Abstract
The mechanism by which cells organize into tissues is fundamental to developmental biology and tissue engineering. Likewise, the disruption of cellular order within tissues is a hallmark of many diseases including cancer and atherosclerosis. Tissue formation is regulated, in part, by a balance between cell–cell cohesion and cell–extracellular matrix (ECM) adhesion. Here, experiments and approaches to alter this balance are discussed, and the nature of this balance in the formation of microvasculature is explored. Using matrices of tailored stiffness and matrix presentation, the role of the mechanical properties and ligand density in angiogenesis has been investigated. Decreasing cell–matrix adhesion by either reducing matrix stiffness or matrix ligand density induces the self-assembly of endothelial cells into network-like structures. These structures are stabilized by the polymerization of the extracellular matrix protein fibronectin. When fibronectin polymerization is inhibited, network formation does not occur. Interestingly, this interplay between substrate mechanics, ECM assembly, and tissue self-assembly is not limited to endothelial cells and has been observed in other cell types as well. These results suggest novel approaches to foster stable cell–cell adhesion and engineer tissues.
Keywords: Endothelial cells, Cell mechanics, Substrate stiffness, Fibronectin
Engineering the Cell–Biomaterial Interface
Much of the biomedical engineering community is focused on the important problem of developing methods, tools, and/or materials to recreate or repair various components of the human body. Recreating or rebuilding even the simplest structures requires knowledge of the structure's composition, and recreating tissues and organs within the body are no exception. The human body can be thought of as a hierarchy of biological structures, scaling from cells to tissues to organs to the human body (Fig. 1). There are approximately 1013 cells in the human body and approximately 200 different cell types (erythrocyte, hepatocyte, osteoblasts, etc.). Each one of these cell types resides in a specific ECM. The ECM is most often defined by the fibrous proteins that it contains. However, it also describes the various soluble molecules, including growth factors, present in the extracellular milieu surrounding cells. The individual components of the extracellular matrix are tissue-specific, and the precise interactions of cells with their ECM are the basis for tissue formation. At the apex of this pyramid (Fig. 1), tissues organize to form organs. The goal of tissue engineering is to produce a functional tissue, and this goal is rooted in the ability to understand and control the interaction of cells with their extracellular matrix and how these interactions affect the formation and maintenance of tissues.
Figure 1.
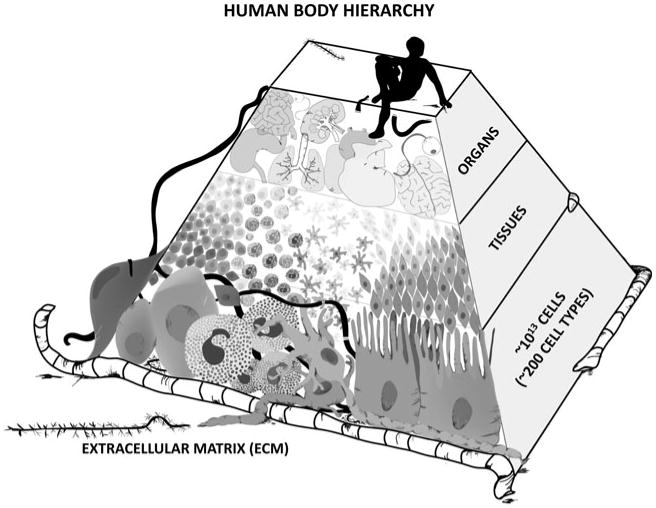
Schematic of the cellular–tissue–organ hierarchy within the human body. Image rendered by Na Young Kim.
Within the biomedical engineering community, much attention has been paid to design principles to guide cell behavior and tissue formation.31 From an engineering perspective, there are three primary tunable parameters in developing cell-instructive scaffolds: chemistry, topography, and mechanics (Fig. 2).
Figure 2.
