Abstract
Conventional assays of viral particle assembly and release are time consuming and laborious. We have developed an enzymatic virus-like particle (VLP) genesis assay that rapid and quantitative and is also versatile and applicable to diverse viruses including HIV-1 and Ebola virus. Using this assay, which has a dynamic range of several orders of magnitude, we show that the efficiency of VLP assembly and release, i.e. the fraction of the expressed protein that is assembled into extracellular particles, is dependent on the absolute level of expression of either HIV-1 Gag or Ebola virus VP40. We also demonstrate that the activity of the antiviral factor tetherin is dependent on the level of HIV-1 Gag expression and the numbers of VLPs generated, and appears to become saturated as these parameters are increased.
Introduction
Virus particle assembly and release is a complex process that is initiated by the synthesis and movement of viral structural proteins and genomes to sites of particle assembly. Subsequently, the virion components coalesce in an ordered manner into viral particles and ultimately depart from the cell. These events are often heavily influenced by host cell machinery and processes. On the one hand the virus can usurp host cell machinery to assist in virion assembly, while on the other, host cells have evolved antiviral restriction factors that can inhibit the release of viral particles.
For some viruses, including HIV-1 and Ebola virus that are the subject of this study, examination of particle assembly and release is facilitated by the use of experimental systems in which a subset of viral proteins - or even a single viral protein species - are expressed. In such instances, virus-like particles (VLPs) that are morphologically similar to authentic virions are generated. For retroviruses, the Gag protein is the major structural component of particles and in most cases the expression of this protein alone in an appropriate cellular context is sufficient to drive the formation of extracellular VLPs (Gottlinger, 2001). Typically, Gag proteins are comprised of the membrane binding matrix (MA) domain as well as capsid (CA) and nucleocapsid (NC) domains that play key roles in assembly though Gag oligomerization and envelopment. In addition, Gag proteins contain sequences (within the C-terminal p6 protein in the case of HIV-1 Gag) that serve as ‘late’ (L-) domains and recruit components of the endosomal sorting complex required for transport (ESCRT) machinery that are required for particle budding from the cell surface (Morita and Sundquist, 2004). In a similar manner to retroviral Gag proteins, the matrix proteins (VP40) of the Filoviridae (e.g. Ebola virus) are capable of generating extracellular VLPs when expressed as the only viral protein in cultured cells (Jasenosky et al., 2001; Timmins et al., 2001). Although retroviral Gag and filoviral VP40 proteins share this intrinsic VLP assembly property, they exhibit no sequence or structural homology, except for their L-domains. VP40 proteins also differ from Gag proteins in that their L-domains are located close to their N-termini and, in contrast to most Gag proteins, VP40 proteins can tolerate fusions at their N-termini rather than their C-termini (Martin-Serrano, Perez-Caballero, and Bieniasz, 2004; Martin-Serrano, Zang, and Bieniasz, 2001).
When first translated, HIV-1 Gag exists primarily in monomeric or low order multimeric states in the cytosol (Kutluay and Bieniasz). In Gag monomers, the N-terminal myristoyl group exhibits a propensity to be occluded within a pocket in the MA domain, while Gag oligomerization induces myristoyl group exposure (Tang et al., 2004; Zhou and Resh, 1996). As such, Gag oligomerization increases the intrinsic membrane binding properties of each Gag monomer. This phenomenon, coupled with the multimerization of membrane binding surfaces that occurs as a result of Gag oligomerization may underlie the apparent cooperativity, or greater efficiency of particle release at higher levels of Gag expression, that characterizes HIV-1 assembly(Hatziioannou et al., 2005; Perez-Caballero et al., 2004). Ebola virus VP40 (EbVP40) also exists in a primarily monomeric state in transfected or infected cells, but assembles into hexamers and/or octamers during particle genesis (Gomis-Ruth et al., 2003; Hartlieb and Weissenhorn, 2006; Scianimanico et al., 2000). It is not known whether EbVP40 assembly exhibits the same cooperative properties as does HIV-1 Gag.
During assembly and budding through the host plasma membrane, a number of enveloped viruses, including HIV-1 and Ebola, can encounter a host defense mechanism that inhibits particle release(Neil, Zang, and Bieniasz, 2008; Van Damme et al., 2008). Tetherin is an interferon induced host cell transmembrane protein that forms parallel dimers and causes retention of virions by physically tethering them to the host cell membrane (Fitzpatrick et al., 2010; Hammonds et al., 2010; Perez-Caballero et al., 2009), It has broad antiviral specificity(Jouvenet et al., 2009; Kaletsky et al., 2009; Sakuma et al., 2009), and as a result, viruses have evolved various mechanisms to counteract it. For example, the HIV-1 Vpu protein antagonizes tetherin, at least in part by reducing its expression at the cell surface(Neil, Zang, and Bieniasz, 2008; Van Damme et al., 2008).
