Summary
Assessment of bone marrow involvement by malignant plasma cells is an important element in the diagnosis and follow-up of patients with multiple myeloma and other plasma cell dyscrasias. Microscope-based differential counts of bone marrow aspirates are used as the primary method to evaluate bone marrow plasma cell percentages. However, multiple myeloma is often a focal process, a fact that impacts the accuracy and reliability of the results of bone marrow plasma cell percentages obtained by differential counts of bone marrow aspirate smears. Moreover, the interobserver and intraobserver reproducibility of counting bone marrow plasma cells microscopically has not been adequately tested. CD138 allows excellent assessment of plasma cell numbers and distribution in bone marrow biopsies. We compared estimates of plasma cell percentages in bone marrow aspirates and in hematoxylin-eosin– and CD138-stained bone marrow biopsy sections (CD138 sections) in 79 bone marrows from patients with multiple myeloma. There was a notable discrepancy in bone marrow plasma cell percentages using the different methods of observation. In particular, there was a relatively poor concordance of plasma cell percentage estimation between aspirate smears and CD138 sections. Estimates of plasma cell percentage using CD138 sections demonstrated the highest interobserver concordance. This observation was supported by computer-assisted image analysis. In addition, CD138 expression highlighted patterns of plasma cell infiltration indicative of neoplasia even in the absence of plasmacytosis. We conclude that examination of CD138 sections should be considered for routine use in the estimation of plasma cell load in the bone marrow.
Keywords: CD138, Multiple myeloma, Bone marrow, Smears, Plasma cell count
1. Introduction
Microscopic examination of the bone marrow plays a pivotal role in establishing the diagnosis of multiple myeloma and other plasma cell dyscrasias, and monitoring therapy. Of importance, it provides information on the level of bone marrow involvement by plasma cells in these disorders. The percentage of plasma cells in the bone marrow, based on the examination of aspirate smears, is one of the criteria used for the diagnosis of multiple myeloma [1,2]. Plasma cell quantitation is also used in evaluating morphologic remission [3] and minimal residual disease in patients with this disease [4]. In a recent study, a high percentage of plasma cells in the bone marrow has been shown to be a good predictor of relapse in cases of treated multiple myeloma [5].
Customarily, the quantitation of bone marrow plasma cell percentages is based on differential counts in Wright-Giemsa–stained bone marrow aspirate smears. This procedure was adopted long ago by clinical hematologists and is still prevalent today. It allows detailed morphologic characterization of individual cells and is considered by some authors to be superior to examination of hematoxylin-eosin–stained bone marrow histologic sections (HE sections) [6]. However, the assessment of aspirate smears alone is often insufficient to accurately convey the overall marrow status in plasma cell neoplasms and does not provide information on tumor distribution within the marrow. Of importance, we only found a single study in the literature addressing the precision of the aspirate smears differential counts [7]. In this article, the authors indicated that from a cytologic viewpoint, the most predictive characteristic of myeloma is plasma cell count, but found that the interobserver variability was high. Conversely, examination of bone marrow sections, which has been traditionally performed by pathologists, is an excellent means to assess plasma cell distribution. Several studies have shown significant discordance in the numbers of plasma cells between bone marrow sections and aspirate smears [6,8–12]. The latter tends to either underestimate or overestimate plasma cell counts [11,12], highlighting the importance of examining bone marrow core biopsies (Bx) in evaluating tumor load in multiple myeloma [6,8–11,13].
The advent of antibody staining has enabled the identification of plasma cells in bone marrow sections with greater specificity than HE stains, an advance that has led to more frequent use of bone marrow sections in the diagnosis and follow-up of multiple myeloma [1,6,11,14,15]. Bone marrow involvement by malignant plasma cells is often focal, more akin to that seen with lymphomas or metastatic tumors than leukemias. The plasma cells may also be variably distributed among bone marrow spicules on the same smear. Moreover, hemodiluted samples can falsely alter the bone marrow plasma cell percentages. This fact casts doubts on the accuracy and reliability of bone marrow differential counts as a primary method of determining bone marrow plasma cell percentages.
