ABSTRACT
Bioavailable levels of trace metals, such as iron and zinc, for bacterial growth in nature are sufficiently low that most microbes have evolved high-affinity binding and transport systems. The microbe Campylobacter jejuni lives in the gastrointestinal tract of chickens, the principal source of human infection. A high-affinity ABC transporter for zinc uptake is required for Campylobacter survival in chicken intestines in the presence of a normal microbiota but not when chickens are raised with a limited microbiota. Mass spectrometric analysis of cecal contents revealed the presence of numerous zinc-binding proteins in conventional chicks compared to the number in limited-microbiota chicks. The presence of a microbiota results in the production of host zinc-binding enzymes, causing a growth restriction for bacteria that lack the high-affinity zinc transporter. Such transporters in a wide range of pathogenic bacteria make them good targets for the development of broad-spectrum antimicrobials.
Importance Zinc is an essential trace element for the growth of most organisms. Quantities of zinc inside cells are highly regulated, as too little zinc does not support growth, while too much zinc is toxic. Numerous bacterial cells require zinc uptake systems for growth and virulence. The work presented here demonstrates that the microbiota in the gastrointestinal tract reduces the quantity of zinc. Without a high-affinity zinc transporter, Campylobacter jejuni, a commensal organism of chickens, is unable to replicate or colonize the gastrointestinal tract. This is the first demonstration of zinc competition between microbiota in the gastrointestinal tract of a host. These results could have profound implications in the field of microbial pathogenesis and in our understanding of host metabolism and the microbiota.
Importance
Zinc is an essential trace element for the growth of most organisms. Quantities of zinc inside cells are highly regulated, as too little zinc does not support growth, while too much zinc is toxic. Numerous bacterial cells require zinc uptake systems for growth and virulence. The work presented here demonstrates that the microbiota in the gastrointestinal tract reduces the quantity of zinc. Without a high-affinity zinc transporter, Campylobacter jejuni, a commensal organism of chickens, is unable to replicate or colonize the gastrointestinal tract. This is the first demonstration of zinc competition between microbiota in the gastrointestinal tract of a host. These results could have profound implications in the field of microbial pathogenesis and in our understanding of host metabolism and the microbiota.
Introduction
Zinc is an essential trace element required for multiple cellular functions, such as enzymatic reactions, DNA synthesis, and gene expression (1). Over 300 enzymes and thousands of transcription factors contain one or more zinc atoms. Mild zinc deficiencies in mammals can lead to an imbalance of the Th1 and Th2 immunity functions, leading to a defect in the Th1 pathway (2). The positive effects of zinc supplementation on boosting the Th1 immune pathway and effects on intestinal bacterial populations make it a common method for treating diarrhea in children and in developing countries (3).
Levels of zinc must be tightly regulated, as too little zinc does not support cellular growth, while too much zinc is toxic (4). Mammalian cells maintain zinc homeostasis by means of transport and export proteins (such as human Zip [hZip] proteins or ZnT-1), sequestration in vesicular compartments, or the zinc-binding protein metallothionein (5-7). Metallothioneins, proteins rich in cysteines, bind multiple atoms of metals, including zinc, and are speculated to protect against metal toxicity and participate in the uptake, transport, and regulation of zinc in biological systems (8-10).
Zinc homeostasis must be tightly regulated in prokaryotic cells as well; however, much less is known about these systems. Under low-zinc conditions, zinc is brought into bacteria through the ZnuABC transporter, which, in many bacteria, is under negative control by the response regulator Zur (11). The ZnuABC transport system is necessary for virulence and host colonization in several bacterial species, including Escherichia coli, Haemophilus spp., Salmonella enterica serovar Typhimurium, and Campylobacter jejuni (12-15).
Campylobacter jejuni is the causative agent of campylobacteriosis in humans, a self-limiting gastroenteritis. Transmission occurs primarily through contaminated poultry products, as C. jejuni asymptomatically colonizes the gastrointestinal tract of chickens. We previously characterized the high-affinity zinc-binding protein (ZnuA) for the ZnuABC transporter in C. jejuni. ZnuA is essential for C. jejuni cecal colonization in a day-of-hatch chick colonization model, presumably due to a limiting concentration of zinc in the intestinal tract (13).
The possibility that available zinc is limiting in animals due to the presence of a gut microbiota was suggested by earlier work showing that conventional animals require more dietary zinc than germfree animals (16). To study zinc utilization by intestinal microbiota, C. jejuni was used as a model commensal bacterium in the chick gastrointestinal tract. A C. jejuni mutant lacking znuA was used to examine zinc competition between C. jejuni and the microbiota in chicks raised conventionally or under germfree conditions.
RESULTS
znuA is not required for C. jejuni replication in the ceca of chicks raised under germfree conditions.
