Abstract
Objective
Preterm labor (PTL) has been associated with an increased thrombin generation in the maternal circulation and amniotic fluid. Tissue factor (TF) is a potent initiator of the coagulation cascade, which can trigger the hemostatic system to generate thrombin. The aims of this study were to determine whether spontaneous PTL with intact membranes is associated with changes in the maternal plasma concentrations and activity of TF and tissue factor pathway inhibitor (TFPI).
Methods
This cross-sectional study included women in the following groups: 1) normal pregnancies (n=86); 2) term pregnancies in spontaneous labor (TIL) (n=67) and not in labor (TNL) (n=88); and 3) patients with spontaneous PTL and intact membranes (n=136) that were classified into three sub-groups: a) PTL without intra-amniotic infection and/or inflammation (IAI) who delivered at term (n=49), b) PTL without IAI who delivered preterm (n=54), and c) PTL with IAI who delivered preterm (n=33). Plasma concentrations of TF and TFPI were measured by ELISA, and their activity was measured by chromogenic assays. Non-parametric statistics were used for analysis.
Results
1) Among women at term, those with spontaneous labor had a higher median maternal plasma TF and a lower median TFPI concentrations than that of those without labor. 2) Patients with PTL had a significantly lower median maternal plasma TFPI concentration than that of normal pregnant women, regardless of the presence of IAI. 3) There was no significant differences in the median maternal plasma TF concentrations between patients with a normal pregnancy and those with PTL. 4) In contrast, the median TF activity was higher among patients with PTL than in women with normal pregnancies, regardless of the presence of IAI or preterm delivery. 5) However, maternal plasma TFPI activity did not differ among the study groups.
Conclusion
Women with preterm parturition, in contrast to those in labor at term, have a higher TF activity and a lower TFPI concentration, without a significant change in the median maternal plasma TF concentration. These observations suggest that the increased thrombin generation reported in patients with PTL may be the result of activation of the extrinsic pathway of the coagulation cascade. In addition, the increased thrombin generation reported in patients with PTL could be due to insufficient anti-coagulation, as reflected by the low maternal plasma TFPI concentration.
Keywords: Thrombin generation, intra-amniotic inflammation, preterm delivery, parturition, TFPI/TF ratio
INTRODUCTION
Preterm labor (PTL) with intact membranes is one of the great obstetrical syndromes and is the clinical manifestation of different underlying mechanisms[1], including the following: intrauterine infection[2–5], uteroplacental ischemia[6–9], uterine over-distention[10–12], cervical disease[13–17], abnormal allograft reaction[7], allergic phenomena[18–20], and endocrine disorders[21,22]. In addition, PTL is associated with an increased thrombin generation. Indeed, patients with PTL have a higher median maternal plasma concentration of thrombin-antithrombin (TAT) III complexes than women with a normal pregnancy[23,24] that is not associated with a history of vaginal bleeding during gestation or the presence of intra-amniotic infection/inflammation (IAI)[23]. Thus, the activation of the maternal coagulation cascade may be associated with an episode of PTL regardless of its underlying mechanism.
Systemic maternal inflammation, which has been reported in patients with PTL[25–28], is associated with increased thrombin generation. Indeed: 1) The administration of tumor necrosis factor (TNF)-α to healthy volunteers induces thrombin generation and the activation of coagulation[29]; 2) Proinflammatory cytokines, such as IL-1β and TNF-α, increase mRNA and protein expression of TF by monocytes[30–37] and macrophages[38] and 3) The blocking of interleukin (IL)-6 by specific antibodies attenuates the activation of coagulation in a chimpanzee model of endotoxemia[39]. This activation of the coagulation cascade by systemic inflammation is mediated through the tissue factor (TF) pathway of the coagulation cascade[38,40–44].
Tissue factor pathway inhibitor (TFPI), the main inhibitor of the TF pathway of coagulation, is a three Kunitz domain glycoprotein which inhibits thrombin generation through the inhibition of activated factor (F) X and the FVIIa/TF complex[45,46]. The mean maternal plasma concentrations of total TFPI remains increase during the first half of pregnancy, remain relatively constant in the second half[47] and to decrease during labor[48]. The changes in plasma TFPI concentrations during inflammation are not well defined. Patients with chronic inflammation such as inflammatory bowel disease[49], chronic liver disease[50] and atherosclerosis[51], have increased plasma concentrations of TFPI; however, low TFPI concentrations have been reported in acute inflammatory processes such as pneumonia[52].
The profile of the maternal plasma TF and TFPI concentrations have different characteristics among several obstetrical syndromes[53–55]. In comparison to patients with a normal pregnancy, the median maternal plasma TF concentration is higher in patients with preeclampsia[53] or preterm prelabor rupture of membranes (PROM)[54], and lower in women with an SGA (small for gestational age) neonate[53]. Of interest, the median maternal plasma TFPI concentration increases during preeclampsia[53,55], which is associated with an exaggerated maternal systemic inflammatory response, decreases in patients with preterm prelabor rupture of membranes[54] and does not change in mothers with SGA fetuses[53]. Currently, there is no report regarding the changes in the maternal plasma TF and TFPI concentrations in patients with PTL. Therefore, the aims of this study were to determine the changes in TF, as well as TFPI maternal plasma concentration and activity in preterm and term labor.
MATERIAL AND METHODS
Study design and population
This cross-sectional study included women in the following groups: 1) normal pregnancies (n=86); 2) term pregnancies in spontaneous labor (TIL) (n=67) and not in labor (TNL) (n=88); and 3) patients with spontaneous PTL and intact membranes (n=136) that were classified into three sub-groups: a) PTL without intra-amniotic infection and/or inflammation (IAI) who delivered at term (n=49), b) PTL without IAI who delivered preterm (n=54), and c) PTL with IAI who delivered preterm (n=33). Patients with multiple gestations or fetuses with congenital and/or chromosomal anomalies were excluded.
Samples and data were retrieved from our bank of biological samples and clinical databases. Many of these samples have been previously employed to study the biology of inflammation, hemostasis, angiogenesis regulation, and growth factor concentrations in normal pregnant women and in those with pregnancy complications.
All women provided written informed consent prior to the collection of maternal blood. The Institutional Review Boards of Wayne State University and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), NIH, DHHS approved the collection and utilization of samples for research purposes.
Clinical definitions
Women with normal pregnancies met the following criteria: 1) no medical, obstetrical, or surgical complications at the time of the study; 2) gestational ages ranging from 20 to 41 weeks; and 3) delivery of a term infant, appropriate for gestational age, without complications. Preterm labor with intact membranes was diagnosed by the presence of at least two regular uterine contractions every 10 minutes associated with cervical change that required hospitalization before 37 completed weeks of gestation. Intra-amniotic infection was defined by the presence of a positive amniotic fluid culture for microorganisms. Intra-amniotic inflammation was defined as an amniotic fluid WBC count ≥ 100 cells/ml. The results of the amniotic fluid analyses were used for clinical management. An SGA neonate was defined as a birth weight below the 10th percentile[56].
Blood sample collection
All blood samples were collected with a vacutainer into 0.109M trisodium citrate anticoagulant solution (BD; San Jose, CA, USA). The samples were centrifuged at 1300g for 10 minutes at 4°C and stored at −70°C until assay.
Immuno and chromogenic assays
Human tissue factor immunoassay
TF concentrations were determined by sensitive and specific immunoassays (American Diagnostica, Greenwich, CT, USA), which recognizes TF-apo, TF, and TF-FVII complexes. The assays were conducted according to the manufacturer’s recommendations. The calculated coefficient of variation (CV) in our laboratory was 5.3%, and the sensitivity was 10 pg/ml.
Human tissue factor activity assay
TF activity was determined by a chromogenic assay (American Diagnostica, Greenwich, CT, USA). The assays were conducted according to the manufacturer’s recommendations. The calculated intra-assay CV was 3.77%, while the inter-assay CV was 6.25%, and the sensitivity of this assay was 0.53 pM.
Human tissue factor pathway inhibitor immunoassay
The concentrations of TFPI in maternal plasma were determined by sensitive and specific immunoassays (American Diagnostica, Greenwich, CT, USA). The TFPI ELISA employs, as the capture antibody, a murine anti-TFPI monoclonal that is directed against the Kunitz-1 domain of the TFPI molecule; therefore, it detects both TFPI-1 and TFPI-2, while measuring the total TFPI plasma concentration. The assay was conducted according to the manufacturer’s recommendations. The calculated CV in our laboratory was 6.6%, and the sensitivity was approximately 10 ng/ml.
