Abstract
The lipopolysaccharide O antigen of Shigella flexneri 2a has two preferred chain lengths, a short (S-OAg) composed of an average of 17 repeated units and a very long (VL-OAg) of about 90 repeated units. These chain length distributions are controlled by the chromosomally encoded WzzB and the plasmid-encoded WzzpHS-2 proteins, respectively. In this study, genes wzzB, wzzpHS-2 and wzy (encoding the O-antigen polymerase) were cloned under the control of arabinose- and rhamnose-inducible promoters to investigate the effect of varying their relative expression levels on O antigen polysaccharide chain length distribution. Controlled expression of the chain length regulators wzzB and wzzpHS-2 revealed a dose-dependent production of each modal length. Increase in one mode resulted in a parallel decrease in the other, indicating that chain length regulators compete to control the degree of O antigen polymerization. Also, when expression of the wzy gene is low, S-OAg but not VL-OAg is produced. Production of VL-OAg requires high induction levels of wzy. Thus, the level of expression of wzy is critical in determining O antigen modal distribution. Western blot analyses of membrane proteins showed comparable high levels of the WzzB and WzzpHS-2 proteins, but very low levels of Wzy. In vivo cross-linking experiments and immunoprecipitation of membrane proteins did not detect any direct interaction between Wzy and WzzB, suggesting the possibility that these two proteins may not interact physically but rather by other means such as via translocated O antigen precursors.
INTRODUCTION
Shigella flexneri is a rod-shaped non-motile Gram-negative bacterium that causes shigellosis, an intestinal disease characterized by inflammation and epithelial cell destruction that results in acute bloody diarrhoea. More than one million deaths a year, affecting mainly young children in developing countries, are attributed to this infection (Jennison & Verma, 2004; Sansonetti, 2001). S. flexneri invades the intestinal epithelium by its basolateral surface, a mechanism mediated by a type III secretion system encoded by the mix/spa genes and by invasion proteins encoded in the ipa operon (Sansonetti & Egile, 1998). Once in the intracellular environment, S. flexneri escapes the phagocytic vacuole and induces actin polymerization at one pole of the bacteria, providing propulsive force to move intracellularly and intercellularly, spreading to adjacent cells, and causing epithelial cell destruction (Bernardini et al., 1989).
Besides invasion proteins, the lipopolysaccharide (LPS) plays an important role in the pathogenesis of S. flexneri infection (Morona et al., 2003; Okada et al., 1991; Sandlin et al., 1995; Van den Bosch et al., 1997). LPS is the major component of the outer membrane of Gram-negative bacteria. It has three structural domains: the inner lipid A region embedded in the membrane, the oligosaccharide core, and the O antigen (OAg), which extends from the cell surface. The S. flexneri OAg is formed by tetrasaccharide repeating units (RU) that are assembled in a complex series of reactions catalysed by a group of proteins bound to the inner membrane. Briefly, sugars are added in a sequential manner to the lipid carrier undecaprenyl phosphate, generating one OAg unit, which is translocated to the periplasmic side of the membrane by the Wzx translocase. The Wzy protein joins the new unit to other pre-existing ones to polymerize the OAg chain. Once polymerized, the OAg chain is linked to a lipid A-core oligosaccharide by the WaaL ligase, and then transported to the outer membrane (Raetz & Whitfield, 2002; Valvano, 2003; Whitfield, 1995). S. flexneri mutants lacking OAg are less virulent in vivo (Okada et al., 1991; Sandlin et al., 1995). The OAg protects the bacteria from the lytic action of serum complement (Hong & Payne, 1997) and plays a role in adherence and internalization to intestinal epithelial cells (Kohler et al., 2002; West et al., 2005).
In S. flexneri 2a, the OAg has a bimodal distribution or two preferred chain lengths, a short OAg (S-OAg) with an average of 17 RU and a very long OAg (VL-OAg) of about 90 RU, which are regulated by the chromosomally encoded WzzB and the plasmid-encoded WzzpHS-2 proteins, respectively (Morona et al., 1995; Stevenson et al., 1995). Mutation of both chain length regulator genes results in a random LPS polymerization pattern. Different reports have shown that not only the presence of OAg but also the chain length distribution is important for full virulence. The VL-OAg plays a key role in resistance to serum complement (Hong & Payne, 1997), but inside epithelial cells VL-OAg compromises the actin-based propulsion as it could mask the IcsA protein that localizes to the bacterial surface (Morona & Van Den Bosch, 2003). Hence, the presence of S-OAg but not of VL-OAg is required intracellularly. This suggests that S. flexneri expresses different OAg chain lengths at different stages of its pathogenic process and underscores the need to better understand the mechanisms leading to the regulation of LPS OAg chain length distribution.
Recently, we demonstrated that S. flexneri 2a modal chain length is growth-regulated through the control of wzy gene expression by the transcription elongation factor RfaH, which allows efficient expression of the genes encoding the proteins for OAg biosynthesis and assembly (Carter et al., 2007). In agreement with an early report (Daniels et al., 1998), we showed that overexpression of the wzy gene alters OAg chain length distribution, suggesting that the levels of Wzy affect not only OAg polymerization but also OAg chain length distribution. We also observed that overexpression of either WzzB or WzzpHS-2 shifts the distribution of chain lengths to the one that is typical of each respective regulator. These results led us to hypothesize that WzzB and WzzpHS-2 compete for the available Wzy. In this study, we cloned the wzy, wzzB and wzzpHS-2 genes under the control of inducible promoters to investigate the effect of the relative levels of each protein on OAg production and chain length distribution under controlled conditions. Our results demonstrate that the level of expression of wzy influences OAg modal distribution and that the two chain length regulators compete to control OAg polymerization.
METHODS
Bacterial strains, plasmids, media and growth conditions
Table 1 summarizes the properties of the bacterial strains and plasmids used in this study. Bacteria were grown aerobically at 37 °C in Luria–Bertani medium (LB) (Bacto tryptone 10 g l−1, Bacto yeast extract 5 g l−1, NaCl 5 g l−1). Solid medium contained 15 g Bacto agar l−1. Media were supplemented with ampicillin 100 μg ml−1, chloramphenicol 20 μg ml−1, kanamycin 50 μg ml−1 or trimethoprim 50 μg ml−1 as appropriate.
Table 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant properties* | Source/reference |
|---|---|---|
| Strains | ||
| 2457T | S. flexneri 2a wild-type | Laboratory stock |
| MSF102 | 2457T ΔwzzB | This work |
| MSF103 | 2457T wzzB-3xFLAG; KmR | This work |
| MSF107 | 2457T ΔwzzpHS-2 : : aph; KmR | This work |
| MSF109 | 2457T wzzpHS-2-3xFLAG; KmR | This work |
| MSF117 | 2457T wzy-3xFLAG | This work |
| MSF114 | 2457T Δwzy : : aph; KmR | This work |
| MSF209 | 2457T ΔwzzB ΔwzzpHS-2 : : aph; KmR | This work |
| MSF487 | 2457T ΔrfaH | Carter et al. (2007) |
| Plasmids | ||
| pBAD24 | Cloning vector, inducible with arabinose; ApR | Guzman et al. (1995) |
| pSCRhaB2 | Cloning vector, inducible with rhamnose; TmR | Cardona & Valvano (2005) |
| pJC75 | rfaH cloned into pGEM-T Easy; ApR | Carter et al. (2007) |
| pJC115 | wzy-3xFLAG cloned into pBAD24; ApR | This work |
| pJC142 | wzzB-3xFLAG cloned into pBAD24; ApR | This work |
| pJC146 | wzzpHS-2-3xFLAG cloned into pSCRhaB2; TmR | This work |
| pJC147 | wzzpHS-2-3xFLAG cloned into pBAD24; ApR | This work |
Ap, ampicillin; Km, kanamycin; Tm, trimethoprim; aph, aminoglycoside phosphotransferase.
