Summary
During definitive erythropoiesis, erythroid precursors undergo differentiation through multiple nucleated states to an enucleated reticulocyte, which loses its residual RNA/organelles to become a mature erythrocyte. Over the course of these transformations, continuous changes in membrane proteins occur, including shifts in protein abundance, rates of expression, isoform prominence, states of phosphorylation, and stability. In an effort to understand when assembly of membrane proteins into an architecture characteristic of the mature erythrocyte occurs, we quantitated the lateral diffusion of the most abundant membrane protein, band 3 (AE1), during each stage of erythropoiesis using single particle tracking. Analysis of the lateral trajectories of individual band 3 molecules revealed a gradual reduction in mobility of the anion transporter as erythroblasts differentiated. Evidence for this progressive immobilization included a gradual decline in diffusion coefficients as determined at a video acquisition rate of 120 frames/s and a decrease in the percentage of compartment sizes >100 nm. Because complete acquisition of the properties of band 3 seen in mature erythrocytes is not observed until circulating erythrocytes are formed, we suggest that membrane maturation involves a gradual and cooperative assembly process that is not triggered by the synthesis of any single protein.
Keywords: band 3 diffusion, single particle tracking, erythrocyte, streptavidin quantum dot, progenitor cells
Introduction
During erythropoiesis, hematopoietic progenitor cells progress through a series of developmental stages into mature erythrocytes (Richmond, et al 2005, Zamai, et al 2004). Thus, after commitment to erythroid differentiation, erythroid burst-forming units (BFU-E) differentiate into erythroid colony-forming units (CFU-E) (Richmond, et al 2005, Spivak 2005, Wickrema, et al 1994), which in turn gradually mature through a series of nucleated states into an orthochromatic erythroblast. The orthochromatic erythroblast then extrudes its nucleus and enters circulation as a reticulocyte (Chasis and Mohandas 2008, Richmond, et al 2005, Wickrema and Crispino 2007). Complete differentiation of the reticulocyte into a mature erythrocyte is finally achieved as reticular networks of polyribosomes are eliminated and the plasma membrane morphs into the classic biconcave disc shape characteristic of the mature red blood cell (Chasis and Mohandas 2008, Richmond, et al 2005, Spivak 2005, Wickrema and Crispino 2007).
During the course of human erythroblast differentiation in culture, membrane-spanning proteins are synthesized and a spectrin-based membrane skeleton is formed that provides strength and stability to the lipid bilayer. Spectrin synthesis begins early in the course of differentiation, but the skeletal protein turns over rapidly until it becomes firmly incorporated into the plasma membrane. Stable association of spectrin with the erythrocyte membrane coincides with synthesis of ankyrin, protein 4.1, and band 3, which collectively organize spectrin and actin into a membrane-anchored cortical cytoskeleton (Chasis, et al 1993, Hanspal, et al 1992a, Hanspal, et al 1992b, Hoffman, et al 2002, Nunomura, et al 2009, Wickrema, et al 1994). Thus, spectrin mRNA first appears in human BFU-Es by day 3, the time when erythropoietin receptors initially appear on the cell surface (Wickrema, et al 1994). Although commitment of CD34+ cells to the erythroid lineage will also have begun by day 3, synthesis of mRNAs for other major erythroid cytoskeletal proteins such as band 3, ankyrin, and protein 4.1 will not have occurred at this early stage of differentiation (Wickrema, et al 1994). Ankyrin and protein 4.1 mRNAs are only observed in a majority of cells by day 7 (proerythroblasts), when only 15% of erythroblasts stain positive for band 3 (Wickrema, et al 1994). Band 3 mRNA, in contrast, reaches its maximal level on day 10 (polychromatic), and expression of the band 3 polypeptide continues until day 15 (orthochromatic) (Zamai, et al 2004). Importantly, in early erythroblasts, ~60% of the cytoskeletal protein adducin, is readily extractable by Triton X-100 detergent, whereas in reticulocytes this soluble population decreases to 30%, and in mature erythrocyte no soluble adducin can be detected (Nehls, et al 1991). Clearly, significant changes in the assembly and organization of membrane proteins occur over the course of erythroblast differentiation (Blikstad, et al 1983, Moon and Lazarides 1984, Woods, et al 1986).
Although much is known about the chronology of synthesis of erythrocyte membrane proteins during erythropoiesis, less is known regarding the timing of organization and assembly of these proteins into their final architecture on the mature erythrocyte membrane. We have recently examined the diffusion of band 3 in mature erythrocytes and have identified subpopulations of the anion transporter that could be attributed to free band 3 and band 3 attached to ankyrin and/or the junctional complex (Kodippili, et al 2009). In the current study, the same diffusion measurements were exploited to characterize the stage-specific association of band 3 with its membrane cytoskeleton during terminal differentiation of human erythroblasts, providing insight into the timing of assembly of membrane proteins into the final architecture characteristic of the mature erythrocyte. The data argue that band 3 only forms its full complement of native interactions late in erythroid differentiation, subsequent to extrusion of the nucleus from the nascent reticulocyte.