The use of CD138 (syndecan-1) as an immunohistochemical marker [16–18] was a major breakthrough in the detection of plasma cell infiltrates in formalin-fixed, paraffin-embedded material. Syndecan-1 is a transmembrane heparan sulfate–rich proteoglycan that consists of 5 glycoaminoglycans and 1 core protein [19–21]. It regulates the activity of heparan-binding growth factors, induces apoptosis, and inhibits growth of myeloma [19]. Synde-can-1 also mediates osteoclast and osteoblast differentiation and myeloma cell adhesion [20–22]. Loss of syndecan-1 from the plasma cell surface may play a critical role in the proliferation and extramedullary dissemination of myeloma or recurrence after therapy [19,23,24]. CD138 expression is observed in terminally differentiated plasma cells, but is lacking on actively proliferative plasmablasts and all earlier stages of B-cell differentiation [25]. Normal epithelial cells [16] and nonhematopoietic tumors, including various carcinomas, melanomas, and mesenchymal tumors, may also express this antigen [26,27]. In addition, nonspecific immunoreactivity has been described in bone marrow extracellular milieu, where shed CD138 tends to accumulate in fibrotic stroma [24]. Nevertheless, in the context of hematopoietic cell identification, CD138 is a highly specific and sensitive [28] marker of normal and neoplastic plasma cells [16–18] and is expressed by lymphoid tumors with plasmacytic differentiation [17,26].
The aim of our study was to compare different microscopy-based methods (Wright-Giemsa–stained aspirate smears, HE sections, and CD138-stained sections [CD138 sections]) used in the quantitation of bone marrow plasma cells, because the bone marrow plasma cell percentage is a parameter used by our clinical colleagues to guide therapeutic decisions, and to determine if the addition of CD138 immunohistochemistry (IHC) offers an advantage in the evaluation of bone marrow plasma cell infiltrates. Many institutions still determine bone marrow plasma cell percentages based upon aspirate smear differential counts. The persistence of this practice attests to the significance of systematically addressing and reporting this issue. It is important to emphasize that in this study, we have not attempted to evaluate the diagnosis of myeloma, which would include the assessment of plasma cell clonality and other laboratory parameters as well as clinical data, but only the methodology by which bone marrow plasma cell quantitation is determined.
2. Materials and methods
2.1. Case selection
Bone marrow samples were retrieved from the files of the University of Florida–Shands Hospital from patients with multiple myeloma, at diagnosis or following therapy, who had simultaneous bone marrow aspirates and Bx performed between July 2001 and March 2004. After the exclusion of cases with inadequate Bx or hemodiluted aspirate smears, 79 diagnostic specimens were selected for this study. All patient identifiers were removed before the study and were randomly assigned an internal code to comply with the guidelines of our institutional review board.
2.2. Immunohistochemistry
Bone marrow Bx were formalin-fixed in IBF (Surgipath, Richmond, IL), decalcified in Rapid-Cal-Immuno (BBC, Stanwood, WA) for 1 hour, washed, and then paraffin embedded. For CD138 staining, 3- to 4-μm tissue sections were mounted on plus slides, dried for 2 hours in a 60°C oven, and then stained using a monoclonal antibody (Serotec, Oxford, UK; 1:40 dilution) according to established protocols in a BenchMark automated immunostainer (Ventana Medical Systems, Tucson, AZ). Heat-induced epitope retrieval was performed with the EDTA-buffered cell conditioning (CC1) retrieval solution. Immunostaining was completed with the iView diaminobenzidine tetrahydrochloride (DAB) detection kit (Ventana), which uses a streptavidin-biotin technique and hematoxylin as a counter-stain. Appropriate positive and negative controls in each run were acceptable.
2.3. Microscopic evaluation
For each case, all available materials were examined. Bone marrow plasma cell percentages were calculated from a 500-cell differential count on conventional Wright-Giemsa–stained bone marrow aspirate smears, which included representative plasma cell–rich areas and were not limited to one spicule, as determined by a complete scan of the entire smear(s). In bone marrow biopsy sections, we did not count individual plasma cells; we estimated the percentage of cellular marrow occupied by plasma cells to the nearest 5% based on the examination of conventional HE sections and CD138 sections. Other parameters assessed on CD138 sections included the dominant pattern of plasma cell distribution in the bone marrow and the characteristics of individual cellular staining such as membrane, Golgi, or nuclear staining. All cases were independently reviewed by 2 hematopathologists without prior knowledge of the clinical data.