Analyses of viral particle assembly and release, and its inhibition by antiviral proteins such as tetherin generally employ assays such as western blotting, metabolic labeling, ELISA or infectivity measurements for the detection of virions. These assays are typically cumbersome, sometimes suffer from a lack of true quantitation, and/or are indirect measures of particle formation. Therefore, to facilitate direct quantitiative analyses of viral particle assembly and release, we developed a rapid and facile luminescence-based assay that quantifies the viral structural proteins in cell lysates and extracellular virus-like particles, providing a simple and direct measurement of virion assembly and release. Specifically, we fused the α-peptide of β-galactosidase to the C-terminus of the HIV-1 Gag protein and to the N-terminus of the Ebola virus Vp40 protein. Thereafter, we measured α-peptides in cell and virion lysates by complementation with the ω-fragment of β-galactosidase and a commercial chemiluminescent enzyme assay. The approach is marginally superior to near-IR quantitative fluorescent western blotting in sensitivity and dynamic range, but requires a fraction of the time and effort. We use this assay to demonstrate that the yield of HIV-1 and Ebola VLPs is not directly proportional to Gag or VP40 expression levels, rather the efficiency with which the viral proteins are assembled and released as VLPs increases as protein expression is increased. Additionally, we demonstrate that the antiviral activity of tetherin is diminished at higher levels of Gag expression, suggesting that it is saturable, consistent with the notion that it acts directly to block virion particle release.
Results
HIV-1 Gag-α is expressed and released as VLPs equivalently to unmodified HIV-1 Gag
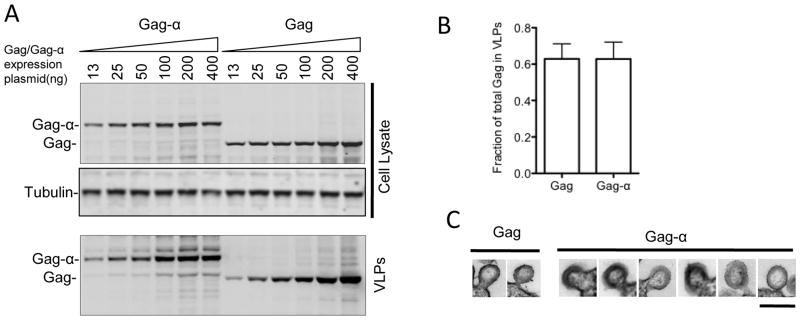
To attempt to quantify the assembly and release of HIV-1 VLPs, we placed a small (6 kDa) αpeptide tag at the C-terminus of the HIV-1 Gag protein in the context of a plasmid expressing a codon optimized gag gene. The α-peptide is a 55-residue sequence from the β-galactosidase enzyme and can complement the inactive ω-fragment when mixed with it, restoring β-galactosidase enzymatic activity. β-galactosidase can be sensitively and accurately detected and quantitated using commercially available chemiluminescent substrates. We first tested whether appending HIV-1 Gag C-terminus with the α-peptide caused any changes in Gag expression or release of VLPs. HIV-1 Gag or Gag-α proteins were transiently expressed at varying levels (13ng to 400ng of transfected plasmid DNA) in 293T cells and protein expression and VLP release were quantitated using western blot analyses of cell and VLP lysates with an anti-Gag antibody. The levels of expression and particle release observed with either Gag or Gag-α were indistinguishable (Fig. 1A,B). Moreover, the particles that formed upon expression of Gag-α were similar in size and morphology to those generated upon expression of unmodified Gag (Fig. 1C). These results suggest that addition of the α-peptide did not alter the overall conformation and functionality of the HIV-1 Gag protein.
Fig. 1.
HIV-1 Gag-α is expressed and released as VLPs equivalently to Gag. (A) 293T cells were transiently transfected with increasing amounts of plasmids expressing either Gag or Gagα (13ng, 25ng, 50ng, 100ng, 200ng and 400ng). Cells were lysed 48 hours post-transfection and VLPs were prepared at the same time from culture supernatants by pelleting through a 20% sucrose cushion. Cell lysates and VLPs were subjected to SDS-PAGE and transferred onto nitrocellulose membranes. Western blots were probed with anti-Gag and anti-Tubulin antibodies. (B) The efficiency with which Gag and Gag-α are released from cells is shown as the fraction of the total Gag in 293T cell cultures that was present in extracellular VLPs following transfection with 500ng of plasmids expressing either Gag or Gag-α. Levels of Gag or Gag-α in VLPs and cell lysates was determined by quantitative western blotting. The mean and standard deviation of 7 experiments is plotted. (C) Gallery of thin-section electron micrographs showing viral like particles budding from 293T cells expressing Gag or Gag-α. Scale bar = 200nm.
