Abstract
Metastasis to bone, liver and lungs is the primary cause of death in breast cancer patients. Our studies have revealed that the novel tumor suppressor Pdcd4 inhibits breast cancer cell migration and invasion in vitro. Loss of Pdcd4 in human nonmetastatic breast cancer cells increased the expression of lysyl oxidase (LOX) mRNA. LOX is a hypoxia-inducible amine oxidase, the activity of which enhances breast cancer cell invasion in vitro and in vivo. Specific inhibition of LOX activity by β-aminopropionitrile or small interfering RNA decreased the invasiveness of T47D and MCF7 breast cancer cells attenuated for Pdcd4 function. Most significantly, loss of Pdcd4 augments hypoxia induction of LOX as well. Conversely, overexpression of Pdcd4 significantly reversed the hypoxia induction of LOX expression in T47D cells attenuated for Pdcd4. However, Pdcd4 did not affect hypoxia-inducible factor-1 (HIF-1) protein expression or HIF-1-responsive element-luciferase activity in response to hypoxia, suggesting that Pdcd4 regulation of LOX occurs through an HIF-independent mechanism. Nevertheless, the loss of Pdcd4 early in cancer progression may have an important role in the increased sensitivity of cancer cells to hypoxia through increased LOX activity and concomitant enhanced invasiveness.
Keywords: PDCD4, lysyl oxidase, breast cancer, hypoxia
Introduction
Metastasis is the primary cause of mortality in breast cancer patients (Kang, 2005). Understanding the mechanisms of breast cancer invasion and metastasis is therefore vital for designing effective therapeutic interventions to prevent the spread of this disease. Increasing evidence suggests that the tumor microenvironment is as important as the intrinsic properties of tumor cells in determining tumor progression and patient prognosis. An important characteristic of the microenvironment of solid tumors is the decreased availability of oxygen, a condition known as hypoxia (Schindl et al., 2002; Yagata et al., 2003; Kronblad et al., 2006). In fact, 25–40% of invasive breast cancer samples score positive for hypoxia markers (Vleugel et al., 2005; Chen et al., 2007; van der Groep et al., 2008). A hypoxic microenvironment results in significant reprograming of the gene expression profile in these tumor cells (Lundgren et al., 2007). Expression of hypoxia-responsive genes affects a wide range of cellular functions including metabolism, cell survival and cancer cell–extracellular matrix interactions through increased angiogenesis, migration and metastasis. Thus, sensitivity to hypoxia is an important determinant for multiple stages of carcinogenesis and for response to antitumor treatments.
Although Pdcd4 (also known as MA-3, TIS, H731 and DUG) was first identified as differentially upregulated during apoptosis, recent experimental evidence establishes it as a novel tumor suppressor (Cmarik et al., 1999; Bitomsky et al., 2004; Goke et al., 2004b; Jansen et al., 2005; Leupold et al., 2007). Pdcd4 is a ubiquitously expressed protein that is lost during carcinogenesis. Pdcd4 suppresses transformation in mouse JB6 cells and tumorigenesis in engineered mouse models (Yang et al., 2001, 2003a; Jansen et al., 2004). In addition, Pdcd4 expression is often decreased in progressed carcinomas of the lung, ovary, breast, colon and prostate (Chen et al., 2003; Jansen et al., 2004; Goke et al., 2004a; Wen et al., 2007; Lu et al., 2008; Li et al., 2009). The only direct molecular role identified thus far for Pdcd4 is as an mRNA-selective regulator of translation. Pdcd4 binds to and inhibits the formation and function of the eIF4F translation initiation complex, which is crucial for efficient cap-dependent mRNA translation; (Yang et al., 2003a, 2004; Zakowicz et al., 2005; LaRonde-LeBlanc et al., 2007; Waters et al., 2007). Although those specific mRNAs the translation of which is inhibited by Pdcd4 are yet unknown, a significant consequence of this direct translational regulation is the indirect but specific downstream effects of Pdcd4 on cell signaling cascades and oncogenic mRNA transcription. For instance, Pdcd4 inhibits AP-1-mediated transcription in a concentration-dependent manner but does not inhibit nuclear factor-κB-mediated transcription (Yang et al., 2001). Recent evidence shows that Pdcd4 regulates transcription of genes involved in microenvironment remodeling. Pdcd4 inhibition of transcription of MAP4K1(HPK1), an upstream activator of the AP-1 signaling pathway, contributes to a decrease in the invasive properties of colon carcinoma cell lines (Yang et al., 2006). Allgayer and co-workers demonstrated that Pdcd4 inhibited urokinase plasminogen-activator receptor transcription to regulate invasion and intravasation in colon carcinoma cells and tissues (Leupold et al., 2007; Mudduluru et al., 2007). More recently, Pdcd4 overexpression was shown to inhibit prostaglandin E2 and interleukin-8-induced breast cancer cell invasion through increased expression of the tissue inhibitor of metalloproteinase-2 (Nieves-Alicea et al., 2008). Thus, Pdcd4 inhibition of the expression of specific oncogenes is functionally significant in tumor progression.
Profiling of genes regulated by Pdcd4 during breast cancer cell progression has implicated lysyl oxidase (LOX). LOX is a copper-dependent amine oxidase the activity of which is vital for the formation and homeostasis of the extracellular matrix (Kagan and Trackman, 1991; Rucker et al., 1998; Kagan and Li, 2003). LOX is expressed as a 50 kDa proenzyme. After glycosylation, the 58 kDa protein is secreted from the cell. On secretion into the extracellular matrix, the proenzyme is processed by procollagen proteases, such as bone-morphogenic protein-1, to yield the 32 kDa mature active form of the enzyme. LOX oxidatively deaminates the amino group of specific peptidyl lysine and hydroxylysine residues of collagen and of lysine in elastin. The resulting peptidyl aldehydes spontaneously condense to form inter- and intramolecular crosslinkages stabilizing the fibrous form of these connective tissue structural proteins. Thus, the normal enzymatic activity of LOX is essential for maintaining tissue structural integrity. Aberrant LOX activity on the other hand can lead to connective tissue disorders including cutis laxa, Menke’s syndrome and ateriosclerosis (Smith-Mungo and Kagan, 1998).
Although little is known about the regulation of LOX in cancer, recent studies indicate that hypoxia in the tumor microenvironment increases LOX expression and activity (Denko et al., 2003; Erler and Giaccia, 2006; Erler et al., 2006; Postovit et al., 2008). This hypoxia-induced LOX enhances in vitro invasion, migration and metastasis in breast cancer (Kirschmann et al., 2002; Payne et al., 2005; Erler et al., 2006). This study provides novel insight into the Pdcd4 suppression of breast cancer cell invasion in response to a hypoxic microenvironment. We demonstrate that this invasion-suppressing function of Pdcd4 is at least in part mediated through the negative regulation of the expression and activity of the hypoxia-responsive extracellular matrix enzyme, LOX.
Results
Pdcd4 inhibits migration and matrigel invasion of breast cancer cells
Consistent with its role as a tumor suppressor, loss of Pdcd4 correlates with increased cancer progression (Jansen et al., 2004). Cell lines such as T47D and MCF7, derived from less aggressive, nonmetastatic breast tumors, demonstrate higher levels of Pdcd4 protein. In contrast, the MDA-MB-231 and Hs578T cell lines that were derived from aggressive tumors and possess a highly invasive phenotype demonstrate negligible levels of Pdcd4.