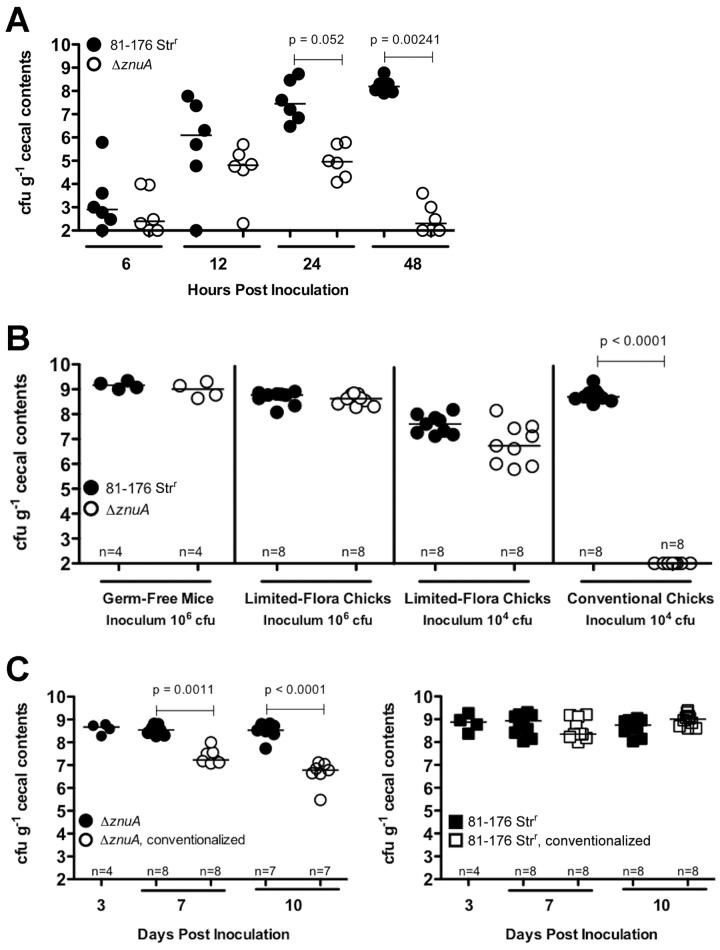
To address whether the colonization defect of C. jejuni ΔznuA in conventional chicks is due to an inability to reach or replicate in the chick ceca, conventional chicks were orally inoculated with either wild-type C. jejuni (strain 81-176 Strr) or the C. jejuni ΔznuA mutant and recovery from the cecal contents was examined for 48 h postinoculation. C. jejuni ΔznuA colonization was significantly attenuated by 12 h, and cells were recovered at levels barely above the level of detection (102 CFU ⋅ g−1 cecal contents) by 48 h postinoculation (Fig. 1A). These data demonstrate that available zinc is sufficiently low in the gastrointestinal tract that microbes lacking a high-affinity uptake system are attenuated for replication and persistent colonization.
FIG 1 .
(A) C. jejuni cecal colonization of conventional day-of-hatch chicks inoculated with 105 CFU of either 81-176 Strr or the ΔznuA mutant at 6, 12, 24, and 48 h postinoculation. Each dot represents the bacterial recovery in a single chick. (B) C. jejuni cecal colonization of germfree mice, germfree chicks, and conventional chicks. The animals were inoculated with the indicated doses of either 81-176 Strr or the ΔznuA mutant, and the cecal contents were plated for C. jejuni recovery after 7 days. Chicks were inoculated on the day of hatching, and germfree mice were inoculated postweaning (1 month old). (C) Conventionalization of ΔznuA mutant-colonized germfree chicks. Day-of-hatch germfree chicks were inoculated with 106 CFU of either wild-type C. jejuni 81-176 Strr or the ΔznuA mutant. Cecal contents were plated on day 3 postinoculation. On day 3, birds colonized with wild-type C. jejuni or the ΔznuA mutant were conventionalized with the cecal contents of age-matched conventional chicks or were not conventionalized (black symbols). Recovery of either the wild type or the ΔznuA mutant from the cecal contents was monitored on days 7 and 13 postinoculation.
We hypothesized that the presence of a microbiota in ceca influences the ability of the ΔznuA mutant to colonize. Non-Campylobacter bacterial species are recovered from chick ceca by 24 h posthatch on nonselective medium, correlating with the decline in the recovery of the ΔznuA mutant (data not shown). To test the contribution of the cecal microbiota in inhibiting colonization by the ΔznuA mutant, chicks were hatched and housed under germfree conditions. The cecal contents of chicks housed under germfree conditions were determined by terminal restriction fragment length polymorphism (tRFLP) analysis, demonstrating a reduction in the quantity and diversity of intestinal flora (see Fig. S1 in the supplemental material). Chicks housed and hatched under germfree conditions will be termed to have “limited microbiota” due to the reduced number of bacteria in their ceca compared to numbers in conventionally raised chicks as analyzed by DAPI (4′,6-diamidino-2-phenylindole) staining with immunofluorescence microscopy (Fig. S2) and by tRFLP analysis (Fig. S1). The cecal microbiota of 7-day-old conventional and limited-microbiota chicks were analyzed by 454 sequencing and are composed of similar bacteria phyla. Both populations are comprised primarily of Firmicutes, more specifically of the Clostridiaceae family (Fig. S3). These data are consistent with previous reports of cecal microbiota composition and horizontal transfer of microbiota in the egg (17, 18) and demonstrate that the limited-microbiota chicks have a reduced number of total bacteria but that they are of a composition similar to that of the conventional chick microbiota.
