Abstract
Background
Although low positive affect (PA) and high negative affect (NA) have been posited to predispose to depressive disorders, little is known about the developmental trajectories of these affects in children at familial risk for mood disorders.
Methods
We examined 202 offspring of mothers who had a history of juvenile-onset unipolar depressive disorder (n = 60) or no history of major psychopathology (n = 80). Offspring participated in up to seven annual, structured laboratory tasks that were designed to elicit PA and NA.
Results
Growth curve analyses revealed that PA increased linearly and similarly for all children from late infancy through age 9. However, there also were individual differences in early PA. Relative to control peers, offspring of mothers with lifetime unipolar depression had consistently lower levels of PA, and this association remained significant even when controlling for current maternal depression and maternal affect displays. Growth curve analyses also revealed a significant linear decrease in NA in children across time; however, there was no significant inter-individual variation either in early NA or rate of change in NA.
Conclusion
Attenuated PA (rather than excessive NA) may be an early vulnerability factor for eventual unipolar depressive disorder in at-risk children and may represent one pathway through which depression is transmitted.
Keywords: Positive affect, negative affect, depression risk, developmental trajectories
There has been increasing interest in the role of positive affect (PA) and negative affect (NA) or affective style in human adjustment and in whether atypical affectivity contributes to risk of affective psychopathology such as depression (e.g., Durbin, Klein, Hayden, Buckley, & Moerk, 2005; Rothbart, Ahadi, & Evans, 2000). In the empirical literature, PA and NA usually refer to individual differences in facial, vocal, and bodily displays and subjective experiences of pleasure and joy versus sadness, fear, distress, and anger, respectively (McCrae & Costa, 1987; Olino, Klein, Durbin, Hayden, & Buckley, 2005). Not surprisingly, clinical depression is associated with high levels of negative affect and low levels of positive affect (e.g., Chorpita & Daleiden, 2002; Joiner, Catanzaro, & Laurent, 1996; Luby, Mrakotsky, Heffelfinger, Brown, & Spitznagel, 2004; Watson et al., 1995). Importantly, however, it has been proposed that the tendency to heightened dysphoria and low positive affect also may be substrates or precursors of clinical depression (Durbin et al., 2005; Meehl, 1975). Meehl (1975) has specifically emphasized that the ability to activate ‘pleasure centers’ in the brain is of great clinical importance and that low hedonic capacity is probably one pathway to depressive illness. If low hedonic capacity and heightened negative affectivity indeed are precursors of depressive disorders, then these attributes should be more evident in populations at elevated risk for clinical depression compared to those at low risk for this outcome.
One population at high risk for depressive disorders is comprised of the young offspring of parents with diagnostic histories of depression (for an overview, see Kovacs & Lopez-Duran, 2010). Because the high-risk period for onset of depressive illness commences in adolescence, studying very young, preadolescent offspring of depressed parents affords the opportunity to characterize true precursors of depressive illness, with important implications for primary prevention. Studies of young children at familial risk for depression indicate that the affect displays of such youngsters differ from that of their low-risk peers, although the findings are somewhat equivocal regarding infants and toddlers. Specifically, cross-sectional, observational studies of 3-year-old and older offspring (or samples with wider age ranges) have consistently reported that at-risk youngsters display lower levels of PA but similar levels of NA than do comparison peers (Dietz et al., 2008; Durbin et al., 2005; Shaw et al., 2006). However, infant/toddler at-risk offspring are either affectively similar to controls, or more likely to display NA and less likely to display PA, with inconsistent between-group differences across time (Feng, Shaw, Skuban, & Lane, 2007; Forbes, Cohn, Allen, & Lewinsohn, 2004; Kochanska, 1991).
The equivocal age-specific findings regarding levels of NA and PA of at-risk versus control offspring could mirror normative variations in affect across the childhood years. However, there are no longitudinal investigations that could shed light on this matter since follow-up studies of affectivity in children at familial risk for depression have spanned no more than about one year (Feng et al., 2007; Forbes et al., 2004). Therefore, the central goal of the present study was to examine developmental trajectories of NA and PA from infancy to late childhood in the context of high- and low-familial risk for depression.
The study of familial depression and offspring’s affective characteristics must also take into account additional variables that may influence the developmental trajectories of interest. One of these variables concerns the possible impact of non-affective disorders in the depressed parent. Depressive disorders are often comorbid with anxiety (Kessler et al., 2003) and substance use disorders (SUD; Swendsen & Merikangas, 2000); these disorders in the depressed parent may adversely affect offspring’s affective style. Additional potentially important variables include whether the parent is depressed (or in remission) at the time that the offspring is assessed and the parent’s own positive and negative affect displays. Current parental depression and the rate and type of parental affect displays may bias offspring’s own manifest affect-related behaviors; however, longitudinal studies have not yet examined these possibilities.