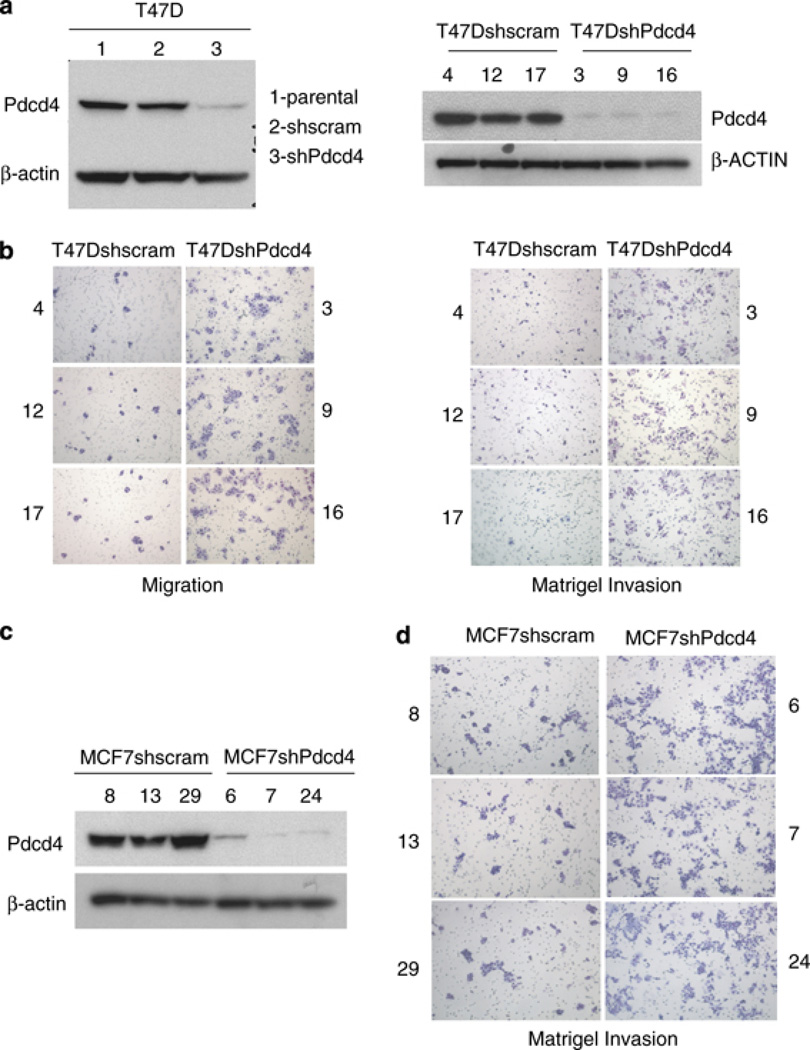
We have previously demonstrated that Pdcd4 inhibits colon cancer cell migration and invasion (Yang et al., 2006; Leupold et al., 2007; Mudduluru et al., 2007). To determine whether Pdcd4 also has an inhibitory role in breast cancer cell progression, we stably expressed short hairpin RNA directed against Pdcd4 in the relatively noninvasive T47D and moderately invasive MCF7 breast cancer cell lines. Parental cells were infected with retrovirus packaged with pFB-Neo-shPdcd4. After antibiotic selection, Pdcd4 downregulation was verified by western blot in pooled (Figure 1a) and single subclones of the cell lines (Figures 1b and c). Cells infected with an short hairpin RNA with a scrambled sequence that does not target Pdcd4, as well as parental T47D and MCF7 cells, were used as controls. The effect of Pdcd4 on tumor cell migration and invasive potential in vitro was assayed using a modified Boyden chamber assay. As expected, cells depleted for Pdcd4 function (T47DshPdcd4 subclones) demonstrated an increase in migration (average of 1.53) and matrigel invasion (average 1.48-fold) compared with control cells (T47Dshscram subclones) (Figure 1b and Supplemental Figure 1). Similar results were obtained with multiple subclones of MCF7shscram and MCF7shPdcd4 cell lines (Figure 1d). In all subsequent text, ‘invasiveness’ or ‘invasive potential’ refers to the ability of cells to invade through matrigel in vitro.
Figure 1.
Pdcd4 inhibits breast cancer cell migration and invasion. (a) Pdcd4 protein depletion on short hairpin RNA (shRNA)-mediated knockdown in T47D cells (pooled) and independent subclones (right panel) was verified by western blot. (b) Pdcd4 depletion enhanced migration and invasion of T47D cells. (c) Pdcd4 protein depletion on shRNA-mediated knockdown in independent subclones of MCF7 cells also resulted in increased matrigel invasion (d).
Pdcd4 inhibits LOX expression
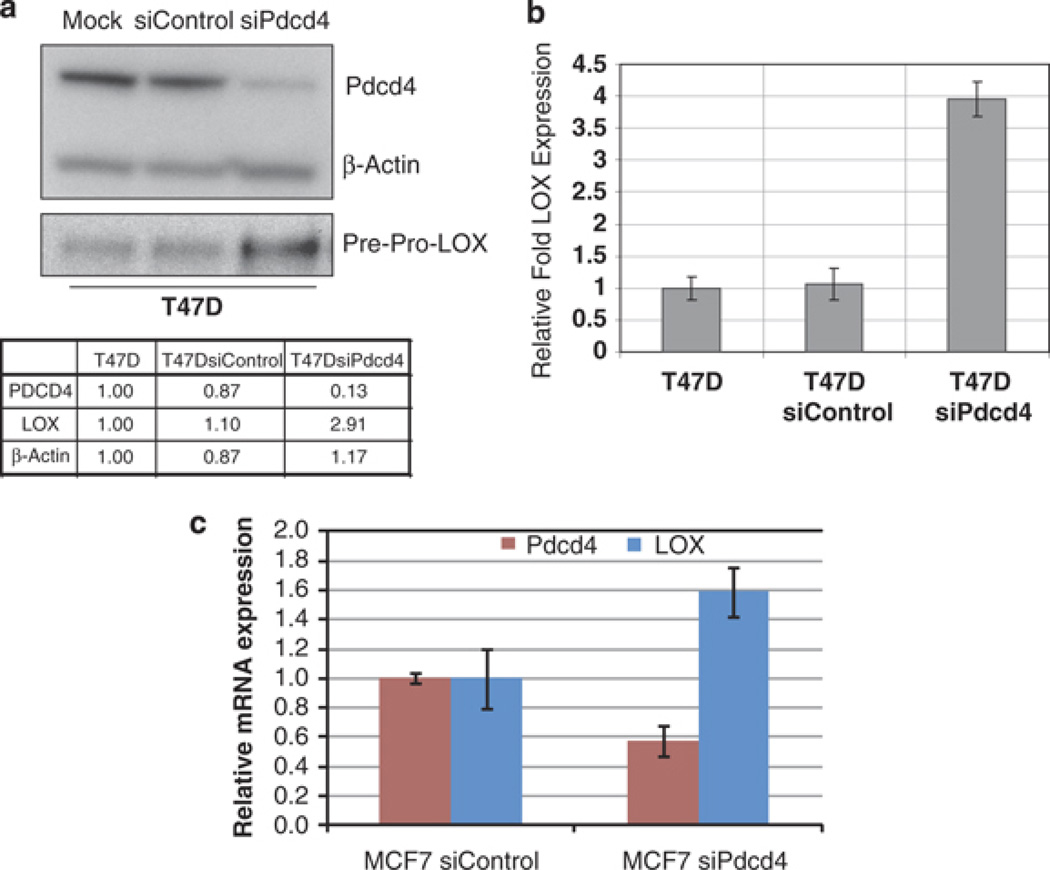
To identify genes that mediate the activity of Pdcd4 in suppressing cancer progression, we used small interfering RNA (siRNA) technology to transiently knockdown Pdcd4 expression in the noninvasive breast cancer cell lines T47D and MCF7. Preliminary microarray profiling identified LOX mRNA as being upregulated by Pdcd4 knockdown (not shown). Western blot and quantitative reverse transcription–PCR (RT–PCR) analysis were conducted to validate the downregulation of Pdcd4 and the concurrent upregulation of LOX mRNA and protein levels. Briefly, cells were transfected with 5 nm siRNA to Pdcd4 or control siRNA and whole-cell extracts were analyzed by SDS–polyacrylamide gel electrophoresis and immunoblotted using antibodies against Pdcd4 and LOX (Figure 2a). LOX is synthesized as a 50 kDa proprotein that becomes gylcosylated (58 kDa) and secreted into the extracellular matrix, where it is processed into active 32 kDa enzyme. We observed an increase in 50 kDa LOX proprotein levels in whole-cell extracts from T47DsiPdcd4 cells. β-Actin levels were used as a loading control.
Figure 2.
Pdcd4 knockdown results in an increase in LOX expression. (a) Western blot with anti-LOX antibody reveals that levels of the LOX pre-proprotein (50 kDa) increase on downregulation of Pdcd4 protein levels in the poorly invasive T47D breast cancer cell line. Relative intensity was calculated using ImageJ and expressed as fold change normalized to the intensity of each protein in lane 1 (T47D parental cell line). Quantitative RT–PCR analysis of LOX mRNA expression in T47D (b) and MCF7 (c) cells transfected with siRNA to Pdcd4, using LOX-specific PCR primers. Expression levels were normalized to 18 s RNA expression. Results were from three independent experiments and plotted as fold expression relative to LOX expression (± s.d.) in the control cell line arbitrarily set at 1.0.
An increase in LOX protein levels with Pdcd4 depletion was seen in cells showing a 3.98-fold (n = 3, P < 0.05) increase in LOX mRNA expression (Figure 2b). Similarly, LOX expression was induced 1.79-fold (n = 3, P < 0.05) in MCF7siPdcd4 cells when compared with corresponding silencing control cells (Figure 2c). These results confirm that Pdcd4 inhibits LOX mRNA and protein expression.
Attenuation of Pdcd4 enhances LOX activity-dependent breast cancer cell migration and invasion
Increased LOX activity has been shown to mediate the invasive phenotype in aggressive breast cancer (Kirschmann et al., 2002; Payne et al., 2005; Erler et al., 2006). Moreover, exogenous overexpression of LOX in breast cancer cell line MCF7 has been shown to induce an invasive phenotype in vitro (Payne et al., 2005). Given the observed increase in in vitro cell migration and invasion in T47DshPdcd4 cells, we hypothesized that this invasive phenotype was mediated by the observed increase in LOX expression. In addition, we asked whether the increase in the intracellular LOX proprotein on Pdcd4 knockdown was accompanied by an increase in extracellular LOX activity. We used β-aminopropionitrile (βAPN), a specific and irreversible inhibitor of LOX activity, to test the predictions.
