Abstract
Urinary schistosomiasis remains a significant burden for Africa and the Middle East. Success of regional control strategies will depend, in part, on what influence local environmental and behavioral factors have on individual risk for primary infection and/or reinfection. Based on experience in a multi-year (1984–1992), school-based Schistosoma haematobium control program in Coast Province, Kenya, we examined risk for infection outcomes as a function of age, sex, pretreatment morbidity, treatment regimen, water contact, and residence location, with the use of life tables and Cox proportional-hazards analysis. After adjustment, location of residence, age less than 12 years, pretreatment hematuria, and incomplete treatment were the significant independent predictors of infection, whereas sex and frequency of water contact were not. We conclude that local physical features and age-related factors play a predominant role in S. haematobium transmission in this setting. In large population-based control programs, treatment allocation strategies may need to be tailored to local conditions on a village-by-village basis.
INTRODUCTION
Urinary schistosomiasis remains a major health burden in disease-endemic areas of Africa and the Middle East, affecting more than 110 million people in rural, agricultural, and peri-urban areas.1-4 Individuals infected by Schistosoma haematobium frequently experience dysuria, pelvic pain, and hematuria, and are at risk of developing bladder cancer or renal failure later in life.4,5 In addition, schistosome infection is significantly associated with anemia, impaired growth, and impaired development and cognition.1 Consequently, schistosomiasis affects not only the health of individuals, but also the economic strength of an affected area.
In Kenya, more than six million people, or approximately 23% of the total population, are infected with urinary or intestinal schistosomiasis.6 Control programs based on oral drug delivery have been developed and partially implemented as a means to control morbidity within these affected populations. 3 However, questions remain about the long-term impact of the programs on parasite transmission. Treatment of the most heavily infected segment of the population, i.e., school age children, has been suggested as the best practical means of reducing contamination of local water by Schistosoma eggs.7 Although treatment has been shown to significantly reduce S. haematobium egg output (by more than 90%) among treated subjects over the short term,8 the actual impact of long-term, population-based treatment programs on year-to-year transmission of schistosomiasis has not been fully explored.
As the basis of this study, an extended, multi-year longitudinal prospective cohort study was carried out within the Msambweni area of Kenya to determine the effect of therapy on transmission of S. haematobium at the community level. Prior surveys in this area established the prevalence of S. haematobium in school age children to be 60–85% with an overall area prevalence of 40–50%.8,9 To determine which factors contributed significantly to the increased risk of infection and reinfection in this area during the eight-year treatment phase of the study, age, sex, water contact, location, snail population, and health status10-12 were individually and jointly assessed for their effect on infection risk with the use of stratified life table analysis and Cox proportional hazard modeling.
The objectives of the survival analysis of the risk of infection or reinfection were 1) to determine whether location of residence (e.g., coastal, inland, or between) was a significant predictor of time to infection; 2) to determine if the type and/or number of observed water contacts were a significant factor determining individual time to infection; 3) to determine if the extent of pre-treatment morbidity was significantly predictive of time to infection, as possibly related to short-term immunity effects; 4) to decide if age and sex significantly impact the rate of infection or reinfection; and 5) to determine if different treatment regimens resulted in differences in time to reinfection.
MATERIALS AND METHODS
Study area and population
The Msambweni study described in this report was a prospective cohort study of school age children conducted from 1984 to 1993.8,13,14 The study was conducted in a nine-village area of Kwale District, Coast Province, Kenya, located 50 km southwest of Mombasa, in a predominantly agricultural region. The nine villages included in the study were Mwaembe, Sawa Sawa, Kisimachande, Vingujini, Vindungeni, Marigiza, Bomani, Milalani, and Nganja (Figure 1). The Indian Ocean forms the eastern boundary of this 25-km2 area, and two small rivers form the northern and southern boundaries, respectively (Figure 1). In this area, limited piped water was available in some of the villages in 1984, but most people depended heavily on natural water sources.15 At the start of the study in 1984, the total area population, which was determined by household census conducted by a team from Division of Vector Borne Diseases, Ministry of Health, Kenya, was 8,957.8 There were 1,624 households in the 9 villages, with an average of 7.3 persons per house. Based on follow-up census surveys performed in 1985, 1987, and 1990, the average population growth rate was 4% per year. Of the 1984 enumerated population, 2,906 of 8,957, or 32% of individuals were of school age (5–20 years) and eligible for participation in the study. It is known that some children were missed because of travel away from the area (to attend outside schools) or because they were temporarily living with relatives residing outside the study villages. Over the nineyear study period, 4,840 study subjects of school age were enrolled in the treatment program. An additional 2,801 provisional study entrants were dropped on the basis of duplicate enrollment (based on yearly updated class lists and periodic census information) or for reasons of 1) identified residence outside the targeted study area; 2) entry age outside the study range (5–20 years); 3) incomplete participation in demography or parasitology segments of the study; or 4) failure to follow-up on the second and subsequent year(s). When compared with those subjects included in the study analysis (n = 4,840), the excluded entrants were significantly more likely to be older (≥ 12 years of age), male, and enrolled in the westernmost school (Milalani) (Table 1).
Figure 1.
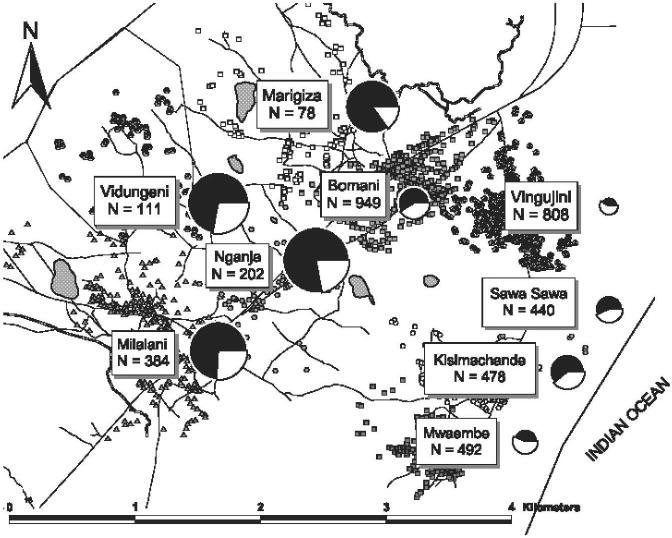
Map of the Msambweni study area on the southern coast of Kenya. Shown are the nine participating villages with their respective numbers of included subjects. To the right of each village label is a pie chart that indicates by internal shading the village prevalence of infection at the outset of the control project, and by relative size the local rate of infection/reinfection over the eight-year follow-up period.
Table 1.