In seeking to characterize children’s developmental trajectories of PA and NA, we expected that our low-risk offspring’s trajectories will mirror previous findings with normative samples. According to such studies, typically developing children generally display increasing PA across the first decade life (Denham & Lehman, 1995; Gaertner, Spinrad, & Eisenberg, 2008; Mathiesen & Tambs, 1999; Rothbart et al., 2000). Concurrently, however, overall NA appears to decline from infancy to the late childhood years, except for an exacerbation of fear around the pre-school years (Denham & Lehman, 1995; Gaertner et al., 2008; Mathiesen & Tambs, 1999; Murphy, Eisenberg, Fabes, Shepard, & Guthrie, 1999; Rothbart et al., 2000). In the present study, we were particularly interested in whether the presence of parental diagnostic history contributes to atypical affective development of the offspring.
Thus, we used longitudinal, laboratory-based, observational data to model trajectories of PA and NA from late infancy to middle/late childhood in offspring of mothers with a history of unipolar depressive disorder or no history of major psychopathology. Based on prior findings (Feng et al., 2007; Shaw et al., 2006), we hypothesized that offspring of mothers with unipolar depression will evidence lower levels of PA across time compared to offspring of normal control mothers, but that these two groups of children will not differ in trajectories of NA.
The present report extends previous publications from our Program Project on risk factors for pediatric- onset depression. Compared to prior publications (Feng et al., 2007; Shaw et al., 2006), the present report includes all available longitudinal observations on the children and explores the influence of parental comorbid non-affective disorders on offspring PA and NA.
Methods
Recruitment and diagnoses
Children and mothers were participants in a Program Project (PP) on risk factors for childhood-onset depression (COD). Adults with a history of COD were recruited from among previous participants in studies of childhood depression or through advertisements in mental health and community settings. Study entry required the presence of a verifiable COD, defined as onset of first depression by the age of 14 years. Adult controls (who were free of lifetime major psychiatric disorder) were recruited by accessing individuals who had participated in studies during their childhood or adolescence as ‘normal controls’, via the Cole directory of households in neighborhoods comparable in socioeconomic status to the COD group, and through advertising in the community. All adult participants were required to be free of major systemic medical disorders and without evidence of mental retardation. Informed consent was provided during the initial screening for study eligibility.
The Psychiatric Evaluation Core of the PP, staffed by experienced professional-level clinical evaluators and independent psychiatrist diagnosticians, was responsible for all psychiatric assessments and verified cases’ life-time diagnostic status via the best-estimate consensus diagnostic procedure (Maziade et al., 1992). Final psychiatric assessments used the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID; First, Spitzer, Gibbon, & Williams, 1995), yielding DSM-IV (American Psychiatric Association, 1994) criteria-based diagnoses.
Participants
Subjects were 140 mothers and 202 offspring, ranging in age from late infancy to 9 years, who had been enrolled in the Parent–Child Interaction Study of the PP. This included 60 mothers with histories of unipolar depressive disorder (DEP) who contributed 117 offspring (M = 1.78; SD = .85) and 80 control mothers who contributed 95 offspring (M = 1.18; SD = .45).
The children (53% boys) were 3.72 years old on average (SD = 2.18) at the initial assessment. Fourteen offspring were assessed before they were 1 year old (M = 11.52 months, SD = .51). Overall, offspring completed 3.03 assessment sessions on average (SD = 1.45; range 1–7). The number of assessments per child did not differ across maternal diagnostic groups. Most children (n = 125, 61.9%) were European American with the remainder being African American (n = 48, 23.8%), biracial (n = 24, 11.9%), or another ethnicity (n = 5, 2.5%). Altogether 47.9% of mothers had some college education or a college degree and 49.3% of them were married at the baseline assessment.
The two maternal groups (DEP versus Controls) did not differ with regard to offspring’s age, sex, and ethnicity. However, there was a difference in maternal education, χ2(1, N = 140) = 10.87, p < .01: 72.5% of control mothers completed some college or had a degree versus 45.0% of DEP mothers. There also was a difference in maternal marital status, χ2 (1, N = 140) = 7.12, p < .01: more control than DEP mothers were married (67.5% vs. 45.0%, respectively).
Affect-eliciting tasks
Children were seen yearly (around their birthday), along with their mothers, for laboratory assessments that lasted about 2 to 2.5 hr. Procedures were videotaped for later coding. The protocol began with a free-play period while mothers completed questionnaires with the examiner in the same room. Following a brief clean-up phase, various age-specific affect-eliciting tasks were administered. Mothers were given instructions and reminder cards to ensure that they proceeded from one task to the next in the appropriate order.