Human tissue factor pathway inhibitor activity assay
TFPI activity was determined by a chromogenic assay (American Diagnostica, Greenwich, CT, USA). The assays were conducted according to the manufacturer’s recommendations. The calculated intra-assay CV was 5.51%, while the inter-assay CV was 8.74% and the sensitivity was 0.017 unit/ml.
Statistical analysis
To test whether our data was normally distributed, we used the Kolmogorov-Smirnov and the Shapiro-Wilk tests. Tissue factor and TFPI plasma concentrations were not normally distributed; thus, Kruskal Wallis and Mann–Whitney U tests were used for comparisons of continuous variables. The Chi-square and Fisher’s exact tests were used to compare categorical variables. The Spearman’s rho test was used to detect a correlation between the concentrations and activity of TF, TFPI, and their ratio to the gestational age at sample collection in women with a normal pregnancy. Multiple logistic regression analysis was performed to study which of the analytes was independently associated with PTL after correction for gestational age at sample collection. A p value < 0.05 was considered statistically significant. Analysis was performed with SPSS package, version 12 (SPSS Inc., Chicago, IL, USA).
RESULTS
The demographic and clinical characteristics of women with normal pregnancies and those with PTL are presented in Table I. Patients with PTL who delivered at term had a lower median maternal age than women with a normal pregnancy. Among patients with PTL, those who delivered preterm (regardless of the presence of IAI) had a lower median gestational age at blood collection than patients with normal pregnancies. Amniocentesis was performed in 83.8% (114/136) of the patients presenting with PTL, of which 14% (16/114) had positive intra-amniotic culture for microorganisms; the frequency of the specific microorganisms is presented in Table II.
Table I.
Demographic and clinical characteristics of the study population
| Normal pregnancy (n= 86) | PTL without IAI (n=54) | PTL with IAI (n=33) | PTL delivered at term (n=49) | |
|---|---|---|---|---|
| Maternal age (years) | 24 (21,27) | 22 (19,29) | 23(19,27) | 20 (18,24)* |
| Gravidity€ | ||||
| 1 | 18 (21.4) | 8 (14.8) | 12 (36.4) | 11 (22.4) |
| 2–5 | 53 (63.1) | 39 (72.2) | 17 (51.5) | 32 (65.3) |
| ≥6 | 13 (15.5) | 7 (13) | 4 (12.1) | 6 (12.2) |
| Parity§ | ||||
| 1 | 46 (54.1) | 30 (55.6) | 24 (72.7) | 31(63.3) |
| 2–5 | 38 (44.7) | 21(38.8) | 6 (18.2) | 18(36.7) |
| ≥6 | 1 (1.2) | 3(5.6) | 2 (6.1) | 0 |
| Ethnic origin£ | ||||
| African-Americans | 67 (80.7) | 42 (79.2) | 27(81.8) | 42 (87.5) |
| Caucasian | 11 (13.3) | 8 (15.1) | 5 (15.2) | 3 (6.2) |
| Hispanic | 2 (2.4) | 3 (5.7) | 0 | 1 (2.1) |
| Asian | 3 (3.6) | 0 | 1 (3) | 2 (4.2) |
| Gestational age at blood collection (weeks) | 31.1 (27.3, 33.6) | 29.5* (25.1,32.2) | 26.1* (24.6,31.6) | 31.4 (29.3,32.5) |
| Gestational age at delivery (weeks) | 39.6 (38.4,40.7) | 31.6* (26.2, 34.7) | 27.9* (25, 33.5) | 38.2* (37.3, 38.9) |
| Cesarean delivery* | 24 (33.8) | 20 (37.7) | 6(18.2) | 0* |
| Neonatal birthweight | 3342.5 (3057.5, 3641.8) | 1690* (880, 2335) | 1040* (642.5, 1755) | 2948* (2710, 3255) |
| SGA | 0 | 5 (9.4)* | 5 (15.1)* | 12 (24.5)* |
Data are presented as median (25–75 percentile) or numbers (%)
p<0.05 in comparison to the normal pregnancy group
PTL- preterm labor; IAI- intraamniotic infection/inflammation; SGA – small for gestational age
Table II.
Prevalence of intra-amniotic microorganisms in patients with preterm labor that underwent diagnostic amniocentesis (n=16)
| Microorganism | Number (%) |
|---|---|
| Mixed flora | 4 (25) |
| Fusobacterium nucleatum | 3 (18.8) |
| Gardnerella Vaginalis | 1 (6.2) |
| Ureaplama Urealiticum | 1 (6.2) |
| Mycoplasma Hominis | 1 (6.2) |
| Streptococcus Agalactia | 1 (6.2) |
| Others | 5 (31.3) |
Data are presented as numbers (%)
Among patients with normal pregnancies, gestational age at sample collection was positively correlated with maternal plasma TFPI activity (r=0.241, p=0.03), but not with TF activity (r=0.17, p=0.15). There was no correlation between TF and TFPI activity in the maternal plasma (r=0.45, p0.72).
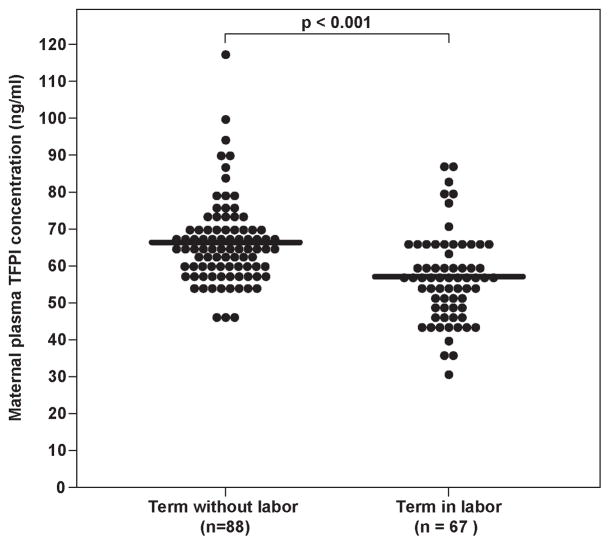
Changes in maternal plasma TF and TFPI concentrations and activity in patients with and without spontaneous labor at term
Patients with spontaneous labor at term had a significantly higher median maternal TF concentration than those women at term not in labor (p<0.001) (Figure 1a). In contrast, there was no significant difference in the median maternal plasma TF activity of patients with and without labor at term (TIL: median: 16.7 pM, range 7.0–31.2, vs. TNL: median 15.3 pM, range 2.9–168.3, p=0.17).
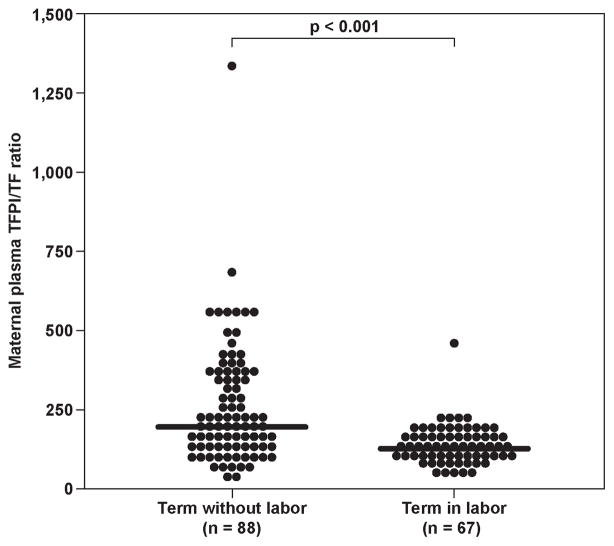
Figure 1.
