Abstract
Context:
Chronic ankle instability (CAI) is a term used to identify a condition associated with recurrent ankle sprains and persistent symptoms. Balance deficits, evaluated using center-of-pressure (COP) force-plate measurements, have been shown to occur in people with CAI.
Objective:
To determine the differential abilities of selected force-plate postural-control measures to assess CAI.
Design:
Case-control study.
Setting:
Laboratory.
Patients or Other Participants:
A total of 63 individuals with CAI (30 men, 33 women: age = 22.3 ± 3.7 years, height = 169.8 ± 9.6 cm, mass = 70.7 ± 14.3 kg) and 46 healthy controls (22 men, 24 women: age = 21.2 ± 4.1 years, height = 173.3 ± 9.2 cm, mass = 69.2 ± 13.2 kg) volunteered.
Intervention(s):
Participants performed 3 10-second trials of quiet, single-limb stance on a force plate under 2 conditions: eyes open and eyes closed.
Main Outcome Measure(s):
Measures of COP area, COP velocity, COP SD, COP range of excursion, percentage of COP range used, time-to-boundary absolute minimum, time-to-boundary mean of the minima, and time-to-boundary SD of the minima were calculated. All measures with the exception of COP area were calculated in both the mediolateral (ML) and anteroposterior directions. For each measure, a receiver operator curve analysis was created, and the corresponding area under the curve was tested. The optimal diagnostic threshold value for each measure was determined, and the corresponding positive and negative likelihood ratios were calculated.
Results:
Three eyes-closed, single-limb force-plate measures (COP ML SD, ML percentage of COP range used, and time-to-boundary absolute minimum) predicted CAI status. However, all 3 measures had positive likelihood ratios associated with only small shifts in the probability of a patient with a positive test having CAI and negative likelihood ratios associated with very small shifts in the probability of a patient with a negative test not having CAI.
Conclusions:
No single force-plate measure was very effective in predicting if an individual had CAI or not.
Keywords: balance, force plate, time to boundary
Key Points.
Selected single-limb, eyes-closed force-plate measures predicted chronic ankle instability status: SD of mediolateral center of pressure, percentage of mediolateral center-of-pressure excursion, and time-to-boundary mediolateral minimum. However, none of the likelihood ratios were clinically meaningful.
Single-limb, quiet-standing force-plate measures of postural control may be more useful for tracking outcome measures in patients with chronic ankle instability than they are for serving as diagnostic tools.
Chronic ankle instability (CAI) is a clinical condition associated with recurrent ankle sprains and persistent symptoms, such as feelings of “giving way,” and can cause significant ankle pain, loss of function, and limitation of movement.1–3 In previous research,4–6 notable differences in single-leg static balance have been shown between those with and without CAI, but conflicting findings abound. Differences in results may be attributed to the variety of static balance measurements used as well as variations in the definition of CAI.
More than 30 different force-plate measurements have been used to evaluate postural-control deficits related to ankle sprain and CAI.4,7,8 Hertel and Olmsted-Kramer7 evaluated postural control in single-leg stance in participants with and without CAI using traditional center-of-pressure (COP) measures, such as mean COP velocity, SD, range, and percentage of range used, and more novel time-to-boundary (TTB) measures, such as the absolute minimum (smallest of the minima), mean of minimum samples, and SD of the minimum samples. All measures were calculated in the mediolateral (ML) and anteroposterior (AP) directions. The CAI group had lower scores for 5 of the 6 TTB measures. Conversely, using traditional force-plate measures, only AP COP velocity was different between the CAI group and the control group. The authors7 concluded that because the TTB measures showed more differences than the traditional measures, the TTB measures might be better able to detect more subtle postural-control deficits associated with CAI. Briefly, the TTB measures are boundary-relevant measures of postural control that assess only the data points at which a volunteer is closest in time to losing his or her balance unless a postural correction is made, whereas the traditional measures are composites that assess all data points in a trial equally.
