Abstract
The development of molecular imaging probes has changed the nature of neurobiological research. Some of the most notable successes have involved the use of biological engineering techniques for the creation of fluorescent protein derivatives for optical imaging, but recent work has also led to a number of bioengineered probes for magnetic resonance imaging (MRI), the preeminent technique for noninvasive investigation of brain structure and function. Molecular MRI agents are beginning to be applied for experiments in the nervous system, where they have the potential to bridge from molecular to systems or organismic levels of analysis. Compared with canonical synthetic small molecule agents, biomolecular or semibiosynthetic MRI contrast agents offer special advantages due to their amenability to molecular engineering approaches, their properties in some cases as catalysts, and their specificity in targeting and ligand binding. Here, we discuss an expanding list of instances where biological engineering techniques have aided in the design of MRI contrast agents and reporter systems, examining both advantages and limitations of these types of probes for studies in the central nervous system.
Keywords: Molecular imaging, neuroimaging, contrast agent, central nervous system, protein engineering
Magnetic resonance imaging (MRI) was recognized soon after its invention as a potentially valuable tool for studying the central nervous system (CNS) and is now used extensively in the diagnosis of neurological disorders and in the study of cognition.1,2 MRI is an attractive tool for examining neurobiology because of its relatively high spatial resolution (<1 mm in humans, <100 μm in animals) and ability to scan body tissues noninvasively. Contrast in MRI results from the distribution and dynamics of nuclear spins in a specimen, usually arising from water protons, as well as the interaction of spins with applied radiofrequency and magnetic field gradient pulses. Contrast can be manipulated using molecular probes, which function as the MRI-equivalent of fluorescent dyes used in optical imaging. Although most MRI agents must be applied at high concentrations >10–6 M (over a million times more than typical nuclear medicine probes), the versatility and precision of MRI and the fact that MRI probes can be sensitized to ligand binding or environmental factors present decisive advantages in contexts such as functional imaging. Research on MRI contrast agents and their application to problems in neuroscience is burgeoning, and there is particular interest in the possibility of finding MRI agents sensitive to time varying components of neurophysiology [reviewed in ref (3)].
The first generation of clinical contrast agents were salts and chemical complexes of paramagnetic metals, such as gadolinium(III) and manganese(II).4−6 Complexes of these ions shorten the longitudinal (T1) and transverse (T2) relaxation times of nearby water molecules, properties that determine, respectively, the rate with which an MRI signal can be repeatedly measured and how long the signal persists during an individual measurement period. Increases in T1 relaxation due to a contrast agent produce image brightening in MRI, whereas increases in T2 relaxation produce image darkening. In either case, contrast is enhanced where the relaxation-based contrast agent localizes. Molecular probes can produce MRI contrast based on alternative mechanisms as well. The so-called chemical exchange saturation transfer (CEST) agents work by providing a probe-specific nuclear magnetic resonance frequency via which radiofrequency irradiation can be applied to diminish the local MRI signal.7 Heteronuclear probes allow MRI to be performed using nuclei other than protons, such as 19F and 129Xe, which are not normally found in living subjects.
Most MRI contrast agents are low molecular weight metal complexes prepared by synthetic chemistry, but notable recent advances have followed from the development of bioengineered macromolecular and supramolecular MRI probes. The biophysical properties that allow some proteins to act as contrast agents were established even before MRI came into use as a clinical imaging tool [reviewed in refs (8 and 9)]. It has also long been known that synthetic relaxation agents experience increases in potency (T1 or T2 relaxivity, denoted r1 or r2, and equal to the slope of relaxation rate vs concentration) upon binding proteins; this follows from the dependence of relaxivity on molecular rotational correlation time. The use of biomolecules and biomolecular conjugates as contrast agents themselves confers additional key advantages, however. The amenability of biomolecules to molecular engineering allows for the facile development of probes with novel functionality such as target binding or ligand responsiveness. With some biomolecular contrast agents, there is the additional possibility of genetically encoding them, for in vivo application as gene reporters or to enable endogenous synthesis in targeted cells. Several of these advantages extend to “hybrid” bioengineered contrast systems, in which biomolecules are designed to interact with synthetic components to produce MRI contrast patterns of physiological interest [reviewed in ref (10)]. The subsequent sections of this review explore the unique properties of bioengineered MRI probes in greater detail, highlighting opportunities to apply the new bioengineered molecular MRI techniques in neurobiological systems.
Amenability of Biomolecular Contrast Agents to Engineering
The discovery of green fluorescent protein (GFP)11 and its homologues in marine organisms led to a dramatic synthesis of genetic and imaging techniques based on light microscopy [reviewed in ref (12)]. Molecular engineering techniques have been applied to construct fluorescent protein-based sensors, to assemble reporters incorporating fluorescent proteins, and to tune the fluorescence properties of GFPs. Nature has been comparatively generous in providing MRI-detectable proteins, candidate “GFPs for MRI.” There are numerous paramagnetic proteins, for instance, which are capable of producing T1 or T2 contrast in MRI. These proteins can be targets for bioengineering techniques in much the way that GFP has been. Molecular engineering can also be applied to design and tune diamagnetic probes or components of hybrid agents.
The most important MRI-detectable protein in neuroscience research is hemoglobin, which is paramagnetic in its unliganded form but diamagnetic when oxygen bound;13 the effect of neurovascular coupling responses on the equilibrium between these two forms underlies the blood oxygen level-dependent effect used for functional MRI (fMRI).14−16 Hemoglobin might seem like a promising starting point for the development of analyte-sensitive MRI molecular imaging probes, and in fact a chemically modified form of the molecule has been applied as an exogenous T2 contrast agent for tissue oxygen tension imaging in vivo.17
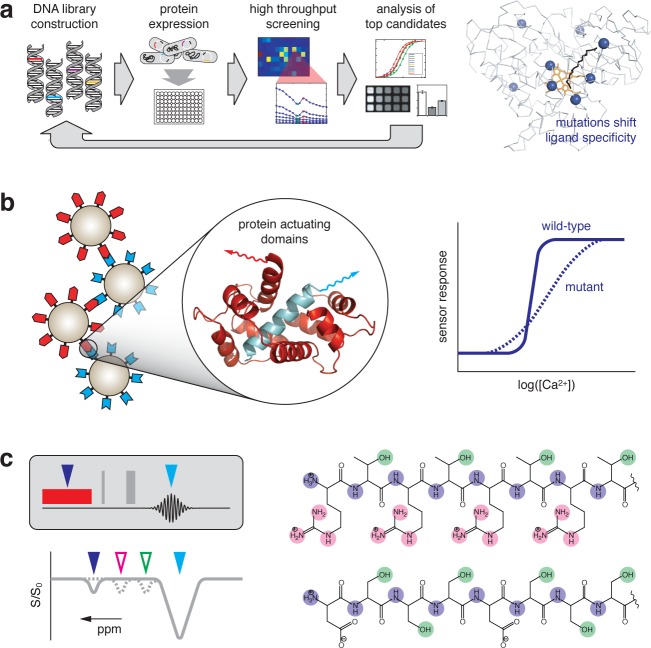
Of various heme-containing molecules, hemoglobin is not the most amenable to further bioengineering, however, in part because of its heterotetrameric structure and also because its binding pocket is too shallow for specific interactions with potential ligands. By applying molecular engineering techniques to another heme protein, a bacterial cytochrome P450 domain (BM3h), Shapiro et al. produced a contrast agent sensitive to the neurotransmitter dopamine (Figure 1a).18 The authors found that binding of a natural ligand, arachadonic acid, alters the T1 relaxivity of BM3h by displacing a water molecule coordinated to the heme iron. They then applied a technique called directed evolution [reviewed in ref (19)], which involves random mutagenesis followed by screening over repeated rounds of optimization, to tune the BM3h binding specificity away from arachidonic acid and toward dopamine. The resulting sensors had dopamine binding affinities of 3.3–8.9 μM and were shown to detect extracellular dopamine in both cell culture and rat brains. Further work was done to enhance the relaxivities of BM3h mutants by substituting the native heme with a high spin manganese(III) protoporphyrin complex.20 Both directed evolution and metal substitution can be generally applicable approaches for bioengineering of MRI contrast agents. Directed evolution in particular is a powerful technique because it does not require a priori knowledge of how mutations affect protein structure and function; the technique can therefore be applied to alter the properties of almost any naturally occurring or artificially constructed biomolecular probe.