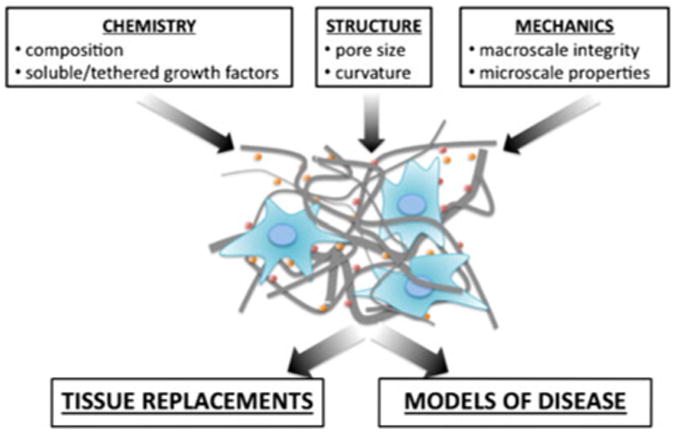
Design parameters for tissue engineering of tissue replacements and disease models.
The chemical environment encountered by cells is perhaps the most studied of the three parameters. This includes the specific composition of the extracellular matrix (both its type and its concentration/density) and the presentation of the extracellular matrix (whether it is soluble, insoluble, uniformly presented, or presented in gradients). The concentration of ECM proteins can alter fundamental behaviors including cell adhesion,26,27 proliferation,1 and cell migration.25 The concentration and presentation of various growth factors are critical regulators of cell growth, motility, and cell–cell adhesion. Because of their roles in regulating fundamental cell behaviors that are central to tissue engineering, historically much attention has been focused on the specific extracellular matrix proteins and growth factors prescribed to cells when engineering the interface between cells and biomaterials.
A second major factor often considered in the engineered design of an instructive cell–material interface is the structure and topography of a given material. The advent of micro and nanofabrication tools and their transition into the world of cell biology has enabled the recreation of ECM-like features into cell culture platforms. There is now significant evidence to show that ridges, grooves, pores, and curvature can all affect basic properties of cell adhesion and migration,37 pointing to an important role for the incorporation of topographical cues in engineering cell-instructive tissue scaffolds.
A third design parameter considered when engineering the interface between a cell and its substrate is the mechanical properties of the material. For centuries, tissue and organ replacements have been designed to be structurally sound and to withstand physiological loads. However, in the past decade, there has been an important recognition that the mechanical properties of a material, in addition to being important at the macroscale, also play a role at the microscale.23 Recent evidence from our lab and others indicates that material stiffness can influence cell–cell adhesion,15,28 cell– substrate adhesion,5,6,25 cell differentiation,11,21 and even the progression of disease.22,34 Therefore, while materials need to be designed to support physiological loads at the macroscale, they must also be tuned to support healthy cell phenotypes.
Engineering Models of Disease
Historically, biomaterials research has been focused on the development of materials and structures for use in the replacement of native organs and tissues for human body repair.2 However, in more recent years, there has been heightened interest in the development of biomaterials and in vitro constructs that mimic the disease state.14 The potential impact of these materials lies in their ability to enable research that investigates factors that contribute to cell dysfunction during disease progression. Compared to animal models, tissue-engineered constructs are easier to manipulate and control. Compared to more conventional polystyrene cell culture dishes, tissue engineering constructs can be tailored with specific chemistries, topographies, and mechanical properties. Scaffolds can be designed to support 3D cell adhesion, present growth factors in specific orientations or gradients, and re-create the mechanical environment to mimic changes that occur during disease progression. To date, tissue engineering and biomaterial research has shown significant promise for use in the study of disease mechanisms. Tailored biomaterial substrates have been used by a number of groups, including our own, to investigate the role of substrate mechanics and chemistry in the formation of vascular networks,3,4 cancer progression,22,34 and the progression of atherosclerosis.6,29
A Role for Matrix Mechanics in Cell–Cell Cohesion
As described above, the current interest in the role of matrix mechanics in affecting cell behavior has gained significant interest in the past several years.10 Pelham and Wang23 were the first to describe a tunable, tractable method to control substrate mechanics using poly-acrylamide polymers almost a decade and a half ago. Since the publication of this landmark paper, a number of investigators have adopted the use of poly-acrylamide substrates as a model material to control the modulus of the substrates presented to cells.10,13,18,26,33 The modulus of poly-acrylamide substrates is tuned based on the ratio of acrylamide to bis-acrylamide. Substrates have been made as compliant as 50 Pa and as stiff as 100 kPa moduli which span a large range of physiological mechanical properties. Because poly-acrylamide substrates are inert to cell adhesion, the poly-acrylamide must be conjugated with cell-adhesive ligands to be amenable as a cell culture platform.25 This provides an additional level of control over the environment presented to the cells, as the specific matrix protein type and density can be tailored.
