Abstract
Context:
The QT interval variability index (QTVI) is a noninvasive measure of beat-to-beat fluctuations of the QT interval as seen from a single electrocardiographic lead. It represents the relationship between the respective variabilities of the QT and R-R intervals. Recently, the QTVI was demonstrated to be an index of vagal cardiac autonomic modulation in resting conditions.
Objective:
To determine whether QTVI varied in athletes at 48 hours, 1 week, and 2 weeks after a concussive head injury.
Design:
Case series.
Setting:
Testing facility.
Patients or Other Participants:
Three athletes with recent concussions and 3 uninjured athletes with similar demographic factors.
Main Outcome Measure(s):
Continuous 3-lead electrocardiograms were obtained in a seated, resting position over 2 successive weeks. Separate, unpaired t tests were performed to determine whether group-visit differences were present in the QTVI at 48 hours, 1 week, or 2 weeks.
Results:
No demographic differences were present between groups. At 48 hours, the QTVI was greater in the concussion group than in the matched controls. At weeks 1 and 2, the QTVI in the concussion group was lower than at 48 hours and not different from that of the control group.
Conclusions:
Vagal cardiac autonomic modulation, as quantified by the QTVI, appeared to be negatively affected in concussed athletes within 48 hours of injury, resolved within 1 week, and remained at control group levels 2 weeks later. Serial assessments of QTVI may be of clinical utility in identifying suspected cases of acute concussion and may provide helpful information for determining when an athlete can return to play safely.
Keywords: autonomic nervous system, heart, cardiovascular system, return to play
Key Points.
The QT interval variability index was higher at rest in concussed participants than in the control group.
This resting change suggests that vagal cardioautonomic modulation was impaired in recently concussed participants.
The QT interval variability index change resolved within 1 week of injury.
By definition, a sport concussion is a complex pathophysiologic process affecting the brain that is induced by traumatic biomechanical forces.1 The nature of the brain injury results in a number of short-term and, in some cases, long-term symptoms. The primary assessment and management of concussion has included comprehensive evaluations designed to identify symptoms from the cognitive, vestibular, and somatic realms; these evaluations are often used in the subsequent determination of playing status.2 To date, the respective assessment techniques have routinely demonstrated efficacy across multiple groups of athletes.
An underexplored area that may have utility in the identification of acute concussion injury is assessment of cardiovascular autonomic function. Linear and nonlinear computations of heart rate variability (HRV), a measure of the autonomically mediated periodicity in a mean heart rate,3 have demonstrated transient, abnormal autonomic responses to exercise or low-intensity provocations in recently concussed athletes that were not present at rest.4,5 Although these findings are limited in their scope and have not been tested on a large scale, the basis for concussion to affect cardiac autonomic modulation during provoked conditions is established. A shortcoming to the assessment of cardiac autonomic modulation in concussion is that the measures of HRV, including low-frequency (LF) and high-frequency (HF) components and the LF/HF ratio, which represent cardiac/baroreceptor, parasympathetic, and sympathovagal balance,3 respectively, have not differentiated the injured from the uninjured at rest.
Recently, the QT interval variability index (QTVI) was found to be a sensitive index of cardiac autonomic modulation; specifically, at rest it was found to represent vagal activity.6,7 The QTVI is a proportion that quantifies the respective variances of the QT and R-R intervals normalized to their means.8 Thus, the measure compares the autonomic mediated depolarization of the ventricular myocardium and sinoatrial node, which are predominantly under sympathetic and vagal control in resting conditions.9 Deranged cardiac autonomic modulation and resting balance in acute concussion may be shown on the QTVI. The purpose of this exploratory study was to determine whether the QTVI varied in recently concussed athletes at rest when compared with control participants within 48 hours of injury presentation and at 1 week and 2 weeks later.
