Abstract
A meta-analysis was performed to understand the role of zinc finger domains in proteins of resistance (R) genes cloned from different crops. We analyzed protein sequences of seventy R genes of various crops in which twenty six proteins were found to have zinc finger domains along with nucleotide binding sites - leucine rice repeats (NBS-LRR) domains. We identified thirty four zinc finger domains in the R proteins of nine crops and were grouped into 19 types of zinc fingers. The size of individual zinc finger domain within the R genes varied from 11 to 84 amino acids, whereas the size of proteins containing these domains varied from 263 to 1305 amino acids. The biophysical analysis revealed that molecular weight of Pi54 zinc finger was lowest whereas the highest one was found in rice Pib zinc finger named as Transposes Transcription Factor (TTF). The instability (R2 = 0.95) and the aliphatic (R2 = 0.94) indices profile of zinc finger domains follows the polynomial distribution pattern. The pairwise identity analysis showed that the Lin11, Isl-1 & Mec-3 (LIM) zinc finger domain of rice blast resistance protein pi21 have 12.3% similarity with the nuclear transcription factor, X-box binding-like 1 (NFX) type zinc finger domain of Pi54 protein. For the first time, we reported that Pi54 (Pi-kh-Tetep), a rice blast resistance (R) protein have a small zinc finger domain of NFX type located on the C-terminal in between NBS and LRR domains of the R-protein. Compositional analysis depicted by the helical wheel diagram revealed the presence of a hydrophobic region within this domain which might help in exposing the LRR region for a possible R-Avr interaction. This domain is unique among all other cloned plant disease resistance genes and might play an important role in broad-spectrum nature of rice blast resistance gene Pi54.
Introduction
The zinc finger proteins are a super family of proteins involved in numerous activities of plant growth and development and are also known to regulate resistance mechanism for various biotic and abiotic stresses [1], [2]. Any small, functional, freely folded domain in which coordination of one or more zinc ions required to stabilize its structure is known as zinc finger [3]. These domains are actively required to regulate various metabolic processes and stress conditions in plants [4], [5], [6]. The Zinc finger domains are widely dispersed in eukaryotic genomes [7], [4], [8], [9] and are actively involved in sequence specific binding to DNA/RNA and contribute in protein-protein recognitions [10]. The presence of zinc finger DNA binding domain in nucleotide binding sites-leucine rich repeats (NBS-LRR) class of proteins determines the regulatory function of this protein in stress conditions. These domains are basically transcription factor in origin which makes the protein as regulator. Zinc finger binds to DNA through the interaction of amino acids at the periphery of the zinc finger with base pairs at the center of the DNA double helix [11]. It is a compact protein domain, and its small size allows it to have close relation with DNA base pairs. Zinc fingers basically bind with nucleic acids for their function or participate in transcriptional or translational regulation processes [12]. These are classified into nine types according to their structural and functional variation. These are C2H2, C8, C6, C3HC4, C2HC, C2HC5, C4, C4HC3 and CCCH (C and H represent cysteine and histidine, respectively) [13], [14], [15], [16], [17]. The presence of zinc finger domain has been reported in many disease resistance genes cloned from various crops. These domains are LSD1 (C2C2), LOL1 (C2C2), Zat 12 (C2H2), Zat 7 (C2H2) & AtNFX1 (NF-X1) of Arabidopsis [18], StZFP1 (C2H2) of potato [8], and OsLSD1 (C2C2), OsLOL1 (C2C2), OsRING-1 (RING H2, RING HC), OsRFP1, OsDOS (CCCH), OsZFP (C2H2) & SRZ1(C2C2) of rice [19], [20]. These reports indicate that zinc finger motifs have important role in imparting host- plant resistance.
The NBS-LRRs are most prevalent class of R- proteins. These are basically three types such as TIR-NBS-LRR (TNL) and CC-NBS-LRR (CNL) and mixed type having either Toll/interleukin-1 receptor-like domain (TIR) or Coiled coil (CC ) domains or both in one protein [21], [22]. Proteins fused with these zinc finger domains make them as zinc finger proteins. Presence of these domains is required for the function of individual proteins under stress conditions. These domains are able to regulate the proteins like switches in rice and poplar [23], [24]. Sometimes, R proteins carry extensions at carboxy-terminus (C- terminal) with a typical WRKY DNA-binding domain and between linkers of NBS and LRR domains, respectively [25], [26].
Rice blast caused by Magnaporthe oryzae is one of the most important biotic stresses of rice resulting into huge yield loss every year [27]. The disease can be effectively managed by the resistance gene deployment. We have earlier identified and cloned a new rice blast resistance gene Pi-kh from indica rice cultivar, Tetep showing resistance to different strains of M. oryzae [28], [29]. The gene was only the third one to be cloned in the series of cloned rice blast resistance genes, after the cloning of Pi-b [30], and Pi-ta genes [31]. The gene was renamed as Pi54, after it was fine mapped at a slightly different location from the Pi-k cluster of the genes [32]. Functional validation of the gene has established that it confers a stable and high-level of resistance against geographically diverse strains of M. oryzae in India [33] and USA [34]. Expression analysis of the gene has revealed that it is induced by pathogen challenge. In turn, the gene was found to induce the synthesis of callose (β-1, 3 glucan) in response to pathogen challenge, suggesting its requirement in the initiation of a defense response cascade in the blast resistant plants [33]. Transcriptional and biochemical analysis revealed that rice transgenic lines containing Pi54 single functional blast resistant gene show activation of a complex defense mechanism after pathogen inoculation [35]. The cloning of orthologue of Pi54 gene has also been achieved from wild species of rice O. rhizomatis [36]. It has also been reported that Pi54 protein contains an NBS-LRR domain in addition to a small zinc finger domain [37], [26].
The R genes are also categorized into separate categories on the basis of status and position of zinc finger domains. These domains are present either at N– terminal or C– terminal of the proteins encoded by R genes and along with NBS-LRR domain, play a crucial role in regulating expression of the genes involved in plant resistance [38], [39]. Many defense proteins of Arabidopsis and rice containing zinc finger domain have been shown to regulate programmed cell death (PCD). Despite having proven role in stress management in plants, the presence and involvement of zinc finger motifs along with NBS-LRR has not been analyzed in relation to plant disease resistance. The structural analysis of zinc finger domain present within these proteins is important for understanding the role of small protein domains that have diverse functions [13], [40].
The objectives of present study were (i) to analyze the presence of zinc finger domains in all the cloned plant disease resistance genes, (ii) to determine the probable structure of zinc finger domain of blast resistance protein Pi54 (iii) computational analysis of biophysical properties of zinc finger domains in the proteins of cloned R genes and (iv) comparative analysis of zinc finger proteins in relation to Pi54 protein.
Results and Discussion
Identification and amino acid composition analysis of zinc finger domains
The amino acid sequence of protein encoded by rice blast resistance gene Pi54 was downloaded and analyzed using various bioinformatics tools along with a careful manual inspection. A small (11AA) zinc finger motif of nuclear transcription factor, X-box binding-like 1 (NFX) type was identified between the positions 253–263 amino acids in this protein. This domain is C– terminal in nature and integrated within LRR. Earlier Pi-kh (Pi54) gene responsible for the expression of this protein was found to be pathogen inducible in nature [35], [33], [26]. Amino acid composition analysis revealed that there are eight amino acids present within this domain with varying properties and mole percentage (Figure 1; Table 1). The amino acids in Pi54_ZnF domain were characterized on the basis of their side chains. These are negatively charged acidic (Glu E), non-polar aliphatic (G, L), polar uncharged (C, Q, S) and positively charged basic (H). The protein characteristics are believed to be related with the composition of amino acids and some of these structural factors were obtained due to the exchange of some amino acids [41].
Figure 1. The structure of Pi54 zinc finger domain.
(A) Positional analysis of the domain showed that this domain is C- terminal in nature. The type of this zinc finger domain is NFX. (B) The amino acids, numbers and their positions in this domain. (C) Chemical structure of individual amino acids. (D) Secondary structure of zinc finger domain (E) Helical wheel diagram of Pi54 zinc finger domain. The helical wheel is a plot of the amino acid residues around a potentially helical segment. The graphical representation showed the clustering of polar and/or non-polar residues toward one face of a helix.
Table 1. Amino acid compositional analysis of Pi54 Zinc finger domain.
| S.No. | Amino Acid | Number | Mol% | R Group |
| 1 | Glu E | 1 | 9.09 | Negatively Charged , Acidic |
| 2 | Gly G | 1 | 9.09 | Non Polar Aliphatic |
| 3 | Leu L | 2 | 18.18 | Non Polar Aliphatic |
| 4 | Cys C | 3 | 27.27 | Polar Uncharged |
| 5 | Gln Q | 1 | 9.09 | Polar Uncharged |
| 6 | Pro P | 1 | 9.09 | Non-polar |
| 7 | Ser S | 1 | 9.09 | Polar Uncharged |
| 8 | His H | 1 | 9.09 | Positively Charged , Basic |
Non-Polar amino acids
The nonpolar amino acids were characterized for having non polar atoms (only carbon and hydrogen) in their side chains. They include Glycine (Gly, G), Ala (Alanine, A), Val (Valine, V), Leu (Leucine, L), Ile (Isoleucine, I), Pro (Proline, P), and Met (Methionine, M). Presence of such residues makes domains more hydrophobic in nature. The hydrophobic amino acid residues can increase the rigidity and hydrophobicity of proteins [42]. Among the hydrophobic residues, Leu belongs to the aliphatic amino acids (Table 1). It has been found that aliphatic amino acids would contribute to the hydrophobic interaction and required to maintain the conformational stability in the inner part of the protein [43]. We identified two Leu residues in small zinc finger domain of Pi54 protein (Figure 1). More number of Leu residues result in higher average hydropathy and aliphatic index [44]. Besides, in Pi54 protein–Gly residues were also identified. Gly is known as the residue responsible to maintain or generate cavity in the inner part of protein structure [41]. These residues make domains more flexible for better folding in different way.