Early experiments using mechanically tunable substrates demonstrated the effects of substrate modulus on migration, proliferation, and adhesion. Later studies have focused on the effects of disease-related tissue stiffening on cell behavior. In our own experiments, we have focused on the role of substrate mechanics and cell-generated traction stresses in tissue assembly and cell–cell cohesion.3,28 Early work using polyacryamide substrates spanning a wide range of moduli (200 Pa–30 kPa) demonstrated that the nature of cell–cell interactions changes with matrix stiffness.28 Pairs of endothelial cells interacting on compliant substrates (E = 500 Pa) tend to remain in contact, while cells on stiffer substrates tend to separate and migrate away from each other. This was the first evidence that matrix stiffness may play a role in endothelial cell–cell cohesion, and it motivated later studies investigating the effects of stiffness on vascular network formation, described below.
The behavior observed in endothelial cells on compliant substrates3,28 does not appear to be limited to endothelial cells. Guo et al. reported that cells in neonatal cardiac tissue aggregates placed on compliant substrates tend to remain in the aggregate, whereas cells in tissue aggregates placed on stiffer substrates tend to migrate out from the aggregate.15 Additionally, when seeded as sub-confluent cultures, fibroblasts on complaint substrates tend to aggregate into “tissue-like” aggregates, whereas cells on stiffer substrates tend to form monolayers. An analogous behavior occurs in mammary epithelial cells as well; cells on compliant substrates form distinct acinar structures, whereas cells on stiffer substrates display disrupted cell–cell contacts.22 Taken together, these studies point to a role of substrate mechanics in mediating cell–cell adhesion.
Endothelial Cell–Substrate Interactions in Angiogenesis
Angiogenesis is the formation of capillaries from pre-existing vascular networks.6 Because of its role in wound healing and tumor formation, it has been widely studied by both the tissue engineering and cancer research communities. Most tissue engineering applications are hindered by the need for vasculature and a blood supply. Therefore, the ability to induce and control angiogenesis is a critical step in the engineering of most tissues. Likewise, during cancer progression, a blood supply is required for the growth of a tumor beyond just a few millimeters in diameter.12 As such, the ability to control the formation of new vasculature could be a viable mechanism for controlling and limiting tumor growth.
Because of the widespread, avid study of angiogenesis, multiple in vitro and in vivo platforms exist to investigate the factors contributing to blood vessel formation and growth. Tubulogenesis and the formation of networks in vitro are common metrics of angiogenic ability. Given sufficient time to secrete their own extracellular matrix, most endothelial cell types will form tubules. However, when plated in collagen gels,9 fibrin gels,32,35 or matrigel19 scaffolds, the assembly of endothelial cells into new vasculature is greatly enhanced and can occur in approximately 24 h.
It is interesting to note that the matrices where tubulogenesis has been reported to occur, including matrigel, collagen and fibrin, are all very compliant compared to glass and tissue culture polystyrene. In all three matrices, the modulus of the material is dependent on the density or concentration of the material. At the concentrations typically used for angiogenesis assays, the modulus of these materials is on the order of hundreds of pascals, compared to the megapascal modulus of polystyrene where networks do not spontaneously form. Because tubulogenesis primarily occurs in compliant matrices, the question that my lab has sought to address is whether the mechanical properties of the extracellular matrix affect angiogenic network formation.