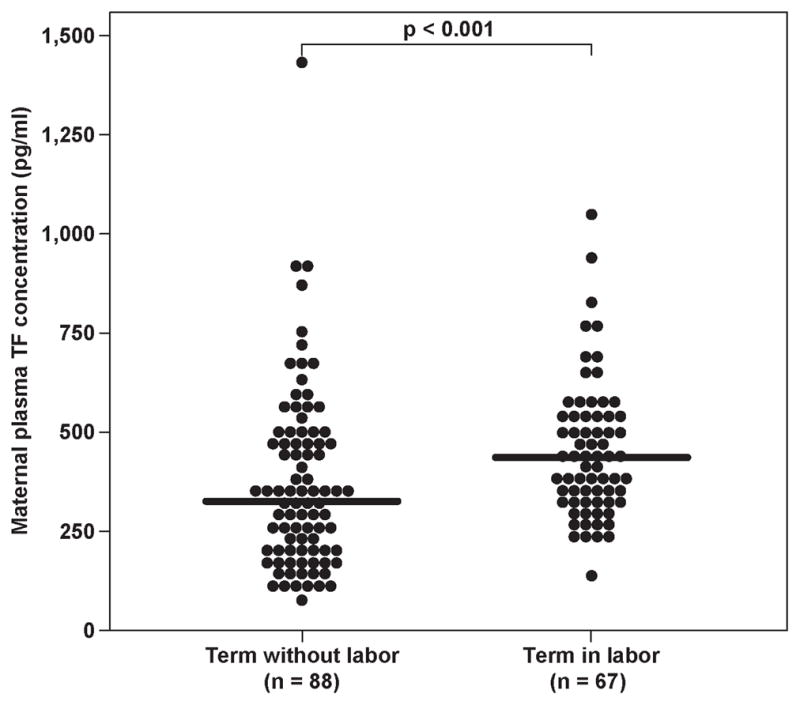
Maternal tissue factor (TF) (TIL: median 432.8 pg/ml, range 130.1–1043.7 vs. TNL: median 320.7 pg/ml, range 70.5–1415.2) (1a) and tissue factor pathway inhibitor (TFPI) (TIL: median 56.6 ng/ml; range 30–87.7, vs. TNL: median 65.3 ng/ml, range 45.5–117.8) (1b) plasma concentrations and the TFPI/ TF ratio (TNL: median 201.4, range 40.1–1326.2 vs. TIL: median 126.2, range 48.3–457.3) (1c) between women with and without spontaneous labor at term.
The median maternal plasma TFPI concentration at term was significantly lower in women in labor, than in those women not in labor (p<0.001) (Figure 1b). The median maternal plasma TFPI activity was not significantly different between patients with, and without labor at term (TNL: median 1.2 unit/ml, range 0.2–1.8 vs. TIL: median 1.2 unit/ml, range 0.5–2.7, p=0.86). The median maternal TFPI/TF ratio was higher in women at term without labor, than in those with active labor (p<0.001) (Figure 1c).
Changes in the maternal plasma TF concentrations and activity in patients with preterm labor
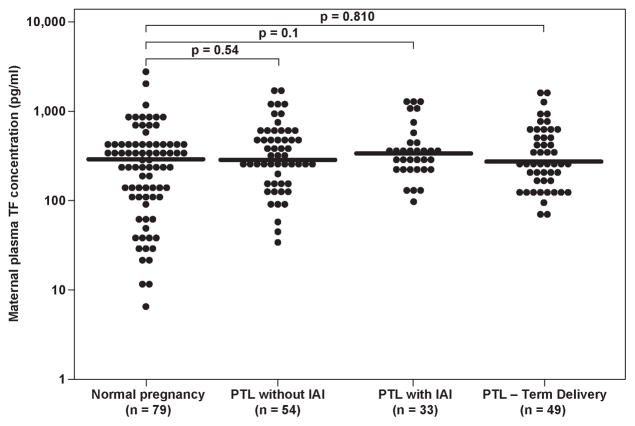
The median maternal plasma TF concentration did not differ significantly among patients with PTL and those with normal pregnancies (Kruskal Wallis, p=0.25) (Figure 2a), nor was it significantly different among the PTL sub-groups (Kruskal Wallis, p=0.51).
Figure 2.
Maternal plasma tissue factor (TF) concentration in women with normal pregnancy and patients with preterm labor according to the presence of intra-amniotic infection/inflammation and those who delivered at term. (Normal pregnancy- median 291.5 pg/ml; range 6.3–2662.2; PTL who delivered at term: median 258.6 pg/ml, range 65.9–1495.3; PTL who delivered preterm without IAI: median 272.97 pg/ml, range 33.5–1774.6; PTL who delivered preterm with IAI: median 345.7pg/ml, range 98.5–1237.0)
The median maternal plasma TF activity was significantly higher among the PTL sub-groups than the normal pregnancy group (Kruskal Wallis, p<0.001). In the post-hoc analysis, all three PTL sub-groups had a higher median maternal plasma TF activity than that of the normal pregnancy group: 1) PTL who delivered at term: median 14.1 pM, range 6.6–65.2 vs. normal pregnancy: median 9.9 pM, range 0.7–37.6, p<0.001; 2) PTL who delivered preterm without IAI: median 12.6 pM, range 7.0–98.7, p<0.001; and 3) PTL who delivered preterm with IAI: median 13.1 pM, range 8.1–51.3, p<0.001. However, the median maternal plasma TF activity did not differ among the PTL sub-groups (Kruskal Wallis, p=0.32).
Changes in the maternal plasma TFPI concentrations and activity in patients with preterm labor
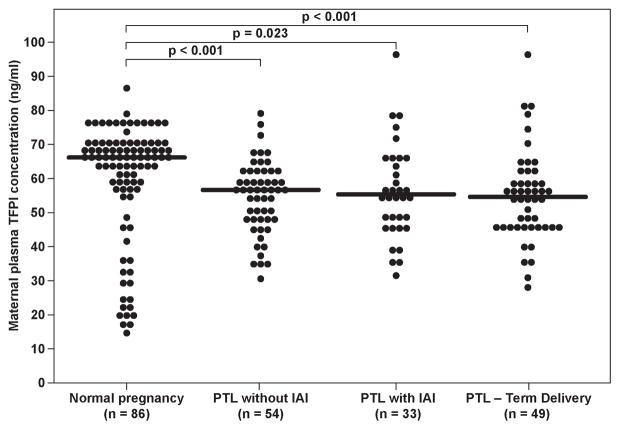
The median maternal plasma TFPI concentration differed significantly among patients with preterm labor, and those with a normal pregnancy (Kruskal Wallis, p=0.001). In the post-hoc analysis, all three PTL sub-groups had a significantly lower median maternal plasma TFPI than that of the normal pregnancy group: 1) PTL who delivered at term: median 54.7 ng/ml, range 28.6–96 vs. normal pregnancy- median 66.1 ng/ml; range 14.3–86.5, p=0.002; 2) PTL who delivered preterm without IAI: median 56.7 ng/ml, range 30.3–78.5, p<0.001; and 3) PTL who delivered preterm with IAI: median 55.2 ng/ml, range 34.7–96.7, p=0.023 (Figure 3). However, the maternal plasma TFPI activity did not differ among patients with PTL and those with normal pregnancy (Kruskal Wallis, p=0.89).
Figure 3.
Maternal plasma tissue factor pathway inhibitor (TFPI) concentrations in women with normal pregnancy and patients with preterm labor according to the presence of intra-amniotic infection/inflammation and those who delivered at term. (Normal pregnancy- median 66.1 ng/ml; range 14.3–86.5; PTL who delivered at term: median 54.7 ng/ml, range 28.6–96; PTL who delivered preterm without IAI: median 56.7 ng/ml, range 30.3–78.5; PTL who delivered preterm with IAI: median 55.2 ng/ml, range 34.7–96.7)
Changes in the maternal plasma TFPI/TF ratio in patients with preterm labor
Patients with PTL had a significantly lower TFPI/TF ratio than in those with a normal pregnancy (Kruskal Wallis, p=0.039). However, none of the comparisons between the groups was significant, after correction for multiple comparisons (PTL without IAI- median 174.4, range 26.8–1665.7 vs. normal pregnancy- median 221.5; range: 21.4–3355.3, p=0.08; PTL with IAI- median 168.6, range 36.9–621.3 vs. normal pregnancy- median 221.5; range 21.4–3355.3, p=0.06; and PTL who delivered at term- median 180.3, range 26.6–890.2 vs. normal pregnancy- median 221.5; range: 21.4–3355.3, p=0.16).
In light of the association between gestational age at sample collection and TFPI activity, we constructed a logistic regression model including an episode of preterm labor as the dependent variable. Maternal age, gestational age at blood draw, maternal plasma TF and TFPI concentration and activity, as well as the interaction between the concentration and activity of each analyte were included as covariates. Maternal plasma TFPI concentration (OR 0.87, 95%CI 0.77–0.98), TF activity (OR 1.14, 95%CI 1.04–1.26), gestational age at blood draw (OR 0.72, 95%CI 0.67–0.79) and maternal age (OR 0.9, 95%CI 0.84–0.97) were all independently associated with an episode of PTL (Table 3).
Table III.
