Abstract
The luciferase protein fragment complementation assay is a powerful tool for studying protein-protein interactions. Two inactive fragments of luciferase are genetically fused to interacting proteins, and when these two proteins interact, the luciferase fragments can reversibly associate and reconstitute enzyme activity. Though this technology has been used extensively in live eukaryotic cells, split luciferase complementation has not yet been applied to studies of dynamic protein-protein interactions in live bacteria. As proof of concept and to develop a new tool for studies of bacterial chemotaxis, fragments of Renilla luciferase (Rluc) were fused to the chemotaxis-associated response regulator CheY3 and its phosphatase CheZ in the enteric pathogen Vibrio cholerae. Luciferase activity was dependent on the presence of both CheY3 and CheZ fusion proteins, demonstrating the specificity of the assay. Furthermore, enzyme activity was markedly reduced in V. cholerae chemotaxis mutants, suggesting that this approach can measure defects in chemotactic signaling. However, attempts to measure changes in dynamic CheY3-CheZ interactions in response to various chemoeffectors were undermined by nonspecific inhibition of the full-length luciferase. These observations reveal an unexpected limitation of split Rluc complementation that may have implications for existing data and highlight the need for great caution when evaluating small molecule effects on dynamic protein-protein interactions using the split luciferase technology.
Introduction
Genetically encoded reporters have revolutionized the study of bacterial protein-protein interactions. Two-hybrid systems, which couple transcriptional activation of a reporter gene to protein binding, have been widely used to characterize novel interaction partners [1]. Protein fragment complementation assays (PCA), in which two inactive fragments of a monomeric reporter, such as β-galactosidase or green fluorescent protein, are fused to interacting proteins, have also been used to measure binding interactions [2], [3]. However, both of these approaches typically suffer from limitations (e.g., lag time of transcriptional activation or fluorescent protein maturation, irreversible signal amplification over time) that preclude real-time analyses of dynamic interactions. Fluorescence and bioluminescence resonance energy transfer (FRET and BRET, respectively) assays can overcome these limitations [4], [5], but are more technically demanding. Thus, a tractable assay that enables quantitative and sensitive measurements of dynamic interactions in real-time would be a powerful addition to the existing toolkit for studies of bacterial protein interactions.
Luciferase protein fragment complementation (a.k.a. split luciferase complementation) is a recently described PCA that has been used to analyze dynamic protein-protein interactions in live mammalian cells [6]–[10]. This assay relies on the division of a bioluminescent luciferase enzyme into two inactive fragments that are genetically fused to interacting proteins. When these two proteins interact, the luciferase fragments can associate, thereby reconstituting luciferase activity. Importantly, unlike fluorescent protein complementation strategies, split luciferase complementation is reversible; it can be used to monitor protein-protein interactions in real-time, unlike two-hybrid approaches and most other protein fragment complementation assays; finally, unlike FRET/BRET, it does not require monitoring of filtered light emissions [3], [10]–[12]. Notably, this technology was recently adapted to monitor the assembly of a stable protein complex in Salmonella [13].
We hypothesized that split luciferase complementation could represent a versatile approach to studying dynamic protein-protein interactions in bacteria. Though several luciferase enzymes have been developed into split reporters, we chose to use recently described fragments of Renilla reniformis luciferase (Rluc) [11] for our assay because the light-emitting substrate of Rluc, coelenterazine (clz), can easily pass through the cell envelope of Gram-negative bacteria [13], [14], unlike the more common luciferase substrate D-luciferin, which requires acidic pH for optimal membrane permeability [15], [16]. As a first application for proof of concept, we fused these fragments to two chemotaxis proteins from Vibrio cholerae, the curved Gram-negative bacterium that causes human cholera.
Classically, bacterial chemotaxis reflects the sensing of external stimuli by membrane-associated receptors that transmit a signal through a cytoplasmic cascade that modulates flagellar rotation [17]. Membrane-embedded methyl-accepting chemotaxis proteins (MCPs) initiate chemotactic signaling upon detecting a change in local chemical gradients. Binding of a chemorepellent (or a decrease in chemoattractant binding) to its cognate MCP induces a conformational change that leads to autophosphorylation of a cytosolic kinase, CheA. CheA subsequently phosphorylates the response regulator CheY, which diffuses across the cell and binds the flagellar motor switch protein FliM. This binding switches the direction of flagellar rotation, which induces random reorientation of the cell. The lifetime of phosphorylated CheY is tightly regulated by the phosphatase CheZ, which hydrolyzes CheY’s phosphate group, thereby terminating the chemotactic signal. Pathway activity is also regulated by methyltransferase and methylesterase proteins (CheR and CheB, respectively), which modulate pathway sensitivity. Collectively, these proteins allow bacteria to maintain their direction (straight swimming) under favorable conditions, and alter their direction (tumbling) under adverse conditions.
V. cholerae encodes an unusually large number of putative MCPs, as well as three potentially independent clusters of downstream signaling proteins (chemotaxis clusters I, II, and III) [18], [19]. Several cluster II genes, such as cheY3 and cheA2, are required for chemotaxis in vitro [20]; however, genes from the other two clusters remain largely uncharacterized, and ligands have not been identified for any of the receptors.