Currently the reference standard for determining if a person has CAI is based on subjective information, such as injury history questionnaires and subjective reporting of repetitive sprains and bouts of feelings of “giving way.”9 Because no single definition is considered the “gold standard” for CAI, it can be very difficult to compare results among different studies. Researchers currently use different subjective questions to classify patients, which can introduce biases into the study. Recall bias and individual interpretation of questions can potentially incorrectly include or exclude participants. Although researchers have identified group differences in various postural-control measures between CAI and healthy control groups, we are unaware of any investigators who have sought to determine if deficits on a specific force-plate measure of postural control can predict whether or not an individual has CAI. If a single force-plate measurement can objectively determine CAI status, we could be less reliant on subjective information for determining CAI status and, thus, be better able to identify patients with CAI for future studies. Therefore, the purpose of our study was to identify the best force-plate measure of postural control in single-limb stance to predict CAI status.
METHODS
A case-control study was selected to compare force-plate measures of postural-control performance in single-limb stance in participants with or without CAI. Receiver operator curve (ROC) analysis was used to identify which postural-control measure was best at predicting CAI status.
Participants
A total of 63 individuals with CAI (30 men, 33 women: age = 22.3 ± 3.7 years, height = 169.8 ± 9.6 cm, mass = 70.7 ± 14.3 kg) and 46 healthy controls (22 men, 24 women: age = 21.2 ± 4.1 years, height = 173.3 ± 9.2 cm, mass = 69.2 ± 13.2 kg) volunteered. Some data from these participants have been previously reported,7,10,11 but the data compiled for this study underwent novel analysis. All volunteers were physically active young adults who participated in some form of physical activity for at least 20 minutes per day, 3 days per week. Inclusion criteria for the CAI group were a history of more than 1 ankle sprain, with the original injury occurring at least 12 months prior, and residual symptoms, as quantified by 4 or more yes responses on the Ankle Instability Instrument.12 Additionally, participants had to have self-reported symptoms of disability due to ankle sprains of 90% or less on the Foot and Ankle Disability Index (FADI) and FADI Sport surveys (FADI = 85.56 ± 8.13, FADI Sport = 44.53 ± 25.44).13 Volunteers were excluded if they had sustained a lower extremity injury, including ankle sprain, within the past 6 weeks or had a history of lower extremity surgery, balance disorder, neuropathy, diabetes, or other conditions known to affect balance. If a participant with CAI reported bilateral ankle instability, the self-reported worst limb was used for analysis. Control participants had no history of ankle sprain in either limb (FADI = 100 ± 0, FADI Sport = 100 ± 0). Limbs chosen for analysis of the control group were randomly matched with the injured limbs of the CAI group based on the involved left and right percentages. The study was approved by the university's institutional review board, and all volunteers signed an informed consent form before data collection.
Instruments
Postural control was assessed with the AccuSway Plus force plate (Advanced Mechanical Technology, Inc, Watertown, MA). Three-dimensional force and moment signals arising from the foot-force-plate interface were filtered using a fourth-order, low zero lag, low-pass filter with a cutoff frequency of 5 Hz. The COP was calculated from the force and moment signals through Balance Clinic software (Advanced Medical Technology, Inc) and sampled at a rate of 50 Hz.
Testing Procedures
Participants performed 3 trials of barefoot, quiet, single-limb stance on each leg, with eyes open and then with eyes closed, on the force plate for 10 seconds each. They were instructed to stand as still as possible during testing, with arms folded across their chests, holding the opposite limb at approximately 45° of knee flexion and 30° of hip flexion, in accordance with a previously established protocol.14 All individuals were given 1 practice trial in each condition to familiarize themselves with the task. If they touched down with the opposite limb, made contact with the stance limb, or were unable to maintain standing posture during the 10-second trial, the trial was terminated and repeated.