Matrix Mechanics in Angiogenesis
To date, the assembly of networks from endothelial cells in vitro has been primarily reported in relatively compliant matrices (E < 1000 Pa) like matrigel and collagen. In addition to be relatively compliant, these extracellular matrices also provide specific chemical cues through integrin-ligand binding in a 3D environment. To determine whether the modulus of the matrix contributes to cell–cell connectivity during network formation independently of the cues provided by the dimensionality and integrin-ligand binding provided by conventional matrices, we employed the mechanically tunable poly-acrylamide substrate described above.3 Thus, changes in matrix stiffness are de-coupled from changes in integrin-ligand density. Using this platform, substrates that ranged in modulus from E = 200 Pa to 10,000 Pa were fabricated and seeded with endothelial cells. This modulus range was chosen because it spans the moduli found for multiple soft tissues including normal mammary tissue and mammary tumor tissue.22 On more compliant substrates (E < 1000 Pa), endothelial cells self-assembled into network structures; cells elongated to form network branches and stable cell–cell connections (Fig. 3). In contrast, cells on stiffer substrates spread with no obvious preference to form cell–cell connections, and network formation did not occur; cells on stiff poly-acrylamide substrates resembled cells on polystyrene dishes (Fig. 3).
Figure 3.
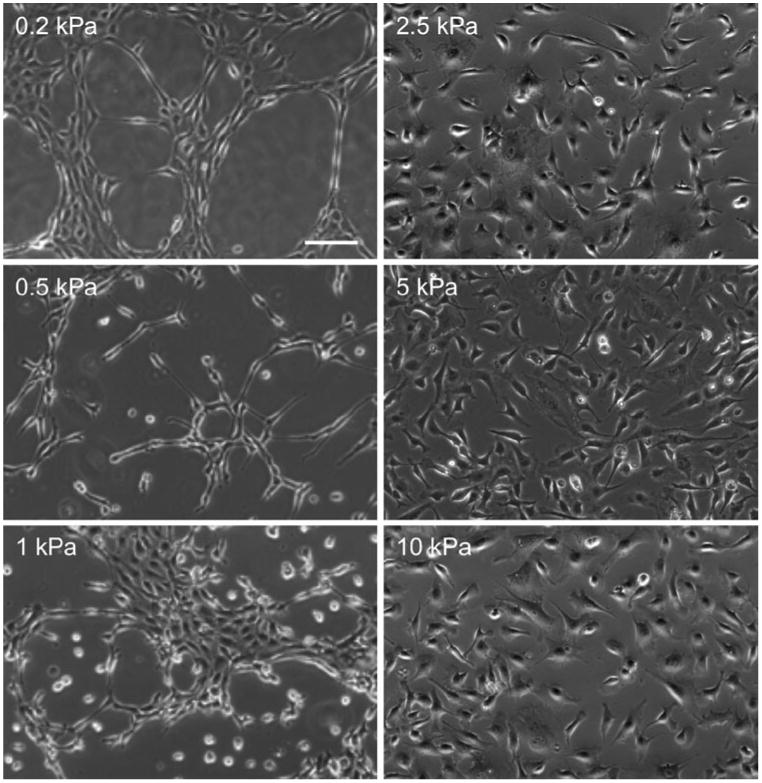
Endothelial cell connectivity is a function of substrate stiffness. Bovine aortic endothelial cells were plated on substrates of E = 0.2, 0.5, 1.0, 2.5, 5.0 and 10.0 kPa. Self-assembly into networks occurs on more compliant substrates (E = 0.2 and 0.5 kPa) and not stiffer substrates (E>1.0 kPa). Scale bar is equal to 100 μm. Images provided by J.P. Califano.
