results correspond to findings that an intervention focused on increasing parental monitoring and responsiveness results in more typical diurnal cortisol rhythms in preschool-aged foster children (Fisher and Stoolmiller, 2008; Fisher et al., 2007).
In contrast, SES risk, perceived discrimination, and harsh parenting were not associated with diurnal cortisol slope. Low SES is frequently thought to be linked to cortisol levels; however, the results from studies examining diurnal cortisol slope are inconsistent in regard to this relationship (Cohen, Doyle, et al., 2006; DeSantis et al., 2007). Moreover, Cohen et al. (Cohen, Doyle, et al., 2006; Cohen, Schwartz, et al., 2006) found that risky health behaviors and psychosocial risk were primarily responsible (up to 94%) for associations between SES and cortisol levels. Thus, perhaps the risky health behaviors and psychosocial risk that frequently coincide with low SES drive the relationship between SES and diurnal cortisol rhythms rather than low SES itself. Indeed, in the current study, greater psychosocial risk was related to flatter cortisol slopes. Likewise, discrimination is often speculated to be associated with dysregulated cortisol rhythms; similar to other studies (e.g., Cohen, Schwartz, et al., 2006; Suglia et al., 2010), though, we found no association. The youths in our study endorsed very few items on the measure of perceived discrimination and might not have experienced the magnitude or chronicity of discrimination that could lead to dysregulated cortisol rhythms. Alternatively, perhaps minority youths are buffered from the negative effects of discrimination as a result of their age or social support. Finally, researchers that have found an association between harsh parenting and cortisol have focused on infancy and early childhood (e.g., Bugental et al., 2003), when harsh parenting occurs more frequently (Straus et al., 1998). Thus, the developmental period in which harsh parenting occurs might be a critical determinant (Shonkoff et al., 2009). Moreover, Deater-Deckard and Dodge (1997) found evidence that the broader, culturally based motives or the underlying meaning of harsh parenting practices is of greater import than harsh parenting practices alone in regard to negative outcomes for children. Thus, the context in which harsh parenting occurs might be essential. Alternatively, the lower internal reliability for our harsh parenting measure might have attenuated the correlation between harsh parenting and the diurnal cortisol slopes (Schmitt, 1996).Little is known regarding why racial/ethnic differences persist after controlling for contextual risk factors such as SES and psychosocial risk. Perhaps researchers have not adequately measured risk and resiliency. For example, most prior researchers (Cohen, Schwartz, et al., 2006; DeSantis et al., 2007; Suglia et al., 2010) only assessed risk factors experienced within the prior year. Examining risk and protective factors across the lifespan, particularly at key developmental periods, might provide more nuanced measures of risk and resiliency (Shonkoff et al., 2009). There might also be differences in temporal exposure to risk. In nonhuman research, intermittent stress exposure has been shown to be associated with resilience in contrast to the negative outcomes associated with chronic stress exposure (Lyons et al., 2010). Moreover, the prenatal period might be particularly relevant. Maternal stress during pregnancy, regardless of gestational period, has been associated with flatter cortisol rhythms (Obel et al., 2005) and with poor infant health (Beijers et al., 2010). Furthermore, African American women have been shown to experience more stressors during pregnancy than Caucasian women, delivering infants with lower average birth weights and increased complications (Oklahoma State Department of Health, 2009). Examining intergenerational risk via differential exposure to prenatal stress and other environmental stressors throughout childhood might provide insight into such diurnal cortisol rhythms.
Genetic differences could also contribute to the disparate diurnal cortisol rhythms of racial/ethnic minorities. Genetic differences are believed to have a greater influence on cortisol production in the morning than in the afternoon or evening (Bartels et al., 2003). Therefore, the finding that African Americans have lower morning cortisol levels compared to Caucasians might reflect genetic differences. Although strong heritability has been demonstrated for morning cortisol levels, Franz et al. (2010) found that, in a study of twins, morning cortisol concentrations are also responsive to environmental factors. Although late afternoon and evening levels are believed to be more strongly influenced by environmental factors (Bartels et al., 2003), our results suggest that African American youths do not differ from Caucasian youths in their evening cortisol levels. In contrast, higher evening levels among African Americans have been reported in adolescence (DeSantis et al., 2007) and adulthood (Cohen, Schwartz, et al., 2006; Hajat et al., 2010; Suglia et al., 2010). Thus, the increased evening cortisol levels typically found in African American adults might not emerge until after middle childhood.