Multiple logistic regression analysis of the association of maternal plasma tissue factor concentrations and activity as well as tissue factor pathway inhibitor and activity and preterm labor
| Factor | OR ( 95% CI) |
|---|---|
| Gestational age at sample collection (wk) | 0.72 (0.67–0.79) |
| Maternal age ( years) | 0.90 (0.84–0.97) |
| TF concentration (pg/ml) | 1.0 (0.99–1.0) |
| TF activity (pM) | 1.14 (1.04–1.25) |
| TF activity by TF concentration | 1.0 (1.0–1.0) |
| TFPI concentration (ng/ml) | 0.87 (0.77–0.98) |
| TFPI activity (unit/ml) | 0.032 (0–8.8) |
| TFPI activity by TFPI concentration | 1.06 (0.97–1.17) |
OR: odds ratio; CI: confidence interval; TF- tissue factor; TFPI- tissue factor pathway inhibitor
DISCUSSION
Principle findings of the study
1) Patients with PTL had a significantly higher median maternal plasma TF activity than that of women with a normal pregnancy, regardless of the presence of IAI or gestational age at delivery.2) Patients with PTL had a significantly lower median maternal plasma TFPI concentration than women with a normal pregnancy, regardless of the presence of IAI or gestational age at delivery. 3) There were no significant differences in the median maternal plasma TF concentrations and TFPI activity between patients in the PTL group with or without IAI, and women with a normal pregnancy. 4) The TFPI/TF ratio was lower in patients with PTL. 5) Maternal plasma TFPI concentration and TF activity were independently associated with the occurrence of an episode of PTL.
The association between inflammation and coagulation
Preterm labor is a syndrome that results from different underlying mechanisms, such as intrauterine infection/inflammation, increased thrombin generation in the maternal circulation, and an increased maternal systemic inflammatory response[1,5]. The coagulation cascade and the inflammatory system can activate and augment the action of each other[38,40,41]. This interaction is particularly important during pregnancy because the placentas of patients with PTL or preterm PROM often have inflammatory lesions as well as thrombotic lesions[6,57].
Maternal inflammation and circulating tissue factor concentration and activity
During acute systemic inflammation (i.e. endotoxemia), there is activation of the coagulation cascade through the TF pathway[38,40,41]. Evidence in support of this view includes the following: 1) Proinflammatory cytokines, such as IL-1β and TNF-α, increase mRNA and protein expression of TF by monocytes[30–37] and macrophages[38]. 2) The administration of low dose endotoxin to healthy volunteers is associated with a 125-fold increase in TF mRNA monocyte concentration[58]. 3) Activated monocytes that express TF on their membrane secrete TF into the plasma in its free form[59–63], or in micro-particles[60].
In the current study, labor at term was associated with a higher median maternal plasma TF concentration, without a significant change in its activity. Preterm labor was associated with an increase in TF activity in the maternal plasma without a significant change in its concentration, in comparison to women with a normal pregnancy, while patients with preterm PROM had an increased maternal plasma TF concentration[54] and activity (unpublished data). A possible explanation for the differences between PTL and preterm PROM may derive from the specific component of the common pathway of parturition, which is activated in each obstetrical syndrome[64]. While preterm PROM is associated with the activation of the decidua and the membranes, myometrial activation is the major component of preterm labor with intact membranes[64]. This is relevant because the decidua and the membranes have a high TF concentration[65–67]. We propose that during labor at term or in preterm PROM, activation of the decidua and membranes may lead to TF shedding into the maternal circulation during preterm PROM and normal labor at term. Conversely, activation of the myometrium may be associated with lower shedding of TF into the maternal circulation. Nevertheless, both PTL and preterm PROM are pathologic processes which are associated with an increased maternal systemic inflammatory response[25,68], that contributes to tissue factor activation in the maternal circulation. However, the physiologic process of labor at term, although defined as inflammatory in nature, is not associated with a similar increase in TF activity.
The effect of the activation of the hemostatic system on maternal inflammation
The activation of the coagulation cascade induces the secretion of proinflammatory cytokines.[38,40,41,44]. Indeed, the expression of TF in sites of injury or on platelets, leads to the generation of the TF/FVIIa/FXa complex, which in turn leads to thrombin generation and activation of the protease-activated receptor (PAR)-2. The latter enhances the production of IL-6[44,69–71]. Evidence in support of this concept includes the observation that the administration of antibodies against the binding site of FXa to TF/FVIIa complexes attenuates tissue damage and thrombosis in baboons with E. coli-induced sepsis[72]. The proposed mechanism of action is that the TF/FVIIa complex cannot activate PAR-2 without the contribution of FXa[72]. Thus, during systemic inflammation, FXa plays a dual role: 1) activation of the prothrombinase complex leading to the generation of thrombin[73–80]; and 2) amplification of the inflammatory process by inducing IL-6 secretion from endothelial cells[81,82] and monocytes[70–72]. The latter may contribute to the systemic maternal inflammation previously reported among patients with PTL[25].
The effect of TF activity on thrombin generation in patients with preterm labor
In this study, we could not demonstrate an increase in the median maternal plasma TF concentration; however, there was an increase in TF activity in the maternal plasma of patients with PTL. Since the assay we used in this study measures TF activity through the generation of FXa in a given sample, increased TF activity reflects elevated FXa generation that leads to the increased thrombin generation previously reported among patients with PTL[23,24,83]. Similarly to the increased thrombin generation, the median maternal plasma TF activity was increased in all sub-groups of PTL, regardless of the presence of intra-amniotic infection/inflammation, or a subsequent preterm delivery.
This observation is relevant given the following findings: 1) patients with preterm labor who subsequently delivered at term had a higher rate of SGA neonates and a higher proportion of placental vascular lesions[84]; and 2) a subset of patients with PTL had vascular lesions in their placenta, [6,85] and 7–20% of inflammatory lesions in the placenta were accompanied by vascular lesions in preterm parturition[6,57]. Thus, the increased TF activity among patients with PTL contributes to a higher generation of FXa that, along with the physiologic increase in the maternal plasma concentrations of FVII and FX during gestation[86–89], may be the underlying mechanism leading to the increased thrombin generation reported in patients with PTL.
Changes in TFPI concentration incomplicated pregnancies
The finding that patients with PTL have a lower median maternal plasma TFPI concentration than in women with normal pregnancies is novel. Moreover, this observation was independently associated with an episode of PTL, Similarly, maternal plasma TFPI concentrations were lower among patients with preterm PROM[54] or a fetal death[90]. In contrast, patients with preeclampsia had a higher median maternal plasma TFPI concentration[53,55].
The process leading to the lower median maternal TFPI plasma concentration observed in patients with PTL is not clear. In contrast to PPROM[54], the lower median plasma TFPI concentration in patients with PTL is not associated with a higher median TF plasma concentration, further supporting a different profile of the activation of the coagulation cascade in these pregnancy complications.
Of interest, thrombin has an inhibitory effect on the production of TFPI by endothelial cells[91], and the increased thrombin generation observed in patients with PTL may be associated with a concomitant reduction in TFPI production by the maternal vascular endothelium. The placenta is an additional source for TFPI (mainly TFPI-2[92–101]), and preterm parturition may be associated with a lower production of TFPI by the placenta, contributing to the low maternal plasma concentrations detected in patients with PTL. Indeed, patients with vascular complications of pregnancy (preeclampsia, eclampsia, placental abruption, fetal growth restriction, and fetal demise) have a lower placental concentration of total TFPI and TFPI mRNA expression than in women with normal pregnancies[102],. However, currently there is no report regarding the placental production of TFPI in patients with PTL or preterm PROM.
Insufficient anti-coagulation as a mechanism for thrombin generation among patients with PTL
The lower median maternal plasma TFPI concentration in patients with PTL, regardless of the presence of infection or gestational age at delivery, suggests that the increased thrombin generation observed among these patients may derive not only from an increased activation of the hemostatic system, but also from insufficient anti-coagulation. The latter can be due to either low concentrations of the anticoagulant proteins, or as a result of an abnormal balance between coagulation factors and their inhibitors.
Low concentration of anti-coagulant proteins in patients with PTL
The activation of factor X is an important step in the coagulation cascade leading to the conversion of prothrombin (factor II) into thrombin. Therefore, the inhibition of FXa by TFPI, protein Z/protein Z dependant protease complex, and antithrombin III[103], leads to a reduction in the generation of thrombin. The findings of the current study regarding the low median maternal plasma TFPI concentration among patients with PTL, and the findings previously reported by our group regarding low concentrations of protein Z/protein Z dependent protease complex in patients with PTL without IAI[104], suggest that the insufficient inhibition of factor Xa may contribute to the increased thrombin generation reported in patients with PTL.