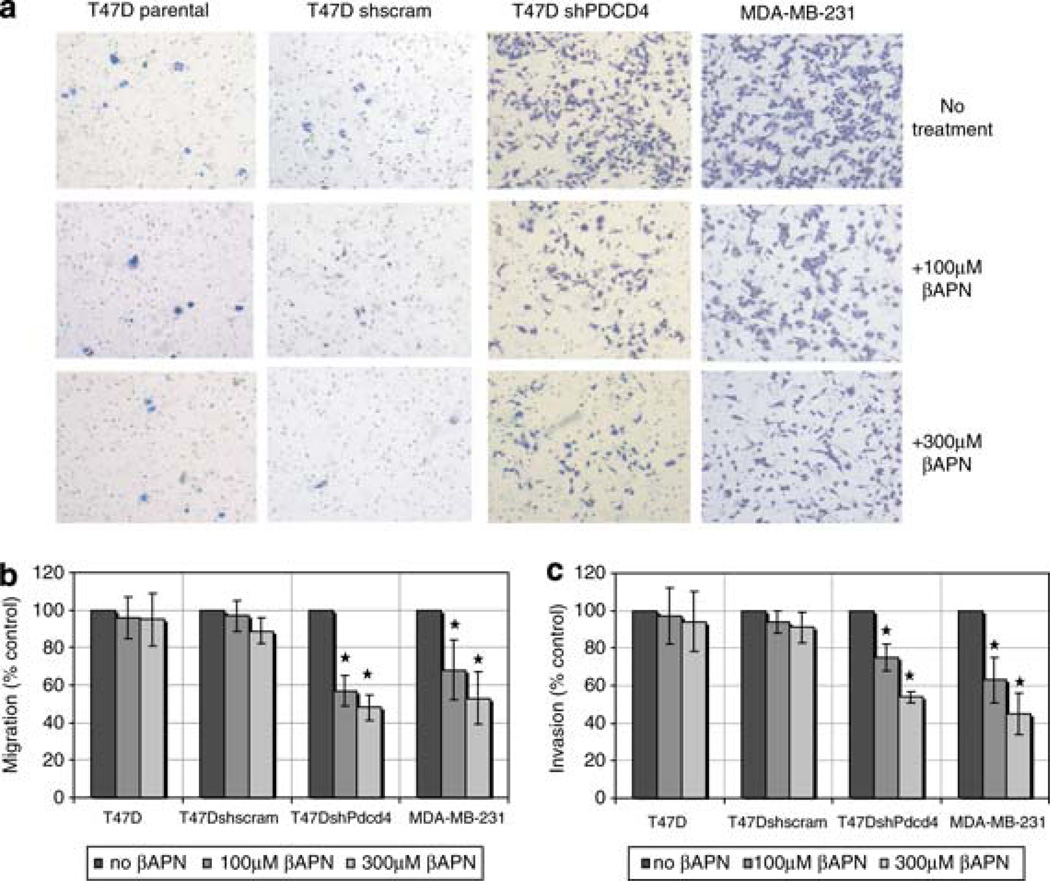
MCF7 and T47D cells stably expressing short hairpin RNA targeting Pdcd4 or scram control short hairpin RNA were analyzed for their migration (not shown) and invasiveness (Supplemental Figure 2 and Figure 3a). As the invasive phenotype of the MDA-MB-231 cell line has been shown to be mediated by LOX (Kirschmann et al., 2002; Erler and Giaccia, 2006; Erler et al., 2006), we used this cell line as a positive control. Cells were either untreated or treated with 100 or 300 µm of LOX inhibitor βAPN. Matrigel-coated or control inserts were used to assay invasion and migration potentials, respectively. Treatment with LOX inhibitor βAPN produced a dose-dependent decrease in migration and matrigel invasion (Figure 3a) of T47DshPdcd4 cells. βAPN treatment did not affect the migration and invasion of parental cells and shcram controls. As expected, βAPN treatment inhibited the migration and invasion of the aggressive MDA-MB-231 cell line.
Figure 3.
Inhibition of LOX activity suppresses migration and invasion in cells attenuated for Pdcd4. (a) Specific inhibition of LOX activity with treatment of LOX inhibitor βAPN (0, 100 or 300 µm) results in decreased migration (b) and matrigel invasion (a, c) of T47D cells attenuated for Pdcd4 function (T47DshPdcd4). The highly invasive and metastatic cell line MDA-MB-231 was used as control. All results were from three independent experiments (n = 6 each) and results were expressed as percentage of control (no βAPN treatment) for each cell line. Statistical significance (*) was established by analysis of variance (P < 0.05).
As different numbers of T47D (2 × 105 cells/well) and MDA-MB-231 (1 × 104 cells/well) cells were seeded to allow for ease of manual counting, βAPN inhibition was quantified as a percentage of control (no βAPN treatment) for each cell line. βAPN (300 µm) treatment inhibited 56% migration and 45% invasion of T47DshPdcd4 cells and this inhibition was comparable to that seen in MDA-MB-231 cells. Similar inhibition of matrigel invasion was observed in MCF7shPdcd4 subclones treated with 300 µm of βAPN (Supplemental Figure 2). These results indicate that the enhanced migration and invasion of cells depleted for Pdcd4 function is at least in part attributable to elevated LOX activity. Thus, Pdcd4 inhibition of LOX may have an important role in suppression of breast cancer progression.
Pdcd4 inhibition of hypoxia-induced breast cancer cell migration and invasion is mediated by LOX repression
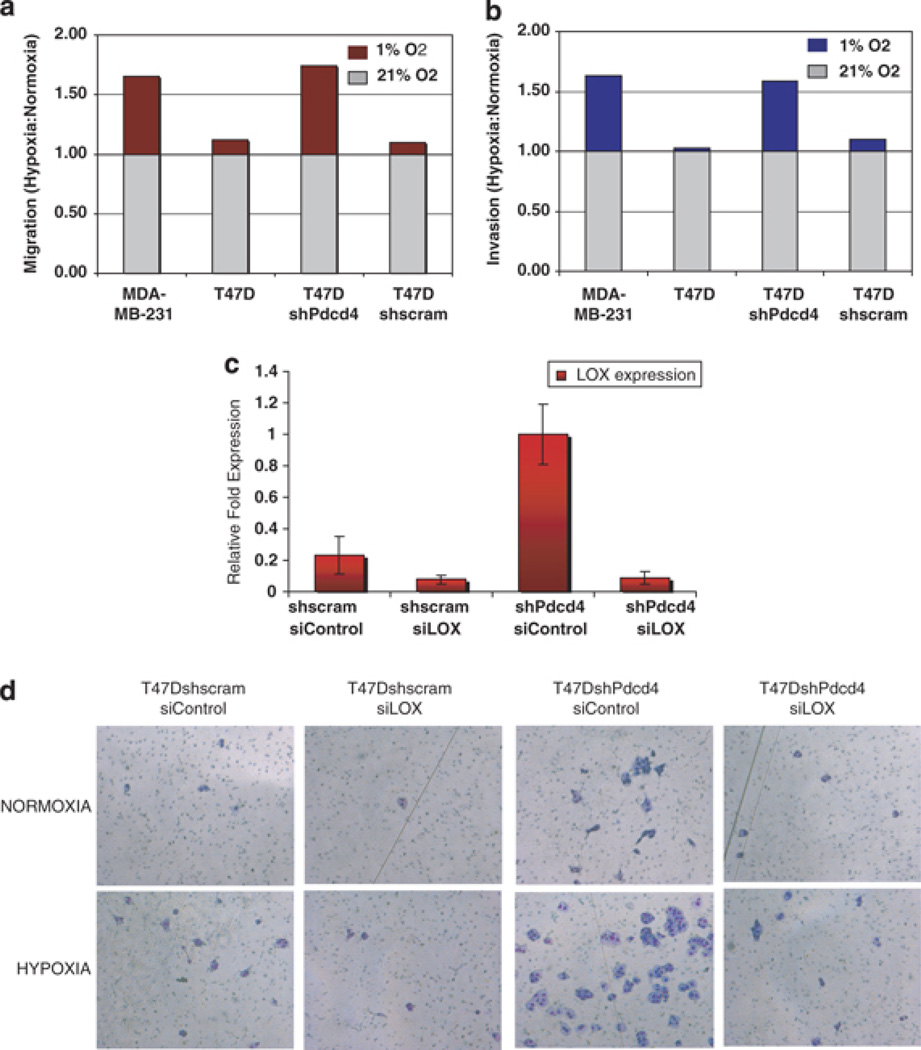
Recent studies have implicated LOX in hypoxia-mediated breast cancer metastasis (Kirschmann et al., 2002; Erler and Giaccia, 2006; Erler et al., 2006; Postovit et al., 2008). Hypoxia response is important in tumor survival and metastasis. The role, if any, of Pdcd4 in hypoxia response is unknown. To determine the effect of Pdcd4 in hypoxia-induced breast cancer cell invasion phenotype, we assayed the migration and invasion of T47DshPdcd4 cells. Poorly invasive T47D parental cells were used as negative control with high Pdcd4 and low LOX levels, whereas MDA-MB-231 cells were used as positive control with low Pdcd4, high LOX and high invasiveness. Serum-starved cells were seeded into 24-well chambers with and without matrigel. Half of the cells were grown in normoxia (21% O2) and the other half were grown in a hypoxic chamber (1% O2). The number of migrated or invaded cells was plotted as a ratio of the number of cells migrated or invaded in hypoxia relative to normoxia for each cell line (Figures 4a and b). T47DshPdcd4 cells demonstrated an enhanced migration and invasive phenotype under hypoxic conditions, whereas the parental T47D and T47Dshscram control cells did not show a significant difference between normoxia and hypoxia. Interestingly, the response in T47DshPdcd4 cells was similar to that in aggressive MDA-MB-231 cells. Similarly, loss of Pdcd4 in MCF7 cells enhanced matrigel invasion under hypoxia when compared with shscram and parental cells (Supplemental Figure 2).
Figure 4.