2.4. Computer-assisted image analysis
To validate the bone marrow plasma cell percentages obtained by the visual estimation of plasma cells in CD138 sections, plasma cell levels in the bone marrow Bx were evaluated using a computer-assisted quantitative IHC on 50 randomly selected cases using the automated cellular imaging system (ACIS) from Chromavision, Inc (currently Clarient, Inc, Aliso Viejo, CA); and the results were compared with the visually based estimations of plasma cells. Briefly, all slides stained with anti-CD138 were scanned with the ACIS digital microscope at Chromavision using an analogue camera digitized for image capture mounted on an automated microscope with multiple objectives for scanning. The images were then reviewed and analyzed by one of the authors (S. Z. A.) with the ACIS image analysis software, which provided histologic reconstruction of the scanned IHC sections for each case. During image analysis, the system calculated staining intensity for each area, discriminating among subtle increments of color, and converted the color into a score. In our study, the system was programmed to differentiate between 3 colors: (1) “brown” (DAB), representing the staining of plasma cells with anti-CD138; (2) “white,” representing clear areas of fat cells and any empty space in the background; and (3) “blue,” representing CD138-negative background elements such as hematopoietic elements, bone marrow stroma, and bone trabeculae. Only regions with cellular marrow and fat within the marrow space were selected for evaluation and scoring. This was achieved by drawing regions around these areas. Bone trabeculae, noncellular (empty) spaces, and areas with high nonspecific background staining were excluded from the analysis as feasible. In each region selected, a score in decimal increments was obtained for each color. Plasma cells were assessed based on their brown color and were reported as the percentage of area they occupied (plasma cell area percentage) based on the color area ratios “brown/(brown + blue).” This calculation is only approximate because the IHC reactivity does not usually encompass all cell components. Since the images were scanned at low resolution, comparisons between montage images on the screen and microscopic view of the CD138 sections were necessary in cases when large amounts of red blood cells contaminated the biopsy specimen and stained with the blue counterstain, or when there were increased amounts of hemosiderin pigment in the bone marrow section.
2.5. Statistical analysis
An intraclass correlation (ICC) analysis [29] was performed to test the interobserver and intraobserver concordance of the bone marrow plasma cell percentages, for all the 79 cases, obtained with the various methods of evaluation (Wright-Giemsa–stained aspirate smears, HE sections, and CD138 sections). Using this method, an ICC value of 1 denotes a perfect interobserver concordance. ICC was also used to test the concordance between the plasma cell area percentage obtained by computer-assisted analysis (ACIS) of the CD138 sections and the bone marrow plasma cell percentages obtained by visual estimation by each of the 2 observers for the 50 randomly selected cases. Scatter plots were prepared with the Prism 4 software (GraphPad Software, Inc, San Diego, CA).
3. Results
3.1. Interobserver concordance
The bone marrow aspirate smears and the HE and CD138 sections from the 79 cases were reviewed microscopically by 2 pathologists independently to determine the reproducibility of morphologic assessment of bone marrow plasma cell percentages. As shown in Table 1, the interobserver concordance was comparable for the aspirate smears (ICC = 0.82) (Fig. 1A) and for the HE sections (ICC = 0.89) (Fig. 1B). However, the plasma cell percentages obtained with CD138 sections had the highest interobserver concordance with an ICC = 0.98 (Fig. 1C). The interobserver concordance based on CD138 was significantly higher than those based on aspirate smears (P = .0001) or HE sections (P = .0001).
Table 1.
Interobserver concordance for the different methods of plasma cell quantitation/estimation
| Aspirate smears | HE sections | CD138 sections | |
|---|---|---|---|
| ICC | 0.82a | 0.89a | 0.98b |
NOTE. Values with the same superscript letter are not significantly different.
Fig. 1.
