Abstract
Post-transplant lymphoproliferative disorder (PTLD) is the most after SOT (liver and lungs) and review cases reported in the literature. common treatment related malignancy that occurs after solid organ Both patients had a bimodal response to therapy with initial transplantation (SOT). PTLD has extended from its initial description eradication of bulky nodal disease with regimens typically used to as an Epstein–Barr virus (EBV)-driven B-cell proliferation to include treat leukemia, but persistence of low-level clonal T-cells in marrow, EBV-negative and non B-lineage cases. T-cell PTLD (T-PTLD) is rare CSF and lung in one case. Pediatr Blood Cancer 2008;50:415–in both adults and children. We report two cases of pediatric T-PTLD 418.
Keywords: flow cytometry, T-cell PTLD, T-cell receptor V-beta
INTRODUCTION
Post-transplant lymphoproliferative disorder (PTLD) was first described in 1981 as an Epstein–Barr virus (EBV)-associated polymorphic B-cell proliferation with either rapidly fatal or relatively slower progressive clinical course arising in patients receiving immunosuppressive therapy following solid organ transplantation (SOT) [1]. A critical step in the pathogenesis of PTLD is believed to be a disruption of the host’s immune response to EBV and impaired immune surveillance against subsequent cell proliferation. PTLD spans a continuum from reversible lymphoid proliferation to irreversible high-grade lymphoma, with corresponding morphological changes ranging from polymorphous to monomorphous and clonality evolution from polyclonal, to oligoclonal and monoclonal. The causative connection between PTLD and immunosuppression is supported by the fact that many PTLDs respond to reduction or cessation of immunosuppressive therapy.
EBV-negative PTLD and proliferations of T-cell or other lineages also occur following SOT, and now are typically included under the umbrella term PTLD. Ambiguity about the pathogenesis of T-PTLD, and the lack of accepted diagnostic criteria may contribute to the rarity and inconsistent characterization of T-PTLD in the literature. Of the 100–200 reported cases of T- and NK-cell PTLD most were single case reports, and very few included more than three cases [2–7]. We report two cases of T-PTLD in children after SOT.
MATERIALS AND METHODS
Patient Data and Specimen Collection
Two recent cases of pediatric T-PTLD prompted us to review the experience at our center, which has a large volume of pediatric SOT patients. No other pediatric cases of T-PTLD were diagnosed between 2001 and 2006. The clinical information and laboratory data were reviewed following the guidelines of the University of Florida Institutional Review Board.
Morphological Evaluation and Immunohistochemistry
Morphological evaluation was based on light microscopy. All tissue stains, including hematoxylin and eosin (H&E), immunohistochemistry, and in situ hybridization (ISH) were performed on formalin-fixed tissue according to standard laboratory procedures.
Flow Cytometric Analysis
Sample preparation from tissue, bone marrow (BM) or cerebrospinal fluid (CSF) was performed as described elsewhere [8]. T-Cell Receptor Vβ analysis was performed according to manufacturer’s instructions. Expression of a single Vβantigen in the majority of T-cells (>40%) is a direct indication of T-cell clonality, while non-reaction to any of the 24 antibodies in the majority of T-cells (>60%), after exclusion of gamma/delta T-cells, is considered indirect evidence of T-cell clonality [13].
Polymerase Chain Reaction (PCR) for TCR-γ Gene Rearrangement
T-cell receptor γ gene rearrangements were evaluated using primers for the four T-gamma gene families (1–4) in a multiplex PCR reaction with a portion of the β-globin gene amplified to ensure DNA integrity [13].