Figure 1.
Bioengineered MRI probes. (a) Directed evolution strategy was used by Shapiro et al. to produce dopamine-sensitive MRI contrast agents from the heme domain of the cytochrome P450 BM3 heme domain (BM3h). The wild-type BM3h gene is randomly mutated to produce a DNA library. The library is transfected into E. coli, and variant proteins are expressed in multiwell format. Cells are lysed and lysates screened by titration with dopamine and the wild-type ligand arachidonic acid. Variants showing the greatest enhancement in dopamine binding and decrement in arichidonate binding are analyzed in purified form to assess ligand responsiveness and relaxivity changes. The process is repeated over multiple cycles to produce progressive improvement in target ligand responsiveness. The right-hand panel shows the distribution of mutations (blue spheres) selected by directed evolution of BM3h (gray Cα trace), depicted in complex with its heme group (orange) and wild type arachidonate ligand (black).18 (b) Rational protein design can be applied to tune properties of a biomolecule-actuated MRI sensor. Calcium-dependent protein–protein interactions drive responsiveness of a superparamagnetic iron-oxide (SPIO) based sensor developed by Atanasijevic et al. Calmodulin (red) and its peptidic binding partner (cyan) are conjugated to two populations of SPIO nanoparticles (left); reversible clustering takes place in the presence of calcium and leads to T2 changes. By mutating the protein domains (inset), the midpoint and cooperativity of the sensor’s response can be altered (schematic graph, right).21 (c) De novo bioengineering produces protein-based chemical exchange saturation transfer (CEST) contrast agents. Probe-specific CEST contrast is produced by an MRI pulse sequence (top left) in which a radiofrequency saturation pulse (red) is delivered at the frequency of an exchangable proton pool associated with the CEST reporter (blue arrowhead); because of chemical exchange, the saturation is transferred to protons at the frequency of bulk water (cyan). MRI signal decreases are observed as a function of frequency, as reflected in the so-called Z-spectrum (graph at bottom left). McMahon et al. showed that peptides can be designed to contain labile proton pools associated with a variety of specific chemical shifts (color coding in structures at right). Each proton pool produces a corresponding signature in the Z-spectra at left (color-coded arrowheads), allowing the molecules to be distinguished by CEST-weighted MRI.26
Rational protein design methods are complementary to screen-based techniques like directed evolution, and have also proved useful in the development of MRI probes. Several groups have constructed contrast agents by conjugating modified proteins to superparamagnetic iron oxide (SPIO) nanoparticles. In one study, Atanasijevic et al. developed an MRI sensor for T2-weighted imaging of calcium ions,21 which are well known as ubiquitous intracellular signaling molecules in the nervous system. The sensor consisted of one set of SPIOs conjugated to the calcium-binding protein calmodulin (CaM) and another conjugated to a target peptide that interacts with CaM only in its calcium bound form. Mixtures of the SPIO populations aggregated in the presence of increased calcium concentrations, producing approximately 5-fold changes in T2-weighted MRI signal in vitro by a mechanism very distinct from earlier synthetic calcium sensors.22,23 Using wild-type CaM, the sensor had a transition midpoint of approximately 1 μM Ca2+, but when rationally designed point mutations were introduced into the interacting protein domains, both the midpoint and cooperativity of the sensor’s calcium-dependent response could be tuned (Figure 1b).21,24 The process of adjusting the properties of a reagent using site-directed mutagenesis is much simpler than the resynthesis that would be required with a more conventional chemical contrast agent and again illustrates the advantage of bioengineering techniques in MRI probe development.
Using biological engineering, MRI probes can also be created from scratch. De novo design was used recently to create diamagnetic metal-free proteins capable of being visualized by the CEST contrast mechanism in living rodent brains.25 Any molecule that contains a labile proton pool in exchange with bulk water can function as a CEST agent, provided that the exchange takes place on an appropriate time scale and that the chemical shift of the bound protons is sufficiently resolved from bulk water. Several amino acid side chains, such as those of lysine, arginine, and tryptophan, contain protons that effectively support CEST contrast and can be incorporated into polypeptides to construct genetically encoded CEST reporters. In the first demonstration of this principle, Gilad et al. designed a lysine rich protein (LRP) that displayed specific contrast in cells expressing the construct.25 Implanted tumors expressing LRPs could be detected by CEST-weighted MRI in rat brains and distinguished from unlabeled tumors. The same group also demonstrated that the magnitude and frequency of protein-associated CEST effects could be tuned by altering the amino acid sequence (Figure 1c).26 McMahon et al. screened an array of peptides composed of combinations of lysine, arginine, and threonine to obtain CEST agents that could be detected differentially. This raises the possibility of performing a form of “multicolor” imaging using several distinct CEST reporters in parallel and exploits both the combinatorial nature and easy synthesis of biopolymers.
Imaging with Biomolecular Catalysts
Efforts to create biosynthetic MRI contrast agents are benefiting not only from the powerful molecular engineering approaches that can be used but also from the unique capabilities of biomacromolecules as imaging agents or components of molecular imaging strategies. Perhaps the most famous example of this is the ability of proteins to act as highly specific catalysts. Enzymes have been used for decades in optical imaging, originally as markers visualized by histology and more recently in combination with bioluminescent and fluorescent substrates for in vivo imaging [reviewed in ref (27)]. Now a host of enyzmes have been explored for their ability to induce contrast in molecular MRI experiments, in conjunction with various mechanisms for coupling MRI contrast to chemical processing (Figure 2). Although some of the enzymes used in these studies are disease-related markers, as opposed to established gene reporters, the strategies used to detect these molecules, and in some cases the enzymes themselves, could be adapted for use in reporter systems or alternative MRI detection specificities as well.
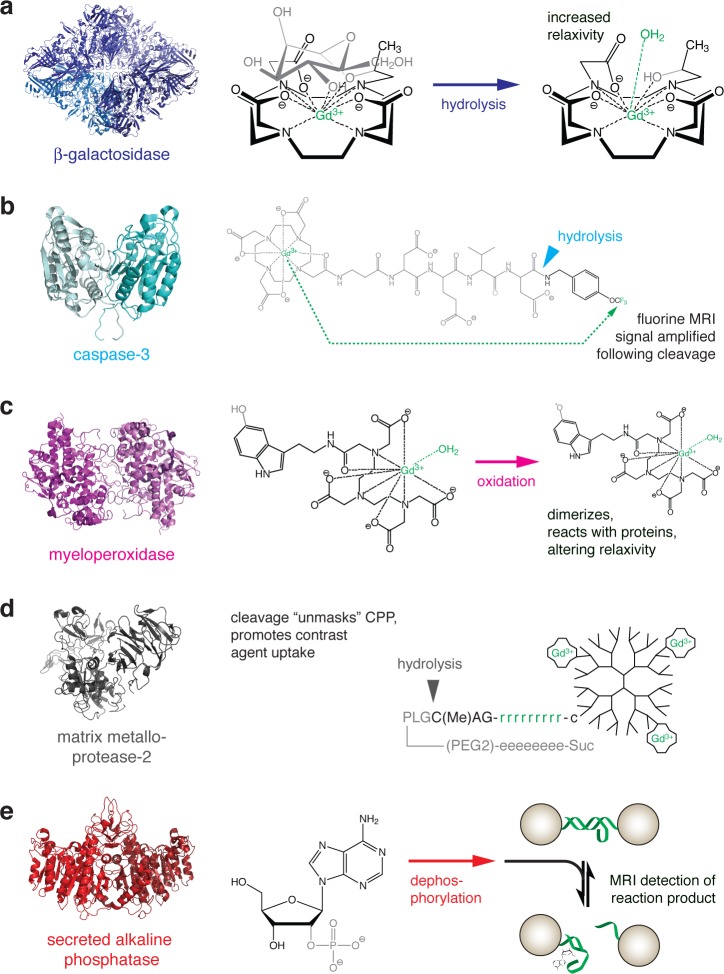
Figure 2.