Changes in the balance between coagulation and anti-coagulation in patients with PTL
The overall balance between the concentration and activity of the coagulation factors and the anti-coagulation proteins is one of the determining factors of thrombin generation. In the normal state, the immunoreactive concentrations of TFPI in the plasma are 500 to 1000 times higher than that of TF[105], suggesting that an excess of anti-coagulant proteins closely controls the coagulation cascade activity. In the current study, although PTL was not associated with a significant change in the median maternal plasma TF concentration, the TFPI/TF ratio was lower than that of normal pregnant women, mainly due to decreased TFPI concentrations. Our group previously reported that patients with preterm PROM[54], as well as those with preeclampsia[53], have a lower median maternal plasma TFPI/TF ratio than that of normal pregnant women. The lower TFPI/TF ratio in patients with preeclampsia occurs despite the increase in the median maternal plasma TFPI concentration observed in these patients. This suggests that the balance between TF and its natural inhibitor may better reflect the overall activity of the TF pathway of coagulation, than the individual concentrations of TF or TFPI. We propose that a subset of patients with PTL have a low anti-coagulation proteins concentration, or a lower ratio between the coagulation factors and their inhibitors, which contributes to the increased thrombin generation observed in these patients.
In conclusion, women with preterm parturition, in contrast to labor at term, have a higher TF activity and a lower TFPI concentration without a significant change in the median maternal plasma TF concentration. These observations suggest that the increased thrombin generation reported in patients with PTL may be the consequence of the activation of the TF pathway of the coagulation cascade. Moreover, the low maternal plasma TFPI concentration suggests that deficient anti-coagulation may be an additional mechanism leading to the increased thrombin generation reported in patients with PTL.
Acknowledgments
This research was supported (in part) by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Reference List
- 1.Romero R, Espinoza J, Mazor M, Chaiworapongsa T. The preterm parturition syndrome. In: Critchley H, Bennett P, Thornton S, editors. Preterm Birth. 1. London: RCOG Press; 2004. pp. 28–60. [Google Scholar]
- 2.Minkoff H. Prematurity: infection as an etiologic factor. Obstet Gynecol. 1983;62:137–44. [PubMed] [Google Scholar]
- 3.Romero R, Mazor M, Wu YK, Sirtori M, Oyarzun E, Mitchell MD, Hobbins JC. Infection in the pathogenesis of preterm labor. Semin Perinatol. 1988;12:262–79. [PubMed] [Google Scholar]
- 4.Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, Sabo V, Athanassiadis AP, Hobbins JC. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol. 1989;161:817–24. doi: 10.1016/0002-9378(89)90409-2. [DOI] [PubMed] [Google Scholar]
- 5.Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev. 2002;8:3–13. doi: 10.1002/mrdd.10008. [DOI] [PubMed] [Google Scholar]
- 6.Arias F, Rodriquez L, Rayne SC, Kraus FT. Maternal placental vasculopathy and infection: two distinct subgroups among patients with preterm labor and preterm ruptured membranes. Am J Obstet Gynecol. 1993;168:585–91. doi: 10.1016/0002-9378(93)90499-9. [DOI] [PubMed] [Google Scholar]
- 7.Romero R, Sepulveda W, Baumann P, Yoon BH, Brandt F, Gomez R, Mazor M, et al. The preterm labor syndrome: Biochemical, cytologic, immunologic, pathologic, microbiologic, and clinical evidence that preterm labor is a heterogeneous disease. Am J Obstet Gynecol. 1993;168:288. [Google Scholar]
- 8.Combs CA, Katz MA, Kitzmiller JL, Brescia RJ. Experimental preeclampsia produced by chronic constriction of the lower aorta: validation with longitudinal blood pressure measurements in conscious rhesus monkeys. Am J Obstet Gynecol. 1993;169:215–23. doi: 10.1016/0002-9378(93)90171-e. [DOI] [PubMed] [Google Scholar]
- 9.Ogunyemi D, Murillo M, Jackson U, Hunter N, Alperson B. The relationship between placental histopathology findings and perinatal outcome in preterm infants. J Matern Fetal Neonatal Med. 2003;13:102–9. doi: 10.1080/jmf.13.2.102.109. [DOI] [PubMed] [Google Scholar]
- 10.Hill LM, Breckle R, Thomas ML, Fries JK. Polyhydramnios: ultrasonically detected prevalence and neonatal outcome. Obstet Gynecol. 1987;69:21–5. [PubMed] [Google Scholar]
- 11.Phelan JP, Park YW, Ahn MO, Rutherford SE. Polyhydramnios and perinatal outcome. J Perinatol. 1990;10:347–50. [PubMed] [Google Scholar]
- 12.Besinger R, Carlson N. The physiology of preterm labor. In: Keith L, Papiernik E, Keith D, Luke B, editors. Multiple Pregnancy: Epidemiology, Gestation and Perinatal Outcome. London: Parthenon Publishing; 1995. p. 415. [Google Scholar]
- 13.Romero R, Mazor M, Gomez R. Cervix, incompetence and premature labor. Fetus. 1993;3:1. [Google Scholar]
- 14.Romero R, Espinoza J, Erez O, Hassan S. The role of cervical cerclage in obstetric practice: can the patient who could benefit from this procedure be identified? Am J Obstet Gynecol. 2006;194:1–9. doi: 10.1016/j.ajog.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romero R. The child is the father of the man. Prenat Neonat Med. 1996;1:8–11. [Google Scholar]
- 16.Heath VC, Southall TR, Souka AP, Elisseou A, Nicolaides KH. Cervical length at 23 weeks of gestation: prediction of spontaneous preterm delivery. Ultrasound Obstet Gynecol. 1998;12:312–7. doi: 10.1046/j.1469-0705.1998.12050312.x. [DOI] [PubMed] [Google Scholar]
- 17.Hassan SS, Romero R, Berry SM, Dang K, Blackwell SC, Treadwell MC, Wolfe HM. Patients with an ultrasonographic cervical length < or =15 mm have nearly a 50% risk of early spontaneous preterm delivery. Am J Obstet Gynecol. 2000;182:1458–67. doi: 10.1067/mob.2000.106851. [DOI] [PubMed] [Google Scholar]
- 18.Holloway JA, Warner JO, Vance GH, Diaper ND, Warner JA, Jones CA. Detection of house-dust-mite allergen in amniotic fluid and umbilical-cord blood. Lancet. 2000;356:1900–2. doi: 10.1016/S0140-6736(00)03265-7. [DOI] [PubMed] [Google Scholar]
- 19.Jones AC, Miles EA, Warner JO, Colwell BM, Bryant TN, Warner JA. Fetal peripheral blood mononuclear cell proliferative responses to mitogenic and allergenic stimuli during gestation. Pediatr Allergy Immunol. 1996;7:109–16. doi: 10.1111/j.1399-3038.1996.tb00117.x. [DOI] [PubMed] [Google Scholar]
- 20.Rudolph MI, Reinicke K, Cruz MA, Gallardo V, Gonzalez C, Bardisa L. Distribution of mast cells and the effect of their mediators on contractility in human myometrium. Br J Obstet Gynaecol. 1993;100:1125–30. doi: 10.1111/j.1471-0528.1993.tb15178.x. [DOI] [PubMed] [Google Scholar]
- 21.Belt AR, Baldassare JJ, Molnar M, Romero R, Hertelendy F. The nuclear transcription factor NF-kappaB mediates interleukin-1beta-induced expression of cyclooxygenase-2 in human myometrial cells. Am J Obstet Gynecol. 1999;181:359–66. doi: 10.1016/s0002-9378(99)70562-4. [DOI] [PubMed] [Google Scholar]