Direct measurement of HIV-1 Gag-α expression and VLP release using β-Galactosidase assays
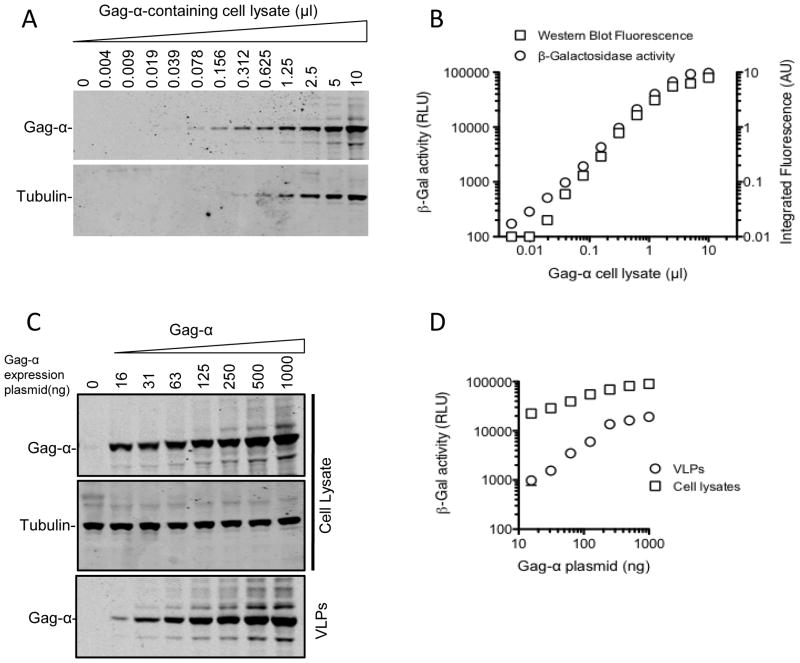
A commonly used method for examining HIV-1 particle assembly and release involves western blot analysis of cell lysates and VLPs with anti-Gag antibodies. We therefore used a quantitative fluorescent western blot approach (LiCOR) as a standard against which to assess the α-peptide based VLP assembly/release assay. To verify that the α-peptide could be sensitively detected in the context of a Gag fusion protein, we first serially diluted lysates of cells expressing Gag-α and subjected replicate aliquots to β-Galalactosidase assay and quantitative fluorescent western blot analysis with an anti-Gag antibody (Fig. 2A, B). Results from the two assays correlated well (Fig. 2B). In fact, at low quantities of cell lysates (<0.04μl) the chemiluminescent α-peptide detection assay was slightly more sensitive, and thus had a commensurately wider dynamic range, than western blot analyses. We next expressed Gag-α at varying levels (by varying the amount of transfected plasmid DNA from 16ng-1000ng) in 293T cells and assayed for α-peptides in cell lysates and VLPs. As expected, we detected a progressive increase in the expression of Gag-α in cell lysates as the amount of transfected Gag-α expression plasmid was increased (Fig. 2C, D). Notably, the amount of Gag-α that was measured in VLPs increased disproportionately to that in cell lysates as the amount of Gag expression plasmid was increased (Fig. 2C, D). Put another way, the efficiency of assembly and release of HIV-1 VLPs appeared to progressively increase as Gag expression was increased (Fig. 2D). The same effect was observed whether western blot or α-peptide assays were used to assess VLP assembly and release.
Fig. 2.
Characteristics of the Gag-α detection assay as compared to western blot analysis. 293T cell lysates expressing Gag-α (500ng of transfected plasmid) were serially diluted and subjected to western blot analysis with anti-Gag and anti-Tubulin antibodies (A, B) and also to β-galactosidase assay for α-peptides (B). Western Blots were quantitated using the Odyssey quantitation software and intensities were assigned arbitrary units. (C) 293T cells were transfected with increasing amounts of Gag-α expression plasmid (16ng, 31ng, 63ng, 125ng, 250ng, 500ng and 1000ng) or empty vector alone. Cells were harvested 48 hours post-transfection and VLPs were pelleted through a 20% sucrose cushion. The cell lysates and VLPs were subjected to western blot analyses (probed with anti-Gag and anti-Tubulin antibodies). (D) Cell lysates and VLPs from (C) were also assayed using the β-galactosidase assay.
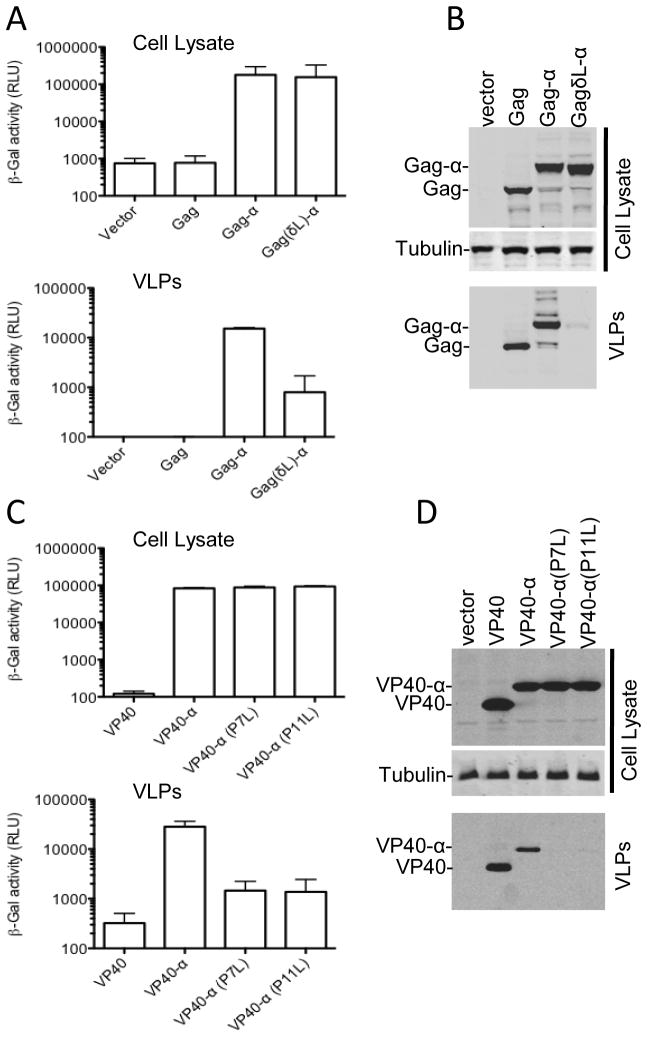
We next sought to verify that the chemiluminscent α-peptide assay genuinely measured VLP release by testing whether the signal was reduced by mutations in the HIV-1 Gag p6 L-domain that block the recruitment of ESCRT proteins required for the efficient budding of viral particles. The wild-type Gag-α gave a strong α-peptide signal in VLPs compared to empty vector or untagged Gag controls (with a signal to background ratio of >100 fold). Conversely, the L-domain mutant Gag-α protein gave a ~20-fold lower signal when VLPs were subjected to analysis (Fig. 3A and 3B,) even though the mutant Gag-α was expressed at a level equivalent to WT Gag-α. Western blot assays confirmed these results, and thus it was clear that Gag-α could assemble and be released as VLPs in an ESCRT-protein dependent manner in the same way as the authentic HIV-1 Gag protein.