Pdcd4 inhibition of LOX suppresses hypoxia-induced breast cancer cell invasion. Migration (a) and matrigel invasiveness (b) of the parental, T47DshPdcd4 and control T47Dshscram cell lines were monitored under normoxic (21% O2) and hypoxic (1% O2) growth conditions. The invasive cell line MDA-MB-231 was used as a positive control. We determined the average number of migratory or invasive cells in three independent experiments and the results are presented as a ratio of the mean number of cells migrating or invading in hypoxia versus normoxia, that is, [Migrationhyp/Migrationnorm] (a) and [Invasionhyp/Invasionnorm] (b). Knockdown of LOX reversed the invasive phenotype of T47DshPdcd4 cells in the matrigel invasion assay. Small interfering RNA to LOX was used to knockdown LOX expression in the T47DshPdcd4 stable cell line. Quantitative PCR analysis of LOX expression was monitored to confirm the knockdown (c). Matrigel invasion assay was performed on control (T47Dshscram) and T47DshPdcd4 cells under both normoxic and hypoxic growth conditions (d).
To determine whether the enhanced breast cancer cell migration and invasion phenotype of T47DshPdcd4 cells under hypoxia was mediated through LOX activity, we knocked down LOX expression with siRNA. T47DshPdcd4 and the control cell lines transiently transfected with 5 nm siLOX (Dharmafect) demonstrated 95% downregulation in LOX mRNA expression as determined by quantitative RT–PCR (Figure 4c). Interestingly, silencing LOX expression in Pdcd4 knockdown cells (T47DshPdcd4siLOX cells) significantly reversed the enhanced matrigel invasion seen in T47DshPdcd4 cells (Figure 4d) under both normoxic and hypoxic growth conditions. This result suggests that increase in LOX activity contributes to the enhanced matrigel invasion in T47D cells attenuated for Pdcd4 function, under both normoxic and hypoxic growth conditions.
Pdcd4 inhibits hypoxia-induced LOX expression
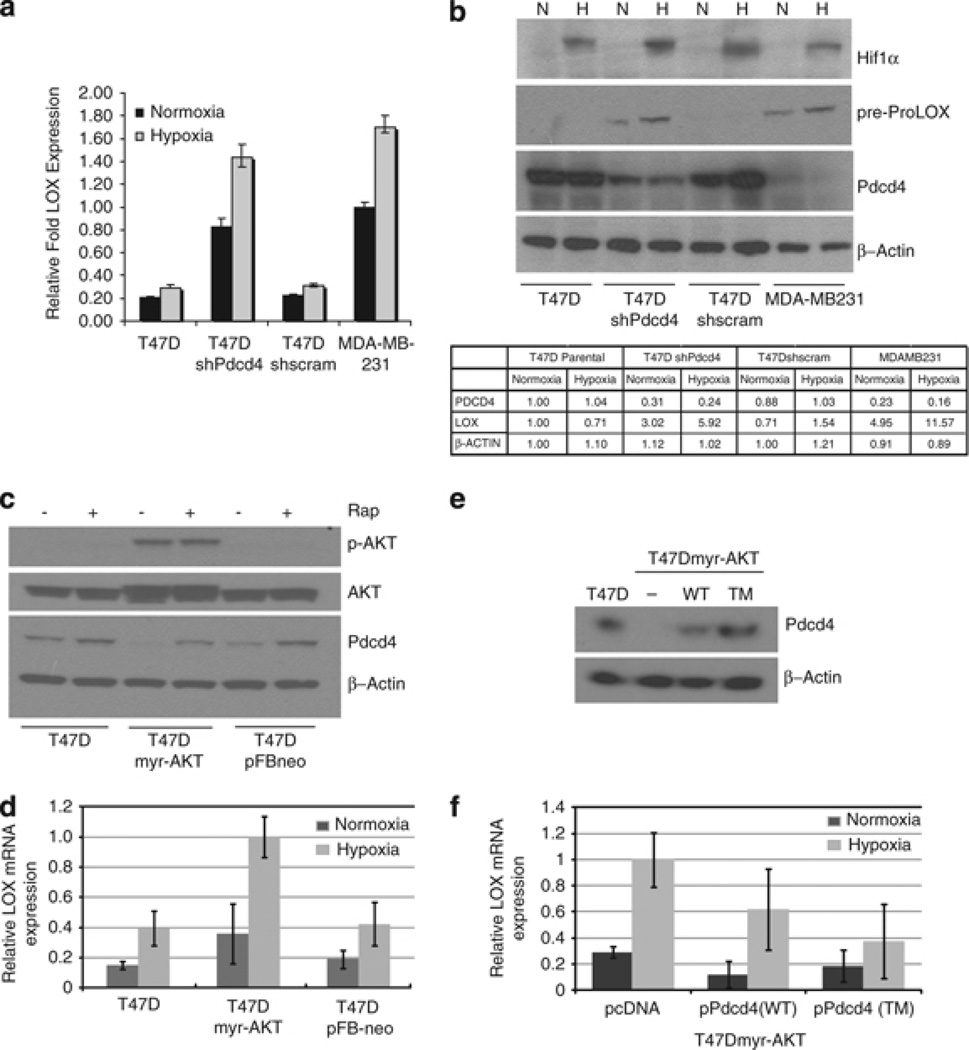
Loss of Pdcd4 enhances LOX activity-dependent invasion of breast cancer cells under hypoxic growth conditions. To determine whether this effect occurred as the result of an increase in LOX expression, we analyzed LOX mRNA and protein levels under normoxia and hypoxia in cells with high or low Pdcd4 protein. Hypoxia did not have a significant effect on LOX mRNA (Figure 5a) and protein (Figure 5b) levels in cells with a relatively high Pdcd4. However, in cell lines with a low Pdcd4 expression, that is, T47DshPdcd4 and MDA-MB-231 cells, LOX expression levels were further enhanced under hypoxic conditions. Similar results were obtained in MCF7shPdcd4 cells (Supplemental Figure 3).
Figure 5.
Hypoxia enhances expression of LOX mRNA and protein in cell lines lacking Pdcd4. Cells were grown under normoxic and hypoxic growth environments. (a) Total RNA was isolated for quantitative reverse transcription (QRT–PCR) analysis to assay LOX gene expression. (b) Total cell lysate was analyzed by SDS–polyacrylamide gel electrophoresis and immunoblotted with anti-Pdcd4 and anti-LOX antibodies. β-Actin expression was monitored as loading control and HIF-1α levels were monitored to ensure hypoxia response. Relative intensity was calculated using ImageJ and expressed as fold change normalized to intensity of each protein in lane 1 (T47D parental cells under normoxia). (c) AKT stable overexpression downregulates Pdcd4 protein. Western blot was performed on whole-cell extracts from T47D, T47Dmyr-AKT and T47DpFBneo control cells grown under normoxia and hypoxia. Rapamycin (20 nm) treatment inhibits AKT-mediated degradation of Pdcd4. (d) Loss of Pdcd4 in T47DMyrAKT results in increased hypoxia-induced LOX expression in T47DmyrAKT cells. (e) Western blot of whole-cell extracts after transient overexpression of degradation-resistant (TM) but not wild-type (WT) Pdcd4 restored Pdcd4 protein levels in T47DmyrAKT cells. (f) Overexpression of Pdcd4 reverses hypoxia-induced LOX expression in cells attenuated for Pdcd4. QRT–PCR analysis of total RNA from Pdcd4-attenuated cells demonstrates that overexpression of degradation-resistant Pdcd4 significantly reverses the elevation of hypoxia-induced LOX expression (mean values ± s.d shown). Results for all quantitative PCR analyses were from three independent experiments. 18 s RNA expression was measured for normalization and results were expressed as fold relative LOX expression in the cell lines indicated. Statistical significance was established by Student’s t-test analysis (P < 0.05).
A hyperactive AKT/mTOR signaling pathway is a hallmark of 30–40% of hormone-responsive breast tumors (Schmitz et al., 2004; Kirkegaard et al., 2005). Akt-phosphorylation of Pdcd4 targets the protein for proteosomal degradation. We attempted reversal of upregulated LOX by reintroducing Pdcd4 both constitutively and through a drug-inducible system (TET On) in MDA-MB-231 cells. This reintroduction of Pdcd4 had only minimal nonsignificant effects on LOX expression in these cells (data not shown). Such a result is not unusual, as cell lines derived from aggressive tumors often develop multiple strategies to shut down or bypass tumor-suppressor expression and function. As we were unable to perform a Pdcd4 rescue experiment in the T47DshPdcd4 cell line, we used a T47D cell line stably overexpressing constitutively active AKT (myr-AKT). Pdcd4 downregulation by myr-AKT (Figure 5c) also resulted in increased LOX expression (Figure 5d). The increase was significant only under hypoxia, suggesting additional mechanisms involved in AKT regulation of LOX expression. Nevertheless, transient overexpression of a degradation-resistant Pdcd4 mutant (pPdcd4-TM) (Figure 5e) significantly reversed hypoxia-induced LOX expression in T47DmyrAKT cells. Thus, Pdcd4 levels limit LOX mRNA expression. AKT-mediated degradation of Pdcd4 during breast cancer progression may have an important role in an increased sensitivity to hypoxia-induced LOX expression.