The Latino youths in our study had diurnal cortisol rhythms that largely (except for lower evening levels) resembled those of the Caucasian youths. In previous studies, Latino adults have shown similarly steep declines in cortisol late in the day despite increased stress (Hajat et al., 2010; Suglia et al., 2010). However, these researchers did not examine potential protective factors such as social support or cultural factors that could account for these differences in evening cortisol levels. Indeed, Latinos have been shown to have strong social support within their families and communities and cultural values that might attenuate the effects of chronic stressors (Gallo et al., 2009). In a study with Mexican-American adults, Mangold et al. (2010) found that higher levels of acculturation were associated with attenuation of the cortisol awakening response, suggesting that a loss of protective cultural values might negatively impact the HPA system.
Our study was limited by several factors. First, salivary cortisol collection was limited to 3 times per day, which precluded an examination of the cortisol awakening response. Second, although the parents reported child wake time and saliva collection times, we did not monitor these times electronically. Thus, we cannot ensure the accuracy of the parent reports. Given the link between parental monitoring and diurnal cortisol slope, perhaps the parents who monitored their children more closely also complied with the saliva collection protocol more accurately; thus, their children might have had steeper slopes (owing to higher morning cortisol levels) compared to children with less parental monitoring. However, even when parental monitoring was included in the analytic model, we found racial/ethnic differences in diurnal cortisol slope and morning cortisol levels. Moreover, we included the length of time between wake time and the morning sampling time as a covariate, but it did not predict diurnal cortisol slope, suggesting that our findings are not merely a lack of compliance. Additionally, the results from studies that have employed electronic monitoring to ensure compliance (e.g., Hajat et al., 2010) were similar to our results in terms of the racial/ethnic differences in diurnal cortisol slope. Third, future researchers might benefit from using longitudinal studies to examine causality; larger racial/ethnic group sample sizes, especially for Asian Americans and Pacific Islanders; less reliance on parent reports by including more child reports and/or observational measures of risk and protective factors; and assessments of body mass index, risky health behaviors (e.g., smoking and exercise), and prenatal and genetic factors.
Despite these limitations, our results offer a unique look into racial/ethnic differences in diurnal cortisol rhythms among a diverse sample of at-risk preadolescent youths. Consistent with studies of adolescents and adults, the African American youths in our study had significantly flatter diurnal cortisol slopes and lower morning levels compared to the Caucasian youths. Overall, the cortisol slopes for the Latino youths did not differ from those of the Caucasian youths, but their evening cortisol levels were significantly lower. SES risk, perceived discrimination, and harsh parenting were not associated with cortisol slope. However, less psychosocial risk and greater parental monitoring was associated with steeper cortisol slopes. The role of parental monitoring and other parenting behaviors on diurnal cortisol rhythms should continue to be examined, especially in high-risk families. To better inform prevention/intervention efforts aimed at decreasing health disparities among racial/ethnic minorities, additional research is needed to assess risk across the lifespan, to examine the developmental trajectory and emergence of disparate cortisol slopes in African American youths, and to disentangle the genetic and environmental contributions on the HPA axis.
Highlights.
Racial/ethnic differences in diurnal cortisol rhythms in preadolescent youth.
African American youths had significantly flatter cortisol slopes than Caucasians.
Latino youths had significantly lower evening cortisol levels than Caucasians.
Psychosocial stress and less parental monitoring linked to flatter cortisol slopes.
Racial/ethnic differences in cortisol remained when contextual risk was controlled.