Materials and methods
Development of erythroblasts and reticulocytes from CD34+ hematopoietic progenitors
Human serum was prepared from blood samples obtained from healthy volunteers following informed consent and approval by the Institutional Review Board at the University of Chicago. Human primary erythroblasts and reticulocytes were derived by in vitro culture of early CD34+ hematopoietic progenitors that were harvested using the isolex300i device (Baxter Healthcare, Inc., Deerfield, IL) from commercially available growth factor mobilized blood samples (AllCells LLC, Emeryville, CA), as described elsewhere (Kang, et al 2008). Briefly, CD34+ cells isolated from growth-factor mobilized peripheral blood were cultured in Isocove’s modified Dulbecco’s medium supplemented with 15% fetal bovine serum (Hyclone, Inc., Logan, UT), 15% human serum, 10 ng/ml interleukin-3 (R & D, Inc., Minneapolis, MN), 2 units/ml erythropoietin (Amgen, Inc., Thousand Oaks, CA), and 50 ng/ml stem cell factor (R & D, Inc., Minneapolis, MN). Cells were fed once on day 3 of culture with the same culture media except during this feeding no interleukin-3 was added and the amount of stem cell factor was reduced to 25 ng/ml. On day 6 of culture cells were further purified by flow cytometric sorting to select for transferrin receptor positive cells (CD71). The cells were labeled with anti-CD71-fluorescein isothiocyanate antibody prior to sorting (BD Bioscience, San Jose, CA). The purity of the population isolated by this method was 98–99% (Kang, et al 2008). Sorted cells were cultured until day 15 in order to promote terminal differentiation into reticulocytes. The feeding of cells was carried out as described previously (Kang, et al 2008). Differentiation of pro-erythroblasts was monitored by flow cytometry (with staining for glycophorin A and CD71 expression) and analyzed by cytospin preparations (using benzidine for detection of haemoglobin and haematoxylin for counterstaining). Although the aforemementioned measures were carefully employed to enrich for erythroblasts of the designated stage, some variation from the average stage of differentiation should be expected in each of the samples.
Labeling of band 3 in intact erythroid progenitors with DIDS-biotin
Erythroblasts were collected at various time points during culture (days 8, 10, 12 and 15) and centrifuged. Pelleted cells were washed 3× with phosphate-buffered saline (PBS), pH 7.4, containing 5 mM glucose and then diluted to 5% v/v in the same buffer. ~2×106 cells were then incubated with 10−11M DIDS (4,4′-di-isothiocyanatostilbene-2,2′-disulphonic acid)-biotin at 37°C for 1.5 h to allow covalent reaction of the compound with a limited number of band 3 molecules on each erythrocyte (usually 1 or 2/cell). Importantly, DIDS-biotin binding to erythrocytes follows a cooperative, single site binding isotherm, suggesting that it does not preferentially label one band 3 population over another (Kodippili, et al 2009). DIDS-biotin labelled cells were then washed 3× with PBS containing 0.1% bovine serum albumin (BSA) to remove unbound reagent, and the cells were incubated at room temperature with streptavidin Q-dot 525. Unbound streptavidin-linked quantum dots were removed by washing twice with 0.1% BSA in PBS, and cells were allowed to settle onto a pre-cleaned, poly-lysine-coated cover slip located within a custom built chamber ready for imaging. Unattached cells were washed from the cover slip with PBS containing 0.1% BSA and 500 µl of PBS were added to the chamber prior to imaging, as described below. Any apoptotic cells (< 2%) were excluded from single particle tracking analysis. Following quantum dot imaging, a bright field image was acquired to enable nucleated and enucleated erythroblasts to be distinguished.
Single Quantum dot fluorescence video microscopy
Tracking of quantum dot-labeled band 3 molecules was performed using an Olympus IX-71 inverted microscope (Olympus, Center Valley, PA, USA), as described previously (Kodippili, et al 2009). Briefly, oblique angle fluorescence imaging was used to excite single quantum dots on the apical surfaces of immobilized erythrocytes. The entire microscope was maintained at 37°C by enclosure in a temperature controlled environment. The Argon ion excitation laser (488 nm emission; Newport, Irvine, CA, USA) was expanded, filtered (488/10 nm line width bandpass filter, Chroma Technology, Rockingham, VT, USA) and directed towards the microscope objective (100× PlanApo, NA 1.45 TIRFM oil immersion, Olympus) parallel but off the optical axis through a dichroic mirror (500 nm Cutoff, Chroma Technology). The resultant fluorescence image was transmitted through the dichroic mirror and an emission filter (525/50 nm bandpass, Chroma Technology), and the image was collected with a cooled CCD camera (XR/Turbo-120z, Stanford Photonics, Inc., Palo Alto, CA). The excitation beam was set barely outside of the condition for total internal reflection, thus allowing for a deeper excitation while still reducing nonspecific background fluorescence in the solution. Data were collected from the dots attached to band 3 on the top of the surface of the cell at 120 frames/s. Each movie was recorded for 1000 frames. The trajectories were collected on a random selection of 400–500 erythroblasts in each sample and only one trajectory was monitored per cell.
Briefly, analysis of the mobility (Ritchie and Kusumi 2003, Tomishige and Kusumi 1999, Tomishige, et al 1998) of band 3 labeled quantum dots yielded a microscopic diffusion coefficient (Dμ), a macroscopic diffusion coefficient (DM), and a measure of the compartment size in which band 3 diffusion is confined (Kodippili, et al 2009). Qualitatively, Dμ refers to the diffusion coefficient measured over very short time spans (commonly <100 ms for band 3), whereas DM refers to the diffusion coefficient over longer time spans (usually 100 to 1000 ms). These diffusion coefficients can in fact differ, because a protein that is tethered to a flexible cytoskeleton will be seen to swing back and forth on a short time scale (Dμ), but remain essentially at the same location on the cell surface over a long time scale (DM). In more technical terms, Dμ constitutes the asymptotic limit of the slope of the mean square displacement of the diffusing particle as a function of time as time approaches zero, whereas DM constitutes the asymptotic slope of the mean square displacement versus time curve as time approaches infinity.
For each stage of erythroblast differentiation, samples from at least two individuals collected on at least two separate days were analyzed. In some cases, data from 5 separate blood draws from up to 3 different donors were analyzed. Because no significant differences were observed between the data sets, data were always combined and presented in a single histogram for each stage of erythroblast differentiation. The distributions of both Dμ and DM were plotted on a log scale and fitted to one or more Gaussian distributions using the program, Origin, where an F-test at a 95% confidence interval was applied to determine the correct number of populations.