Data Processing
To calculate TTB measures, the foot was modeled as a rectangle to allow for separation of the AP and ML components of COP, as suggested by van Wegen et al.15 The COP data files were processed using a custom MATLAB software program (The Math Works, Inc, Natick, MA).8 For each COP ML data point, the COP ML position and velocity (depicted with a subscript “i”) were used to calculate TTBML. If the COP MLi was moving medially, the distance between COP MLi and the medial border of the foot was calculated. This distance was then divided by the corresponding velocity of COP MLi to calculate the time it would take the COP MLi to reach the medial border of the foot if it was to continue moving in the same direction with no acceleration or deceleration. If the COP MLi was moving laterally, the distance between COP MLi and the lateral border of the foot was calculated and divided by the corresponding velocity of COP MLi. Thus, a time series of TTBML measures was generated. A time series of corresponding TTBAP measures was similarly generated by determining the time it would take COP APi to reach either the anterior or posterior boundary of the foot.8
A typical TTB series shows a sequence of peaks and valleys, with each valley representing an instant in time when the participant is close, in the time domain, to losing his or her balance if a postural correction is not made. We identified TTB measures at the valleys, or minima, in each trial. The valleys in the data may be viewed as points of potential postural instability, whereas the peaks represent points of postural stability. To identify these minima, derivatives of the TTB measures were computed using first-order finite difference equations. The first derivative values were used to identify minima and maxima, and the second derivative values were used to compute the minima. Because of problems associated with accurately calculating derivatives, the software performed a local search around the derivative-identified minima to precisely locate the minima.8
The traditional COP-based dependent variables included the mean velocity (total COP excursion length in centimeters divided by the time of the trial [10 seconds]), SD of COP excursions, COP area (95% confidence ellipse), range of COP excursions (distance between the minimum and maximum COP positions), and percentage of available range used (range divided by width or length of the foot, respectively) in the ML and AP directions.7 The TTB dependent variables were the absolute minima, mean of the minima, and SD of the minima in the AP and ML directions.
Statistical Analysis
An ROC analysis was used to determine whether or not a measurement was useful for evaluation purposes. The ROC curve used the sensitivity and specificity values of the individual force-plate measurements to determine the diagnostic accuracy of the experimental test, in comparison with the reference standard, for diagnosing the condition of interest. Thus, for each dependent variable, sensitivity and specificity were calculated. The reference standard was CAI status, as determined by our subjective inclusion and exclusion criteria. An ROC curve was constructed for each dependent variable, plotting sensitivity versus 1 – specificity. The score with the combination of highest sensitivity and lowest 1 – specificity, determined to be the most “northwest” point on the ROC curve, was designated as the threshold value. Statistical significance of the predictive ability of the threshold value was assessed by area-under-the-curve (AUC) analysis. An AUC value of 1.0 indicates perfect accuracy of discriminating ankle groups, whereas a value less than or equal to 0.50 indicates poor predictive accuracy.16 Significance was set at P ≤ .05. For each measure, positive and negative likelihood ratios (LRs) were also calculated using the threshold values.
RESULTS
Outcome measures for each group are reported in Table 1. The optimum threshold values, sensitivity, specificity, and LRs for each measure are shown in Table 2. Three measures were predictors of CAI status, based on the ROC analysis: (1) eyes-closed COP SD in the ML direction (Figure 1), (2) eyes-closed percentage of COP range used in the ML direction (Figure 2), and (3) eyes-closed TTB absolute minimum in the ML direction (Figure 3). None of the other measures had significant AUC results (P > .05).
Table 1.