Characteristics of Msambweni urinary schistosomiasis control study population 1984–1992, according to baseline infection status*
| Characteristics | Uninfected at baseline (n = 2,506) |
Infected at baseline (n = 2,334) |
χ2 (df) | P | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Age, years | ||||||
| 5–11 | 945 | 37.7 | 1,063 | 45.5 | 30.6 (1) | < 0.001 |
| 12–20 | 1,561 | 62.3 | 1,271 | 54.5 | ||
| Sex | ||||||
| Male | 1,323 | 52.8 | 1,150 | 49.3 | 6.0 (1) | 0.01 |
| Female | 1,183 | 47.2 | 1,184 | 50.7 | ||
| Village | ||||||
| Vingujini | 671 | 26.8 | 353 | 15.1 | 185.9 (8) | < 0.0001 |
| Bomani | 579 | 23.1 | 575 | 24.6 | ||
| Mwaembe | 351 | 14.0 | 223 | 9.6 | ||
| Sawa Sawa | 259 | 10.3 | 257 | 11.0 | ||
| Marigiza | 45 | 1.8 | 71 | 3.0 | ||
| Vindungeni | 67 | 2.7 | 84 | 3.6 | ||
| Kisimachande | 263 | 10.5 | 306 | 13.1 | ||
| Nganja | 80 | 3.2 | 164 | 7.0 | ||
| Mililani | 191 | 7.6 | 301 | 12.9 | ||
| School | ||||||
| Msambweni Secondary | 211 | 8.4 | 122 | 5.2 | 224.0 (8) | < 0.0001 |
| Msambweni Primary | 391 | 15.6 | 531 | 22.8 | ||
| Vingujini Primary | 421 | 16.8 | 319 | 13.7 | ||
| Jomo Kenyatta | 485 | 19.4 | 643 | 27.5 | ||
| Milalani Primary | 115 | 4.6 | 384 | 16.5 | ||
| Nursery School 6 | 57 | 2.3 | 33 | 1.4 | ||
| Nursery School 7 | 29 | 1.2 | 12 | 0.5 | ||
| Nursery School 8 | 87 | 3.5 | 60 | 2.6 | ||
| Not in school | 18 | 0.7 | 12 | 0.5 | ||
| Treatment | ||||||
| None | 1,051 | 41.9 | 0 | 0.0 | 3,038.3 (4) | < 0.0001 |
| Praziquantel | 5 | 0.2 | 1,023 | 43.8 | ||
| Three doses of metrifonate | 2 | 0.1 | 752 | 32.2 | ||
| One or two doses of metrifonate | 12 | 0.5 | 226 | 9.7 | ||
| Missed, placebo, unknown | 21 | 0.8 | 106 | 4.5 | ||
| Hematuria | ||||||
| None | 1,549 | 61.8 | 496 | 21.3 | 1,833.5 (5) | < 0.0001 |
| Trace | 24 | 1.0 | 106 | 4.5 | ||
| 1+ | 80 | 3.2 | 571 | 24.5 | ||
| 2+ | 29 | 1.2 | 390 | 16.7 | ||
| 3+ | 18 | 0.7 | 644 | 27.6 | ||
Column percentages were calculated for each sub-group category within the separate characteristics listed. df = degrees of freedom.
Students from eight Msambweni area schools (as well as some not attending school) were involved in the study. Four of the schools were primary schools, one was a secondary school, and three were nursery schools. Of the targeted school age children in the community (both in school and not attending school), 79% were ultimately enrolled in the study, screened for infection by standard urine filtration, and treated as indicated. To keep track of the children over time, school lists were updated yearly and the census records were updated in 1987 and in 1990. In any year, repeated school and home visits were made to find the children who were absent. This yielded up to 10% of that year’s missing children. After three unsuccessful follow-up attempts, the child was scored as absent for that year. During the course of the study, a significant number of missing children did return and rejoin the study. However, because the interval infection and treatment status of these re-entrants was unknown, for purposes of the survival analysis presented here, the outcomes of such subjects were considered censored after their first departure from the study.
Ethical considerations
This study was performed under a protocol reviewed and approved by the human investigations review boards of University Hospitals of Cleveland and the Kenya Medical Research Institute, Nairobi. Consent for participation in the study was obtained from the children’s parents or guardians, with subsequent assent obtained from the participating children before examination.
Outcomes measured
The primary outcomes of the Msambweni study were infection and reinfection with S. haematobium among school age children. Testing for infection was performed on a yearly basis in June–July of each year by technicians from the Division of Vector Borne Diseases of the Kenyan Ministry of Health. Infection was identified and quantified using membrane filtration of 10-mL midday urine sample, which was collected from 10:00 am to 1:00 pm.16 The 10-mL urine sample was divided into two stirred 5-mL aliquots and were passed through 12-μm pore Nuclepore filters (Nuclepore, Pleasanton, CA). Hematuria was evaluated in semi-quantitative fashion using reagent strips (Hemastix®; Ames, Bie and Bernsten, Copenhagen, Denmark), and results were ranked as negative, trace, 1+, 2+, or 3+ according to the manufacturer’s instructions. Egg count quality and accuracy were checked and standardized by supervisors who reread approximately 10% of the slides each day. A light S. haematobium infection was categorized as detection of 1–99 eggs/10 mL of urine, a moderate infection as 100–399 eggs/10 mL, and a heavy infection as ≥ 400 eggs/10 mL.17 (For consistent comparison between our publications, this system of intensity classification has been used in all reports of our Coast Province S. haematobium studies, although it is different from the current World Health Organization WHO) definition of light infection as < 50 eggs/10 mL of urine and heavy infection as ≥ 50 eggs/10 ml).3 All subjects identified as infected were offered U.S. Food and Drug Administration–approved standard therapy. For years 1–3 of the project, the yearly treatment of each participant was assigned (on a random basis) as either praziquantel (40 mg/kg once) or metrifonate (10 mg/kg in three divided doses).8,18 After year 4 of the project, only praziquantel was used for treatment, and all metrifonatetreated subjects changed to this agent for their drug therapy.
During the course of the project, individuals were considered at risk for reinfection if they were positive for S. haematobium infection during their first examination and then underwent a fully curative regimen of anti-schistosomal drugs (either praziquantel or metrifonate)8 resulting in a follow-up egg count of zero on re-examination. A reinfected subject was defined as a one who was positive for S. haematobium on his or her initial examination, then was negative on the next examination, and then was positive for infection on any subsequent examination. In contrast, a new infection was recorded for a subject who was negative on an initial examination, then was positive in a later year. In this study, due to work force limitations, no interval assessment of effective cure rates (short-term clearance of initial egg counts after treatment) was possible between the one-year follow-up surveys. As such, results of annual treatments were scored as cure for treated individuals who changed from egg positive to egg negative between subsequent years. Those who were classified as non-cure were those who remained egg positive. In subsequent analysis, those who changed from egg negative to egg positive between yearly examinations were aggregated into a combined infected/reinfected category to reflect all infections newly acquired during a given 12-month interval.