Results
Characterization of human erythroblasts during terminal differentiation in culture
Human proerythroblasts, derived from BFU-Es, changed morphology, granularity, and haemoglobin content gradually during differentiation to orthochromatic erythroblasts and then reticulocytes. Indeed, both forward light scatter (a measure of cell diameter) and side light scatter (a measure of internal cell granularity/complexity) decreased monotonically from day 8 through day 15 of culture (data not shown). Day 8 erythroblasts corresponded to a basophilic stage, as demonstrated by their prominent nuclei, comparatively large size, elevated levels of transferrin receptor (CD71), increasing numbers of glycophorin A, and low concentrations of haemoglobin (Fig. 1A). Day 10 erythroblasts, in contrast, expressed greater amounts of haemoglobin, higher levels of glycophorin A, and were at the polychromatic stage of differentiation. Day 12 cells corresponded to the orthochromatic stage of differentiation, as evidenced by their smaller size, greater expression of haemoglobin and glycophorin A, and sustained concentration of transferrin receptor. Furthermore, by day 12 the cells had completed their proliferative phase and were in the process of exiting the cell cycle and beginning to enter the enucleation phase of their differentiation program. By day 15, the majority of cells had lost their granularity, synthesized copious amounts of haemoglobin, eliminated many of their transferrin receptors, and maintained their levels of glycophorin A. Furthermore, ~50% of the cells have extruded their nuclei, giving rise to nascent reticulocytes (Fig.1B).
Fig 1.
Development of human primary erythroblasts and reticulocytes in vitro. CD34+ early hematopoietic cells were cultured under precise conditions to promote their commitment and differentiation into erythroblasts and reticulocytes. On Day 6 of culture cells were sorted by flow cytometry to further enrich for transferrin receptor (CD71) positive cells and recultured as indicated in Materials and Methods. Cultured cells were analysed by (A) Flow cytometry to determine the levels of glycophorin A and CD71 in order to monitor the progression of the differentiation program. Plots from flow cytometry analyses are shown (days 8, 10, 12 and 15) along with appropriate isotype controls (left panels) for each of the days indicated. (B) Photomicrographs of a representative selection of haematoxylin/benzidine stained cells on days 8, 10, 12 and 15 depict increasing haemoglobinization as the differentiation program reaches the reticulocyte stage. Enucleated cells (nascent reticulocytes) are depicted by arrows on day 15. In general, ~50% of the erythroblasts will have enucleated by day 15. A photomicrograph of mature RBCs prepared after removal of the buffy coat from a healthy donor is also depicted. Bar = 20 µm
Labeling of band 3
The specificity of the DIDS-biotin conjugate (Fig S1) for band 3 was established by reacting the conjugate with intact erythroblasts, isolating their membranes, separating component proteins by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE), blotting the proteins onto nitrocellulose paper, and visualizing the location of biotinylated polypeptides by staining with streptavidin-horseradish peroxidase. As seen in Fig S1B, only one polypeptide with apparent Mr ~100 kDa was labeled with streptavidin in erythroblasts from day 8 cells culture. Given that band 3 (immunoblotted in Fig S1C) is the only known receptor for DIDS on human erythrocytes, and because band 3 has the molecular weight of the protein stained in Fig S1B (i.e. 101 kDa), it was concluded that band 3 is selectively labeled by the DIDS-biotin conjugate in intact erythroblasts. This result was, in fact, not surprising, because the same DIDS-biotin conjugate was shown to label only band 3 when added to mature erythrocytes (Kodippili, et al 2009).
Band 3 mobility in day 8 erythroblasts
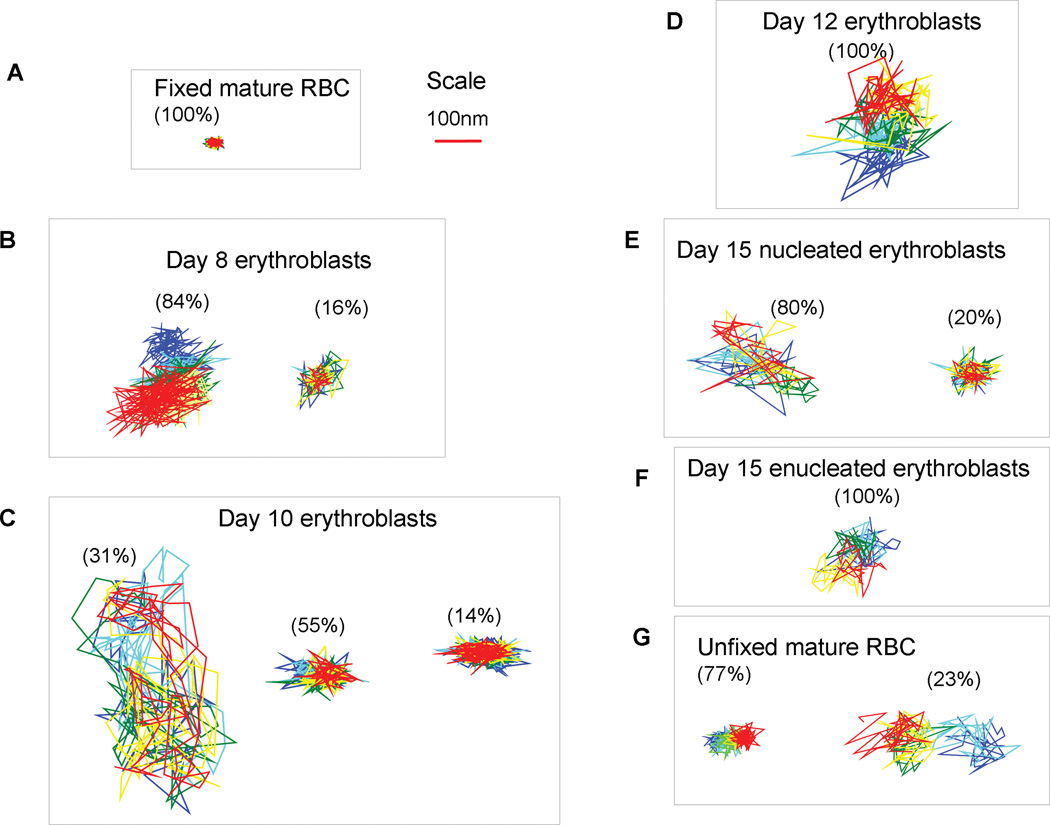
Typical trajectories of band 3 in intact day 8 erythroblasts are shown in Fig. 2. Following the procedures described by Kodippili, et al (2009), these trajectories were analyzed for: i) their diffusion coefficients over short time spans (yielding a microscopic diffusion coefficient, Dμ), ii) their asymptotic diffusion coefficients over longer time spans (yielding a macroscopic diffusion coefficient, DM), and iii) the sizes of the compartments in which any restricted diffusion was confined. The distribution of these diffusion coefficients for a single band 3 molecule from each of ~140 different day 8 erythroblasts is shown in Fig. 3. The distribution of microscopic diffusion coefficients (Dμ) revealed two peaks with mean values of 6.8×10−11 cm2/s (84%) and 2.4×10−12 cm2/s (16%) (Fig. 3, Table 1). The macroscopic diffusion coefficients (DM) were also characterized by two Gaussian distributions with mean values of 1.8×10−12 cm2/s (88%) and 4.5×10−11 cm2/s (12%); i.e. similar to mature RBCs except for the presence of the minor population of fast diffusing molecules (Figure 3, Table 1). The large shift in distribution of microscopic diffusion coefficients towards faster diffusing populations suggests that band 3 encountered fewer barriers during diffusion in day 8 erythroblasts than in mature erythrocytes.