Construction of mutant strains and plasmids
Mutagenesis of the wzy, wzzB and wzzpHS-2 genes was performed by the method of Datsenko & Wanner (2000) as described previously (Carter et al., 2007). The sequences of the oligonucleotide primers used in this study are available upon request. The cloning of the wzzB-3xFLAG and wzzpHS-2-3xFLAG fusions was carried out by PCR amplification using genomic DNA from strains MSF103 and MSF109, respectively. The amplicons were cloned into pGEM-T Easy as recommended by the supplier (Promega), followed by EcoRI digestion. The released fragments were cloned into pBAD24 (Guzman et al., 1995), also digested with EcoRI. Insert orientation was confirmed by PCR. The cloning of the wzy-3xFLAG fusion was carried out by PCR amplification of genomic DNA from strain MSF117. The amplicon was digested with KpnI and ligated to pBAD24, also digested with KpnI. Insert orientation was confirmed by PCR. Cloning of the wzzpHS-2 gene into the rhamnose-inducible promoter plasmid pSCRhaB2 (Cardona & Valvano, 2005) was performed by amplifying the wzzpHS-2 coding sequence from plasmid pHS-2 using specific primers with NdeI restriction sites. The plasmid vector and the PCR product were digested with NdeI, purified, and subsequently ligated. Insert orientation was confirmed by PCR. Plasmid constructs were checked by DNA sequencing.
Construction of wzy-3xFLAG, wzzB-3xFLAG and wzzpHS-2-3xFLAG chromosomal translational fusions
An oligonucleotide encoding three copies of the FLAG epitope was added in-frame to the 3′ end of the wzy, wzzB and wzzpHS-2 genes to construct strains expressing these proteins as C-terminal 3xFLAG fusions. Mutagenesis was carried out according to the method described by Uzzau et al. (2001) using PCR products. The oligonucleotides encoding the epitope tag and downstream kanamycin resistance gene cassette for the mutation of wzy, wzzB and wzzpHS-2 genes were obtained by PCR. The sequences of the oligonucleotide primers used in this study are available upon request. S. flexneri 2457T carrying pKD46 (Datsenko & Wanner, 2000) was transformed by electroporation with the PCR product generated using plasmid pSUB11 as a template (Uzzau et al., 2001). Transformants were plated on LB agar containing kanamycin and the insertion was confirmed by PCR.
Membrane protein preparation
Strains expressing the proteins of interest were grown overnight at 37 °C and subcultured in 50 ml LB at a dilution of 1 : 1000. Growth was continued with vigorous aeration for 4–5 h until an OD600 of 1.0 was reached. The cells were collected by centrifugation at 5900 g for 10 min and the pellet was washed three times with 20 mM phosphate buffer, pH 6.5. Then the cells were resuspended in 20 mM phosphate buffer supplemented with 1 mM PMSF and 1 mM EDTA. The samples were sonicated on ice in a Microson XL2000 Ultrasonic Cell Disruptor (Misonix) until a clear lysate was observed. Cell fractions were centrifuged at 8000 g at 4 °C for 5 min, then the supernatant was centrifuged a second time at 20 000 g at 4 °C for 60 min. The pellet containing total membrane proteins was resuspended in 20 mM phosphate buffer pH 6.5 and stored at −20 °C until used. A sample of the membrane preparation was used for protein quantification using the Coomassie Plus reagent (Pierce).
Western blot analysis
Samples from total membrane preparations were mixed with 3X protein tracking dye, incubated for 30 min at 45 °C, and separated on a 14 % SDS-PAGE gel. Proteins were transferred to a nitrocellulose membrane and the membrane was blocked according to standard procedures. The membrane was incubated either with anti-FLAG M2 mAbs (Sigma) to detect the Wzy-3xFLAG and WzzpHS-2-3xFLAG fusions, or with anti-Wzz affinity-purified polyclonal rabbit antibodies to detect the WzzB protein. Goat anti-mouse horseradish peroxidase (HRP)-conjugated (Rockland) or goat anti-rabbit HRP-conjugated (Pierce) were used as secondary antibodies, respectively. Detection was performed using the SuperSignal West Pico chemiluminiscent substrate (Pierce).
Induction assays
Strains carrying the inducible plasmids with the cloned wzy, wzzB or wzzpHS-2 genes were grown overnight at 37 °C and subcultured in 10 ml LB at a dilution of 1 : 1000. The medium was supplemented with arabinose or rhamnose to induce, or with glucose to repress, the expression of the genes. Growth was continued with aeration until an OD600 of 1.0 was reached. Cells were collected and LPS samples were prepared.
LPS analysis
LPS was prepared as described elsewhere (Marolda et al., 2006a). Briefly, culture samples were adjusted to an OD600 of 2.0 in a final volume of 1.5 ml LB medium. Cells were centrifuged and the pellets were suspended in 100 μl lysis buffer containing proteinase K. LPS was separated on 14 % acrylamide gels using a Tricine/SDS buffer system (Lesse et al., 1990) and visualized by silver staining (Marolda et al., 2006b). Gel loading was normalized so that each sample represented the same number of bacterial cells: each well was loaded with LPS from approximately 1×108 c.f.u. Densitometry analysis was performed using the UN-SCANT-IT gel software (Silk Scientific). The ratio of the average intensity of the bands corresponding to the S-OAg or VL-OAg relative to the intensity of the total OAg bands was calculated by quantifying the pixels in a narrow window across the centre of each lane. The densitometry analysis was calibrated using a range of loading volumes of S. flexneri 2a 2457T LPS, as described by Bravo et al. (2008).
In vivo protein cross-linking
In vivo cross-linking with dithio-bis(succinimidylpropionate) (DSP) and disuccinimidyl glutarate (DSG) was performed as described by Daniels & Morona (1999) with minor changes. Briefly, 25 ml cultures that had reached an OD600 of 1.0 were centrifuged, the pellet was washed in cross-linking buffer (150 mM NaCl, 20 mM sodium phosphate, pH 7.2) and concentrated 10-fold in the same buffer followed by cross-linking with 0.5 mM DSP or DSG for 45 min at 37 °C. Then, DSP or DSG was quenched by washing twice with 20 mM Tris/HCl, pH 7.5. Cells were then harvested and either stored at −20 °C or immediately used to obtain membrane fractions as described above. Protein samples were separated on a Novex 4–12 % Tris-Glycine gel (Invitrogen) and analysed by Western blotting as described above except that either IRDye 680 goat anti-mouse IgG or IRDye 800 goat anti-rabbit IgG (Rockland Immunochemicals) was used as the secondary antibody and detection was performed by infrared imaging using the Odyssey Infrared Imager (Li-Cor Biosciences).