RESULTS
Case Reports
Case 1
This patient underwent bilateral lung transplant at 4 months of age for congenital surfactant C deficiency. Post-transplant immunosuppression included tacrolimus and prednisone. Thirty-two months following lung transplant, a CT scan revealed multiple mesenteric and retroperitoneal masses, with the largest approximately 10 × 8 × 4 cm. Biopsy of the mesenteric mass showed T-cell PTLD. A low level (approximately 1%) of T-cells with the same aberrant immunophenotype present in the abdominal tumor was detected in the marrow. She was EBV-seropositive pretransplant, but had also received multiple red blood cell transfusions. Quantitative PCR for EBV was performed after the transplant and reported as negative. Following diagnosis of T-PTLD, the patient was treated with three cycles of cyclophosphamide (600 mg/m2) plus a 5-day pulse of prednisone (2 mg/kg/day) given every 21 days with continuation of reduced dose tacrolimus [9]. She had an initial significant decrease in size of the abdominal masses, but re-evaluation after cycle #3 showed regrowth of the abdominal adenopathy. She then received four-drug acute lymphoblastic leukemia (ALL)-like induction chemotherapy with vincristine, daunorubicin, PEG asparaginase and prednisone with intrathecal methotrexate [10]. She had a dramatic response to this therapy with complete disappearance of abdominal disease. She continued to receive chemotherapy as given in the augmented BFM regimen [11], but persisted with low-level presence of the abnormal T-cell population in BM specimens 9 months following diagnosis. Therapy was complicated by multiple infectious and gastrointestional complications. The family elected to discontinue intensive chemotherapy. She received several brief pulses of high dose steroids and was continued on reduced doses of immunosuppression with tacrolimus and prednisone with addition of sirolimus. Twenty months following diagnosis of PTLD, she has ongoing problems with lung rejection and gastrointestional problems with intermittent low-level persistence of clonal T-cells in the marrow and liver, but no mass disease.
Case 2
At age 4 years, a previously healthy girl had a cadaveric liver transplant for end stage liver disease due to idiopathic Budd–Chiari syndrome. She received tacrolimus for post-transplant immunosuppression, without significant episodes of rejection. Five years post-transplant, she developed abdominal pain, and hepatosplenomegaly; imaging studies revealed multiple abdominal masses, including one of 11 × 8 × 4 cm. Retroperitoneal lymph node and liver biopsies showed T-PTLD with low-level presence of the same T-cell clone in the marrow and CSF, and clinical and radiological evidence of interstitial lung involvement. Her pre-transplant EBV status was unknown. ALL induction therapy, as given in case #1 [10], was complicated by pancreatitis, and posterior reversible encephalopathy syndrome. Following completion of induction chemotherapy she had complete resolution of abdominal nodal disease, but the abnormal T-cell clone persisted at a low-level in the marrow and CSF in conjunction with interstitial lung disease. Following 4 weeks of augmented BFM consolidation therapy (without asparaginase) [11], she had persistent disease in the BM, CSF, and biopsy-proven lung involvement. She then received two 4-day courses of fludarabine (30 mg/m2/day) and high dose Ara C (2 g/m2/day), and attained a complete remission. She continues to receive ALL maintenance therapy and remains in complete remission 10 months following diagnosis of T-PTLD.
Diagnosis of T-PTLD
The histological appearance of the abdominal masses from both patients is very similar, with diffuse lymphoid proliferation effacing normal nodal architecture (Fig. 1). The proliferating cells were predominantly small to medium size lymphocytes with slightly irregular nuclei, inconspicuous nucleoli and abundant pale stained cytoplasm. Prominent vascular proliferation was present in both cases (Fig. 1 insets). These proliferating cells are almost exclusively CD4 (+) T-cells with dim CD3 and negative for CD8 and the pan-B-cell marker CD79a. The liver biopsy in Case 2 demonstrated a similar T-cell proliferation (not shown). Both tumors were EBV-negative based on in-situ hybridization with EBV-encoded RNA (not shown).
Fig. 1.
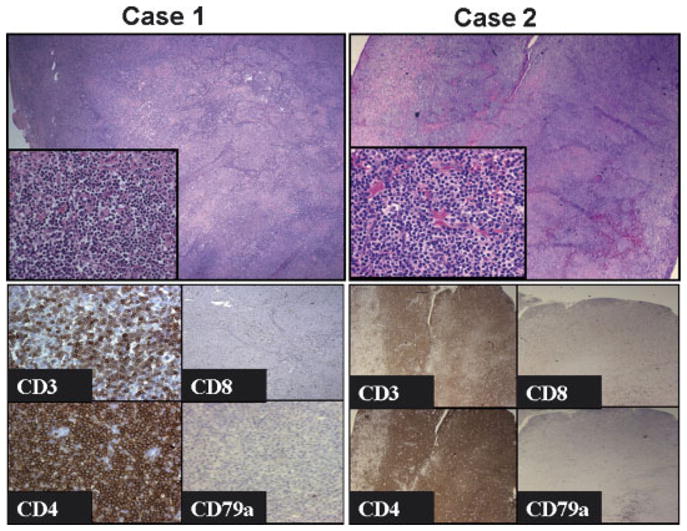
Abdominal masses from Case 1 (left panel) and Case 2 (right panel) show similar morphological features with diffuse lymphoid proliferation effacing normal nodal architecture (top panels 100×; insets 400×). Immunohistochemical stains (bottom panels) show that both tumors are composed of CD3+ CD4+ T-cells that lack expression of CD8 and CD79a (case 1 400×; case 2 200×).