One Pro residue was also identified in the zinc finger domain of Pi54 protein. This residue contains a non-polar, uncharged R group (Figure 1). The Pro residue can only adopt a few configurations due to their pyrolidine ring and has the lowest conformational entropy. It thus restricts the configurations allowed for the preceding residue. Because of the presence of this residue rigidity and conformation in protein structure have been reported [45]. The Pro residue has been used to increase the protein stability in the several mutational studies and hence an increase in the stability of such domains of disease resistant proteins might be important for rice plant to resist more against M. oryzae [46].
Polar, uncharged amino acids
The polar nature of the side chain means that these amino acids are ready to interact with water (hydrophilic) and can form hydrogen bonds [42]. There are four numbers of polar uncharged amino acids (Cysteine C, Glutamine Q, Serine S and sometimes Histidine H with pK of 6.5) in small zinc finger domain of the Pi54 protein (Figure 1). These are the amino acids which possess oxygen, sulfur and/or nitrogen in the side chain and hence polar, but cannot have their side chain ionized and thus do not carry an overall charge. The amino acid Glutamine is known as thermolabile amino acids due to its tendency to undergo deamination at high temperature [41]. This indicates that presence of such residue makes the zinc finger domain of Pi54 more stable in various stress conditions; hence might help in maintaining the ability of rice plants to resist against the incoming M. oryzae pathogen under varied climatic conditions. The amino acid residue Serine is known as the best residues for interacting with the water molecules surrounding protein due to its hydrophilic nature [47]. The water molecules that are ready for interaction with these residues for hydrogen bond formation have been reported to release at high temperature. Hence, the protein structure around water-binding site changed to unstable which might increase the instability of proteins [48].
The side group of another polar, uncharged residue Cysteine present in zinc finger domain of Pi54 protein contains a sulfur atom (Figure 1). The sulfur group in Cysteine comes at the end of the hydrocarbon chain and therefore, has the potential to be more reactive. Cysteine is also known as thermolabile amino acids because it undergoes oxidation at high temperature like Methionine [49]. There are three Cysteine residues in Pi54 zinc finger domain. The proteins of maize, rice, and tomato contain 1.62%–1.69% cysteine whereas the yeast contained least cysteine (1.21%). More number of cysteine residues indicates existence of short-range intra-polypeptide chains interactions which plays important role in evolution [50]. Among the prokaryotes, Cyanobacteria, E. coli, Psuedomonas aeruginosa, and Rhodobactor sphaeroides contained 1.03%–1.13% cysteine. The cysteine contents of proteins of different species may be as low as 0.4%–0.5% in Archea. The extreme halophil Haloarcula marismortui and the thermophil Thermus aquaticus were reported to have the lowest 0.49% and 0.41%, respectively cysteine among the investigated species. The C-(X)2-C motifs present at position 253–260 of Pi54 protein are important domains of metal binding proteins [51].
Polar, charged amino acids
The negatively charged, acidic R group containing Glutamic acid and positively charged, basic amino acid, Histidine were also been identified in zinc finger domain of the Pi54 protein (Figure 1). The polar side chains of these residues can also carry a positive charge or negative charge and are therefore highly hydrophilic in nature. The charged amino acids would contribute to the electrostatic interaction, which is an important force for maintaining conformational stability in the outer part of the proteins [52], [53]. The conformational stability to expose the LRR domain for interaction with Avr proteins of the pathogen M. oryzae is necessary for R gene interactions [54]. Since the zinc finger domain of Pi54 protein is C– terminal in nature and integrated with LRR, it might be playing an important role in protein-protein interaction in rice-M. oryzae pathosystem. The charged amino acid residues are less labile and also retain the hydrogen bonding capacity. The charged residues may be involved in location of ion pairs within molecular structures which also appears to be important in determining protein stability [55]. The presence of these residues at the protein surface, increase ion interactions and enhances occurrence of salt bridges and ion pairs [56], [55]. These amino acids are important for the flexibility of the proteins [6].
The Helical wheel diagram gives a view of a helix from a protein sequence looking down the axis of the helix. It is useful for highlighting the amphipathicity and other properties of residues around a helix. The hydrophobic region of Pi54-ZnF domain which constitutes of two Leucine residues, one Proline and one Glycine has been represented by a helical wheel diagram (Figure 1). The helical wheel is a plot of the amino acid residues around a potentially helical segment. The method was developed to find helices with a hydrophobic face buried away from a polar solvent, with the graphical representation showing the clustering of polar and/or non- polar residues toward one face of a helix. Earlier, the helical wheel facilitated the identification of potential helical segments in protein sequences [57]. Besides, its applicability has also been expanded to designer proteins such as leucine zipper proteins [58] and studying trans-membrane proteins like G-protein- coupled receptors [59].
Comparative analysis of proteins of R genes cloned from different crops
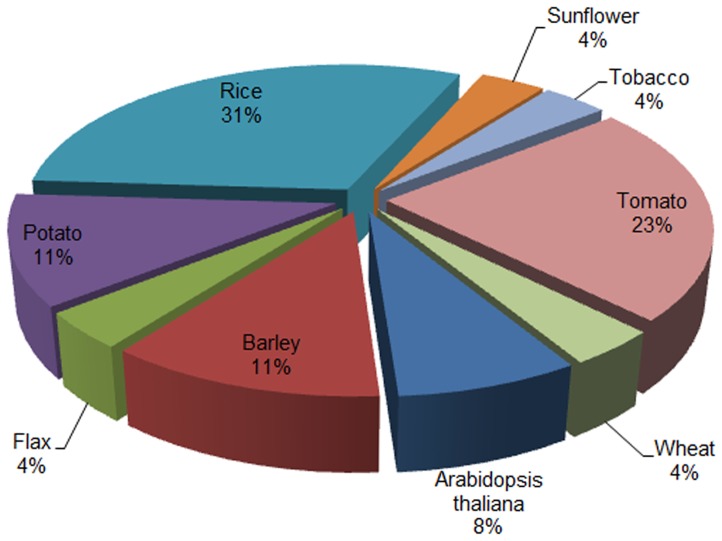
We analyzed protein sequences of 70 R genes cloned from different crops to delineate their zinc finger domain (Table S1 and Supplementary References, 1–69). The cloned disease resistance genes of the thirteen crops (Arabidopsis; Supplementary References (1–16), Barley (17–22), Pepper (23), Potato (24–27), Rice (28–43), Sunflower (44), Tomato (45–58), Tobacco (59), Wheat (60–63), Flax (64–66), Maize (67), Lettuce (68) and Beet (69)) were analyzed for identification and characterization of zinc finger domains in their respective proteins. The types of the proteins encoded by these R genes were varied in nature. The ‘SMART’ analysis revealed that twenty six genes contain zinc finger domains of different types, varying sizes and at different positions in their respective proteins. We found twenty six R genes from nine crops having nineteen types of zinc finger (Table 2). The R genes of rice represent 31% of all the zinc finger containing cloned R genes followed by tomato which has 23% proteins containing zinc finger domains. The R genes of wheat, sunflower, flax and tobacco were found to have zinc finger domains and constitute 4% of all the cloned R genes. Barley and potato represents 11% while Arabidopsis showed 8% share in this study of cloned R genes (Figure 2; Table S2). All zinc finger containing rice blast resistance genes encoded the NBS-LRR type of proteins except Pi36 and Pi5-1 which contains the CC-NBS-LRR type of proteins. The sunflower gene pI8 was also found to contain zinc finger domain but is a CC-NBS-LRR type of protein. Tobacco R gene ‘N’ encoded the TIR-NBS-LRR type of protein also have the zinc finger domains. The six R genes of Tomato have zinc finger domain in their NBS-LRR, LZ-NBS-LRR and LRR types of the proteins (Table 2).