Outcome Measures
| Outcome Measure | Control Mean ± SD | CAI Mean ± SD |
| Center-of-pressure area, cm2 | ||
| Eyes open | 6.49 ± 2.83 | 6.69 ± 3.03 |
| Eyes closed | 25.20 ± 7.26 | 28.46 ±10.66 |
| Center-of-pressure mean velocity, m/s | ||
| Mediolateral, eyes open | 0.99 ± 0.28 | 1.01 ±0.26 |
| Anteroposterior, eyes open | 0.81 ± 0.22 | 0.87 ± 0.26 |
| Mediolateral, eyes closed | 2.05 ± 0.41 | 2.21 ± 0.50 |
| Anteroposterior, eyes closed | 1.92 ± 0.58 | 2.08 ± 0.64 |
| SD of center of pressure, cm2 | ||
| Mediolateral, eyes open | 0.19 ± 0.04 | 0.20 ± 0.04 |
| Anteroposterior, eyes open | 0.26 ± 0.06 | 0.28 ± 0.08 |
| Mediolateral, eyes closed | 0.42 ± 0.06 | 0.45 ± 0.08 |
| Anteroposterior, eyes closed | 0.48 ± 0.11 | 0.53 ±0.13 |
| Center-of-pressure range, cm | ||
| Mediolateral, eyes open | 0.91 ± 0.17 | 0.94 ±0.18 |
| Anteroposterior, eyes open | 1.22 ± 0.28 | 1.30 ±0.37 |
| Mediolateral, eyes closed | 1.67 ±0.18 | 1.75 ±0.27 |
| Anteroposterior, eyes closed | 2.41 ± 0.56 | 2.63 ± 0.68 |
| Range of center of pressure used, % | ||
| Mediolateral, eyes open | 9.50 ± 1.76 | 10.09 ± 2.01 |
| Anteroposterior, eyes open | 4.85 ± 1.11 | 5.17 ± 1.50 |
| Mediolateral, eyes closed | 17.41 ± 1.88 | 18.74 ± 2.65 |
| Anteroposterior, eyes closed | 9.60 ± 2.16 | 10.50 ± 2.72 |
| Time-to-boundary absolute minimum, s | ||
| Mediolateral, eyes open | 1.08 ± 0.27 | 1.10 ± 0.29 |
| Anteroposterior, eyes open | 3.67 ± 1.00 | 3.58 ± 1.17 |
| Mediolateral, eyes closed | 0.52 ± 0.09 | 0.49 ± 0.11 |
| Anteroposterior, eyes closed | 1.58 ± 0.51 | 1.46 ± 0.50 |
| Time-to-boundary mean minimum, s | ||
| Mediolateral, eyes open | 4.02 ± 1.38 | 4.10±1.31 |
| Anteroposterior, eyes open | 12.24 ± 3.59 | 12.26 ± 3.46 |
| Mediolateral, eyes closed | 1.97 ± 0.54 | 1.86 ± 0.51 |
| Anteroposterior, eyes closed | 5.35 ± 1.64 | 4.95 ± 1.40 |
| SD of time-to-boundary minimum, s | ||
| Mediolateral, eyes open | 3.16 ± 1.68 | 3.08 ± 1.25 |
| Anteroposterior, eyes open | 7.76 ± 2.70 | 8.03 ± 2.50 |
| Mediolateral, eyes closed | 1.75 ± 0.68 | 1.70 ± 0.73 |
| Anteroposterior, eyes closed | 3.35 ± 1.19 | 3.08 ± 0.92 |
Abbreviation: CAI, chronic ankle instability
Table 2.