Time-lapse microscopy of endothelial cells on compliant substrates indicated that cells exhibited significant shape changes during network formation, elongating in the direction of adjacent cells to form networks (Fig. 4). Additionally, this elongation and contact occurred over very long distances (> 300 μm), where cells on opposite sides of a ring within a network extended protrusions toward each other. These protrusion events were followed by proliferation, elongation, and directed migration toward the opposing protrusion to ultimately form connections.
Figure 4.
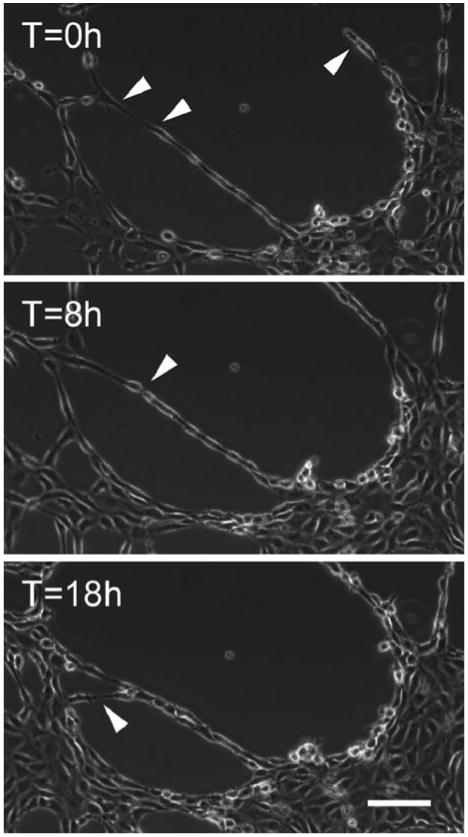
Time-lapse images of endothelial cells during network formation. Note the formation of connection over across large gaps, indicated by arrows. Images provided by J.P. Califano.
The mechanism by which cells sense other cells to connect remains unclear. Our prior work indicates that pairs of cells have the ability to mechanically communicate through compliant substrates.28 Individual cells exert traction stresses that create strains in the substrate that are detected by an adjacent cell. In response to these strains, the cells migrate toward each other. It remains unclear if the mechanical communication observed for cell pairs also occurs in developing networks, and the exact intracellular signaling mechanism by which cell-created substrate strains are detected by adjacent cells has not been extensively studied yet. However, several other groups have investigated cell response to imposed strains and the mechanisms of mechanosensing.7,29,36 When fibronectin-coated beads are bound to a cell and restrained using an optical trap, the cell-bead binding strength increases and focal adhesions grow.7 Additionally, it has been shown that focal adhesion kinase (FAK) activity is required for the formation of focal adhesions and reorientation of cell movements due to strains imposed on the substrate through micropipette manipulation.36 These data suggest that one mechanism by which cells mechanically sense each other is through the growth of focal adhesions, the reorganization of the cytoskeleton, and the subsequent reorientation of the cell.
It is well established that cells on more compliant matrices exhibit less spreading and are more weakly adhered to the substrate.5,38 Decreased cell–matrix adhesion on more compliant substrates may in fact support increased cell–cell adhesion due to a proposed balance between cell–cell and cell–matrix adhesion that optimizes the mechanical feedback to cells. If cells are unable to adhere well to a substrate, then cell–cell adhesion is enhanced to enable the cells to assemble their cytoskeleton and spread. This same hypothesis has been explored in the context of integrin and cadherin adhesion as a function of ligand density to show that cells are more likely to dissociate from an aggregate if the substrate is sufficiently adhesive or integrin expression is increased.20,24,30 To further explore this hypothesis in the context of our network formation assay, we altered cell–substrate adhesion by decreasing the density of collagen type I conjugated to the poly-acrylamide gels.3 Interestingly, when the collagen density was decreased on stiffer substrates, endothelial cell–cell connectivity increased. While formal endothelial cell networks did not form, there was increased cell aggregation and cell–cell contact that was reminiscent of immature networks. These data suggest that cell–cell connectivity is governed in part by cell–matrix adhesion, and a balance between the two may exist.