Water contact and exposure
Observation of human water contact was carried out at 42 defined contact sites at seasonal surface ponds and on the two watersheds of the Mkurumji and Lukungwi Rivers. Three local villagers, who had at least a primary education background and who were familiar with the study area, were recruited to monitor the water sites. The observations were performed at the different sites in rotation for half-day intervals (from 9:00 am to 1:00 pm or 1:00 pm to 6:00 pm) on all seven days of the week. Factorial latin square design19 was used to determine the randomized rotation schedule for the sites, which provided representative coverage for each site for each portion of the day during each month. During the assigned observation period, observers recorded each member of the community who came into contact with water at that particular site. The information recorded on the entry form included the name, sex, date, village of residence, household number, site number, the extent of contact, the times of entering and exiting the water, the activity carried out during the water contact, and the observer’s identification. To control for performance quality, spot visits were performed during the observation periods by a supervisor from the Division of Vector Borne Diseases, with crosschecking of the data entry forms for accuracy and completeness.
Data handling and analysis
All record forms were kept at the project headquarters and checked for accuracy and reliability before data entry onto a microcomputer spreadsheet. From 1987 on, information was directly entered from worksheets into laptop computers. Data analysis was performed by transfer of clinical and demographic information into a Reliant Unix 5.4 database program (Siemens, Milpitas, CA) in the Department of Epidemiology and Biostatistics at Case Western Reserve University. Statistical analysis was performed using SPSS version 9 (SPSS Inc., Chicago, IL) on the mainframe computer or using CRUNCH statistical package (Crunch Software, Oakland, CA) on an IBM (White Plains, NY) PC microcomputer. Chi-square tests and t-tests were performed for group difference comparisons. Cox proportional hazards models coded in SPSS were used to model risk of infection and/or reinfection. The following covariates were evaluated in the Cox models: age, sex, village of residence, school, number of water contacts, treatment type, and hematuria status at baseline, as well as their interaction terms.
RESULTS
Prevalence and incidence of schistosomiasis
The overall prevalence of infection and reinfection for the 1984–1992 period is shown in Figure 2a. Pre-treatment prevalence of infection started at 67% and decreased to 21% after treatment was initiated. Despite repeated annual treatment visits, school age prevalence of infection did not decrease below 14% between 1984 and 1992. Age-stratified analysis showed that overall children in the older (12–20 years) age groups had less infection than those in the younger (5–11 years) age groups, although at the outset of the program in 1984, the older children had a higher prevalence (71%) compared with the young (63%), as shown in Figure 2b. After the first year of treatment, prevalence of infection for the 12–20-year-old group was 15% compared with 27% for the 5–11-year-old group, and this trend continued in each of the subsequent years of the study. Sex-specific prevalence of infection by year is shown in Figure 2c. At the start of the program, females had a higher prevalence of infection (71%) compared with males (63%) (χ2 = 25.6, P < 0.001). In 1985, after the first year of therapy, prevalence decreased to 23% for females and 19% for males. This male:female pattern was inconstant, however, and over the course of the nine years of the study, there were some years during which males had a higher prevalence than females (e.g., 1988, 1989, and 1990).
Figure 2.
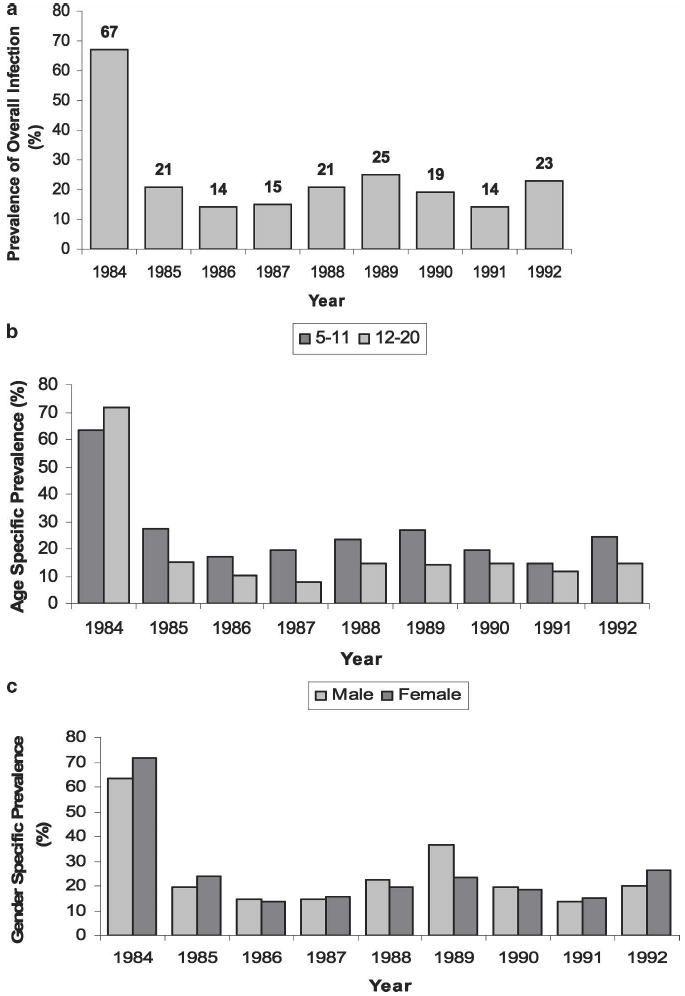
Prevalence of schistosomiasis infection in the Msambweni study area, by study year. After baseline testing in 1984, a program of annual drug treatment was implemented and continued until 1992. a, yearly prevalence for the total population. b, yearly prevalence among age-stratified subgroups. c, yearly prevalence among sex-stratified subgroups.
Infection prevalence by village location before treatment in 1984 was quite variable. As shown in Figure 1, at the outset of the study, Marigiza had the highest prevalence of infection (85%) among school age children, whereas Vingujini had the lowest (48%). Additional characteristics of the study population are shown in Table 1, and further defined according to the subjects’ infection status at baseline. As shown in Table 1, those who were uninfected at baseline were significantly different with respect to their distribution of age groups, sex, village of residence, school attended, and hematuria status compared with entrants who were infected.
Overall incidence of infection, defined as any one-year interval conversion from negative to positive egg count status, is shown by year in Figure 3a for the at-risk study population. In 1985, the incidence of infection was 22%, but was lower in each subsequent calendar year. However, an apparent increase in overall incidence of infection was observed in 1988 and again in 1990, 1991, and 1992 (Figure 3a), suggesting an increase in transmission during each of the preceding 12- month periods. Figure 3b and c show, respectively, the rate of newly detected infection and the rate of reinfection (after cure), by calendar year. As with the overall trend for infection incidence, both new infection rates and reinfection rates varied substantially across the years.
Figure 3.