Fig. 3.
Verification that the α-peptide/β-galactosidase assay measures bona fide HIV-1 release and can also be applied to Ebola virus. (A and B) 293T cells were transiently transfected with 500ng of plasmids expressing either Gag, Gag-α or GagδL-α. After 48 hours cell lysates and VLPs were subjected to β-galactosidase assay (A) and western blot analysis (B) with anti-Gag and anti-Tubulin antibodies. Graphs represent mean and standard deviation of three experiments. (C and D) 293T cells were transiently transfected with 500ng of plasmids expressing either Ebola virus VP40, VP40-α, VP40-α (P7L) or VP40-α (P11L). After 48 hours cell lysates and VLPs were subjected to β-galalactosidase assay (C) and western blot analysis (D) with anti-Myc and anti-Tubulin antibodies. Graphs represent mean and standard deviation of two experiments.
In order to test the versatility of this approach for measuring VLP assembly and release, we placed sequences encoding the α-peptide at the N-terminus of Ebola virus VP40 protein. As was the case with HIV-1 Gag, we could easily detect and quantify Ebola VLP assembly and release, (Fig. 3C), with signal to background ratios of ~100. Similarly, mutating either of the PTAP or PPXY motifs in the VP40 L-domain (P7L or P11L) led to a decrease of ~20-fold in VLP (α-peptide) signal as compared to WT VP40-α (Fig. 3C). Again, the results obtained using the chemiluminescent assay were concordant with those obtained by western Blot (Fig. 3D) and with previous reports (Martin-Serrano, Zang, and Bieniasz, 2001). However, the chemiluminsecent assay was more rapid and allowed the residual VLP release observed with the L-domain mutant to be detected and quantified (Fig. 3C).
Cooperativity in HIV-1 and Ebola VLP assembly
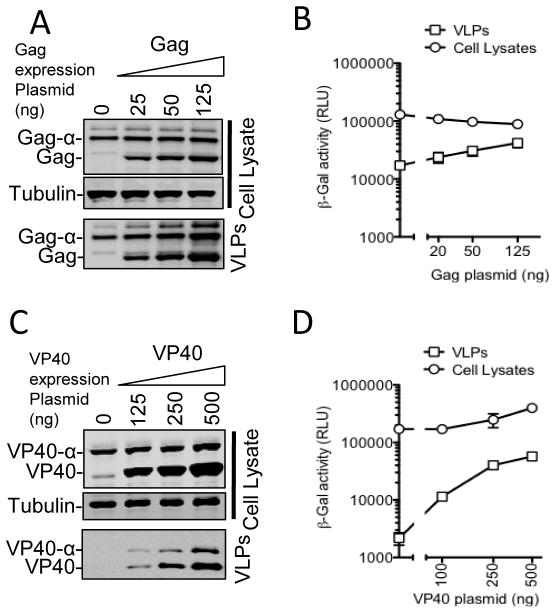
The apparently disproportionate increase in the level of VLP-associated α-peptides as Gag-α expression was increased (Fig. 2C and 2D) is consistent with the notion that assembly of Gag proteins and generation of VLPs is cooperative. To demonstrate this property more definitively, we expressed a fixed amount of Gag-α alone, or with an increasing amount of untagged Gag. If the probability that individual molecule of Gag is incorporated into a VLP is increased by the presence of other molecules of Gag, then the level of Gag-α molecules in VLPs should increase as untagged Gag levels are increased, even if the level of expression of Gag-α is held constant. In fact, as expression of untagged Gag was increased, the fraction of Gag-α that was released in VLPs was increased (Fig. 4A and 4B). We next tested whether the assembly Ebola VP40 molecules into VLPs exhibited similar cooperative properties. For this, we expressed a fixed amount of VP40-α in the absence or presence of an increasing concentration of untagged VP40. As was the case for HIV-1 Gag, we observed an increase in the efficiency of VP40-α incorporation into extracellular particles as the levels of untagged VP40 were increased (Fig 4C and 4D). In addition, the untagged VP40 appeared to marginally increase the levels of VP40-α protein that were detected in cells, suggesting the possibility that VP40 acquires greater stability as it is assembled into particles within cells. Thus, concentration dependence might be a general characteristic of the assembly of diverse enveloped virus structural proteins.
Fig. 4.
Cooperativity in HIV-1 and Ebola virus particle assembly/release. (A and B). 293T cells were transfected with a fixed amount (25ng) of a Gag-α expression plasmid along with increasing amounts (0ng, 25ng, 50ng and 125ng) of a plasmid expressing untagged Gag. Cells and VLPs were subjected to western blot analysis with anti-Gag and anti-Tubulin antibodies (A) and β-galactosidase assay (B). (C and D) 293T cells were transfected with a fixed amount (50ng) of a plasmid expressing VP40-α along with increasing amounts (0ng 125ng, 250ng and 500ng) of plasmid expressing untagged VP40. Cells and VLPs were subjected to western blot analysis with anti-Myc and anti-Tubulin antibodies (C) and β-galactosidase assay (D).