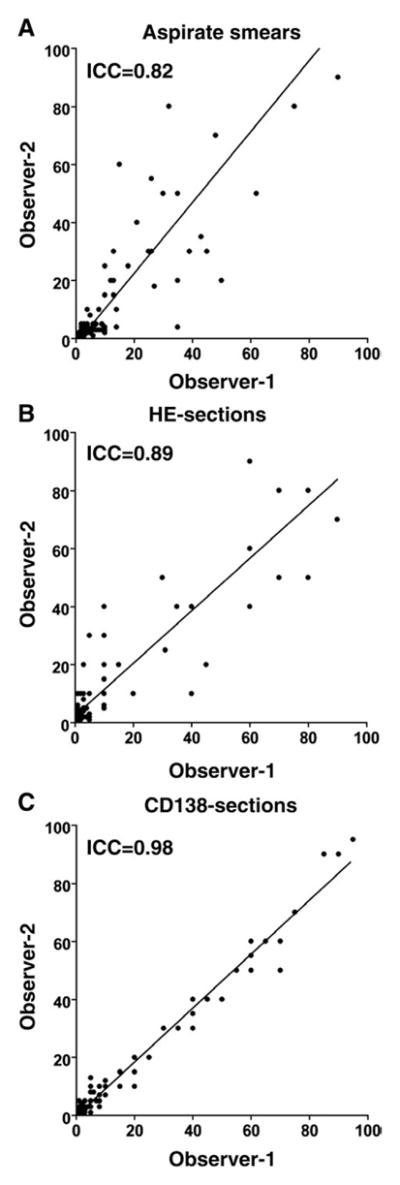
Scatter plots of interobserver results of bone marrow plasma cell percentage estimation using different methods of evaluation. Bone marrow plasma cell percentages obtained form aspirate smears (A) and HE sections (B) showed lower concordance than those from CD138 sections (C).
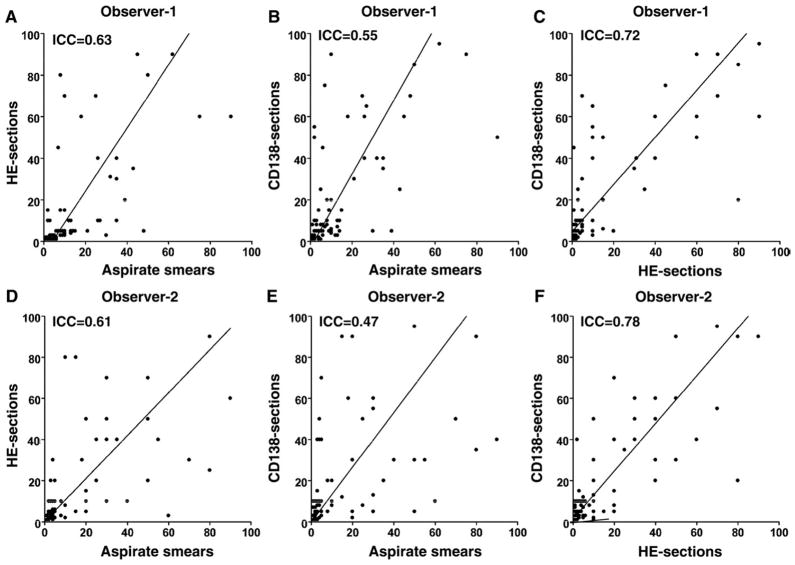
3.2. Intraobserver concordance
For both observers, as shown in Table 2 and Fig. 2, there was a poor intraobserver concordance in bone marrow plasma cell percentage results obtained by 3 different methods of evaluation, most notably between aspirate smears and CD138 sections (ICC = 0.55 for observer 1 and ICC = 0.47 for observer 2).
Table 2.
Intraobserver concordance between different methods of evaluation
| Aspirate smears vs HE sections | Aspirate smears vs CD138 sections | HE sections vs CD138 sections | |
|---|---|---|---|
| ICC (observer 1) | 0.63 | 0.55 | 0.72 |
| ICC (observer 2) | 0.61 | 0.47 | 0.78 |
Fig. 2.
Scatter plots of intraobserver results of bone marrow plasma cell percentage estimation using different methods of evaluation. For both observers (1 and 2), there was a poor concordance in bone marrow plasma cell percentages, most notably between aspirate smears and CD138 sections (B and E). Bone marrow plasma cell percentages tended to be higher in CD138 sections than in aspirate smear or HE sections (B, C, E and F).
In most cases, as shown in Fig. 2, individual observer’s assessment of plasma cell percentage using different evaluation methods resulted in higher plasma cell percentage with CD138 sections than with aspirate smears or HE preparations (Fig. 2B, C, E, and F). However, in some cases, higher values of plasma cell percentage were recorded using aspirate smears or HE preparations. Overall, individual observers using different methods of evaluation recorded a high (>25%), and in some cases extreme, difference in plasma cell percentage in 30 of 79 cases, with the highest overall plasma cell percentage obtained by CD138 sections in 16 cases, by bone marrow aspirate smears in 8 cases, and by HE sections in 6 cases.