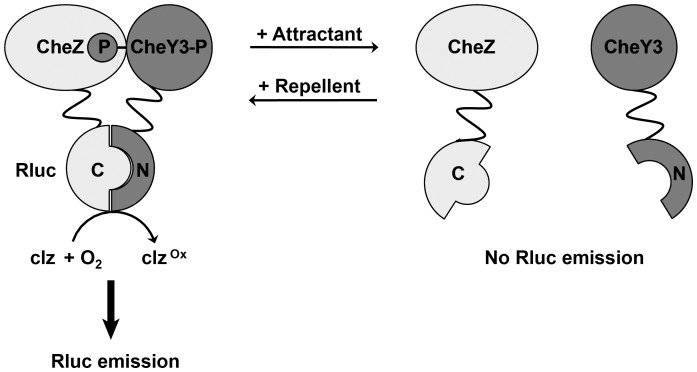
Progress in studies of bacterial chemotaxis has been hindered by a dependence on low-throughput and/or semi-quantitative chemotaxis assays (i.e., capillary assays, soft-agar swarm plate assays) [21], [22]. An assay based on the split luciferase technology could provide a sensitive, quantitative, and rapid microplate-based approach to studying bacterial chemotaxis, with particular utility for characterizing novel chemoeffectors. Importantly, elegant BRET and FRET analyses of chemotaxis by Berg and colleagues have demonstrated that the interaction of CheY and CheZ proteins in E. coli is directly proportional to chemotaxis pathway activity [14], [23]. However, due to their relative technical complexity, these assays are not widely used or easily adapted to a high-throughput format. We hypothesized that fusion of Rluc fragments to homologous proteins from V. cholerae would enable a direct measure of chemotactic responses and provide a more tractable platform for chemoeffector characterization (Figure 1).
Figure 1. Design of an Rluc-based PCA for studies of Vibrio cholerae chemotaxis.
CheY3 and CheZ proteins fused to inactive Rluc N- and C-terminal fragments, respectively, associate upon CheY3 phosphorylation, reconstituting full-length Rluc, which oxidizes the light-emitting substrate coelenterazine (clz).
Here, we demonstrate that an Rluc-based PCA can be used to measure CheY3-CheZ interactions in V. cholerae and that this approach can quantify differences in chemotactic signaling. However, nonspecific inhibition of Rluc activity by small molecule effectors compromises the utility of this technique in measuring dynamic protein-protein interactions. These findings uncover a critical limitation of split luciferase complementation that may have broad implications for existing and future applications of this technology.
Materials and Methods
Growth Conditions and Media
V. cholerae and E. coli were grown at 37°C in LB medium or on LB agar plates supplemented as needed with the following antibiotics: 5 µg/mL chloramphenicol and 200 µg/mL streptomycin (V. cholerae); 20 µg/mL chloramphenicol (E. coli).
Strains and Plasmids
A complete list of the strains, plasmids, and primers used in this study can be found in the Supplemental Information (Table S1, S2, and S3). The V. cholerae El Tor clinical isolate C6706 was used to generate all V. cholerae mutant strains. The E. coli strains DH5αλpir and SM10λpir were used for cloning and for conjugation, respectively, and MG1655 was used for bioluminescence assays.
Strain SR28 (Δche2, a deletion which encompasses the entire V. cholerae chemotaxis cluster II) was generated using standard allele exchange techniques as previously described [24]. Strains SR33 (Δche1che2che3) and ΔcheY3cheZ were similarly prepared using derivatives of plasmid pCVD442 [25]. Strain SR33 was created by consecutive deletions of chemotaxis clusters I, II, and III using plasmids pSR1157, pSR1020, and pSR1158, respectively. Strain ΔcheY3cheZ was created by simultaneously deleting the adjacent cheY3 and cheZ genes of chemotaxis cluster II using plasmid pCVD442-ΔcheY3cheZ. Plasmids pSR1157, pSR1020, pSR1158, and pCVD442-ΔcheY3cheZ were conjugated into V. cholerae using E. coli strain SM10λpir [26].
Plasmid pSR1157 was constructed by PCR amplification of the up- and downstream regions flanking genes vc1394 and vc1406, respectively, using primer pairs che1-cc/che1-dd and che1-aa/che1-bb and V. cholerae C6706 genomic DNA as template. Equal amounts of these PCR products were combined and used as template in a third PCR reaction using primers che1-aa and che1-dd. The resulting product was digested with XbaI and ligated into similarly digested pCVD442 plasmid to generate pSR1157. Plasmid pSR1020 was constructed as previously described [24]. Plasmid pSR1158 was constructed by PCR amplification of the up- and downstream regions flanking genes vca1088 and vca1096, respectively, using primer pairs che3-cc/che3-dd and che3-aa/che3-bb and V. cholerae C6706 genomic DNA as template. Equal amounts of these PCR products were combined and used as template in a third PCR reaction using primers che3-aa and che3-dd. The resulting product was digested with XbaI and ligated into similarly digested pCVD442 plasmid to generate pSR1158.
Plasmid pCVD442-ΔcheY3cheZ was constructed by PCR amplification of the regions flanking the cheY3 (vc2065) and cheZ (vc2064) genes of chemotaxis cluster II using V. cholerae C6706 genomic DNA as template. Primers SKH-51 and SKH-52 were used to amplify the region downstream of cheY3, and primers SKH-53 and SKH-54 were used to amplify the region upstream of cheZ. Equal amounts of these PCR products were then combined and used as template in a final PCR reaction with primers SKH-51 and SKH-54. The resulting product was digested with XbaI and ligated into similarly digested pCVD442 plasmid to generate pCVD442-ΔcheY3cheZ.