Limited-microbiota chicks were inoculated with either wild-type C. jejuni or the C. jejuni ΔznuA mutant. After 7 days, both the wild type and the ΔznuA mutant were able to colonize the chick ceca at similar levels in limited-microbiota chicks (Fig. 1B). This was not specific to chicks, as both the wild type and the ΔznuA mutant were able to colonize germfree mice at similar efficiencies (Fig. 1B). These results demonstrate that the microbiota influences whether the C. jejuni ΔznuA mutant colonizes the chick cecum and suggest that intestinal zinc levels differ between conventional and limited-microbiota chicks.
Conventionalization of C. jejuni ΔznuA-colonizing limited-flora chicks excludes C. jejuni colonization.
To ascertain whether the microbiota compete with the ΔznuA mutant, limited-microbiota chicks colonized with the ΔznuA mutant were conventionalized with age-matched conventional chick cecal contents. There was a significant (P < 0.0001) reduction in the recovery of the ΔznuA mutant in the conventionalized chicks compared to the unconventionalized chicks (Fig. 1C). Exclusion by the microbiota was specific for the ΔznuA mutant and not for wild-type C. jejuni (Fig. 1C). This suggests that the conventional chick cecal microbiota have a direct antagonistic effect on ΔznuA mutant colonization, presumably due to zinc competition.
Cecal microbiota restrict available zinc in cecal contents and the subsequent growth of C. jejuni ΔznuA.
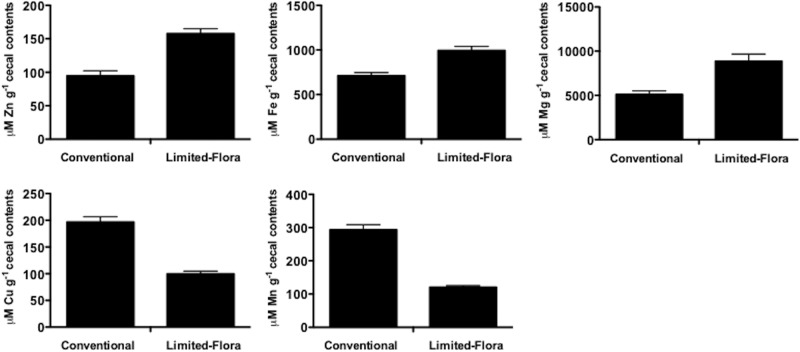
To test if the effect of ΔznuA exclusion in conventional chicks correlates to zinc levels, we measured the amount of total zinc in the cecal contents of conventional and limited-microbiota chicks with an inductively coupled plasma high-resolution mass spectrometer (ICP-HRMS). There was significantly less zinc in the conventional cecal contents of 7-day-old chicks than in the cecal contents of limited-microbiota chicks (Fig. 2). There was also less iron and magnesium in the conventional chick cecal contents than in the limited-microbiota chicks. However, the opposite trend was observed for copper and manganese ions (Fig. 2). Conventional rats have lower available copper, calcium, phosphorous, and magnesium levels than germfree rats, while manganese levels were the same between the two groups (19). The reason for differences in copper and manganese levels observed between the limited-microbiota chicks in our study and germfree rats is unknown but may reflect (i) differences in the species in and the diets of the limited-microbiota chicks or (ii) the few bacterial species in these chicks.
FIG 2 .
ICP-HRMS (inductively coupled plasma-high resolution mass spectrometer) analysis of zinc, iron, magnesium, copper, and manganese atoms in conventional and germfree cecal contents.
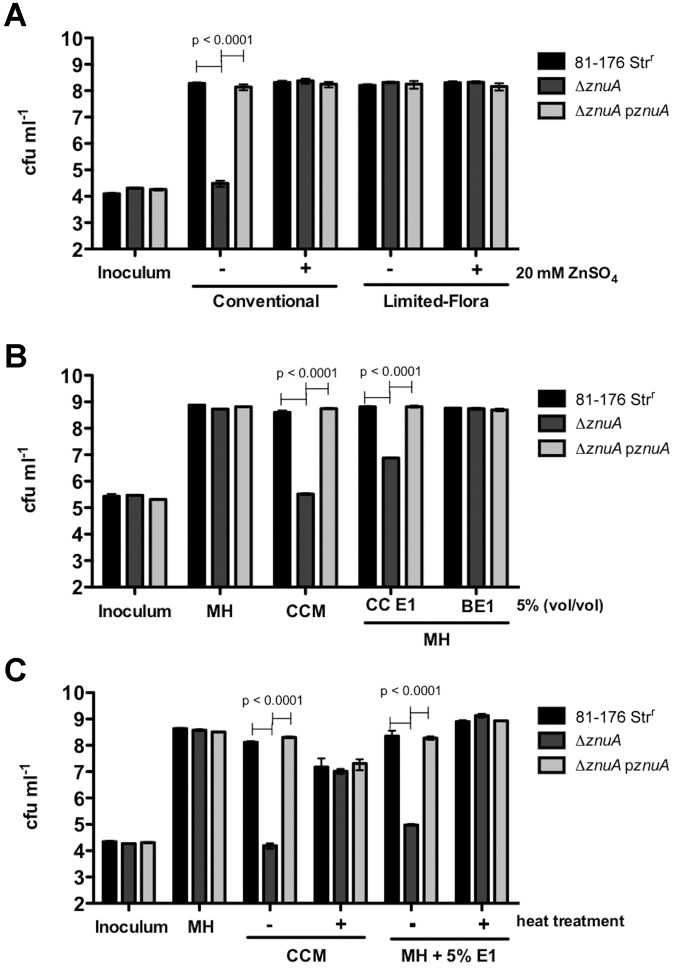
We hypothesized that the ΔznuA mutant was unable to colonize conventional birds due to an inability to replicate in the zinc-limiting cecal contents. To examine specific factors limiting the growth of the ΔznuA mutant in chick cecal contents, a cecal-content medium was developed. Chick cecal contents from both limited-microbiota and conventional birds were isolated, diluted in phosphate-buffered saline (PBS), and filter sterilized to make a medium termed chicken cecal medium (CCM). CCM prepared from both conventional and limited-microbiota birds was inoculated with either the wild type, the ΔznuA mutant, or the ΔznuA mutant complemented with a plasmid-borne znuA gene (pznuA) and incubated for 24 h. The ΔznuA mutant was attenuated for growth in the conventional CCM but not in the limited-microbiota CCM. Attenuation was alleviated by adding 20 µM ZnSO4 to the conventional CCM, demonstrating that the growth defect of the ΔznuA mutant in this medium is zinc dependent (Fig. 3A).