Sensitivity, Specificity, and Likelihood
| Variable | Cutoff Value | Sensitivity | Specificity | Positive Likelihood Ratio | Negative Likelihood Ratio | Area | P Value |
| Center-of-pressure area, cm2 | |||||||
| Eyes open | 5.55 | 0.63 | 0.50 | 1.26 | 0.74 | 0.52 | 0.76 |
| Eyes closed | 31.01 | 0.37 | 0.88 | 2.97 | 0.72 | 0.59 | 0.16 |
| Center-of-pressure mean velocity, m/s | |||||||
| Mediolateral, eyes open | 0.97 | 0.54 | 0.57 | 1.24 | 0.81 | 0.52 | 0.68 |
| Anteroposterior, eyes open | 0.78 | 0.64 | 0.52 | 1.33 | 0.70 | 0.55 | 0.34 |
| Mediolateral, eyes closed | 2.23 | 0.46 | 0.78 | 2.12 | 0.69 | 0.60 | 0.09 |
| Anteroposterior, eyes closed | 2.00 | 0.56 | 0.63 | 1.50 | 0.70 | 0.57 | 0.23 |
| SD of center of pressure, cm2 | |||||||
| Mediolateral, eyes open | 0.18 | 0.78 | 0.41 | 1.33 | 0.54 | 0.56 | 0.26 |
| Anteroposterior, eyes open | 0.23 | 0.78 | 0.37 | 1.23 | 0.60 | 0.57 | 0.23 |
| Mediolateral, eyes closeda | 0.47 | 0.41 | 0.83 | 2.37 | 0.71 | 0.61 | 0.04 |
| Anteroposterior, eyes closed | 0.56 | 0.43 | 0.83 | 2.47 | 0.69 | 0.60 | 0.07 |
| Center-of-pressure range, cm | |||||||
| Mediolateral, eyes open | 0.86 | 0.67 | 0.48 | 1.28 | 0.70 | 0.56 | 0.31 |
| Anteroposterior, eyes open | 1.39 | 0.40 | 0.80 | 2.03 | 0.75 | 0.56 | 0.31 |
| Mediolateral, eyes closed | 1.91 | 0.24 | 0.96 | 5.53 | 0.80 | 0.58 | 0.18 |
| Anteroposterior, eyes closed | 2.75 | 0.40 | 0.83 | 2.28 | 0.73 | 0.59 | 0.11 |
| Range of center of pressure used, % | |||||||
| Mediolateral, eyes open | 9.09 | 0.71 | 0.48 | 1.37 | 0.60 | 0.58 | 0.15 |
| Anteroposterior, eyes open | 5.72 | 0.35 | 0.83 | 2.01 | 0.79 | 0.55 | 0.38 |
| Mediolateral, eyes closeda | 18.80 | 0.48 | 0.78 | 2.19 | 0.67 | 0.65 | 0.01 |
| Anteroposterior, eyes closed | 10.69 | 0.48 | 0.74 | 1.82 | 0.71 | 0.60 | 0.09 |
| Time-to-boundary absolute minimum, s | |||||||
| Mediolateral, eyes open | 0.91 | 0.32 | 0.80 | 1.62 | 0.85 | 0.50 | 0.95 |
| Anteroposterior, eyes open | 3.50 | 0.57 | 0.57 | 1.31 | 0.76 | 0.54 | 0.47 |
| Mediolateral, eyes closeda | 0.46 | 0.52 | 0.80 | 2.67 | 0.59 | 0.63 | 0.03 |
| Anteroposterior, eyes closed | 1.71 | 0.75 | 0.41 | 1.27 | 0.62 | 0.57 | 0.19 |
| Time-to-boundary mean minimum, s | |||||||
| Mediolateral, eyes open | 4.12 | 0.62 | 0.43 | 1.09 | 0.88 | 0.48 | 0.77 |
| Anteroposterior, eyes open | 12.13 | 0.62 | 0.48 | 1.18 | 0.80 | 0.49 | 0.85 |
| Mediolateral, eyes closed | 1.49 | 0.32 | 0.87 | 2.38 | 0.79 | 0.56 | 0.31 |
| Anteroposterior, eyes closed | 5.45 | 0.67 | 0.51 | 1.36 | 0.65 | 0.57 | 0.20 |
| SD of time-to-boundary minimum, s | |||||||
| Mediolateral, eyes open | 1.74 | 0.14 | 0.93 | 2.10 | 0.92 | 0.49 | 0.83 |
| Anteroposterior, eyes open | 8.29 | 0.68 | 0.46 | 1.25 | 0.70 | 0.47 | 0.61 |
| Mediolateral, eyes closed | 1.61 | 0.59 | 0.60 | 1.47 | 0.69 | 0.54 | 0.47 |
| Anteroposterior, eyes closed | 3.60 | 0.71 | 0.41 | 1.22 | 0.69 | 0.55 | 0.38 |
aBold font indicates significance at the .05 level.