Strategies for MRI-based detection of enzyme activity. For each example, the structure of the enzyme is shown at left, and the reaction catalyzed is shown at right. The chemical moieties most directly affected by the enzyme are shown in gray, and molecular components most directly responsible for MRI contrast are shown in green. (a) Gadolinium-containing substrate for β-galactosidase. Enzyme activity cleaves off a sugar moiety, increasing exposure of the gadolinium atom to interaction with a water molecule and consequently increasing T1 relaxivity.28 (b) Peptide-based probe for 19F MRI-based detection of caspase-3 activity. Prior to cleavage, the relaxation enhancement caused by the gadolinium chelate at left prevents detection of an 19F signal arising from the trifluoromethyl group at the right. Action of the enzyme cleaves the gadolinium-containing fragment and relieves the intramolecular relaxation effect, allowing an 19F signal to be detected.30 (c) Myeloperoxidase oxidizes the 5-hydroxy group of a serotonin-conjugated gadolinium chelate. The resulting free radical species tends to dimerize and react with proteins, resulting in compounds with longer τR and higher relaxivity.31 (d) A gadolinium-bearing dendrimer is conjugated to a peptide containing a poly-d-arginine cell penetrating domain, “masked” by an oppositely charged poly-d-glutamate domain. Action of matrix metalloprotease-2 or -9 cleaves the peptide, unmasking the polyarginine fragment and promoting accumulation of the contrast agent in nearby cells.37 (e) Detection of the reporter enzyme secreted alkaline phosphatase (SEAP) is performed using a nanoparticle-based T2 MRI sensor that detects adenosine, a product of SEAP-mediated dephosphorylation of 2′-adenosine monophosphate (left). Removal of adenosine by transport or further enzymatic processes reverses the contrast change mediated by the sensor.40
One of the earliest demonstrations of reporter enzyme detection using MRI was the mapping of the β-galactosidase (β-gal) activity in whole frog embryos with a β-gal-sensitive T1 contrast agent (Figure 2a).28 In this work, Louie et al. created a Gd3+ macrocyclic attached to a galactose group, joined by a linker that is cleavable by β-gal. Enzymatic hydrolysis of the contrast agent exposes the paramagnetic ion to water molecules, resulting in an increase in T1 relaxivity. Because the contrast agent was injected intracellularly at an early stage in embryogenesis, numerous structures, including the head, could be visualized in the presence of β-gal activity in these experiments. Although β-gal is perhaps the most widely used reporter enzyme in biological research, the difficulty of delivering the contrast agent has impeded efforts to apply the technique for gene expression mapping in additional contexts.
In another example involving a Gd3+-based enzyme substrate, a lipid-modified MRI probe was developed by Himmelreich et al. and used to detect intracellular lipase activity in cells.29 Prior to enzyme processing, the agent was insoluble and did not alter the MRI signal. However, once internalized via phagocytosis by cells expressing lipases, the fatty acid chains were cleaved from the compound, solubilizing the Gd3+ chelate and enhancing T1 contrast. A completely different mechanism also involving hydrolysis of a gadolinium compound was used to monitor activity of the protease caspase-3 in a recent study. Mizukami et al. created a probe consisting of gadolinium-tetraazacyclododecanetetraacetic acid (Gd-DOTA) conjugated to the peptide sequence DEVD and an 19F-containing group potentially detectable by heteronuclear MRI (Figure 2b).30 The short peptide brings the Gd3+ in close proximity to the fluorine atom, promoting relaxation of the 19F signal on a time scale too short for measurement by MRI. The action of the enzyme cleaves off the fluorinated moiety and removes the intramolecular relaxation effect, allowing the 19F MRI signal to be detected. Although 19F MRI is a relatively insensitive technique in general, the enzyme can provide amplification by processing many copies of the substrate; in vitro the caspase-3 reaction could be effectively monitored.
Qualitatively different enzyme-catalyzed chemical reactions, involving neither cleavage nor hydrolysis, can also potentially become the basis for MRI detection schemes. Rodriguez et al. synthesized Gd3+ chelates that could be oxidized by myeloperoxidase (MPO),31 an enzymatic marker associated with inflammation. Oxidized contrast agents tended to oligomerize and cross-link via phenolic side groups of tyrosine residues, increasing r1 by up to 1.5-fold, due to the dependence of relaxivity on the time scale for molecular motion (Figure 2c). MPO activity has also been coupled to the aggregation of serotonin-functionalized SPIOs.32 The phenolic group acts as the electron donor group and is converted to a tyrosyl radical when MPO reduces hydrogen peroxide. Formation of tyrosyl radicals by peroxidases induced cross-linking and aggregation of the nanoparticles, creating T2 changes. A somewhat related strategy could be used to detect the enzyme tyrosinase, which catalyzes serial oxidation steps that lead to the formation of melanin polymers, which in turn promotes paramagnetic metal ion accumulation in cells and consequent MRI changes.33 The activity of overexpressed tyrosinase recapitulates the process whereby neuromelanin forms in cells of the substantia nigra in the brain, a region known for high iron content and T2 MRI contrast.34
Enzymatic detection schemes for MRI have been developed around nanoparticle contrast agents as well as small molecules. Nanoparticle T1 and T2 contrast agents are particularly advantageous because of their high relaxivities.35 In one of the first examples of this, in vivo expression of an engineered transferrin (Tf) receptor in mice was detected using Tf-conjugated SPIOs.36 The Tf-SPIOs are transported into the cell by receptor-mediated endocytosis, allowing tumor cells to be imaged and tracked. A similar accumulation-based mechanism has also been demonstrated with SPIOs coated with a “masked” version of a cell penetrating peptide (CPP).37 Matrix metalloproteins (MMPs) are proteases with activities highly linked to tumor invasion and metastasis.38 Olsen et al. used an MMP substrate peptide consisting of a polyarginine CPP sequence separated by the MMP cleavage site from a polyglutamate stretch expected to block CPP function;37,39 peptides were conjugated to fluorescent and Gd-DOTA-labeled dendrimers (Figure 2d). The action of MPP-2 or MMP-9 cut off the polyglutamate masking region, allowing the peptides to be taken up by cells due to the unmasked CPP function. The authors observed accumulation of the probe at the invasive edges of MMP-expressing tumors by fluorescence, as well as T1 relaxation changes of up to approximately 30%. The use of dendrimers reduced the clearance rate of Gd-DOTA and apparently limited its nonspecific uptake by tissues, thus improving the contrast between MMP-expressing structures and other tissue.
Another nanoparticle-based enzyme detection scheme was developed by Westmeyer et al., who applied an MRI sensor to detect enzymatic turnover catalyzed by secreted alkaline phosphatase (SEAP),40 a reporter used previously to visualize gene expression patterns in the brain. Bioengineering of an MRI technique around a secreted enzyme obviates the need for intracellular delivery, in contrast to strategies for β-gal detection.28,41−44 Further, the use of a contrast agent to detect products of the enzyme, as opposed to functioning as substrates themselves, facilitates dynamic studies of enzyme activity since the contrast agent is not irreversibly modified over time. An unnatural nucleotide 2′-adenosine monophosphate is processed by SEAP into adenosine (Ado), which is then detected by an Ado sensor for MRI (Figure 2e). Ado acts on the sensor by inducing disaggregation of SPIO nanoparticles cross-linked by a switchable DNA aptamer;45 the aggregation state influences changes in T2 relaxivity which can be detected by MRI. A nanoparticle T2 contrast agent based on the enzymatic degradation of a polymer coating has also been explored as a possible gene reporter component.46 In this strategy, commercial SPIOs coated with a relatively thick coat of dextran (Feridex) were substrates for the enzyme dextranase. Enzymatic digestion of the nanoparticle coating exposed the iron oxide core to water, producing T2 changes both in vitro and in vivo.