Fig 2.
Representative trajectories of DIDS-biotin labeled band 3 on human erythroblasts. Cells were reacted with DIDS-biotin conjugate, labeled with streptavidin-quantum dots, and imaged by fluorescence video microscopy to monitor the diffusion of individual band 3 molecules. Each sample was imaged at least 100 consecutive frames at a speed of 120 frames/s on (A) fixed mature RBCs, (B) day 8 erythroblasts, (C) day 10 erythroblasts, (D) day 12 erythroblasts, (E) day 15 nucleated erythroblasts, (F) day 15 enucleated erythroblasts, and (G) unfixed mature RBCs at 37° C. The approximate percentages of each cell population that displayed the displayed characteristic trajectory are also indicated.
Fig 3.
Distributions of the logarithms of the microscopic (Dμ) and macroscopic (DM) diffusion coefficients. Primary erythroblasts on various days of culture and mature RBCs as indicated were analyzed in order to determine the microscopic and macroscopic diffusion coefficients (see Materials and Methods for details). Dotted lines represent the means of the major populations of microscopic and macroscopic diffusion coefficients of mature RBC.
Table 1.
Microscopic and macroscopic diffusion data of day 8, 10, 12 and 15 erythroblasts and mature RBCs
| RBC type | Dμ (cm2/s) | DM (cm2/s) | Percent immobile† |
Percent constrained‡ |
Percent free |
|---|---|---|---|---|---|
| Fixed (n= 134)¶ |
(6.8 ± 0.1††) ×10−14 (100%)¶¶ |
(5.5 ± 0.2) ×10−14 (100%) |
95‡‡ | 0 | 5 |
| Day 8 erythroblasts (n=296) |
(2.4 ± 0.5) ×10−12 (16%) (6.8 ± 1.0) ×10−11 (84%) |
(1.8 ± 0.1) ×10−12(88%) (4.5 ± 0.7) ×10−11(12%) |
25 | 37 | 38 |
| Day 10 erythroblasts (n=436) |
(2.2 ± 0.4) ×10−12 (14%) (6.3 ± 1.2) ×10−11 (55%) (1.2 ± 0.5) ×10−9 (31%) |
(2.6 ± 0.2) ×10−12 (80%) (3.7 ± 0.5) ×10−10 (20%) |
24 | 34 | 42 |
| Day 12 erythroblasts (n=540) |
(5.5 ± 0.7) ×10−11 (100%) | (2.8 ± 0.1) ×10−12 (92%) (3.1 ± 0.6) ×10−10 (8%) |
18 | 38 | 44 |
| Day 15 erythroblasts nucleated (n=413) |
(2.4 ± 0.8) ×10−12 (20%) (1.2 ± 0.1) ×10−10 (80%) |
(2.9 ± 0.2) ×10−12 (100%) |
30 | 31 | 39 |
| Day 15 erythroblasts Enucleated (n=157) |
(3.6 ± 0.4) ×10−11 (100%) | (1.1 ± 0.1) ×10−12 (100%) |
25 | 36 | 39 |
| Mature RBC (n= 626) |
(1.4 ± 0.4) ×10−11 (77%) (2.2 ± 0.1) ×10−10 (23%) |
(1.7 ± 0.1) ×10−12 (100%) |
24 | 30 | 46 |
The totally immobile fraction is defined as the fraction of band 3 molecules that were found to have a DM value less than or equal to the slowest 95% of DM values measured for band 3 on fixed erythrocytes (DMImm); i.e. the fraction of band 3 that diffuses similar to band 3 on fixed cells.
Percent constrained includes all trajectories that were classified as undergoing confined or hop diffusion and for which DM > DMImm.
Total number of trajectories analysed.
Errors are given as the standard error of the mean.
Defined as such.
Reflects the fraction of total area contained in each peak of the Gaussian fit to the distribution. For those distributions that fit best with a single peak it is understood that 100% of the area is contained within that peak
Band 3 mobility in day 10 erythroblasts
Typical trajectories of band 3 on intact day 10 erythroblasts are shown in Fig. 2 and the distributions of their diffusion coefficients obtained upon analysis of 436 different trajectories are displayed in Fig. 3. Analysis of the distribution of Dμ values revealed three Gaussian distributions centred at 2.2×10−12 (14%), 6.3×10−11 (55%) and 1.2×10−9 cm2/s (31%). Interestingly, the slowest of these populations (Dμ= 2.2×10−12 cm2/s) diffused ~6 times slower than the slowest population present in mature erythrocytes, whereas the fastest diffusing population moved ~5 times faster than the fastest band 3 population in mature erythrocytes. Moreover, day 10 progenitor cells exhibited a third population of band 3 with a mean Dμ of 6.3×10−11 cm2/s that diffuses ~4.5 times faster than the slow population in mature red cells. These data suggested that short term constraints on the motion of band 3 in day 10 progenitor cells were also significantly different from those on band 3 in mature cells and that the tethers that anchor most of the band 3 to the membrane skeleton in these immature erythroblasts were significantly less well established than comparable tethers in mature erythrocytes.