Across the age span of offspring in this study (from late infancy through age 9), a series of 4–5 tasks were used to elicit positive and negative affect, such as joy, surprise, and pleasure versus anxiety, distress, frustration, anger, or sadness, respectively (Shaw et al., 2006). Each task series was tailored to the child’s developmental level. The negative affects included anxiety, fear, distress, disappointment, frustration, and more rarely anger or sadness. Fear, general anxiety, or performance anxiety was elicited using developmentally appropriate stimuli (e.g., a wiggle ball, jack-in-the-box for younger children and ‘Jenga,’ ‘Don’t Break the Ice’). Anxiety provocation for older children also included an anticipated speech task (the child was asked to develop but did not get to actually give a speech to a group of younger children about what they might expect when they begin school). Frustration, impatience, or anger was elicited using stimuli and games slightly above the child’s developmental level (e.g., puzzles, naming tasks, completing mazes). For example, both ‘Jenga’ and ‘Don’t Break the Ice’ are games that call for the participants to remove pieces one at a time and each successive removed piece increases the likelihood that the structure will fail and the participant loses the game. Older children had to complete puzzles that required reproducing target images in brief periods of time (two to three minutes) or were engaged in a naming task which required generating as many responses as possible that fit a specific kind – such as things that have wheels.
Affect expression coding
Videotapes were coded for each 10-second interval by raters who were blind to maternal diagnostic grouping. Ratio scores were then computed across toys, reflecting the proportion of time that the affect was expressed across the entire protocol. Approximately 15% of the tapes were coded twice; resultant kappas (reported below) reflect reliability with master coder (Shaw et al., 2006).
Child affective displays
Positive emotional displays included physical gestures (e.g., hugging or kissing mom), smiling and laughing, and verbal comments that were positive in both content and tone (e.g., ‘Cool!’, ‘This is fun!’). Negative emotional displays included facial (e.g., frowning) and verbal expressions of frustration/disappointment with the task (e.g., ‘I can’t’, ‘I don’t know how’), asking mother for help in a distressed, upset, or frustrated tone of voice, and comments that were both negative in content and tone (e.g., ‘It’s scary’, ‘Stop it’, ‘This is boring’). Affective displays were coded regardless of situation, and thus provide a global index of propensity to display PA and NA (Durbin et al., 2005; Durbin, Hayden, Klein, & Olino, 2007). Intraclass correlations for average scores of child affect displays across all intervals were .68 for positivity and .70 for negativity.
Maternal affect displays
PA expression included physical gestures (e.g., smiling, hugging) or verbal statements that were positive in content and tone (Feng et al., 2007). NA expression included negative verbal comments and intrusive and harsh behavior directed towards the child. Intraclass correlations for average maternal affect display scores across all intervals were .88 for PA and .77 for NA displays.
Data analysis
Children’s trajectories of PA and NA were modeled using Mplus 6 (Muthén & Muthén, 1998–2010). The MLM framework was used to estimate linear growth models. To accommodate multiple assessments for a given offspring and multiple offspring of the same mother, we implemented the TWOLEVEL and COMPLEX analysis options in Mplus to correct standard errors. Offspring affect was regressed on age at assessment; age was centered such that the intercept reflected the mean level at age 1 to correspond to general trends in the literature (Durbin et al., 2005; Forbes et al., 2004). These models accommodate varied numbers of observations, with cases contributing one observation influencing the mean level of affectivity at the respective, specific age.
Unconditional growth models estimate trajectories using intercept and slope parameters with their respective mean and variance estimates. The intercept is the estimated level of the outcome variable at an identified location of the growth trajectory (in these analyses the intercept reflected age 1 levels). The variance estimate of the intercept reflects whether there is significant variation in the outcome variable at age 1. The mean value of the slope is the estimated average rate of change over time in the data. The variance estimate of the slope parameter reflects whether there is significant variation in the rate of change in levels of the outcome variable. Thus, this modeling approach examines cross-sectional variability in the outcome at the intercept value and longitudinal variability in the outcome for the slope. We examined predictors of intercept and/or slope only when those parameters had significant variance (Raudenbush & Bryk, 2002).
Results
Table 1 displays the characteristics (mean, standard deviation) of children’s observed PA and NA for each age of assessment by maternal diagnostic group. The values in Table 1 were not adjusted for within-family clustering.
Table 1.
Rates of observed positive affect (PA) and negative affect (NA) by age in at-risk and control offspring
| Offspring’s age in years | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Affect display | < 1 M (SD) |
1 M (SD) |
2 M (SD) |
3 M (SD) |
4 M (SD) |
5 M (SD) |
6 M (SD) |
7 M (SD) |
8 M (SD) |
9 M (SD) |
| At-risk offspring | ||||||||||
| PA | .10 (.07) | .09 (.08) | .10 (.09) | .24 (.14) | .19 (.12) | .19 (.10) | .22 (.08) | .20 (.09) | .22 (.13) | .21 (.13) |
| NA | .06 (.08) | .10 (.13) | .15 (.18) | .08 (.08) | .03 (.04) | .03 (.04) | .04 (.05) | .03 (.04) | .04 (.04) | .04 (.04) |
| n | 9 | 27 | 39 | 55 | 41 | 44 | 30 | 33 | 19 | 8 |
| Control offspring | ||||||||||
| PA | .08 (.03) | .15 (.16) | .18 (.09) | .24 (.12) | .21 (.12) | .22 (.10) | .23 (.12) | .26 (.14) | .28 (.13) | .21 (.13) |
| NA | .04 (.01) | .11 (.13) | .10 (.11) | .04 (.04) | .03 (.04) | .03 (.04) | .03 (.05) | .03 (.05) | .02 (.03) | .04 (.04) |
| n | 3 | 19 | 32 | 50 | 45 | 44 | 37 | 33 | 19 | 9 |
Note: There were 684 observations from 237 children from 162 families. Mean age during infancy was 11.56 months (SD = 5.37 months). At-risk = maternal history of depressive disorder; control = no maternal history of major psychiatric disorder.