Phenotypically abnormal T cells in Case 1 and Case 2 exhibited similar aberrant antigen expression pattern via FCM, namely down-regulated CD3, partial loss of CD7, up-regulated CD2 and aberrant expression of HLA-DR (data not shown). Clonal T-cell proliferation was suggested by FCM analysis of TCR Vβ usage either directly (Case 1), or indirectly (Case 2). Clonality was confirmed by amplification of distinct products via TCR-γ chain gene PCR in both cases (not shown).
DISCUSSION
Pediatric T-cell PTLD is very rare, with only 17 other cases previously described in the literature. We report two cases of T-PTLD with large abdominal nodal masses arising in children following SOT. The T-cell lineage was established based on immunohistochemistry and FCM analysis with T-cell monoclonality confirmed by FCM analysis of Vβ family usage and by PCR analysis of TCR-γ chain gene rearrangement. In contrast to most cases of B-lineage PTLD, these two T-PTLD cases were EBV-negative. This is consistent with data from the literature, with 42 of 69 (60%) T-PTLD cases being EBV-negative based on Southern blot hybridization with a fragment of the EBV genome or in-situ hybridization or EBV RNA. Table I shows characteristics of the 14 cases of pediatric T-PTLD that arose following SOT, including the cases described herein.
TABLE I.
Pediatric T-Cell PTLD After Solid Organ Transplant
| Transplant
|
Diagnosis of PTLD
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age and sex | Organ | Immu. supp | After Tx | After PTLD | Disease type | Location | EBV status | Clonality assay | Refs. | |
| 1 | 4 M/f | Lung | Tac, Pred | 3 Y | 15 M-A | PTL (CD4+) | GI, blood, lung | Neg | FCM+PCR | Yang |
| 2 | 4 Y/f | Liver | Tac | 5 Y | 5 M-A | PTL (CD4+) | GI, liver, BM, CSF | Neg | FCM+PCR | Yang |
| 3 | 5 Y/f | Multi-organ | Tac, cellcept Pred | 2 Y | 5 M-A | PTL (CD3+) | Graft-GI | Neg | SB | [14] |
| 4 | 1 Y/m | Liver | CsA, Pred | 3 M | 1 M | Polymorphous | Brain | Neg | MNR | [15] |
| 5 | 4 Y/m | Liver | Tac | 6 Y | 12 M-A | PTL (CD8+) | GI, UR, spleen, brain, pleura | Pos. | PCR | [16] |
| 6 | 9 D/f | Heart | CsA, Aza | 5 Y | 2 M | HSGD | BM, liver | Neg | SB+PCR | [17] |
| 7 | 15 Y/m | Heart | CsA, Aza, Pred, ATG | 4 M | 3 M | PTL (CD8+) AILD-like? | Blood | Pos | SB | [18] |
| 8 | 15 Y/f | Liver | Tac, Pred | 4 Y | 17 M-A | PTL (CD4+) | GI | Pos | PCR | [19] |
| 9 | 1 M/m | Heart | NR | 14 Y | NR | ALCL | Neck LNs | Pos | PCR | [20] |
| 10 | 12 Y/m | Kidney | Aza, Pred | 10 Y | Living | LBL | NR | Neg | SB | [21] |
| 11 | 14 Y/m | Kidney | Aza, Pred | 11 Y | 5 M | Diffuse large cell | Renograft, CSF | Neg | SB | [21] |
| 12 | 5 Y/m | Heart | CsA, Aza, Pred | 6 Y | Days | PTL (CD43) | Spleen | Pos | SB | [5] |
| 13 | 4 Y/f | Kidenys | CsA, siroli | 2 Y (4Y) | 1 Y (3Y) | B-PTLD (node) T-PTLD (liver, BM) | Liver, BM, LNs | Pos | PCR | [22] |
| 14 | 14 Y/f | Kidney | CsA | 28 Y | 1 4D | Agg. LGL-like CD8+ | BM | Neg | SB | [4] |
m, male; f, female; Y, years; M, months; D, days; LNs, lymph nodes; Tx, transplant; CsA, cyclosporine A; Aza, azathioprine; Pred, prednisone; Tac, tacrolimus; Cellcept, mycophenolate mofetil; MTX, methotrexate; Siroli, sirolimus; -A, alive; NR, not reported; HSGD, hepatosplenic gamma/delta lymphoma; LBL, lymphoblastic lymphoma; FCM, flow cytometry; SB, Southern blot; PCR, polymerase chain reaction; MNR, method not reported.