The rluc gene was cloned from the pRL-null vector (Promega). The cheY3 and cheZ genes were cloned from V. cholerae C6706 genomic DNA. Split Rluc complementation fragments RlucN and RlucC corresponded to amino acids 1–110 and 111–311, respectively. A T2A mutation was introduced in RlucN to preserve the amino acid sequence used in the original split Rluc construct [11], [27]. Fusions to CheY3 and CheZ were constructed by PCR using primers with complementary overhangs. Following initial amplification of each chemotaxis gene and Rluc fragment, a second round of PCR was used to anneal cheY3 with rlucN and cheZ with rlucC. A ribosome-binding site was introduced upstream of cheZ (and the rlucC fragment of the control plasmid) to enable tandem assembly of both Rluc fusions (and the Rluc fragments alone) into a single expression vector. The cheY3-rlucN and cheZ-rlucC inserts were sequentially introduced into pBAD33 (chloramphenicol resistant) under an arabinose-inducible promoter using the KpnI/XbaI and XbaI/SphI restriction sites, respectively, to give plasmid pYNZC. The rlucN and rlucC inserts were cloned into pBAD33 using the same restriction sites to give plasmid pRlucN-RlucC. Plasmid pRluc was constructed by PCR amplification of the rluc gene using primers SKH-71 and SKH-76 followed by digestion with KpnI/SphI and ligation into similarly digested pBAD33. The final constructs were confirmed by DNA sequencing and transformed into V. cholerae and E. coli via electroporation.
Preparation of Cells for Bioluminescence Assays
An overnight culture of V. cholerae or E. coli was prepared in 2-mL LB supplemented with chloramphenicol and inoculated with cells from a frozen glycerol stock. The culture was incubated at 37°C with shaking at 250–275 rpm. The following day, the overnight culture was diluted 1∶100 in 5 mL fresh LB-chloramphenicol containing 0.2% (v/v) L-arabinose, which conferred optimal restoration of swarming activity in ΔcheY3cheZ V. cholerae cells, and grown for 3 h at 37°C on a rotary shaker to an OD∼1. The culture was washed twice with phosphate buffered saline (PBS; pH 7), adjusted to an OD∼0.4–0.5, and aliquoted into a white 96-well plate in triplicate.
Bioluminescence Assays
Aliquots of native coelenterazine (clz) or benzyl-coelenterazine (clz-h) (NanoLight) dissolved in ethanol were stored at −80°C. Solutions (30 mM in distilled water) of D-glucose (American Bioanalytical), L-serine, L-alanine, L-arginine, D-mannose, and nickel chloride (Sigma) were prepared and used at a concentration of 1 mM. Prior to each assay, a single aliquot of clz/clz-h was thawed, diluted in PBS to a final concentration of 250 µM, and incubated in the dark at room temperature for 1 h to stabilize the clz/clz-h signal. To measure changes in split Rluc complementation resulting from small molecule-induced effects on CheY3-CheZ binding, we established conditions that would provide a stable split Rluc signal. Because the kinetics of Rluc luminescence are characterized by a rapid drop in signal intensity over time [14], obtaining a stable baseline is essential for comparing pre- and post-stimulation values. We determined that incubating cells with clz for 30 min at room temperature prior to initiating the bioluminescence read produced a consistently stable split Rluc signal. Thus, clz/clz-h was added to a final concentration of 7.5 µM to the PBS-washed cells, which were subsequently incubated in the dark for 30 min at room temperature to allow the luciferase-generated signal to stabilize. The total luminescence of each well was then measured every minute for a total of 5 min with an integration time of 1 sec using a SpectraMax L Luminescence Microplate Reader (Molecular Devices). Immediately thereafter, a single chemoeffector (or an equal volume of distilled water) was manually injected into each well and a second luminescence read was initiated as before. Data analysis was performed using Microsoft Excel.
Western Blot Analysis
For analysis of CheY3-RlucN/CheZ-RlucC expression levels in different genetic backgrounds, 1 mL of cells from cultures of equal OD was centrifuged for 1 min at 15 krpm and frozen at −80°C. The following day, cells were resuspended in 150 µL 50 mM Tris buffer (pH 8) containing 1% (v/v) sodium dodecyl sulfate and incubated at 95°C for 5 min. Cells were lysed by sonication (10 1-sec pulses at an output setting of 5; Fisher Scientific Model 60 Sonic Dismembrator), treated with 4X NuPAGE LDS sample buffer (Life Technologies) containing 4 mM dithiothreitol, and incubated at 65°C for 10 min prior to SDS-PAGE and immunoblotting with the anti-Rluc antibody (1∶100,000 dilution; mouse monoclonal, clone 5B11.2; Millipore). Protein bands were detected using a horseradish peroxidase-conjugated secondary antibody (1∶2000 dilution; goat anti-mouse IgG-HRP, sc-2005; Santa Cruz Biotechnology) and chemiluminescent reagents (Pierce).
Results
Split Rluc Complementation Measures CheY3-CheZ Interaction in Vibrio cholerae
To explore the utility of split Rluc complementation for probing dynamic protein-protein interactions in V. cholerae, we applied the technology toward the goal of developing a novel chemotaxis assay that could in principle enable rapid, quantitative measurements of chemotaxis in response to chemotactic stimuli. Since previous BRET- and FRET-based analyses of chemotactic signaling in E. coli [14], [23] demonstrated that the interaction of the CheY and CheZ proteins is directly proportional to chemotaxis pathway activity, we reasoned that fusion of Rluc fragments to homologous proteins from V. cholerae (CheY3 and CheZ, respectively) would enable a direct measure of chemotactic responses. Notably, unlike existing chemotaxis assays, we envisioned that split Rluc complementation performed in a microplate-based format might also provide a tractable platform for high-throughput studies of V. cholerae chemotaxis.