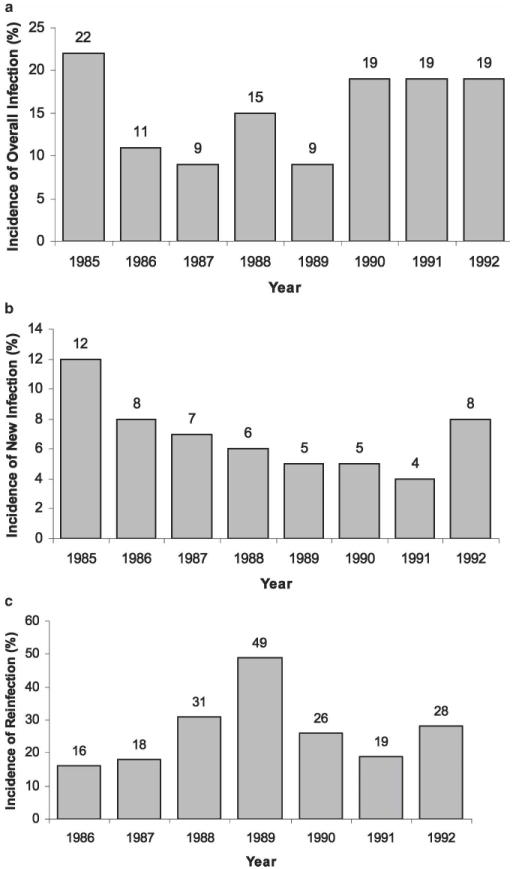
Incidence of schistosomiasis infection and reinfection in the Msambweni study area, by year. Annual rate of infections per 100 population at risk is shown as a percentage for each year, as indicated by the numbers above each bar. a, combined incidence of new infection and reinfection between years 2 and 9 of the study (1985–1992). b, incidence of new infection among previously uninfected subjects, c, incidence of reinfection among treated subjects in the years after a documented parasitologic cure.
Time to infection or reinfection
To better capture the extended multi-year risk of infection and reinfection during the treatment phase of the study, we next performed stratified survival analysis of these two outcomes. For the variable sex, males had longer time to failure than females. The unadjusted survival curves for the two sex groups were significantly different for infection/reinfection among the total population (P = 0.031) and borderline for reinfection among the baseline infected population (P = 0.061), but not significant for the new infection among the baseline uninfected population (P = 0.464).
For the age variable, the older subjects (12–20 years of age) had a longer time to failure than the younger subjects (5–11 years of age). The differences were statistically significant for total infection/reinfection (P < 0.001) and for reinfection (P < 0.001), but borderline for new infection among the baseline uninfected population (P = 0.071). Among groups stratified according to numbers of observed water contacts (high > 10 contacts versus low ≤ 10 contacts), a higher number of water contacts was not significantly associated with a reduced time to failure; observed frequency of water contact was not significantly associated with cumulative risk for either infection or reinfection by univariate survival analysis (relative hazard = 1.02, 95% confidence interval = 0.76, 1.28).
Among those with hematuria on entry into the program, those without hematuria had the longest time to failure, and those with a 3+ hematuria had the shortest time to failure. The differences between the hematuria groups were significant for all infection/reinfection outcomes (P < 0.001).
Among the baseline-infected subjects, reinfection hazard could be further stratified according to their documented treatment (i.e., analysis of treatment as given). Praziquantel therapy had the longest time to reinfection, whereas incomplete (one or two) metrifonate dosing had the shortest time to failure. The differences were significant for both the total (P < 0.001) and baseline-infected (P = 0.002) sub-population.
In examining the impact of location of home residence, among the villages studied, Vingujini, an initially lowprevalence coastal village, had the longest time to reinfection, whereas Milalani, an inland high-prevalence village with limited access to piped water, had the shortest time to failure. Village-related differences in infection risk were highly significant overall, whether for total infection/reinfection (P < 0.001), reinfection (P < 0.001), or for new infection (P < 0.001). Kaplan-Meier survival curves for infection/reinfection by village are shown in Figure 4. As shown, the time until infection differed by village, with Bomani, Sawa Sawa, Vingujini, and Mwaembe having longer infection-free survival times compared with the other five villages. Median unadjusted survival time for Bomani, Sawa Sawa, Vingujini, and Mwaembe were 8.53, 8.16, 8.00, 7.34 years, respectively, and the median unadjusted survival time for Kisimachande, Marigiza, Nganja, Vindungeni, Milalani were 5.91, 4.59, 4.08, 4.06, and 2.90 years, respectively.
Figure 4.
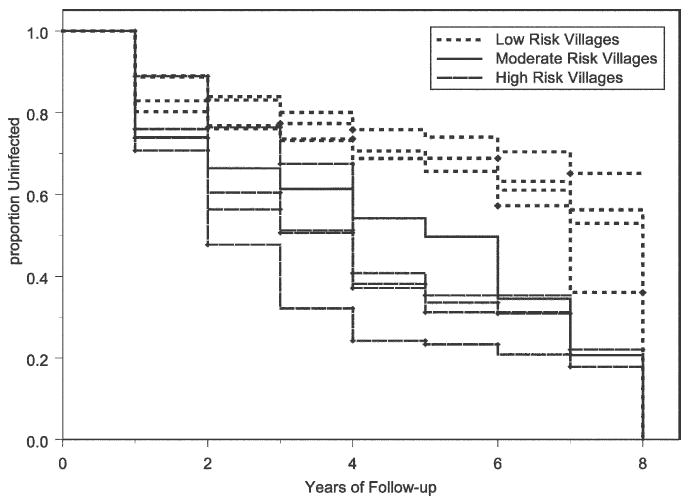
Kaplan-Meier plot of time to infection or reinfection in the nine Msambweni study villages over an eight-year follow-up period. The hazard of infection was consistently different between villages. Low risk was observed in Mwambe, Vingujini, Bowmani and Sawa Sawa, intermediate risk in Kisimachande, and high risk in the inland villages of Milalani, Marigiza, Vindungeni, and Nganja.
Multivariable analysis
To adjust for possible confounding or interaction among the subject attributes believed to be contributing to infection risk, multivariable Cox proportional hazards modeling was next used to re-evaluate their adjusted significance in predicting the time to infection for the whole population, the time to new infection for those without prior infection, and the time to reinfection for those with successfully treated infection. The subject’s number of observed water contacts and school did not significantly contribute to the models, and therefore are not presented in the final adjusted models. The final model for time to reinfection indicated that age, village location, treatment, hematuria status, and interaction between sex and village were significant predictors for infection (Table 2). Those who were young, female, residing in Nganja, missed treatment, and had a severe (3+) level of hematuria had higher risks of infection compared with the rest of their cohort. Significant interaction was noted between sex and village attributes in Bowmani, with a borderline significant effect in the village of Nganja. Within the total population, the covariates significantly associated with risk of either infection or reinfection were the same as the significant covariates for risk of reinfection among previously treated subjects.
Table 2.