Figure 1.
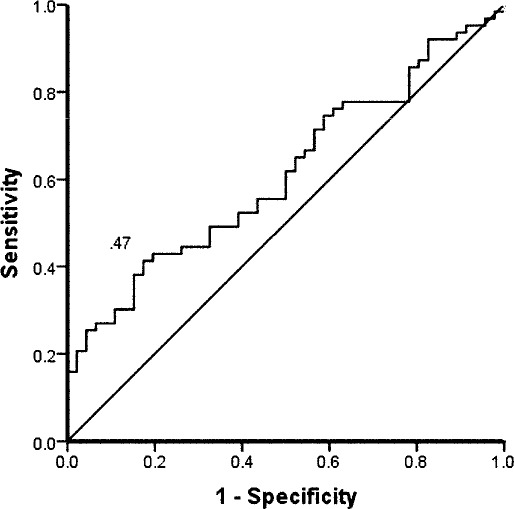
Receiver operating curve for the SD of center of pressure, mediolateral, eyes closed, injured foot. Sensitivity was 0.41, specificity was 0.83, and cutoff point was 0.47 cm2. Positive likelihood ratio value was 2.37, and negative likelihood ratio was 0.71.
Figure 2.
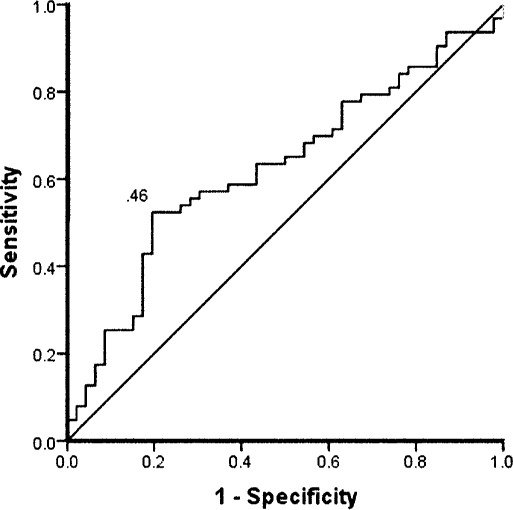
Receiver operating curve for the percentage of center-of-pressure range, mediolateral, eyes closed, injured foot. Sensitivity was 0.48, specificity was 0.78, and cutoff point was 18.80%. Positive likelihood ratio was 2.19, and negative likelihood ratio was 0.67.
Figure 3.
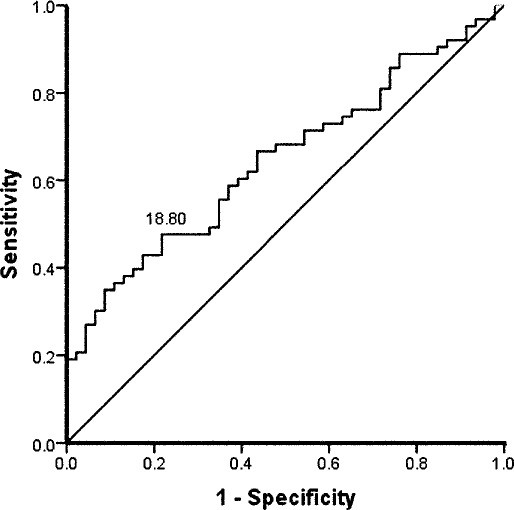
Receiver operating curve for the time-to-boundary minimum, mediolateral, eyes closed, injured foot. Sensitivity was 0.52, specificity was 0.80, and cutoff point was 0.46 seconds. Positive likelihood ratio was 2.67, and negative likelihood ratio was 0.59.