METHODS
Participants
Three athletes with concussion and 3 in-season, demographically matched (ie, sex, age, height, mass) and sport position–matched (ie, assumption of similar fitness) control participants volunteered for this pilot study. Requirements were that the athlete either have a confirmed diagnosis of concussion by the clinical staff and not be taking any medications with known actions on the cardiovascular system, including fast-acting inhalers (concussion group), or be in season with no acute medical illness and be willing to participate (control group). A player suspected of sustaining a head injury was identified by the sports medicine staff (ie, certified athletic trainers and team physician, on the field or in the clinic) and removed from athletic activity for further clinical evaluation and confirmation using accepted practices of concussion assessment. These included review of somatic symptoms and a computerized neurocognitive test battery (Concussion Resolution Index; HeadMinder, Inc, New York, NY) for comparison with the preseason assessment. The study procedures were approved by the university institutional review board, and all volunteers provided written informed consent before the study began.
Procedures
The initial study observation was completed 48 hours after either concussion or clinical presentation of concussion and repeated at 1 week and 2 weeks after the initial visit. At each visit, 3 electrodes were placed in standardized fashion (ie, modified limb leads were placed distal to the midclavicle bilaterally and precordial lead V5) for 5 minutes of continuous heart rate monitoring. Data from lead II were monitored and collected during test conditions and used for offline analysis. After instrumentation and before data collection, participants remained seated in the upright position at quiet rest for approximately 20 minutes to acclimate to the testing facility. To offset contamination by circadian variability, data collection occurred between 3:00 and 6:30 pm for all study visits. Efforts were made to begin subsequent visits within 30 minutes of the time of the initial visit.
Measures
The electrocardiographic signal was captured at 500 Hz with a 12-bit analog-to-digital converter using a customized Lab VIEW program (National Instruments Corporation, Austin, TX). The digital signal was filtered with a zero-lag, fourth-order Butterworth low-pass filter with default cutoff frequencies of 6 Hz (high-pass filter) and 100 Hz (low-pass filter). The QT interval was calculated as the elapsed time from the onset of the Q wave to the termination of the T wave (QTe). The QTe interval was corrected for heart rate using the Bazett correction formula. The QTVI was calculated from 60 seconds of artifact-free data using WinCPRS software (Absolute Aliens Oy, Turku, Finland) as follows:
where QTev is the QT interval variance, QTem is the mean QT interval, R-Rv is the R-R interval variance, and R-Rm is the mean R-R interval.7
Statistical Analysis
Data are reported as group mean±SD. All data were analyzed using Statview (version 5.0; SAS Institute Inc, Cary, NC). The a priori level of significance was set at P ≤ .05. Independent variables group (control, concussion) and visit (48 hours, 1 week, 2 weeks) were combined to create a concatenate variable with 6 categorical levels (control at 48 hours, control at 1 week, control at 2 weeks, concussion at 48 hours, concussion at 1 week, concussion at 2 weeks). Separate, unpaired t tests were performed to identify demographic (age, height, mass; with regard to sex, both groups consisted of 2 males and 1 female) and group-visit differences in heart rate, corrected heart rate, and QTVI.
RESULTS
Because of differences in symptom presentation and subsequent clinical action, the 3 concussed athletes were evaluated on days 2, 3, and 5, respectively, after each one's suspected date of injury. No group differences were present between demographic variables, heart rate, or QT interval corrected for heart rate at any visit (Table 1). Based on clinical assessment, 2 of the concussed athletes were removed from athletic participation for 10 and 14 days, respectively. The other athlete was excluded from participation for the remainder of the season due to residual neurocognitive symptoms. The present injury was the first concussion for each of the 3 athletes. Control athletes reported no history of concussion and were free of all injuries during the study. Self-reported general and somatic symptoms for each visit are reported (Table 2). At 48 hours, a higher QTVI was observed in the concussion group than in the control group (−0.4±0.4 versus −1.7±0.4, P=0.016). In the concussion group at 1 week (−1.8±0.3 versus −0.4±0.4, P<.01) and 2 weeks (−1.7±0.3 versus −0.4±0.4, P=.013), QTVI was less than at 48 hours. The QTVI in the concussion group at 1 week (−1.8±0.3 versus −1.7±0.3, P=.67) and 2 weeks (−1.7±0.3 versus −1.6±0.1, P=43) was not different from that of the control group (Figure).