Trajectories of PA and NA in offspring
Trajectories of affect were estimated using growth models with individual affect display scores as outcomes. Linear growth curves were estimated for PA and NA separately with age at assessment, centered at age 1, as the predictor variable.
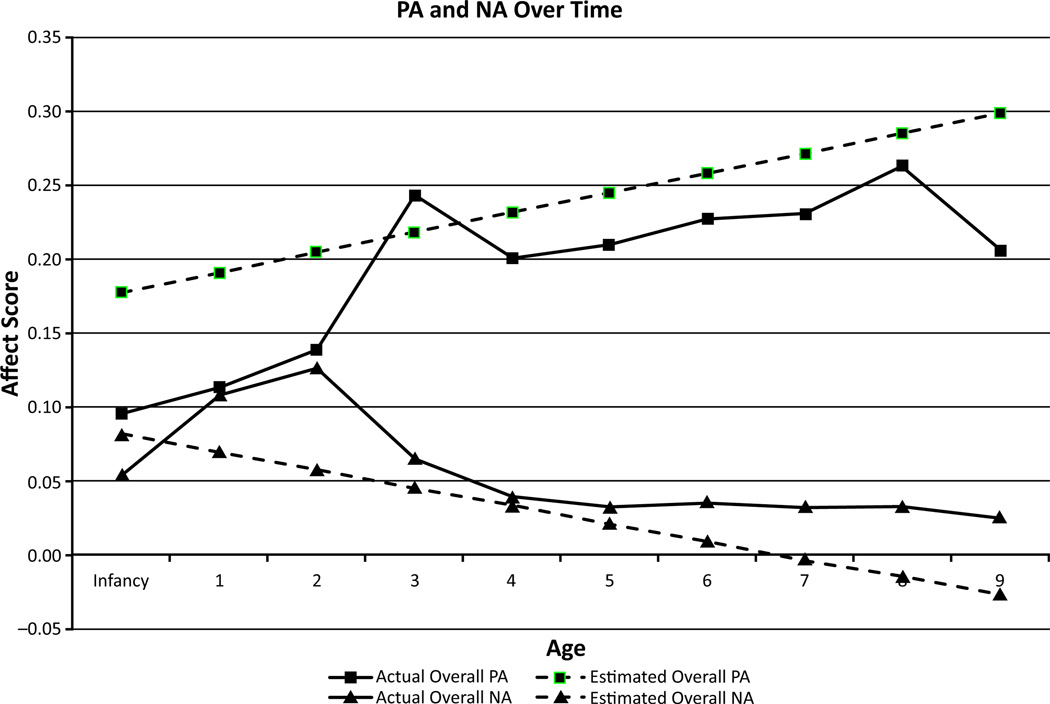
A preliminary analysis of the unconditional growth model for PA revealed that the variance for the slope parameter was not significant. Therefore, the model was re-estimated fixing the variance in the association between age and PA to be 0 (see Hox, 1995). For this more parsimonious model, the estimated PA level at age 1 was significantly greater than 0 (value = .19, SE = .007, t = 28.11, p < .001) and there was significant estimated variance in initial PA (value = .003, SE = .001, t = 3.55, p < .001). Further, there was a significant increase of PA as a function of age (value = .014, SE = .002, t = 5.62, p < .001). In other words, PA increased linearly through childhood at similar rates for all children (Figure 1). However, there were individual differences in initial levels of PA, suggesting that there might be predictable, systematic variance as a function of factors such as maternal psychopathology.
Figure 1.
Estimated and actual values for positive affect (PA) and negative affect (NA) displays from infancy through age 9 (N = 202). Estimated values are plotted based on the results of the multilevel modeling analyses. Actual values for each age are the actual average values for offspring at that specific age
A preliminary analysis of the unconditional growth model for NA revealed that the variances for the intercept and slope parameter were not significant. Therefore, the model was re-estimated fixing the variance in the intercept and the association between age and NA to be 0 (Hox, 1995). For this more parsimonious model, initial level of NA was significantly greater than 0 (value = .07, SE = .005, t = 13.47, p < .001) and significantly decreased as a function of age (value = −.012, SE = .002, t = −6.62, p < .001). In other words, offspring displayed similar amounts of NA initially and had similar reductions in NA across development.