DISCUSSION
Currently, CAI status is most often identified only through subjective measures such as injury history or self-reported symptoms on questionnaires. The purpose of our study was to identify objective force-plate measures that could categorize individuals as having CAI. Although differences were noted in the force-plate measures between the CAI and healthy groups, our most important finding was that no single force-plate measure of postural control in single-limb stance was effective in conclusively predicting whether an individual had CAI or not. This was evident from the highest positive LR of 2.67, a value that is considered to show only small and sometimes clinically important results in posttest probability that the target condition is present.17
All 3 of the significant measures (COP SD, percentage of COP range used, and TTB absolute minimum) were performed with eyes closed and represented ML excursions. This information in and of itself is valuable to clinicians because it shows a pattern of impaired postural control in the ML direction in the absence of vision in patients with CAI. Unfortunately, the positive LRs associated with these 3 measures ranged only from 2.19 to 2.67. A positive finding on a diagnostic test with a positive LR between 2 and 5 is thought to demonstrate a small shift in the probability of a patient having the target disorder and to only sometimes yield clinically important results. Additionally, none of the measures we evaluated had clinically meaningful negative LRs (all were >0.5), and, therefore, no single measurement would be useful in ruling out CAI status.
Currently, several force-plate measurements have been shown7,8 to detect group differences between participants previously screened as CAI and controls. However, the present study shows that those measures cannot be effectively used to determine CAI status as an individual diagnostic tool. Postural-control measures, such as those we evaluated, appear to be more effectively used as outcome measures to track changes in health status (rather than as diagnostic tools).11
The lack of significant results may reflect the possibility that people with CAI use a variety of compensatory mechanisms to maintain balance. We used quiet standing in single-limb stance, which is a relatively easy task for many otherwise-healthy individuals. The sensitivity of traditional COP postural-control measurements has been questioned when detecting deficits associated with CAI.18 In a 2006 study,9 TTB measures appeared to show the most sensitivity in demonstrating differences between groups with and without CAI. In order to determine compensations, more difficult postural tasks, such as time to stabilization,4 should be considered because more demanding tasks may cause greater compensation. Alternately, combining force-plate measures with other evaluations, such as 3-dimensional kinematics of the entire lower quarter, may provide a more comprehensive assessment of balance performance. Such measures may allow us to group individuals by compensation patterns so that force-plate comparisons can be made. Classifying the degree of functional impairment may also reveal different compensation patterns.
Our study has a limitation in relation to spectrum bias because we only compared the balance performance in healthy and CAI participants. We did not compare the CAI individuals with those experiencing other foot and ankle conditions who may also present with balance deficits. Regardless of balance-performance measures, taking a thorough foot and ankle injury history should always remain a central component of the diagnosis of CAI.
In conclusion, measures of eyes-closed COP SD ML, percentage of COP ML range used, and TTB ML absolute minimum predicted CAI status, but based on the LRs associated with these measures, we determined that no single force-plate measure was clinically valuable in predicting CAI status. With regard to CAI, force-plate measures of postural control in single-limb quiet standing may be more effective as a means of tracking outcome measures than as diagnostic tools.