Table 2. Summary of various Zinc finger domains present in cloned R genes.
| S No. | Crop name | Name of R gene | Protein length (aa) | Zinc finger type | Size of ZnF domain | Position of ZnF protein | Number of ZnF domains | Accession number | Disease resistance type | References |
| 1 | Arabidopsis | SSI4 | 1055 | DBF | 52 | 910–961 | 1 | AAN86124.1 | TIR-NBS-LRR | [92] |
| 2 | Arabidopsis | RCY1 | 361 | RAD18 | 22 | 48–69 | 2 | NP_001077963.1 | RLKs | [93] |
| PMZ | 28 | 129–156 | ||||||||
| 3 | Barley | Mla1 | 958 | BED | 49 | 386–434 | 1 | AAG37354.1 | CC-NBS-LRR | [94] |
| 4 | Barley | Mla12 | 961 | BED | 43 | 893–935 | 1 | AAO43441.1 | CC-NBS-LRR | [95] |
| 5 | Barley | Rpg1 | 837 | TTF | 84 | 145–228 | 2 | AAM76922.1 | RLKs | [96] |
| C3H1 | 21 | 543–563 | ||||||||
| 6 | Flax | M | 1305 | Rad18 | 16 | 290–305 | 1 | AAB47618.1 | NBS-LRR | [97] |
| 7 | Potato | Rx2 | 912 | BED | 47 | 346–392 | 1 | CAB55838.1 | NBS-LRR | [98] |
| 8 | Potato | Gpa2Rx1 | 937 | BED | 47 | 346–392 | 1 | CAB50786.1 | NBS-LRR | [99] |
| 9 | Potato | Gro 1.4 | 1136 | CHCC | 41 | 113–153 | 2 | AAP44390.1 | TIR-NBS-LRR | [100] |
| CDGSH | 31 | 688–718 | ||||||||
| 10 | Rice | Pib | 1251 | U1 | 35 | 546–580 | 2 | BAA76282.2 | NBS-LRR | [30] |
| TTF | 83 | 60–142 | ||||||||
| 11 | Rice | Pi-ta | 928 | UBP | 43 | 537–579 | 1 | AAK00132.1 | NBS-LRR | [31] |
| 12 | Rice | Pi36 | 1056 | CHCC | 40 | 845–884 | 1 | ABI64281.1 | CC-NBS-LRR | [101] |
| 13 | Rice | Pi54 | 330 | NFX | 11 | 253–263 | 1 | AAY33493.1 | NBS-LRR | [26] |
| 14 | Rice | Piz-t | 1033 | PMZ | 20 | 645–664 | 1 | ABC73398.1 | NBS-LRR | [102] |
| 15 | Rice | Pi21 | 263 | LIM | 39 | 11–49 | 1 | BAG72124.1 | NBS-LRR | [76] |
| 16 | Rice | Pi5-1 | 1025 | TAZ | 67 | 749–815 | 1 | ACJ54697.1 | CC-NBS-LRR | [78] |
| 17 | Rice | Pi-2 | 974 | UBP | 45 | 74–118 | 1 | ABC94597 | NBS-LRR | [102] |
| 18 | Sunflower | pI8 | 1279 | CHCC | 45 | 292–236 | 3 | AAT08955.1 | CC-NBS-LRR | [103] |
| GATA | 43 | 754–796 | ||||||||
| C2C2 | 31 | 1083–1113 | ||||||||
| 19 | Tobacco | N | 1128 | C2H2 | 30 | 568–597 | 1 | BAD12594 | TIR-NBS-LRR | [104] |
| 20 | Tomato | Mi-1 | 1257 | UBR1 | 56 | 1064–1119 | 1 | AAC97933 | NBS-LRR | [105] |
| 21 | Tomato | Sw5-e | 1241 | UBP | 37 | 42–78 | 1 | AAG31017 | LZ-NBS-LRR | [106] |
| 22 | Tomato | Cf-9 | 863 | PMZ | 20 | 160–179 | 1 | AAA65235.1 | LRR | [107] |
| 23 | Tomato | I2C | 373 | U1 | 27 | 127–153 | 1 | AAB63276 | NBS-LRR | [15] |
| 24 | Tomato | Hero | 1283 | ZZ | 44 | 171–214 | 2 | CAD29729 | NBS-LRR | [108] |
| 25 | Tomato | Cf-4 | 806 | ZNF_C4 | 39 | 46–84 | 1 | CAA05268.1 | LRR | [109] |
| 26 | Wheat | Lr10 | 636 | C2C2 | 38 | 32–69 | 2 | AAC49629.1 | RLKs | [110] |
| ZZ | 39 | 238–276 |
Figure 2. The distribution of zinc finger domains across different crops.
The cloned R genes of various crops were found to have zinc finger domain in their proteins. The number of zinc finger domains represented by cloned R genes of each crop is shown in pie chart.
Analysis of zinc finger domain in R proteins
The zinc finger domains encoded by these proteins were used for further analysis. R genes of nine crops were further distributed into five families of eudicots and monocots representing their zinc finger domains (Table S2). Thirty four zinc finger sequences were found in twenty six R genes (Table S3). The domain architecture of these proteins depicts different types, size, position and number of zinc finger domains (Figure 3; Table 2). The blast resistance gene Pi54 contain NFX type zinc finger domain in their protein. This domain is smallest and distinct from all others, though the smallest protein was encoded by Pi21 of rice (Figure 3). The NFX1-type zinc finger proteins are a group of the human NFX1 transcription factors. This protein was identified as a protein that represses class II MHC (major histocompatibility complex) gene [60]. The NFX1-type zinc fingers containing proteins are found in protists, fungi, animals and plants. The NFX type zinc finger domain containing proteins are known to be involved in growth and survival of plants by managing reactive oxygen species (ROS), salicylic acid (SA), and also in biotic stress and abscisic acid (ABA) responses [61]. The only plant homologue AtNFXL1 of the NFX1 gene has been experimentally confirmed and found that it plays a crucial role in different stress responses [62], [63], [64]. In the present analysis, five genes were found to have two zinc finger domains whereas three zinc finger domains were obtained in one gene only. Twenty genes were found to contain only one zinc finger domains in their respective proteins (Table 2).
Figure 3. The architecture of zinc finger domains containing R proteins.
The scale (0–1300) represents the size of proteins and their domains. The position and number of zinc finger domains in each R genes are also represented in the scale. The black broken lines indicate the zinc finger domains in individual R-proteins.
Characteristic features of zinc finger domains in R-proteins
Zinc finger domain was deduced in a total of eight blast resistance genes of rice (Table 2). These genes were found to have ten different types of zinc finger domains in their respective proteins. The R- gene Pib encoded NBS-LRR protein along with two zinc finger domains U1 and TTF. Eight types of zinc fingers such as U1, TTF, UBP, CHCC, NFX, PMZ, LIM and TAZ were found in the rice R proteins (Table 2). The zinc finger type UBP was deduced in blast resistance genes Pi-ta and Pi-2. The size of zinc finger domains varies from 11 to 84 amino acid residues. The NFX, LIM and TAZ types were only found in the R genes cloned from rice. The U1-ZnF of Pib was reported in I2C gene of tomato whereas TTF was found in barley Rpg1 protein. The PMZ of rice deduced in Piz-t protein having length (20 AA) similar to PMZ of Tomato R protein Cf-9. This zinc finger domain is also present in RCY1 protein of barley. The LIM zinc finger deduced in Pi21 protein of rice is smallest protein amongst all the proteins of cloned genes in plants, whereas the zinc finger domain deduced in Pi54 protein was found to be the smallest amongst all plant proteins cloned till date. The Pib gene encoded protein of rice was found to have largest zinc finger domain TTF (83AA) among all the cloned R genes of rice though TTF of barley R gene Rpg1 is largest (84 AA) among all the crops (Table 2, S3).
Among other crops, tomato contains six types of zinc finger domains like UBR1, UBP, PMZ, U1, ZZ and C4 in their disease resistance genes. The smallest zinc finger domain of tomato is PMZ (20AA) which was deduced in Cf-9 protein and is similar to the rice PMZ domain of blast resistance gene Piz-t. The UBR1 zinc finger of tomato protein Mi-1 is largest amongst all other cloned resistance proteins of tomato (Table 2, S3). Similarly, cloned R proteins of potato were found to have three types of zinc finger domains such as BED, CHCC and CDGSH. Of these, the zinc finger domain BED has also been deduced in Mla1& Mla12 genes of barley.
Biophysical characterization of zinc finger domains of R-proteins
The Protparam analysis revealed that these R-proteins have varying numbers and types of amino acids (Table S1, S3). This analysis also includes the molecular weight, theoretical PI, instability index, aliphatic index and hydropathicity index of all zinc finger domains present in R genes (Table S1, S4). The molecular weight of Pi54 zinc finger NFX is 1189.3 Daltons. It was lowest among all, whereas the highest one was 9905 Daltons in Pib zinc finger TTF (Table S4). The isoelectric point (pI) of zinc fingers of cloned R- genes ranged from 4.0 (UBP of Sw5-e) to 10.66 (PMZ of RCY1). The isoelectric point (pI) is the pH at which the surface of protein is covered with charge but net charge of protein is zero. The pI of Pi54 zinc finger domain was found to be 5.24 (Table S4) which indicates that this domain is slightly acidic in nature. The calculated isoelectric point (pI) will be useful because at this pI, solubility is least and mobility in an electro focusing system is zero [65].
The instability status of zinc finger proteins
The instability index value for the R gene zinc finger domains ranged from 6.37 to 120.89. The instability index of Pi54 zinc finger domain was 120.89. This value indicates its highly unstable nature among all the cloned R genes. The zinc finger domain is arranged in their increasing order of stability profile which follows the polynomial trends (R2 = 0.95) (Figure 4; Table S4). The instability index affords an estimate of the stability of protein in a test tube. This method assigns a weight value of instability, which can be used to compute an instability index. A protein whose instability index smaller than 40 is predicted as stable where as a value above 40 predicts that the protein may be unstable [66].