As noted for day 8 erythroblasts, band 3 diffusion in day 10 erythroblasts revealed two populations of molecules with mean macroscopic diffusion coefficients (DM) values of 3.7×10−10 cm2/s (20%) and 2.6×10−12 cm2/s (80%). Curiously, the faster population was not detected in normal mature RBCs, suggesting that long term constraints on a fraction of band 3 molecules are also different in day 10 erythroblasts from those in normal mature cells. Although all proteins necessary for assembly of the mature erythrocyte membrane skeleton are thought to be present at this stage of erythropoiesis (Blikstad, et al 1983, Chang, et al 1976, Chen, et al 2009, Hanspal, et al 1992a, Hanspal, et al 1992b, Hanspal and Palek 1987, Moon and Lazarides 1984, Wickrema, et al 1994), the above data suggest that proper assembly of these components into an architecture characteristic of the mature cell remains incomplete at this stage of differentiation.
Band 3 mobility in day 12 erythroblasts
Typical trajectories of band 3 in intact day 12 erythroblasts are shown in Fig. 2. Analysis of the microscopic diffusion coefficients revealed that the three populations of band 3 seen on day 10 had largely converged into a single broad population by day 12 with a mean Dμ of 5.5×10−11 cm2/s (Fig. 3, Table 1). The macroscopic diffusion coefficients (DM), however, remain characterized by two Gaussian distributions with mean values of 2.8×10−12 cm2/s (92%) (i.e. similar to mature RBCs) and 3.1×10−10 cm2/s (8%) (i.e. similar to the faster population in day 10 erythroblasts; Figure 3, Table 1). Thus, the continued bimodal distribution of macroscopic diffusion coefficients on day 12 suggests the persistence of some band 3 molecules that remain unanchored to the spectrin/actin skeleton.
Band 3 mobility in nucleated and enucleated day 15 erythroblasts
Examination of the trajectories (Fig. 2) of band 3 in intact nucleated erythroblasts on day 15 of differentiation revealed a pattern not dramatically different from day 12 erythroblasts except for the emergence of a small population of slower diffusing molecules (Dμ = 2.4×10−12 cm2/s; ~20%) (Fig.3, Table 1). Not surprisingly, a concomitant decline in the fraction of highly mobile band 3 (Dμ > 10−9 cm2/s) was also seen as the cell matured, suggesting once again a gradual anchoring of band 3 as differentiation approaches completion. The macroscopic diffusion coefficient of band 3 in day 15 nucleated cells exhibited a mean value of 2.9×10−12 cm2/s; i.e. only marginally faster than the value in mature RBCs.
Day 15 enucleated erythroblasts display only a single population of band 3 with a mean Dμ of 3.6×10−11 cm2/s and a mean DM of 1.1×10−12 cm2/s (Figure 3, Table 1). Interestingly, these diffusion coefficients were both significantly slower than the corresponding Dμ and DM values of the predominant population of band 3 in the day 15 nucleated cells (vide supra), suggesting that significant changes in band 3 mobility occur rather abruptly during erythroblast enucleation.
Analysis of compartment size distributions
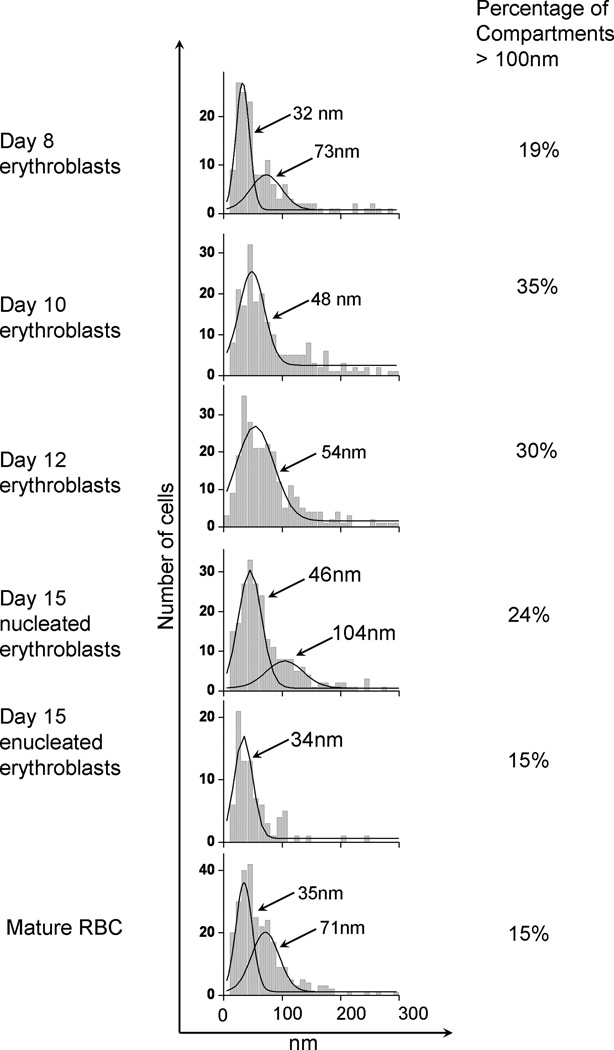
Analysis of the trajectories of band 3 in the various erythroblasts also permitted estimation of the membrane compartment sizes in which the protein’s diffusion is confined. The distribution of these compartment sizes is shown in Fig. 4. Although the median compartment size of the major population of band 3 appeared to first increase and then gradually decrease as the cell matured, the majority of the polypeptide still appeared to diffuse within a compartment of ~30 to 55 nm on a side. Because these dimensions correspond closely with those of a spectrin corral in mature erythrocytes (Lee and Discher 2001), it is tempting to speculate that the majority of band 3 molecules, even in relatively early erythroblasts, is constrained primarily by a spectrin/actin network not too different from that found in mature red blood cells. Obviously, a smaller population of band 3 in the same erythroblasts encounters significantly fewer barriers than those experienced in mature erythrocytes (see % of compartments >100 nm, Fig.4), suggesting that patches of incomplete spectrin assembly also exist in the same early-stage cells and that these regions of incomplete network formation decline in number as the erythroblast matures.