While traction stresses and cell–substrate adhesion strength may contribute to the ability of cells to find each other on a compliant substrate, it is unclear which intracellular or extracellular factors determine whether cells remain in contact. Because fibronectin matrix synthesis has been reported to stabilize networks in vivo, we investigated fibronectin polymerization as a function of stiffness in our in vitro system.3 Interestingly, fibronectin localized to the network structures. Moreover, we determined that the fibronectin fibrils were secreted from the cells and were not necessarily derived from the serum in the media. When fibronectin polymerization was inhibited, cells migrated toward each other, but networks did not form. These data indicate that while substrate mechanics may drive cells together, fibronectin polymerization stabilizes endothelial cell–cell connections. These data also present the interesting possibility that the nature of cell– substrate adhesion mediated by matrix mechanics can alter how cells secrete and polymerize matrix proteins.
Implications of Tissue Stiffness in Angiogenesis: Future Directions
The need for viable vasculature remains a critical hurdle in most tissue engineering applications. While significant emphasis has been placed on the growth factors required to induce angiogenesis, more recent data suggest that matrix mechanics can drive the self-assembly of endothelial cell networks.3,6 These results emphasize the need to consider the mechanical properties of a biomaterial to elicit a particular cell response, namely infiltration of new capillary networks. The data described above suggest that more compliant matrices foster cell–cell adhesion and the assembly of networks better than stiffer matrices.
These data may also have ramifications for our understanding of angiogenesis in tumor formation. Vasculature within solid tumors is typically abnormal compared to vasculature in healthy tissue. It is often more tortuous and hyper-permeable, lacking structural integrity.17 Therapeutically, there is growing interest in normalizing tumor vasculature to improve drug delivery into the tumor and fortify the barrier preventing metastatic cell escape into the vasculature.16 Given that most solid tumors are stiffer than healthy tissue8,22 and matrix stiffness can lead to the disruption of cell–cell contacts,3,28 tumor stiffness may be one underlying causes of the tortuous, permeable structure of the tumor vasculature. We speculate that one approach to normalize tumor vasculature may be to disrupt matrix deposition and cross-linking in the tumor stroma, however, this is an area that requires additional investigation.
Acknowledgments
This paper is based on the 2010 Rita Schaffer Memorial Lecture that I presented in October 2010 at the Annual BMES Meeting in Austin, TX. The Rita Schaffer Young Investigator Award is given annually “to a young investigator whose originality and ingenuity in a published work are recognized by the Awards Committee.” I was nominated, in part, for work performed by my graduate student, Joseph Califano, published in Cellular and Molecular Bioengineering.3 I have been told that the success of a young independent investigator often depends on the success of the first one or two graduate students to join the lab. I am grateful to have so many talented graduate students, including Joe, in my lab while so early in my career.
My path to an independent career has been paved by excellent mentoring I have received throughout my training. As an undergraduate at MIT, I had the privilege of working in Doug Lauffenburger's lab under the direct supervision of then graduate student, now associate professor, Anand Asthagiri. Experiencing the excitement for research cultivated in the Lauffenburger Lab initiated and solidified my own decision to pursue a career in academia—the enthusiasm was contagious. As a graduate student at the University of Pennsylvania, I worked with Dan Hammer. Dan's keen physical insights and mentoring style inspired success, and I am extremely grateful for having had the opportunity to receive my PhD under his guidance. While a graduate student, I was a visiting scholar at the University of Rochester in the lab of Rick Waugh. Despite not being one of Rick's students, he treated me like one of his research-family and I am thankful for his generosity. As a postdoctoral associate, I worked at the University of Rochester School of Medicine and Dentistry at the Aab Cardiovascular Research Institute under the guidance of Brad Berk and Keigi Fujiwara. Working in a center focused on cardiovascular health exposed me to research problems tied very closely to human disease and truly helped me stay at the interface of biology, medicine, and engineering. Each one of these research experiences has shaped my scientific abilities and inspired my career. My hope, going forward, is that I can inspire my own students in the same way my mentors have inspired me.
