Abstract
Context:
In a recent study, we were unable to measure lidocaine in the human calf at a 5-mm depth via iontophoresis. We surmised that this might be due to a lack of epinephrine in the compound. Because epinephrine is a vasoconstrictor, it might allow the drug to pass beyond the capillaries and be delivered to the deeper tissues.
Objective:
To determine if iontophoresis could deliver lidocaine with epinephrine 5 mm under the surface of human skin, as measured by microdialysis.
Design:
Descriptive laboratory study.
Setting:
Therapeutic modalities research laboratory.
Patients or Other Participants:
Ten volunteers (5 males, 5 females; age, 15–28 years) with less than 5 mm of adipose tissue in the area we measured and with no allergies to lidocaine participated. The measurement area had been free of any injury, swelling, or infection for at least 3 months before the study.
Intervention(s):
We inserted a microdialysis probe 0.5 cm under the skin of the right lower leg. Next, microdialysis was performed through this area for 60 minutes, which allowed local skin blood flow to return to baseline. We then performed iontophoresis at 40 mA/min using 2 mL of 2% lidocaine. Iontophoresis was performed over this area for 10.5 minutes to collect the lidocaine samples. After this stage, the electrode was left in place for another 50 minutes for a total of 60 minutes.
Main Outcome Measure(s):
The samples of the drug were analyzed via reverse-phase high-performance liquid chromatography (RP-HPLC) in the chemistry department.
Results:
The RP-HPLC analysis confirmed the presence of lidocaine in all 10 participants. The mean concentration of lidocaine detected at the 5-mm depth was calculated as 3.63 mg/mL (greater than 18% of delivered concentration).
Conclusions:
We found that 2% lidocaine can be delivered up to 5 mm below the surface of the skin when the drug compound contains epinephrine and when passive delivery occurs for at least 50 minutes after the active delivery has terminated.
Keywords: drug delivery, diffusion, passive diffusion
Key Points.
Iontophoresis can be absorbed in tissues to a depth of at least 5 mm.
Microdialysis is an effective tool with which to measure absorption of iontophoretic medication.
Iontophoresis is a method of local transfer (phoresis) or delivery of ionized (ionto) medicated and nonmedicated solutions into the skin and through local microcirculation. It involves the use of electric energy for ion transfer through the skin and is implemented using the traditional wire programmable devices and newer wireless or nonprogrammable patches.1 Iontophoresis is used widely in physical medicine and rehabilitation. In 2009, one company reported more than 3.8 million treatments (Empi administrator, oral communication, 2009).
Researchers have doubts about the success of iontophoresis in delivering medication to the desired treatment area. One of their major concerns involves the ability of the medication to penetrate the skin and reach the tendon, muscle, bursa, etc.2–4 The literature includes studies in which investigators have measured delivery depths as shallow as 3 mm to as deep as 15 mm into human tissue5–8 and as deep as 17 mm in monkeys.9
One way of determining how much drug compound has reached the target tissue from iontophoresis is to use microdialysis. Microdialysis is a method of studying extracellular fluid composition and response to exogenous agents using an approximately 30-gauge tubular probe with a dialysis membrane and fluid flow rates of 1 to 3 μL/min into tissues.10 It includes the sampling of extracellular fluid either to assess the concentration of local chemical components or to perfuse drugs directly into small clusters of cells. Neuroscientists use microdialysis to study the release of neurotransmitters in the brain,11 exercise physiologists to measure the rate of sweating,12 and medical doctors to deliver drugs to organs and to measure blood flow.11 We have used microdialysis to measure drug delivery via iontophoresis.
As we have pointed out, researchers have reported a wide range of depths that iontophoresis has reached, but not all measuring techniques were optimal.3–5,8,9 Reaching the target tissue is critical when the goal is to deliver medication to the inflamed or injured site. Microdialysis has been shown to be effective in measuring chemical compounds in tissues. Therefore, the purpose of our study was to determine if iontophoresis using the following variables could deliver 2% lidocaine with epinephrine 5 mm past the skin's surface: 40 mA/min, 10 minutes, 50 minutes posttreatment diffusion time. We hypothesized that the introduction of epinephrine into the lidocaine and the longer passive delivery of the drug would result in the drug being absorbed in the tissues at depths equal to 5 mm.
METHODS
Design
This descriptive study included the independent variable of depth and the dependent variable of volume of the drug compound recovered at a depth of 5 mm.
Participants
Five male and 5 female (ages, 15–28 years) volunteers participated in the study. The treatment area had been free of any injury, swelling, or infection for at least 3 months before the study, and no participant had an allergy to lidocaine. Participants had less than 5-mm skin thickness in the treatment area. All participants provided written informed consent, and the study was approved by the institutional review board at Brigham Young University.