Table 1.
Participant Characteristics (Mean ± SD) a
| Characteristic | Concussion Group | Control Group |
| Age, y | 19±2 | 19±2 |
| Height, cm | 178 ± 10 | 180 ± 13 |
| Mass, kg | 80±17 | 81 ± 14 |
| Sex | 2 males, 1 female | 2 males, 1 female |
| Heart rate, beats/min | ||
| 48 h | 58±8 | 62±6 |
| 1 wk | 61±4 | 61±7 |
| 2wk | 66±6 | 58±6 |
| QT interval corrected for heart rate, ms | ||
| 48 h | 377±21 | 369±34 |
| 1 wk | 403±32 | 398±38 |
| 2wk | 353±35 | 396±34 |
aNo differences were noted between groups in any of the characteristics (P>.05)
Table 2.
Self-Reported Symptoms (No. of Reports/No. in Group)
| Time | Concussion Group | Control Group |
| 48 h | Photosensitivity (2/3) | None |
| Headaches (2/3) | ||
| Nausea (2/3) | ||
| Dizziness (3/3) | ||
| Neck/back pain (1/3) | ||
| Vertigo (1/3) | ||
| 1 wk | Photosensitivity (1/3) | None |
| Headaches (2/3) | ||
| Dizziness (1/3) | ||
| Neck/back pain (1/3) | ||
| Vertigo (1/3) | ||
| 2wk | None | None |
Figure.
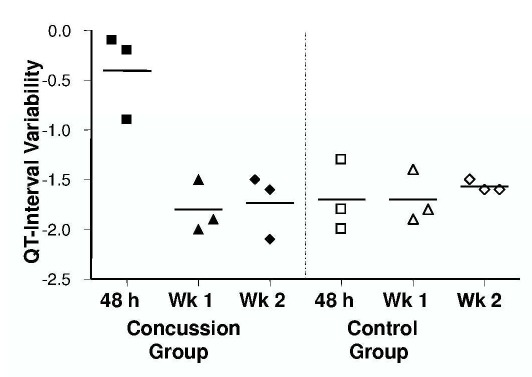
QT interval variability at rest in 3 athletes with concussion and 3 uninjured control participants at 48 hours, 1 week, and 2 weeks after injury. Shapes at each time point represent individual participants; horizontal lines indicate group means.
DISCUSSION
The findings from this exploratory study demonstrate a higher QTVI within 48 hours of injury presentation in recently concussed athletes than in uninjured, matched control participants. The QTVI returned to the control group level within 1 week and remained at this level for the 2-week postinjury follow-up, suggesting that the impairment had resolved. The magnitude of change in the concussion group at 48 hours was profound: 75% greater than the nearest control group or concussion group week 1 or 2 observation. Individually, the smallest QTVI noted at 48 hours was still 36% greater than any other individual observation. To the best of our knowledge, this is the first demonstration of a resting, noninvasive measure of cardiovascular or autonomic function to differentiate a person with acute concussion from one without an injury. These findings suggest that cardiac autonomic modulation is temporarily negatively affected by a concussion, which in turn affects the balance between autonomic mediated depolarization of the ventricular myocardium and the sinoatrial node.8
The QTVI has been used primarily in models of cardiac disease.10–12 Healthy volunteers have negative QTVIs; increases toward a positive value serve as a predictor of fatal ventricular arrhythmias and sudden cardiac death.13 At this time, we do not contend that people with acute or subacute concussion are at risk for aberrant cardiac outcomes, although these results suggest a transient period of cardiac electrical instability. Recently, the QTVI was demonstrated to reflect autonomic state (eg, rest, provoked conditions).6 Those authors determined that the QTVI at rest reflects vagal modulation of heart rate. Conversely, during provocation, the QTVI was directly related to sympathetic modulation. We speculate that our finding of increased QTVI at rest within 48 hours of a concussion is a consequence of vagal dysfunction.