Multivariable Cox proportional hazards analysis of risk of reinfection during the 1984–1992 Msambweni school-based schistosomiasis control program: multiply-adjusted risk-ratios according to subject characteristic*
| Characteristic | RR | 95% CI | P |
|---|---|---|---|
| Age category, years | |||
| 12–20 | 1.00 | Reference | |
| 5–11 | 1.82 | 1.51–2.19 | < 0.0001 |
| Sex | |||
| Male | 1.00 | Reference | |
| Female | 1.32 | 0.77–2.24 | 0.30 |
| Village of residence | |||
| Vingujini | 1.00 | Reference | |
| Bomani | 1.64 | 1.02–2.64 | 0.04 |
| Mwaembe | 1.31 | 0.69–2.47 | 0.40 |
| Sawa Sawa | 1.43 | 0.81–2.52 | 0.21 |
| Maigiza | 2.92 | 1.18–7.20 | 0.02 |
| Vindungeni | 3.45 | 1.72–6.92 | 0.0005 |
| Kisimachande | 1.81 | 1.07–3.07 | 0.03 |
| Nganja | 3.78 | 2.24–6.36 | < 0.0001 |
| Milalani | 3.23 | 1.97–5.29 | < 0.0001 |
| Treatment in first year | |||
| Praziquantel | 1.00 | Reference | |
| None | NA | NA | NA |
| Three doses of metrifonate | 1.16 | 0.97–1.39 | 0.09 |
| One or two doses of metrifonate | 1.48 | 1.08–2.01 | 0.01 |
| Missed, placebo, unknown | 2.14 | 1.35–3.40 | 0.001 |
| Hematuria at entry | |||
| None | 1.00 | Reference | |
| Trace | 1.21 | 0.75–1.94 | 0.43 |
| 1+ | 1.30 | 1.00–1.71 | 0.05 |
| 2+ | 1.39 | 1.05–1.84 | 0.02 |
| 3+ | 1.63 | 1.26–2.11 | 0.0002 |
| Sex/village interaction | |||
| Male × Vingujini | 1.00 | Reference | |
| Female × Bomani | 0.42 | 0.22–0.82 | 0.01 |
| Female × Mwaembe | 1.30 | 0.58–2.88 | 0.52 |
| Female × Sawa Sawa | 1.03 | 0.49–2.17 | 0.93 |
| Female × Marigiza | 0.79 | 0.24–2.61 | 0.71 |
| Female × Vindungeni | 0.53 | 0.17–1.62 | 0.27 |
| Female × Kisimachande | 1.42 | 0.72–2.79 | 0.30 |
| Female × Nganja | 0.49 | 0.23–1.05 | 0.07 |
| Female × Milalani | 1.08 | 0.56–2.08 | 0.80 |
RR = relative risk; CI = confidence interval; NA = not applicable.
Results of the adjusted Cox proportional hazards model for time until new infection are shown in Table 3. Since this cohort was not considered to be infected at baseline, they were not given treatment for schistosomiasis, and therefore treatment was not a factor in the model. Overall, age, village location, and hematuria status were significant predictors for new infections. Those who were young, residing in Milalani, and had a 1+ hematuria had the highest risk of infection among the cohort of baseline uninfected. No significant interaction was detected between the predictors modeled.
Table 3.
Multivariable Cox proportional hazards analysis of risk of new infection during the 1984–1992 Msambweni school-based schistosomiasis control program: multiply-adjusted risk-ratios according to subject characteristic*
| Characteristic | RR | 95% CI | P |
|---|---|---|---|
| Age category, years | |||
| 12–20 | 1.00 | Reference | |
| 5–11 | 1.80 | 1.38–2.33 | < 0.0001 |
| Village of residence | |||
| Vingujini | 1.00 | Reference | |
| Bomani | 1.45 | 1.05–1.99 | 0.02 |
| Mwaembe | 1.49 | 1.04–2.13 | 0.02 |
| Sawa Sawa | 1.65 | 1.11–2.44 | 0.01 |
| Vindungeni | 4.66 | 2.49–8.84 | < 0.0001 |
| Kisimachande | 2.92 | 2.05–4.16 | < 0.0001 |
| Nganja | 4.51 | 2.69–7.53 | < 0.0001 |
| Milalani | 5.28 | 3.55–7.74 | < 0.0001 |
| Hematuria at entry | |||
| None | 1.00 | Reference | |
| Trace | 1.80 | 0.74–4.39 | 0.21 |
| 1+ | 2.82 | 1.97–4.04 | < 0.0001 |
| 2+ | 1.97 | 0.92–4.20 | 0.09 |
| 3+ | 2.61 | 1.07–6.35 | 0.03 |
RR = relative risk; CI = confidence interval.
DISCUSSION
Relevant to the debate over the implementation of largescale, population-based treatment programs for schistosomiasis in sub-Saharan Africa,20,21 the present retrospective analysis provides information on the factors that are significantly associated with infection and reinfection risk during the course of a long-term (nine-year) control program. Our study took place in Kwale District, in an area of coastal Kenya that is highly endemic for S. haematobium infection. Our multivariable proportional hazards model indicated that village of residence, age, hematuria status, and a village-sex interaction were independent predictors of infection risk in the face of a continuing, school-based, age-targeted mass treatment campaign. Of these, village of residence was estimated to have the greatest effect on risk for infection, suggesting that very local environmental factors may need to be considered for the optimum design of schistosomiasis control programs.
Schistosomiasis is essentially tied to local water-use behaviors. 22 Previous studies have indicated that younger children engage in more high-risk behaviors when in contact with water, e.g., submerging a larger portion of their body in water or remaining in water for a longer duration.23 In contrast to these previous studies, our analysis indicated that the number of water contacts (and the interaction of age by number of water contacts) were not significant predictors of infection risk over an extended period of observation. This suggests that there is a non-linear feature of water contact, in which quality (categorized by location) outweighs the influence of quantity of exposure in terms of schistosomiasis risk on a multi-village scale. Nevertheless, in examining the occurrence of both new infection and reinfection, schistosomiasis risk remained lower for older children (12–20 years of age) throughout the nine years of the study. Beyond behavioral factors, older children may have a lower risk for infection because of an acquired immunity that is not found in younger children, as has been suggested by earlier studies on immune response and reinfection.24-26
Among the three models developed, village of residence was consistently a significant predictor of infection and reinfection. The study villages most at risk for infection were the ones with no piped water and persistently high snail (and human) infection rates, such as Milalani and Nganja.27,28 In a overview of the Msambweni project by Muchiri and others in 1996,13 analysis of village characteristics indicated that individuals from Mwaembe, Kisimachande, Vingujini, Sawa Sawa, and Bomani villages who had access to piped water had overall shorter and fewer contacts than the residents in other villages that had only borehole wells and surface water as their main sources of water. These findings complement the results of the survival analysis, for the villages with piped water were the same ones that had the lowest risk of infection/reinfection. Studies have also shown that distance from home to a water source plays an important role in risk of infection.12,29 Such results suggest that when initiating a treatment program, characteristics of the village of residence should be considered a factor when deciding where and when to treat. Furthermore, beyond broad-based drug treatment, adequate provision of safe water supply may be necessary for long-term control of S. haematobium transmission in high-risk areas.20,30,31
Hematuria status upon study entry was also a significant predictor of infection/reinfection risk. Hematuria was evaluated as a possible proxy for acquired immunity in which higher levels of hematuria associated with higher levels of S. haematobium exposure could be an indicator of short-lived immunity. Results of our analysis indicated that hematuria was not an effective substitute for acquired immunity because it was shown that the risk of infection increased as the severity of pre-treatment hematuria increased. We concluded that dipstick detection of individuals with high levels of hematuria (or the visual identification or self-report of gross hematuria) 32,33 could identify a subset of individuals at significantly greater risk of infection/reinfection in this setting.