- 22.Allport VC, Pieber D, Slater DM, Newton R, White JO, Bennett PR. Human labour is associated with nuclear factor-kappaB activity which mediates cyclo-oxygenase-2 expression and is involved with the ‘functional progesterone withdrawal’. Mol Hum Reprod. 2001;7:581–6. doi: 10.1093/molehr/7.6.581. [DOI] [PubMed] [Google Scholar]
- 23.Chaiworapongsa T, Espinoza J, Yoshimatsu J, Kim YM, Bujold E, Edwin S, Yoon BH, Romero R. Activation of coagulation system in preterm labor and preterm premature rupture of membranes. J Matern Fetal Neonatal Med. 2002;11:368–73. doi: 10.1080/jmf.11.6.368.373. [DOI] [PubMed] [Google Scholar]
- 24.Elovitz MA, Baron J, Phillippe M. The role of thrombin in preterm parturition. Am J Obstet Gynecol. 2001;185:1059–63. doi: 10.1067/mob.2001.117638. [DOI] [PubMed] [Google Scholar]
- 25.Gervasi MT, Chaiworapongsa T, Naccasha N, Blackwell S, Yoon BH, Maymon E, Romero R. Phenotypic and metabolic characteristics of maternal monocytes and granulocytes in preterm labor with intact membranes. Am J Obstet Gynecol. 2001;185:1124–9. doi: 10.1067/mob.2001.117681. [DOI] [PubMed] [Google Scholar]
- 26.Dudley DJ. Immunoendocrinology of preterm labor: the link between corticotropin-releasing hormone and inflammation. Am J Obstet Gynecol. 1999;180:S251–S256. doi: 10.1016/s0002-9378(99)70711-8. [DOI] [PubMed] [Google Scholar]
- 27.Pitiphat W, Gillman MW, Joshipura KJ, Williams PL, Douglass CW, Rich-Edwards JW. Plasma C-reactive protein in early pregnancy and preterm delivery. Am J Epidemiol. 2005;162:1108–13. doi: 10.1093/aje/kwi323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khoury J, Henriksen T, Seljeflot I, Morkrid L, Froslie KF, Tonstad S. Effects of an antiatherogenic diet during pregnancy on markers of maternal and fetal endothelial activation and inflammation: the CARRDIP study. BJOG. 2007;114:279–88. doi: 10.1111/j.1471-0528.2006.01187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Poll T, Buller HR, ten CH, Wortel CH, Bauer KA, van Deventer SJ, Hack CE, Sauerwein HP, Rosenberg RD, ten Cate JW. Activation of coagulation after administration of tumor necrosis factor to normal subjects. N Engl J Med. 1990;322:1622–7. doi: 10.1056/NEJM199006073222302. [DOI] [PubMed] [Google Scholar]
- 30.Semeraro N, Lattanzio A, Montemurro P, Papanice M, De Lucia O, De Bellis G, Giordano D. Mechanisms of blood clotting activation in inflammation: the role of mononuclear phagocytes. Int J Tissue React. 1985;7:313–20. [PubMed] [Google Scholar]
- 31.Salgado A, Boveda JL, Monasterio J, Segura RM, Mourelle M, Gomez-Jimenez J, Peracaula R. Inflammatory mediators and their influence on haemostasis. Haemostasis. 1994;24:132–8. doi: 10.1159/000217093. [DOI] [PubMed] [Google Scholar]
- 32.Oeth P, Parry GC, Mackman N. Regulation of the tissue factor gene in human monocytic cells. Role of AP-1, NF-kappa B/Rel, and Sp1 proteins in uninduced and lipopolysaccharide-induced expression. Arterioscler Thromb Vasc Biol. 1997;17:365–74. doi: 10.1161/01.atv.17.2.365. [DOI] [PubMed] [Google Scholar]
- 33.Almdahl SM, Osterud B. Experimental gram-negative septicemia: thromboplastin generation in mononuclear phagocytes from different anatomical sites. Thromb Res. 1987;47:37–46. doi: 10.1016/0049-3848(87)90238-6. [DOI] [PubMed] [Google Scholar]
- 34.Heyderman RS, Klein NJ, Daramola OA, Hammerschmidt S, Frosch M, Robertson BD, Levin M, Ison CA. Induction of human endothelial tissue factor expression by Neisseria meningitidis: the influence of bacterial killing and adherence to the endothelium. Microb Pathog. 1997;22:265–74. doi: 10.1006/mpat.1996.0112. [DOI] [PubMed] [Google Scholar]
- 35.Semeraro N, Colucci M. Tissue factor in health and disease. Thromb Haemost. 1997;78:759–64. [PubMed] [Google Scholar]
- 36.Bouwman JJ, Visseren FL, Bosch MC, Bouter KP, Diepersloot RJ. Procoagulant and inflammatory response of virus-infected monocytes. Eur J Clin Invest. 2002;32:759–66. doi: 10.1046/j.1365-2362.2002.01041.x. [DOI] [PubMed] [Google Scholar]
- 37.Gando S, Kameue T, Matsuda N, Hayakawa M, Morimoto Y, Ishitani T, Kemmotsu O. Imbalances between the levels of tissue factor and tissue factor pathway inhibitor in ARDS patients. Thromb Res. 2003;109:119–24. doi: 10.1016/s0049-3848(03)00151-8. [DOI] [PubMed] [Google Scholar]
- 38.Levi M, van der Poll T, ten Cate H. Tissue factor in infection and severe inflammation. Semin Thromb Hemost. 2006;32:33–9. doi: 10.1055/s-2006-933338. [DOI] [PubMed] [Google Scholar]
- 39.van der Poll T, Levi M, Hack CE, ten CH, van Deventer SJ, Eerenberg AJ, de Groot ER, Jansen J, Gallati H, Buller HR, et al. Elimination of interleukin 6 attenuates coagulation activation in experimental endotoxemia in chimpanzees. J Exp Med. 1994;179:1253–9. doi: 10.1084/jem.179.4.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Esmon CT, Fukudome K, Mather T, Bode W, Regan LM, Stearns-Kurosawa DJ, Kurosawa S. Inflammation, sepsis, and coagulation. Haematologica. 1999;84:254–9. [PubMed] [Google Scholar]
- 41.Esmon CT. The interactions between inflammation and coagulation. Br J Haematol. 2005;131:417–30. doi: 10.1111/j.1365-2141.2005.05753.x. [DOI] [PubMed] [Google Scholar]
- 42.Levi M, ten CH, Bauer KA, van der PT, Edgington TS, Buller HR, van Deventer SJ, Hack CE, ten Cate JW, Rosenberg RD. Inhibition of endotoxin-induced activation of coagulation and fibrinolysis by pentoxifylline or by a monoclonal anti-tissue factor antibody in chimpanzees. J Clin Invest. 1994;93:114–20. doi: 10.1172/JCI116934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levi M, van der Poll T, Buller HR. Bidirectional relation between inflammation and coagulation. Circulation. 2004;109:2698–704. doi: 10.1161/01.CIR.0000131660.51520.9A. [DOI] [PubMed] [Google Scholar]
- 44.Levi M, van der Poll T. Two-way interactions between inflammation and coagulation. Trends Cardiovasc Med. 2005;15:254–9. doi: 10.1016/j.tcm.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 45.Broze GJ, Jr, Girard TJ, Novotny WF. Regulation of coagulation by a multivalent Kunitz-type inhibitor. Biochemistry. 1990;29:7539–46. doi: 10.1021/bi00485a001. [DOI] [PubMed] [Google Scholar]
- 46.Broze GJ, Jr, Warren LA, Novotny WF, Higuchi DA, Girard JJ, Miletich JP. The lipoprotein-associated coagulation inhibitor that inhibits the factor VII-tissue factor complex also inhibits factor Xa: insight into its possible mechanism of action. Blood. 1988;71:335–43. [PubMed] [Google Scholar]
- 47.Sarig G, Blumenfeld Z, Leiba R, Lanir N, Brenner B. Modulation of systemic hemostatic parameters by enoxaparin during gestation in women with thrombophilia and pregnancy loss. Thromb Haemost. 2005;94:980–5. doi: 10.1160/TH05-03-0212. [DOI] [PubMed] [Google Scholar]
- 48.Uszynski M, Zekanowska E, Uszynski W, Kuczynski J. Tissue factor (TF) and tissue factor pathway inhibitor (TFPI) in amniotic fluid and blood plasma:implications for the mechanism of amniotic fluid embolism. Eur J Obstet Gynecol Reprod Biol. 2001;95:163–6. doi: 10.1016/s0301-2115(00)00448-6. [DOI] [PubMed] [Google Scholar]
- 49.Souto JC, Martinez E, Roca M, Mateo J, Pujol J, Gonzalez D, Fontcuberta J. Prothrombotic state and signs of endothelial lesion in plasma of patients with inflammatory bowel disease. Dig Dis Sci. 1995;40:1883–9. doi: 10.1007/BF02208650. [DOI] [PubMed] [Google Scholar]