I would like to thank the following agencies for funding our research: the National Institutes of Health, the National Science Foundation, the American Heart Association, the American Federation for Aging Research, and the Cornell Nanobiotechnology Center.
Footnotes
Conflict of Interest: The author declares no conflicting or competing interests.
References
- 1.Asthagiri AR, Reinhart CA, Horwitz AF, Lauffenburger DA. The role of transient ERK2 signals in fibronectin- and insulin-mediated DNA synthesis. J Cell Sci. 2000;113(Pt 24):4499–4510. doi: 10.1242/jcs.113.24.4499. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia SK. Tissue engineering for clinical applications. Biotechnol J. 2010;5(12):1309–1323. doi: 10.1002/biot.201000230. [DOI] [PubMed] [Google Scholar]
- 3.Califano JP, Reinhart-King CA. A balance of substrate mechanics and matrix chemistry regulates endothelial cell network assembly. Cell Mol Bioeng. 2008;1(2–3):122–132. [Google Scholar]
- 4.Califano JP, Reinhart-King CA. The effects of substrate elasticity on endothelial cell network formation and traction force generation. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:3343–3345. doi: 10.1109/IEMBS.2009.5333194. [DOI] [PubMed] [Google Scholar]
- 5.Califano JP, Reinhart-King CA. Substrate stiffness and cell area predict cellular traction stresses in single cells and cells in contact. Cell Mol Bioeng. 2010;3(1):68–75. doi: 10.1007/s12195-010-0102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Califano JP, Reinhart-King CA. Exogenous and endogenous force regulation of endothelial cell behavior. J Biomech. 2010;43(1):79–86. doi: 10.1016/j.jbiomech.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Choquet D, Felsenfeld DP, Sheetz MP. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell. 1997;88(1):39–48. doi: 10.1016/s0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- 8.Cukierman E, Bassi DE. Physico-mechanical aspects of extracellular matrix influences on tumorigenic behaviors. Semin Cancer Biol. 2010;20(3):139–145. doi: 10.1016/j.semcancer.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis GE, Bayless KJ, Mavila A. Molecular basis of endothelial cell morphogenesis in three-dimensional extracellular matrices. Anat Rec. 2002;268(3):252–275. doi: 10.1002/ar.10159. [DOI] [PubMed] [Google Scholar]
- 10.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310(5751):1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 11.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 12.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1(1):27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 13.Gaudet C, Marganski WA, Kim S, Brown CT, Gunderia V, Dembo M, Wong JY. Influence of type I collagen surface density on fibroblast spreading, motility, and contractility. Biophys J. 2003;85(5):3329–3335. doi: 10.1016/S0006-3495(03)74752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grayson WL, Martens TP, Eng GM, Radisic M, Vunjak-Novakovic G. Biomimetic approach to tissue engineering. Semin Cell Dev Biol. 2009;20(6):665–673. doi: 10.1016/j.semcdb.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo WH, Frey MT, Burnham NA, Wang YL. Substrate rigidity regulates the formation and maintenance of tissues. Biophys J. 2006;90(6):2213–2220. doi: 10.1529/biophysj.105.070144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001;7(9):987–989. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 17.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 18.Kandow CE, Georges PC, Janmey PA, Beningo KA. Polyacrylamide hydrogels for cell mechanics:steps toward optimization and alternative uses. Methods Cell Biol. 2007;83:29–46. doi: 10.1016/S0091-679X(07)83002-0. [DOI] [PubMed] [Google Scholar]