Maternal diagnostic grouping and offspring PA
We examined predictors of the PA intercept because the unconditional growth model revealed significant variance for this parameter (Table 2). We estimated the associations between maternal DEP and estimated offspring PA at age 1. We also controlled for the effects of maternal education and marital status in these analyses. Specifically, educational attainment and marital status were entered as time-varying covariates (i.e., level 1 predictors) in the models, reflecting fluctuations in these variables over the course of the study. In this analysis, offspring’s initial PA was associated with maternal DEP.
Table 2.
Univariate and multivariate modeling of initial levels of positive affect in offspring
| Variable | Univariate analyses | ||
|---|---|---|---|
| Maternal diagnostic history | B | SE | t |
| Reference (healthy controls) | .210 | .015 | 13.87*** |
| Depressive disordera | −.032 | .013 | −2.43* |
| Multivariate models of comorbidity |
|||
| B | SE | t | |
| Comorbidity with anxiety disorder | |||
| Reference (healthy control) | .212 | .016 | 13.64*** |
| Depressive but no anxiety disordera | −.028 | .018 | −1.49 |
| Depressive and anxiety disordera | −.037 | .015 | −2.53** |
| Comorbidity with substance use disorder | |||
| Reference (healthy control) | .205 | .015 | 13.49*** |
| Depressive but no substance use disordera | −.047 | .021 | −2.19* |
| Depressive and substance use disordera | −.025 | .014 | −1.79 |
p < .05;
p < .01.
Note: Values reflect beta coefficients. Analyses adjusted for the effects of maternal education and marital status.
Values marked by ‘a’ refer to differences from the Reference group.
We also fit models that included maternal DEP and comorbid anxiety, and separately, maternal DEP and comorbid substance use, to estimate offspring’s PA at age 1. These models were implemented by creating sets of dummy-coded predictors (i.e., maternal DEP only vs. healthy controls; maternal DEP with comorbid psychopathology vs. healthy controls). The results are summarized in Table 2. As can be seen, maternal MDD with comorbid anxiety disorder was significantly associated with lower levels of offspring PA, while maternal DEP free of comorbid anxiety disorder was not significantly associated with lower levels of offspring PA. Maternal DEP with comorbid substance use disorder was not associated with offspring PA, while maternal DEP without this comorbidity was associated with lower levels of offspring PA.
We also examined whether maternal depression at the time of child assessment (29.0% of total assessments) or maternal expression of PA and NA impacted on offspring’s affect displays. We found that: (a) current maternal DEP was not associated with offspring’s PA (B = −.004, SE = .003, t = −1.24, p = ns) while lifetime history of DEP continued to signal low offspring PA (B = −.031, SE = .012, t = −2.47, p < .05), (b) maternal PA displays and history of DEP both were significantly associated with offspring’s PA (B = .28, SE = .05, t = 5.61, p < .001; B = −.026, SE = .012, t = −2.22, p < .05, respectively), and (c) maternal NA displays were not significantly associated with offspring’s PA (B = .37, SE = .26, t = 1.41, p = ns) while lifetime depression history continued to signal lower levels of PA in offspring (B = −.034, SE = .013, t = −2.63, p < .01). In a final model, we examined the effects of all covariates simultaneously (current maternal DEP, maternal PA and NA, and DEP history). The results indicated that current maternal depressive episode (B = −.004, SE = .003, t = −1.17, p = ns) and maternal NA (B = .42, SE = .24, t = 1.72, p = ns) were not associated with offspring’s PA. However, maternal PA (B = .28, SE = .049, t = 5.69, p < .001) and lifetime DEP (B = −.027, SE = .012, t = −2.28, p < .05) continued to exert significant effects on offspring’s PA displays. In other words, children’s displays of positive affect were facilitated by mothers who also displayed positive affect but were attenuated by mothers who had a history of depressive disorder.
Discussion
To the best of our knowledge, the present study is the first to have characterized in a developmental framework the longitudinal trajectories of positive and negative affect displays of young children at familial risk for depressive disorders. We reasoned that, if low levels of positive affectivity and high levels of negative affectivity are precursors of clinical depression, as has been proposed, then these attributes should be detectable in a population known to be at highly elevated risk for eventual clinical depression, namely the young offspring of mothers with depression histories. We modeled children’s trajectories of affect using observational ratings during structured laboratory tasks at yearly intervals and examined the potential impact of selected maternal characteristics.
We found that the children (regardless of maternal diagnostic status) evidenced the expected normative developmental changes from early infancy through late childhood, namely, increasing positive affectivity and decreasing negative affectivity. However, compared to the children of healthy mothers, young offspring at high risk for depressive disorder consistently displayed significantly lower levels of PA across development. This association was strong and persisted after adjusting for maternal affect displays and presence of maternal depressive episode during each laboratory assessment. Our finding therefore complements and extends the results of cross-sectional studies in which children with a family history of depression have been uniformly found to display lower levels of positive affect than typically developing peers. This finding has been reported for 3- to 4-year-olds (n = 100) (Durbin et al., 2005) as well as for 8- to 17-year-olds (Dietz et al., 2008).