Bioengineering of Targeted Imaging Agents
The promise of applying contrast agents for diagnosis of disease, both inside and outside the brain, drives a major portion of molecular imaging research. The disadvantageous sensitivity of MRI compared with positron emission tomography is compensated in many cases by MRI’s superior resolution, and low sensitivity is not necessarily a barrier in cases where substantial amount of imaging agent can be delivered to sites of action, e.g., in the bloodstream. Many targeted MRI probes developed to date include biological components, in large part because of the high specificity imparted by macromolecular ligands. To produce targeted contrast agents, biomolecular domains can be chemically conjugated to passive synthetic contrast agents such as paramagnetic metal complexes, SPIOs, and dendrimers (Figure 3). An early example was offered by Sipkins et al., who used monoclonal antibodies labeled with paramagnetic liposomes to target the angiogenesis marker integrin αvβ3.47 The anti-integrin antibody was conjugated to the liposomes through biotin–avidin conjugation. They demonstrated that the integrin-targeted liposome could be used to image angiogenic vasculature and distinguish between benign and malignant tumor phenotypes in rabbits, a distinction that could not be made with conventional MR imaging techniques. An integrin binding partner, VCAM-1, has also been targeted in a more recent approach that enabled the detection of acute endothelial inflammation in mouse ears48 and brains.49 Here, a monoclonal antibody against VCAM-1 was conjugated to SPIO microparticles, which are individually detectable by MRI. In another study, an intravascular agent designed for the detection of fibrotic lesions was prepared by conjugating a gadolinium chelate to a collagen binding peptide identified by phage display.50−53 The use of phage display or related affinity-based polypeptide screening methods to produce targeting motifs for molecular imaging agents represents a prime example of how biological engineering techniques can advance this field.
Figure 3.
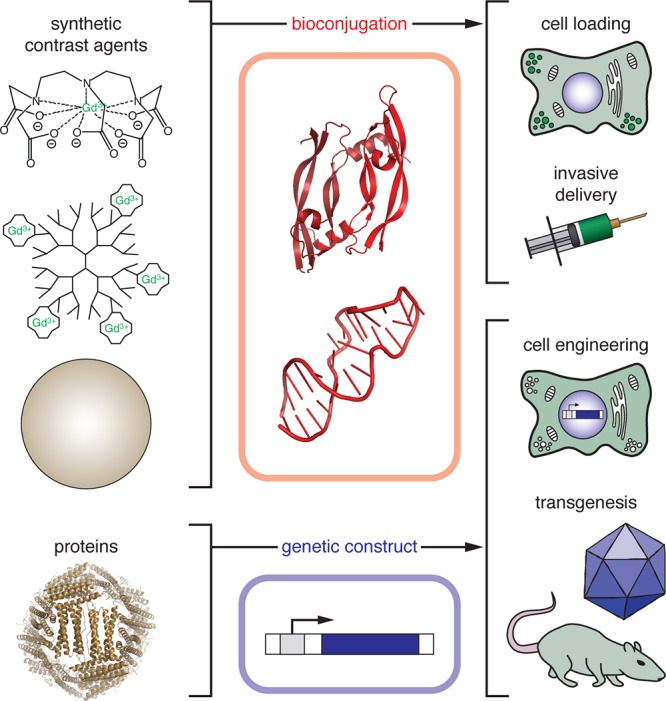
Biomolecule-based targeting of MRI contrast agents. Bioengineering approaches are used to target both synthetic and genetically encoded MRI contrast agents to the nervous system or elsewhere. Synthetic contrast agents (top) may be conjugated to macromolecular domains (proteins, nucleic acids, or oligosaccharides) for targeted delivery. The resulting semisynthetic agents are introduced into experimental subjects either by loading and introducing cells (e.g., for cell tracking or monitoring) or by direct injection into the bloodstream or brain. Protein-based agents (bottom) can be targeted for endogenous production using genetic constructs. DNA vectors are used to engineer cells to express the contrast agent, or to induce contrast agent expression directly in animals via viral-mediated gene transduction or transgenesis.
Targeted MRI probes of potential utility in basic neuroscience have been formed by conjugating contrast agents to proteins that are spontaneously taken up and transported along neural fibers. Early attempts at this were based on conjugation of wheat germ agglutinin (WGA), a predominantly anterograde tracer, to SPIOs.54−56 Using WGA-SPIO conjugates, bidirectional slow axonal transport could be visualized as migrating hypointensity in rodent peripheral nerves, but analogous transport could not be seen in the CNS. Recently, however, Wu et al. achieved successful tract tracing results in the brain using a gadolinium–DOTA conjugate of the cholera toxin B subunit (CTB),57 an established retrograde tract tracer.58 Injections of the MRI tracer into the somatosensory cortex of rats produced hyperintense T1-weighted MRI signal in projections arising from thalamic nuclei and injections into olfactory bulb labeled olfactory regions of the cortex.57 The comparative success of the Gd-based tracer compared with WGA–SPIO conjugates for tract tracing in the CNS suggests the potential for modifications of larger size to interfere with properties of proteins to which they are conjugated.
Biological macromolecules other than proteins have also been used to engineer targeted imaging agents for in vivo MRI. In one study, the so-called glyconanoparticles consisting of iron oxides modified with carbohydrates were created to bind the endothelial marker proteins E and P-selectin.59 Like VCAM-1 and integrin-targeted conjugates, these imaging agents were also able to detect vascular hallmarks of cerebral inflammation in rodent brains. Oligonucleotide aptamers attached to MRI contrast agents also permit targeting to protein epitopes. In one study, an aptamer-conjugated SPIO contrast agent was used to detect the coagulation factor thrombin in vitro; detection of concentrations as low as 25 nM was reported.45 The possibility of producing aptamers against a wide variety of targets using systematic evolution of ligands by exponential enrichment (SELEX) technology60,61 may lead to further examples of nucleic acid-based molecular MRI agents. Even more exciting is the possibility of targeting oligonucleotide conjugates to DNA and RNA molecules themselves, especially in the CNS. In a series of papers, Liu and co-workers have described MRI experiments in which oligonucleotide conjugated SPIOs were applied to detect specific gene transcripts in rodent brains, in an MRI-based version of in situ hybridization mapping.62−64 The authors found contrast patterns dependent on the oligonucleotide targeting sequences they used and on stimuli delivered in conjunction with the MRI probes. This approach requires that the contrast agents permeate the blood–brain barrier (BBB), as well as individual cell membranes, and evidence of both was reported.65
The difficulty of delivering contrast agents past the BBB poses a unique challenge for targeted molecular imaging of most ligands in the brain.66 A particularly interesting solution to this problem is the possibility of producing the contrast agents directly within the brain, using DNA constructs which may be targeted using genetic techniques. Although genetic approaches are not yet on the horizon for clinical molecular MRI, basic scientific applications may benefit considerably from the fusion of imaging with genetic technologies [reviewed in refs (10 and 67)]. The metalloprotein ferritin (Ft) stores endogenous iron as a crystalline ferrihydrite core which has been shown to influence MRI contrast where it is naturally expressed. Genove et al. showed that viral-mediated overexpression of Ft in the mouse brain produces clear T2 signal changes by enhancing iron loading in the transduced neurons,68 and Cohen et al. showed altered T2 contrast patterns in transgenic mice expressing Ft as a marker for endothelial cells in the brain and elsewhere.69,70 Although Ft is similar in size and iron content to synthetic SPIOs, its relaxivity is over 100 times lower due to the relatively amorphous and hydrated mineral structure of its core.71 Strategies have been explored to improve Ft relaxivity, such as coexpressing the transferrin receptor to transport more iron into cells.72 Additional genetically encodable MRI reporters have also been explored, including ion transporters such as MagA,73,74 a protein from magnetotactic bacteria. Several studies have also demonstrated changes in MRI due to the expression of diamagnetic proteins, including the lysine-rich CEST reporters described above,25,26,75,76 and GFP, which was detected by magnetization transfer-weighted imaging.77 An advantage of genetically encoded diamagnetic agents is that their expression and resultant contrast do not require association with or accumulation of a paramagnetic cofactor.
Limitations of Bioengineered MRI Probes
Advances have been made in the design of new MRI contrast agents that could eventually further our understanding of the brain and its diseases. Most MRI probes, however, have not progressed past the proof-of-principle stage for basic science applications and are certainly not yet suitable for clinical use. Many agents are limited by their low sensitivities,66 as demonstrated by the high probe concentrations applied in published studies. Although low sensitivity is a problem for all MRI contrast agents, it is more of a challenge for existing biomolecular contrast agents than for synthetic agents. The problem is exemplified by protein T1 agents like BM3h, which has a relaxivity of 1.23 mM–1s–1 at 21 °C and 4.7 T,18 compared with 3–5 mM–1s–1 for Gd3+ compounds under similar conditions.4 The relaxivity of Ft is also much lower than that of synthetic SPIO contrast agents, ∼1 (mM Fe)−1s–1 for Ft (78) vs 50–200 (mM Fe)−1s–1 for typical SPIOs.79 Protein-based CEST agents also offer relatively low sensitivity, compared with synthetic CEST agents that incorporate chelated lanthanides.80 With today’s technology, a synthetic MRI contrast agent is therefore likely to be applicable at lower concentration and with lesser risk of physiological disruption or toxicity than a biomolecular probe. This is one reason why much effort has focused on detection systems in which small molecule synthetic contrast agents function as responsive substrates for enzymes that provide amplification as well as desirable targeting and specificity properties.