Fig 4.
Distributions of the compartment sizes determined by analysis of individual trajectories of labeled band 3 molecules on intact mature human fixed, unfixed mature RBC, human day 8, 10, 12, 15 nucleated 15 enucleated erythroblasts. The percentage of compartments above 100 nm is also indicated. As indicated in the figure, the percentage of compartments larger than 100 nm decrease as erythroblasts differentiate into mature red cells.
Discussion
Although a number of studies have examined the chronology of synthesis of erythrocyte membrane proteins during erythroid differentiation (Chen, et al 2009, Wickrema, et al 1994), little information is available regarding the timing of assembly of these proteins into functional membrane complexes. Data from the laboratories of Chasis/Mohandas (Chasis, et al 1993, Chen, et al 2009, Lee, et al 2004), Hanspal (Hanspal, et al 1992a, Hanspal, et al 1992b, Hanspal and Palek 1987), Koury (Koury, et al 1987, Koury, et al 1989) and Wickrema (Wickrema, et al 1994, Wickrema, et al 1992) suggest that several major cytoskeletal proteins (e.g. spectrin, actin, adducin, and ankyrin) are synthesized early in erythropoiesis, but are turned over rapidly until band 3, and perhaps protein 4.1 and protein 4.2 are synthesized (Chang, et al 1976, Koch, et al 1975, Nunomura, et al 2009, Peters, et al 1992). It has consequently been hypothesized that incorporation of band 3 into the lipid bilayer provides a stable nucleation site onto which the aforementioned peripheral membrane proteins can assemble (Campanella, et al 2008, Hanspal, et al 1992b), thereby triggering formation of the architecture characteristic of the mature erythrocyte. The data presented here, however, question whether the appearance of either band 3, protein 4.1 or protein 4.2 in the membrane is sufficient to trigger immediate assembly of the mature membrane structure. Thus, band 3, protein 4.1 and protein 4.2 are largely present by day 10 (polychromatic stage), however, the properties of band 3 diffusion (i.e. Dμ, DM, and compartment size) do not acquire their final characteristics until the mature erythrocyte is formed. Consistent with this hypothesis, changes in the attachment of glycophorin A and adducin to the spectrin/actin skeleton also occur continuously during differentiation (Lee, et al 2004, Nehls, et al 1991, Zamai, et al 2004), and membrane remodeling is even known to occur throughout enucleation and subsequent organelle exocytosis/degradation (Koury, et al 1989, Lee, et al 2004, Zweig, et al 1981). This more gradual model of membrane assembly should not be surprising, given that the exocytosis of intracellular material commonly requires different membrane components than those present in mature red blood cells (Fader, et al 2005, Johnstone 2006), and these will have to be eliminated late in the differentiation process.
One unanticipated observation in the present work concerns the magnitude of the change in band 3 diffusion that accompanies nuclear extrusion. Thus, comparison of nucleated and enucleated day 15 cells from the same culture shows a change in band 3 Dμ of nearly an order of magnitude (from 1.2×10−10 to 3.6×10−11 cm2/s), a three-fold change in DM (from 2.9 to 1.1 ×10−12 cm2/s), and the loss of a 104 nm compartment in which band 3 diffuses. These data suggest that the membrane material eliminated during enucleation is significantly different from the membrane material retained in the nascent reticulocyte (Koury, et al 2005, Lee, et al 2004), and that the components removed during enucleation must be eliminated to complete the final assembly of the mature membrane’s architecture.
A few discontinuities can be noted in the data (e.g. the unexpected but small increase in the mobility of band 3 seen in the transition from day 8 to 10 erythroblasts; Fig 3). Although each panel in Fig 3 was derived from analysis of 300 to 400 cells from multiple donors, it is conceivable that this transient increase in band 3 mobility embedded within a continuous trend towards decreasing mobility could be an experimental artifact. Alternatively, because the erythroblast membrane must remodel from an actin- and tubulin-dominated skeleton to a spectrin-dominated skeleton some time during maturation, if this transformation were to occur between days 8 and 10, the transient increase in band 3 mobility would not be unexpected. It will obviously be important to examine more closely the nature of membrane remodeling that occurs over this time span in order to evaluate whether such discontinuities have a basis in biology.
In conclusion, the data presented above demonstrate that changes in band 3 diffusion occur gradually and continuously during erythroblast differentiation, and do not appear to be associated with any specific stage of erythropoiesis. With the exception of the small increase in mobility between day 8 and day 10 of erythroblast differentiation, the mobility of band 3 decreases monotonically until the mature red cell is formed (see Fig. 2). Thus, values of Dμ and DM both decline as erythroblasts mature (Fig. 3) and the percentage of compartment sizes >100 nm also decreases over the same time frame (Fig. 4). Whether this decline in band 3 mobility derives from a gradual assembly of the spectrin/actin skeleton (Hanspal, et al 1992a, Hanspal and Palek 1987), a protracted attachment of band 3 to adducin (Anong, et al 2009, Nehls, et al 1991) or ankyrin (Hanspal, et al 1992a, Hanspal and Palek 1987), the elimination of membrane surface area devoid of normal skeletal structures during organelle extrusion (Koury, et al 2005, Lee, et al 2004), or the activation of regulatory signals (e.g. kinases) (Jacobs-Helber, et al 2002, Mahmud, et al 2002, Uddin, et al 2004) that prevent formation of a stable membrane skeleton until organelle extrusion is complete will obviously require further scrutiny.
Supplementary Material
Acknowledgements
The authors would like to thank Kati Giger for helpful discussions.
This work was supported by the following grants: NIH grant GM24417-29 to P.S.L.; NSF Grant #0646633 to KR; Giving Tree Foundation to AW.