FIG 3 .
(A) Growth in CCM (chicken cecal medium). 81-176 Strr, the ΔznuA mutant, and a ΔznuA pznuA mutant were grown in conventional CCM or limited-microbiota CCM statically with and without 20 µM ZnSO4 for 24 h. The cultures were diluted and plated for C. jejuni recovery, and growth was reported as CFU ⋅ ml−1. Each culture was done in triplicate, and the results shown are representative of three separate trials. (B) Growth in the presence of zinc-binding proteins from conventional chick cecal contents (CC). E1, first elution fraction; BE1, first elution buffer. (C) Growth of 81-176 Strr, the ΔznuA mutant, and the ΔznuA pznuA mutant after 24 h in MH broth was as described in panel B but with heat treatment as indicated. The cultures were diluted and plated for C. jejuni recovery, and growth is reported as numbers of CFU ⋅ ml−1. Each culture was done in triplicate, and shown are representative results of three separate trials.
Zinc-binding proteins inhibit the growth of C. jejuni ΔznuA.
Zinc-binding proteins in the cecal contents of conventional birds were isolated by immobilized metal chromatography and eluted in a solution of 0.1 M sodium phosphate to discern whether a specific component of the cecal milieu was responsible for the ΔznuA mutant’s growth inhibition. Growth of the ΔznuA mutant was attenuated in the presence of the cecal content zinc-binding proteins in Mueller-Hinton (MH) broth (Fig. 3B). These results are similar to the attenuation observed in conventional CCM (Fig. 3B). This suggests that the zinc-binding proteins in the cecal contents of chicks have an inhibitory effect on the ΔznuA mutant’s growth, even in a rich medium containing zinc. Growth restriction of the mutant by chick cecal contents was sensitive to heat treatment, consistent with the conclusion that zinc-binding proteins are responsible for the poor growth of the mutant (Fig. 3C).
Zinc-binding proteins isolated from the cecal contents of conventional chicks by immobilized metal-affinity chromatography were identified by mass spectrometry (Table 1). The majority of the proteins isolated from the chick cecal contents identified in this screen were chick digestive proteins known to bind zinc. Further, proteins present in the conventional CCM and not in the limited-microbiota CCM separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) were isolated and sequenced by mass spectrometry (Table 1). Several of the proteins identified as zinc-binding proteins by immobilized metal chromatography described above in the chick cecum were also identified in this experiment. This demonstrates that the conventional CCM contains several zinc-binding proteins that the limited-microbiota CCM does not. Presumably, the presence of these zinc-binding proteins inhibits the growth of the ΔznuA mutant in the conventional CCM but not in the limited-microbiota CCM.
TABLE 1 .
Identification of zinc-binding proteins in chick cecal contentsa
| Protein | Source | Conventional cecal contents |
Germfree cecal contents |
Zinc-binding protein E1 |
Zinc-binding protein |
|---|---|---|---|---|---|
| Aminopeptidase Ey | Gallus gallus | + | − | + | + |
| Ovalbumin | Gallus gallus | − | − | + | + |
| Ovotransferrin | Gallus gallus | − | − | + | + |
| Procarboyxpeptidase A | Gallus gallus | + | − | + | + |
| Superoxide dismutase A | Gallus gallus | + | − | − | + |
| Transthyretin chain | Gallus gallus | + | − | + | |
| Trypsin | Gallus gallus | + | − | − | |
| Glutamate dehydrogenase NADP |
Clostridium phytofermentans | + | − | − |
Zinc-binding proteins isolated from the cecal contents of conventional chicks by immobilized metal-affinity chromatography and identified by mass spectrometry. A plus symbol denotes the presence of the protein in the indicated sample. Descriptions of the identified proteins are given. Samples of conventional and limited-flora CCM were separated by SDS-PAGE, and proteins present in conventional CCM and absent in germfree CCM were isolated and identified by mass spectrometry. Proteins known to bind zinc are indicated.