Pdcd4 regulation of LOX occurs by an HIF-independent mechanism
Previous studies have demonstrated a hypoxia-inducible factor-1 (HIF-1)-responsive element (HRE) in the LOX promoter (Erler et al., 2006). Interestingly, HIF-1α protein is not induced under normoxia in cells depleted of Pdcd4, whereas hypoxia response was fairly normal in all cell lines as evidenced by hypoxia-responsive HIF-1α protein expression (Figure 6a). To further confirm this result, we tested hypoxia-induced transcriptional response at varying Pdcd4 levels. T47D (parental, shPdcd4 and shscram) cell lines were transiently transfected with the HRE-luciferase reporter, the luciferase expression of which is driven by the HIF-responsive element (HRE) in the promoter. As seen in Figure 6a, HRE-luciferase expression was low in all three cell lines under normoxic growth conditions. Moreover, the level of induction of HRE-luc expression under hypoxia was similar, regardless of the Pdcd4 levels in these cells. Having previously shown that Pdcd4 specifically inhibits AP-1-dependent transcription, we tested the tumor promoter-induced transactivation of a transiently transfected AP-1-luciferase reporter expression in all three cell lines (Figure 6b). As expected, loss of Pdcd4 rendered T47DshPdcd4 cells more susceptible to 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced AP-1 transactivation. Collectively, these results suggest that Pdcd4 inhibits LOX expression through an HIF-1-independent mechanism.
Figure 6.
Pdcd4 regulates LOX expression by an HIF-1-independent mechanism. Hypoxia response was monitored by expression of HRE-luciferase reporter transfected into T47D parental, shscram and shPdcd4 cell lines grown under normoxic and hypoxic conditions (a). Response to TPA treatment was monitored using the AP-1-luciferase reporter transiently transfected into the above cell lines (b). Cells were then treated for 18 h with dimethylsulfoxide or TPA. Results were from three independent transfections (n = 9). Proposed model for the role of Pdcd4 in hypoxia induction of LOX (c). Pdcd4 suppresses breast cancer cell invasion by inhibiting the expression of LOX (i). Pdcd4 depletion enhances LOX expression and LOX activity-dependent invasive potential of T47D cells under normoxia (ii). Loss of Pdcd4 increases sensitivity to hypoxia-induced breast cancer cell migration, invasion and, possibly, metastasis through HRE-independent regulation of LOX expression (iii).
Discussion
This study identifies LOX, an extracellular matrix remodeling enzyme, as a target of Pdcd4. Knockdown and rescue experiments demonstrate that Pdcd4 negatively regulates LOX gene expression under both normoxic and hypoxic growth conditions. Loss of Pdcd4 in nonaggressive cells (T47D, MCF7) increases their sensitivity to hypoxic growth conditions. This increased sensitivity to hypoxia is reflected in enhanced expression of LOX and LOX activity-dependent increased invasiveness.
LOX has been shown to have contradictory roles in cancer progression (Payne et al., 2007). Whereas the overexpression of the LOX gene and the increased activity of LOX enzyme have been implicated in enhanced invasion and breast cancer metastasis, the 18 kDa LOX propeptide, a by-product of the processing of the 50 kDa LOX proenzyme, functions as a tumor suppressor in H-RAS-mediated tumorigenesis (Jeay et al., 2003; Min et al., 2007). The increased LOX enzyme activity contributes to the increase in migration and invasion observed in T47D shPdcd4 cells, as βAPN, an inhibitor of LOX activity, inhibits the invasion. Because βAPN also inhibits other members of the LOX multigene family comprised of LOX-like enzymes (LOXL, LOXL-3, LOXL-4) (Payne et al., 2007), we validated our results by specifically inhibiting the expression of the LOX gene using a siRNA to LOX. The results are most consistent with a mechanism in which LOX overexpression and activity under hypoxic growth conditions promote tumor cell invasion. Interestingly, both T47DshPdcd4 and MCF7shPdcd4 cells inhibited for LOX activity show residual matrigel invasion relative to controls. This suggests that other Pdcd4-targeted genes also contribute to regulating invasion.
Previous studies have demonstrated an HIF-1-responsive element in the LOX promoter (Smith-Mungo and Kagan, 1998; Erler et al., 2006). However, our results demonstrate that hypoxia alone cannot significantly induce LOX expression in T47D cells. This is not because of a lack of hypoxia response in T47D cells, as both stabilization of the HIF-1α protein and HRE-luciferase reporter expression occur as expected. Interestingly, loss of Pdcd4 does not affect HIF-1α protein stabilization or HRE-luciferase expression in response to hypoxia. Thus, Pdcd4 may regulate only a subset of hypoxia-response genes. A further identification of any such genes is currently in progress.
Together, these results are consistent with a model wherein hypoxia synergizes with Pdcd4-regulated HIF-1-independent mechanisms to upregulate LOX. Interestingly, LOX expression was recently reported to be only partially HIF-1α responsive in cell lines (Postovit et al., 2008). In metastatic breast tumor cells, LOX expression is no longer under HIF-1 regulation (Postovit et al., 2008). We postulate that these HIF-1-independent mechanisms are mediated through Pdcd4-regulated transcription factors such as SP-1 and AP-1 that may bind cis elements in the transcriptional promoter of LOX. As expected, loss of Pdcd4 does enhance tumor promoter TPA-induced AP-1 luciferase reporter expression (Figure 6b). Interestingly both SP-1- and AP-1-binding sites are present and conserved in the LOX promoter in mice, rats and humans (Csiszar et al., 1996; Tan et al., 1996). Thus, according to our model (Figure 6c), under normoxic growth conditions, Pdcd4 inhibits LOX expression and therefore the invasive potential of cells. Loss of Pdcd4 during tumor progression results in a moderate increase in LOX expression and tumor cell invasion. However, this effect is further enhanced when tumors are exposed to a hypoxic microenvironment. The model thus proposes a tantalizing notion that loss of Pdcd4 early during carcinogenesis could be indicative of a later adaptive response to hypoxia leading to tumor invasion and metastasis.
The only direct molecular function identified for Pdcd4 is its role in the inhibition of mRNA translation (Yang et al., 2003a, 2004). Pdcd4 binds to and inhibits the function of the mammalian mRNA initiation factor eIF4A. The RNA helicase activity of eIF4A is essential for efficient translation of mRNAs that possess a complex 5′UTR secondary structure. Binding of Pdcd4 inhibits this RNA helicase activity and prevents efficient translation initiation. Although the ‘direct’ mRNA targets of Pdcd4 are as yet unknown, those genes the transcription of which is negatively regulated by Pdcd4 are considered as ‘indirect’ targets. Thus, LOX seems to be an indirect functionally significant transcriptional target of Pdcd4. We speculate that Pdcd4 inhibits translation of an as yet unidentified transcription factor (or an upstream activating kinase) that in turn activates LOX gene expression. Analysis of the promoter of LOX will take us a step closer to identifying the molecular mechanism of regulation by Pdcd4.
Three molecular mechanisms directly or indirectly regulate Pdcd4 expression and function, namely, AKT kinase activity (Dorrello et al., 2006; Schmid et al., 2008), transforming growth factor-β signaling (Zhang et al., 2006; Davis et al., 2008) and microRNA miR21 activity (Asangani et al., 2008; Lu et al., 2008; Zhu et al., 2008). It is interesting to note that all three of these molecular mechanisms can be influenced by a hypoxic microenvironment (Ivan et al., 2008; Kulshreshtha et al., 2008). A recent study by Atsawasuwan and co-workers shows that the mature LOX enzyme can negatively regulate transforming growth factor-β signaling in bone (Atsawasuwan et al., 2008). Interestingly, transforming growth factor-β-induced apoptosis in hepatocellular carcinoma was shown to be mediated by Pdcd4 (Zhang et al., 2006). In addition to transforming growth factor-β-mediated regulation, Pdcd4 mRNA translation is negatively regulated by microRNA miR21. Hypoxia has also been shown to regulate the expression of a specific set of microRNAs, among them miR21 (Kulshreshtha et al., 2007). Finally, increased AKT-kinase activity targets Pdcd4 protein for ubiquitin-mediated proteosomal degradation. AKT signaling mediates angiogenesis and cancer progression through HIF-1-dependent mechanisms in certain cancers, but not all (Arsham et al., 2004; Bedogni et al., 2005; Li et al., 2005). However, as we did not observe a decrease in Pdcd4 mRNA expression or protein levels in response to hypoxia alone in the T47D parental cell line, the role of these pathways in Pdcd4 regulation of LOX in breast cancer cells is unclear. Further examination of the correlation of AKT activity and miR21, Pdcd4 and LOX expression in the context of hypoxia-mediated breast carcinogenesis is thus required. Nonetheless, the inhibitory effect of Pdcd4 on induction of LOX seems to be functionally significant for cancer cell invasiveness in vitro.
In summary, these findings are the first to implicate loss of Pdcd4 in an enhanced hypoxic response in breast cancer cells. This observation adds to the already demonstrated regulation of tumor microenvironment by Pdcd4. Hypoxia has been shown not only to interfere with radiation therapy but also to enhance metastasis in various cancers including breast. The identification of Pdcd4 as a modulator of hypoxic response in tumors suggests its utility for prognosis of disease outcome. Thus, stabilization of Pdcd4 protein or increased expression of Pdcd4 may prove to be viable options for preventing tumor progression and metastasis in response to hypoxia.