Therefore, attenuated capacity for PA across development (rather than high levels of NA) may represent one source of vulnerability to depressive disorder. This possibility specifically was suggested by Meehl (1975) and has been reiterated across the years by others (e.g., Durbin et al., 2005). Recent neuroimaging studies of young offspring at familial risk for depression also have yielded findings that converge with and support the current results. Specifically, Monk et al. (2008) and Gotlib et al. (2010) have reported that, compared to controls, young offspring at familial risk for depression evidence reduced neural activation in brain regions (particularly in areas of the ventral striatum) that have been associated with reward processing and positive affect. Low hedonic tone is believed to increase the risk of depression by attenuating reactivity to external reinforces or reward experiences, as well as by attenuating the positive (and functionally useful) cognitive bias that is characteristic of typical youths (Hayden, Klein, Durbin, & Olino, 2006; Forbes, Shaw, & Dahl, 2007). Low PA also may contribute to depression risk by compromising the effectiveness of self-regulatory responses to distress that draw upon the capacity for pleasure (Kovacs & Lopez-Duran, 2010).
Maternal history of anxiety disorders exacerbated the detrimental effect of maternal depression on offspring’s positive affectivity as compared to levels of PA in control children. At first glance, these results suggested that the adverse effect of maternal depression was due solely to those mothers who also had a history of comorbid anxiety disorders. However, a closer look at the data revealed the existence of a linear additive trend: maternal depression (without anxiety) lowered children’s PA levels by 14% and maternal depression with comorbid anxiety lowered those levels by 19%. Consequently, in these analysis, the non-significant difference in PA between offspring of mothers with depression (but no anxiety) and control offspring is likely to reflect limited statistical power, as most (68%) depressed mothers had anxiety disorders.
Unexpectedly, substance use comorbidity partially mitigated the detrimental effect of maternal depressive diagnosis on offspring’s PA: positive affectivity was reduced by 23% in children of depressed mothers without substance use disorders, while presence of substance use comorbidity reduced children’s PA by only 13%. Possibly, self-medication among mothers with histories of depression may attenuate their own affective difficulties and result in more appropriate maternal affective responses. However, because Durbin et al. (2005) did not find such an association in their study of at-risk children, there is a need for further work with larger sample sizes to clarify this issue.
Consistent with prior work, children’s negative affect displays mirrored a relatively homogenous, linearly decreasing trajectory from late infancy to late childhood. This trajectory may reflect the growing ability for more effective self-regulation of negative affect with age, along with increasing awareness of socially appropriate emotion displays (e.g., Sallquist et al., 2009). Because children did not manifest inter-individual heterogeneity in either initial level of NA or inter-individual changes in NA, our models prohibited direct probes of the associations between NA and maternal diagnostic grouping. However, in previous analyses on a subset of this sample, no significant associations were detected between maternal depression and offspring’s displays of negative affect (Feng et al., 2007; Shaw et al., 2006).
The implications of our findings are constrained by several limitations of our design. First, assessments of positive and negative affectivity were based on behavioral observations, which is likely to be only a partial index of affective capacity or internal experience. Further, our coding schema focused on the frequency of a given affect, while emotional intensity also may be an important marker of vulnerability to depressive disorders. Although a developmental approach has informed our protocol, testing children at different ages requires changes in stimuli, which may not be of comparable ‘strength’ or validity. Second, because at-risk offspring were ascertained by virtue of having a mother with juvenile-onset mood disorder, our findings do not speak to the impact of adult-onset parental depression or the effects of paternal psychopathology on offspring’s affective development (e.g., Phares & Compas, 1992). Relatedly, our ‘high-risk design’ itself is limited to offspring of COD mothers, which may limit generalizability of the findings to other high-risk populations. Third, our work focuses on identifying a vulnerability marker for depression indexed by family history of disorder, rather than examining associations between a vulnerability marker and youth depression. While some work is available to support this claim (e.g., Dougherty, Klein, Durbin, Hayden, & Olino, 2010), further longitudinal work is needed to bolster this association. Further, the plots of actual and predicted values of NA from the growth models suggested that changes in this dimension may be better represented by non-linear (e.g., quadratic, cubic) patterns. While analyses that considered such growth patterns (not presented herein) did not provide greater clarity, our final model of NA may not be the most optimal.
Importantly, while our findings are consistent with a presumed pathway to clinical depression, and the literature on the affective characteristics of children at familial risk for depression, the offspring in our sample were too young to have evidenced the eventual outcome of interest, namely: onset of a diagnosable depressive disorder. As a partial remedy, we conducted a post-hoc test of maternal reports of their children’s depressive symptoms when offspring were between ages 8 and 9. We had ratings for 67 offspring; if 2 scores were available, we selected the higher score. At-risk children were rated by their mothers as having significantly higher levels of depressive symptoms by ages 8–9 than were control children (t = 4.08, p < .001). While this simple comparison is not sufficient to link atypical affectivity to eventual clinical depression, the results do suggest the early appearance of symptoms in at-risk children that are known to constitute depressive syndromes. Further, depressive symptoms in somewhat older children have been linked directly to increased risk of subsequent depressive disorder (Kovacs & Lopez- Duran, 2010).