Instruments
We used an iontophoresis unit (ActivaTek, Salt Lake City, UT) to deliver the lidocaine with epinephrine. One of the 2 electrodes (Trivarion; ActivaTek) carried the drug to the treatment site, and the other was used as a dispersive electrode. We used an infusion pump (model Pump 11 VPF; Harvard Apparatus, Holliston, MA) to perform the microdialysis. The microdialysis probes were manufactured in our university's laboratory (Figure 1, and see figure 3 in Coglianese et al15)
Figure 1.
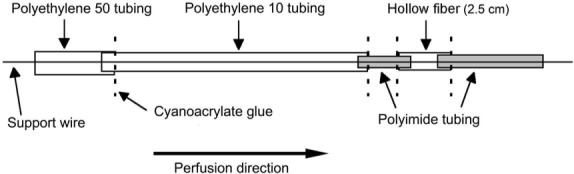
Construction of the microdialysis probe used to collect the drug compound.
Procedures
We used ethylene oxide to gas sterilize each probe before the study. The chief data collector (M.C.) donned surgical gloves before preparing the site. The skin was cleaned with a povidone-iodine swab and an alcohol preparation wipe. Before handling the spinal needle or the probe, the data collector also donned sterile gloves. A 27-gauge spinal needle was placed 5 mm under the skin at the thickest aspect of the gastrocnemius muscle of the right leg. Five centimeters of skin separated the entrance and exit sites. The microdialysis probe was inserted through the guide cannula, which was placed horizontally in the dermis. Next, the cannula was removed from the leg, and the probe was left in place. After placement, microdialysis was performed through the leg for 60 minutes to allow for a recovery period because of probe insertion and related soft tissue trauma. This permitted local skin blood flow to return to base-line. The probe was perfused continuously with 0.9% bacteriostatic saline at a rate of 5 μL/min with the infusion pump.
Drug Delivery.
After probe insertion and the recovery period, the drug-delivery electrode was prepared with 2 mL of 2% lidocaine (positive charge) and epinephrine solution. It was placed on the skin directly over the microdialysis probe. The 14-cm2 delivery electrode had a 2.0-mL reservoir. The larger dispersive electrode (negative charge) was 37 cm2 and was placed 6 in (15.24 cm) proximal to the drug delivery site on the same leg. After the electrode leads were affixed to the skin, the unit was turned on (Figure 2). The iontophoresis (activator) unit was set at a current charge of 4 mA/min to deliver a total of 40 mA in approximately 10.5 minutes. The unit ramped up the current for 30 seconds until it reached 4 mA. At the end of the treatment, the activator beeped and automatically shut off. During the treatment, perfusate from the intramuscular microdialysis probe was collected in a collecting vial (1.5-mL microcentrifuge, Safe-Lock tube; Eppendorf, Hamburg, Germany) for analysis. At the conclusion of the treatment, the dispersive electrode was removed from the participant, and the treatment site was cleaned of any residue; however, the active electrode remained in place for 50 minutes for passive delivery. The intramuscular microdialysis probe was removed carefully immediately after the treatment ended, and portal sites were treated with triple antibiotic ointment and, if necessary, were covered with a self-adhesive bandage. Before leaving the laboratory, each participant received a basic wound-care guide and our contact information.
Figure 2.
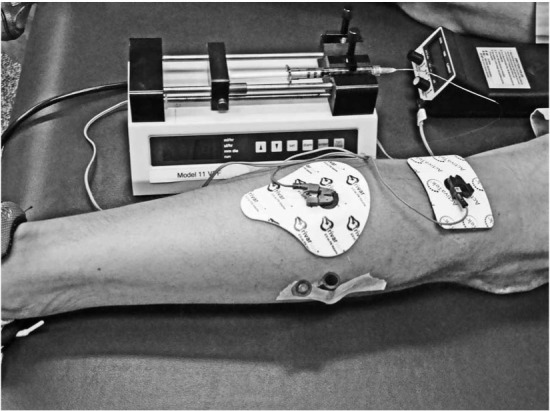
The experimentation setup displaying the microdialysis probe, epindorph container, iontophoresis unit, and microdialysis device.