Acknowledgements
Funding for this research was provided by the following grants: HD045894, NICHD, NIH, U.S. PHS; MH059780, MH020012, and MH078105, NIMH, NIH, U.S. PHS; and DA023920, NIDA, NIH, U.S. PHS. The authors thank the families who participated in the study; John Landsverk, Cynthia Connelly, and their colleagues at the Child and Adolescent Services Research Center in San Diego; and Alice Graham, Kristen Greenly, and Matthew Rabel.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler NE, Rehkopf DH. U.S. disparities in health: Descriptions, causes, and mechanisms. Annu. Rev. Public Health. 2008;29:235–252. doi: 10.1146/annurev.publhealth.29.020907.090852. [DOI] [PubMed] [Google Scholar]
- Bartels M, de Geus EJC, Kirschbaum C, Sluyter F, Boomsma DI. Heritability of daytime cortisol level in children. Behav. Genet. 2003;33:421–433. doi: 10.1023/a:1025321609994. [DOI] [PubMed] [Google Scholar]
- Beijers R, Jansen J, Riksen-Walraven M, de Weerth C. Maternal prenatal anxiety and stress predict infant illnesses and health complaints. Pediatrics. 2010;126:e401–e409. doi: 10.1542/peds.2009-3226. [DOI] [PubMed] [Google Scholar]
- Bugental DB, Martorell GA, Barraza V. The hormonal costs of subtle forms of infant maltreatment. Horm. Behav. 2003;43:237–244. doi: 10.1016/s0018-506x(02)00008-9. [DOI] [PubMed] [Google Scholar]
- Capaldi DM, Wilson J. Monitor and Parent-Child Relationship Questionnaire. 1998. Unpublished instrument available from the Oregon Social Learning Center. [Google Scholar]
- Cardona PG, Nicholson BC, Fox RA. Parenting among Hispanic and Anglo-American mothers with young children. J. Soc. Psychol. 2000;140:357–365. doi: 10.1080/00224540009600476. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention CDC health disparities and inequalities report— United States, 2011. Morbidity Mortality Weekly Rep. 2011;60(Suppl.):1–114. [PubMed] [Google Scholar]
- Chamberlain P, Reid JB. Parent observation and report of child symptoms. Behav. Assess. 1987;9:97–109. [Google Scholar]
- Chrousos GP. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Baum A. Socioeconomic status is associated with stress hormones. Psychosom. Med. 2006;68:414–420. doi: 10.1097/01.psy.0000221236.37158.b9. [DOI] [PubMed] [Google Scholar]
- Cohen S, Schwartz JE, Epel E, Kirschbaum C, Sidney S, Seeman T. Socioeconomic status, race, and diurnal cortisol decline in the coronary artery risk development in young adults (CARDIA) study. Psychosom. Med. 2006;68:41–50. doi: 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- Davies PT, Sturge-Apple ML, Cicchetti D, Cummings EM. Adrenocortical underpinnings of children’s psychological reactivity to interparental conflict. Child Dev. 2008;79:1693–1706. doi: 10.1111/j.1467-8624.2008.01219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, Vreugdenhil E, Oitzl MS, Joels A. Brain corticosteroid receptor balance in health and disease. Endocr. Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- Deater-Deckard K, Dodge KA. Externalizing behavior problems and discipline revisited: Nonlinear effects and variation by culture, context, and gender. Psychol. Inq. 1997;8:161–175. [Google Scholar]
- DeSantis AS, Adam EK, Doane LD, Mineka S, Zinbarg RE, Craske MG. Racial/ethnic differences in cortisol diurnal rhythms in a community sample of adolescents. J. Adolesc. Health. 2007;41:3–13. doi: 10.1016/j.jadohealth.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Dougherty LR, Klein DN, Rose S, Laptook RS. Hypothalamic-pituitary-adrenal axis reactivity in the preschool-age offspring of depressed parents: Moderation by early parenting. Psychol. Sci. 2011;22:650–658. doi: 10.1177/0956797611404084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd JB, Simanek AM, Aiello AE. Socio-economic status, cortisol and allostatic load: a review of the literature. Int. J. Epidemiol. 2009;38:1297–1309. doi: 10.1093/ije/dyp277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressendörfer RA, Kirschbaum C, Rohde W, Stahl F, Strasburger CJ. Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. J. Steroid Biochem. Mol. Biol. 1992;43:683–692. doi: 10.1016/0960-0760(92)90294-s. [DOI] [PubMed] [Google Scholar]