Immunoprecipitation assays
Membrane fractions were prepared as described above. Triton X-100 was added at a final concentration of 1 %; the mix was incubated for 1 h at 4 °C with rotation and centrifuged at 20 000 g at 4 °C for 30 min. The supernatant was recovered and used for the assay. Agarose-Protein G beads (Roche) were centrifuged and washed with PBS, mixed with 5 μg anti-FLAG M2 mAb per sample, incubated for 2 h at 4 °C with rotation and finally washed three times with PBS. Membrane proteins were added to the beads and incubated for 2 h at 4 °C. Beads were centrifuged, washed three times with PBS, and proteins were eluted with 0.2 M glycine/HCl buffer pH 2.8. Proteins were separated on a 12 % SDS-PAGE gel and analysed by Western blotting as described above.
RESULTS
The level of expression of wzy determines the bimodal distribution of the Shigella flexneri 2a O antigen
We previously reported that when the wild-type strain S. flexneri 2a 2457T was transformed with the wzy gene cloned in a multicopy plasmid, OAg synthesis was displaced towards the production of high-molecular-mass molecules and, hence, the LPS profile did not show a clear bimodal distribution (Carter et al., 2007). This result suggested that Wzy levels could not only be important for OAg polymerization, but also contribute to OAg modal distribution. To test this hypothesis, we cloned the wzy gene under the control of the arabinose-inducible promoter (plasmid pJC115) and introduced it by transformation into the S. flexneri wzy null mutant strain MSF114. In contrast to the wild-type (Fig. 1, lane 1), the wzy mutant synthesized LPS composed of lipid A-core substituted with a high amount of one OAg unit (Fig. 1, lane 2). An identical phenotype was obtained when expression of the wzy gene encoded by pJC115 was repressed by glucose (Fig. 1, lane 3). When wzy expression was induced by the addition of 0.02 % arabinose, low-molecular-mass OAg molecules, as well as a small amount of the S-OAg distribution, were produced, but no VL-OAg was detected (Fig. 1, lane 4). High wzy gene expression by induction with 0.2 % arabinose resulted in the production of both modal distributions (Fig. 1, lane 5) plus additional bands in the intermediate region. Although a higher amount of low-molecular-mass (1–2 RU) molecules were observed, the LPS phenotype was very similar to the wild-type. Therefore, this experiment confirms that the level of wzy gene expression influences OAg chain length distribution in S. flexneri 2a 2457T.
Fig. 1.
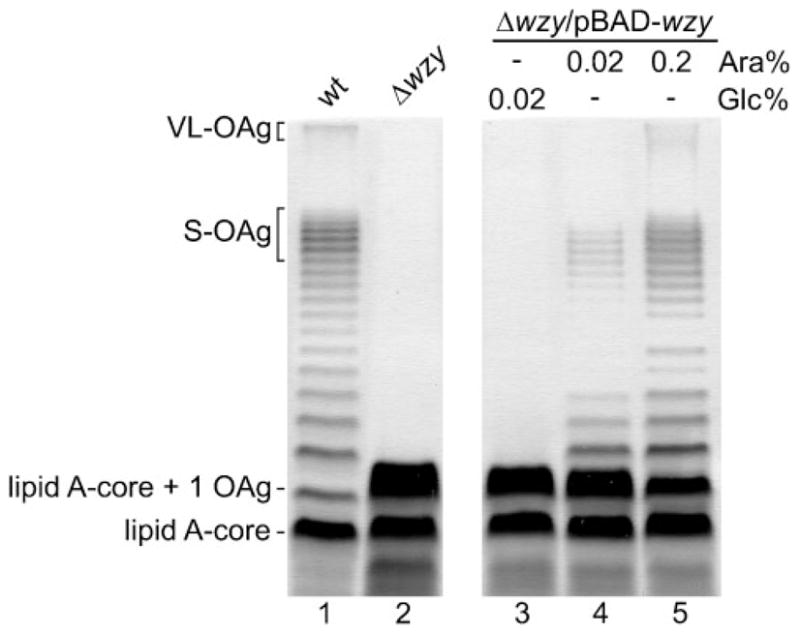
The level of wzy expression affects OAg modal distribution in S. flexneri 2a. LPS profiles of wild-type and Δwzy strains grown in LB medium (lanes 1 and 2), and LPS profile of the Δwzy/pBAD-wzy strain grown under repressing (Glc) (lane 3) and inducing (Ara) conditions (lanes 4 and 5) to an OD600 of 1.0. LPS samples from equal numbers of bacterial cells were loaded in each lane and analysed by SDS-Tricine-PAGE on a 14 % acrylamide gel followed by silver staining. Strains are: 2457T (wild-type, wt), MSF114 (2457T Δwzy) and MSF114/pJC115 (2457T Δwzy/wzy).
The chain length regulators compete to control the degree of polymerization
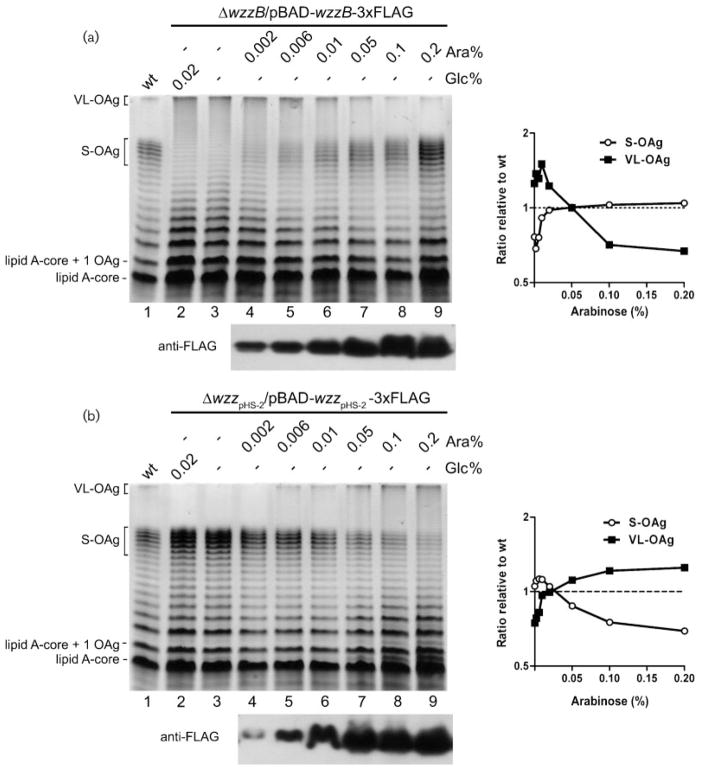
We previously showed that overexpression of the chain lengh regulators WzzB or WzzpHS-2 in the wild-type S. flexneri strain increases the proportion of S-OAg or VL-OAg, respectively. Our results suggested that WzzB would be more efficient in controlling the degree of polymerization than WzzpHS-2 (Carter et al., 2007). To analyse further the effect of varying the expression of each chain length regulator we constructed pJC142 and pJC147, containing the wzzB-3xFLAG and wzzpHS-2-3xFLAG gene fusions, respectively, cloned into pBAD24 under the control of the arabinose-inducible promoter. Each plasmid was introduced into the respective wzzB (MSF102) and wzzpHS-2 (MSF107) mutants and LPS samples from bacteria grown in LB medium supplemented with different concentrations of arabinose were examined. When strain MSF102/pJC142 was grown under repressing conditions (0.02 % glucose) the LPS lacked S-OAg (Fig. 2a, left panel, lane 2). The same LPS profile was obtained in a medium lacking both glucose and arabinose (Fig. 2a, lane 3). As expression of pBAD-wzzB was induced by the addition of arabinose, a dose-dependent increase of the S-OAg modal distribution was observed, with a concomitant decrease of the VL-OAg (Fig. 2a, lanes 4–9). Fig. 2(b) shows LPS profiles of strain MSF107/pJC147. In the presence of glucose or in the absence of glucose and arabinose, the LPS lacked the VL-OAg distribution (Fig. 2b, left panel, lanes 2 and 3). Under inducing conditions, a dose-dependent augmentation of VL-OAg with a simultaneous reduction of S-OAg was observed (Fig. 2b, lanes 4–9). Densitometry analyses of the lanes in the gels (Fig. 2a and b, right panels) allowed us to determine that the wild-type LPS phenotype (ratio relative to wild-type=1) was obtained in strain MSF102/pJC142 grown in LB medium supplemented with 0.05 % arabinose, and in strain MSF107/pJC147 grown in LB supplemented with 0.02 % arabinose. Under these induction conditions, comparable levels of the WzzB and WzzpHS-2 proteins were detected by Western blot analyses of membrane proteins (Fig. 2a and b, bottom). These results suggest that the two regulators are equally efficient in controlling OAg chain length distribution.