Data Analysis
The samples, which were taken during and 50 minutes after the iontophoresis treatment, were analyzed via reverse-phase high-performance liquid chromatography (RP-HPLC) in the chemistry department. A previously established protocol for detection and quantification of lidocaine using RP-HPLC was followed.13 The 2% lidocaine standard and several diluted concentrations (1%, 0.5%, and 0.25%) also were analyzed via RP-HPLC. The results from these various concentrations were used to generate a standard curve. Our objective was not only to determine if the drug penetrated the skin and tissues up to 5 mm deep but also to quantify the percentage of lidocaine at that depth. The percentage of lidocaine in our samples was calculated using the line of best fit from the standard curve (R2 = 0.9964). The results from the 10 participants were averaged, and the mean was used in the equation (y = 22583Ln(x) + 38281, where y is milliabsorbance units (mAU) and x is the percentage of lidocaine in the standard solution) from the standard curve to calculate the percentage of lidocaine detected.
Statistical Analysis
The percentage of lidocaine detected was analyzed using 1-way analysis of variance to determine if it was different from zero. The α level was set a priori at .05. We used SPSS (version 17; SPSS Inc, Chicago, IL) for statistical analysis.
RESULTS
The RP-HPLC analysis confirmed the presence of lidocaine in all 10 participants (Figure 3). The mean area under the lidocaine curve for the 10 participants sampled was 15440.79 mAU/s, with an SD of 550.76 mAU/s and a coefficient of variation of 3.6%. Using the mean area under the lidocaine peak for all 10 participants and the equation from the standard curve, the mean volume of lidocaine recovered at the 5-mm depth was calculated as 3.63 mg/mL (greater than 18% of the delivered 20 mg/mL). This recovered volume was determined to be different from 0 (F1,19 = 1.84333, P = .001).
Figure 3.
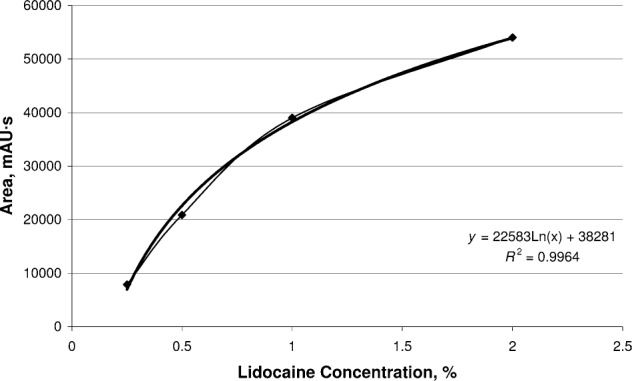
Standard curve for 2% lidocaine with epinephrine.
DISCUSSION
We detected 3.63 mg/mL mean volume of the 2% lidocaine at a 5-mm depth in the tissue. Because lidocaine is known to have therapeutic effects at less than 1.0 μg/mL, it might have a therapeutic effect at a depth of 5 mm when it is delivered at a 2% concentration with epinephrine (40 mA/min dosage, with electrode left in place 50 minutes posttreatment).14
Addition of Epinephrine
In a previous study, we detected 1% lidocaine using microdialysis at a depth of 3 mm below the surface of the skin, but we did not detect it at 5 mm.15 During the present study, we found lidocaine at a 5-mm depth below the surface of the skin. We attribute these results to 3 things that we did differently compared with the previous study: (1) epinephrine (a vasoconstrictor) was included in the lidocaine compound; (2) the drug-delivery electrode remained in place for 50 minutes after the treatment, as opposed to 30 minutes; and (3) a higher concentration of lidocaine (2%) was used.
Including epinephrine in the drug solution is important because it causes vasoconstriction of the blood vessels. We believe that lidocaine has a difficult time passing through the superficial layers of the tissue owing to absorption in the superficial bloodstream. Introducing epinephrine, a vasoconstrictor, might allow drugs to pass through the bloodstream and be absorbed in the deeper tissues (such as at 5 mm).
One of the limitations in our previous study15 was the absence of epinephrine in the lidocaine. In that study we hypothesized that because epinephrine causes vasoconstriction, it might have enabled the drug to reach the microprobe at the 5-mm depth,6,7,16 and we stated that researchers should consider using lidocaine with epinephrine in future studies involving lidocaine detection at depths greater than 3 mm. We have corrected for that limitation and believe that few limitations occurred in this study.
The molecular weight of the drug also should be considered. Epinephrine weighs 183.21 Da, whereas 2% lidocaine weighs 288.82 Da. This means that much of the epinephrine would pass through the skin and have the vasoconstriction action on the capillaries before the lidocaine passed into the area.