3.3. Bone marrow plasma cell quantitation by ACIS
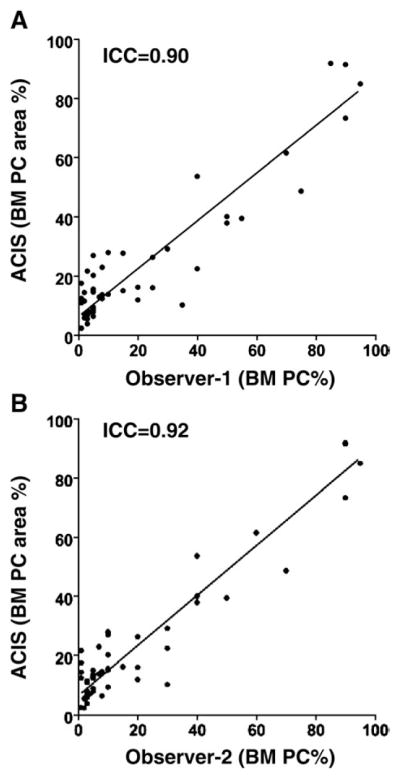
All montage images from 50 specimens were analyzed by a single pathologist using the ACIS software to provide an objective assessment of bone marrow plasma cell percentages. The number of regions selected for scoring ranged from 6 to 47 per case, and the analysis was accomplished in 15 to 30 minutes per case. The concordance between the plasma cell area percentage data derived from the semiautomated image analysis and the results of visual estimation of CD138 expression was higher, with an ICC = 0.90 for observer 1 and ICC = 0.92 for observer 2 (Fig. 3), than the concordance between CD138 sections and either the aspirate smears or the HE sections (Fig. 2). As shown in Fig. 3, with low-level involvement by plasma cells, the plasma cell area percentage obtained by the ACIS was higher than that recorded by visual estimation. Conversely, with very high levels of plasma cell involvement, the scores attained by ACIS tended to be lower than those obtained by visual estimation.
Fig. 3.
Scatter plots of results obtained with the ACIS-assisted analysis and visual estimation of CD138 sections (n = 50) for 2 observers (1 and 2).
3.4. Patterns of plasma cell distribution and cellular staining
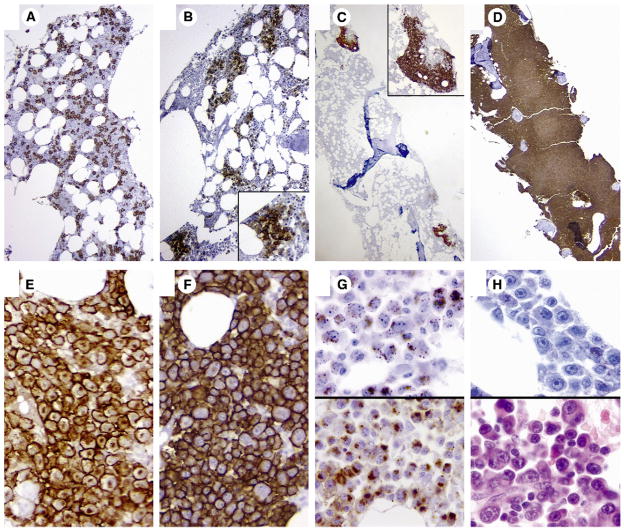
Based on the CD138 staining in the 79 cases with multiple myeloma included in the study, we identified 4 distinct morphologic patterns of plasma cell distribution, similar to what was reported in previous studies using κ and λ light chain IHC [1]. These included interstitial patterns, micro-aggregates, large nodules, and diffuse patterns (Fig. 4A–D). Based on the dominant pattern of the plasma cell distribution, more than half (52%) of the cases had an interstitial pattern, 23% had microaggregates, 17% had large nodules, and 8% had diffuse infiltrates of plasma cell distribution. In 25% of cases, more than one pattern was present in the same section. In 12 cases (15%), CD138 highlighted microaggregates or isolated plasma cell nodules where the overall bone marrow plasma cell percentage was not increased (<5%). In 6 cases, the diffuse pattern of plasma cell distribution was associated with high levels of bone marrow involvement, which showed the least interobserver discordance (Fig. 1C).