DISCUSSION
There is a significant amount of knowledge regarding the competition for iron between bacteria and their hosts (as reviewed in reference 20), but less attention has been paid to the mechanisms that limit zinc for microbes growing in association with hosts. Competition for zinc is suggested by the fact that several bacterial species require a functional high-affinity ZnuABC transport system for colonization and/or virulence, including C. jejuni, E. coli, Salmonella enterica, Proteus mirabilis, Haemophilus influenzae, and Brucella abortus (13-15, 21-23). The presence of the microbiota affects metabolic functions and subsequent zinc utilization, of which little is known. Beyond uncovering basic new knowledge about an important biological process, the value of understanding the role of ZnuABC function in the interaction of pathogenic bacteria and their hosts is enhanced by the fact that a Salmonella znuABC mutant strain provides protection against lethal challenge in a mouse model (24). The ZnuABC transporter is required for Salmonella persistence during an inflammatory response due to zinc limitation resulting from expression of the metal-binding antimicrobial protein calprotectin by neutrophils. znuA+ Salmonella had a significant growth advantage over ΔznuA Salmonella in the inflamed intestines of colonized mice. When infection was established without inflammation or in mice lacking the genes for calprotectin, the growth advantage of the ZnuA-expressing Salmonella was lost (25). Growth limitation of incoming microbes by limiting essential elements, such as zinc or iron, has been termed “nutritional immunity” (26). Inflammation is not a major outcome from infection of chickens by C. jejuni (27; L. M. Gielda, N. Iovine, K. Eaton, and V. DiRita, unpublished data), but our work demonstrates that the microbiota can also contribute to limiting the bioavailability of micronutrients. These studies highlight the importance of zinc acquisition for both pathogenic and commensal organisms in the intestines and demonstrate how basic metabolic activities of pathogens are as critical to their association with hosts as are frank virulence traits, such as adhesins, toxins, and invasion systems.
Lower quantities of trypsin, aminopeptidase Ey, and procarboxypeptidase A in limited-microbiota chicks suggest that there are metabolic changes dependent on the presence of a microbiota (Table 1). This alteration in host metabolism may explain the higher level of zinc in limited-microbiota cecal contents, as several of the digestive proteins identified in the conventional cecal contents bind zinc. The decrease in zinc-binding proteins, or a decreased rate of metabolism or cell proliferation in the intestines (functions that require the use of zinc), would cause an increase in available zinc. However, the effects of the microbiota on digestive functions of the host are poorly understood.
Intestinal bacteria have been implicated in the degradation of secreted digestive enzymes, including trypsin, chymotrypsin, glycoside hydrolases, and aminopeptidase N (28-30). In a recent study, conventionalization of neonatal gnotobiotic pigs resulted in higher aminopeptidase N expression than in germfree pigs (30). However, enzymatic activity was reduced in conventionalized and E. coli-infected pigs, suggesting protein destabilization by the resident bacteria, which was supported by E. coli degradation of purified aminopeptidase in vitro (30). Our work demonstrated an abundance of aminopeptidase Ey in conventional chicks compared to that in limited-flora chicks, correlating with the increase of aminopeptidase N expression in the aforementioned study (30). Although deactivation of the protein was not observed in conventionalized chicks, differences between the microbiota compositions of chicks and pigs may explain the observation, as Lactobacillus fermentum and Klebsiella pneumoniae do not deactivate aminopeptidase N activity as well as E. coli does (30). These studies demonstrate that the host metabolism may be altered based on the bacterial enzymatic activities and composition of the residing microbiota.
The presence of ovotransferrin in the chicken cecal contents, a protein found in high abundance in chicken egg whites capable of binding ions such as iron and zinc may deprive C. jejuni ΔznuA of zinc necessary for growth. Ovotransferrin has been shown to have antibacterial effects against Pseudomonas, E. coli, Streptococcus mutans, Staphylococcus aureus, Bacillus cereus, and Salmonella enterica serovar Enteritidis (31-33). Research into whether growth inhibition of the ΔznuA C. jejuni mutant in chick cecal contents is due to the presence of a specific microbiota-dependent zinc-binding protein, competition with the dense microbiota population, or alterations in the host immune or metabolic functions is under way.
We have demonstrated that quantities of zinc in the gastrointestinal tract are reduced in conventional chicks compared to limited-flora chicks, suggesting that the microbiota affect the availability of this trace element. Without its high-affinity zinc transporter, C. jejuni is unable to compete in conventional chicks. Further research is needed to understand how the microbiota-dependent metabolic changes of the host affect the availability of zinc and other micronutrients for commensal organisms and for incoming pathogens as well.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
A streptomycin-resistant variant of C. jejuni 81-176 (81-176 Strr) was used as the parental wild-type strain. An isogenic deletion of znuA was previously described (13)
C. jejuni was grown on Mueller-Hinton (MH) agar containing 10 µg ml−1 trimethoprim (TMP) in a microaerobic atmosphere (10% CO2, 5% O2, 85% N2) in a tri-gas incubator at 37°C. Inoculations for animal infections and growth experiments were performed by resuspending C. jejuni from 24-h growths on MH agar plates in PBS. Growth experiments were carried out in 96-well plates in 200 µl of static MH broth under the same atmospheric conditions.