- Dube SR, Fairweather D, Pearson WS, Felitti VJ, Anda RF, Croft JB. Cumulative childhood stress and autoimmune diseases in adults. Psychosom. Med. 2009;71:243–250. doi: 10.1097/PSY.0b013e3181907888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW, Kim P, Ting A, Tesher H, Shannis D. Cumulative risk, maternal responsiveness, and allostatic load among young adolescents. Dev. Psychol. 2007;43:341–351. doi: 10.1037/0012-1649.43.2.341. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Stoolmiller M. Intervention effects on foster parent stress: Associations with child cortisol levels. Dev. Psychopathol. 2008;20:1003–1021. doi: 10.1017/S0954579408000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PA, Stoolmiller M, Gunnar MR, Burraston BO. Effects of a therapeutic intervention for foster preschoolers on diurnal cortisol activity. Psychoneuroendocrinology. 2007;32:892–905. doi: 10.1016/j.psyneuen.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty EG, Thompson R, Litrownik AJ, Zolotor AJ, Dubowitz H, Runyan DK, English DJ, Everson MD. Adverse childhood exposures and reported child health at age 12. Acad. Pediatr. 2009;9:150–156. doi: 10.1016/j.acap.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Flinn MV, England BG. Social economics of childhood glucocorticoid stress response and health. Am. J. Phys. Anthropol. 1997;102:33–53. doi: 10.1002/(SICI)1096-8644(199701)102:1<33::AID-AJPA4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Fomby P, Cherlin AJ. Family instability and child well-being. Am. Sociol. Rev. 2007;72:181–204. doi: 10.1177/000312240707200203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz CE, York TP, Eaves LJ, Mendoza SP, Hauger RL, Hellhammer DH, Jacobson KC, Levine S, Lupien SJ, Lyons MJ, Prom-Wormley E, Xian H, Kremen WS. Genetic and environmental influences on cortisol regulation across days and contexts in middle-aged men. Behav. Genet. 2010;40:467–479. doi: 10.1007/s10519-010-9352-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30:1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Gallo LC, Penedo FJ, Espinosa de los Monteros K, Arguelles W. Resiliency in the face of disadvantage: do Hispanic cultural characteristics protect health outcomes? J. Pers. 2009;77:1707–1746. doi: 10.1111/j.1467-6494.2009.00598.x. [DOI] [PubMed] [Google Scholar]
- Gunnar MR. Quality of early care and buffering of neuroendocrine stress reactions: potential effects on the developing human brain. Prev. Med. 1998;27:208–211. doi: 10.1006/pmed.1998.0276. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Brodersen L, Nachmias M, Buss M, Rigatuso J. Stress reactivity and attachment security. Dev. Psychobiol. 1996;29:191–204. doi: 10.1002/(SICI)1098-2302(199604)29:3<191::AID-DEV1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27:199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development. Dev. Psychopathol. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Gustafsson PE, Anckarsäter H, Lichtenstein P, Nelson N, Gustafsson PA. Does quantity have a quality all its own? Cumulative adversity and up- and down-regulation of circadian salivary cortisol levels in healthy children. Psychoneuroendocrinology. 2010;35:1410–1415. doi: 10.1016/j.psyneuen.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Hajat A, Diez-Roux A, Franklin TG, Seeman T, Shrager S, Ranjit N, Castro C, Watson K, Sanchez B, Kirschbaum C. Socioeconomic and race/ethnic differences in daily salivary cortisol profiles: the Multi-Ethnic Study of Atherosclerosis. Psychoneuroendocrinology. 2010;35:932–943. doi: 10.1016/j.psyneuen.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch SL, Dohrenwend BP. Distribution of traumatic and other stressful life events by race/ethnicity, gender, SES, and age: A review of the research. Am. J. Community Psychol. 2007;40:313–332. doi: 10.1007/s10464-007-9134-z. [DOI] [PubMed] [Google Scholar]
- Hawkins AO, Danielson CK, de Arellano MA, Hanson RF, Ruggiero KJ, Smith DW, Saunders BE, Kilpatrick DG. Ethnic/racial differences in the prevalence of injurious spanking and other child physical abuse in a national survey of adolescents. Child Maltreat. 2010;15:242–249. doi: 10.1177/1077559510367938. [DOI] [PubMed] [Google Scholar]
- Holman EA, Silver RC, Waitzkin H. Traumatic events in primary care patients: a study in an ethnically diverse sample. Arch. Fam. Med. 2000;9:802–810. doi: 10.1001/archfami.9.9.802. [DOI] [PubMed] [Google Scholar]
- Johnson EO, Kamilaris TC, Chrousos GP, Gold PW. Mechanisms of stress: A dynamic overview of hormonal and behavioral homeostasis. Neurosci. Biobehav. Rev. 1992;16:115–130. doi: 10.1016/s0149-7634(05)80175-7. [DOI] [PubMed] [Google Scholar]
- Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci. Biobehav. Rev. 2010;35:2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Mickelson KD, Williams DR. The prevalence, distribution, and mental health correlates of perceived discrimination in the United States. J. Health Soc. Behav. 1999;40:208–230. [PubMed] [Google Scholar]
- Knight RG, Williams S, McGee R, Olaman S. Psychometric properties of the Centre for Epidemiologic Studies Depression Scale (CES-D) in a sample of women in middle life. Behav. Res. Ther. 1997;35:373–380. doi: 10.1016/s0005-7967(96)00107-6. [DOI] [PubMed] [Google Scholar]
- Koch FS, Ludvigsson J, Sepa A. Parents’ psychological stress over time may affect children’s cortisol at age 8. J. Pediatr. Psychol. 2010;35:950–959. doi: 10.1093/jpepsy/jsp132. [DOI] [PubMed] [Google Scholar]
- Kumari M, Shipley M, Stafford M, Kivimaki M. Association of diurnal patterns in salivary cortisol with all-cause and cardiovascular mortality: findings from the Whitehall II study. J. Clin. Endocrinol. Metab. 2011;96:1478–1485. doi: 10.1210/jc.2010-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsverk J, Carrilio T, Connelly CD, Ganger WC, Slymen DJ. Healthy Families San Diego clinical trial: technical report. San Diego Children’s Hospital and Health Center, Child and Adolescent Services Research Center; San Diego, CA: 2002. [Google Scholar]
- Lucas-Thompson RG, Goldberg WA. Family relationships and children’s stress responses. Adv. Child. Dev. Behav. 2011;40:243–299. doi: 10.1016/b978-0-12-386491-8.00007-4. [DOI] [PubMed] [Google Scholar]
- Luecken LJ, Kraft A, Hagan MJ. Negative relationships in the family-of-origin predict attenuated cortisol in emerging adults. Horm. Behav. 2009;55:412–417. doi: 10.1016/j.yhbeh.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons DM, Parker KJ, Schatzberg AF. Animal models of early life stress: implications for understanding resilience. Dev. Psychobiol. 2010;52:616–624. doi: 10.1002/dev.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie MJ, Nicklas E, Brooks-Gunn J, Waldfogel J. Who spanks infants and toddlers? Evidence from the fragile families and child well-being study. Child Youth Serv. Rev. 2011;33:1364–1373. doi: 10.1016/j.childyouth.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangold D, Wand G, Javors M, Mintz J. Acculturation, childhood trauma and the cortisol awakening response in Mexican-American adults. Horm. Behav. 2010;58:637–646. doi: 10.1016/j.yhbeh.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez CR, Ruth B, Goldman M. Perceived Discrimination—Youth. 2002. Unpublished instrument available from the Oregon Social Learning Center. [Google Scholar]
- Matthews KA, Gallo LC. Psychological perspectives on pathways linking socioeconomic status and physical health. Annu. Rev. Psychol. 2011;62:501–530. doi: 10.1146/annurev.psych.031809.130711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum TJ, Sorocco KH, Fritsch T. Mental health and diurnal salivary cortisol patterns among African American and European American female dementia family caregivers. Am. J. Geriatr. Psychiatry. 2006;14:684–693. doi: 10.1097/01.JGP.0000225109.85406.89. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur. J. Pharmacol. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E. Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychol. Sci. 2010;21:848–856. doi: 10.1177/0956797610370161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol. Bull. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics . Health, United States, 2009: with special feature on medical technology. Author; Hyattsville, MD: 2010. [PubMed] [Google Scholar]
- Nijm J, Kristenson M, Olsson AG, Jonasson L. Impaired cortisol response to acute stressors in patients with coronary disease: Implications for inflammatory activity. J. Intern. Med. 2007;262:375–384. doi: 10.1111/j.1365-2796.2007.01817.x. [DOI] [PubMed] [Google Scholar]
- Obel C, Heegaard M, Henriksen TB, Secher NJ, Olsen J, Levine S. Stress and salivary cortisol during pregnancy. Psychoneuroendocrinology. 2005;30:647–656. doi: 10.1016/j.psyneuen.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Oklahoma State Department of Health Stressors, social support and pregnancy outcomes among African American and white mothers. Oklahoma Pregn. Risk Assess. Monitor Sys. 2009;13:1–6. [Google Scholar]