Tetherin is a saturable host cell restriction factor
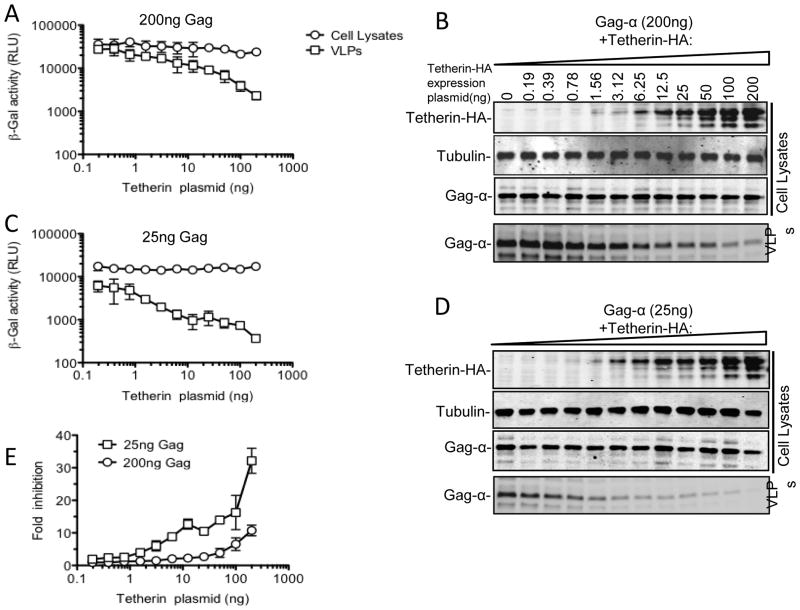
Tetherin is a host antiviral protein whose expression leads to the retention of virion particles by infected cells and inhibition of viral dissemination. Current models of the mechanism by which tetherin functions invoke a scenario in which the tetherin protein directly blocks particle release via the action of two membrane anchors that partition between the virion and host cell membranes. If this model is correct, then increasing the expression of Gag to a sufficiently high level might saturate the available tetherin, facilitating particle release. The development of a rapid quantitative VLP release assay with a large dynamic range provided an opportunity to easily investigate this question. Therefore, we examined the ability of varying levels of tetherin to inhibit particle release at high and low levels of HIV-1 Gag-α expression (200ng or 25ng of transfected Gag-α expression plasmid, respectively, Fig. 5). When the higher amount of Gag-α was expressed, tetherin was clearly less effective at inhibiting particle release than when lower levels of Gag-α were expressed (compare Fig. 5A and B with Fig 5C and D). Indeed, at lower levels of Gag-α expression, only 10–20ng of cotransfected tetherin expression plasmid was required to inhibit particle release by 10-fold (Fig. 5E). Conversely, 200ng of cotransfected tetherin expression plasmid was required to achieve the same level of inhibition when the higher level of Gag-α was expressed (Fig 5A and 5B).
Fig. 5.
Effect of Tetherin on HIV-1 VLP release is dependent on Gag expression level. 293T cells were transfected either with one of two fixed amounts (200ng (A and B) and 25ng (C and D) of a plasmid expressing Gag-α along with increasing amounts (0 to 200ng) of a tetherin expression plasmid. Cells and VLPs were subjected to β-galactosidase assay (A and C) and western blot analysis (B and D) with anti-Gag, anti-Tubulin and anti-HA antibodies. The graph in (E) shows the fold reduction in Gag-α VLP yield (as measured by β-galactosidase assay) attributable to tetherin for each amount of Gag-α expression plasmid (200ng and 25ng).
Discussion
The lack of a rapid and quantitative system to specifically measure the assembly and release of viral particles is a significant inconvenience in studies of this process. Therefore, we set out to develop a novel assay to detect VLP formation sensitively, quantitatively and accurately, in experiments involving many samples, and in a significantly shorter time (2 hours) compared to other conventional methods (1–2 days). As expression of retrovirus Gag and filovirus VP40 proteins is sufficient for the assembly of bonafide extracellular VLPs, placing an easily quantifiable tag on them enabled direct quantification of expression and VLP assembly. We decided to use a small 55 amino acid α-peptide from β-galactosidase enzyme as the tag. This has at least two advantages. First, the tag is small (6kDa) and should, therefore, be less likely to cause major changes in the folding of virals proteins and assembly into VLPs. This prediction proved accurate; α-peptide tagged HIV-1 Gag and Ebola VP40 proteins assembled into extracellular particles as efficiently as untagged counterparts. Second, the tagged protein could be quantified using a very simple α-complementation assay which dramatically reduced the time and effort required to measure VLP generation. Overall, the assay fulfilled the desired criteria.