For the design of our Rluc-based PCA, we used recently described Rluc fragments that provided sensitive, quantitative measurements of dynamic protein interactions with a high signal-to-noise ratio in mammalian cells [11]. Plasmids were constructed encoding the V. cholerae chemotaxis cluster II genes cheY3 and cheZ fused to the N- and C-terminal fragments of Rluc (rlucN and rlucC), respectively, by a 14-amino acid flexible linker. RlucN was fused to either the N- or C-terminus of CheY3, while RlucC was fused to the C-terminus of CheZ; structural comparisons to homologous proteins from E. coli suggested this fusion strategy would yield the best split Rluc complementation upon binding of CheY3 to CheZ. These constructs, as well as various control constructs, were introduced into wild-type and ΔcheY3cheZ V. cholerae and evaluated for their ability to reconstitute luciferase activity.
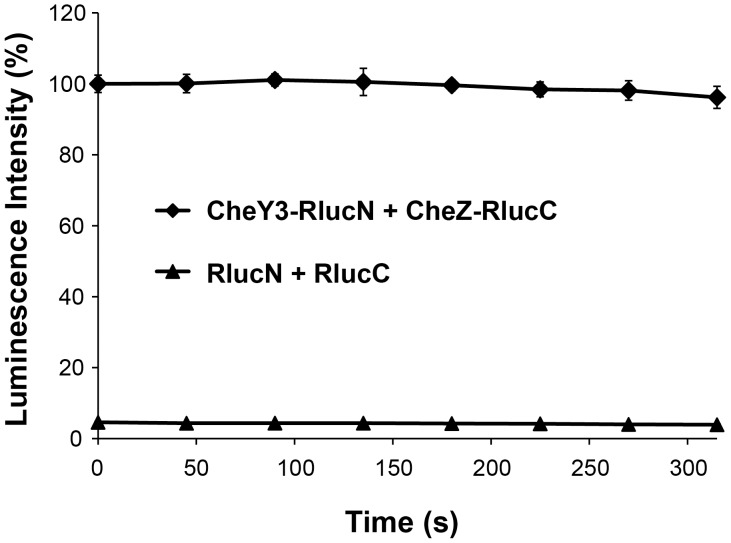
The luciferase activity of cells co-expressing CheY3-RlucN and CheZ-RlucC far exceeded that generated by cells co-expressing unfused RlucN and RlucC (∼20-fold difference in luminescence intensity; Figure 2), suggesting that split Rluc complementation is dependent on CheY3-CheZ interaction. Expression of individual CheY3-RlucN or CheZ-RlucC fusion proteins produced negligible luciferase activity (data not shown). Cells expressing the RlucN-CheY3/CheZ-RlucC fusion pair grew poorly and were not analyzed further. Thus, the CheY3-RlucN/CheZ-RlucC construct, pYNZC, was used for all subsequent experiments. To further maximize reporter activity, expression of fusion proteins was induced with 0.2% arabinose, and the Rluc substrate that yielded the highest luminescence (i.e., clz) was selected (Figure S1). Notably, co-expression of CheY3-RlucN and CheZ-RlucC in wild-type V. cholerae generated luciferase activity comparable to that observed in the ΔcheY3cheZ mutant, demonstrating that the endogenous CheY3 and CheZ proteins do not significantly inhibit split Rluc complementation.
Figure 2. Split Rluc complementation is dependent on fusing Rluc N- and C-terminal fragments to CheY3 and CheZ.
Luminescence generated by wild-type V. cholerae co-expressing CheY3-RlucN and CheZ-RlucC or unfused RlucN and RlucC. Data are reported as a percentage of the highest initial signal intensity and represent the average of three technical replicates.
CheY3-CheZ Interaction is Markedly Reduced in V. cholerae Chemotaxis Mutants
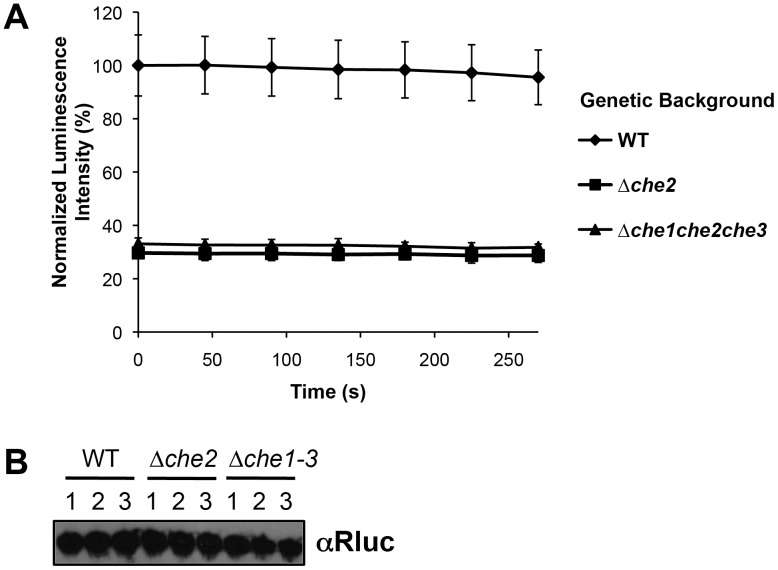
We hypothesized that pYNZC could be used to measure changes in CheY3-CheZ interaction, and thus chemotactic signaling, in V. cholerae mutants compromised for chemotaxis. We introduced pYNZC into Δche2, a strain lacking all cluster II chemotaxis genes (including cheY3 and cheZ), and Δche1che2che3, which lacks all three chemotaxis clusters. Because cluster II genes are required for chemotaxis under laboratory conditions [20], [24], both of these strains are non-chemotactic and were thus predicted to have significantly fewer CheY3-CheZ interactions than wild-type V. cholerae. As expected, the luciferase activities of both Δche2 and Δche1che2che3 were markedly lower than wild-type cells (∼70% reduction in luminescence intensity; Figure 3A). Immunoblotting confirmed that these differences were not due to variable expression of the luciferase fragments from pYNZC in these different genetic backgrounds (Figure 3B). Since Δche2 and Δche1che2che3 exhibited approximately equal luminescence intensities (Figure 3A), the additional chemotaxis signaling proteins encoded by cluster II (e.g., cheA2, cheW1) are presumably the primary mediators of CheY3-CheZ binding under these conditions. However, a low level of split Rluc complementation was still observed in these strains, raising the possibility that some interactions between CheY3 and CheZ can occur despite the absence of chemotaxis proteins that ostensibly regulate CheY3 phosphorylation (e.g., CheA2). Indeed, other factors, such as CheY acetylation [28], have been shown to affect chemotactic signaling in E. coli and may stimulate CheY3-CheZ binding in V. cholerae independent of other chemotaxis machinery. Still, the difference in reporter activity between wild-type and non-chemotactic strains appears to validate use of split Rluc complementation as an indicator of chemotactic signaling in different V. cholerae genetic backgrounds.