Figure 4. Instability index profile of identified zinc finger domain across cloned R gene.
The data of 26 zinc finger R genes with their zinc finger types were included in this analysis.
The aliphatic index of zinc finger domains of R proteins
The aliphatic index is defined as the relative volume of a protein occupied by aliphatic side chains. The aliphatic index of the zinc finger sequences ranged from 30.0 to 122.58. The very high aliphatic index of zinc finger sequences indicates that these domains are stable at wide temperature range [67]. The aliphatic index of Pi54 zinc finger domain ZnF_NFX was recorded as 70.91. This value indicates that the NFX domain of Pi54 is thermo-stable as well as flexible in nature. The high aliphatic index 122.58 was recorded for CDGSH zinc finger domain of Gro 1-4 gene of potato. The lower thermal stability of ZZ zinc finger domain of Lr10 gene of wheat indicates that this domain is very flexible than others. The aliphatic index profile follows the polynomial distribution pattern (R2 = 0.94) (Figure 5; Table S4). The aliphatic index (AI) occupied by aliphatic side chains (A, V, I and L) is regarded as a positive factor which is defined as the relative volume of a protein for the increase of thermal stability of globular proteins [68], [69]. The very high aliphatic index of zinc finger sequences indicates that these domains are stable at wide temperature range [67].
Figure 5. Aliphatic index profile of identified zinc finger domain across cloned R gene.
The data of 26 zinc finger R genes with their zinc finger types were included in this analysis.
Analysis of Grand Average of Hydropathy of zinc finger domains of R-proteins
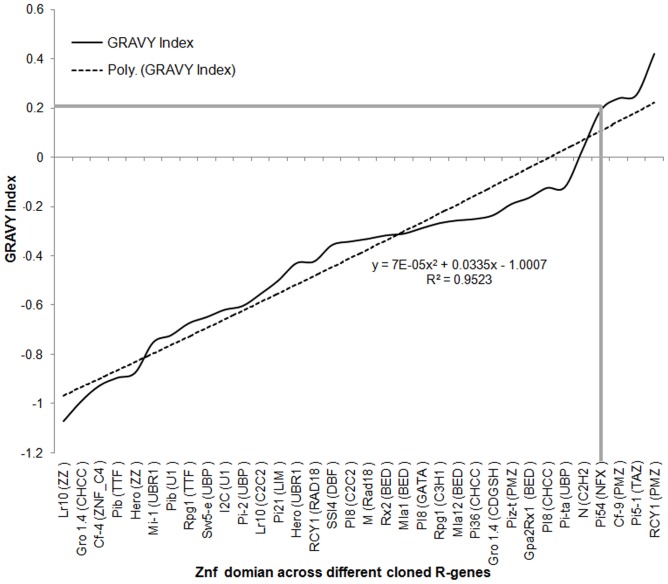
The Grand Average of Hydropathy (GRAVY) indices of zinc finger domain of cloned R genes ranged from −1.072 to 0.421 (Figure 6; Table S4). GRAVY values determined to provide a view of the hydrophobicity of the whole protein [70]. The GRAVY values usually vary in the range of ±2. Positive scores indicate hydrophobicity and negative scores indicate hydrophilicity. This low range of GRAVY value indicates the possibility of better interaction with water [67], [71]. The GRAVY value for Pi54 ZnF domain was calculated as 0.191 (Figure 6) which indicates that this domain is hydrophobic in nature. This nature of the domain of R proteins makes them available for better interaction with their Avr counterparts as reported in case of Pto protein of tomato [72]. GRAVY values calculated for this domain of all the protein also follows the linear distribution pattern ((R2 = 0.95). The majority of zinc finger domains were hydrophilic in nature and some were found to hydrophobic.
Figure 6. Grand Average of hydropathicity index profile of identified zinc finger domain across cloned R gene.
The data of 26 zinc finger R genes with their zinc finger types were included in this analysis.
Phylogenetic relationship among zinc finger motifs
To establish a relationship between thirty four taxa of zinc finger domains, a maximum parsimony tree was constructed to identify the significant correlation among the different and highly divergent zinc fingers present in R genes. Fourteen possible groups of taxa having sequence similarities between each other were obtained (Figure 7). Further, percentage identity plot was calculated between each taxa which results a significant association with maximum parsimony inference between T1 & T2 (40%) and T16 & T17 (80.8%). The taxa T16 & T17 belong to potato R genes Rx2 and Gpa2/Rx1. Both these genes contained BED zinc finger domains. The size of zinc finger domains in both the proteins is similar (47AA) but the size of both the NBS-LRR proteins is 912 and 937 AA, respectively (Table 2). This zinc finger domain was first reported in 2000 after two drosophila proteins named BEAF and DREF were identified [23]. These domains are probably involved in regulatory function of transcriptional control in plant disease resistance proteins [73]. Similarly the T1 & T2 taxa were represented by rice (Piz-t) and tomato (Cf-9). These are having sequence similarities between PMZ zinc fingers. The PMZ zinc finger size is 20 AA in both the proteins though the size of the protein in both the genes was 1033 AA (NBS-LRR) and 863 AA (LRR), respectively (Figure 7; Table 2). The PMZ (Plant Mutator Transposase) zinc finger is basically a transcription factor that is induced during the senescence and pathogen infection. These domains are present in AN1 like protein families. The PMZ domain containing proteins are induced by the abscisic acid and chitin stimuli [74], [75]. There are four groups of taxa having more than 10% parsimony inference between each other. These are T9 & T10 (10.2%), T20 & T21 (11.3%), T22 & T23 (10.8%) and T30 & T31 (11.6%). However, eight groups of taxa having less than 10% sequence similarities between each other were also obtained. The parsimony inference was not observed between zinc finger domain of Pi54 and other genes. These findings indicate that the zinc finger domain of Pi54 protein is distinct amongst all the analyzed highly divergent zinc finger sequences of the proteins of plant disease resistance genes.
Figure 7. Maximum parsimony tree of 34 R genes containing zinc finger proteins.
The bootstrap consensus tree (1000 replicates) is taken to represent the relationship between the taxa. Branches corresponding to partitions reproduced in less than 50% bootstrap replicates are collapsed. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) is shown next to the branches. The name of R gene, zinc finger types and crops are given on the termini of branches.
Identity plot of zinc fingers of cloned R- genes in relation to Pi54 gene
This analysis encompasses the identity between the zinc finger domains of blast resistance gene Pi54 and the genes cloned from other crops (Figure 8). The blast resistance genes cloned from rice were found to be closer to Pi54. The rice blast resistance protein pi21 is the smallest protein amongst all the zinc finger proteins which contains the LIM zinc finger domain [76]. This domain showed 12.3% sequence similarity (AA sequences) with zinc finger domain of Pi54 protein. The LIM domain is also a new functional motif deduced in pi21 gene of rice and it contains a cysteine-rich motif of CX2 - CX17–19HX2CX2CX2CX16–20CX2–3C. It was reported that LIM- containing proteins have been implicated in the transcriptional regulation of cell-differentiation and growth regulation and serve as the site for protein-protein interaction [17]. The similarity in amino acid sequences of zinc finger domains between Pi54 and pi21 supports our hypothesis that the LRR integrated NFX zinc finger of Pi54 might be involved in protein-protein interaction. We obtained 2.9% similarity between TAZ zinc finger domain of Pi5-1 and NFX domain of Pi54 proteins. The transcriptional adapter zinc binding (TAZ) domains are important sites for protein-protein interactions [77]. The similarity obtained between TAZ zinc finger domains of Pi5-1 also supports our hypothesis. It was also reported that both the genes Pi5-1 [78] and Pi54 [26], [33], [35] expressed constitutively at a basal level in both transgenic as well as susceptible native lines up to 48 hours post inoculation and induced by M. oryzae infection in later hours. The pathogen inducible nature of both the genes also supports this analysis that similar amino acids in these proteins might play some important role in R-Avr interactions. The TTF zinc finger domain of Pib protein showed only 1.2% similarity with Pi54. The UBP zinc finger of Pi-z, PMZ zinc finger domain of Piz-t, UBP zinc finger of Pita, U1 zinc finger of Pib and CHCC of Pi36 protein were found to have no similarity with Pi54 zinc finger domain. The Lr10 disease resistance protein of wheat was found to have two zinc finger domains namely C2C2 and ZZ and have 10.5% and 7.6% similarity with zinc finger domain of Pi54 protein, respectively. The C2C2 zinc finger domain is reported as novel zinc finger in many disease resistance proteins of various crops like Arabidopsis (LSD1& LOL1) and its homologs in rice OsLSD1 and OsLOL1 that negatively regulates programmed cell death (PCD) and plant defense signaling pathways [19], [30]. The similarity found between pairwise sequence alignment of zinc finger domains C2C2 and NFX showed that both are actively involved in negative regulation of up or down stream defense events. It has already been reported that NFX domains actively worked as negative regulators of various regulatory mechanisms which improves the physiological status of plants and supports growth and survival under various stress conditions. The expression of such type of zinc finger containing rice blast resistance gene Pi54 was found under biotic stress conditions [64], [79], [35], [33], [26]. The ZZ zinc finger domain is a type of protein domain that was named because of its ability to bind two zinc ions [80]. The ZZ zinc finger domains containing proteins are found to be involved in protein-protein interactions and generation of hypersensitive responses [81]. The similarity between amino acid sequences of ZZ and NFX domains further supports the results of Pi54 zinc finger analysis. Tobacco R gene N having C2H2 zinc finger domain showed 6.6% similarity with NFX of Pi54. The C2H2 zinc finger proteins were mainly related to the plant development regulation or involved in various types of stress responses [82]. The one of the largest transcription factor families in eukaryotes are constituted by Cys2/His2-type zinc finger proteins [83]. Many stress-responsive C2H2-type zinc finger proteins have been identified in various plant species. Several studies have reported that C2H2-type zinc finger proteins are responsible for both the activation of some stress-related genes and enhanced tolerance to salt, dehydration, and/or cold stresses [82]. The C2H2 zinc finger containing proteins are also reported for their ROS scavenging nature and enhanced expression of defense response genes in plants [84]. Hence, C2H2 zinc finger of N protein having similarity with Pi54 zinc finger also supports our results.