Impaired cardiac autonomic modulation after concussion is probably a functional consequence of short-term changes in cerebral cellular metabolism. According to the Walker et al14 convulsive theory, a concussive insult to the brain results in hyperexcitability due to widespread neuronal membrane depolarization, which has since been characterized by a significant outflow of neurometabolites and excitatory neurotransmitters.15,16 Experimental evidence from brain injury models has demonstrated lateralized cortical autonomic outflow,17,18 such that direct electrical stimulation (producing an efferent output) of the left insular cortex results in cardiac parasympathetic effects (eg, bradycardia and preserved HRV) and regulation of baroreflex sensitivity, whereas stimulation of the right insular cortex results in generalized sympathetic cardiovascular responses (eg, faster heart rate, loss of HRV, peripheral vasoconstriction).19,20 Similarly, left insular cortex lesions (eg, those associated with stroke) increase basal cardiac sympathetic tone and decrease HRV21 and are associated with an increased risk of adverse cardiac outcomes,22 all of which may be explained by ablation of parasympathetic outflow.
Previous reports4,23 of autonomic control in concussed athletes suggest the presence of exaggerated sympathetic control of heart rate during exercise based on group differences in the LF band or the LF/HF ratio. From an applied autonomics perspective, the LF band of HRV has been related to both sympathetic and parasympathetic control of HR.24,25 Recently, the LF band of HRV was shown to reflect the baroreflex and not sympathetic cardiac control in the supine position.26 Empirical evidence and inconsistencies in these outcomes contribute to lingering questions about their accuracy and reliability during exercise.27–32 We must appreciate the fact that elevated heart rates during exercise are due more to metabolic demand than to sympathetic control, let alone autonomic influence. Thus, evidence of exaggerated sympathetic control of heart rate from HRV variables in concussed athletes, although probable, remains debatable and may be more easily described with low-intensity provocations such as an isometric handgrip test, during which heart rate responses are lower than in an exercise assessment.5
From a clinical perspective, the immediate application of our findings is limited, because standard electrocardiographic technology is not equipped to perform the QTVI algorithm. This observation is preliminary and must be replicated on a larger scale before a definitive conclusion about or association between QTVI and concussion can be made. It is possible that the resolution of QTVI occurred at some point between our 48-hour and 1-week observations, but given our design, we were not able to capture the exact time. Investigations with a larger and more diverse sampling are warranted to verify this finding and to facilitate a further understanding of the causes and implications of concussive head trauma on cardiac autonomic modulation. Future efforts to assess cardiac autonomic modulation after concussion should use a resting and low-intensity provocation in a serial manner over the first 10 to 14 days and, if possible, include preseason, preinjury data for comparison. Applying this technique during provoked conditions will require high-resolution digital electrocardiography to minimize signal artifact. This will reduce the need for noise-reduction filters, which can affect the temporal nature of the data. Our concussed athletes did not report any cardiovascular or abnormal symptoms to the research staff during this study; however, the presence of other cognitive and somatic symptoms precluded their involvement in sport-related activities.
CONCLUSIONS
Concussive head trauma appears to induce a short-term period of cardiac electrical instability, with the QTVI increased by 48 hours after injury presentation. With future verification, these findings may have clinical use in identifying suspected cases of acute concussion. Measuring cardiac autonomic modulation has potential use as a complement to the concussive screening battery. Furthermore, serial application of this testing model may be beneficial in the decision-making process for safe return to play.
Acknowledgments
The study was conducted in cooperation with the Department of Veterans Affairs Rehabilitation Research and Development Center of Excellence on the Medical Consequences of Spinal Cord Injury (#B4162C) and the Vidda Foundation.