Figure 3. Rluc PCA detects reduced CheY3-CheZ interaction in V. cholerae chemotaxis mutants.
(A) Luminescence generated by wild-type ( ),Δche2 (
),Δche2 ( ), and Δche1che2che3 (
), and Δche1che2che3 ( ) V. cholerae co-expressing CheY3-RlucN and CheZ-RlucC. Data from three biological replicates were normalized by optical density, averaged, and reported as a percentage of the highest initial signal intensity. (B) Western blot analysis of CheY3-RlucN/CheZ-RlucC expression in samples from (A). Only the CheZ-RlucC fusion protein was detected by the monoclonal anti-Rluc antibody.
) V. cholerae co-expressing CheY3-RlucN and CheZ-RlucC. Data from three biological replicates were normalized by optical density, averaged, and reported as a percentage of the highest initial signal intensity. (B) Western blot analysis of CheY3-RlucN/CheZ-RlucC expression in samples from (A). Only the CheZ-RlucC fusion protein was detected by the monoclonal anti-Rluc antibody.
Nonspecific Inhibition of Rluc Precludes Analysis of Dynamic CheY3-CheZ Interactions
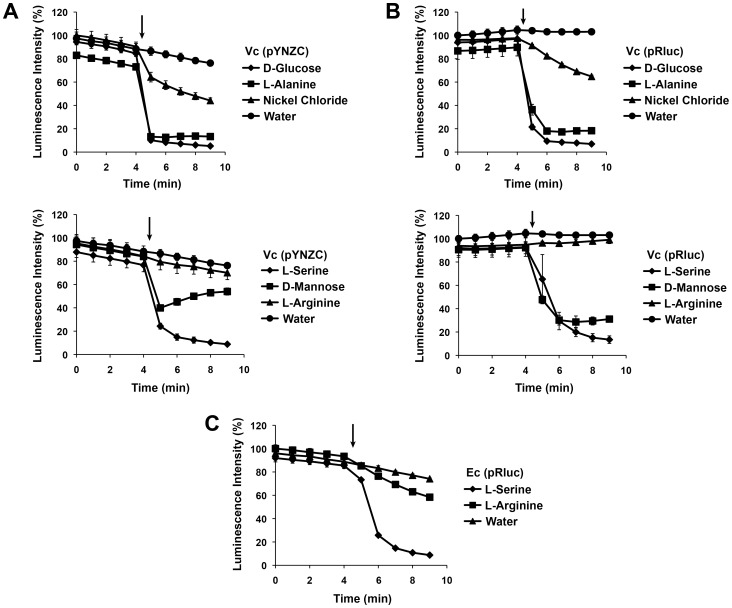
To assess the utility of split Rluc complementation for monitoring changes in protein-protein interactions in V. cholerae, we investigated the effects of chemoeffector addition on pYNZC reporter activity. Based on the BRET- and FRET-based assays for studying CheY-CheZ interactions in E. coli [14], [23], we expected the luciferase activity generated by cells co-expressing CheY3-RlucN and CheZ-RlucC to be proportional to chemotaxis pathway activity and to reflect the reversible association of CheY3 and CheZ proteins. Thus, applying a chemoattractant to those cells should decrease CheY3 phosphorylation and the extent of CheY3-CheZ binding, resulting in a rapid loss of luminescence. To test this, we treated wild-type V. cholerae expressing these fusion proteins from pYNZC with several well-characterized chemoattractants, including L-serine and D-glucose [29]. These molecules stimulated a rapid (within one minute) and significant decrease in cellular luminescence relative to a water-only control (Figure 4A). Maximal effects were observed following addition of these factors at 1 mM; however, addition of known attractants at much lower concentrations (∼1 µM) also reduced luminescence (data not shown). Thus, chemoattractant addition appeared to reduce the interaction between CheY3 (presumably as CheY3-P) and CheZ as expected, suggesting that split Rluc complementation was indeed reversible.
Figure 4. Apparent chemoattractant response of Rluc PCA reflects nonspecific inhibition of full-length Rluc.
Luminescence generated by wild-type V. cholerae co-expressing CheY3-RlucN and CheZ-RlucC from pYNZC (A) or unfused, full-length Rluc from pRluc (B) was measured before and after treatment with 1 mM D-glucose, L-alanine, or nickel chloride (top panel) and 1 mM L-serine, D-mannose, or L-arginine (bottom panel) or an equal volume of water. Luminescence generated by wild-type E. coli expressing full-length Rluc from pRluc was measured before and after treatment with L-serine, L-arginine, or water (C). Reagent addition is denoted by an arrow. Data are reported as a percentage of the highest initial signal intensity and represent the average of three technical replicates.