Chicken housing.
All animals were humanly treated and given access to food and water under conditions set forth by the University of Michigan Committee on the Use and Care of Animals (UCUCA). Conventional chicks were hatched in a specific-pathogen-free environment and were given immediate access to water. Conventional chicks were fed a diet consisting of LabDiet 5065 chick diet S-G.
To generate limited-microbiota chicks, 1 day prior to hatch, eggs were transferred to the Germ-Free Life Facility at the University of Michigan. To sterilize the surfaces of the eggs, they were dipped in a 10% solution of bleach for 30 s, followed with 3 washes in sterilized water. The eggs were then transferred to germfree isolators with 85% relative humidity and a temperature of over 90°F until hatch. The birds were fed autoclaved LabDiet 5065 with silicon dioxide and extra vitamins. The feeds for conventional and germfree-environment-raised birds have similar compositions and contain similar quantities of zinc (data not shown).
tRFLP.
tRFLP analysis was performed on the cecal contents of 7-day-old conventional chicks and chicks raised in germfree facilities. Four chicks per group were analyzed. Cecal tissues were homogenized and DNA was extracted using a DNeasy blood and tissue kit (Qiagen). DNA was amplified using illustra puReTaq Ready-To-Go PCR beads (GE Healthcare). Primers for this reaction were 6-carboxyfluorescein (FAM)-labeled 5′ AGAGTTTGATCCTGGCTCAG 3′ and 5′ GGTTACCTTGTTACGACTT 3′. PCR mixtures were purified using a QIAquick PCR purification kit (Qiagen) and digested with MspI (NEB) and HhaI (NEB). Digests were purified using the QIAquick nucleotide removal kit and sequenced (UM Sequencing Core). Although bacteria were not recovered on nonselective blood agar plates, the tRFLP results demonstrated that the chicks hatched in the germfree facility contained bacterial species and are referred to as having “limited microbiota.” Bacterial transfer to eggs from hen cloacae and vaginal contamination have been reported (34).
Sequencing.
DNA isolated and amplified from cecal tissue of infected chicks used in tRFLP analysis was used for sequencing. Samples were sent to Research and Testing LLC, and the sequences were classified using RDP Classifier.
Immunofluorescence microscopy.
Cecal cross sections were immersed in 10% neutral buffered formalin, embedded in paraffin, cut in 5-µm sections, and placed onto microscope slides. The tissue was deparaffinized in xylene, washed three times in PBS, and incubated for 2 h with DAPI (Invitrogen; 1:10,000 dilution). The sections were washed three times with PBS, fixed using ProLong gold antifade reagents (Invitrogen), and visualized.
Chicken infections.
Day-of-hatch chicks were orally inoculated with C. jejuni. On the days indicated in the figures, the birds were euthanized using isoflurane and their ceca were removed. Cecal contents were diluted, plated on MH agar containing 10 µg ml−1 TMP and 2 mg ml−1 streptomycin, and incubated for 2 days under C. jejuni growing conditions.
Inductively coupled plasma mass spectrometry.
Cecal contents were diluted in 1% HNO3 and run on a Thermo Scientific Finnigan Element ICP-HRMS using instrument-standard conditions.
Isolation of zinc-binding proteins.
Zinc-binding proteins were isolated as previously described (35). Chelating Sepharose fast-flow gel was purchased from GE Healthcare and used for the matrix for immobilized metal. Sepharose gel was packed onto a column and washed with distilled water. A 0.1% ZnSO4 solution was applied until metal appeared in the eluate, as detected by the 4-(2-pyridylzol) resorcinol (PAR) assay (see below). Solutions of 50 mM Tris-HCl buffer (pH 8.0), 0.1 M sodium phosphate (pH 6.5), and 0.1 M sodium acetate (pH 6.5) were used as the washing buffer and the first and second elution buffers, respectively.
Fractions were run on 10% SDS-PAGE separation gels according to standard procedures. SDS-PAGE gels were stained by Coomassie blue staining, and proteins were isolated for sequencing and identification by mass spectrometry.
PAR assay.
The PAR assay was performed as previously described (13, 36). Briefly, 4-(2-pyridylzol) resorcinol (PAR) was used to quantify the amount of zinc in a given sample. Equal amounts (by volume) of 0.2 mM PAR were mixed, and results were read with a spectrophotometer at an optical density of 490 nm. The quantity of zinc was determined by comparison to a standard curve of ZnSO4.
SUPPLEMENTAL MATERIAL
tRFLP (terminal restriction fragment length polymorphism) chromatogram results from cecal tissue from 7-day-old conventional and limited-microbiota chicks digested with either HhaI or MspI. Chromatogram results are averages of results for four chicks per group. Download Figure S1, PDF file, 0.2 MB.
DAPI-stained cecal tissue from 7-day-old conventional and limited-microbiota chicks. The abundance and diversity of flora in limited-microbiota chicks are evident (compared to those in conventional chicks). Download Figure S2, JPG file, 0.3 MB.