Fig. 2.
Increasing expression of chain regulators generates a dose-dependent shift in OAg distribution in S. flexneri 2a. (a) Left panel: LPS analysis of the wild-type strain grown in LB medium (lane 1), and of the ΔwzzB/pBAD-wzzB strain grown under repressing conditions (Glc) (lane 2), with no sugars added (lane 3) and under different inducing conditions (Ara) (lanes 4–9). All cultures were grown to an OD600 of 1.0. LPS samples from equal numbers of bacterial cells were loaded in each lane and analysed by SDS-Tricine-PAGE on a 14 % acrylamide gel followed by silver staining. Bottom: Western blot analysis of WzzB-3xFLAG expression under the same inducing conditions. Right panel: densitometry analysis of the gel performed using the UN-SCANT-IT software (b) Left panel: LPS analysis of the ΔwzzpHS-2/pBAD-wzzpHS-2 strain grown under the same conditions as in (a). Bottom: Western blot analysis of Wzz pHS-2-3xFLAG expression under the same inducing conditions. Right panel: densitometry analysis of the gel. The graphs show the ratio between the average intensity of the bands corresponding to the S-OAg or VL-OAg and the intensity of the total OAg bands in each lane, relative to the wild-type grown in LB medium (lane 1). Strains: 2457T (wild-type, wt), MSF102/pJC142 (2457T ΔwzzB/wzzB-3xFLAG) and MSF107/pJC147 (2457T ΔwzzpHS-2/wzzpHS-2-3xFLAG).
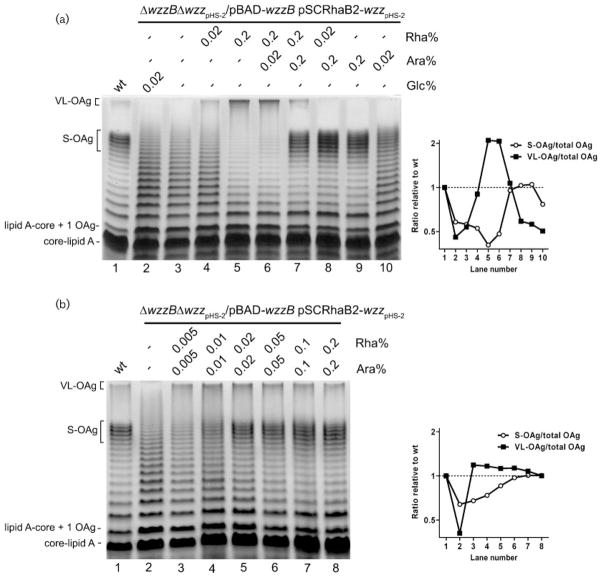
To avoid the possibility of a contribution of the chain regulators encoded either in the chromosome or on plasmid pHS-2 in the single mutants, we constructed strain MSF209, a double mutant carrying deletions of both wzzB and wzzpHS-2 genes. MSF209 was simultaneously transformed with pJC146 carrying a wzzpHS-2-3xFLAG fusion under the control of the rhamnose-inducible promoter and pJC142 (pBAD-wzzB-3xFLAG). The wild-type LPS phenotype was recovered only when both wzzB and wzzpHS-2 genes were highly expressed by the co-induction of their respective promoters (0.2 % arabinose for wzzB and 0.2 % rhamnose for wzzpHS-2) (Fig. 3a, lane 7). To further investigate this observation, the transformed strain was grown under increasing inducing conditions, at the same concentrations of arabinose and rhamnose inducers (Fig. 3b). Densitometric analysis of the lanes in the gel confirmed that the wild-type LPS phenotype was obtained at a concentration of 0.2 % of both inducers (Fig. 3b, right panel). Altogether, these data confirm the competition between WzzB and WzzpHS-2 to control polymer elongation, and underscore the importance of the proper balance between the cellular levels of the chain length regulators.
Fig. 3.
Competition between the chain length regulators determines OAg modal distribution. (a) Left panel: LPS profiles of the wild-type strain grown in LB medium (lane 1), and of strain ΔwzzB ΔwzzpHS-2/pBAD-wzzB/pSCRhaB-wzzpHS-2 grown under repressing conditions (Glc) (lane 2), with no sugars added (lane 3) and under different inducing conditions (Ara, Rha) (lanes 4–10). All cultures were grown to an OD600 of 1.0. LPS samples from equal numbers of bacterial cells were loaded in each lane and analysed by SDS-Tricine-PAGE on a 14 % acrylamide gel followed by silver staining. Right panel: Densitometric analysis of the gel. The graph shows the ratio between the average intensity of the bands corresponding to the S-OAg or VL-OAg and the intensity of the total OAg bands in each lane, relative to the wild-type grown in LB medium (lane 1). (b) Left panel: LPS profiles of strain ΔwzzB ΔwzzpHS-2/pBAD-wzzB/pSCRhaB-wzzpHS-2 grown under increasing inducing conditions (Ara, Rha). Right panel: densitometric analysis of the gel. Strains: 2457T (wild-type, wt) and MSF209/pJC142/pJC146 (2457T ΔwzzB ΔwzzpHS-2/wzzB wzzpHS-2).