Though initially promising, several aspects of the CheY3-CheZ response to chemoattractant addition were inconsistent with published reports of bacterial chemotactic signaling and prompted us to question the significance of these data. First, not all chemoeffectors had the anticipated effect on split Rluc complementation. For example, L-arginine, a strong V. cholerae chemoattractant [29], [30], did not decrease luciferase activity (Figure 4A). Furthermore, some common bacterial repellents, such as nickel chloride, cobalt chloride, and butyric acid [31], [32], which were expected to stimulate CheY3 phosphorylation, enhance binding to CheZ, and thus increase split Rluc complementation, instead elicited an “attractant-like” response (e.g., Figure 4A, nickel chloride). Finally, the signal recovery typically observed shortly after attractant addition in BRET- and FRET-based assays [14], [23], which reflects MCP adaptation to local chemical gradients, was notably absent from our split Rluc data. In light of these issues, we began to suspect that our Rluc PCA was not accurately reporting changes in CheY3-CheZ interactions in response to chemotactic stimuli.
To explore the possibility that certain chemoeffectors might nonspecifically inhibit Rluc activity, generating a false “attractant-like” response, we monitored the effect of chemoeffector treatment upon cells expressing the full-length enzyme. Remarkably, we observed a decrease in luminescence that mirrored nearly exactly the response of cells co-expressing CheY3-RlucN and CheZ-RlucC (compare Figure 4A and Figure 4B), thereby confirming our suspicion that nonspecific inhibition of Rluc was compromising the significance of the split Rluc data. Notably, we observed a similar inhibition profile in E. coli expressing the full-length luciferase (Figure 4C), indicating that this phenomenon is manifest in other bacteria. Furthermore, nonspecific inhibition of Rluc activity was observed regardless of the order of reagent addition. For example, pretreatment of cells expressing CheY3-RlucN/CheZ-RlucC or the full-length enzyme with L-serine followed immediately by clz addition and measurement of Rluc activity decreased luminescence intensity by at least 65% relative to cells pretreated with water or L-arginine. Thus, we were unable to validate the reversibility of CheY3-CheZ-mediated split Rluc complementation, precluding its utility as a tool for evaluating dynamic chemotactic responses.
Discussion
We sought to develop a new method for quantitatively probing real-time protein-protein interactions in live bacteria using split luciferase complementation, a technique that has been widely applied to study protein-protein interactions in mammalian cells [3]. As proof of concept, we chose to develop an Rluc-based PCA for V. cholerae chemotaxis by fusing two inactive Rluc fragments to the chemotaxis response regulator CheY3 and its cognate phosphatase CheZ. In E. coli, BRET and FRET have been used to study the transient, phosphorylation-dependent interaction of CheY and CheZ proteins, which is directly proportional to chemotaxis pathway activity [14], [23]. We hypothesized that split Rluc complementation could provide an alternative means of studying these interactions in V. cholerae, establishing a rapid, highly tractable, microplate-based assay for bacterial chemotaxis with the potential for high-throughput applications.
Consistent with a previous study that used Gaussia princeps luciferase complementation to detect assembly of a stable protein complex in Salmonella [13], we found that fusion of Rluc fragments to known chemotaxis interaction partners enabled reconstitution of an active luciferase in V. cholerae cells (Figure 2). Furthermore, we discovered that the CheY3-CheZ-dependent signal was reduced in chemotaxis-deficient V. cholerae strains, suggesting that this system serves as a reporter of basal chemotactic signaling (Figure 3). However, our attempts to measure changes in chemotactic signaling in response to the addition of known chemoattractants, which should decrease CheY3-CheZ interactions, and hence, the associated split Rluc signal, were compromised by the unexpectedly broad small molecule inhibition profile of the full-length Rluc enzyme (Figure 4). These findings demonstrate the need for great caution in interpreting chemical effects on dynamic protein-protein interactions using split Rluc complementation.
Importantly, we performed our assays under conditions that yield a relatively stable split Rluc signal in order to more easily compare enzyme activity before and after chemoeffector addition. We determined that pre-incubation of cells with clz for 30 minutes prior to measuring luminescence was typically sufficient to achieve a stable signal (e.g., Figure 2 and Figure 3A), though a gradual decrease in Rluc activity was sometimes still observed despite this incubation period (e.g., Figure 4A). In mammalian cell-based Rluc PCAs, bioluminescence is typically measured immediately following clz addition because maximal luminescence occurs after an initial burst in Rluc activity (typically within the first minute of clz addition) [10], [11]. Though we obtained similar results using this approach (i.e., pretreatment of cells with L-serine followed by clz addition significantly decreased luminescence intensity relative to cells pretreated with water or L-arginine), we found that pre-incubation of the cells with clz facilitated more quantitative comparisons between different chemoeffectors. However, nonspecific inhibition of full-length Rluc was evident regardless of the order of reagent addition.
Chemical inhibition of luciferases, which has been previously documented, can complicate their use as both transcriptional and biochemical reporters [33]–[36]. Even so, we were greatly surprised by the breadth of the inhibition we observed, as the majority of the compounds we tested appeared to reduce Rluc activity. Notably, these inhibitory compounds (i.e., L-serine, L-alanine, D-glucose, D-mannose, and nickel chloride) are quite structurally dissimilar from clz, the Rluc substrate, suggesting that their inhibitory effects most likely result from some nonspecific mode of inhibition, rather than from direct competition with clz for access to the enzyme’s active site. For example, it is possible these molecules compromise protein fold, photonic processes, or form inhibitory aggregates that decrease the luminescent signal [33].