Fig. 4.
Architectural patterns of bone marrow infiltration by plasma cells highlighted by CD138 immunohistochemical stain: (A) interstitial (×200), (B) microaggregates (×100), (C) large nodules (×20), and (D) diffuse (×20). Cytologic patterns of CD138 staining (×1000): intense membranous staining with (E) or without (F) Golgi staining and granular pattern with and without Golgi staining (G). No CD138 expression in plasma cells that demonstrate plasmablastic morphology (H).
At the cellular level, the CD138-expressing plasma cells showed varied patterns of staining (Fig. 4E–H). The most common pattern was intense membranous staining with or without associated diffuse weak cytoplasmic and/or Golgi staining. Few cases exhibited cytoplasmic granular staining with or without associated Golgi staining. In 2 cases (2.5%), the plasma cells demonstrated classic “plasmablastic” morphology and were negative for CD138. These cells were recognized as plasma cells by their morphology and the assessment of κ and λ light chain restriction by IHC or in situ hybridization (data not shown). In these cases, the estimated bone marrow plasma cell percentage was based on the κ- or λ-stained section.
4. Discussion
Numerous prognostic factors have been reported in patients with multiple myeloma [5,30]. These include β2-microglobulin, mature plasma cell morphology, plasmablastic morphology, interleukin 6, cytogenetics [31], the fraction of immunophenotypically aberrant plasma cell [32], DNA content [33], the plasma cell labeling index, and the bone marrow plasma cell percentage [5]. The latter is purported to be the best predictor of relapse in cases of treated multiple myeloma [5].
Bone marrow plasma cell percentage has been traditionally assessed by aspirate smear differential counts, and pathologists often rely on HE sections for an estimation of plasma cell infiltrates. However, the precision and reproducibility of the counts obtained by bone marrow aspirate smears had not been adequately tested [7]. Although we did overall scan and performed differential counts in aspirate smears, in tissue sections, we chose to solely estimate bone marrow plasma cell percentage rather than counting individual plasma cells because of the inherent difficulty in performing differential cell counts in these preparations. This was particularly true in HE sections in which abnormal plasma cells can be difficult to differentiate from other hematopoietic cells. Even in CD138-stained preparations, performing an accurate cell count may not be practical. We have shown that when compared with standard Wright-Giemsa–stained smears or HE sections, examination of CD138 sections was the most precise method of bone marrow plasma cell quantitation. Moreover, CD138 often unmasked a higher plasma cell percentage than those estimated in aspirate smears or HE sections, as has been shown recently by other investigators [28], and can highlight a pattern of distribution indicative of neoplastic infiltration/recurrence in the absence of increased overall bone marrow plasma cell percentage [4]. This finding may be of particular importance in the context of posttherapeutic hypocellular marrows where the plasma cell infiltrates may be small and arranged in microaggregates [4], which may lead to sampling error.
The assessment of plasma cell percentage in aspirate smears and HE sections showed lower interobserver concordance than that obtained with anti-CD138 staining. Plasma cells are readily recognized in bone marrow aspirate smears. However, there may be variability in plasma cell distribution between different bone marrow aspirate smears and between different spicules in the same preparation because of the frequent focal nature of the disease. In fact, the rather common nodular and microaggregate patterns of involvement most dramatically illustrate the focal nature of plasma cell infiltrates, which could have contributed to the lower concordance of results between smears and CD138 sections. In addition, hemodilution can lead to sampling errors. HE-stained preparations may also be problematic in recognizing plasma cells. This is particularly true when there is interstitial infiltration by plasma cells, the most frequent pattern of involvement. In these preparations, it may be difficult to differentiate plasma cells from other hematopoietic cells unless they form large nodules or diffusely replace the marrow.
In several cases, the plasma cell percentage estimates based on HE sections were higher than those observed using CD138 sections. Most likely, the reason for this discrepancy was the lack of typical membranous and Golgi staining pattern of plasma cells with anti-CD138. The underestimation of plasma cells with the granular pattern of staining is a common pitfall with cursory examination of bone marrow sections, and this pattern requires evaluation at high power for maximum detection. By contrast, plasma cells with the intense membranous and/or Golgi staining are easily recognized. Rare cases that were CD138 negative were usually associated with a plasmablastic morphology, and the cells were recognized as plasma cells by immunoglobulin κ or λ light chain expression.