Wzy is scarcely expressed in the bacterial membrane
As described above, high expression of the wzy gene (Fig. 1) as well as of both genes encoding the chain length regulators (Fig. 3) is required to produce the bimodal distribution of the OAg. With the aim of detecting the native levels of the Wzy, WzzB and WzzpHS-2 proteins, we constructed C-terminal FLAG epitope translational fusion derivatives of Wzy, WzzB and WzzpHS-2, resulting in strains MSF117 (wzy-3xFLAG), MSF103 (wzzB-3xFLAG) and MSF109 (wzzpHS-2-3xFLAG). Total membrane protein fractions of each of these strains were analysed by Western blotting using the anti-FLAG mAb. The results showed a dramatic difference in the amount of Wzy compared to either chain regulator. While the Wzy level was very low, the WzzB and WzzpHS-2 proteins were highly expressed at similar levels in the bacterial membrane (Fig. 4, lanes 3–5). Densitometric analyses of the bands showed that the amount of WzzB is 6-fold higher than that of Wzy, whereas the amount of WzzpHS-2 is 7.5-fold higher (not shown). Lanes 4 and 5 of the Western blots in Fig. 4 also showed high-molecular-mass bands; these could correspond to WzzB or WzzpHS-2 oligomeric forms, or to aggregates formed as consequence of the mild denaturation conditions used.
Fig. 4.
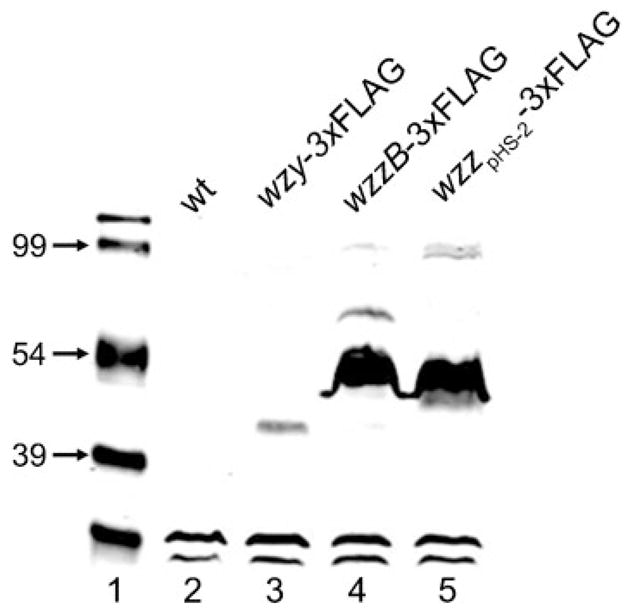
Protein levels of Wzy, WzzB and WzzpHS-2 FLAG fusions in total membrane extracts of S. flexneri 2a. After SDS-PAGE, proteins were detected by Western blotting with anti-FLAG mAbs. The lower, fast-migrating bands in each lane correspond to unspecific reaction of the Ab. Strains: 2457T (wild-type, wt), MSF117 (2457T wzy-3xFLAG), MSF103 (2457T wzzB-3xFLAG) and MSF109 (2457T wzzpHS-2-3xFLAG).
Cross-linking experiments do not reveal physical interactions between Wzy and WzzB
Several authors have proposed that proteins involved in OAg synthesis (Wzx, Wzz, Wzy, WecA) form a multi-protein complex in the bacterial inner membrane (Daniels & Morona, 1999; Daniels et al., 2002; Guo et al., 2006; Marolda et al., 2006b; Morona et al., 1994; Purins et al., 2008). Since our results indicated that the level of Wzy affects modal distribution, we attempted to demonstrate protein–protein interactions between Wzy and WzzB. Thus, we carried out in vivo cross-linking experiments using DSP or DGS as reactive agents, which react with lysine residues in the proteins and have spacing arms of 12 Å and 7.7 Å (1.2 and 0.77 nm), respectively. After in vivo cross-linking, bacterial cells were lysed and membrane proteins were resolved by SDS-PAGE followed by Western blotting. Detection was performed by infrared imaging using the Odyssey Infrared Imager (Li-Cor Biosciences), which allows the simultaneous detection of two signals. Anti-FLAG and anti-Wzz antibodies were used to detect the 3xFLAG epitope in the Wzy-3xFLAG fusion protein and the WzzB protein, respectively. The secondary antibodies IRDye 680 goat anti-mouse IgG and IRDye 800 goat anti-rabbit IgG recognize the anti-FLAG and anti-Wzz antibodies, giving a red or green signal, respectively. Co-localization of the proteins is detected as a yellow signal. Fig. 5 shows that without cross-linking, WzzB in the wild-type strain (lane 2) and WzzB and Wzy-3xFLAG in strain MSF117 (lane 3) are found as monomers. After cross-linking with DSP or DGS, no co-localization of Wzy and WzzB was observed in the wild-type strain (lanes 4 and 5), but bands of higher molecular mass were detected, which could correspond to oligomeric forms (dimers* or trimers**) of WzzB or to an interaction of WzzB with another protein(s) of similar molecular mass. Alternatively, the larger slow-migrating bands could correspond to aggregates formed by the mild denaturation conditions used. Similar results were obtained in strain MSF117 (lanes 6 and 7). In vivo cross-linking performed in a strain lacking the wzzpHS-2 gene to favour the possible interaction between Wzy and WzzB, and in vitro cross-linking experiments, gave the same results (not shown). To confirm these results, immunoprecipitation of membrane proteins obtained from the wild-type strain and strains carrying fusions WzzB-3xFLAG and Wzy-3xFLAG was performed using anti-FLAG M2 mAbs. After immunoprecipitation, Western blot analyses were carried out. As shown in Fig. 6, no co-immunoprecipitation of WzzB and Wzy was detected, further confirming the lack of protein–protein interaction between Wzy and WzzB under our experimental conditions.
Fig. 5.
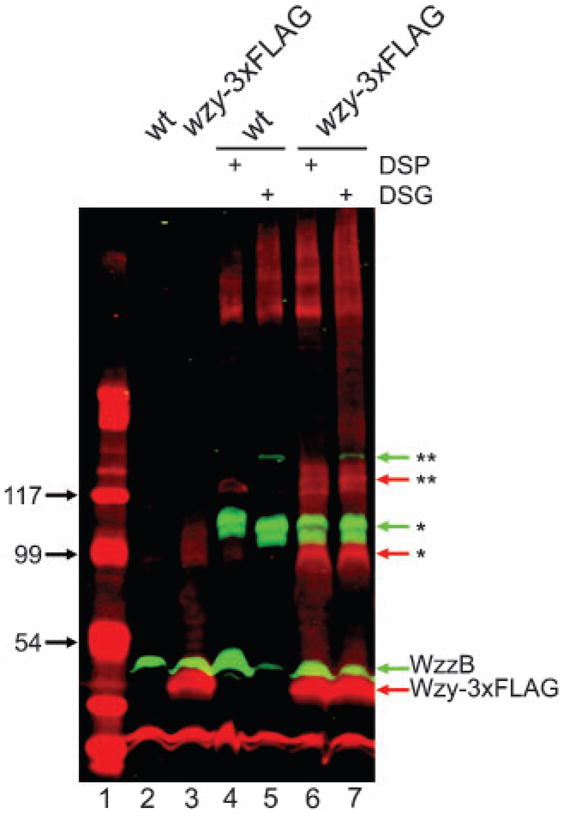
In vivo cross-linking assays do not detect protein–protein interaction between WzzB and Wzy. Cross-linking was performed using DSP (12 Å) or DSG (7.7 Å) as reactive agents, then membrane fractions were obtained and proteins were separated by SDS-PAGE (14 %) followed by Western blotting. Detection was performed by infrared imaging as described in Methods. The green signal corresponds to the WzzB protein, and the red signal to the Wzy protein. Co-localization of proteins (not visualized) should be seen as a yellow signal. High-molecular-mass bands corresponding to possible oligomers are symbolized as follows: * dimer, ** trimer. Strains: 2457T (wild-type, wt) and MSF117 (2457T wzy-3xFLAG).