- 50.Berrettini M, Malaspina M, Parise P, Lucarelli G, Kisiel W, Nenci GG. A simple chromogenic substrate assay of tissue factor pathway inhibitor activity in plasma and serum. Am J Clin Pathol. 1995;103:391–5. doi: 10.1093/ajcp/103.4.391. [DOI] [PubMed] [Google Scholar]
- 51.Novo G, Caplice N, Tantillo R, Bonura F, Simari R, Novo S. TFPI antigen and activity levels in patients with asymptomatic atherosclerosis and target organ acute and chronic complications. Int Angiol. 2005;24:366–71. [PubMed] [Google Scholar]
- 52.Sandset PM, Andersson TR. Coagulation inhibitor levels in pneumonia and stroke: changes due to consumption and acute phase reaction. J Intern Med. 1989;225:311–6. doi: 10.1111/j.1365-2796.1989.tb00087.x. [DOI] [PubMed] [Google Scholar]
- 53.Erez O, Hoppensteadt D, Than NG, Fareed J, Mazaki-Tovi S, Espinoza J, Chaiworapongsa T, Yoon BH, Hassan S, Gotsch F, et al. Tissue Factor and its Natural Inhibitor in Preeclampsia and SGA. J Matern Fetal Neonatal Med. 2008;21:855–9. doi: 10.1080/14767050802361872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Erez O, Espinoza, Chaiworapongsa T, Gotsch F, Kusanovic JP, Than NG, Mazaki-Tovi S, Papp Z, Yoon BH, Hoppensteadt D, et al. A link between a hemostatic disorder and preterm PROM: a role for tissue factor and tissue factor pathway inhibitor. J Matern Fetal Med. 2008;21:732–44. doi: 10.1080/14767050802361807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abdel Gader AM, Al-Mishari AA, Awadalla SA, Buyuomi NM, Khashoggi T, Al-Hakeem M. Total and free tissue factor pathway inhibitor in pregnancy hypertension. Int J Gynaecol Obstet. 2006;95:248–53. doi: 10.1016/j.ijgo.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 56.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–8. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 57.Arias F, Victoria A, Cho K, Kraus F. Placental histology and clinical characteristics of patients with preterm premature rupture of membranes. Obstet Gynecol. 1997;89:265–71. doi: 10.1016/S0029-7844(96)00451-6. [DOI] [PubMed] [Google Scholar]
- 58.Franco RF, de JE, Dekkers PE, Timmerman JJ, Spek CA, van Deventer SJ, van DP, van KL, van GB, ten CH, et al. The in vivo kinetics of tissue factor messenger RNA expression during human endotoxemia: relationship with activation of coagulation. Blood. 2000;96:554–9. [PubMed] [Google Scholar]
- 59.Osterud B. Cellular interactions in tissue factor expression by blood monocytes. Blood Coagul Fibrinolysis. 1995;6 (Suppl 1):S20–S25. [PubMed] [Google Scholar]
- 60.Butenas S, Bouchard BA, Brummel-Ziedins KE, Parhami-Seren B, Mann KG. Tissue factor activity in whole blood. Blood. 2005;105:2764–70. doi: 10.1182/blood-2004-09-3567. [DOI] [PubMed] [Google Scholar]
- 61.Bach RR, Moldow CF. Mechanism of tissue factor activation on HL-60 cells. Blood. 1997;89:3270–6. [PubMed] [Google Scholar]
- 62.Egorina EM, Sovershaev MA, Bjorkoy G, Gruber FX, Olsen JO, Parhami-Seren B, Mann KG, Osterud B. Intracellular and surface distribution of monocyte tissue factor: application to intersubject variability. Arterioscler Thromb Vasc Biol. 2005;25:1493–8. doi: 10.1161/01.ATV.0000168413.29874.d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rivers RP, Hathaway WE, Weston WL. The endotoxin-induced coagulant activity of human monocytes. Br J Haematol. 1975;30:311–6. doi: 10.1111/j.1365-2141.1975.tb00547.x. [DOI] [PubMed] [Google Scholar]
- 64.Romero R, Goncalves LF, Chaiworapongsa T, Kusanovic JP, Espinoza J. Mechanisms of preterm labor and preterm premature rupture of the membranes. In: Kurjak A, Chervenak F, editors. Textbook of Perinatal Medicine. 2. Taylor and Francis; 2006. pp. 1379–1393. [Google Scholar]
- 65.Lockwood CJ, Krikun G, Rahman M, Caze R, Buchwalder L, Schatz F. The role of decidualization in regulating endometrial hemostasis during the menstrual cycle, gestation, and in pathological states. Semin Thromb Hemost. 2007;33:111–7. doi: 10.1055/s-2006-958469. [DOI] [PubMed] [Google Scholar]
- 66.Lockwood CJ, Krikun G, Schatz F. The decidua regulates hemostasis in human endometrium. Semin Reprod Endocrinol. 1999;17:45–51. doi: 10.1055/s-2007-1016211. [DOI] [PubMed] [Google Scholar]
- 67.Lockwood CJ, Krikun G, Schatz F. Decidual cell-expressed tissue factor maintains hemostasis in human endometrium. Ann N Y Acad Sci. 2001;943:77–88. doi: 10.1111/j.1749-6632.2001.tb03793.x. [DOI] [PubMed] [Google Scholar]
- 68.Gervasi MT, Chaiworapongsa T, Naccasha N, Pacora P, Berman S, Maymon E, Kim JC, Kim YM, Yoshimatsu J, Espinoza J, et al. Maternal intravascular inflammation in preterm premature rupture of membranes. J Matern Fetal Neonatal Med. 2002;11:171–5. doi: 10.1080/jmf.11.3.171.175. [DOI] [PubMed] [Google Scholar]
- 69.Chi L, Li Y, Stehno-Bittel L, Gao J, Morrison DC, Stechschulte DJ, Dileepan KN. Interleukin-6 production by endothelial cells via stimulation of protease-activated receptors is amplified by endotoxin and tumor necrosis factor-alpha. J Interferon Cytokine Res. 2001;21:231–40. doi: 10.1089/107999001750169871. [DOI] [PubMed] [Google Scholar]
- 70.Ahamed J, Ruf W. Protease-activated receptor 2-dependent phosphorylation of the tissue factor cytoplasmic domain. J Biol Chem. 2004;279:23038–44. doi: 10.1074/jbc.M401376200. [DOI] [PubMed] [Google Scholar]
- 71.Camerer E, Huang W, Coughlin SR. Tissue factor- and factor X-dependent activation of protease-activated receptor 2 by factor VIIa. Proc Natl Acad Sci U S A. 2000;97:5255–60. doi: 10.1073/pnas.97.10.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Welty-Wolf KE, Carraway MS, Ortel TL, Ghio AJ, Idell S, Egan J, Zhu X, Jiao JA, Wong HC, Piantadosi CA. Blockade of tissue factor-factor X binding attenuates sepsis-induced respiratory and renal failure. Am J Physiol Lung Cell Mol Physiol. 2006;290:L21–L31. doi: 10.1152/ajplung.00155.2005. [DOI] [PubMed] [Google Scholar]
- 73.Hawiger J. Formation and regulation of platelet and fibrin hemostatic plug. Hum Pathol. 1987;18:111–22. doi: 10.1016/s0046-8177(87)80330-1. [DOI] [PubMed] [Google Scholar]
- 74.Tracy PB, Rohrbach MS, Mann KG. Functional prothrombinase complex assembly on isolated monocytes and lymphocytes. J Biol Chem. 1983;258:7264–7. [PubMed] [Google Scholar]
- 75.Bajaj SP, Joist JH. New insights into how blood clots: implications for the use of APTT and PT as coagulation screening tests and in monitoring of anticoagulant therapy. Semin Thromb Hemost. 1999;25:407–18. doi: 10.1055/s-2007-994943. [DOI] [PubMed] [Google Scholar]
- 76.ten Cate H, Bauer KA, Levi M, Edgington TS, Sublett RD, Barzegar S, Kass BL, Rosenberg RD. The activation of factor X and prothrombin by recombinant factor VIIa in vivo is mediated by tissue factor. J Clin Invest. 1993;92:1207–12. doi: 10.1172/JCI116691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Camerer E, Kolsto AB, Prydz H. Cell biology of tissue factor, the principal initiator of blood coagulation. Thromb Res. 1996;81:1–41. doi: 10.1016/0049-3848(95)00209-x. [DOI] [PubMed] [Google Scholar]
- 78.Butenas S, Mann KG. Blood coagulation. Biochemistry (Mosc ) 2002;67:3–12. doi: 10.1023/a:1013985911759. [DOI] [PubMed] [Google Scholar]