Figure 8. Identity plot of zinc finger domains.
Identity plot of zinc fingers represents the identity analysis between amino acid sequences of Pi54 zinc finger domain and zinc finger domains present in cloned R genes of various crops.
The R gene of sunflower pI8 was found to be having three zinc finger domains viz C2C2, CHCC and GATA. Two zinc finger domains C2C2 and CHCC showed 6.4 and 4.4% similarities with NFX domain of Pi54 protein, respectively. These domains in pI8 protein were also present at -C terminal like that of the zinc finger domain of Pi54 protein. The CHCC zinc finger domains of pI8 protein are the variants of the classical zinc fingers like C2C2 and C2H2 [85]. The formation of CHCC is due to replacement of one cysteine residue by histidine residue. These are short zinc-finger domains conserved from fungi to humans with a consensus sequence of Cx8Hx14Cx2C. Both the domains, NFX and CHCC are short in length, have three cysteine and one histidine residues and structural fold comprising a β-hairpin followed by a short α-helix that adopt two different conformations. This makes the structure of such type of zinc fingers highly divergent from other eukaryotic zinc fingers and these motifs are reported to have a group of DNA binding proteins from Archea [86]. However, the third zinc finger domain GATA of this gene showed no similarity with NFX-Pi54. There were no significant similarity between Pi54 and other zinc finger domains of barley proteins. The zinc finger domains of Tomato disease resistance proteins, viz., Hero, I2C, Sw5-e and Mi-1 have 5.6% and 3.4%, 2.7% and 1.7% similarity with Pi54 protein, respectively. The zinc finger domains of Arabidopsis and Potato R proteins showed 5.7 and 2.1% sequence similarities with NFX zinc finger domain of Pi54 protein, respectively. However, no similarity was found between Rad18 zinc finger domain of Flax M protein and NFX of Pi54 (Figure 8).
An identity matrix shows the proportion of identical residues between all of the sequences in the alignment as they are originally aligned. A total of 561 combinations of pairwise sequence identity were generated with the given data sets, out of which 90.55% of the combinations shows less than 10% sequence identity (which represents a significant statistical support of zinc finger sequence divergence) whereas 8.73% of the combinations shows an identity between 10–25% and the highest pairwise sequence identity (80.8%) exist between Gpa2Rx1_BED-Potato & Rx2_BED-Potato followed by Cf-9_PMZ-Tomato & Piz-t_PMZ-Rice (40.0%), Mla1_BED-Barley & Rx2_BED-Potato (38.7%) and Mla1_BED-Barley & Gpa2Rx1_BED-Potato (36.7%).
Pairwise identity matrix created from the zinc finger proteins, zinc finger domains and 11AA trimmed sequence aligned with Pi54_NFX-Rice is given in Figure 9. The analysis showed that Pi21 protein has 0.5% identity with Pi54. In case of zinc finger domain, LIM zinc finger of pi21gene of rice showed maximum identity (10%) with the zinc finger domain (NFX) of Pi54 protein (Figure 9). The 15 zinc finger domain sequences analyzed in the present study did not show any identity with zinc finger domain of Pi54 protein (Figure 9). A total of fifteen zinc finger domains of proteins (Pib, Mla-1, GPa2Rx1, Rx2, Sw5-e, Pi5-1, Gro1.4, I2C, RCY1, pI8 & N) were found with increasing identity with zinc finger domain of Pi54 blast resistance protein. Three zinc finger domains of wheat (Lr10_ZZ-wheat, Lr10_C2C2-wheat), and rice (pi21_LIM-rice) were found to be very close to NFX zinc finger domain of Pi54 protein. These domains are known to be actively involved in protein-protein interaction and as a potential regulator of various regulatory mechanisms, besides helping to maintain the physiological status of these proteins in various abiotic and biotic stresses [17], [64], [79]. After alignment of the eleven amino acids of the Pi54 zinc finger with other zinc finger domains as trimmed sequence, we found that LIM zinc finger domain of rice blast resistance pi21 protein showed maximum similarity (more than 40%) with zinc finger domain of rice blast resistance protein Pi54. The TTF zinc finger of Pib, UBR1 of Mla1, BED of the GPa2Rx1 and UBP of Sw5-e showed 10% identity to the Pi54 zinc finger while TAZ zinc finger of Pi5-1 showed 20% identity. The CHCC zinc finger of pI8, UBR1 of Hero, DBF of SSI4 and BED zinc finger of Mla1 showed 30% identity. The C4 zinc finger of Cf-4, and TTF of Rpg1 protein showed no (0%) identity with the Pi54 zinc finger (Figure 9).
Figure 9. Pair-wise percentage identity profile.
The Graphical representation showed the pair wise percentage identity profile of zinc finger domains of cloned R genes with respect to NFX domain of Pi54 gene. This analysis includes the comparison between the complete protein sequences of zinc finger containing R-proteins, Zinc finger domains of these proteins and 11 amino acid trimmed sequences showed similarity with Pi54 zinc finger domains. The X-axis of the graph represents the zinc finger containing cloned R genes across different crops whereas Y-axis represent the percentage identity profile between zinc fingers with respect to Pi54.
Conclusions
This study presents an analysis of plant disease resistance protein sequences in which zinc finger domains were found to be present along with other previously described domains like NBS and LRRs. The Pi54 gene conferring durable resistance to blast disease in rice encodes a NBS-LRR protein along with a typical zinc finger domain. The zinc finger domain of Pi54 protein is NFX type and located on the C-terminal in between NBS and LRR domains of the R-protein. Compositional analysis depicted by the helical wheel diagram revealed the presence of a hydrophobic region within this domain which might help in exposing the LRR region for a possible R-Avr interaction. We also found the presence of different types of Zinc finger domains in rice blast resistance and in other plant disease resistance proteins. Maximum numbers of zinc finger domains were found in the proteins of disease resistance genes cloned from rice and tomato. Many disease resistance genes like pI8 (Sunflower), Pib (Rice), Lr10 (Wheat), Gro1-4 (Potato), RCY1 (Arabidopsis) and Rpg1 (Barley) contains multiple number of zinc finger domains. The Pi54 protein contains the smallest zinc finger domain, despite the fact that smallest protein among these plant disease resistance proteins is pi21, another blast resistance gene. Instability, aliphatic and hydropathicity profile of these zinc finger domains gave a representation of the biochemical features of these proteins. We identified thirty four zinc finger domains in twenty six plant disease resistance proteins. These were found to be of nineteen different types of zinc fingers belonging to nine crops of five different families. Resistance proteins are known to play a crucial role in pathogen resistance by utilizing NBS and LRR domains for receiving signals from the pathogen. However, the presence of zinc finger domains, in combination with NBS-LRR domains in resistance proteins may reflect a major role of these domains in host- pathogen interaction.
Materials and Methods
In- silico analysis of zinc finger motif
The in-silico examination was performed to deduce the zinc finger domain in cloned R genes. The protein sequences of more than 70 genes present in National Centre for Biotechnology Information (NCBI) database (www.ncbi.nlm.nih.gov) were downloaded and analyzed (Table S1). The Expasy proteomic tool SMART (Simple Modular Architecture Research Tool) (www.smart.embl-heidelberg.de) was used for the identification of zinc finger motif [87], [88]. The identified zinc finger domain sequences were used for multiple sequence alignment using Clustal X version 1.83 (www.clustal.org) and Bio-edit 2.0 (http://www.mbio.ncsu.edu/bioedit) using default parameters [89], [90].
Biophysical characterization
The structural and functional prediction of Pi-kh (Pi54) and other ZnF domains were studied using Expasy proteomic tool Protparam (http://us.expasy.org/tools/protparam.html) [70]. The physico-chemical parameters like Molecular weight, theoretical pI, instability index [66], aliphatic index [91] and grand average of hydropathicity (GRAVY) [70] were computed using Expasy's ProtParam Proteomics server. The Grand Average hydropathy (GRAVY) value for a peptide or protein is calculated as the sum of hydropathy values of all the amino acids, divided by the number of residues in the sequence.