A characteristic that was not significantly associated with multiply adjusted risk of reinfection was sex. In a study conducted by Fulford and others11 in Kenya, researchers found that in some communities, females had far more water contact than males across most age groups, while in other villages the sexes had almost identical patterns of contact. It is likely that due to sex role differences, exposure to S. haematobium differed somewhat between males and females in our study. Results of interaction analysis suggest that although males tend to have less infection overall than females, within certain villages, females may have had less exposure to the parasite than males.
Within the infected population, the completion of assigned treatment was a significant predictor of reduced reinfection risk. This study and our previous analyses8 have suggested that praziquantel and full metrifonate regimens were not significantly different in their treatment effects. However, those subjects who had questionable adherence to their assigned protocol were at greater risk of subsequent infection. This suggests a certain advantage in choosing the single-dose regimen of praziquantel for mass therapy, although, in the future drug resistance issues may change the balance of factors in choosing which drug to use. Currently, metrifonate is not commercially available because of limited demand, but it remains an essential drug that may need to be recalled if praziquantel resistance becomes a factor.34,35 The long-term effects of multiple treatments or treatment crossover could not be evaluated in our study because the analysis only dealt with treatment in the year of entry and not the effect of multiple treatments.
There are both strengths and limitations to the present study. The large sample size of the study cohort and the use of survival analysis to capture long-term outcomes10 provide greater power to detect those identifiable features within the population that are significantly associated with infection risk. The rural, mixed-agriculture/fishing setting is typical for locations to be targeted in new national schistosomiasis control programs. Because the study was done over a nine-year period, individual yearly variation in transmission is less of a factor in determining overall infection outcomes. The Msambweni area includes a number of diverse village settings, which may mean that the findings are more generalizable. However, there are several limitations that may have affected the analysis of the data. First, there is possible selection bias due to subjects’ incomplete participation and incomplete follow- up. Some individuals entered the study for one or two years, then had no data for several years, but later re-entered the study. Since we did not know the infection status or treatment history of these individuals during the years they were absent, these individuals were coded as censored, and, consequently, some potentially useful information was lost. Not all school age children were in the study, and it is known that in school-based programs, children are not missing-atrandom. 36 Rather, absentees during one visit are significantly more likely to be missing in a follow-up visit. Children who are not in the study were the ones who were frequently not in school, which gave them an opportunity to be in greater contact with water. In addition, those who do not go to school are of unknown status, i.e., they may be the ones who are too ill to go to school or the ones who are healthy and working in the fields. Within the study group, a cohort effect is also likely. Since children were followed over a period of nine years, it is likely they would have had the same general history of environmental exposure. Because of the cohort effect, results of the study may not be fully applicable to other treatment programs, or even future populations within the same geographical region. Another limitation of the study is the incomplete sensitivity of urine filtration for light infection; it is not known if a portion of those who were scored as uninfected at start of the study did not in fact have light infections.37,38 Therefore, we cannot be certain that these individuals were actually newly infected in later follow-ups. Likewise, we may have missed some new infections due to false-negative testing of those lightly infected in follow-up years. These effects would, respectively, overestimate and underestimate the true rate of infection and contribute to the variance in our risk estimates. Finally, because of our once-a-year follow-up strategy, rapid reinfection will have been missed, and the exact date of infection/reinfection would not be known in second and later years, and, as such, the interval nature of the data poses statistical limitations for distinguishing potentially important group-wise differences.
Our findings in this long-term, school-based schistosomiasis control project indicate several important factors that should be addressed in implementing new national schistosomiasis control programs in sub-Saharan Africa. Our school-based, age-targeted intervention, similar to that currently recommended in WHO guidelines,3 did not effectively interrupt transmission within high-risk communities. This suggests that without further intervention to modify schistosome exposure or transmission, there will be an indefinite need for continuing drug delivery in these areas.20,21,39 Age and hematuria can be used to identify individuals at high risk for infection or reinfection, and possibly sex, although sex results are not consistent across all areas, most likely due to differences in sexspecific water use behavior. Good adherence to a treatment protocol is an effective marker of reduced risk for reinfection in later years. However, this also indicates that extra efforts should be made to enroll at-risk children who are not in school36 (and possibly high-risk adults), to achieve optimal levels of community control. We recently reported that multiple treatments given during childhood are associated with lower levels of S. haematobium-associated morbidity among adults examined 10 or more years after their last treatment, even if intervening reinfection had occurred.40 It will be important to identify those factors that encourage multi-year participation in targeted population-based schistosomiasis control, so that control of infection-associated morbidity can be fully optimized. In locations where the risk for reinfection is substantially higher, additional strategies aimed at more aggressive re-treatment or reduction of transmission would be appropriate. In future operational research, there is a need to define the readily identifiable, distinguishing features of such high risk locations, (whether by water quality and snail habitat, 28 by type of water use,15 by access to alternative sources, or by distance from high or low risk sources,29) so as to provide simple criteria to adapt intervention strategies within the context of large-scale schistosomiasis control programs.
Acknowledgments
We thank the people of Mwaembe, Kisimachande, Sawa Sawa, Vingujini, Bomani, Nganja, Milalani, Marigiza, and Vindungeni villages for their ready participation with this project. We also thank Henry Kinyanjui, Peter Mungai, Saidi Tosha, Iddi Masemo, Malick Ndzovu, Wallace Saha Ndune, Fredrick Thiongo, Bob Sturrock, Ralph Klumpp, and the late Peter Dalton for their extensive efforts in the fieldwork that contributed to the success of this project. This work is published with the kind permission of the Director of Medical Services, Ministry of Health, Kenya.
Financial support: This research was supported by grants from the Edna McConnell Clark Foundation, the Rockefeller Foundation/World Health Organization–Tropical Disease Research joint funding venture, and the National Institutes of Health (AI15351, AI45473 [National Institute of Allergy and Infectious Diseases], and TW/ES01543 (Fogarty International Center).