For neuroimaging applications, a second confounding issue is the difficulty of delivering contrast agents noninvasively to the brain.81 Because almost all MRI contrast agents are polar, charged compounds, BBB permeability is not spontaneously achieved. For applications in humans, the problem of trans-BBB delivery has almost entirely prevented CNS applications of MRI contrast agents, except for imaging cerebrovascular parameters. In animals, BBB disruption using hyperosmotic shock or ultrasound has been used to deliver agents ranging from small molecules82−84 to nanoparticles85−87 into the brain parenchyma. Although they are not yet common techniques in the clinic, both osmotic shock88 and focused ultrasound-mediated89 BBB disruption techniques have also been applied in human or nonhuman primate subjects. Even using these methods, however, larger molecules are difficult to deliver. A systematic study of ultrasound-mediated delivery of fluorescent dextrans of varying size found for instance that a 3 kD molecule (comparable to a short peptide) could be delivered at approximately 5-fold higher doses than a 70 kD molecule (comparable to a medium-sized protein).90 Molecules in the megadalton size range were not effectively delivered. For bioengineered protein probes or nanoparticle bioconjugates, compared with “conventional” small molecule MRI contrast agents, the BBB delivery problem clearly poses a particular challenge therefore. One way to bypass the need for trans-BBB delivery is to use genetic targeting to the brain, in conjunction with protein-based agents. Although this is an exciting approach to use in transgenic animals, it cannot yet be contemplated in humans, ruling out clinical applications. Moreover, because of the relatively low sensitivity afforded by existing genetically encoded contrast agents, achieving high enough expression levels to produce desired MRI contrast levels in cells or tissue is not easy.
Both the sensitivity and delivery limitations of bioengineered MRI probes may reflect the current “state of play,” as opposed to theoretical constraints, however. Biomolecular relaxation agents for instance, can in principle reach relaxivity levels considerably higher than current synthetic contrast agents.91−94 High T1 relaxivity depends on the interplay between electronic relaxation and solvent interaction parameters that might indeed be easier to optimize in macromolecular agents than with synthetic probes, due to the amenability of biomolecules to tuning and screening approaches. Large magnetic moments characteristic of synthetic SPIOs might also be achieved in biological systems and have naturally occurring precedents in the magnetosomes of magnetotactic bacteria,95 as well as mineral deposits found in several vertebrate species.96−99 Improving the sensitivity of biomolecular MRI contrast agents will also enable them to be used at concentrations where immunogenicity, an intrinsic property of these types of probes, will be less significant. Meanwhile, brain delivery of macromolecules could be enhanced by fusion to so-called Trojan horse vehicles, like Tf, which are themselves proteins and have been shown to deliver cargo to the brain.100 In conjunction with improved genetically encoded contrast agents, trans-BBB viral delivery strategies may also one day prove effective for noninvasive brain delivery biosynthetic MRI probes. Some types of viruses have been shown to cross the BBB spontaneously101−103 and could serve as vectors for this purpose, at least in animals.
Conclusions
In the wake of the genetic and genomic revolutions, the expansion of biology-based technologies into multiple spheres of investigation has been dramatic. Protein therapeutics are becoming widespread, and some are being actively investigated for CNS applications. In the area of molecular neuroimaging, which seeks to monitor neural structure and function at a molecular level for both research and clinical purposes, bioengineering methods are having impact largely via the development of new probes. New concepts for the design of molecular MRI agents have emerged from the use of biomolecules as templates or building blocks. Strategies for discovering and enhancing contrast agents have been borrowed from the palette of macromolecular engineering techniques available to researchers across the life sciences. Although there are substantial limitations to the currently available molecular MRI methods and clinical applications are in most cases still remote, the bioengineering approaches recently demonstrated in this field provide a strong foundation for continued innovation of MRI-based measurement modalities that could over time change the way the brain is studied in healthy and diseased states.
Glossary
Abbreviations
- Ado
adenosine
- β-gal
β-galactosidase
- BBB
blood–brain barrier
- CaM
calmodulin
- CPP
cell penetrating peptide
- CNS
central nervous system
- CEST
chemical exchange saturation transfer
- CTB
cholera toxin B subunit
- BM3h
cytochrome P450 BM3 heme domain
- Ft
ferritin
- fMRI
functional magnetic resonance imaging
- Gd-DOTA
gadolinium-tetraazacyclododecanetetraacetic acid
- GFP
green fluorescent protein
- T1
longitudinal relaxation time
- r1
longitudinal relaxivity
- LRP
lysine rich protein
- MRI
magnetic resonance imaging
- MMP
matrix metalloprotease
- MPO
myeloperoxidase
- SEAP
secreted alkaline phosphatase
- SPIO
superparamagnetic iron oxide
- Tf
transferrin
- T2
transverse relaxation time
- r2
transverse relaxivity
- WGA
wheat germ agglutinin
This work was supported by NIH grants DP2-OD002114 and R01-DA028299 to A.J. and an NSERC Post-Graduate Scholarship to V.H.
The authors declare no competing financial interest.
References
- Gadian D. G. (1995) NMR and Its Applications to Living Systems, Oxford University Press, Oxford, UK. [Google Scholar]
- Buxton R. B. (2009) Introduction to Functional Magnetic Resonance Imaging: Principles and Techniques, Cambridge University Press, New York. [Google Scholar]
- Jasanoff A. (2007) MRI contrast agents for functional molecular imaging of brain activity. Curr. Opin. Neurobiol. 17, 593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauffer R. B. (1987) Paramagnetic metal complexes as water proton relaxation agents for NMR imaging: theory and design. Chem. Rev. 87, 901–927. [Google Scholar]
- Toth E., Helm L., and Merbach A. (2002) Relaxivity of MRI Contrast Agents (Krause W., Ed.) pp 61–101, Springer, Berlin. [Google Scholar]
- Schwert D., Davies J., and Richardson N. (2002) Non-Gadolinium-Based MRI Contrast Agents (Krause W., Ed.) pp 165–199, Springer, Berlin. [Google Scholar]
- Ward K. M.; Aletras A. H.; Balaban R. S. (2000) A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST). J. Magn. Reson. 143, 79–87. [DOI] [PubMed] [Google Scholar]
- Mildvan A. S. (1974) Mechanism of enzyme action. Annu. Rev. Biochem. 43, 357–399. [DOI] [PubMed] [Google Scholar]
- Burton D. R.; Forsen S.; Karlstrom G.; Dwek R. A. (1979) Proton relaxation enhancement (PRE) in biochemistry: critical survey. Prog. Nucl. Magn. Reson. Spectrosc. 13, 1–45. [Google Scholar]
- Westmeyer G. G.; Jasanoff A. (2007) Genetically controlled MRI contrast mechanisms and their prospects in systems neuroscience research. Magn. Reson. Imaging 25, 1004–1010. [DOI] [PubMed] [Google Scholar]