Phylogenetic analyses
The predicted zinc finger domains of R-proteins were further undertaken for phylogenetic analysis. The aligned sequences were inspected and adjusted manually to minimize the number of gaps and insertions. The manual adjustments were based on the sequence similarities. The phylogenetic tree was constructed according to the Neighbor-Joining method and visualized by MEGA program version 4.0 [56]. To validate the reproducibility of the branching pattern, bootstrap analysis (1000 replicates) and distance analysis were performed.
Comparative pairwise identity profiling of Pi54 zinc finger domain across different crops
For the pairwise identity between the zinc finger domain of blast resistance gene Pi54 and the genes cloned from other crops, 2-D pairwise identity data matrix was generated using Bio-Edit version 5.0.6 [89]. Further, 2-D pairwise identity data matrix was resolved with respect to Pi54 to delineate its comparative status in relation to other zinc finger domains across different crops.
Supporting Information
List of References for Supplementary files.
(DOCX)
The cloned plant disease resistance genes and their specific features.
(DOCX)
Distribution of Zinc finger R-proteins across different crops.
(DOCX)
Details of different Zinc finger domains across various crops cloned R genes.
(DOCX)
Biophysical parameters of identified Zinc finger domains across various cloned R genes.
(DOCX)
Acknowledgments
Authors are thankful to Mr. Niraj Kumar of Xplorigen Technologies, New Delhi for his invaluable contribution in statistical data analyses, making graphics and Mr. Deepak Kumar Gupta for his help in domain architecture analysis of zinc finger proteins.
Funding Statement
Financial assistance received from the Department of Biotechnology (F.No. BT/AB/FG-2(PH-II)/2009-3A), Government of India, National Agricultural Innovation Project-Indian Council of Agricultural Research (C4/C1071) by TRS is gratefully acknowledged. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Feurtado JA, Huang D, Wicki-Stordeur L, Hemstock LE, Potentier MS, et al. (2011) The Arabidopsis C2H2 zinc finger Indeterminate Domain1/Enhydrous promotes the transition to germination by regulating light and hormonal signaling during seed maturation. The Plant Cell 23: 1772–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Giri J, Vij S, Dansana PK, Tyagi AK (2011) Rice A20/AN1 zinc-finger containing stress-associated proteins (SAP1/11) and a receptor-like cytoplasmic kinase (OsRLCK253) interact via A20 zinc-finger and confer abiotic stress tolerance in transgenic Arabidopsis plants. New Phytol 191: 721–732. [DOI] [PubMed] [Google Scholar]
- 3. Laity JH, Lee BM, Wright PE (2001) Zinc finger proteins: New insights into structural and functional diversity. Curr Opin Struct Biol 11: 39–46. [DOI] [PubMed] [Google Scholar]
- 4. Gourcilleau D, Lenne C, Armenise C, Moulia B, Julien J, et al. (2011) Phylogenetic study of plant Q-type C2H2 zinc finger proteins and expression analysis of poplar genes in response to osmotic, cold and mechanical stresses. DNA Research 18: 77–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kodaira K, Qin F, Tran LP, Maruyama K, Kidokoro S, et al. (2011) Arabidopsis Cys2/His2 zinc-finger proteins AZF1 and AZF2 negatively regulate abscisic acid-repressive and auxin-inducible genes under abiotic stress conditions. Plant Physiol 157: 742–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Parthasarathy S, Murthy MR (2000) Protein thermal stability: Insights from atomic displacement parameters (B values). Protein Engg 13: 9–13. [DOI] [PubMed] [Google Scholar]
- 7. Boocock GRB, Marit MR, Rommens JM (2006) Phylogeny, sequence conservation, and functional complementation of the SBDS protein family. Genomics 87: 758–771. [DOI] [PubMed] [Google Scholar]
- 8. Emerson RO, Thomas JH (2009) Adaptive evolution in zinc finger transcription factors. PLoS Genet 5: e1000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Englbrecht CC, Schoof H, Böhm S (2004) Conservation, diversification and expansion of C2H2 zinc finger proteins in the Arabidopsis thaliana genome. BMC Genomics 5: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mackay JP, Crossley M (1998) Zinc fingers are sticking together. Trends Biochem Sci 23: 1–4. [DOI] [PubMed] [Google Scholar]
- 11. Klug A, Rhodes D (1987) ‘Zinc fingers’: a novel protein motif for nucleic acid recognition. Trends Biochem Sci 12: 464–469. [Google Scholar]
- 12. Klug A (2005) Towards therapeutic applications of engineered zinc finger proteins. FEBS Lett 579: 892–894. [DOI] [PubMed] [Google Scholar]
- 13. Berg JM, Shi Y (1996) The galvanization of biology: A growing appreciation for the roles of zinc. Science 271: 1081–1085. [DOI] [PubMed] [Google Scholar]
- 14. Jenkins TH, Li J, Scutt CP, Gilmartin PM (2004) Analysis of members of the Silene latifolia Cys2/His2 zinc-finger transcription factor family during dioecious flower development and in a novel stamen-defective mutant ssf1. Planta 220: 559–571. [DOI] [PubMed] [Google Scholar]
- 15. Ori N, Eshed Y, Paran I, Presting G, Aviv D, et al. (1997) The I2C family from the wilt disease resistance locus I2 belongs to the nucleotide binding, leucine-rich repeat superfamily of plant resistance genes. Plant Cell 9: 521–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schumann U, Prestele J, O'Geen H, Brueggeman R, Wanner G, et al. (2007) Requirement of the C3HC4 zinc RING finger of the Arabidopsis PEX10 for photorespiration and leaf peroxisome contact with chloroplasts. Proc Natl Acad Sci U S A 104: 1069–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Takatsuji H (1999) Zinc-finger proteins: The classical zinc finger emerges in contemporary plant science. Plant Mol Biol 39: 1073–1078. [DOI] [PubMed] [Google Scholar]
- 18. Ciftci-Yilmaz S, Mittler R (2008) The zinc finger network of plants. Cell Mol Life Sci 65: 1150–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dietrich RA, Richberg MH, Schmidt R, Dean C, Dangl JL (1997) A novel zinc finger protein is encoded by the Arabidopsis LSD1 gene and functions as a negative regulator of plant cell death. Cell 88: 685–694. [DOI] [PubMed] [Google Scholar]
- 20. Epple P, Mack AA, Morris VRF, Dangl JL (2003) Antagonistic control of oxidative stress-induced cell death in arabidopsis by two related, plant-specific zinc finger proteins. Proc Natl Acad Sci U S A 100: 6831–6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Meyers BC, Chin DB, Shen KA, Sivaramakrishnan S, Lavelle DO, et al. (1998) The major resistance gene cluster in lettuce is highly duplicated and spans several megabases. Plant Cell 10: 1817–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Głowacki S, Macioszek VK, Kononowicz AK (2011) R proteins as fundamentals of plant innate immunity. Cell Mol Biol Lett 16: 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aravind L (2000) The BED finger, a novel DNA-binding domain in chromatin-boundary-element-binding proteins and transposases. Trends Biochem Sci 25: 421–423. [DOI] [PubMed] [Google Scholar]
- 24. Tuskan GA, DiFazio S, Jansson S, Bohlmann J, Grigoriev I, et al. (2006) The genome of black cottonwood, Populus trichocarpa (torr. & gray). Science 313: 1596–1604. [DOI] [PubMed] [Google Scholar]
- 25. Deslandes L, Olivier J, Peeters N, Feng DX, Khounlotham M, et al. (2003) Physical interaction between RRS1-R, a protein conferring resistance to bacterial wilt, and PopP2, a type III effector targeted to the plant nucleus. Proc Natl Acad Sci U S A 100: 8024–8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sharma TR, Madhav MS, Singh BK, Shanker P, Jana TK, et al. (2005) High-resolution mapping, cloning and molecular characterization of the Pi-kh gene of rice, which confers resistance to Magnaporthe grisea . Mol Genet Genomics 274: 569–578. [DOI] [PubMed] [Google Scholar]
- 27. Sharma TR, Rai AK, Gupta SK, Vijyan J, Devanna BN, et al. (2012) Rice Blast Management through Host Resistance: Retrospect and Prospects. Agric Res 1: 37–52. [Google Scholar]
- 28. Sharma TR, Shanker P, Singh BK, Jana TK, Madhav MS, et al. (2005) Molecular mapping of rice blast resistance gene Pi-kh in rice variety Tetep. J Plant Biochem Biotechnol 14: 127–133. [Google Scholar]
- 29. Sonah H, Deshmukh RK, Singh VP, Gupta DK, Singh NK, et al. (2011) Genomic resources in horticultural crops: Status, utility and challenges. Biotechnol Adv 29: 199–209. [DOI] [PubMed] [Google Scholar]
- 30. Wang ZX, Yano M, Yamanouchi U, Iwamoto M, Monna L, et al. (1999) The Pib gene for rice blast resistance belongs to the nucleotide binding and leucine-rich repeat class of plant disease resistance genes. Plant J 19: 55–64. [DOI] [PubMed] [Google Scholar]