Footnotes
Author contributions: P.S.L, G.C.K. and A.W. designed the research; G.C.K synthesized the DIDS-biotin and performed the single particle tracking experiments; G.C.K., J.S., G.E.K and K.R. analyzed the diffusion data; A.W provided human erythroblast cultures and interpreted the cellular data; H.L performed the flow cytometry; P.S.L. A.W. and G.C.K wrote the paper.
References
- Anong WA, Franco T, Chu HY, Weis TL, Devlin EE, Bodine DM, An XL, Mohandas N, Low PS. Adducin Forms a Bridge Between the Erythrocyte Membrane and its Cytoskeleton and Regulates Membrane Cohesion. Blood. 2009;114:1904–1912. doi: 10.1182/blood-2009-02-203216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blikstad I, Nelson WJ, Moon RT, Lazarides E. Synthesis and Assembly of Spectrin During Avian Erythropoiesis - Stoichiometric Assembly but Unequal Synthesis of Alpha-Spectrin and Beta-Spectrin. Cell. 1983;32:1081–1091. doi: 10.1016/0092-8674(83)90292-1. [DOI] [PubMed] [Google Scholar]
- Campanella ME, Chu HY, Wandersee NJ, Peters LL, Mohandas N, Gilligan DM, Low PS. Characterization of Glycolytic Enzyme Interactions with Murine Erythrocyte Membranes in Wild-type and Membrane Protein Knockout Mice. Blood. 2008;112:3900–3906. doi: 10.1182/blood-2008-03-146159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H, Langer PJ, Lodish HF. Asynchronous Synthesis of Erythrocyte-Membrane Proteins. Proceedings of the National Academy of Sciences of the United States of America. 1976;73:3206–3210. doi: 10.1073/pnas.73.9.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasis JA, Mohandas N. Erythroblastic Islands: Niches for Erythropoiesis. Blood. 2008;112:470–478. doi: 10.1182/blood-2008-03-077883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasis JA, Coulombel L, Conboy J, McGee S, Andrews K, Kan YW, Mohandas N. Differentiation-Associated Switches in Protein 4.1 Expression - Synthesis of Multiple Structural Isoforms During Normal Human Erythropoiesis. Journal of Clinical Investigation. 1993;91:329–338. doi: 10.1172/JCI116189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Liu J, Heck S, Chasis JA, An XL, Mohandas N. Resolving the Distinct Stages in Erythroid Differentiation Based on Dynamic Changes in Membrane Protein Expression During Erythropoiesis. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:17413–17418. doi: 10.1073/pnas.0909296106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fader CM, Savina A, Sanchez D, Colombo MI. Exosome secretion and red cell maturation: Exploring Molecular Components Involved in the Docking and Fusion of Multivesicular Bodies in K562 Cells. Blood Cells Molecules and Diseases. 2005;35:153–157. doi: 10.1016/j.bcmd.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Hanspal M, Palek J. Synthesis and Assembly of Membrane Skeletal Proteins in Mammalian Red-Cell Precursors. Journal of Cell Biology. 1987;105:1417–1424. doi: 10.1083/jcb.105.3.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanspal M, Hanspal JS, Kalraiya R, Liu SC, Sahr KE, Howard D, Palek J. Asynchronous Synthesis of Membrane Skeletal Proteins During Terminal Maturation of Murine Erythroblasts. Blood. 1992a;80:530–539. [PubMed] [Google Scholar]
- Hanspal M, Hanspal JS, Kalraiya R, Palek J. The Expression and Synthesis of the Ban-3 Protein Initiates the Formation of a Stable Membrane Skeleton in Murine Rauscher-Transformed Erythroid-Cells. European Journal of Cell Biology. 1992b;58:313–318. [PubMed] [Google Scholar]
- Hoffman JF, Wickrema A, Potapova O, Milanick M, Yingst DR. Na Pump Isoforms in Human Erythroid Progenitor Cells and Mature Erythrocytes. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:14572–14577. doi: 10.1073/pnas.222539999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs-Helber SM, Abutin RM, Tian C, Bondurant M, Wickrema A, Sawyer ST. Role of JunB in Erythroid Differentiation. Journal of Biological Chemistry. 2002;277:4859–4866. doi: 10.1074/jbc.M107243200. [DOI] [PubMed] [Google Scholar]
- Johnstone RM. Exosomes Biological Significance: A concise review. Blood Cells Molecules and Diseases. 2006;36:315–321. doi: 10.1016/j.bcmd.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Kang JA, Zhou Y, Weis TL, Liu H, Ulaszek J, Satgurunathan N, Zhou L, van Besien K, Crispino J, Verma A, Low PS, Wickrema A. Osteopontin Regulates Actin Cytoskeleton and Contributes to Cell Proliferation in Primary Erythroblasts. Journal of Biological Chemistry. 2008;283:6997–7006. doi: 10.1074/jbc.M706712200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch PA, Gartrell JE, Gardner FH, Carter JR. Biogenesis of Erythrocyte-Membrane Proteins Invivo Studies in Anemic Rabbits. Biochimica Et Biophysica Acta. 1975;389:162–176. doi: 10.1016/0005-2736(75)90394-6. [DOI] [PubMed] [Google Scholar]
- Kodippili GC, Spector J, Sullivan C, Kuypers FA, Labotka R, Gallagher PG, Ritchie K, Low PS. Imaging of the Diffusion of Single Band 3 Molecules on Normal and Mutant Erythrocytes. Blood. 2009;113:6237–6245. doi: 10.1182/blood-2009-02-205450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koury MJ, Bondurant MC, Rana SS. Changes in Erythroid Membrane-Proteins During Erythropoietin-Mediated Terminal Differentiation. Journal of Cellular Physiology. 1987;133:438–448. doi: 10.1002/jcp.1041330304. [DOI] [PubMed] [Google Scholar]