- Shimomura O.; Johnson F. H.; Saiga Y. (1962) Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J. Cell Comp. Physiol. 59, 223–239. [DOI] [PubMed] [Google Scholar]
- Giepmans B. N.; Adams S. R.; Ellisman M. H.; Tsien R. Y. (2006) The fluorescent toolbox for assessing protein location and function. Science 312, 217–224. [DOI] [PubMed] [Google Scholar]
- Pauling L.; Coryell C. D. (1936) The magnetic properties and structure of hemoglobin, oxyhemoglobin and carbonmonoxyhemoglobin. Proc. Natl. Acad. Sci. U.S.A. 22, 210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S.; Lee T. M.; Kay A. R.; Tank D. W. (1990) Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl. Acad. Sci. U.S.A. 87, 9868–9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S.; Tank D. W.; Menon R.; Ellermann J. M.; Kim S. G.; Merkle H.; Ugurbil K. (1992) Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc. Natl. Acad. Sci. U.S.A. 89, 5951–5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong K. K.; Belliveau J. W.; Chesler D. A.; Goldberg I. E.; Weisskoff R. M.; Poncelet B. P.; Kennedy D. N.; Hoppel B. E.; Cohen M. S.; Turner R.; Cheng H. M.; Brady T. J.; Rosen B. R. (1992) Dynamic magnetic-resonance-imaging of human brain activity during primary sensory stimulation. Proc. Natl. Acad. Sci. U.S.A. 89, 5675–5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun P. Z.; Schoening Z. B.; Jasanoff A. (2003) In vivo oxygen detection using exogenous hemoglobin as a contrast agent in magnetic resonance microscopy. Magn. Reson. Med. 49, 609–614. [DOI] [PubMed] [Google Scholar]
- Shapiro M. G.; Westmeyer G. G.; Romero P. A.; Szablowski J. O.; Küster B.; Shah A.; Otey C. R.; Langer R.; Arnold F. H.; Jasanoff A. (2010) Directed evolution of a magnetic resonance imaging contrast agent for noninvasive imaging of dopamine. Nat. Biotechnol. 28, 264–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brustad E. M.; Arnold F. H. (2011) Optimizing non-natural protein function with directed evolution. Curr. Opin. Chem. Biol. 15, 201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelyveld V. S.; Brustad E.; Arnold F. H.; Jasanoff A. (2011) Metal-substituted protein MRI contrast agents engineered for enhanced relaxivity and ligand sensitivity. J. Am. Chem. Soc. 133, 649–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atanasijevic T.; Shusteff M.; Fam P.; Jasanoff A. (2006) Calcium-sensitive MRI contrast agents based on superparamagnetic iron oxide nanoparticles and calmodulin. Proc. Natl. Acad. Sci. U.S.A. 103, 14707–14712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. A.; Hesketh R. T.; Metcalfe J. C.; Feeney J.; Morris P. G. (1983) Intracellular calcium measurements by 19F NMR of fluorine-labeled chelators. Proc. Natl. Acad. Sci. U.S.A. 80, 7178–7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W. H.; Fraser S. E.; Meade T. J. (1999) A calcium-sensitive magnetic resonance imaging contrast agent. J. Am. Chem. Soc. 121, 1413–1414. [Google Scholar]
- Green D. F.; Dennis A. T.; Fam P. S.; Tidor B.; Jasanoff A. (2006) Rational design of new binding specificity by simultaneous mutagenesis of calmodulin and a target peptide. Biochemistry 45, 12547–12559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilad A. A.; McMahon M. T.; Walczak P.; Winnard P. T.; Raman V.; van Laarhoven H. W.; Skoglund C. M.; Bulte J. W.; van Zijl P. C. (2007) Artificial reporter gene providing MRI contrast based on proton exchange. Nat. Biotechnol. 25, 217–219. [DOI] [PubMed] [Google Scholar]
- McMahon M. T.; Gilad A. A.; DeLiso M. A.; Berman S. M.; Bulte J. W.; van Zijl P. C. (2008) New ″multicolor″ polypeptide diamagnetic chemical exchange saturation transfer (DIACEST) contrast agents for MRI. Magn. Reson. Med. 60, 803–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescher J. A.; Contag C. H. (2010) Guided by the light: visualizing biomolecular processes in living animals with bioluminescence. Curr. Opin. Chem. Biol. 14, 80–89. [DOI] [PubMed] [Google Scholar]
- Louie A. Y.; Huber M. M.; Ahrens E. T.; Rothbacher U.; Moats R.; Jacobs R. E.; Fraser S. E.; Meade T. J. (2000) In vivo visualization of gene expression using magnetic resonance imaging. Nat. Biotechnol. 18, 321–325. [DOI] [PubMed] [Google Scholar]
- Himmelreich U.; Aime S.; Hieronymus T.; Justicia C.; Uggeri F.; Zenke M.; Hoehn M. (2006) A responsive MRI contrast agent to monitor functional cell status. NeuroImage 32, 1142–1149. [DOI] [PubMed] [Google Scholar]
- Mizukami S.; Takikawa R.; Sugihara F.; Hori Y.; Tochio H.; Wälchli M.; Shirakawa M.; Kikuchi K. (2007) Paramagnetic relaxation-based 19F MRI probe to detect protease activity. J. Am. Chem. Soc. 130, 794–795. [DOI] [PubMed] [Google Scholar]
- Rodriguez E.; Nilges M.; Weissleder R.; Chen J. W. (2010) Activatable magnetic resonance imaging agents for myeloperoxidase sensing: mechanism of activation, stability, and toxicity. J. Am. Chem. Soc. 132, 168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez J. M.; Simeone F. J.; Tsourkas A.; Josephson L.; Weissleder R. (2004) Peroxidase substrate nanosensors for MR imaging. Nano Lett. 4, 119–122. [Google Scholar]
- Weissleder R.; Simonova M.; Bogdanova A.; Bredow S.; Enochs W. S.; Bogdanov A. (1997) MR imaging and scintigraphy of gene expression through melanin induction. Radiology 204, 425–429. [DOI] [PubMed] [Google Scholar]
- Haacke E. M.; Cheng N. Y.; House M. J.; Liu Q.; Neelavalli J.; Ogg R. J.; Khan A.; Ayaz M.; Kirsch W.; Obenaus A. (2005) Imaging iron stores in the brain using magnetic resonance imaging. Magn. Reson. Imaging 23, 1–25. [DOI] [PubMed] [Google Scholar]
- Wang Y.-X.; Hussain S.; Krestin G. (2001) Superparamagnetic iron oxide contrast agents: physicochemical characteristics and applications in MR imaging. Eur. Radiol. 11, 2319–2331. [DOI] [PubMed] [Google Scholar]
- Weissleder R.; Moore A.; Mahmood U.; Bhorade R.; Benveniste H.; Chiocca E. A.; Basilion J. P. (2000) In vivo magnetic resonance imaging of transgene expression. Nat. Med. 6, 351–354. [DOI] [PubMed] [Google Scholar]
- Olson E. S.; Jiang T.; Aguilera T. A.; Nguyen Q. T.; Ellies L. G.; Scadeng M.; Tsien R. Y. (2010) Activatable cell penetrating peptides linked to nanoparticles as dual probes for in vivo fluorescence and MR imaging of proteases. Proc. Natl. Acad. Sci. U.S.A. 107, 4311–4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeblad M.; Werb Z. (2002) New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2, 161–174. [DOI] [PubMed] [Google Scholar]
- Jiang T.; Olson E. S.; Nguyen Q. T.; Roy M.; Jennings P. A.; Tsien R. Y. (2004) Tumor imaging by means of proteolytic activation of cell-penetrating peptides. Proc. Natl. Acad. Sci. U.S.A. 101, 17867–17872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westmeyer G. G.; Durocher Y.; Jasanoff A. (2010) A secreted enzyme reporter system for MRI. Angew. Chem., Int. Ed. 49, 3909–3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanaoka K.; Kikuchi K.; Terai T.; Komatsu T.; Nagano T. (2008) A Gd3+-based magnetic resonance imaging contrast agent sensitive to beta-galactosidase activity utilizing a receptor-induced magnetization enhancement (RIME) phenomenon. Chemistry 14, 987–995. [DOI] [PubMed] [Google Scholar]