- 19.Kubota Y, Kleinman HK, Martin GR, Lawley TJ. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J Cell Biol. 1988;107(4):1589–1598. doi: 10.1083/jcb.107.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lauffenburger DA, Griffith LG. Who's got pull around here? Cell organization in development and tissue engineering. Proc Natl Acad Sci USA. 2001;98(8):4282–4284. doi: 10.1073/pnas.081083698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6(4):483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 22.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8(3):241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Pelham RJ, Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci USA. 1997;94(25):13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Powers MJ, Griffith LG. Adhesion-guided in vitro morphogenesis in pure and mixed cell cultures. Microsc Res Tech. 1998;43(5):379–384. doi: 10.1002/(SICI)1097-0029(19981201)43:5<379::AID-JEMT4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 25.Reinhart-King CA. Endothelial cell adhesion and migration. Methods Enzymol. 2008;443:45–64. doi: 10.1016/S0076-6879(08)02003-X. [DOI] [PubMed] [Google Scholar]
- 26.Reinhart-King CA, Dembo M, Hammer DA. Endothelial cell traction forces on RGD-derivatized polyacrylamide substrata. Langmuir. 2003;19(5):1573–1579. [Google Scholar]
- 27.Reinhart-King CA, Dembo M, Hammer DA. The dynamics and mechanics of endothelial cell spreading. Biophys J. 2005;89(1):676–689. doi: 10.1529/biophysj.104.054320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reinhart-King CA, Dembo M, Hammer DA. Cell–cell mechanical communication through compliant substrates. Biophys J. 2008;95(12):6044–6051. doi: 10.1529/biophysj.107.127662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reinhart-King CA, Fujiwara K, Berk BC. Physiologic stress-mediated signaling in the endothelium. Methods Enzymol. 2008;443:25–44. doi: 10.1016/S0076-6879(08)02002-8. [DOI] [PubMed] [Google Scholar]
- 30.Ryan PL, Foty RA, Kohn J, Steinberg MS. Tissue spreading on implantable substrates is a competitive outcome of cell–cell vs. cell–substratum adhesivity. Proc Natl Acad Sci USA. 2001;98(8):4323–4327. doi: 10.1073/pnas.071615398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwarz US, Bischofs IB. Physical determinants of cell organization in soft media. Med Eng Phys. 2005;27(9):763–772. doi: 10.1016/j.medengphy.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 32.Stephanou A, Meskaoui G, Vailhe B, Tracqui P. The rigidity in fibrin gels as a contributing factor to the dynamics of in vitro vascular cord formation. Microvasc Res. 2007;73(3):182–190. doi: 10.1016/j.mvr.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Tse JR, Engler AJ. Preparation of hydrogel substrates with tunable mechanical properties. Curr Protoc Cell Biol. 2010;Chapter 10 doi: 10.1002/0471143030.cb1016s47. Unit 10.16. [DOI] [PubMed] [Google Scholar]
- 34.Ulrich TA, de Juan Pardo EM, Kumar S. The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells. Cancer Res. 2009;69(10):4167–4174. doi: 10.1158/0008-5472.CAN-08-4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vailhe B, Ronot X, Tracqui P, Usson Y, Tranqui L. In vitro angiogenesis is modulated by the mechanical properties of fibrin gels and is related to alpha(v)beta3 integrin localization. In Vitro Cell Dev Biol Anim. 1997;33(10):763–773. doi: 10.1007/s11626-997-0155-6. [DOI] [PubMed] [Google Scholar]
- 36.Wang HB, Dembo M, Hanks SK, Wang Y. Focal adhesion kinase is involved in mechanosensing during fibroblast migration. Proc Natl Acad Sci USA. 2001;98(20):11295–11300. doi: 10.1073/pnas.201201198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Y, Leong KW. Nanoscale surfacing for regenerative medicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2(5):478–495. doi: 10.1002/wnan.74. [DOI] [PubMed] [Google Scholar]
- 38.Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, Zahir N, Ming W, Weaver V, Janmey PA. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskelet. 2005;60(1):24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]