- Koury MJ, Koury ST, Kopsombut P, Bondurant MC. In vitro Maturation of Nascent Reticulocytes to Erythrocytes. Blood. 2005;105:2168–2174. doi: 10.1182/blood-2004-02-0616. [DOI] [PubMed] [Google Scholar]
- Koury ST, Koury MJ, Bondurant MC. Cytoskeletal Distribution and Function During the Maturation and Enucleation of Mammalian Erythroblasts. Journal of Cell Biology. 1989;109:3005–3013. doi: 10.1083/jcb.109.6.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JCM, Discher DE. Deformation - Enhanced Fluctuations in the Red Cell Skeleton with Theoretical Relations to Elasticity, Connectivity, and Spectrin Unfolding. Biophysical Journal. 2001;81:3178–3192. doi: 10.1016/S0006-3495(01)75954-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JCM, Gimm JA, Lo AJ, Koury MJ, Krauss SW, Mohandas N, Chasis JA. Mechanism of Protein Sorting During Erythroblast Enucleation: Role of Cytoskeletal Connectivity. Blood. 2004;103:1912–1919. doi: 10.1182/blood-2003-03-0928. [DOI] [PubMed] [Google Scholar]
- Mahmud DL, G-Amlak M, Deb DK, Platanias LC, Uddin S, Wickrema A. Phosphorylation of Forkhead Transcription Factors by Erythropoietin and Stem Cell Factor Prevents Acetylation and Their Iinteraction with Coactivator p300 in Erythroid Progenitor Cells. Oncogene. 2002;21:1556–1562. doi: 10.1038/sj.onc.1205230. [DOI] [PubMed] [Google Scholar]
- Moon RT, Lazarides E. Biogenesis of the Avian Erythroid Membrane Skeleton - Receptor-Mediated Assembly and Stabilization of Ankyrin (Goblin) and Spectrin. Journal of Cell Biology. 1984;98:1899–1904. doi: 10.1083/jcb.98.5.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehls V, Drenckhahn D, Joshi R, Bennett V. Adducin in Erythrocyte Precursor Cells of Rats and Humans - Expression and Compartmentalization. Blood. 1991;78:1692–1696. [PubMed] [Google Scholar]
- Nunomura W, Parra M, Hebiguchi M, Sawada K, Mohandas N, Takakuwa Y. Marked Difference in Membrane-Protein-Binding Properties of the Two Isoforms of Protein 4.1R Expressed at Early and Late Stages of Erythroid Differentiation. Biochemical Journal. 2009;417:141–148. doi: 10.1042/BJ20081372. [DOI] [PubMed] [Google Scholar]
- Peters LL, White RA, Birkenmeier CS, Bloom ML, Lux SE, Barker JE. Changing Patterns in Cytoskeletal Messenger-RNA Expression and Protein-Synthesis During Murine Erythropoiesis Invivo. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:5749–5753. doi: 10.1073/pnas.89.13.5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond TD, Chohan M, Barber DL. Turning Cells Red: Signal Transduction Mediated by Erythropoietin. Trends in Cell Biology. 2005;15:146–155. doi: 10.1016/j.tcb.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Ritchie K, Kusumi A. Single-Particle Tracking Image Microscopy. Biophotonics, Pt A. 2003;360:618–634. doi: 10.1016/s0076-6879(03)60131-x. [DOI] [PubMed] [Google Scholar]
- Spivak JL. The anaemia of cancer: Death by a Thousand Cuts. Nature Reviews Cancer. 2005;5:543–555. doi: 10.1038/nrc1648. [DOI] [PubMed] [Google Scholar]
- Tomishige M, Kusumi A. Compartmentalization of the Erythrocyte Membrane by the Membrane Skeleton: Intercompartmental Hop Diffusion of Band 3. Molecular Biology of the Cell. 1999;10:2475–2479. doi: 10.1091/mbc.10.8.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomishige M, Sako Y, Kusumi A. Regulation Mechanism of the Lateral Diffusion of Band 3 in Erythrocyte Membranes by the Membrane Skeleton. Journal of Cell Biology. 1998;142:989–1000. doi: 10.1083/jcb.142.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin S, Ah-Kang J, Ulaszek J, Mahmud D, Wickrema A. Differentiation Stage-Specific Activation of p38 Mitogen-Activated Protein Kinase Isoforms in Primary Human Erythroid Cells. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:147–152. doi: 10.1073/pnas.0307075101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickrema A, Crispino JD. Erythroid and Megakaryocytic Transformation. Oncogene. 2007;26:6803–6815. doi: 10.1038/sj.onc.1210763. [DOI] [PubMed] [Google Scholar]
- Wickrema A, Krantz SB, Winkelmann JC, Bondurant MC. Differentiation and Erythropoietin Receptor Gene-Expression in Human Erythroid Progenitor Cells. Blood. 1992;80:1940–1949. [PubMed] [Google Scholar]
- Wickrema A, Koury ST, Dai CH, Krantz SB. Changes in Cytoskeletal Proteins and Their Messenger-Rnas During Maturation of Human Erythroid Progenitor Cells. Journal of Cellular Physiology. 1994;160:417–426. doi: 10.1002/jcp.1041600304. [DOI] [PubMed] [Google Scholar]
- Woods CM, Boyer B, Vogt PK, Lazarides E. Control of Erythroid-Differentiation - Asynchronous Expression of the Anion Transporter and the Peripheral Components of the Membrane Skeleton in Aev- and S13-Transformed Cells. Journal of Cell Biology. 1986;103:1789–1798. doi: 10.1083/jcb.103.5.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamai L, Burattini S, Luchetti F, Canonico B, Ferri P, Melloni E, Gonelli A, Guidotti L, Papa S, Falcieri E. In vitro Apoptotic Cell Death During Erythroid Differentiation. Apoptosis. 2004;9:235–246. doi: 10.1023/B:APPT.0000018805.63663.a5. [DOI] [PubMed] [Google Scholar]
- Zweig SE, Tokuyasu KT, Singer SJ. Member-Associated Changes During Erythropoiesis - on the Mechanism of Maturation of Reticulocytes to Erythrocytes. Journal of Supramolecular Structure and Cellular Biochemistry. 1981;17:163–181. doi: 10.1002/jsscb.380170207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.