- Cui W. N.; Liu L.; Kodibagkar V. D.; Mason R. P. (2010) S-Gal, a novel 1H MRI reporter for beta-galactosidase. Magn. Reson. Med. 64, 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arena F.; Singh J.; Gianolio E.; Stefania R.; Aime S. (2011) beta-Gal gene expression MRI reporter in melanoma tumor cells. Design, synthesis, and in vitro and in vivo testing of a Gd(III) containing probe forming a high relaxivity, melanin-like structure upon beta-Gal enzymatic activation. Bioconjugate Chem. 22, 2625–2635. [DOI] [PubMed] [Google Scholar]
- Keliris A.; Ziegler T.; Mishra R.; Pohmann R.; Sauer M. G.; Ugurbil K.; Engelmann J. (2011) Synthesis and characterization of a cell-permeable bimodal contrast agent targeting beta-galactosidase. Bioorg. Med. Chem. 19, 2529–2540. [DOI] [PubMed] [Google Scholar]
- Yigit M. V.; Mazumdar D.; Lu Y. (2008) MRI detection of thrombin with aptamer functionalized superparamagnetic iron oxide nanoparticles. Bioconjugate Chem. 19, 412–417. [DOI] [PubMed] [Google Scholar]
- Granot D.; Shapiro E. M. (2011) Release activation of iron oxide nanoparticles: (REACTION) a novel environmentally sensitive MRI paradigm. Magn. Reson. Med. 65, 1253–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipkins D. A.; Cheresh D. A.; Kazemi M. R.; Nevin L. M.; Bednarski M. D.; Li K. C. (1998) Detection of tumor angiogenesis in vivo by alphaVbeta3-targeted magnetic resonance imaging. Nat. Med. 4, 623–626. [DOI] [PubMed] [Google Scholar]
- Tsourkas A.; Shinde-Patil V. R.; Kelly K. A.; Patel P.; Wolley A.; Allport J. R.; Weissleder R. (2005) In vivo imaging of activated endothelium using an anti-VCAM-1 magnetooptical probe. Bioconjugate Chem. 16, 576–581. [DOI] [PubMed] [Google Scholar]
- McAteer M. A.; Sibson N. R.; von zur Muhlen C.; Schneider J. E.; Lowe A. S.; Warrick N.; Channon K. M.; Anthony D. C.; Choudhury R. P. (2007) In vivo magnetic resonance imaging of acute brain inflammation using microparticles of iron oxide. Nat. Med. 13, 1253–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caravan P.; Das B.; Dumas S.; Epstein F. H.; Helm P. A.; Jacques V.; Koerner S.; Kolodziej A.; Shen L.; Sun W. C.; Zhang Z. (2007) Collagen-targeted MRI contrast agent for molecular imaging of fibrosis. Angew. Chem., Int. Ed. 46, 8171–8173. [DOI] [PubMed] [Google Scholar]
- Nair S. A.; Kolodziej A. E.; Bhole G.; Greenfield M. T.; McMurry T. J.; Caravan P. (2008) Monovalent and bivalent fibrin-specific MRI contrast agents for detection of thrombus. Angew. Chem., Int. Ed. 47, 4918–4921. [DOI] [PubMed] [Google Scholar]
- Overoye-Chan K.; Koerner S.; Looby R. J.; Kolodziej A. F.; Zech S. G.; Deng Q.; Chasse J. M.; McMurry T. J.; Caravan P. (2008) EP-2104R: A fibrin-specific gadolinium-based MRI contrast agent for detection of thrombus. J. Am. Chem. Soc. 130, 6025–6039. [DOI] [PubMed] [Google Scholar]
- Kolodziej A. F.; Nair S. A.; Graham P.; McMurry T. J.; Ladner R. C.; Wescott C.; Sexton D. J.; Caravan P. (2012) Fibrin specific peptides derived by phage display: characterization of peptides and conjugates for imaging. Bioconjugate Chem. 23, 548–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enochs W. S.; Schaffer B.; Bhide P. G.; Nossiff N.; Papisov M.; Bogdanov A.; Brady T. J.; Weissleder R. (1993) MR-imaging of slow axonal-transport in-vivo. Exp. Neurol. 123, 235–242. [DOI] [PubMed] [Google Scholar]
- Petropoulos A. E.; Schaffer B. K.; Cheney M. L.; Enochs S.; Zimmer C.; Weissleder R. (1995) MR imaging of neuronal transport in the guinea pig facial nerve: initial findings. Acta Oto-Laryngol. 115, 512–516. [DOI] [PubMed] [Google Scholar]
- van Everdingen K. J.; Enochs W. S.; Bhide P. G.; Nossiff N.; Papisov M.; Bogdanov A.; Brady T. J.; Weissleder R. (1994) Determinants of in vivo MR imaging of slow axonal transport. Radiology 193, 485–491. [DOI] [PubMed] [Google Scholar]
- Wu C. W. H.; Vasalatiy O.; Liu N.; Wu H. T.; Cheal S.; Chen D. Y.; Koretsky A. P.; Griffiths G. L.; Tootell R.; Ungerleider L. (2011) Development of a MR-visible compound for tracing neuroanatomical connections in vivo. Neuron 70, 229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercelli A.; Repici M.; Garbossa D.; Grimaldi A. (2000) Recent techniques for tracing pathways in the central nervous system of developing and adult mammals. Brain Res. Bull. 51, 11–28. [DOI] [PubMed] [Google Scholar]
- van Kasteren S. I.; Campbell S. J.; Serres S.; Anthony D. C.; Sibson N. R.; Davis B. G. (2009) Glyconanoparticles allow pre-symptomatic in vivo imaging of brain disease. Proc. Natl. Acad. Sci. U.S.A. 106, 18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellington A. D.; Szostak J. W. (1990) In vitro selection of RNA molecules that bind specific ligands. Nature 346, 818–822. [DOI] [PubMed] [Google Scholar]
- Stoltenburg R.; Reinemann C.; Strehlitz B. (2007) SELEX: a revolutionary method to generate high-affinity nucleic acid ligands. Biomol. Eng. 24, 381–403. [DOI] [PubMed] [Google Scholar]
- Liu C. H.; Huang S.; Cui J.; Kim Y. R.; Farrar C. T.; Moskowitz M. A.; Rosen B. R.; Liu P. K. (2007) MR contrast probes that trace gene transcripts for cerebral ischemia in live animals. FASEB J. 21, 3004–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C. H.; Ren J. Q.; Yang J.; Liu C. M.; Mandeville J. B.; Rosen B. R.; Bhide P. G.; Yanagawa Y.; Liu P. K. (2009) DNA-based MRI probes for specific detection of chronic exposure to amphetamine in living brains. J. Neurosci. 29, 10663–10670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C. H.; Ren J. Q.; You Z.; Yang J.; Liu C. M.; Uppal R.; Liu P. K. (2012) Noninvasive detection of neural progenitor cells in living brains by MRI. FASEB J. 26, 1652–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C. H.; You Z. R.; Ren J. Q.; Kim Y. R.; Eikermann-Haerter K.; Liu P. K. (2008) Noninvasive delivery of gene targeting probes to live brains for transcription MRI. FASEB J. 22, 1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelyveld V. S.; Atanasijevic T.; Jasanoff A. (2010) Challenges for molecular neuroimaging with MRI. Int. J. Imaging Syst. Technol. 20, 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilad A. A.; Winnard P. T.; van Zijl P. C. M.; Bulte J. W. M. (2007) Developing MR reporter genes: promises and pitfalls. NMR Biomed. 20, 275–290. [DOI] [PubMed] [Google Scholar]
- Genove G.; DeMarco U.; Xu H.; Goins W. F.; Ahrens E. T. (2005) A new transgene reporter for in vivo magnetic resonance imaging. Nat. Med. 11, 450–454. [DOI] [PubMed] [Google Scholar]
- Cohen B.; Dafni H.; Meir G.; Harmelin A.; Neeman M. (2005) Ferritin as an endogenous MRI reporter for noninvasive imaging of gene expression in C6 glioma tumors. Neoplasia 7, 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B.; Ziv K.; Plaks V.; Israely T.; Kalchenko V.; Harmelin A.; Benjamin L. E.; Neeman M. (2007) MRI detection of transcriptional regulation of gene expression in transgenic mice. Nat. Med. 13, 498–503. [DOI] [PubMed] [Google Scholar]
- Meldrum F. C.; Heywood B. R.; Mann S. (1992) Magnetoferritin: in vitro synthesis of a novel magnetic protein. Science 257, 522–523. [DOI] [PubMed] [Google Scholar]