Fig. 6.
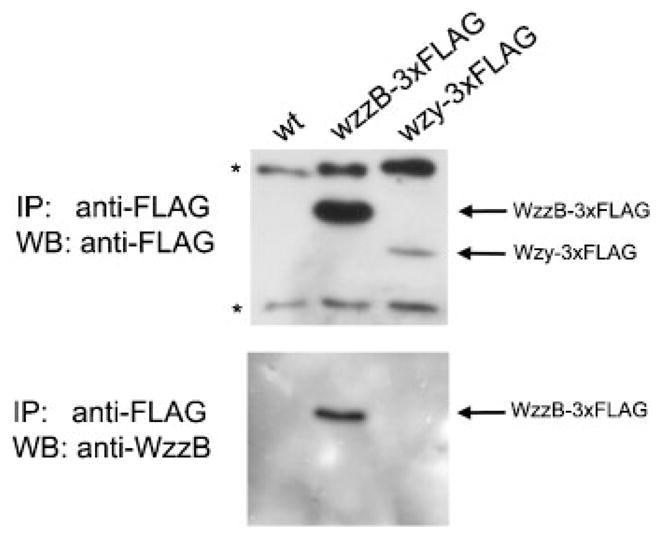
Immunoprecipitation assays do not detect co-immunoprecipitation of WzzB and Wzy. Immunoprecipitation (IP) was done using Agarose-Protein G beads and anti-FLAG antibody. Immunoprecipitated proteins were separated by SDS-PAGE (12 %) followed by Western blot analysis (WB). Nitrocellulose membrane was first incubated with anti-FLAG antibody, stripped and then incubated with anti-WzzB antibody. Asterisks represent unspecifically precipitated proteins. Strains: 2457T (wild-type, wt), MSF103 (2457T wzzB-3xFLAG) and MSF117 (2457T wzy-3xFLAG).
DISCUSSION
The LPS OAg is an important virulence determinant for many bacteria. In S. flexneri, not only OAg production but also the chain length distribution of the OAg molecules play a role in resistance to the innate immune system as well as in invasion of epithelial cells (Hong & Payne, 1997). However, the mechanisms that regulate S. flexneri bimodal OAg distribution are not clear. Three models to explain chain length regulation have been proposed: Bastin et al. (1993) suggested that Wzz would function as a molecular clock, switching Wzy activity between polymerization and transfer of the OAg to WaaL. In a different but not mutually exclusive model, Morona et al. (1995) suggested that Wzz functions as a molecular chaperone that facilitates the interaction between Wzy and WaaL, thus controlling the extension of the OAg chain. A new model has been proposed by Tocilj et al. (2008), in which Wzz would associate with Wzy in a membrane complex. Wzz would function as a scaffold that aids in OAg polymerization, and different Wzz oligomer sizes would give rise to modal distributions. However, a recent report by Larue et al. (2009) studying full-length Wzz homologues embedded in lipid membranes showed that all the proteins display the same hexameric state rather than different oligomeric forms.
Most studies aimed at describing a mechanism for chain length regulation have been conducted in bacteria that possess OAgs with a single modal distribution, including S. flexneri serotype Y and different serotypes of Escherichia coli, where VL-OAg production is not apparent (Bastin et al., 1993; Marolda et al., 2006b, 2008). Much less work has been performed in S. flexneri 2a, which exhibits a bimodal distribution of the OAg controlled by the chromosomally encoded WzzB and plasmid-encoded WzzpHS-2 proteins (Stevenson et al., 1995).
Previous work by Daniels et al. (1998) demonstrated that overexpression of Wzy in S. flexneri serotype Y produced LPS with unregulated OAg chain length distribution, and that a modal chain length distribution was restored by supplying the wzz gene in a low-copy-number plasmid. Here we show that Wzy levels are critical not only for OAg polymerization but also to determine the proper modal distribution of the S. flexneri 2a OAg. Our data show that when wzy expression is low, S-OAg but not VL-OAg is produced. Production of the VL-OAg distribution seemingly requires higher induction levels of wzy (Fig. 1). These experiments suggest that the level of Wzy protein must be tightly regulated to ensure proper LPS expression. In fact, the amount of Wzy is regulated at both the transcriptional and post-transcriptional levels. We recently reported that wzy gene expression in S. flexneri 2a 2457T is regulated during bacterial growth by the transcriptional elongation factor RfaH (Carter et al., 2007). In addition, the wzy gene lacks a detectable ribosome-binding site and has four rare codons in the translation initiation region (Daniels et al., 1998), suggesting that its protein product is poorly expressed. This could explain why previous attempts to detect the wild-type Wzy were unsuccessful. In this study, we were able to detect the wild-type Wzy protein in membrane preparations by incubation of the protein samples at 45 °C for 30 min, rather than boiling, and confirmed that Wzy is scarcely expressed in the bacterial membrane.
We previously showed that overexpression of either chain length regulator in S. flexneri 2a 2457T increases the modal distribution corresponding to that expected for each respective regulator (Carter et al., 2007). Also, our results suggested that WzzB is more efficient than WzzpHS-2 in controlling the degree of polymerization. However, a drawback in our previous work was that the genes encoding both regulators were expressed from their native promoters in a multicopy plasmid. Moreover, the promoter activity of a wzzB–lacZ fusion was ten times higher than that of a wzzpHS-2–lacZ fusion. Taking this into account, here we investigated the role of each regulator under controlled expression conditions using inducible promoters, in mutant strains lacking wzzB, wzzpHS-2, or both. Analysis of OAg profiles at different concentrations of inducer revealed a dose-dependent production of each modal length. Increase in one mode resulted in a parallel decrease in the other, indicating that WzzB and WzzpHS-2 compete to control the degree of polymerization. Our data also suggest that WzzpHS-2 would be slightly more efficient than WzzB in controlling the degree of polymerization (Fig. 2). Competition between the chain length regulators was supported by the results obtained when a double ΔwzzB ΔwzzpHS-2 mutant was transformed simultaneously with the wzzB gene cloned under the control of an arabinose-inducible promoter and the wzzpHS-2 gene cloned under the control of a rhamnose-inducible promoter (Fig. 3). Our results also show that WzzB and WzzpHS-2 are highly expressed in vivo, in comparison to Wzy (Fig. 4), and illustrate the importance of a proper balance between the levels of Wzy, WzzB and WzzpHS-2 to establish the correct chain length distribution of the OAg.