Footnotes
Authors' addresses: Sudtida Satayathum, Department of Pediatrics, Case Western Reserve University School of Medicine, 11400 Euclid Avenue, Cleveland, OH 44106, Telephone: 216-844-6283, Fax: 216-844-6233, ssata@hotmail.com. Eric M. Muchiri, Division of Vector Borne Diseases, Ministry of Health, PO 20750, Nairobi, Kenya, Telephone: 254-20-2725833, Fax: 254-20-2720030, schisto@wananchi.com. John H. Ouma, Biomedical Sciences and Technology Programme, Maseno University, Private Bag, Maseno, Kenya, Telephone: 254-733-725721, Fax: 254-20-2725833, ouma@wananchi.com. Christopher C. Whalen, Department of Epidemiology and Biostatistics, Case Western Reserve University School of Medicine, 10900 Euclid Avenue, Cleveland, OH 44106, Telephone: 216-368-4192, Fax: 216-368-3970, ccw@po.cwru.edu. Charles H. King, Center for Global Health and Diseases, Case Western Reserve University School of Medicine, Wolstein 4126, 10900 Euclid Avenue, Cleveland, OH 44106-7286, Telephone: 216-368-4818, Fax: 216-368-4825, chk@cwru.edu.
References
- 1.King CH, Dickman K, Tisch DJ. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability- related outcomes in endemic schistosomiasis. Lancet. 2005;365:1561–1569. doi: 10.1016/S0140-6736(05)66457-4. [DOI] [PubMed] [Google Scholar]
- 2.King CH. Disease in schistosomiasis haematobia. In: Mahmoud AAF, editor. Schistosomiasis. London: Imperial College Press; 2001. pp. 265–296. [Google Scholar]
- 3.WHO. Prevention and control of schistosomiasis and soiltransmitted helminthiasis: report of a WHO expert committee. World Health Organ Tech Rep Ser. 2002;912:2–5. [PubMed] [Google Scholar]
- 4.van der Werf MJ, de Vlas SJ, Brooker S, Looman CW, Nagelkerke NJ, Habbema JD, Engels D. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop. 2003;86:125–139. doi: 10.1016/s0001-706x(03)00029-9. [DOI] [PubMed] [Google Scholar]
- 5.Chen MG, Mott KE. Progress in assessment of morbidity due to Schistosoma haematobium infection. Trop Dis Bull. 1989;86:R1–R36. [Google Scholar]
- 6.Chitsulo L, Engels D, Montresor A, Savioli L. The global status of schistosomiasis and its control. Acta Trop. 2000;77:41–51. doi: 10.1016/s0001-706x(00)00122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warren KS. Selective primary health care: strategies for control of disease in the developing world. I. Schistosomiasis. Rev Infect Dis. 1982;4:715–726. doi: 10.1093/clinids/4.3.715. [DOI] [PubMed] [Google Scholar]
- 8.King CH, Lombardi G, Lombardi C, Greenblatt R, Hodder S, Kinyanjui H, Ouma J, Odiambo O, Bryan PJ, Muruka J, Magak P, Weinert D, Mackay W, Ransohoff D, Houser H, Koech D, Siongok TK, Mahmoud AAF. Chemotherapy-based control of schistosomiasis haematobia. I. Metrifonate versus praziquantel in control of intensity and prevalence of infection. Am J Trop Med Hyg. 1988;39:295–305. doi: 10.4269/ajtmh.1988.39.295. [DOI] [PubMed] [Google Scholar]
- 9.Hodder SL, Mahmoud AAF, Sorenson K, Weinert DM, Stein RL, Ouma JH, Koech D, King CH. Predisposition to urinary tract epithelial metaplasia in Schistosoma haematobium infection. Am J Trop Med Hyg. 2000;63:133–138. doi: 10.4269/ajtmh.2000.63.133. [DOI] [PubMed] [Google Scholar]
- 10.Etard JF, Borel E, Segala C. Schistosoma haematobium infection in Mauritania: two years of follow-up after a targeted chemotherapy: a life-table approach of the risk of reinfection. Parasitology. 1990;100:399–406. doi: 10.1017/s0031182000078689. [DOI] [PubMed] [Google Scholar]
- 11.Fulford AJ, Ouma JH, Kariuki HC, Thiongo FW, Klumpp R, Kloos H, Sturrock RF, Butterworth AE. Water contact observations in Kenyan communities endemic for schistosomiasis: methodology and patterns of behaviour. Parasitology. 1996;113:223–241. doi: 10.1017/s0031182000082007. [DOI] [PubMed] [Google Scholar]
- 12.Amazigo UO, Anago-Amanze CI, Okeibunor JC. Urinary schistosomiasis among school children in Nigeria: consequences of indigenous beliefs and water contact activities. J Biosoc Sci. 1997;29:9–18. doi: 10.1017/s0021932097000096. [DOI] [PubMed] [Google Scholar]
- 13.Muchiri EM, Ouma JH, King CH. Dynamics and control of Schistosoma haematobium transmission in Kenya: an overview of the Msambweni Project. Am J Trop Med Hyg. 1996;55:127–134. doi: 10.4269/ajtmh.1996.55.127. [DOI] [PubMed] [Google Scholar]
- 14.King CH, Muchiri EM, Ouma JH. Evidence against rapid emergence of praziquantel resistance in Schistosoma haematobium, Kenya. Emerg Infect Dis. 2000;6:585–594. doi: 10.3201/eid0606.000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.el Kholy H, Arap Siongok TK, Koech D, Sturrock RF, Houser H, King CH, Mahmoud AA. Effects of borehole wells on water utilization in Schistosoma haematobium endemic communities in Coast Province, Kenya. Am J Trop Med Hyg. 1989;41:212–219. doi: 10.4269/ajtmh.1989.41.212. [DOI] [PubMed] [Google Scholar]
- 16.Peters PAS, Mahmoud AAF, Warren KS, Ouma JH, Siongok TKA. Field studies of a rapid, accurate means of quantifying Schistosoma haematobium eggs in urine samples. Bull World Health Organ. 1976;54:159–162. [PMC free article] [PubMed] [Google Scholar]
- 17.Warren KS, Mahmoud AAF, Muruka JF, Whittaker LR, Ouma JH, Arap Siongok TK. Schistosomiasis haematobia in Coast Province, Kenya. Relationship between egg output and morbidity. Am J Trop Med Hyg. 1979;28:864–870. [PubMed] [Google Scholar]
- 18.King CH, Lombardi G, Lombardi C, Greenblatt R, Hodder S, Kinyanjui H, Ouma J, Odiambo O, Bryan PJ, Muruka J, Magak P, Weinert D, Mackay W, Ransohoff D, Houser H, Koech D, Siongok TK, Mahmoud AAF. Chemotherapy-based control of schistosomiasis haematobia. II. Metrifonate vs. praziquantel in control of infection-associated morbidity. Am J Trop Med Hyg. 1990;42:587–595. doi: 10.4269/ajtmh.1990.42.587. [DOI] [PubMed] [Google Scholar]