Previously, few other studies have described luminescence-based assays of modified viral proteins to study viral assembly. In one such study, firefly luciferase was fused to EbVP40, but the fusion protein was not enzymatically active in VLPs (McCarthy, Licata, and Harty, 2006). In another study Gaussia luciferase was fused to the C-terminus of the Z-protein of Lassa virus but the signal to noise ratio in that assay was only ~8-fold (Capul and de la Torre, 2008). Others have reported the use of an HIV-1 Gag-luciferase fusion protein to study particle formation in yeast (Sakuragi et al., 2006). However, chemiluminescent signals obtained from this protein were very low, consistent with our own experience that HIV-1 Gag-Luciferase fusion proteins are poorly incorporated into VLPs in mammalian cells (unpublished observations). Recently, a Vpr-firefly luciferase fusion has been used to monitor HIV-1 particle release in a baculovirus-based Gag and Vpr expression system (Gonzalez et al., 2011). However this is obviously a non-native context, with highly overexpressed Gag and Vpr proteins. Finally, the α-peptide has previously been inserted at an internal location in the HIV-1 Gag protein in order to monitor proteolytic processing (Jochmans et al., 2010). Overall, this report is the first time that a direct enzyme based quantification of viral structural proteins has been used to monitor viral particle assembly and release in mammalian cells.
The approach and assay described herein is flexible. Indeed, it can be used to study the assembly and release of two unrelated viruses, namely Ebola and HIV-1 (Fig. 3). Moreover, it has a large dynamic range (two to three orders of magnitude) and has comparable sensitivity to western blot analysis (Fig. 2A and 2B). We could directly quantify the effect of L-domain mutations on particle release, further demonstrating the utility and authenticity of the measurements. We applied this rapid assay to investigate two aspects of viral particle assembly and release in a quantitative manner. First, it has been previously shown that the assembly of HIV-1 Gag protein into particles is more efficient (i.e. a greater fraction of Gag found in extracellular virions as opposed to cells) when Gag is expressed at higher levels(Hatziioannou et al., 2005; Perez-Caballero et al., 2004). We quantified this phenomenon in two ways. First, by increasing the level of HIV-1 Gag-α expression we found that the fraction of Gag-α that was found in VLPs could be increased by up to 5-fold (Fig 2C, D). Second, by holding the level of Gag-α constant and increasing the level of unmodified Gag, the fraction of Gag-α that was incorporated into virions could be increased by 3.5 fold. (Fig. 4A and 4B). A similar effect was seen for Ebola virus VP40, where an increase in intracellular concentration of unmodified VP40 lead to an approximately 10-fold increase in the fraction of the α-VP40 protein that was present in extracellular VLPs. This apparent cooperativity in the generation of VLPs upon enhanced expression of viral structural proteins in viruses as diverse as HIV-1 Gag or Ebola virus VP40 suggests the possibility that it might be a general phenomenon. The mechanistic underpinning of this effect would likely lie in increased multimer formation as a consequence of increased viral protein concentration. Such multimerization of viral proteins could result in conformation changes that increase the intrinsic affinity of each viral protein monomer for other monomers, or (in the case of enveloped viruses) the affinity of each monomer for membranes. Alternatively, it is possible that multimerization of proteins with weak membrane binding proteins could result in complexes with higher membrane binding avidity. In the case of HIV-1, it appears that the intrinsic membrane binding affinity of Gag is regulated by multimerization, in part due to the presence of a myristoyl switch, whereby the N-terminal myristate is preferentially concealed in a hydrophobic pocket in the monomeric state, and exposed in the multimeric state(Tang et al., 2004).
A second way in which viral structural protein expression levels can affect the efficiency with which extracellular particles are generated occurs as a consequence of the expression of the antiviral protein, tetherin. Recent studies have shown that tetherin is incorporated into the lipid envelope of HIV-1 particles and is directly responsible for virion tethering to the plasma membranes(Fitzpatrick et al., 2010; Perez-Caballero et al., 2009). Using the assay developed herein, we could precisely quantitate the reduction in physical particle yield attributable to tetherin expression, and demonstrate that tetherin can be saturated by increasing the expression of HIV-1 Gag. Indeed, the ability of tetherin to inhibit HIV-1 particle production varied dramatically according to the level at which the HIV-1 Gag protein was expressed. The saturability of tetherin and its alleviation by increasing the level of tetherin expression provide further support to the notion that tetherin acts by directly tethering virions on the cell surface.
Because the efficiency of particle assembly/release can, in part, be determined by the expression level of viral structural proteins, caution is warranted in interpreting data in which manipulations that affect particle yield also affect viral protein expression levels. Often, investigators ‘normalize’ measurements of extracellular viral particle yield to viral protein expression level in cells to obtain a measurement of particle formation efficiency. However, the findings reported herein suggest that experimental manipulations that only alter viral protein expression levels can also alter the intrinsic efficiency of virion assembly and release, leading to misleading conclusions if data is normalized in the aforementioned way.
In conclusion, we have developed a quantitative, sensitive and easily performed assay to study particle formation of two diverse viruses. The assay principle should be broadly applicable to studies of the assembly of many viruses, and with slight modifications might be amenable to high throughput screening. It provides a potentially valuable tool for understanding the complex process of virion assembly and a possible approach to rapidly screen for chemical inhibitors of viral assembly and release as potential therapeutics.