- 31. Bryan GT, Wu K, Farrall L, Jia Y, Hershey HP, et al. (2000) A single amino acid difference distinguishes resistant and susceptible alleles of the rice blast resistance gene Pi-ta . Plant Cell 12: 2033–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sharma TR, Rai AK, Gupta SK, Singh NK (2010) Broad-spectrum Blast Resistance Gene Pi-kh Cloned from Rice Line Tetep Designated as Pi54 . J Plant Biochem Biotechnol 19: 87–89. [Google Scholar]
- 33. Rai AK, Kumar SP, Gupta SK, Gautam N, Singh NK, et al. (2011) Functional complementation of rice blast resistance gene Pi-kh (Pi54) conferring resistance to diverse strains of Magnaporthe oryzae . J Plant Biochem Biotechnol 20: 55–65. [Google Scholar]
- 34. Costanzo S, Jia Y (2010) Sequence variation at the rice blast resistance gene Pi-km locus: Implications for the development of allele specific markers. Plant Science 178: 523–530. [Google Scholar]
- 35. Gupta SK, Rai AK, Kanwar SS, Chand D, Singh NK, et al. (2012) The single functional blast resistance gene Pi54 activates a complex defence mechanism in rice. J Exp Bot 63: 757–772. [DOI] [PubMed] [Google Scholar]
- 36. Das A, Soubam D, Singh PK, Thakur S, Singh NK, et al. (2012) A novel blast resistance gene, Pi54rh cloned from wild species of rice, Oryza rhizomatis confers broad spectrum resistance to Magnaporthe oryzae . Funct Integr Genomics 12: 215–228. [DOI] [PubMed] [Google Scholar]
- 37. Madhav MS, Plaha P, Singh NK, Sharma TR (2009) Molecular Characterization of a Genomic Fragment Containing Pi-kh Gene from the Genomic Library of indica Rice Line Tetep. J Phytopathol 157: 322–324. [Google Scholar]
- 38. Eulgem T, Somssich IE (2007) Networks of WRKY transcription factors in defense signaling. Curr Opin Plant Biol 10: 366–371. [DOI] [PubMed] [Google Scholar]
- 39. Ulker B, Somssich IE (2004) WRKY transcription factors: From DNA binding towards biological function. Curr Opin Plant Biol 7: 491–498. [DOI] [PubMed] [Google Scholar]
- 40. Klug A, Schwabe JW (1995) Protein motifs 5. zinc fingers. FASEB J 9: 597–604. [PubMed] [Google Scholar]
- 41. Zhou X, Wang Y, Pan Y, Li W (2007) Differences in amino acids composition and coupling patterns between mesophilic and thermophilic proteins. Amino Acids 34: 25–33. [DOI] [PubMed] [Google Scholar]
- 42. Yu H, Li J, Zhang D, Yang Y, Jiang W, et al. (2008) Improving the thermostability of N-carbamyl-d-amino acid amidohydrolase by error-prone PCR. Appl Microbiol Biotechnol 82: 279–285. [DOI] [PubMed] [Google Scholar]
- 43. Pack SP, Yoo YJ (2004) Protein thermostability: Structure-based difference of amino acid between thermophilic and mesophilic proteins. J Biotechnol 111: 269–277. [DOI] [PubMed] [Google Scholar]
- 44. Lawrence GJ, Finnegan EJ, Ayliffe MA, Ellis JG (1995) The L6 gene for flax rust resistance is related to the Arabidopsis bacterial resistance gene RPS2 and the tobacco viral resistance gene N . Plant Cell 7: 1195–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Watanabe K, Iwabuchi K, Sun J, Tsuji Y, Tani T, et al. (2009) RAD18 promotes DNA double-strand break repair during G1 phase through chromatin retention of 53BP1. Nucleic Acids Res 37: 2176–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Van den Burg B, Vriend G, Veltman OR, Venema G, Eijsink VGH (1998) Engineering an enzyme to resist boiling. Proc Natl Acad Sci U SA 95: 2056–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mattos C (2002) Protein-water interactions in a dynamic world. Trends Biochem Sci 27: 203–208. [DOI] [PubMed] [Google Scholar]
- 48. Xu J, Baase WA, Quillin ML, Baldwin EP, Matthews BW (2001) Structural and thermodynamic analysis of the binding of solvent at internal sites in T4 lysozyme. Protein Science 10: 1067–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Russell RJ, Hough DW, Danson MJ, Taylor GL (1994) The crystal structure of citrate synthase from the Thermophilic archaeon, Thermoplasma acidophilum . Structure 2: 1157–1167. [DOI] [PubMed] [Google Scholar]
- 50. Cammack R, Johnson CE, Hall DO, Rao KK (1971) The state of the iron atoms in hydroxylase iron-sulphur proteins. Biochem J 125: 18–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Miseta A, Csutora P (2000) Relationship between the occurrence of cysteine in proteins and the complexity of organisms. Mol Biol Evol 17: 1232–1239. [DOI] [PubMed] [Google Scholar]
- 52. Dill KA (1990) Dominant forces in protein folding. Biochemistry 29: 7133–7155. [DOI] [PubMed] [Google Scholar]
- 53. Kumar S, Nei M, Dudley J, Tamura K (2008) MEGA: biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief Bioinformatics 9: 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Flor HH (1971) Current status of the gene-for- gene concept. Annu Rev Phytopathol 9: 275–296. [Google Scholar]
- 55. Xiao L, Honig B (1999) Electrostatic contributions to the stability of hyperthermophilic proteins. J Mol Biol 289: 1435–1444. [DOI] [PubMed] [Google Scholar]
- 56. Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24: 1596–1599. [DOI] [PubMed] [Google Scholar]
- 57. Schiffer M, Edmundson AB (1967) Use of helical wheels to represent the structures of proteins and to identify segments with helical potential. Biophys J 7: 121–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kamtekar S, Schiffer JM, Xiong H, Babik JM, Hecht MH (1993) Protein design by binary patterning of polar and nonpolar amino acids. Science 262: 1680–1685. [DOI] [PubMed] [Google Scholar]
- 59. Baldwin JM (1993) The probable arrangement of the helices in G protein-coupled receptors. EMBO J 12: 1693–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Song Z, Krishna S, Thanos D, Strominger JL, Ono SJ (1994) A novel cysteine-rich sequence-specific DNA-binding protein interacts with the conserved X-box motif of the human major histocompatibility complex class II genes via a repeated cys-his domain and functions as a transcriptional repressor. J Exp Med 180: 1763–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Müssig C, Schröder F, Usadel B, Lisso J (2010) Structure and putative function of NFX1-like proteins in plants. Plant Biology 12: 381–394. [DOI] [PubMed] [Google Scholar]
- 62. Asano T, Yasuda M, Nakashita H, Kimura M, Yamaguchi1 K, et al. (2008) The AtNFXL1 gene functions as a signaling component of the type A trichothecene-dependent response. Plant Signal Behav 3: 991–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Larkindale J, Vierling E (2008) Core genome responses involved in acclimation to high temperature. Plant Physiol 146: 748–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lisso J, Altmann T, Müssig C (2006) The AtNFXL1 gene encodes a NF-X1 type zinc finger protein required for growth under salt stress. FEBS Lett 580: 4851–4856. [DOI] [PubMed] [Google Scholar]
- 65. Arora N, Banerjee AK, Mutyala S, Murty US (2009) Comparative characterization of commercially important xylanase enzymes. Bioinformation 3: 446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Guruprasad K, Reddy BV, Pandit MW (1990) Correlation between stability of a protein and its dipeptide composition: A novel approach for predicting in vivo stability of a protein from its primary sequence. Protein Engg 4: 155–161. [DOI] [PubMed] [Google Scholar]
- 67. Roy S, Maheshwari N, Chauhan R, Sen NK, Sharma A (2011) Structure prediction and functional characterization of secondary metabolite proteins of Ocimum. Bioinformation 6: 315–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Liao H, Yeh W, Chiang D, Jernigan RL, Lustig B (2005) Protein sequence entropy is closely related to packing density and hydrophobicity. Protein Eng Des Sel 18: 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ponnuswamy PK, Muthusamy R, Manavalan P (1982) Amino acid composition and thermal stability of proteins. Int J Biol Macromol 4: 186–190. [Google Scholar]
- 70. Kyte J, Doolittle RF (1982) A simple method for displaying the hydropathic character of a protein. J Mol Biol 157: 105–132. [DOI] [PubMed] [Google Scholar]
- 71. Sahay A, Shakya M (2010) In-silico analysis and homology modelling of antioxidant proteins of spinach. J Proteomics Bioinform 3: 148–154. [Google Scholar]
- 72. Xing W, Zou Y, Liu Q, Liu J, Luo X, et al. (2007) The structural basis for activation of plant immunity by bacterial effector protein AvrPto. Nature 449: 243–247. [DOI] [PubMed] [Google Scholar]
- 73. Hacquard S, Petre B, Frey P, Hecker A, Rouhier N, et al. (2011) The poplar-poplar rust interaction: Insights from genomics and transcriptomics. Mol Plant Microbe Interact 25: 279–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Breeze E, Harrison E, McHattie S, Hughes L, Hickman R, et al. (2011) High-resolution temporal profiling of transcripts during Arabidopsis leaf senescence reveals a distinct chronology of processes and regulation. The Plant Cell 23: 873–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Barth C, Moeder W, Klessig DF, Conklin PL (2004) The timing of senescence and response to pathogens is altered in the ascorbate-deficient Arabidopsis mutant vitamin C-1. Plant Physiol 134: 1784–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Fukuoka S, Saka N, Koga H, Ono K, Shimizu T, et al. (2009) Loss of function of a proline-containing protein confers durable disease resistance in rice. Science 325: 998–1001. [DOI] [PubMed] [Google Scholar]