While there is a general impression that T-PTLD is very difficult to cure, several recently reported cases, including those described in this report, demonstrate that these tumors can be very treatment responsive. This may be due to use of different chemotherapy regimens than those typically used to treat B-PTLD, such as the intensive ALL-type treatments we employed, and/or the use of different strategies for immunosuppression. Most T-PTLDs are not EBV-driven; thus, reduction of immunosuppression may not be effective as a sole treatment strategy, and may be less critical for management of T-PTLD than it is in EBV-driven B-PTLDs. However, the follow-up on several cases, including those we describe, is short and it will be important to gather information on a larger number of cases with longer follow-up to make definitive conclusions regarding the outcome of T-PTLD.
It is important to note that both of our pediatric T-PTLD cases exhibited a bimodal response to therapy, with initial eradication of the bulk nodal disease with regimens typically used to treat ALL, but persistence of low level clonal T-cells in marrow, CSF and lung (1 case). Case #1 showed prolonged disease control with modification of immunosuppression, including introduction of sirolimus, which may have anti-tumor activity [12]. While we are cautious to make conclusions based on small patient numbers, different strategies of treatment may be needed in patients with T-PTLD than those that are often successful in treatment of B-lineage-PTLD. Multi-institutional trials are needed to define the optimal diagnostic evaluation and management strategy for T-PTLD.
Acknowledgments
We thank Professor Yoshihiko Hoshida of Department of Pathology, Osaka University Medical School, Suita, Osaka, Japan for providing additional clinical information for their 12 case series cited in this article.
References
- 1.Hanto DW, Sakamoto K, Purtilo DT, et al. The Epstein-Barr virus in the pathogenesis of posttransplant lymphoproliferative disorders. Clinical, pathologic, and virologic correlation. Surgery. 1981;90:204–213. [PubMed] [Google Scholar]
- 2.Draoua HY, Tsao L, Mancini DM, et al. T-cell post-transplantation lymphoproliferative disorders after cardiac transplantation: A single institutional experience. Br J Haematol. 2004;127:429–432. doi: 10.1111/j.1365-2141.2004.05212.x. [DOI] [PubMed] [Google Scholar]
- 3.Rajakariar R, Bhattacharyya M, Norton A, et al. Post transplant T-cell lymphoma: A case series of four patients from a single unit and review of the literature. Am J Transplant. 2004;4:1534–1538. doi: 10.1111/j.1600-6143.2004.00521.x. [DOI] [PubMed] [Google Scholar]
- 4.Hanson MN, Morrison VA, Peterson BA, et al. Posttransplant T-cell lymphoproliferative disorders—an aggressive, late complication of solid-organ transplantation. Blood. 1996;88:3626–3633. [PubMed] [Google Scholar]
- 5.Waller EK, Ziemianska M, Bangs CD, et al. Characterization of posttransplant lymphomas that express T-cell-associated markers: Immunophenotypes, molecular genetics, cytogenetics, and hetero-transplantation in severe combined immunodeficient mice. Blood. 1993;82:247–261. [PubMed] [Google Scholar]
- 6.Dror Y, Greenberg M, Taylor G, et al. Lymphoproliferative disorders after organ transplantation in children. Transplantation. 1999;67:990–998. doi: 10.1097/00007890-199904150-00010. [DOI] [PubMed] [Google Scholar]
- 7.Hoshida Y, Li T, Dong Z, et al. Lymphoproliferative disorders in renal transplant patients in Japan. Int J Cancer. 2001;91:869–875. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1125>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 8.Yuan CM, Douglas-Nikitin VK, Ahrens KP, et al. DRAQ5-based DNA content analysis of hematolymphoid cell subpopulations discriminated by surface antigens and light scatter properties. Cytometry B Clin Cytom. 2004;58:47–52. doi: 10.1002/cyto.b.20000. [DOI] [PubMed] [Google Scholar]