454 sequencing of two conventional and two limited-microbiota chicks after 7 days. Analysis of phylum composition demonstrates a high abundance of Firmicutes in both groups, with a higher abundance of Proteobacteria in limited-microbiota chicks. Mapping by the unweighted-pair group method using average linkages (UPGMA) based on Morisita-Horn statistics of relatedness shows distinct groups between conventional and limited-microbiota chicks based on the composition of the microbiota. Download Figure S3, PDF file, 1.4 MB.
ACKNOWLEDGMENTS
L.M.G. was supported by the University of Michigan Genetics Training Program and its training grant 5-T32-GM07544 from the National Institute of General Medicine Services. This work is supported by grants to V.J.D. from the USDA and NIAID.
Animal experiments were reviewed and approved by the University Committee on Use and Care of Animals at Michigan (UCUCA approval number 10462).
We thank Ted Huston, Department of Geological Sciences, W. M. Keck Elemental Geochemistry Laboratory, University of Michigan, for the ICP analyses. We also thank Kathryn Eaton, Sara Poe, and the staff of germfree animal housing (University of Michigan) for their assistance in the germfree facility.
Footnotes
Citation Gielda LM, DiRita VJ. 2012. Zinc competition among the intestinal microbiota. mBio 3(4):00171-12. doi:10.1128/mBio.00171-12.
REFERENCES
- 1. Berg JM, Shi Y. 1996. The galvanization of biology: a growing appreciation for the roles of zinc. Science 271:1081–1085 [DOI] [PubMed] [Google Scholar]
- 2. Prasad AS, et al. 2007. Zinc supplementation decreases incidence of infections in the elderly: effect of zinc on generation of cytokines and oxidative stress. Am. J. Clin. Nutr. 85:837–844 [DOI] [PubMed] [Google Scholar]
- 3. Bhutta ZA, Darmstadt GL, Hasan BS, Haws RA. 2005. Community-based interventions for improving perinatal and neonatal health outcomes in developing countries: a review of the evidence. Pediatrics 115:519–617 [DOI] [PubMed] [Google Scholar]
- 4. Costello LC, Liu Y, Franklin RB, Kennedy MC. 1997. Zinc inhibition of mitochondrial aconitase and its importance in citrate metabolism of prostate epithelial cells. J. Biol. Chem. 272:28875–28881 [DOI] [PubMed] [Google Scholar]
- 5. Coyle P, Philcox JC, Carey LC, Rofe AM. 2002. Metallothionein: the multipurpose protein. Cell. Mol. Life Sci. 59:627–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haase H, Beyersmann D. 2002. Intracellular zinc distribution and transport in C6 rat glioma cells. Biochem. Biophys. Res. Commun. 296:923–928 [DOI] [PubMed] [Google Scholar]
- 7. McMahon RJ, Cousins RJ. 1998. Regulation of the zinc transporter ZnT-1 by dietary zinc. Proc. Natl. Acad. Sci. U. S. A. 95:4841–4846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blindauer CA, et al. 2001. A metallothionein containing a zinc finger within a four-metal cluster protects a bacterium from zinc toxicity. Proc. Natl. Acad. Sci. U. S. A. 98:9593–9598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kelly EJ, Quaife CJ, Froelick GJ, Palmiter RD. 1996. Metallothionein I and II protect against zinc deficiency and zinc toxicity in mice. J. Nutr. 126:1782–1790 [DOI] [PubMed] [Google Scholar]
- 10. Palmiter RD. 2004. Protection against zinc toxicity by metallothionein and zinc transporter 1. Proc. Natl. Acad. Sci. U. S. A. 101:4918–4923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hantke K. 2001. Bacterial zinc transporters and regulators. Biol. Met. 14:239–249 [DOI] [PubMed] [Google Scholar]
- 12. Campoy S, et al. 2002. Role of the high-affinity zinc uptake znuABC system in Salmonella enterica serovar Typhimurium virulence. Infect. Immun. 70:4721–4725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Davis LM, Kakuda T, DiRita VJ. 2009. A Campylobacter jejuni znuA orthologue is essential for growth in low-zinc environments and chick colonization. J. Bacteriol. 191:1631–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lu D, Boyd B, Lingwood CA. 1997. Identification of the key protein for zinc uptake in Hemophilus influenzae. J. Biol. Chem. 272:29033–29038 [DOI] [PubMed] [Google Scholar]
- 15. Patzer SI, Hantke K. 1998. The ZnuABC high-affinity zinc uptake system and its regulator zur in Escherichia coli. Mol. Microbiol. 28:1199–1210 [DOI] [PubMed] [Google Scholar]
- 16. Smith JC, Jr, McDaniel EG, McBean LD, Doft FS, Halsted JA. 1972. Effect of microorganisms upon zinc metabolism using germfree and conventional rats. J. Nutr. 102:711–719 [DOI] [PubMed] [Google Scholar]
- 17. Lan PT, Hayashi H, Sakamoto M, Benno Y. 2002. Phylogenetic analysis of cecal microbiota in chicken by the use of 16S rDNA clone libraries. Microbiol. Immunol. 46:371–382 [DOI] [PubMed] [Google Scholar]