REFERENCES
- 1.McCrory P, Meeuwisse W, Johnston K. Consensus statement on concussion in sport: the 3rd International Conference on Concussion in Sport held in Zurich, November 2008. J Athl Train. 2009;44(4):434–448. doi: 10.4085/1062-6050-44.4.434. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guskiewicz K, Bruce SL, Cantu RC. National Athletic Trainers' Association position statement: management of sport-related concussion. J Athl Train. 2004;39(3):280–297. et al. [PMC free article] [PubMed] [Google Scholar]
- 3.van Ravenswaaij-Arts CM, Kollee LA, Hopman JC, Stoelinga GB, van Geijn HP. Heart rate variability. Ann Intern Med. 1993;118(6):436–447. doi: 10.7326/0003-4819-118-6-199303150-00008. [DOI] [PubMed] [Google Scholar]
- 4.Gall B, Parkhouse W, Goodman D. Heart rate variability of recently concussed athletes at rest and exercise. Med Sci Sports Exerc. 2004;36(8):1269–1274. doi: 10.1249/01.mss.0000135787.73757.4d. [DOI] [PubMed] [Google Scholar]
- 5.La Fountaine MF, Heffernan KS, Gossett JD, Bauman WA, De Meersman RE. Transient suppression of heart rate complexity in concussed athletes. Auton Neurosci. 2009;148(1–2):101–103. doi: 10.1016/j.autneu.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Piccirillo G, Magri D, Ogawa M. Autonomic nervous system activity measured directly and QT interval variability in normal and pacing-induced tachycardia heart failure dogs. J Am Coll Cardiol. 2009;54(9):840–850. doi: 10.1016/j.jacc.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piccirillo G, Cacciafesta M, Lionetti M. Influence of age, the autonomic nervous system and anxiety on QT-interval variability. Clin Sci (Lond). 2001;101(4):429–438. [PubMed] [Google Scholar]
- 8.Browne KF, Zipes DP, Heger JJ, Prystowsky EN. Influence of the autonomic nervous system on the Q-T interval in man. Am J Cardiol. 1982;50(5):1099–1103. doi: 10.1016/0002-9149(82)90425-8. [DOI] [PubMed] [Google Scholar]
- 9.Mine T, Shimizu H, Hiromoto K. Beat-to-beat QT interval variability is primarily affected by the autonomic nervous system. Ann Noninvasive Electrocardiol. 2008;13(3):228–233. doi: 10.1111/j.1542-474X.2008.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atiga WL, Calkins H, Lawrence JH, Tomaselli GF, Smith JM, Berger RD. Beat-to-beat repolarization lability identifies patients at risk for sudden cardiac death. J Cardiovasc Electrophysiol. 1998;9(9):899–908. doi: 10.1111/j.1540-8167.1998.tb00130.x. [DOI] [PubMed] [Google Scholar]
- 11.Maison-Blanche P, Coumel P. Changes in repolarization dynamicity and the assessment of the arrhythmic risk. Pacing Clin Electrophysiol. 1997;20(10, pt 2):2614–2624. doi: 10.1111/j.1540-8159.1997.tb06111.x. [DOI] [PubMed] [Google Scholar]
- 12.Piccirillo G, Magri D, Matera S. QT variability strongly predicts sudden Cardiac death in asymptomatic subjects with mild or moderate left ventricular systolic dysfunction: a prospective study. Eur Heart J. 2007;28(11):1344–1350. doi: 10.1093/eurheartj/ehl367. [DOI] [PubMed] [Google Scholar]
- 13.Berger RD. QT interval variability: is it a measure of autonomic activity? J Am Coll Cardiol. 2009;54(9):851–852. doi: 10.1016/j.jacc.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Walker AE, Kollros JJ, Case TJ. The physiological basis of concussion. J Neurosurg. 1944;1:103–116. [Google Scholar]
- 15.Giza CC, Hovda DA. The neurometabolic cascade of concussion. J Athl Train. 2001;36(3):228–235. [PMC free article] [PubMed] [Google Scholar]