It has been proposed that the enzymes involved in the assembly of OAg form a multiprotein complex in the bacterial inner membrane. Studies by Morona’s group (Daniels & Morona, 1999; Morona et al., 1995) suggested that Wzz recruits a protein complex consisting of Wzy, WaaL and the nascent polysaccharide chain. However, it was demonstrated later that modality of the OAg chain can be observed in the absence of WaaL (Feldman et al., 2005). More recently, genetic evidence has suggested that the Wzx protein in E. coli forms a putative complex together with Wzz and Wzy (Marolda et al., 2006b). This notion is supported by functional studies performed with purified proteins, which suggest that E. coli Wzz would interact with Wzy (Tocilj et al., 2008), but this interaction has not been directly and unequivocally demonstrated. Our attempts to detect protein–protein interaction between Wzy and WzzB by cross-linking and immunoprecipitation assays were unsuccessful (Figs 5 and 6). Similar attempts using Wzz, WaaL, and Wzx in the E. coli K-12 system did not afford any evidence of cross-linking (C. L. Marolda & M. A. Valvano, unpublished). Nonetheless, our results demonstrate that WzzB could form oligomeric complexes, most probably homodimers, in agreement with Stenberg et al. (2005), who performed mass spectrometry of E. coli WzzB oligomers and concluded that they consist exclusively of WzzB. Our results indicating that the amount of Wzy is much smaller than that of both chain length regulators, together with the apparent absence of protein–protein interactions between WzzB and Wzy, are not consistent with the recent model proposing that Wzz oligomers serve as a scaffold for multiple Wzy polymerase molecules to facilitate OAg polymerization (Morona et al., 2009; Tocilj et al., 2008). Besides protein–protein interaction, Wzz and Wzy could also interact via contacts mediated by the OAg chains. Evidence from in vivo cross-linking and immunoprecipitation demonstrated that the OAg may physically interact with a putative protein complex in Pseudomonas aeruginosa (Daniels et al., 2002). Other studies in E. coli have suggested Wzz–OAg interaction in vitro (Guo et al., 2006; Tang et al., 2007). These observations are consistent with recent data from Larue et al. (2009) suggesting that Wzz proteins could act by regulating the flow of lipid-linked O-repeat units into the polymerization reaction. Our data indicating a role for Wzy in modulating the modal length of the OAg chain in competition with two different Wzz forms may also be explained by a differential level of interaction of each Wzz with the nascent OAg polymer. Further investigations are required to unequivocally determine whether OAg interacts with Wzz, Wzy, or both, in vivo.
Acknowledgments
This work was supported by grant ADI-08/2006 from Conicyt-World Bank (to I. C.) and by a grant from the Canadian Institutes of Health Research (to M. A. V.). M. A. V. holds a Canada Research Chair in Infectious Diseases and Microbial Pathogenesis.
Abbreviations
- DSG
disuccinimidyl glutarate
- DSP
dithio-bis(succinimidylpropionate)
- RU
repeating unit(s)
- S-OAg
short O-antigen
- VL-OAg
very long O antigen
References
- Bastin DA, Stevenson G, Brown PK, Haase A, Reeves PR. Repeat unit polysaccharides of bacteria: a model for polymerization resembling that of ribosomes and fatty acid synthetase, with a novel mechanism for determining chain length. Mol Microbiol. 1993;7:725–734. doi: 10.1111/j.1365-2958.1993.tb01163.x. [DOI] [PubMed] [Google Scholar]
- Bernardini ML, Mounier J, d’Hauteville H, Coquis-Rondon M, Sansonetti PJ. Identification of icsA, a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc Natl Acad Sci U S A. 1989;86:3867–3871. doi: 10.1073/pnas.86.10.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo D, Silva C, Carter JA, Hoare A, Alvarez SA, Blondel CJ, Zaldivar M, Valvano MA, Contreras I. Growth-phase regulation of lipopolysaccharide O-antigen chain length influences serum resistance in serovars of Salmonella. J Med Microbiol. 2008;57:938–946. doi: 10.1099/jmm.0.47848-0. [DOI] [PubMed] [Google Scholar]
- Cardona ST, Valvano MA. An expression vector containing a rhamnose-inducible promoter provides tightly regulated gene expression in Burkholderia cenocepacia. Plasmid. 2005;54:219–228. doi: 10.1016/j.plasmid.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Carter JA, Blondel CJ, Zaldivar M, Alvarez SA, Marolda CL, Valvano MA, Contreras I. O-antigen modal chain length in Shigella flexneri 2a is growth-regulated through RfaH-mediated transcriptional control of the wzy gene. Microbiology. 2007;153:3499–3507. doi: 10.1099/mic.0.2007/010066-0. [DOI] [PubMed] [Google Scholar]
- Daniels C, Morona R. Analysis of Shigella flexneri wzz (Rol) function by mutagenesis and cross-linking: wzz is able to oligomerize. Mol Microbiol. 1999;34:181–194. doi: 10.1046/j.1365-2958.1999.01591.x. [DOI] [PubMed] [Google Scholar]
- Daniels C, Vindurampulle C, Morona R. Overexpression and topology of the Shigella flexneri O-antigen polymerase (Rfc/Wzy) Mol Microbiol. 1998;28:1211–1222. doi: 10.1046/j.1365-2958.1998.00884.x. [DOI] [PubMed] [Google Scholar]
- Daniels C, Griffiths C, Cowles B, Lam JS. Pseudomonas aeruginosa O-antigen chain length is determined before ligation to lipid A core. Environ Microbiol. 2002;4:883–897. doi: 10.1046/j.1462-2920.2002.00288.x. [DOI] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman MF, Wacker M, Hernandez M, Hitchen PG, Marolda CL, Kowarik M, Morris HR, Dell A, Valvano MA, Aebi M. Engineering N-linked protein glycosylation with diverse O antigen lipopolysaccharide structures in Escherichia coli. Proc Natl Acad Sci U S A. 2005;102:3016–3021. doi: 10.1073/pnas.0500044102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Lokko K, Zhang Y, Yi W, Wu Z, Wang PG. Overexpression and characterization of Wzz of Escherichia coli O86 : H2. Protein Expr Purif. 2006;48:49–55. doi: 10.1016/j.pep.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Guzman LM, Belin D, Carson MJ, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M, Payne SM. Effect of mutations in Shigella flexneri chromosomal and plasmid-encoded lipopolysaccharide genes on invasion and serum resistance. Mol Microbiol. 1997;24:779–791. doi: 10.1046/j.1365-2958.1997.3731744.x. [DOI] [PubMed] [Google Scholar]