Materials and Methods
Plasmids
To generate plasmids expressing α-peptide tagged HIV-1 Gag proteins (Gag-α), sequences encoding the 55-residue α-peptide of β-galactosidase (MSSNSLAVVLQRRDWENPGVTQLNRLAAHPPFASWRNSEEARTDRPSQQLRSNLGE) were amplified, using PCR, from the plasmid, pProLabel-N (Clontech). This sequence was inserted at the 3′ end of HIV-1 Gag coding sequences in place of GFP in pCR3.1SynGag-GFP or an an L-domain mutant thereof (pCR3.1SynGagLD-GFP) between the NotI and XhoI sites (Perez-Caballero et al., 2004). To generate plasmids expressing N-terminally α-peptide tagged Ebola virus VP40 protein (VP40-α) and a corresponding L-domain mutant (P7L and P11L), the same sequence was inserted into pCR3.1MycEbVP40 (Martin-Serrano, Perez-Caballero, and Bieniasz, 2004) between EcoRI and BamHI sites. Plasmids expressing Tetherin-HA and Vpu-HA have been described previously (Jouvenet et al., 2009; Neil et al., 2006). Integrity of all the constructs were confirmed by restriction digestion and sequencing.
Chemiluminescent VLP release assay
293T cells were maintained in Dulbecco’s Modified Eagle Medium supplemented with 10% fetal bovine serum and gentamycin. For virus release assays, cells were seeded at 1X105 cells/well in 24-well plates and transfected using polyethyleneimine (PEI) at a DNA:PEI ratio of 1:4. At 48 hours post-transfection supernatants were collected and clarified by centrifugation at 1000 rpm for 5 minutes. The cleared supernatants (350–400μl) were filtered (0.2μm), layered over 600μl of 20% sucrose in 1X PBS and centrifuged at 14,000 rpm in an Eppendorf 5417R microfuge for 90 minutes at 4°C. The pellets containing viral like particles (VLPs) were resuspended in 1X Reporter Lysis Buffer (NEB Cat. No. B3321S). Cell lysates were prepared by incubating the cells in 1X lysis buffer with rocking for 30 minutes at room temperature. VLPs and cell lysates were subjected to cheminluminescent β-galactosidase assay for α-peptides according to manufacturer’s instructions (ProLabel Detection kit, Clontech). The reactions were developed for 1 hour at room temperature in the dark and luminescence was read using a luminometer (Dynex technologies).
Western Blot analyses
Cell lysates and VLPs were separated on 4–12% acrylamide gels (Novex) and proteins transferred to nitrocellulose membranes which were then probed with antibodies against HIV-1 Gag (183-H12-5C), tubulin (Santa Cruz), and the HA (Santa Cruz) or Myc (9E10) epitope tags. The blots were then probed with appropriate IR Dye (680 or 800CW, LiCOR) conjugated secondary antibodies. The blots were scanned using the LICOR Odyssey IR imager and signals quantified using the Odyssey quantification software.
Electron Microscopy
293T cells were transfected with plasmids expressing Gag or Gag-α (5μg) along-with a plasmid expressing GFP (0.5μg) using PEI. Forty hours later cells were harvested and fixed with 2% paraformaldehyde and 2.5% glutaraldehyde in 0.1M cacodylate buffer (0.1M sodium cacodylate [pH 7.4], 35mM sucrose, 4mM CaCl2). The cells were first stained with 1% OsO4 and 1% K4FeCN6 in 0.1M cacodylate buffer and dehydrated with 50% ethanol. After subsequent staining with 2% uranyl acetate in 70% ethanol the cells were dehydrated in graded ethanol (70%–100%) and embedded in epoxy resin (Electron Microscopy Sciences). Thin sections were stained with uranyl acetate. Electron micrographs were taken on a transmission electron microscope (FEI TECNAI G2 Spirit BioTwin Transmission Electron).
Acknowledgments
We thank members of the Bieniasz laboratory for advice. This work was supported by a grant from the NIH (R01 AI50111) to PDB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Capul AA, de la Torre JC. A cell-based luciferase assay amenable to high-throughput screening of inhibitors of arenavirus budding. Virology. 2008;382(1):107–14. doi: 10.1016/j.virol.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick K, Skasko M, Deerinck TJ, Crum J, Ellisman MH, Guatelli J. Direct restriction of virus release and incorporation of the interferon-induced protein BST-2 into HIV-1 particles. PLoS Pathog. 2010;6(3):e1000701. doi: 10.1371/journal.ppat.1000701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomis-Ruth FX, Dessen A, Timmins J, Bracher A, Kolesnikowa L, Becker S, Klenk HD, Weissenhorn W. The matrix protein VP40 from Ebola virus octamerizes into pore-like structures with specific RNA binding properties. Structure. 2003;11(4):423–33. doi: 10.1016/S0969-2126(03)00050-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez G, DaFonseca S, Errazuriz E, Coric P, Souquet F, Turcaud S, Boulanger P, Bouaziz S, Hong SS. Characterization of a novel type of HIV-1 particle assembly inhibitor using a quantitative luciferase-Vpr packaging-based assay. PLoS One. 2011;6(11):e27234. doi: 10.1371/journal.pone.0027234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlinger HG. The HIV-1 assembly machine. AIDS. 2001;15(Suppl 5):S13–20. doi: 10.1097/00002030-200100005-00003. [DOI] [PubMed] [Google Scholar]