The enumeration of plasma cells on bone marrow sections is more challenging than that based on aspirate smears. However, the concordance of the bone marrow plasma cell percentage estimates using CD138 sections among 2 pathologists was excellent. Assuming that the IHC preparations are adequate, we expect these results to apply to a broader pathology community as well. Furthermore, the possible advantages of the aspirate smears are offset by the inability to assess plasma cell distribution within the marrow spaces. This is of particular importance in posttherapeutic cases in which the average plasma cell counts could be low but the distribution may indicate significant focal residual/recurrent disease.
The cases evaluated in this study reflect the spectrum of material received in our laboratory on a daily basis; and because many represented samples from treated patients, the number of plasma cells was frequently low (less than 10% in more than half of the cases). We did not consider that this low number of plasma cells is necessarily an impediment. On the contrary, quantitative errors may be clinically irrelevant when a marrow contains a large number of plasma cells. By contrast, following therapy, when residual marrow plasma cells frequently represent less than 10% of the marrow cellularity, critical therapeutic decisions may be based on the number of these cells as assessed on smears.
Although the ACIS-assisted quantitation of bone marrow plasma cell area percentage showed a good correlation with the visual estimation of plasma cell percentage in the CD138 sections, it was somewhat time consuming. However, with very high levels of plasma cell involvement, plasma cell area percentage scores obtained by ACIS tended to be lower than the visual estimates of bone marrow plasma cell percentage using anti-CD138. With the ACIS, the plasma cell quantitation was based on color ratios, which reflected the amount of brown (anti-CD138 stain) as the fraction of the cellular marrow. The system does not actually compute the percentage of labeled cells but rather assesses the relative area of positive stain. Because CD138 is often only expressed in the cell membrane, unstained cellular components such as plasma cell nuclei and cytoplasm would result in a relatively lower plasma cell area percentage score. Conversely, with low-level involvement by plasma cells, the plasma cell area percentage scores obtained by the ACIS were rather higher than those obtained by visual estimation. This suggests that the ACIS may be more sensitive in detecting plasma cell percentage than the visual method, typically when the latter is performed at a low microscopic magnification and in cases where plasma cells display a predominantly granular staining pattern. However, the discrepancies between the ACIS system and the visual examination may have also been due to certain artifacts. For example, any brown background staining (eg, iron pigment, CD138-stained stroma) may be misinterpreted as plasma cells and scored incorrectly, falsely elevating the plasma cell percentage. In addition, the ACIS images were obtained at low resolution, which in some cases of unclear structures required visual confirmation by microscopy. Newer technology that produces images at a higher resolution and improves the accuracy and scoring time could be more suited for this type of analysis and may impact favorably on inter-observer variability.
Flow cytometry (FCM) is another technique used in plasma cell evaluation that has been shown to be a useful tool in the characterization of malignant plasma cells and the diagnosis of multiple myeloma [32,34]. The advantage of FCM is that it may help in the detection of small immunophenotypically abnormal/clonal populations of plasma cells, which in conjunction with the other methods provides a comprehensive evaluation of the burden of plasma cells. However, FCM may not be an accurate method for overall plasma cell quantitation. It is greatly dependent on the quality of the bone marrow aspirate specimen, and it generally tends to underestimate bone marrow plasma cell percentages [35]. The focal nature of multiple myeloma, hemodilution, and/or selective plasma cell loss during cell processing and analysis is typically what leads to plasma cell underestimation by FCM as compared with morphologic review.
The combined use of bone marrow aspirate smears and HE sections and CD138 IHC is beneficial in obtaining the most clinically relevant bone marrow plasma cell percentage. Our results indicate that bone marrow Bx sections stained with anti-CD138 antibody provide an excellent assessment of plasma cell load and infiltration and should be used in the management of patients with plasma cell dyscrasias, particularly when there is a low level of bone marrow involvement. Very little data exist on the clinical utility of plasma cell quantitation by CD138 IHC [4,17,18,24]. Further studies correlating the bone marrow plasma cell percentages obtained by CD138 IHC with clinical and laboratory parameters may be warranted.