- Orsi JM, Margellos-Anast H, Whitman S. Black-White health disparities in the United States and Chicago: a 15-year progress analysis. Am. J. Public Health. 2010;100:349–356. doi: 10.2105/AJPH.2009.165407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendry P, Adam EK. Associations between parents’ marital functioning, maternal parenting quality, and maternal emotion and child cortisol levels. Int. J. Behav. Dev. 2007;31:218–231. [Google Scholar]
- Pinderhughes EE, Dodge KA, Bates JE, Petit GS, Zelli A. Discipline responses: influences of parents’ socioeconomic status, ethnicity, beliefs about parenting, stress, and cognitive-emotional processes. J. Fam. Psychol. 2000;14:380–400. doi: 10.1037//0893-3200.14.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl. Psychol Meas. 1977;1:385–401. [Google Scholar]
- Raphael JL, Zhang Y, Liu H, Giardino AP. Parenting stress in US families: implications for pediatric healthcare utilization. Child Care Health Dev. 2010;36:216–224. doi: 10.1111/j.1365-2214.2009.01052.x. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero M, Munck A. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schmidt-Reinwald A, Pruessner JC, Hellhammer DH, Federenko I, Rohleder N, Schürmeyer TH, Kirschbaum C. The cortisol response to awakening in relation to different challenge tests and a 12-hour cortisol rhythm. Life Sci. 1999;64:1635–1660. doi: 10.1016/s0024-3205(99)00103-4. [DOI] [PubMed] [Google Scholar]
- Schmitt N. Uses and abuses of coefficient alpha. Psychol. Assess. 1996;8:350–353. [Google Scholar]
- Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA. 2009;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- Sondik EJ, Huang DT, Klein RJ, Satcher D. Progress toward the healthy people 2010 goals and objectives. Annu. Rev. Public Health. 2010;31:271–281. doi: 10.1146/annurev.publhealth.012809.103613. [DOI] [PubMed] [Google Scholar]
- Spence NJ, Adkins DE, Dupre ME. Racial differences in depression trajectories among older women: socioeconomic, family, and health influences. J. Health Soc. Behav. 2011;52:444–459. doi: 10.1177/0022146511410432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone AA, Schwartz JE, Smyth J, Kirschbaum C, Cohen S, Hellhammer D, Grossman S. Individual differences in the diurnal cycle of salivary free cortisol: A replication of flattened cycles for some individuals. Psychoneuroendocrinology. 2001;26:295–306. doi: 10.1016/s0306-4530(00)00057-3. [DOI] [PubMed] [Google Scholar]
- Straus MA, Hamby SL, Boney-McCoy S, Sugarman DB. The Revised Conflict Tactics Scales (CTS2): development and preliminary psychometric data. J. Fam. Iss. 1996;17:283–316. [Google Scholar]
- Straus MA, Hamby SL, Finkelhor D, Moore DW, Runyan D. Identification of child maltreatment with the parent-child conflict tactics scales: development and psychometric data for a national sample of American parents. Child Abuse Negl. 1998;22:249–270. doi: 10.1016/s0145-2134(97)00174-9. [DOI] [PubMed] [Google Scholar]
- Suglia SF, Staudenmayer J, Cohen S, Enlow MB, Rich-Edwards JW, Wright RJ. Cumulative stress and cortisol disruption among Black and Hispanic pregnant women in an urban cohort. Psychol. Trauma. 2010;4:326–334. doi: 10.1037/a0018953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoits PA. Stress and health: major findings and policy implications. J. Health Soc. Behav. 2010;51(Suppl.):41–53. doi: 10.1177/0022146510383499. [DOI] [PubMed] [Google Scholar]
- Tolma EL, Oman RF, Vesely SK, Aspy CB, Beebe L, Fluhr J. Parental youth assets and sexual activity: differences by race/ethnicity. Am. J. Health Behav. 2011;35:513–524. doi: 10.5993/ajhb.35.5.1. [DOI] [PubMed] [Google Scholar]
- Walsemann KM, Geroniumus AT, Gee GC. Accumulating disadvantage over the live course: evidence from a longitudinal study investigating the relationship between educational advantage in youth and health in middle age. Res. Aging. 2008;30:169–199. [Google Scholar]
- Weinrott M, Bauske B, Patterson GR. Systematic replication of a social learning approach to parent training. In: Bates SL, Sjoden PO, Dockens WS III, editors. Trends in behavior therapy. Academic Press; New York: 1979. pp. 331–351. [Google Scholar]
- Williams DR, Jackson PB. Social sources of racial disparities in health. Health Aff. 2005;24:325–334. doi: 10.1377/hlthaff.24.2.325. [DOI] [PubMed] [Google Scholar]