Passive Delivery of the Drug Posttreatment
In a related study, Smutok et al3 did not show any measure-able amounts of dexamethasone or dexamethasone phosphate in the antecubital veins of humans after iontophoresis treatment of the wrist. Variables were 2.5 mL or 4 mg/mL at 4 mA for 10 minutes and 4 mA for 20 minutes. The antecubital vein is superficial, and some might surmise that the drug would be delivered to this depth. However, others might argue that the drug reached the target tissues without being absorbed into the bloodstream. Additional possible explanations for the results of Smutok et al could be that the drug electrode was not left on long enough after the treatment or that drug detection was performed too quickly posttreatment.
On the basis of their model, Anderson et al7 suggested that for equivalent iontophoretic dosages, the key element that enables passive diffusion is the length of time for which the drug is applied. According to them,7 it is the time the drug is applied rather than the current amplitude that determines penetration depth. Our results concurred with their premise. However, we also believe that passive delivery of the medication posttreatment should be considered. In our previous study,15 in which we did not discover any lidocaine at the 5-mm depth, we only left the electrode on for 30 minutes posttreatment. In this study, we increased the passive diffusion time posttreatment by 20 minutes.
Use of 2% Lidocaine
Researchers3–5,8,9,14,17 have suggested that drug concentrations used for iontophoresis usually range from 2% to 5% aqueous solution or ointment. The drug solution should contain relatively low concentrations of medication. According to Henley14 and Ciccone,18 an increased concentration does not appear to increase the amount of the drug delivered; yet we found the opposite to be true. In our previous study,15 in which we used 1 % lidocaine, we did not find the drug at the 5-mm depth in the tissues; however, when we used 2% lidocaine in this study, we collected it at the 5-mm depth. Our methods are novel because the drug actually is collected in a microdialysis probe as the drug passes through the tissue. However, we still believe that passive delivery posttreatment and the effect of epinephrine on the local vasculature played major roles in allowing us to collect the drug at this depth.
As stated earlier, information provided by an administrator at the leading company indicated that sufficient devices and electrodes were sold to provide nearly 4 million treatments in 2009. Given this large number, investigators should determine if iontophoresis indeed delivers the medication deep enough to reach the injured site and have an effect.
Methods of Determining Depth
The patients in a study by Gurney et al8 were undergoing anterior cruciate ligament repairs using the semitendinosis. Thus, the investigators measured whether the dexamethasone reached the tissue by dividing skinfold measurements, which had been taken immediately before the study, by 2. Their skinfold range was 6 to 30 mm (3–15 mm when divided by 2). Costello and Jeske6 measured 10 mm in the gluteus muscles of rabbits to find the numbing effect of lidocaine. Anderson et al7 used an agarose gel model to measure doses of dexamethasone 12 mm deep in the gel. Wieder19 used acetic acid iontophoresis to decrease the size of myositis ossificans of the quadriceps muscle to show the positive effects of iontophoresis. Both Baskurt et al20 and Demirtaş and Oner17 were able to decrease the symptoms of lateral epicondylitis in patients when using iontophoresis with naproxen and sodium diclofenac, respectively. Each of these studies can be categorized as ranging from the most objective8 to the most subjective.17,20 Our previous study15 and present studies using microdialysis are considered objective because we measured the amount of drug that actually was absorbed in the tissues and reached the target site.
CONCLUSIONS
Much conflicting research exists regarding the effectiveness and depth of drug delivery via iontophoresis. Some of this conflict is due to less-than-ideal treatment variables, sampling, and research methods. However, using microdialysis, we determined that 2% lidocaine combined with epinephrine could be detected at a 5-mm depth in the human leg. We discovered that the drug penetrated to the depth at a concentration of greater than 18% of the original drug concentration. Based on our results, we suggest that 2% lidocaine can be delivered up to 5 mm below the skin's surface when the drug compound contains epinephrine and when passive delivery occurs for at least 50 minutes after the active delivery has terminated (variables: 40 mA/min, 4 mA, 10.5 minutes). Future research could involve testing topical patches; other frequencies that might more easily penetrate the skin; other depths; and other medications, such as the more commonly used dexamethasone.18