- Jennison AV, Verma NK. Shigella flexneri infection: pathogenesis and vaccine development. FEMS Microbiol Rev. 2004;28:43–58. doi: 10.1016/j.femsre.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Kohler H, Rodrigues SP, McCormick BA. Shigella flexneri interactions with the basolateral membrane domain of polarized model intestinal epithelium: role of lipopolysaccharide in cell invasion and in activation of the mitogen-activated protein kinase ERK. Infect Immun. 2002;70:1150–1158. doi: 10.1128/IAI.70.3.1150-1158.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larue K, Kimber MS, Ford R, Whitfield C. Biochemical and structural analysis of bacterial O antigen chain length regulator proteins reveals a conserved quaternary structure. J Biol Chem. 2009;284:7395–7403. doi: 10.1074/jbc.M809068200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesse AJ, Campagnari AA, Bittner WE, Apicella MA. Increased resolution of lipopolysaccharides and lipooligosaccharides utilizing tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J Immunol Methods. 1990;126:109–117. doi: 10.1016/0022-1759(90)90018-q. [DOI] [PubMed] [Google Scholar]
- Marolda CL, Lahiry P, Vines E, Saldias S, Valvano MA. Micromethods for the characterization of lipid A-core and O-antigen lipopolysaccharide. Methods Mol Biol. 2006a;347:237–252. doi: 10.1385/1-59745-167-3:237. [DOI] [PubMed] [Google Scholar]
- Marolda CL, Tatar LD, Alaimo C, Aebi M, Valvano MA. Interplay of the Wzx translocase and the corresponding polymerase and chain length regulator proteins in the translocation and periplasmic assembly of lipopolysaccharide O antigen. J Bacteriol. 2006b;188:5124–5135. doi: 10.1128/JB.00461-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marolda CL, Haggerty ER, Lung M, Valvano MA. Functional analysis of predicted coiled-coil regions in the Escherichia coli K-12 O-antigen polysaccharide chain length determinant Wzz. J Bacteriol. 2008;190:2128–2137. doi: 10.1128/JB.01746-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morona R, Van Den Bosch L. Lipopolysaccharide O antigen chains mask IcsA (VirG) in Shigella flexneri. FEMS Microbiol Lett. 2003;221:173–180. doi: 10.1016/S0378-1097(03)00210-6. [DOI] [PubMed] [Google Scholar]
- Morona R, Mavris M, Fallarino A, Manning PA. Characterization of the rfc region of Shigella flexneri. J Bacteriol. 1994;176:733–747. doi: 10.1128/jb.176.3.733-747.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morona R, van den Bosch L, Manning PA. Molecular, genetic, and topological characterization of O-antigen chain length regulation in Shigella flexneri. J Bacteriol. 1995;177:1059–1068. doi: 10.1128/jb.177.4.1059-1068.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morona R, Daniels C, Van Den Bosch L. Genetic modulation of Shigella flexneri 2a lipopolysaccharide O antigen modal chain length reveals that it has been optimized for virulence. Microbiology. 2003;149:925–939. doi: 10.1099/mic.0.26141-0. [DOI] [PubMed] [Google Scholar]
- Morona R, Purins L, Tocilj A, Matte A, Cygler M. Sequence–structure relationships in polysaccharide co-polymerase (PCP) proteins. Trends Biochem Sci. 2009;34:78–84. doi: 10.1016/j.tibs.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Okada N, Sasakawa C, Tobe T, Yamada M, Nagai S, Talukder KA, Komatsu K, Kanegasaki S, Yoshikawa M. Virulence-associated chromosomal loci of Shigella flexneri identified by random Tn5 insertion mutagenesis. Mol Microbiol. 1991;5:187–195. doi: 10.1111/j.1365-2958.1991.tb01839.x. [DOI] [PubMed] [Google Scholar]
- Purins L, Van Den Bosch L, Richardson V, Morona R. Coiled-coil regions play a role in the function of the Shigella flexneri O-antigen chain length regulator WzzpHS2. Microbiology. 2008;154:1104–1116. doi: 10.1099/mic.0.2007/014225-0. [DOI] [PubMed] [Google Scholar]
- Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandlin RC, Lampel KA, Keasler SP, Goldberg MB, Stolzer AL, Maurelli AT. Avirulence of rough mutants of Shigella flexneri: requirement of O antigen for correct unipolar localization of IcsA in the bacterial outer membrane. Infect Immun. 1995;63:229–237. doi: 10.1128/iai.63.1.229-237.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti PJ. Microbes and microbial toxins: paradigms for microbial–mucosal interactions. III. Shigellosis: from symptoms to molecular pathogenesis. Am J Physiol Gastrointest Liver Physiol. 2001;280:G319–G323. doi: 10.1152/ajpgi.2001.280.3.G319. [DOI] [PubMed] [Google Scholar]
- Sansonetti PJ, Egile C. Molecular bases of epithelial cell invasion by Shigella flexneri. Antonie Van Leeuwenhoek. 1998;74:191–197. doi: 10.1023/a:1001519806727. [DOI] [PubMed] [Google Scholar]
- Stenberg F, Chovanec P, Maslen SL, Robinson CV, Ilag LL, von Heijne G, Daley DO. Protein complexes of the Escherichia coli cell envelope. J Biol Chem. 2005;280:34409–34419. doi: 10.1074/jbc.M506479200. [DOI] [PubMed] [Google Scholar]
- Stevenson G, Kessler A, Reeves PR. A plasmid-borne O-antigen chain length determinant and its relationship to other chain length determinants. FEMS Microbiol Lett. 1995;125:23–30. doi: 10.1111/j.1574-6968.1995.tb07330.x. [DOI] [PubMed] [Google Scholar]
- Tang KH, Guo H, Yi W, Tsai MD, Wang PG. Investigation of the conformational states of Wzz and the Wzz. O-antigen complex under near-physiological conditions. Biochemistry. 2007;46:11744–11752. doi: 10.1021/bi701181r. [DOI] [PubMed] [Google Scholar]
- Tocilj A, Munger C, Proteau A, Morona R, Purins L, Ajamian E, Wagner J, Papadopoulos M, Van Den Bosch L, et al. Bacterial polysaccharide co-polymerases share a common framework for control of polymer length. Nat Struct Mol Biol. 2008;15:130–138. doi: 10.1038/nsmb.1374. [DOI] [PubMed] [Google Scholar]
- Uzzau S, Figueroa-Bossi N, Rubino S, Bossi L. Epitope tagging of chromosomal genes in Salmonella. Proc Natl Acad Sci U S A. 2001;98:15264–15269. doi: 10.1073/pnas.261348198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvano MA. Export of O-specific lipopolysaccharide. Front Biosci. 2003;8:s452–s471. doi: 10.2741/1079. [DOI] [PubMed] [Google Scholar]
- Van den Bosch L, Manning PA, Morona R. Regulation of O-antigen chain length is required for Shigella flexneri virulence. Mol Microbiol. 1997;23:765–775. doi: 10.1046/j.1365-2958.1997.2541625.x. [DOI] [PubMed] [Google Scholar]
- West NP, Sansonetti P, Mounier J, Exley RM, Parsot C, Guadagnini S, Prévost MC, Prochnicka-Chalufour A, Delepierre M, et al. Optimization of virulence functions through glucosylation of Shigella LPS. Science. 2005;307:1313–1317. doi: 10.1126/science.1108472. [DOI] [PubMed] [Google Scholar]
- Whitfield C. Biosynthesis of lipopolysaccharide O antigens. Trends Microbiol. 1995;3:178–185. doi: 10.1016/s0966-842x(00)88917-9. [DOI] [PubMed] [Google Scholar]