- 79.Lu G, Broze GJ, Jr, Krishnaswamy S. Formation of factors IXa and Xa by the extrinsic pathway: differential regulation by tissue factor pathway inhibitor and antithrombin III. J Biol Chem. 2004;279:17241–9. doi: 10.1074/jbc.M312827200. [DOI] [PubMed] [Google Scholar]
- 80.Rodgers GM, Shuman MA. Prothrombin is activated on vascular endothelial cells by factor Xa and calcium. Proc Natl Acad Sci U S A. 1983;80:7001–5. doi: 10.1073/pnas.80.22.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Busch G, Seitz I, Steppich B, Hess S, Eckl R, Schomig A, Ott I. Coagulation factor Xa stimulates interleukin-8 release in endothelial cells and mononuclear leukocytes: implications in acute myocardial infarction. Arterioscler Thromb Vasc Biol. 2005;25:461–6. doi: 10.1161/01.ATV.0000151279.35780.2d. [DOI] [PubMed] [Google Scholar]
- 82.Daubie V, Cauwenberghs S, Senden NH, Pochet R, Lindhout T, Buurman WA, Heemskerk JW. Factor Xa and thrombin evoke additive calcium and proinflammatory responses in endothelial cells subjected to coagulation. Biochim Biophys Acta. 2006 doi: 10.1016/j.bbamcr.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 83.Gomez R, Athayde N, Pacora P, Mazor M, Yoon BH, Romero R. Increased Thrombin in Intrauterine Inflammation. Am J Obstet Gynecol. 1998;178:S62. [Google Scholar]
- 84.Espinoza J, Kusanovic JP, Kim CJ, Kim YM, Kim JS, Hassan SS, Gotsch F, Goncalves LF, Erez O, Friel L, et al. An episode of preterm labor is a risk factor for the birth of a small-for-gestational-age neonate. Am J Obstet Gynecol. 2007;196:574–5. doi: 10.1016/j.ajog.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim YM, Bujold E, Chaiworapongsa T, Gomez R, Yoon BH, Thaler HT, Rotmensch S, Romero R. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2003;189:1063–9. doi: 10.1067/s0002-9378(03)00838-x. [DOI] [PubMed] [Google Scholar]
- 86.Beller FK, Ebert C. The coagulation and fibrinolytic enzyme system in pregnancy and in the puerperium. Eur J Obstet Gynecol Reprod Biol. 1982;13:177–97. doi: 10.1016/0028-2243(82)90028-4. [DOI] [PubMed] [Google Scholar]
- 87.Stirling Y, Woolf L, North WR, Seghatchian MJ, Meade TW. Haemostasis in normal pregnancy. Thromb Haemost. 1984;52:176–82. [PubMed] [Google Scholar]
- 88.Bremme KA. Haemostatic changes in pregnancy. Best Pract Res Clin Haematol. 2003;16:153–68. doi: 10.1016/s1521-6926(03)00021-5. [DOI] [PubMed] [Google Scholar]
- 89.Brenner B. Haemostatic changes in pregnancy. Thromb Res. 2004;114:409–14. doi: 10.1016/j.thromres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 90.Erez O, Gotsch F, Mazaki-Tovi S, Vaisbuch E, Kusanovic JP, Kim CJ, Chaiwerapongsa T, Hoppensteadt D, Fareed J, Than NG, et al. Evidence of Maternal Platelet Activation, Excessive Thrombin Generation, and High Amniotic Fluid Tissue Factor Immunoreactivity and Functional Activity in Patients with Fetal Death. Journal of Maternal-Fetal and Neonatal Medicine 2009. J Matern Fetal Neonatal Med. 2009 doi: 10.1080/14767050902853117. [DOI] [PubMed] [Google Scholar]
- 91.Bilsel AS, Onaran N, Moini H, Emerk K. Long-term effect of 17beta-estradiol and thrombin on tissue factor pathway inhibitor release from HUVEC. Thromb Res. 2000;99:173–8. doi: 10.1016/s0049-3848(00)00228-0. [DOI] [PubMed] [Google Scholar]
- 92.Salem HT, Seppala M, Chard T. The effect of thrombin on serum placental protein 5 (PP5): is PP5 the naturally occurring antithrombin III of the human placenta? Placenta. 1981;2:205–9. doi: 10.1016/s0143-4004(81)80003-3. [DOI] [PubMed] [Google Scholar]
- 93.Jones GR, Davey MW, Sinosich M, Grudzinskas JG. Specific interaction between placental protein 5 and heparin. Clin Chim Acta. 1981;%19;110:65–70. doi: 10.1016/0009-8981(81)90301-6. [DOI] [PubMed] [Google Scholar]
- 94.Iino M, Foster DC, Kisiel W. Quantification and characterization of human endothelial cell-derived tissue factor pathway inhibitor-2. Arterioscler Thromb Vasc Biol. 1998;18:40–6. doi: 10.1161/01.atv.18.1.40. [DOI] [PubMed] [Google Scholar]
- 95.Sprecher CA, Kisiel W, Mathewes S, Foster DC. Molecular cloning, expression, and partial characterization of a second human tissue-factor-pathway inhibitor. Proc Natl Acad Sci U S A. 1994;91:3353–7. doi: 10.1073/pnas.91.8.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Udagawa K, Miyagi Y, Hirahara F, Miyagi E, Nagashima Y, Minaguchi H, Misugi K, Yasumitsu H, Miyazaki K. Specific expression of PP5/TFPI2 mRNA by syncytiotrophoblasts in human placenta as revealed by in situ hybridization. Placenta. 1998;19:217–23. doi: 10.1016/s0143-4004(98)90011-x. [DOI] [PubMed] [Google Scholar]
- 97.Udagawa K, Yasumitsu H, Esaki M, Sawada H, Nagashima Y, Aoki I, Jin M, Miyagi E, Nakazawa T, Hirahara F, et al. Subcellular localization of PP5/TFPI-2 in human placenta: a possible role of PP5/TFPI-2 as an anti-coagulant on the surface of syncytiotrophoblasts. Placenta. 2002;23:145–53. doi: 10.1053/plac.2001.0774. [DOI] [PubMed] [Google Scholar]
- 98.Kamei S, Kazama Y, Kuijper JL, Foster DC, Kisiel W. Genomic structure and promoter activity of the human tissue factor pathway inhibitor-2 gene. Biochim Biophys Acta. 2001;1517:430–5. doi: 10.1016/s0167-4781(00)00298-0. [DOI] [PubMed] [Google Scholar]
- 99.Hube F, Reverdiau P, Iochmann S, Trassard S, Thibault G, Gruel Y. Demonstration of a tissue factor pathway inhibitor 2 messenger RNA synthesis by pure villous cytotrophoblast cells isolated from term human placentas. Biol Reprod. 2003;68:1888–94. doi: 10.1095/biolreprod.102.011858. [DOI] [PubMed] [Google Scholar]
- 100.Butzow R, Virtanen I, Seppala M, Narvanen O, Stenman UH, Ristimaki A, Bohn H. Monoclonal antibodies reacting with placental protein 5: use in radioimmunoassay, Western blot analysis, and immunohistochemistry. J Lab Clin Med. 1988;111:249–56. [PubMed] [Google Scholar]
- 101.Kisiel W, Sprecher CA, Foster DC. Evidence that a second human tissue factor pathway inhibitor (TFPI-2) and human placental protein 5 are equivalent. Blood. 1994;84:4384–5. [PubMed] [Google Scholar]
- 102.Aharon A, Lanir N, Drugan A, Brenner B. Placental TFPI is decreased in gestational vascular complications and can be restored by maternal enoxaparin treatment. J Thromb Haemost. 2005;3:2355–7. doi: 10.1111/j.1538-7836.2005.01564.x. [DOI] [PubMed] [Google Scholar]
- 103.Colman RW, Clowes AW, George JN, Goldhaber SZ, Marder VJ. Overview of hemostasis. In: Colman RW, Marder VJ, Clowes AW, George JN, Goldhaber SZ, editors. Hemostasis and Thrombosis Basic Principles and Clinical Practice. 5. Philadelphia: Lipincott Williams & Wilkins; 2009. pp. 3–16. [Google Scholar]
- 104.Kusanovic JP, Espinoza J, Romero R, Hoppensteadt D, Nien JK, Kim CJ, Erez O, Soro E, Fareed J, Edwin S, et al. Plasma protein Z concentrations in pregnant women with idiopathic intrauterine bleeding and in women with spontaneous preterm labor. J Matern Fetal Neonatal Med. 2007;20:453–63. doi: 10.1080/14767050701398272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shimura M, Wada H, Wakita Y, Nakase T, Hiyoyama K, Nagaya S, Mori Y, Shiku H. Plasma tissue factor and tissue factor pathway inhibitor levels in patients with disseminated intravascular coagulation. Am J Hematol. 1997;55:169–74. doi: 10.1002/(sici)1096-8652(199707)55:4<169::aid-ajh1>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]