- Deans A. E.; Wadghiri Y. Z.; Bernas L. M.; Yu X.; Rutt B. K.; Turnbull D. H. (2006) Cellular MRI contrast via coexpression of transferrin receptor and ferritin. Magn. Reson. Med. 56, 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurkiya O.; Chan A. W. S.; Hu X. (2008) MagA is sufficient for producing magnetic nanoparticles in mammalian cells, making it an MRI reporter. Magn. Reson. Med. 59, 1225–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldhawk D. E.; Lemaire C.; McCreary C. R.; McGirr R.; Dhanvantari S.; Thompson R. T.; Figueredo R.; Koropatnick J.; Foster P.; Prato F. S. (2009) Magnetic resonance imaging of cells overexpressing MagA, an endogenous contrast agent for live cell imaging. Mol. Imaging 8, 129–139. [PubMed] [Google Scholar]
- van Zijl P. C. M.; Zhou J.; Mori N.; Payen J. F.; Wilson D.; Mori S. (2003) Mechanism of magnetization transfer during on-resonance water saturation. A new approach to detect mobile proteins, peptides, and lipids. Magn. Reson. Med. 49, 440–449. [DOI] [PubMed] [Google Scholar]
- Zhou J. Y.; Payen J. F.; Wilson D. A.; Traystman R. J.; van Zijl P. C. M. (2003) Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat. Med. 9, 1085–1090. [DOI] [PubMed] [Google Scholar]
- Perez-Torres C. J.; Massaad C. A.; Hilsenbeck S. G.; Serrano F.; Pautler R. G. (2009) In vitro and in vivo magnetic resonance imaging (MRI) detection of GFP through magnetization transfer contrast (MTC). NeuroImage 50, 375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossuin Y.; Muller R. N.; Gillis P.; Bartel L. (2005) Relaxivities of human liver and spleen ferritin. Magn. Reson. Imaging 23, 1001–1004. [DOI] [PubMed] [Google Scholar]
- Jung C. W.; Jacobs P. (1995) Physical and chemical properties of superparamagnetic iron oxide MR contrast agents: ferumoxides, ferumoxtran, ferumoxsil. Magn. Reson. Imaging 13, 661–674. [DOI] [PubMed] [Google Scholar]
- Zhang S.; Merritt M.; Woessner D. E.; Lenkinski R. E.; Sherry A. D. (2003) PARACEST agents: modulating MRI contrast via water proton exchange. Acc. Chem. Res. 36, 783–790. [DOI] [PubMed] [Google Scholar]
- Vykhodtseva N.; McDannold N.; Hynynen K. (2008) Progress and problems in the application of focused ultrasound for blood-brain barrier disruption. Ultrasonics 48, 279–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman-Goldstein S. M.; Barnett P. A.; McCormick C. I.; Szumowski J.; Shannon E. M.; Ramsey F. L.; Mass M.; Neuwelt E. A. (1994) Effects of Gd-DTPA after osmotic BBB disruption in a rodent model: toxicity and MR findings. J. Comput. Assisted Tomogr. 18, 731–736. [DOI] [PubMed] [Google Scholar]
- Norman A. B.; Bertram K. J.; Thomas S. R.; Pratt R. G.; Samaratunga R. C.; Sanberg P. R. (1991) Magnetic resonance imaging of rat brain following in vivo disruption of the cerebral vasculature. Brain Res. Bull. 26, 593–597. [DOI] [PubMed] [Google Scholar]
- Hynynen K.; McDannold N.; Sheikov N. A.; Jolesz F. A.; Vykhodtseva N. (2005) Local and reversible blood-brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. NeuroImage 24, 12–20. [DOI] [PubMed] [Google Scholar]
- Neuwelt E. A.; Weissleder R.; Nilaver G.; Kroll R. A.; Roman-Goldstein S.; Szumowski J.; Pagel M. A.; Jones R. S.; Remsen L. G.; McCormick C. I. (1994) Delivery of virus-sized iron oxide particles to rodent CNS neurons. Neurosurgery 34, 777–784. [DOI] [PubMed] [Google Scholar]
- Muldoon L. L.; Sandor M.; Pinkston K. E.; Neuwelt E. A. (2005) Imaging, distribution, and toxicity of superparamagnetic iron oxide magnetic resonance nanoparticles in the rat brain and intracerebral tumor. Neurosurgery 57, 785–796. [DOI] [PubMed] [Google Scholar]
- Hynynen K.; McDannold N.; Vykhodtseva N.; Raymond S.; Weissleder R.; Jolesz F. A.; Sheikov N. (2006) Focal disruption of the blood-brain barrier due to 260-kHz ultrasound bursts: a method for molecular imaging and targeted drug delivery. J. Neurosurg. 105, 445–454. [DOI] [PubMed] [Google Scholar]
- Neuwelt E. A.; Rapoport S. I. (1984) Modification of the blood-brain barrier in the chemotherapy of malignant brain tumors. Fed. Proc. 43, 214–219. [PubMed] [Google Scholar]
- McDannold N.; Arvanitis C. D.; Vykhodtseva N.; Livingstone M. S. (2012) Temporary disruption of the blood-brain barrier by use of ultrasound and microbubbles: safety and efficacy evaluation in rhesus macaques. Cancer Res. 10.1158/0008-5472.CAN-12-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J. J.; Wang S. G.; Tung Y. S.; Morrison B.; Konofagou E. E. (2010) Molecules of various pharmacologically-relevant sizes can cross the ultrasound-induced blood-brain barrier opening in vivo. Ultrasound Med. Biol. 36, 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caravan P.; Cloutier N. J.; Greenfield M. T.; McDermid S. A.; Dunham S. U.; Bulte J. W.; Amedio J. C.; Looby R. J.; Supkowski R. M.; Horrocks W. D.; McMurry T. J.; Lauffer R. B. (2002) The interaction of MS-325 with human serum albumin and its effect on proton relaxation rates. J. Am. Chem. Soc. 124, 3152–3162. [DOI] [PubMed] [Google Scholar]
- Caravan P.; Parigi G.; Chasse J. M.; Cloutier N. J.; Ellison J. J.; Lauffer R. B.; Luchinat C.; McDermid S. A.; Spiller M.; McMurry T. J. (2007) Albumin binding, relaxivity, and water exchange kinetics of the diastereoisomers of MS-325, a gadolinium(III)-based magnetic resonance angiography contrast agent. Inorg. Chem. 46, 6632–6639. [DOI] [PubMed] [Google Scholar]
- Caravan P. (2009) Protein-targeted gadolinium-based magnetic resonance imaging (MRI) contrast agents: design and mechanism of action. Acc. Chem. Res. 42, 851–862. [DOI] [PubMed] [Google Scholar]
- Zech S. G.; Eldredge H. B.; Lowe M. P.; Caravan P. (2007) Protein binding to lanthanide(III) complexes can reduce the water exchange rate at the lanthanide. Inorg. Chem. 46, 3576–3584. [DOI] [PubMed] [Google Scholar]
- Faivre D.; Schuler D. (2008) Magnetotactic bacteria and magnetosomes. Chem. Rev. 108, 4875–4898. [DOI] [PubMed] [Google Scholar]
- Walcott C.; Gould J. L.; Kirschvink J. L. (1979) Pigeons have magnets. Science 205, 1027–1029. [DOI] [PubMed] [Google Scholar]
- Kuterbach D. A.; Walcott B.; Reeder R. J.; Frankel R. B. (1982) Iron-containing cells in the honey bee (Apis mellifera). Science 218, 695–697. [DOI] [PubMed] [Google Scholar]
- Beason R. C.; Nichols J. E. (1984) Magnetic orientation and magnetically sensitive material in a transequatorial migratory bird. Nature 309, 151–153. [Google Scholar]
- Zoeger J.; Dunn J. R.; Fuller M. (1981) Magnetic material in the head of the common Pacific dolphin. Science 213, 892–894. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M. (2008) Re-engineering biopharmaceuticals for delivery to brain with molecular Trojan horses. Bioconjugate Chem. 19, 1327–1338. [DOI] [PubMed] [Google Scholar]
- Wang T.; Town T.; Alexopoulou L.; Anderson J. F.; Fikrig E.; Flavell R. A. (2004) Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat. Med. 10, 1366–1373. [DOI] [PubMed] [Google Scholar]
- Koenig S.; Gendelman H. E.; Orenstein J. M.; Dal Canto M. C.; Pezeshkpour G. H.; Yungbluth M.; Janotta F.; Aksamit A.; Martin M. A.; Fauci A. S. (1986) Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science 233, 1089–1093. [DOI] [PubMed] [Google Scholar]
- Fink D. J.; Glorioso J. C. (1997) Engineering herpes simplex virus vectors for gene transfer to neurons. Nat. Med. 3, 357–359. [DOI] [PubMed] [Google Scholar]