Materials and methods
Cell culture
The noninvasive breast cancer cell lines T47D, MCF7 and the invasive cell line MDA-MB-231 were obtained from ATCC. Cells were grown in complete Dulbecco’s modified Eagle’s medium (DMEM) (10% fetal bovine serum, 25 µg/ml gentamicin, 2 mm l-glutamate). Individual subclones of each stable cell line were obtained and analyzed for Pdcd4 levels similar to the corresponding parental cells. Cells were either grown under normoxia (21% O2) or incubated in hypoxic chambers with 1% O2, with 5 % CO2 at 37 °C for the times indicated.
Plasmid and retroviral vector design
For stable knockdown of Pdcd4, shPdcd4 (5′-GATCCCGGTGGCTGGAACATCTATTTTCAAGAGAAATAGATGTTCCAGCCACCTTTTA-3′) and shScram (5′-GATCCCTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAATTTTA-3′) sequences were cloned into BglII and HindIII sites in pSuper.retro.puro. For creating T47D myr-AKT cells, we used pSRMSVtkneo originated in Philip Tsichlis’ laboratory and generously provided by Phillip Dennis, NCI. Myristylated AKT fused to a C-terminal hemagglutinin epitope tag was cut from the donor vector by SalI and NotI digest and inserted into pFB-Neo (Stratagene, Santa Clara, CA, USA). For stably overexpressing Pdcd4, we amplified human Pdcd4 cDNA with primers designed to add restriction sites to both the 5′ and 3′ ends of the gene. We chose EcoRI and XhoI sites to allow for insertion of the gene in correct orientation into pcDNA3.1.
Retroviral packaging and transduction
GP2-293 packaging cells (Clonetech, Mountain View, CA, USA) were seeded at 6 × 106 cells in 100 mm collagen I-coated dishes (BD Biosciences, San Jose, CA, USA). At 24 h after seeding, the medium was removed and replaced with 5 ml DMEM without antibiotics. Transfection complexes were prepared by adding 60 µl TransIT-293 transfection reagent (Mirus, Madison, WI, USA), 10 µg appropriate vector and 10 µg of the retroviral vector pVSV-G (Clonetech) to Optimem I media (Invitrogen, Carlsbad, CA, USA), with a final volume of 1 ml. Viral supernatants were collected between 48 and 72 h after transfection and were then cleared of cellular debris by filtration through a 0.45 µm polyvinylidene difluoride syringe filter.
T47D cells were seeded at 1 × 106 cells in 100 mm dishes. The following day, the medium was removed and replaced with 10 ml of cleared viral supernatant containing 5 µg/ml polybrene. After 24 h of viral infection, the viral supernatants were removed and cells were reseeded in DMEM containing 10% fetal bovine serum, gentamicin, and 1 µg/ml puromycin or 800 µg/ml neomycin as appropriate. After 2 weeks of antibiotic selection, pooled clones were maintained in complete DMEM with 500 ng/ml puromycin or 400 µg/ml neomycin. Clonal T47DshPdcd4, T47Dshscram, MCF7shPdcd4 and MCF7shscram stable cell lines were generated by single-cell dilution. Relative levels of Pdcd4 overexpression were determined by western blot. Clones exhibiting the relative Pdcd4 expression to parental cell lines for shscram and those exhibiting maximal Pdcd4 knockdown with shPdcd4 were chosen for further analysis.
RNA interference
T47D and MCF7 breast cancer cells were transfected with siRNA specifically designed to silence the Pdcd4 gene (siPdcd4) or a nonsilencing control. The sequence of Pdcd4-specific siRNA was 5′-GGUGGCUGGAACAUCUAUU-3′ and that of silencing control was 5′-TTCTCCGAACGTGTCACGT-3′. Short hairpin RNA against Pdcd4 (shPdcd4) and shScram control (shscram) were designed using the above sequences as a basis. The siRNA to LOX was obtained from Dharmacon (Lafayette, CO, USA). Cells were transfected with siRNA using either Fugene6 (Roche, Indianapolis, IN, USA) or Dharmafect1 (Dharmacon). Establishment of stable knockdown of Pdcd4 in T47D cells is described above.
Migration and invasion assays
The modified Boyden chamber/matrigel assay was performed according to the manufacturer’s directions (BD Biosciences). For in vitro migration assays, we used chambers with control inserts that lacked the matrigel coating. For ease of counting migrated and invaded cells, different numbers of total cells were plated for the T47D and MDA-MB-231 cells. Briefly, 2 × 105 T47D cells/well and 1 × 104 MDA-MB-231 cells/well were resuspended in serum-free DMEM and seeded in triplicate in matrigel-coated and uncoated control inserts. DMEM with 10% serum was used as a chemoattractant in the lower chamber. Chambers were incubated under normoxic (21% O2) or hypoxic (1% O2) conditions for 24 h. For treatment with βAPN, the inhibitor was added to cells 24 h before serum deprivation and also to the lower chamber throughout the experiment. After 24 h, the cells that did not migrate or invade were removed by scraping with a cotton swab. The migrated and invaded cells were fixed and stained with the Diff-Quik stain kit (Dade Behring, Newark, DE, USA). Cells were photographed and counted for quantification. Results were calculated as the mean number of cells of triplicate measurements that migrated or invaded to the lower chamber and described as fold migration or invasion relative to the control parental cell line. The number of invaded or migrated cells per insert was determined by counting the center field plus up to four surrounding, nonoverlapping fields. The center field is depicted in the figures. Statistical significance was established by analysis of variance (P < 0.05).
Immunobloting
Indicated cells (1 × 106 cells/100 mm dish) were grown under normoxic (21% O2) or hypoxic (1% O2) growth conditions for 24 h. Whole-cell extracts were prepared by lysing cells with 300 µl of nondenaturing lysis buffer (20 mm HEPES-KOH at pH 7.6, 100 mm KCl, 0.5 mm EDTA, 20% glycerol, 0.5% Triton X-100, 1× protease inhibitor cocktail) for 30 min at 4 °C. A volume of 30 µg of total lysate was used for western blot analysis except in the case of detecting LOX proenzyme, in which case, 80 µg of total protein was analyzed by SDS–polyacrylamide gel electrophoresis. β-Actin levels were monitored as a loading control. Polyclonal antibody to LOX was used at a concentration of 1:1000 (Payne et al., 2005). Polyclonal antibody to Pdcd4 has been described before (Rockland Immunochemicals, Gilbertsville, MD, USA; Schmid et al., 2008). Monoclonal antibodies to HIF-1α and β-actin were obtained from BD Biosciences and Sigma (St Louis, MO, USA), respectively, and used according to the manufacturer’s recommendation. Densitometry data were obtained using NIH ImageJ (Bethesda, MD, USA), and the relative intensity of detected bands was normalized to intensity in lane 1 for each protein blot.
Luciferase reporter assays
Cell lines indicated in the figure legends were transiently transfected with either 4xAP-1-luciferase or HRE-luciferase reporter plasmids as described previously (Melillo et al., 1999; Yang et al., 2003b, 2006). Cells (1 × 104 cells/well in 24-well plate) were transiently transfected with 0.25 µg of reporter plasmid (pcDNA3.1–4xAP1-Luc or pcDNA-HRE-Luc), along with 10 ng of phRL-TK (Renilla luciferase) control plasmid using Fugene 6 as the transfection reagent. To test hypoxia response, transfected cells were incubated under either normoxic or hypoxic growth conditions for 24 h. For TPA treatments, transfected cells were serum starved in DMEM with 0.2% fetal bovine serum for 24 h to lower the AP-1 background to basal levels. Cells were then treated with either TPA (10 ng/ml) or dimethylsulfoxide for an additional 24 h. Cells were then lysed in 100 µl of 1× passive lysis buffer (Promega, Madison, WI, USA). Aliquots (25 µl) of lysates were analyzed for luminescent signal with a Microtiter Plate Luminometer (Dynex Technologies, Chantilly, VA, USA). Luciferase activity of each sample was normalized against Renilla luciferase monitored for transfection efficiency. Results (average light units) were expressed as mean ± s.d. Experiments were repeated three times in sextuplicate, and representative data are shown as percentage control.
RT–PCR and quantitative RT–PCR
Cells were grown in appropriate growth conditions and treated with LOX inhibitor βAPN, where indicated. Total RNA was isolated using Triozol and further purified using the RNeasy kit (Qiagen, Valencia, CA, USA). A volume of 1 µg of total cellular mRNA was reverse transcribed into cDNA using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA) and oligo(dT) primer. For RT–PCR and quantitative RT–PCR for LOX, Pdcd4 and CA-9 gene primers were obtained from Dharmacon. CA-9 expression was monitored as a surrogate for hypoxia (Pastorek et al., 1994; Ivanov et al., 1998). Expression of 18s RNA (primers from Ambion, Austin, TX, USA) was used for normalization. RT–PCR products were run on 0.8% agarose gels and imaged using GEL Logic imaging (Rochester, NY, USA). Relative intensity was measured using KODAK MI software (Rochester, NY, USA) and normalized to intensity in lane 1 for each gene. Quantitative RT–PCR was carried out by SYBR Green I-based detection using the iQ5 Real-Time PCR Detection System (Bio-Rad). ΔCt values were defined as LOX Ct minus 18 s RNA Ct and averaged for each triplicate sample. Statistical significance was determined by Student’s t-test analysis (P < 0.05).