REFERENCES
- 1.Belanger AY. Therapeutic Electrophysical Agents: Evidence Behind Practice. 2nd ed. Baltimore, MD: Wolters Kluwer/Lippincott Williams & Wilkins; 2010. p. 251. [Google Scholar]
- 2.Knight KL, Draper DO. Therapeutic Modalities: The Art and Science. Baltimore, MD: Wolters Kluwer/Lippincott Williams & Wilkins; 2007. pp. 170–175. [Google Scholar]
- 3.Smutok MA, Mayo MF, Gabaree CL, Ferslew KE, Panus PC. Failure to detect dexamethasone phosphate in the local venous blood postcathodic iontophoresis in humans. J Orthop Sports Phys Ther. 2002;32(9):461–468. doi: 10.2519/jospt.2002.32.9.461. [DOI] [PubMed] [Google Scholar]
- 4.Aygül R, Ulvi H, Karatay S, Deniz O, Varoglu AO. Determination of sensitive electrophysiologic parameters at follow-up of different steroid treatments of carpal tunnel syndrome. J Clin Neurophysiol. 2005;22(3):222–230. [PubMed] [Google Scholar]
- 5.Blackford J, Doherty TJ, Fernslew KE, Panus PC. Iontophoresis of dexamethasone-phosphate into the equine tibiotarsal joint. J Vet Pharmacol Ther. 2000;23(4):229–236. [PubMed] [Google Scholar]
- 6.Costello CT, Jeske AH. Iontophoresis: applications in transdermal medication delivery. Phys Ther. 1995;75(6):554–563. doi: 10.1093/ptj/75.6.554. [DOI] [PubMed] [Google Scholar]
- 7.Anderson CR, Morris RL, Boeh SD, Panus PC, Sembrowich WL. Effects of iontophoresis current magnitude and duration on dexamethasone deposition and localized drug retention. Phys Ther. 2003;83(2):161–170. [PubMed] [Google Scholar]
- 8.Gurney B, Wascher D, Eaton L, Benesh E, Lucak J. The effect of Skin thickness and time in the absorption of dexamethasone in human tendons using iontophoresis. J Orthop Sports Phys Ther. 2008;38(5):238–245. doi: 10.2519/jospt.2008.2648. [DOI] [PubMed] [Google Scholar]
- 9.Glass JM, Stephen RL, Jacobson SC. The quantity and distribution of radiolabeled dexamethasone delivered to tissue by iontophoresis. Int J Dermatol. 1980;19(9):519–525. doi: 10.1111/j.1365-4362.1980.tb00380.x. [DOI] [PubMed] [Google Scholar]
- 10.Stedman's Medical Dictionary for the Health Professions and Nursing. 5th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2005. p. 922. [Google Scholar]
- 11.Oshima T, Kashiki K, Toyooka H, Masuda A, Amaha K. Cutaneous iontophoretic application of condensed lidocaine. Can J Anaesth. 1994;41(8):677–679. doi: 10.1007/BF03015620. [DOI] [PubMed] [Google Scholar]
- 12.Lee K, Mack GW. Role of nitric oxide in methacholine-induced sweating and vasodilation in human skin. J Appl Physiol. 2006;100(4):1355–1360. doi: 10.1152/japplphysiol.00122.2005. [DOI] [PubMed] [Google Scholar]
- 13.Liawruangrath S, Liawruangrath B, Pibool P. Simultaneous determination of tolperisone and lidocaine by high performance liquid chromatography. J Pharm Biomed Anal. 2001;26(5–6):865–872. doi: 10.1016/s0731-7085(01)00462-9. [DOI] [PubMed] [Google Scholar]
- 14.Henley EJ. Transcutaneous drug delivery: iontophoresis, phonophoresis. Crit Rev Phys Rehabil Med. 1991;2:139–151. [Google Scholar]
- 15.Coglianese M, Draper DO, Shurtz J, Mark G. Microdialysis and delivery of iontophoresis-driven lidocaine into the human gastrocnemius muscle. J Athl Train. 2011;46(3):270–276. doi: 10.4085/1062-6050-46.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banga AK, Panus PC. Clinical applications of iontophoretic devices in rehabilitation medicine. Crit Rev Phys Rehabil Med. 1998;10(2):147–179. [Google Scholar]
- 17.Demirtaş RN, Oner C. The treatment of lateral epicondylitis by iontophoresis of sodium salicylate and sodium diclofenac. Clin Rehabil. 1998;12(1):23–29. doi: 10.1191/026921598672378032. [DOI] [PubMed] [Google Scholar]
- 18.Ciccone CD. Does acetic acid iontophoresis accelerate the resorption of calcium deposits in calcific tendinitis of the shoulder? Phys Ther. 2003;83(1):68–74. [PubMed] [Google Scholar]
- 19.Wieder DL. Treatment of traumatic myositis ossificans with acetic acid iontophoresis. Phys Ther. 1992;72(2):133–137. doi: 10.1093/ptj/72.2.133. [DOI] [PubMed] [Google Scholar]
- 20.Baskurt F, Ozcan A, Algun C. Comparison of effects of phonophoresis and iontophoresis of naproxen in the treatment of lateral epicondylitis. Clin Rehabil. 2003;17(1):96–100. doi: 10.1191/0269215503cr588oa. [DOI] [PubMed] [Google Scholar]