- 77. De Guzman RN, Wojciak JM, Martinez-Yamout MA, Dyson HJ, Wright PE (2005) CBP/p300 TAZ1 domain forms a structured scaffold for ligand binding. Biochemistry 44: 490–497. [DOI] [PubMed] [Google Scholar]
- 78. Lee S, Song M, Seo Y, Kim H, Ko S, et al. (2009) Rice Pi5-mediated resistance to Magnaporthe oryzae requires the presence of two coiled-coil-nucleotide-binding-leucine-rich repeat genes. Genetics 181: 1627–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Krishna SS, Majumdar I, Grishin NV (2003) Structural classification of zinc fingers: Survey and summary. Nucleic Acids Res 31: 532–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ponting CP, Blake DJ, Davies KE, Kendrick-Jones J, Winder SJ (1996) ZZ and TAZ: New putative zinc fingers in dystrophin and other proteins. Trends Biochem Sci 21: 11–13. [PubMed] [Google Scholar]
- 81. Kang X, Chong J, Ni M (2005) Hypersensitive to red and blue 1, a ZZ-type zinc finger protein, regulates phytochrome B–Mediated red and cryptochrome-mediated blue light responses. The Plant Cell 17: 822–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kim S, Park J, Park S, Mitchell TK, Lee Y (2010) Identification and analysis of in planta expressed genes of Magnaporthe oryzae . BMC Genomics 11: 104–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kubo K, Sakamoto A, Kobayashi A, Rybka Z, Kanno Y, et al. (1998) Cys2/His2 zinc-finger protein family of petunia: Evolution and general mechanism of target-sequence recognition. Nucleic Acids Res 26: 608–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sun S, Guo S, Yang X, Bao Y, Tang H, et al. (2010) Functional analysis of a novel Cys2/His2-type zinc finger protein involved in salt tolerance in rice. J Exp Bot 61: 2807–2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Matthews JM, Sunde M (2002) Zinc fingers–folds for many occasions. IUBMB Life 54: 351–355. [DOI] [PubMed] [Google Scholar]
- 86. Cornilescu CC, Porter FW, Zhao KQ, Palmenberg AC, Markley JL (2008) NMR structure of the mengovirus leader protein zinc-finger domain. FEBS Lett 582: 896–900. [DOI] [PubMed] [Google Scholar]
- 87. Letunic I, Doerks T, Bork P (2012) SMART 7: recent updates to the protein domain annotation resource. Nucleic Acids Res 40: 302–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Schultz J, Milpetz F, Bork P, Ponting CP (1998) SMART, a simple modular architecture research tool: Identification of signaling domains,. Proc Natl Acad Sci U S A 95: 5857–5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hall TA (1999) BioEdit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp Ser 41: 95–98. [Google Scholar]
- 90. Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25: 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ikai A (1980) Thermostability and aliphatic index of globular proteins. J Biochem 88: 1895–1898. [PubMed] [Google Scholar]
- 92. Shirano Y, Kachroo P, Shah J, Klessig DF (2002) A gain-of-function mutation in an Arabidopsis Toll Interleukin1 receptor-nucleotide binding site-leucine-rich repeat type R gene triggers defense responses and results in enhanced disease resistance. Plant Cell 14: 3149–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Takahashi H, Miller J, Nozaki Y, Takeda M, Shah J, et al. (2002) RCY1, an Arabidopsis thaliana RPP8/HRT family resistance gene, conferring resistance to cucumber mosaic virus requires salicylic acid, ethylene and a novel signal transduction mechanism. Plant J 32: 655–667. [DOI] [PubMed] [Google Scholar]
- 94. Zhou F, Kurth J, Wei F, Elliott C, Valè G, et al. (2001) Cell-Autonomous Expression of Barley Mla1 Confers Race-Specific Resistance to the Powdery Mildew Fungus Via a Rar1-Independent Signaling Pathway. The Plant Cell 13: 337–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Shen Q, Zhou F, Bieri S, Haizel T, Shirasu K, et al. (2003) Recognition specificity and RAR1/SGT1 dependence in barley Mla disease resistance genes to the powdery mildew fungus. Plant Cell 15: 732–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Brueggeman R, Rostoks N, Kudrna D, Kilian A, Han F, et al. (2002) The barley stem rust-resistance gene Rpg1 is a novel disease-resistance gene with homology to receptor kinases. Proc Natl Acad Sci U S A 99: 9328–9333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Anderson PA, Lawrence GJ, Morrish BC, Ayliffe MA, Finnegan EJ, et al. (1997) Inactivation of the flax rust resistance gene M associated with loss of a repeated unit within the leucine-rich repeat coding region. The Plant Cell 9: 641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bendahmane A, Querci M, Kanyuka K, Baulcombe DC (2000) Agrobacterium transient expression system as a tool for the isolation of disease resistance genes: Application to the Rx2 locus in potato. Plant J 21: 73–81. [DOI] [PubMed] [Google Scholar]
- 99. van der Biezen EA, Freddie CT, Kahn K, Parker JE, Jones JDG (2002) Arabidopsis RPP4 is a member of the RPP5 multigene family of TIR-NB-LRR genes and confer downy mildew resistance through multiple signalling components. Plant J 29: 439–451. [DOI] [PubMed] [Google Scholar]
- 100. Paal J, Henselewski H, Muth J, Meksem K, Menéndez CM, et al. (2004) Molecular cloning of the potato Gro1-4 gene conferring resistance to pathotype Ro1 of the root cyst nematode Globodera rostochiensis, based on a candidate gene approach. Plant J 38: 285–297. [DOI] [PubMed] [Google Scholar]
- 101. Liu X, Lin F, Wang L, Pan Q (2007) The in-silico map-based cloning of Pi36, a rice coiled-coil nucleotide-binding site leucine-rich repeat gene that confers race-specific resistance to the blast fungus. Genetics 176: 2541–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zhou B, Qu S, Liu G, Dolan M, Sakai H, et al. (2006) The eight amino-acid differences within three leucine-rich repeats between Pi2 and Piz-t resistance proteins determine the resistance specificity to Magnaporthe grisea . Mol Plant Microbe Interact 19: 1216–1228. [DOI] [PubMed] [Google Scholar]
- 103. Radwan O, Mouzeyar S, Nicolas P, Bouzidi MF (2005) Induction of a sunflower CC-NBS-LRR resistance gene analogue during incompatible interaction with Plasmopara halstedii . J Exp Bot 56: 567–575. [DOI] [PubMed] [Google Scholar]
- 104. Konagaya K, Matsushita Y, Kasahara M, Nyunoya H (2004) Members of 14-3-3 protein isoforms interacting with the resistance gene product N and the elicitor of tobacco mosaic virus. J Gen Plant Pathol 70: 221–231. [Google Scholar]
- 105. Vos P, Simons G, Jesse T, Wijbrandi J, Heinen L, et al. (1998) The tomato Mi-1 gene confers resistance to both root-knot nematodes and potato aphids. Nature Biotechnol 16: 1365–1369. [DOI] [PubMed] [Google Scholar]
- 106. Brommonschenkel SH, Frary A, Frary A, Tanksley SD (2000) The broad-spectrum tospovirus resistance gene Sw5-e of tomato is a homolog of the root-knot nematode resistance gene Mi . Mol Plant-Microbe Interact 13: 1130–1138. [DOI] [PubMed] [Google Scholar]
- 107. Jones DA, Thomas CM, Hammond-Kosack KE, Balint-Kurti PJ, Jones JD (1994) Isolation of the tomato Cf-9 gene for resistance to Cladosporium fulvum by transposon tagging. Science 266: 789–793. [DOI] [PubMed] [Google Scholar]
- 108. Ernst K, Kumar A, Kriseleit D, Kloos D, Phillips MS, et al. (2002) The broad-spectrum potato cyst nematode resistance gene (hero) from tomato is the only member of a large gene family of NBS-LRR genes with an unusual amino acid repeat in the LRR region. Plant J 31: 127–136. [DOI] [PubMed] [Google Scholar]
- 109. Parniske M, Hammond-Kosack KE, Golstein C, Thomas CM, Jones DA, et al. (1997) Novel disease resistance specificities result from sequence exchange between tandemly repeated genes at the Cf-4/9 locus of tomato. Cell 91: 821–832. [DOI] [PubMed] [Google Scholar]
- 110. Feuillet C, Schachermayr G, Keller B (1997) Molecular cloning of a new receptor-like kinase gene encoded at the Lr10 disease resistance locus of wheat. Plant J 11: 45–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of References for Supplementary files.
(DOCX)
The cloned plant disease resistance genes and their specific features.
(DOCX)
Distribution of Zinc finger R-proteins across different crops.
(DOCX)
Details of different Zinc finger domains across various crops cloned R genes.
(DOCX)
Biophysical parameters of identified Zinc finger domains across various cloned R genes.
(DOCX)