Supplementary Material
Acknowledgements
We thank Drs Giovanni Melillo and Annamaria Rapisarda (Tumor Hypoxia Laboratory, NCI-Frederick) for helpful suggestions and for the use of reagents and hypoxia chambers. We also thank members of the Gene Regulation Section, Laboratory of Cancer Prevention, for helpful discussions. This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
References
- Arsham AM, Plas DR, Thompson CB, Simon MC. Akt and hypoxia-inducible factor-1 independently enhance tumor growth and angiogenesis. Cancer Res. 2004;64:3500–3507. doi: 10.1158/0008-5472.CAN-03-2239. [DOI] [PubMed] [Google Scholar]
- Asangani IA, Rasheed SA, Nikolova DA, Leupold JH, Colburn NH, Post S, et al. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27:2128–2136. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- Atsawasuwan P, Mochida Y, Katafuchi M, Kaku M, Fong KS, Csiszar K, et al. Lysyl oxidase binds transforming growth factor-beta and regulates its signaling via amine oxidase activity. J Biol Chem. 2008;283:34229–34240. doi: 10.1074/jbc.M803142200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedogni B, Welford SM, Cassarino DS, Nickoloff BJ, Giaccia AJ, Powell MB. The hypoxic microenvironment of the skin contributes to Akt-mediated melanocyte transformation. Cancer Cell. 2005;8:443–454. doi: 10.1016/j.ccr.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Bitomsky N, Bohm M, Klempnauer KH. Transformation suppressor protein Pdcd4 interferes with JNK-mediated phosphorylation of c-Jun and recruitment of the coactivator p300 by c-Jun. Oncogene. 2004;23:7484–7493. doi: 10.1038/sj.onc.1208064. [DOI] [PubMed] [Google Scholar]
- Chen HH, Su WC, Lin PW, Guo HR, Lee WY. Hypoxia-inducible factor-1alpha correlates with MET and metastasis in node-negative breast cancer. Breast Cancer Res Treat. 2007;103:167–175. doi: 10.1007/s10549-006-9360-3. [DOI] [PubMed] [Google Scholar]
- Chen Y, Knosel T, Kristiansen G, Pietas A, Garber ME, Matsuhashi S, et al. Loss of PDCD4 expression in human lung cancer correlates with tumour progression and prognosis. J Pathol. 2003;200:640–646. doi: 10.1002/path.1378. [DOI] [PubMed] [Google Scholar]
- Cmarik JL, Min H, Hegamyer G, Zhan S, Kulesz-Martin M, Yoshinaga H, et al. Differentially expressed protein Pdcd4 inhibits tumor promoter-induced neoplastic transformation. Proc Natl Acad Sci USA. 1999;96:14037–14042. doi: 10.1073/pnas.96.24.14037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar K, Entersz I, Trackman PC, Samid D, Boyd CD. Functional analysis of the promoter and first intron of the human lysyl oxidase gene. Mol Biol Rep. 1996;23:97–108. doi: 10.1007/BF00424435. [DOI] [PubMed] [Google Scholar]
- Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 2008;454:56–61. doi: 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denko NC, Fontana LA, Hudson KM, Sutphin PD, Raychaudhuri S, Altman R, et al. Investigating hypoxic tumor physiology through gene expression patterns. Oncogene. 2003;22:5907–5914. doi: 10.1038/sj.onc.1206703. [DOI] [PubMed] [Google Scholar]
- Dorrello NV, Peschiaroli A, Guardavaccaro D, Colburn NH, Sherman NE, Pagano M. S6K1- and betaTRCP-mediated degradation of PDCD4 promotes protein translation and cell growth. Science. 2006;314:467–471. doi: 10.1126/science.1130276. [DOI] [PubMed] [Google Scholar]
- Erler JT, Bennewith KL, Nicolau M, Dornhofer N, Kong C, Le QT, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- Erler JT, Giaccia AJ. Lysyl oxidase mediates hypoxic control of metastasis. Cancer Res. 2006;66:10238–10241. doi: 10.1158/0008-5472.CAN-06-3197. [DOI] [PubMed] [Google Scholar]
- Goke R, Barth P, Schmidt A, Samans B, Lankat-Buttgereit B. Programmed cell death protein 4 suppresses CDK1/cdc2 via induction of p21(Waf1/Cip1) Am J Physiol Cell Physiol. 2004a;287:C1541–C1546. doi: 10.1152/ajpcell.00025.2004. [DOI] [PubMed] [Google Scholar]
- Goke R, Gregel C, Goke A, Arnold R, Schmidt H, Lankat-Buttgereit B. Programmed cell death protein 4 (PDCD4) acts as a tumor suppressor in neuroendocrine tumor cells. Ann N Y Acad Sci. 2004b;1014:220–221. doi: 10.1196/annals.1294.024. [DOI] [PubMed] [Google Scholar]
- Ivan M, Harris AL, Martelli F, Kulshreshtha R. Hypoxia response and microRNAs: no longer two separate worlds. J Cell Mol Med. 2008;12:1426–1431. doi: 10.1111/j.1582-4934.2008.00398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov SV, Kuzmin I, Wei MH, Pack S, Geil L, Johnson BE, et al. Down-regulation of transmembrane carbonic anhydrases in renal cell carcinoma cell lines by wild-type von Hippel-Lindau transgenes. Proc Natl Acad Sci USA. 1998;95:12596–12601. doi: 10.1073/pnas.95.21.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen AP, Camalier CE, Colburn NH. Epidermal expression of the translation inhibitor programmed cell death 4 suppresses tumorigenesis. Cancer Res. 2005;65:6034–6041. doi: 10.1158/0008-5472.CAN-04-2119. [DOI] [PubMed] [Google Scholar]
- Jansen AP, Camalier CE, Stark C, Colburn NH. Characterization of programmed cell death 4 in multiple human cancers reveals a novel enhancer of drug sensitivity. Mol Cancer Ther. 2004;3:103–110. [PubMed] [Google Scholar]
- Jeay S, Pianetti S, Kagan HM, Sonenshein GE. Lysyl oxidase inhibits ras-mediated transformation by preventing activation of NF-kappa B. Mol Cell Biol. 2003;23:2251–2263. doi: 10.1128/MCB.23.7.2251-2263.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan HM, Li W. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem. 2003;88:660–672. doi: 10.1002/jcb.10413. [DOI] [PubMed] [Google Scholar]
- Kagan HM, Trackman PC. Properties and function of lysyl oxidase. Am J Respir Cell Mol Biol. 1991;5:206–210. doi: 10.1165/ajrcmb/5.3.206. [DOI] [PubMed] [Google Scholar]
- Kang Y. Functional genomic analysis of cancer metastasis: biologic insights and clinical implications. Expert Rev Mol Diagn. 2005;5:385–395. doi: 10.1586/14737159.5.3.385. [DOI] [PubMed] [Google Scholar]
- Kirkegaard T, Witton CJ, McGlynn LM, Tovey SM, Dunne B, Lyon A, et al. AKT activation predicts outcome in breast cancer patients treated with tamoxifen. J Pathol. 2005;207:139–146. doi: 10.1002/path.1829. [DOI] [PubMed] [Google Scholar]
- Kirschmann DA, Seftor EA, Fong SF, Nieva DR, Sullivan CM, Edwards EM, et al. A molecular role for lysyl oxidase in breast cancer invasion. Cancer Res. 2002;62:4478–4483. [PubMed] [Google Scholar]
- Kronblad A, Jirstrom K, Ryden L, Nordenskjold B, Landberg G. Hypoxia inducible factor-1alpha is a prognostic marker in premenopausal patients with intermediate to highly differentiated breast cancer but not a predictive marker for tamoxifen response. Int J Cancer. 2006;118:2609–2616. doi: 10.1002/ijc.21676. [DOI] [PubMed] [Google Scholar]
- Kulshreshtha R, Davuluri RV, Calin GA, Ivan M. A microRNA component of the hypoxic response. Cell Death Differ. 2008;15:667–671. doi: 10.1038/sj.cdd.4402310. [DOI] [PubMed] [Google Scholar]
- Kulshreshtha R, Ferracin M, Wojcik SE, Garzon R, Alder H, Agosto-Perez FJ, et al. A microRNA signature of hypoxia. Mol Cell Biol. 2007;27:1859–1867. doi: 10.1128/MCB.01395-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRonde-LeBlanc N, Santhanam AN, Baker AR, Wlodawer A, Colburn NH. Structural basis for inhibition of translation by the tumor suppressor Pdcd4. Mol Cell Biol. 2007;27:147–156. doi: 10.1128/MCB.00867-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leupold JH, Yang HS, Colburn NH, Asangani I, Post S, Allgayer H. Tumor suppressor Pdcd4 inhibits invasion/intravasation and regulates urokinase receptor (u-PAR) gene expression via Sp-transcription factors. Oncogene. 2007;26:4550–4562. doi: 10.1038/sj.onc.1210234. [DOI] [PubMed] [Google Scholar]
- Li T, Li D, Sha J, Sun P, Huang Y. MicroRNA-21 directly targets MARCKS and promotes apoptosis resistance and invasion in prostate cancer cells. Biochem Biophys Res Commun. 2009;383:280–285. doi: 10.1016/j.bbrc.2009.03.077. [DOI] [PubMed] [Google Scholar]