- 9.Balfour IC, Wall D, Luisiri A, et al. Cyclosphosphamide/prednisone for combination immunosuppression and therapy of lymphoproliferative disease. J Heart Lung Transplant. 1999;18:492–495. doi: 10.1016/s1053-2498(98)00072-2. [DOI] [PubMed] [Google Scholar]
- 10.Panosyan EH, Seibel NL, Martin-Aragon S, et al. Asparaginase antibody and asparaginase activity in children with higher-risk acute lymphoblastic leukemia: Children’s Cancer Group Study CCG-1961. J Pediatr Hematol Oncol. 2004;26:217–226. doi: 10.1097/00043426-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Nachman J, Sather HN, Gaynon PS, et al. Augmented Berlin-Frankfurt-Munster therapy abrogates the adverse prognostic significance of slow early response to induction chemotherapy for children and adolescents with acute lymphoblastic leukemia and unfavorable presenting features: A report from the Children’s Cancer Group. J Clin Oncol. 1997;15:2222–2230. doi: 10.1200/JCO.1997.15.6.2222. [DOI] [PubMed] [Google Scholar]
- 12.Janus A, Robak T, Smolewski P. The mammalian target of the rapamycin (mTOR) kinase pathway: Its role in tumourigenesis and targeted antitumour therapy. Cell Mol Biol Lett. 2005;10:479–498. [PubMed] [Google Scholar]
- 13.Madeiros LJ, Carr J. Overview of the role of molecular methods in the diagnosis of malignant lymphomas. Arch Pathol Lab Med. 1999;123:1189–1207. doi: 10.5858/1999-123-1189-OOTROM. [DOI] [PubMed] [Google Scholar]
- 14.Berho M, Viciana A, Weppler D, et al. T cell lymphoma involving the graft of a multivisceral organ recipient. Transplantation. 1999;68:1135–1139. doi: 10.1097/00007890-199910270-00013. [DOI] [PubMed] [Google Scholar]
- 15.Collins MH, Montone KT, Leahey AM, et al. Post-transplant lymphoproliferative disease in children. Pediatr Transplant. 2001;5:250–257. doi: 10.1034/j.1399-3046.2001.005004250.x. [DOI] [PubMed] [Google Scholar]
- 16.George TI, Jeng M, Berquist W, et al. Epstein-Barr virus-associated peripheral T-cell lymphoma and hemophagocytic syndrome arising after liver transplantation: Case report and review of the literature. Pediatr Blood Cancer. 2005;44:270–276. doi: 10.1002/pbc.20231. [DOI] [PubMed] [Google Scholar]
- 17.Kraus MD, Crawford DF, Kaleem Z, et al. T gamma/delta hepatosplenic lymphoma in a heart transplant patient after an Epstein-Barr virus positive lymphoproliferative disorder: A case report. Cancer. 1998;82:983–992. doi: 10.1002/(sici)1097-0142(19980301)82:5<983::aid-cncr26>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 18.Leblond V, Sutton L, Dorent R, et al. Lymphoproliferative disorders after organ transplantation: A report of 24 cases observed in a single center. J Clin Oncol. 1995;13:961–968. doi: 10.1200/JCO.1995.13.4.961. [DOI] [PubMed] [Google Scholar]
- 19.Lundell R, Elenitoba-Johnson KS, Lim MS. T-cell posttransplant lymphoproliferative disorder occurring in a pediatric solid-organ transplant patient. Am J Surg Pathol. 2004;28:967–973. doi: 10.1097/00000478-200407000-00019. [DOI] [PubMed] [Google Scholar]
- 20.Pitman SD, Rowsell EH, Cao JD, et al. Anaplastic large cell lymphoma associated with Epstein-Barr virus following cardiac transplant. Am J Surg Pathol. 2004;28:410–415. doi: 10.1097/00000478-200403000-00018. [DOI] [PubMed] [Google Scholar]
- 21.van Gorp J, Doornewaard H, Verdonck LF, et al. Posttransplant T-cell lymphoma. Report of three cases and a review of the literature. Cancer. 1994;73:3064–3072. doi: 10.1002/1097-0142(19940615)73:12<3064::aid-cncr2820731227>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 22.Yin CC, Medeiros LJ, Abruzzo LV, et al. EBV-associated B- and T-cell posttransplant lymphoproliferative disorders following primary EBV infection in a kidney transplant recipient. Am J Clin Pathol. 2005;123:222–228. [PubMed] [Google Scholar]