- 19.Pocock SJ. Clinical Trials, A Practical Approach. New York: John Wiley & Sons; 1983. [Google Scholar]
- 20.Utzinger J, Bergquist R, Xiao SH, Singer BH, Tanner M. Sustainable schistosomiasis control: the way forward. Lancet. 2003;362:1932–1934. doi: 10.1016/S0140-6736(03)14968-9. [DOI] [PubMed] [Google Scholar]
- 21.Savioli L, Engels D, Roungou JB, Fenwick A, Endo H. Schistosomiasis control. Lancet. 2004;363:658. doi: 10.1016/S0140-6736(04)15603-1. [DOI] [PubMed] [Google Scholar]
- 22.King CH. Epidemiology of schistosomiasis: determinants of transmission of infection. In: Mahmoud AAF, editor. Schistosomiasis. London: Imperial College Press; 2001. pp. 115–132. [Google Scholar]
- 23.Klumpp RK, Webbe G. Focal, seasonal and behavioural patterns of infection and transmission of Schistosoma haematobium in a farming village at the Volta Lake, Ghana. J Trop Med Hyg. 1987;90:265–281. [PubMed] [Google Scholar]
- 24.Sturrock RF, Kimani R, Cottrell BJ, Butterworth AE, Seitz HM, Siongok TK, Houba V. Observations on possible immunity to reinfection among Kenyan schoolchildren after treatment for Schistosoma mansoni. Trans R Soc Trop Med Hyg. 1983;77:363–371. doi: 10.1016/0035-9203(83)90166-9. [DOI] [PubMed] [Google Scholar]
- 25.Hagan P, Blumenthal UJ, Chaudri M, Greenwood BM, Hayes RJ, Hodgson I, Kelly C, Knight M, Simpson AJ, Smithers SR. Resistance to reinfection with Schistosoma haematobium in Gambian children: analysis of their immune responses. Trans R Soc Trop Med Hyg. 1987;81:938–946. doi: 10.1016/0035-9203(87)90359-2. [DOI] [PubMed] [Google Scholar]
- 26.Etard JF, Audibert M, Dabo A. Age-acquired resistance and predisposition to reinfection with Schistosoma haematobium after treatment with praziquantel in Mali. Am J Trop Med Hyg. 1995;52:549–558. doi: 10.4269/ajtmh.1995.52.549. [DOI] [PubMed] [Google Scholar]
- 27.Sturrock RF, Kinyanjui H, Thiongo FW, Tosha S, Ouma JH, King CH, Koech D, Siongok TK, Mahmoud AA. Chemotherapy- based control of schistosomiasis haematobia. 3. Snail studies monitoring the effect of chemotherapy on transmission in the Msambweni area, Kenya. Trans R Soc Trop Med Hyg. 1990;84:257–261. doi: 10.1016/0035-9203(90)90278-m. [DOI] [PubMed] [Google Scholar]
- 28.Kariuki HC, Clennon JA, Brady MS, Kitron U, Sturrock RF, Ouma JH, Ndzovu ST, Mungai P, Hoffman O, Hamburger J, Pellegrini C, Muchiri EM, King CH. Distribution patterns and cercarial shedding of Bulinus nasutus and other snails in the Msambweni area, Coast Province, Kenya. Am J Trop Med Hyg. 2004;70:449–456. [PubMed] [Google Scholar]
- 29.Clennon JA, King CH, Muchiri EM, Kariuki HC, Ouma JH, Mungai P, Kitron U. Spatial patterns of urinary schistosomiasis infection in a highly-endemic area of coastal Kenya. Am J Trop Med Hyg. 2004;70:443–448. [PubMed] [Google Scholar]
- 30.Asaolu SO, Ofoezie IE. The role of health education and sanitation in the control of helminth infections. Acta Trop. 2003;86:283–294. doi: 10.1016/s0001-706x(03)00060-3. [DOI] [PubMed] [Google Scholar]
- 31.Gurarie D, King CH. Heterogeneous model of schistosomiasis transmission and long-term control: the combined influence of spatial variation and age-dependent factors on optimal allocation of drug therapy. Parasitology. 2005;130:49–65. doi: 10.1017/s0031182004006341. [DOI] [PubMed] [Google Scholar]
- 32.Guyatt H, Brooker S, Lwambo NJ, Siza JE, Bundy DA. The performance of school-based questionnaires of reported blood in urine in diagnosing Schistosoma haematobium infection: patterns by age and sex. Trop Med Int Health. 1999;4:751–757. doi: 10.1046/j.1365-3156.1999.00483.x. [DOI] [PubMed] [Google Scholar]
- 33.Lengeler C, Utzinger J, Tanner M. Questionnaires for rapid screening of schistosomiasis in sub-Saharan Africa. Bull World Health Organ. 2002;80:235–242. [PMC free article] [PubMed] [Google Scholar]
- 34.Cioli D. Praziquantel: is there real resistance and are there alternatives? Curr Opin Infect Dis. 2000;13:659–663. doi: 10.1097/00001432-200012000-00014. [DOI] [PubMed] [Google Scholar]
- 35.Feldmeier H, Chitsulo L. Therapeutic and operational profiles of metrifonate and praziquantel in Schistosoma haematobium infection. Arzneimittelforschung. 1999;49:557–565. doi: 10.1055/s-0031-1300462. [DOI] [PubMed] [Google Scholar]
- 36.Talaat M, Omar M, Evans D. Developing strategies to control schistosomiasis morbidity in nonenrolled school-age children: experience from Egypt. Trop Med Int Health. 1999;4:551–556. doi: 10.1046/j.1365-3156.1999.00439.x. [DOI] [PubMed] [Google Scholar]
- 37.Warren KS, Arap Siongok TK, Hauser HB, Ouma JH, Peters PAS. Quantification of infection with Schistosoma haematobium in relation to epidemiology and selective population chemotherapy. I. Minimal number of daily egg counts in urine necessary to establish intensity of infection. J Infect Dis. 1978;138:849–855. doi: 10.1093/infdis/138.6.849. [DOI] [PubMed] [Google Scholar]
- 38.Savioli S, Hatz C, Dixon H, Kisumku UM, Mott KE. Control of morbidity due to Schistosoma haematobium on Pemba Island: egg excretion and hematuria as indicators of infection. Am J Trop Med Hyg. 1990;43:289–295. doi: 10.4269/ajtmh.1990.43.289. [DOI] [PubMed] [Google Scholar]
- 39.Miguel E, Kremer M. Worms: identifying impacts on education and health in the presence of treatment externalities. Econometrica. 2004;72:159–217. [Google Scholar]
- 40.Ouma JH, King CH, Muchiri EM, Mungai P, Koech DK, Ireri E, Magak P, Kadzo H. Late benefits 10-18 years after drug therapy for infection with Schistosoma haematobium in Kwale District, Coast Province, Kenya. Am J Trop Med Hyg. 2005;73:359–364. [PubMed] [Google Scholar]