REFERENCES
- 1.Hertel J. Functional anatomy, pathomechanics, and pathophysiology of lateral ankle instability. J Athl Train. 2002;37(4):364–375. [PMC free article] [PubMed] [Google Scholar]
- 2.Hubbard TJ, Kramer LC, Denegar CR, Hertel J. Contributing factors to chronic ankle instability. Foot Ankle Int. 2007;28(3):343–354. doi: 10.3113/FAI.2007.0343. [DOI] [PubMed] [Google Scholar]
- 3.Kirby AB, Beall DP, Murphy MP, Ly JQ, Fish JR. Magnetic resonance imaging findings of chronic lateral ankle instability. Curr Prob Diagn Radiol. 2005;34(5):196–203. doi: 10.1067/j.cpradiol.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Ross SE, Guskiewicz KM, Yu B. Single-leg jump-landing stabilization times in subjects with functionally unstable ankles. J Athl Train. 2005;40(4):298–304. [PMC free article] [PubMed] [Google Scholar]
- 5.Rozzi SL, Lephart SM, Sterner R, Kuligowski L. Balance training for persons with functionally unstable ankles. J Orthop Sports Phys Ther. 1999;29(8):478–486. doi: 10.2519/jospt.1999.29.8.478. [DOI] [PubMed] [Google Scholar]
- 6.Willems T, Witvrouw E, Verstuyft J, Vaes P, De Clercq D. Proprioception and muscle strength in subjects with a history of ankle sprains and chronic instability. J Athl Train. 2002;37(4):487–493. [PMC free article] [PubMed] [Google Scholar]
- 7.Hertel J, Olmsted-Kramer LC. Deficits in time-to-boundary measures of postural control with chronic ankle instability. Gait Posture. 2007;25(1):33–39. doi: 10.1016/j.gaitpost.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Hertel J, Olmsted-Kramer LC, Challis JH. Time-to-boundary measures of postural control during single leg quiet standing. J Appl Biomech. 2006;22(1):67–73. doi: 10.1123/jab.22.1.67. [DOI] [PubMed] [Google Scholar]
- 9.Caulfield B, Monaghan K, Kaminski T, Hertel J. Do we need to standardize the way in which we classify those with chronic ankle instability? J Orthop Sports Phys Ther. 2006;36(11) [Google Scholar]
- 10.McKeon PO, Hertel J. Spatiotemporal postural control deficits are present in those with chronic ankle instability. BMC Musculoskeletal Disord. 2008;9:76. doi: 10.1186/1471-2474-9-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKeon PO, Ingersoll CD, Kerrigan DC, Saliba E, Bennett BC, Hertel J. Balance training improves function and postural control in those with chronic ankle instability. Med Sci Sports Exerc. 2008;40(10):1810–1819. doi: 10.1249/MSS.0b013e31817e0f92. [DOI] [PubMed] [Google Scholar]
- 12.Docherty CL, Gansneder BM, Arnold BL, Hurwitz SR. Development and reliability of the ankle instability instrument. J Athl Train. 2006;41(2):154–158. [PMC free article] [PubMed] [Google Scholar]
- 13.Hale SA, Hertel J. Reliability and sensitivity of the Foot and Ankle Disability Index in subjects with chronic ankle instability. J Athl Train. 2005;40(1):35–40. [PMC free article] [PubMed] [Google Scholar]
- 14.Hertel J, Buckley WE, Denegar CR. Serial testing of postural control after acute lateral ankle sprain. J Athl Train. 2001;36(4):363–368. [PMC free article] [PubMed] [Google Scholar]
- 15.van Wegen EE, van Emmerik RE, Riccio GE. Postural orientation: age-related changes in variability and time-to-boundary. Hum Mov Sci. 2002;21(1):61–84. doi: 10.1016/s0167-9457(02)00077-5. [DOI] [PubMed] [Google Scholar]
- 16.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 17.Sackett D, Haynes R, Tugwell P, Guyatt G. Clinical Epidemiology: A Basic Science for Clinical Medicine. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1991. pp. 119–139. [Google Scholar]
- 18.Holme E, Magnusson SP, Becher K, Bieler T, Aagaard P, Kjaer M. The effect of supervised rehabilitation on strength, postural sway, position sense and re-injury risk after acute ankle ligament sprain. Scand J Med Sci Sports. 1999;9(2):104–109. doi: 10.1111/j.1600-0838.1999.tb00217.x. [DOI] [PubMed] [Google Scholar]