- Hammonds J, Wang JJ, Yi H, Spearman P. Immunoelectron microscopic evidence for Tetherin/BST2 as the physical bridge between HIV-1 virions and the plasma membrane. PLoS Pathog. 2010;6(2):e1000749. doi: 10.1371/journal.ppat.1000749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartlieb B, Weissenhorn W. Filovirus assembly and budding. Virology. 2006;344(1):64–70. doi: 10.1016/j.virol.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Hatziioannou T, Martin-Serrano J, Zang T, Bieniasz PD. Matrix-induced inhibition of membrane binding contributes to human immunodeficiency virus type 1 particle assembly defects in murine cells. J Virol. 2005;79(24):15586–9. doi: 10.1128/JVI.79.24.15586-15589.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasenosky LD, Neumann G, Lukashevich I, Kawaoka Y. Ebola virus VP40-induced particle formation and association with the lipid bilayer. J Virol. 2001;75(11):5205–14. doi: 10.1128/JVI.75.11.5205-5214.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochmans D, Anders M, Keuleers I, Smeulders L, Krausslich HG, Kraus G, Muller B. Selective killing of human immunodeficiency virus infected cells by non-nucleoside reverse transcriptase inhibitor-induced activation of HIV protease. Retrovirology. 2010;7:89. doi: 10.1186/1742-4690-7-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouvenet N, Neil SJ, Zhadina M, Zang T, Kratovac Z, Lee Y, McNatt M, Hatziioannou T, Bieniasz PD. Broad-spectrum inhibition of retroviral and filoviral particle release by tetherin. J Virol. 2009;83(4):1837–44. doi: 10.1128/JVI.02211-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaletsky RL, Francica JR, Agrawal-Gamse C, Bates P. Tetherin-mediated restriction of filovirus budding is antagonized by the Ebola glycoprotein. Proc Natl Acad Sci U S A. 2009;106(8):2886–91. doi: 10.1073/pnas.0811014106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutluay SB, Bieniasz PD. Analysis of the initiating events in HIV-1 particle assembly and genome packaging. PLoS Pathog. 2010;6(11):e1001200. doi: 10.1371/journal.ppat.1001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Serrano J, Perez-Caballero D, Bieniasz PD. Context-dependent effects of L domains and ubiquitination on viral budding. J Virol. 2004;78(11):5554–63. doi: 10.1128/JVI.78.11.5554-5563.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Serrano J, Zang T, Bieniasz PD. HIV-1 and Ebola virus encode small peptide motifs that recruit Tsg101 to sites of particle assembly to facilitate egress. Nat Med. 2001;7(12):1313–9. doi: 10.1038/nm1201-1313. [DOI] [PubMed] [Google Scholar]
- McCarthy SE, Licata JM, Harty RN. A luciferase-based budding assay for Ebola virus. J Virol Methods. 2006;137(1):115–9. doi: 10.1016/j.jviromet.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Morita E, Sundquist WI. Retrovirus budding. Annu Rev Cell Dev Biol. 2004;20:395–425. doi: 10.1146/annurev.cellbio.20.010403.102350. [DOI] [PubMed] [Google Scholar]
- Neil SJ, Eastman SW, Jouvenet N, Bieniasz PD. HIV-1 Vpu promotes release and prevents endocytosis of nascent retrovirus particles from the plasma membrane. PLoS Pathog. 2006;2(5):e39. doi: 10.1371/journal.ppat.0020039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil SJ, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451(7177):425–30. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- Perez-Caballero D, Hatziioannou T, Martin-Serrano J, Bieniasz PD. Human immunodeficiency virus type 1 matrix inhibits and confers cooperativity on gag precursor-membrane interactions. J Virol. 2004;78(17):9560–3. doi: 10.1128/JVI.78.17.9560-9563.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Caballero D, Zang T, Ebrahimi A, McNatt MW, Gregory DA, Johnson MC, Bieniasz PD. Tetherin inhibits HIV-1 release by directly tethering virions to cells. Cell. 2009;139(3):499–511. doi: 10.1016/j.cell.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma T, Noda T, Urata S, Kawaoka Y, Yasuda J. Inhibition of Lassa and Marburg virus production by tetherin. J Virol. 2009;83(5):2382–5. doi: 10.1128/JVI.01607-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuragi S, Sakuragi J, Morikawa Y, Shioda T. Development of a rapid and convenient method for the quantification of HIV-1 budding. Microbes Infect. 2006;8(7):1875–81. doi: 10.1016/j.micinf.2006.02.027. [DOI] [PubMed] [Google Scholar]
- Scianimanico S, Schoehn G, Timmins J, Ruigrok RH, Klenk HD, Weissenhorn W. Membrane association induces a conformational change in the Ebola virus matrix protein. EMBO J. 2000;19(24):6732–41. doi: 10.1093/emboj/19.24.6732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C, Loeliger E, Luncsford P, Kinde I, Beckett D, Summers MF. Entropic switch regulates myristate exposure in the HIV-1 matrix protein. Proc Natl Acad Sci U S A. 2004;101(2):517–22. doi: 10.1073/pnas.0305665101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmins J, Scianimanico S, Schoehn G, Weissenhorn W. Vesicular release of ebola virus matrix protein VP40. Virology. 2001;283(1):1–6. doi: 10.1006/viro.2001.0860. [DOI] [PubMed] [Google Scholar]
- Van Damme N, Goff D, Katsura C, Jorgenson RL, Mitchell R, Johnson MC, Stephens EB, Guatelli J. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe. 2008;3(4):245–52. doi: 10.1016/j.chom.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Resh MD. Differential membrane binding of the human immunodeficiency virus type 1 matrix protein. J Virol. 1996;70(12):8540–8. doi: 10.1128/jvi.70.12.8540-8548.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]