Despite its limitations, the present study represents an important contribution to a growing literature on at-risk youths, suggesting that lower than typical level of positive affectivity or hedonic capacity is a developmental correlate of familial depression risk. Thereby, this attribute may well be a specific and early marker of vulnerability for unipolar depression. This proposition can be validated in future studies by linking lower levels of hedonic capacity to eventual depressive disorders in at-risk youth. Confirmatory findings will have important implications for primary and secondary prevention and underscore that assessment of the capacity for positive affect should play a role in traditional case selection methods. Further, fostering positive affect or hedonic capacity deserves to be investigated as a component of interventions that seek to prevent depressive disorder in youths.
Key points.
Clinical depression is associated with low positive affect (PA) and high negative affect (NA). However, it has been proposed that these characteristics may exist prior to, and represent risk factors for, depression.
A history of parental depression is a known risk factor for depression in the offspring. If low PA and high NA are additional risk factors, these characteristics should be evident in juveniles at familial risk for depression.
This follow-up study found that offspring at high familial risk for depressive disorders and control offspring both showed the expected developmental trajectory from infancy up to age 9 of increasing PA and decreasing NA, but differed in one regard: children of mothers who had a history of juvenile-onset depression manifested consistently lower levels of PA.
Reduced PA may be one source of developmental vulnerability to familial depression and could be a potential target in efforts to prevent pediatric depression.
Acknowledgements
This study was supported by Grant Number P01 MH056193 and R01 MH085722 (PI: MK).
Abbreviations
- PA
positive affect
- NA
negative affect
- COD
childhood-onset depression
Footnotes
Conflict of interest statement: No conflicts declared.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th edn. Washington, DC: Author; 1994. rev. [Google Scholar]
- Chorpita BF, Daleiden EL. Tripartite dimensions of emotion in a child clinical sample: Measurement strategies and implications for clinical utility. Journal of Consulting and Clinical Psychology. 2002;70:1150–1160. doi: 10.1037//0022-006x.70.5.1150. [DOI] [PubMed] [Google Scholar]
- Denham SA, Lehman EB. Continuity and change in emotional components of infant temperament. Child Study Journal. 1995;25:289–309. [Google Scholar]
- Dietz LJ, Birmaher B, Williamson DE, Silk JS, Dahl RE, Axelson DA, Ehmann M, Ryan ND. Mother–child interactions in depressed children and children at high risk and low risk for future depression. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:574–582. doi: 10.1097/CHI.0b013e3181676595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty LR, Klein DN, Durbin CE, Hayden EP, Olino TM. Temperamental positive and negative emotionality and children’s depressive symptoms: A longitudinal prospective study from age three to age ten. Journal of Social and Clinical Psychology. 2010;29:462–488. [Google Scholar]
- Durbin C, Hayden EP, Klein DN, Olino TM. Stability of laboratory-assessed temperamental emotionality traits from ages 3 to 7. Emotion. 2007;7:388–399. doi: 10.1037/1528-3542.7.2.388. [DOI] [PubMed] [Google Scholar]
- Durbin CE, Klein DN, Hayden EP, Buckley ME, Moerk KC. Temperamental emotionality in preschoolers and parental mood disorders. Journal of Abnormal Psychology. 2005;114:28–37. doi: 10.1037/0021-843X.114.1.28. [DOI] [PubMed] [Google Scholar]
- Feng X, Shaw DS, Skuban EM, Lane T. Emotional exchange in mother–child dyads: Stability, mutual influence, and associations with maternal depression and child problem behavior. Journal of Family Psychology. 2007;21:714–725. doi: 10.1037/0893-3200.21.4.714. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders – patient edition (SCID-I/D, version 2.0) New York: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- Forbes EE, Cohn JF, Allen NB, Lewinsohn PM. Infant affect during parent– infant interaction at 3 and 6 months: Differences between mothers and fathers and influence of parent history of depression. Infancy. 2004;5:61–84. doi: 10.1207/s15327078in0501_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes E, Shaw D, Dahl R. Alterations in reward-related decision making in boys with recent and future depression. Biological Psychiatry. 2007;61:633–639. doi: 10.1016/j.biopsych.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Gaertner BM, Spinrad TL, Eisenberg N. Focused attention in toddlers: Measurement, stability, and relations to negative emotion and parenting. Infant and Child Development. 2008;17:339–363. doi: 10.1002/ICD.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Hamilton JP, Cooney RE, Singh MK, Henry ML, Joormann J. Neural processing of reward and loss in girls at risk for major depression. Archives of General Psychiatry. 2010;67:380–387. doi: 10.1001/archgenpsychiatry.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden EP, Klein DN, Durbin CE, Olino TM. Low positive emotionality at age three predicts depressogenic cognitions in seven year old children. Development and Psychopathology. 2006;18:409–423. doi: 10.1017/S0954579406060226. [DOI] [PubMed] [Google Scholar]