We considered the possibility that low concentrations of these molecules might not inhibit full-length Rluc, but still be potent enough to affect chemotactic signaling and elicit a meaningful response from the Rluc PCA. However, chemoeffector concentrations at the detection limit of the assay (∼1 µM) resulted in significant Rluc inhibition (data not shown), rendering the system untenable. Furthermore, the lack of a response to L-arginine (Figure 4A), a strong V. cholerae chemoattractant that should in principle decrease CheY3-RlucN/CheZ-RlucC interactions and the corresponding luminescent signal, suggests that the binding of these two fusion proteins may be irreversible. While this possibility is unexpected, there may be factors specific to this application (e.g., altered folding kinetics of Rluc fragments in bacteria, unforeseen effects of Rluc fusions on CheY3-CheZ phosphotransfer) that compromise the previously demonstrated reversibility of split luciferase complementation observed in eukaryotes.
Split luciferase is a powerful tool for measuring protein-protein interactions in live cells. However, our observations demonstrate an essential control for these studies: when investigating the effects of small molecules on dynamic protein-protein interactions, cells expressing the full-length luciferase must also be tested to ensure that compounds of interest truly inhibit protein interactions and not the activity of the reconstituted luciferase. A review of the split luciferase literature revealed that this control is seldom performed. Thus, we urge caution when applying this system to identify small molecule effectors of dynamic protein-protein interactions.
Supporting Information
Split Rluc signal is influenced by inducer concentration and Rluc substrate. Luminescence generated by wild-type V. cholerae co-expressing CheY3-RlucN and CheZ-RlucC following induction with either 0.01% ( ) or 0.2% (
) or 0.2% ( ) L-arabinose and treatment with native coelenterazine (clz) (A), or induction with 0.2% L-arabinose and treatment with either clz (
) L-arabinose and treatment with native coelenterazine (clz) (A), or induction with 0.2% L-arabinose and treatment with either clz ( ) or benzyl-coelenterazine (clz-h,
) or benzyl-coelenterazine (clz-h,  ) (B). Data are reported as a percentage of the highest initial signal intensity and represent the average of three technical replicates.
) (B). Data are reported as a percentage of the highest initial signal intensity and represent the average of three technical replicates.
(TIF)
Strains used in this study.
(DOCX)
Plasmids used in this study.
(DOCX)
Primers used in this study.
(DOCX)
Acknowledgments
We thank Stephen Michnick for providing the DNA sequences of the Rluc fusion constructs designed by his lab and for helpful comments. We are also grateful to Howard Berg, Jennifer Prescher, and the members of the Waldor lab for helpful discussions.
Funding Statement
This research was funded by the National Institutes of Health (NIH R37 AI-42347) and the Howard Hughes Medical Institute (www.hhmi.org). S.R. was funded by a post-doctoral fellowship from the Villum Kann Rasmussen Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bouveret E, Brun C (2012) Bacterial interactomes: from interactions to networks. Methods Mol Biol 804: 15–33. [DOI] [PubMed] [Google Scholar]
- 2. Stynen B, Tournu H, Tavernier J, Van Dijck P (2012) Diversity in genetic in vivo methods for protein-protein interaction studies: from the yeast two-hybrid system to the Mammalian split-luciferase system. Microbiol Mol Biol Rev 76: 331–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Villalobos V, Naik S, Piwnica-Worms D (2007) Current state of imaging protein-protein interactions in vivo with genetically encoded reporters. Annu Rev Biomed Eng 9: 321–349. [DOI] [PubMed] [Google Scholar]
- 4. Ciruela F (2008) Fluorescence-based methods in the study of protein-protein interactions in living cells. Curr Opin Biotechnol 19: 338–343. [DOI] [PubMed] [Google Scholar]
- 5. Sourjik V, Vaknin A, Shimizu TS, Berg HC (2007) In vivo measurement by FRET of pathway activity in bacterial chemotaxis. Methods Enzymol 423: 365–391. [DOI] [PubMed] [Google Scholar]
- 6. Ozawa T, Kaihara A, Sato M, Tachihara K, Umezawa Y (2001) Split luciferase as an optical probe for detecting protein-protein interactions in mammalian cells based on protein splicing. Anal Chem 73: 2516–2521. [DOI] [PubMed] [Google Scholar]
- 7. Paulmurugan R, Gambhir SS (2003) Monitoring protein-protein interactions using split synthetic Renilla luciferase protein-fragment-assisted complementation. Anal Chem 75: 1584–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kaihara A, Kawai Y, Sato M, Ozawa T, Umezawa Y (2003) Locating a protein-protein interaction in living cells via split Renilla luciferase complementation. Anal Chem 75: 4176–4181. [DOI] [PubMed] [Google Scholar]
- 9. Luker KE, Smith MC, Luker GD, Gammon ST, Piwnica-Worms H, et al. (2004) Kinetics of regulated protein-protein interactions revealed with firefly luciferase complementation imaging in cells and living animals. Proc Natl Acad Sci U S A 101: 12288–12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Remy I, Michnick SW (2006) A highly sensitive protein-protein interaction assay based on Gaussia luciferase. Nat Methods 3: 977–979. [DOI] [PubMed] [Google Scholar]