- 18. Zhu XY, Zhong T, Pandya Y, Joerger RD. 2002. 16S rRNA-based analysis of microbiota from the cecum of broiler chickens. Appl. Environ. Microbiol. 68:124–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reddy BS, Wostmann BS, Pleasants JR. 1965. Iron, copper, and manganese in germfree and conventional rats. J. Nutr. 86:159–168 [DOI] [PubMed] [Google Scholar]
- 20. Wandersman C, Delepelaire P. 2004. Bacterial iron sources: from siderophores to hemophores. Annu. Rev. Microbiol. 58:611–647 [DOI] [PubMed] [Google Scholar]
- 21. Kim S, Watanabe K, Shirahata T, Watarai M. 2004. Zinc uptake system (znuA locus) of Brucella abortus is essential for intracellular survival and virulence in mice. J. Vet. Med. Sci. 66:1059–1063 [DOI] [PubMed] [Google Scholar]
- 22. Patzer SI, Hantke K. 2000. The zinc-responsive regulator zur and its control of the znu gene cluster encoding the ZnuABC zinc uptake system in Escherichia coli. J. Biol. Chem. 275:24321–24332 [DOI] [PubMed] [Google Scholar]
- 23. Nielubowicz GR, Smith SN, Mobley HL. 2010. Zinc uptake contributes to motility and provides a competitive advantage to Proteus mirabilis during experimental urinary tract infection. Infect. Immun. 78:2823–2833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pesciaroli M, et al. 2011. An attenuated Salmonella enterica serovar Typhimurium strain lacking the ZnuABC transporter induces protection in a mouse intestinal model of Salmonella infection. Vaccine 29:1783–1790 [DOI] [PubMed] [Google Scholar]
- 25. Liu JZ, et al. 2012. Zinc sequestration by the neutrophil protein calprotectin enhances Salmonella growth in the inflamed gut. Cell Host Microbe 11:227–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kehl-Fie TE, Skaar EP. 2010. Nutritional immunity beyond iron: a role for manganese and zinc. Curr. Opin. Chem. Biol. 14:218–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hermans D, et al. 2012. A tolerogenic mucosal immune response leads to persistent Campylobacter jejuni colonization in the chicken gut. Crit. Rev. Microbiol. 38:17–29 [DOI] [PubMed] [Google Scholar]
- 28. Hoskins LC, et al. 1985. Mucin degradation in human colon ecosystems. Isolation and properties of fecal strains that degrade ABH blood group antigens and oligosaccharides from mucin glycoproteins. J. Clin. Invest. 75:944–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Malis F, Fric P, Stĕpánková R, Kruml J. 1976. Trypsin and chymotrypsin activity of the intestinal content in germfree, monoassociated and conventional rabbits. Physiol. Bohemoslov. 25:71–74 [PubMed] [Google Scholar]
- 30. Willing BP, Van Kessel AG. 2009. Intestinal microbiota differentially affect brush border enzyme activity and gene expression in the neonatal gnotobiotic pig. J. Anim. Physiol. Anim. Nutr. (Berl.) 93:586–595 [DOI] [PubMed] [Google Scholar]
- 31. Abdallah FB, Chahine JM. 1999. Transferrins, the mechanism of iron release by ovotransferrin. Eur. J. Biochem. 263:912–920 [DOI] [PubMed] [Google Scholar]
- 32. Ko KY, Mendoncam AF, Ismail H, Ahn DU. 2009. Ethylenediaminetetraacetate and lysozyme improves antimicrobial activities of ovotransferrin against Escherichia coli O157:H7. Poult. Sci. 88:406–414 [DOI] [PubMed] [Google Scholar]
- 33. Valenti P, et al. 1983. Studies of the antimicrobial activity of ovotransferrin. Int. J. Tissue React. 5:97–105 [PubMed] [Google Scholar]
- 34. Miyamoto T, et al. 1997. Salmonella enteritidis contamination of eggs from hens inoculated by vaginal, cloacal, and intravenous routes. Avian Dis. 41:296–303 [PubMed] [Google Scholar]
- 35. Yip TT, Hutchens TW. 1996. Immobilized metal ion affinity chromatography. Methods Mol. Biol. 59:197–210 [DOI] [PubMed] [Google Scholar]
- 36. Hunt JB, Neece SH, Ginsburg A. 1985. The use of 4-(2-pyridylazo) resorcinol in studies of zinc release from Escherichia coli aspartate transcarbamoylase. Anal. Biochem. 146:150–157 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
tRFLP (terminal restriction fragment length polymorphism) chromatogram results from cecal tissue from 7-day-old conventional and limited-microbiota chicks digested with either HhaI or MspI. Chromatogram results are averages of results for four chicks per group. Download Figure S1, PDF file, 0.2 MB.
DAPI-stained cecal tissue from 7-day-old conventional and limited-microbiota chicks. The abundance and diversity of flora in limited-microbiota chicks are evident (compared to those in conventional chicks). Download Figure S2, JPG file, 0.3 MB.
454 sequencing of two conventional and two limited-microbiota chicks after 7 days. Analysis of phylum composition demonstrates a high abundance of Firmicutes in both groups, with a higher abundance of Proteobacteria in limited-microbiota chicks. Mapping by the unweighted-pair group method using average linkages (UPGMA) based on Morisita-Horn statistics of relatedness shows distinct groups between conventional and limited-microbiota chicks based on the composition of the microbiota. Download Figure S3, PDF file, 1.4 MB.