- Hox JJ. Applied multilevel analysis. Amsterdam: TT-Publikaties; 1995. [Google Scholar]
- Joiner TE, Jr, Catanzaro SJ, Laurent J. Tripartite structure of positive and negative affect, depression, and anxiety in child and adolescent psychiatric inpatients. Journal of Abnormal Psychology. 1996;105:401–409. doi: 10.1037//0021-843x.105.3.401. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. The epidemiology of major depressive disorder: Results from the National Comorbidity Study Replication (NCS-R) Journal of the American Medical Association. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kochanska G. Patterns of inhibition to the unfamiliar in children of normal and affectively ill mothers. Child Development. 1991;62:250–263. doi: 10.1111/j.1467-8624.1991.tb01529.x. [DOI] [PubMed] [Google Scholar]
- Kovacs M, Lopez-Duran N. Prodromal symptoms and atypical affectivity as predictors of major depression in juveniles: Implications for prevention. Journal of Child Psychology and Psychiatry. 2010;51:472–496. doi: 10.1111/j.1469-7610.2010.02230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby JL, Mrakotsky C, Heffelfinger A, Brown K, Spitznagel E. Characteristics of depressed preschoolers with and without anhedonia: Evidence for a melancholic depressive subtype in young children. American Journal of Psychiatry. 2004;161:1998–2004. doi: 10.1176/appi.ajp.161.11.1998. [DOI] [PubMed] [Google Scholar]
- Mathiesen KS, Tambs K. The EAS Temperament Questionnaire – factor structure, age trends, reliability, and stability in a Norwegian sample. Journal of Child Psychology and Psychiatry. 1999;40:431–439. [PubMed] [Google Scholar]
- Maziade M, Roy MA, Fournier JP, Cliché D, Mérette C, Caron C, Garneau Y, Montgrain N, Shriqui C, Dion C, Nicole L, Potvin A, Lavallée JC, Pirès A, Raymond V. Reliability of best-estimate diagnosis in genetic linkage studies of major psychoses: Results from the Quebec pedigree studies. American Journal of Psychiatry. 1992;149:1674–1686. doi: 10.1176/ajp.149.12.1674. [DOI] [PubMed] [Google Scholar]
- McCrae RR, Costa PT., Jr Validation of a five-factor model of personality across instruments and observers. Journal of Personality and Social Psychology. 1987;52:81–90. doi: 10.1037//0022-3514.52.1.81. [DOI] [PubMed] [Google Scholar]
- Meehl PE. Hedonic capacity: Some conjectures. Bulletin of the Menninger Clinic. 1975;39:295–307. [PubMed] [Google Scholar]
- Monk CS, Klein RG, Telzer EH, Schroth EA, Mannuzza S, Moulton JL, Guardino M, Masten CL, McClure-Tone EB, Fromm S, Blair RJ, Pine DS, Ernst M. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. American Journal of Psychiatry. 2008;165:90–98. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- Murphy BC, Eisenberg N, Fabes RA, Shepard S, Guthrie IK. Consistency and change in children’s emotionality and regulation: A longitudinal study. Merrill-Palmer Quarterly. 1999;45:413–444. [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. 6th edn. Los Angeles, CA: Muthén & Muthén; 1998–2010. [Google Scholar]
- Olino TM, Klein DN, Durbin CE, Hayden EP, Buckley ME. The structure of extraversion in preschool aged children. Personality and Individual Differences. 2005;39:481–492. [Google Scholar]
- Phares V, Compas BE. The role of fathers in child and adolescent psychopathology: Make room for daddy. Psychological Bulletin. 1992;111:387–412. doi: 10.1037/0033-2909.111.3.387. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- Rothbart MK, Ahadi SA, Evans DE. Temperament and personality: Origins and outcomes. Journal of Personality and Social Psychology. 2000;78:122–135. doi: 10.1037//0022-3514.78.1.122. [DOI] [PubMed] [Google Scholar]
- Sallquist JV, Eisenberg N, Spinrad TL, Reiser M, Hofer C, Zhou Q, et al. Positive and negative emotionality: Trajectories across six years and relations with social competence. Emotion. 2009;9:15–28. doi: 10.1037/a0013970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw DS, Sherrill J, Huffman D, Schonberg MA, Lukon J, Obrosky S, Kovacs M. Responsivity to offspring’s expression of emotion among childhood-onset depressed mothers. Journal of Clinical Child and Adolescent Psychology. 2006;35:490–503. doi: 10.1207/s15374424jccp3504_1. [DOI] [PubMed] [Google Scholar]
- Swendsen JD, Merikangas KR. The comorbidity of depression and substance use disorders. Clinical Psychology Review. 2000;20:173–189. doi: 10.1016/s0272-7358(99)00026-4. [DOI] [PubMed] [Google Scholar]
- Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA. Testing a tripartite model: I, Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. Journal of Abnormal Psychology. 1995;104:3–14. doi: 10.1037//0021-843x.104.1.3. [DOI] [PubMed] [Google Scholar]