- 11. Stefan E, Aquin S, Berger N, Landry CR, Nyfeler B, et al. (2007) Quantification of dynamic protein complexes using Renilla luciferase fragment complementation applied to protein kinase A activities in vivo. Proc Natl Acad Sci U S A 104: 16916–16921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Villalobos V, Naik S, Bruinsma M, Dothager RS, Pan MH, et al. (2010) Dual-color click beetle luciferase heteroprotein fragment complementation assays. Chem Biol 17: 1018–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wille T, Blank K, Schmidt C, Vogt V, Gerlach RG (2012) Gaussia princeps luciferase as a reporter for transcriptional activity, protein secretion, and protein-protein interactions in Salmonella enterica serovar Typhimurium. Appl Environ Microbiol 78: 250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shimizu TS, Delalez N, Pichler K, Berg HC (2006) Monitoring bacterial chemotaxis by using bioluminescence resonance energy transfer: absence of feedback from the flagellar motors. Proc Natl Acad Sci U S A 103: 2093–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wood KV, DeLuca M (1987) Photographic detection of luminescence in Escherichia coli containing the gene for firefly luciferase. Anal Biochem 161: 501–507. [DOI] [PubMed] [Google Scholar]
- 16. Jawhara S, Mordon S (2004) In vivo imaging of bioluminescent Escherichia coli in a cutaneous wound infection model for evaluation of an antibiotic therapy. Antimicrob Agents Chemother 48: 3436–3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Armitage JP (1999) Bacterial tactic responses. Adv Microb Physiol 41: 229–289. [DOI] [PubMed] [Google Scholar]
- 18. Butler SM, Camilli A (2005) Going against the grain: chemotaxis and infection in Vibrio cholerae . Nat Rev Microbiol 3: 611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boin MA, Austin MJ, Hase CC (2004) Chemotaxis in Vibrio cholerae . FEMS Microbiol Lett 239: 1–8. [DOI] [PubMed] [Google Scholar]
- 20. Lee SH, Butler SM, Camilli A (2001) Selection for in vivo regulators of bacterial virulence. Proc Natl Acad Sci U S A 98: 6889–6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Adler J (1973) A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli . J Gen Microbiol 74: 77–91. [DOI] [PubMed] [Google Scholar]
- 22. Armstrong JB, Adler J, Dahl MM (1967) Nonchemotactic mutants of Escherichia coli . J Bacteriol 93: 390–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sourjik V, Berg HC (2002) Receptor sensitivity in bacterial chemotaxis. Proc Natl Acad Sci U S A 99: 123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ringgaard S, Schirner K, Davis BM, Waldor MK (2011) A family of ParA-like ATPases promotes cell pole maturation by facilitating polar localization of chemotaxis proteins. Genes Dev 25: 1544–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Donnenberg MS, Kaper JB (1991) Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun 59: 4310–4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miller VL, Mekalanos JJ (1988) A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR . J Bacteriol 170: 2575–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Loening AM, Fenn TD, Wu AM, Gambhir SS (2006) Consensus guided mutagenesis of Renilla luciferase yields enhanced stability and light output. Protein Eng Des Sel 19: 391–400. [DOI] [PubMed] [Google Scholar]
- 28. Barak R, Yan J, Shainskaya A, Eisenbach M (2006) The chemotaxis response regulator CheY can catalyze its own acetylation. J Mol Biol 359: 251–265. [DOI] [PubMed] [Google Scholar]
- 29. Freter R, O’Brien PC (1981) Role of chemotaxis in the association of motile bacteria with intestinal mucosa: chemotactic responses of Vibrio cholerae and description of motile nonchemotactic mutants. Infect Immun 34: 215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Butler SM, Nelson EJ, Chowdhury N, Faruque SM, Calderwood SB, et al. (2006) Cholera stool bacteria repress chemotaxis to increase infectivity. Mol Microbiol 60: 417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Porter SL, Wadhams GH, Armitage JP (2011) Signal processing in complex chemotaxis pathways. Nat Rev Microbiol 9: 153–165. [DOI] [PubMed] [Google Scholar]
- 32. Tso WW, Adler J (1974) Negative chemotaxis in Escherichia coli . J Bacteriol 118: 560–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Auld DS, Southall NT, Jadhav A, Johnson RL, Diller DJ, et al. (2008) Characterization of chemical libraries for luciferase inhibitory activity. J Med Chem 51: 2372–2386. [DOI] [PubMed] [Google Scholar]
- 34. Thorne N, Auld DS, Inglese J (2010) Apparent activity in high-throughput screening: origins of compound-dependent assay interference. Curr Opin Chem Biol 14: 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thorne N, Inglese J, Auld DS (2010) Illuminating insights into firefly luciferase and other bioluminescent reporters used in chemical biology. Chem Biol 17: 646–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Herbst KJ, Allen MD, Zhang J (2009) The cAMP-dependent protein kinase inhibitor H-89 attenuates the bioluminescence signal produced by Renilla luciferase. PLoS One 4: e5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Split Rluc signal is influenced by inducer concentration and Rluc substrate. Luminescence generated by wild-type V. cholerae co-expressing CheY3-RlucN and CheZ-RlucC following induction with either 0.01% ( ) or 0.2% (
) or 0.2% ( ) L-arabinose and treatment with native coelenterazine (clz) (A), or induction with 0.2% L-arabinose and treatment with either clz (
) L-arabinose and treatment with native coelenterazine (clz) (A), or induction with 0.2% L-arabinose and treatment with either clz ( ) or benzyl-coelenterazine (clz-h,
) or benzyl-coelenterazine (clz-h,  ) (B). Data are reported as a percentage of the highest initial signal intensity and represent the average of three technical replicates.
) (B). Data are reported as a percentage of the highest initial signal intensity and represent the average of three technical replicates.
(TIF)
Strains used in this study.
(DOCX)
Plasmids used in this study.
(DOCX)
Primers used in this study.
(DOCX)