- Li YM, Zhou BP, Deng J, Pan Y, Hay N, Hung MC. A hypoxia-independent hypoxia-inducible factor-1 activation pathway induced by phosphatidylinositol-3 kinase/Akt in HER2 overexpressing cells. Cancer Res. 2005;65:3257–3263. doi: 10.1158/0008-5472.CAN-04-1284. [DOI] [PubMed] [Google Scholar]
- Lu Z, Liu M, Stribinskis V, Klinge CM, Ramos KS, Colburn NH, et al. MicroRNA-21 promotes cell transformation by targeting the programmed cell death 4 gene. Oncogene. 2008;27:4373–4379. doi: 10.1038/onc.2008.72. [DOI] [PubMed] [Google Scholar]
- Lundgren K, Holm C, Landberg G. Hypoxia and breast cancer: prognostic and therapeutic implications. Cell Mol Life Sci. 2007;64:3233–3247. doi: 10.1007/s00018-007-7390-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melillo G, Sausville EA, Cloud K, Lahusen T, Varesio L, Senderowicz AM. Flavopiridol, a protein kinase inhibitor, down-regulates hypoxic induction of vascular endothelial growth factor expression in human monocytes. Cancer Res. 1999;59:5433–5437. [PubMed] [Google Scholar]
- Min C, Kirsch KH, Zhao Y, Jeay S, Palamakumbura AH, Trackman PC, et al. The tumor suppressor activity of the lysyl oxidase propeptide reverses the invasive phenotype of Her-2/neu-driven breast cancer. Cancer Res. 2007;67:1105–1112. doi: 10.1158/0008-5472.CAN-06-3867. [DOI] [PubMed] [Google Scholar]
- Mudduluru G, Medved F, Grobholz R, Jost C, Gruber A, Leupold JH, et al. Loss of programmed cell death 4 expression marks adenoma-carcinoma transition, correlates inversely with phosphorylated protein kinase B, and is an independent prognostic factor in resected colorectal cancer. Cancer. 2007;110:1697–1707. doi: 10.1002/cncr.22983. [DOI] [PubMed] [Google Scholar]
- Nieves-Alicea R, Colburn NH, Simeone AM, Tari AM. Programmed cell death 4 inhibits breast cancer cell invasion by increasing tissue inhibitor of metalloproteinases-2 expression. Breast Cancer Res Treat. 2008;114:203–209. doi: 10.1007/s10549-008-9993-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorek J, Pastorekova S, Callebaut I, Mornon JP, Zelnik V, Opavsky R, et al. Cloning and characterization of MN, a human tumor-associated protein with a domain homologous to carbonic anhydrase and a putative helix-loop-helix DNA binding segment. Oncogene. 1994;9:2877–2888. [PubMed] [Google Scholar]
- Payne SL, Fogelgren B, Hess AR, Seftor EA, Wiley EL, Fong SF, et al. Lysyl oxidase regulates breast cancer cell migration and adhesion through a hydrogen peroxide-mediated mechanism. Cancer Res. 2005;65:11429–11436. doi: 10.1158/0008-5472.CAN-05-1274. [DOI] [PubMed] [Google Scholar]
- Payne SL, Hendrix MJ, Kirschmann DA. Paradoxical roles for lysyl oxidases in cancer—a prospect. J Cell Biochem. 2007;101:1338–1354. doi: 10.1002/jcb.21371. [DOI] [PubMed] [Google Scholar]
- Postovit LM, Abbott DE, Payne SL, Wheaton WW, Margaryan NV, Sullivan R, et al. Hypoxia/reoxygenation: a dynamic regulator of lysyl oxidase-facilitated breast cancer migration. J Cell Biochem. 2008;103:1369–1378. doi: 10.1002/jcb.21517. [DOI] [PubMed] [Google Scholar]
- Rucker RB, Kosonen T, Clegg MS, Mitchell AE, Rucker BR, Uriu-Hare JY, et al. Copper, lysyl oxidase, and extracellular matrix protein cross-linking. Am J Clin Nutr. 1998;67:996S–1002S. doi: 10.1093/ajcn/67.5.996S. [DOI] [PubMed] [Google Scholar]
- Schindl M, Schoppmann SF, Samonigg H, Hausmaninger H, Kwasny W, Gnant M, et al. Overexpression of hypoxia-inducible factor 1alpha is associated with an unfavorable prognosis in lymph node-positive breast cancer. Clin Cancer Res. 2002;8:1831–1837. [PubMed] [Google Scholar]
- Schmid T, Jansen AP, Baker AR, Hegamyer G, Hagan JP, Colburn NH. Translation inhibitor Pdcd4 is targeted for degradation during tumor promotion. Cancer Res. 2008;68:1254–1260. doi: 10.1158/0008-5472.CAN-07-1719. [DOI] [PubMed] [Google Scholar]
- Schmitz KJ, Otterbach F, Callies R, Levkau B, Holscher M, Hoffmann O, et al. Prognostic relevance of activated Akt kinase in node-negative breast cancer: a clinicopathological study of 99 cases. Mod Pathol. 2004;17:15–21. doi: 10.1038/modpathol.3800002. [DOI] [PubMed] [Google Scholar]
- Smith-Mungo LI, Kagan HM. Lysyl oxidase: properties, regulation and multiple functions in biology. Matrix Biol. 1998;16:387–398. doi: 10.1016/s0945-053x(98)90012-9. [DOI] [PubMed] [Google Scholar]
- Tan RS, Taniguchi T, Harada H. Identification of the lysyl oxidase gene as target of the antioncogenic transcription factor, IRF-1, and its possible role in tumor suppression. Cancer Res. 1996;56:2417–2421. [PubMed] [Google Scholar]
- van der Groep P, Bouter A, Menko FH, van der Wall E, van Diest PJ. High frequency of HIF-1alpha overexpression in BRCA1 related breast cancer reast. Cancer Res Treat. 2008;111:475–480. doi: 10.1007/s10549-007-9817-z. [DOI] [PubMed] [Google Scholar]
- Vleugel MM, Greijer AE, Shvarts A, van der Groep P, van Berkel M, Aarbodem Y, et al. Differential prognostic impact of hypoxia induced and diffuse HIF-1alpha expression in invasive breast cancer. J Clin Pathol. 2005;58:172–177. doi: 10.1136/jcp.2004.019885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters LC, Veverka V, Bohm M, Schmedt T, Choong PT, Muskett FW, et al. Structure of the C-terminal MA-3 domain of the tumour suppressor protein Pdcd4 and characterization of its interaction with eIF4A. Oncogene. 2007;26:4941–4950. doi: 10.1038/sj.onc.1210305. [DOI] [PubMed] [Google Scholar]
- Wen YH, Shi X, Chiriboga L, Matsahashi S, Yee H, Afonja O. Alterations in the expression of PDCD4 in ductal carcinoma of the breast. Oncol Rep. 2007;18:1387–1393. [PubMed] [Google Scholar]
- Yagata H, Harigaya K, Suzuki M, Nagashima T, Hashimoto H, Ishii G, et al. Comedonecrosis is an unfavorable marker in node-negative invasive breast carcinoma. Pathol Int. 2003;53:501–506. doi: 10.1046/j.1440-1827.2003.01514.x. [DOI] [PubMed] [Google Scholar]
- Yang HS, Cho MH, Zakowicz H, Hegamyer G, Sonenberg N, Colburn NH. A novel function of the MA-3 domains in transformation and translation suppressor Pdcd4 is essential for its binding to eukaryotic translation initiation factor 4A. Mol Cell Biol. 2004;24:3894–3906. doi: 10.1128/MCB.24.9.3894-3906.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HS, Jansen AP, Komar AA, Zheng X, Merrick WC, Costes S, et al. The transformation suppressor Pdcd4 is a novel eukaryotic translation initiation factor 4A binding protein that inhibits translation. Mol Cell Biol. 2003a;23:26–37. doi: 10.1128/MCB.23.1.26-37.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HS, Jansen AP, Nair R, Shibahara K, Verma AK, Cmarik JL, et al. A novel transformation suppressor, Pdcd4, inhibits AP-1 transactivation but not NF-kappaB or ODC transactivation. Oncogene. 2001;20:669–676. doi: 10.1038/sj.onc.1204137. [DOI] [PubMed] [Google Scholar]
- Yang HS, Knies JL, Stark C, Colburn NH. Pdcd4 suppresses tumor phenotype in JB6 cells by inhibiting AP-1 transactivation. Oncogene. 2003b;22:3712–3720. doi: 10.1038/sj.onc.1206433. [DOI] [PubMed] [Google Scholar]
- Yang HS, Matthews CP, Clair T, Wang Q, Baker AR, Li CC, et al. Tumorigenesis suppressor Pdcd4 down-regulates mitogen-activated protein kinase kinase kinase kinase 1 expression to suppress colon carcinoma cell invasion. Mol Cell Biol. 2006;26:1297–1306. doi: 10.1128/MCB.26.4.1297-1306.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakowicz H, Yang HS, Stark C, Wlodawer A, Laronde-Leblanc N, Colburn NH. Mutational analysis of the DEAD-box RNA helicase eIF4AII characterizes its interaction with transformation suppressor Pdcd4 and eIF4GI. RNA. 2005;11:261–274. doi: 10.1261/rna.7191905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Ozaki I, Mizuta T, Hamajima H, Yasutake T, Eguchi Y, et al. Involvement of programmed cell death 4 in transforming growth factor-beta1-induced apoptosis in human hepatocellular carcinoma. Oncogene. 2006;25:6101–6112. doi: 10.1038/sj.onc.1209634. [DOI] [PubMed] [Google Scholar]
- Zhu S, Wu H, Wu F, Nie D, Sheng S, Mo YY. MicroRNA-21 targets tumor suppressor genes in invasion and metastasis. Cell Res. 2008;18:350–359. doi: 10.1038/cr.2008.24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.