- 16.Hovda DA, Lee SM, Smith ML. The neurochemical and metabolic cascade following brain injury: moving from animals to man. J Neurotrauma. 1995;12(5):903–906. doi: 10.1089/neu.1995.12.903. [DOI] [PubMed] [Google Scholar]
- 17.Oppenheimer S. The anatomy and physiology of cortical mechanisms of cardiac control. Stroke. 1993;24(12 suppl):I3–I5. [PubMed] [Google Scholar]
- 18.Verberne AJ, Owens NC. Cortical modulation of the cardiovascular system. Prog Neurobiol. 1998;54(2):149–168. doi: 10.1016/s0301-0082(97)00056-7. [DOI] [PubMed] [Google Scholar]
- 19.Oppenheimer SM, Gelb A, Girvin JP, Hachinski VC. Cardiovascular effects of human insular cortex stimulation. Neurology. 1992;42(9):1727–1732. doi: 10.1212/wnl.42.9.1727. [DOI] [PubMed] [Google Scholar]
- 20.Zamrini EY, Meador KJ, Loring DW. Unilateral cerebral inactivation produces differentialleftJright heart rate responses. Neurology. 1990;40(9):1408–1411. doi: 10.1212/wnl.40.9.1408. [DOI] [PubMed] [Google Scholar]
- 21.Oppenheimer SM, Kedem G, Martin WM. Left-insular cortex lesions perturb cardiac autonomic tone in humans. Clin Auton Res. 1996;6(3):131–140. doi: 10.1007/BF02281899. [DOI] [PubMed] [Google Scholar]
- 22.Laowattana S, Zeger SL, Lima JA, Goodman SN, Wittstein IS, Oppen-heimer SM. Left insular stroke is associated with adverse cardiac outcome. Neurology. 2006;66(4):477–483. doi: 10.1212/01.wnl.0000202684.29640.60. [DOI] [PubMed] [Google Scholar]
- 23.Gall B, Parkhouse WS, Goodman D. Exercise following a sport induced concussion. Br J Sports Med. 2004;38(6):773–777. doi: 10.1136/bjsm.2003.009530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213(4504):220–222. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- 25.Pagani M, Lombardi F, Guzzetti S. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res. 1986;59(2):178–193. doi: 10.1161/01.res.59.2.178. [DOI] [PubMed] [Google Scholar]
- 26.Moak JP, Goldstein DS, Eldadah BA. Supine low-frequency power of heart rate variability reflects baroreflex function, not cardiac sympathetic innervation. Heart Rhythm. 2007;4(12):1523–1529. doi: 10.1016/j.hrthm.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arai Y, Saul JP, Albrecht P. Modulation of cardiac autonomic activity during and immediately after exercise. Am J Physiol. 1989;256(1, pt 2):H132–H141. doi: 10.1152/ajpheart.1989.256.1.H132. [DOI] [PubMed] [Google Scholar]
- 28.Borresen J, Lambert MI. Autonomic control of heart rate during and after exercise: measurements and implications for monitoring training status. Sports Med. 2008;38(8):633–646. doi: 10.2165/00007256-200838080-00002. [DOI] [PubMed] [Google Scholar]
- 29.Breuer HW, Skyschally A, Schulz R, Martin C, Wehr M, Heusch G. Heart rate variability and circulating catecholamine concentrations during steady state exercise in healthy volunteers. Br Heart J. 1993;70(2):144–149. doi: 10.1136/hrt.70.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakamura Y, Yamamoto Y, Muraoka I. Autonomic control of heart rate during physical exercise and fractal dimension of heart rate variability. J Appl Physiol. 1993;74(2):875–881. doi: 10.1152/jappl.1993.74.2.875. [DOI] [PubMed] [Google Scholar]
- 31.Perini R, Orizio C, Baselli G, Cerutti S, Veicsteinas A. The influence of exercise intensity on the power spectrum of heart rate variability. Eur J Appl Physiol Occup Physiol. 1990;61(1–2):143–148. doi: 10.1007/BF00236709. [DOI] [PubMed] [Google Scholar]
- 32.Shin K, Minamitani H, Onishi S, Yamazaki H, Lee M. The power spectral analysis of heart rate variability in athletes during dynamic exercise, part II. Clin Cardiol. 1995;18(11):664–668. doi: 10.1002/clc.4960181114. [DOI] [PubMed] [Google Scholar]