Acknowledgments
We would like to thank Dr Karen Yamamoto from Clarient, Inc, for her help with scanning the slides and useful discussions, and Ms Elaine Dooley for her technical support.
Footnotes
Presented in part at the 93rd Annual Meeting of the United States and Canadian Academy of Pathology, Vancouver, British Columbia, March 2004.
References
- 1.Sukpanichnant S, Cousar JB, Leelasiri A, et al. Diagnostic criteria and histologic grading in multiple myeloma: histologic and immunohistologic analysis of 176 cases with clinical correlation. Hum Pathol. 1994;25:308–18. doi: 10.1016/0046-8177(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 2.Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121:749–57. [PubMed] [Google Scholar]
- 3.Smith A, Wisloff F, Samson D. Guidelines on the diagnosis and management of multiple myeloma, 2005. Br J Haematol. 2006;132:410–51. doi: 10.1111/j.1365-2141.2005.05867.x. [DOI] [PubMed] [Google Scholar]
- 4.Wei A, Westerman D, Feleppa F, et al. Bone marrow plasma cell microaggregates detected by immunohistology predict earlier relapse in patients with minimal disease after high-dose therapy for myeloma. Haematologica. 2005;90:1147–9. [PubMed] [Google Scholar]
- 5.Rajkumar SV, Fonseca R, Dispenzieri A, et al. Methods for estimation of bone marrow plasma cell involvement in myeloma: predictive value for response and survival in patients undergoing autologous stem cell transplantation. Am J Hematol. 2001;68:269–75. doi: 10.1002/ajh.10003. [DOI] [PubMed] [Google Scholar]
- 6.Buss DH, Prichard RW, Hartz JW, et al. Comparison of the usefulness of bone marrow sections and smears in diagnosis of multiple myeloma. Hematol Pathol. 1987;1:35–43. [PubMed] [Google Scholar]
- 7.Milla F, Oriol A, Aguilar J, et al. Usefulness and reproducibility of cytomorphologic evaluations to differentiate myeloma from monoclonal gammopathies of unknown significance. Am J Clin Pathol. 2001;115:127–35. doi: 10.1309/34D8-V2KU-23UL-VFBW. [DOI] [PubMed] [Google Scholar]
- 8.Bartl R, Frisch B, Burkhardt R, et al. Bone marrow histology in myeloma: its importance in diagnosis, prognosis, classification and staging. Br J Haematol. 1982;51:361–75. doi: 10.1111/j.1365-2141.1982.tb02791.x. [DOI] [PubMed] [Google Scholar]
- 9.Terpstra WE, Lokhorst HM, Blomjous F, et al. Comparison of plasma cell infiltration in bone marrow biopsies and aspirates in patients with multiple myeloma. Br J Haematol. 1992;82:46–9. doi: 10.1111/j.1365-2141.1992.tb04592.x. [DOI] [PubMed] [Google Scholar]
- 10.Bartl R, Frisch B, Fateh-Moghadam A, et al. Histologic classification and staging of multiple myeloma. A retrospective and prospective study of 674 cases. Am J Clin Pathol. 1987;87:342–55. doi: 10.1093/ajcp/87.3.342. [DOI] [PubMed] [Google Scholar]
- 11.Pileri S, Poggi S, Baglioni P, et al. Histology and immunohistology of bone marrow biopsy in multiple myeloma. Eur J Haematol Suppl. 1989;51:52–9. doi: 10.1111/j.1600-0609.1989.tb01493.x. [DOI] [PubMed] [Google Scholar]
- 12.Markey GM, Kettle P, Morris TC, et al. Quantitation of monoclonal plasma cells in bone marrow biopsies in plasma cell dyscrasia. Anal Cell Pathol. 2003;25:167–71. doi: 10.1155/2003/821978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sailer M, Vykoupil KF, Peest D, et al. Prognostic relevance of a histologic classification system applied in bone marrow biopsies from patients with multiple myeloma: a histopathological evaluation of biopsies from 153 untreated patients. Eur J Haematol. 1995;54:137–46. doi: 10.1111/j.1600-0609.1995.tb00204.x. [DOI] [PubMed] [Google Scholar]
- 14.Thiele J, Arenz B, Klein H, et al. Differentiation of plasma cell infiltrates in the bone marrow. A clinicopathological study on 80 patients including immunohistochemistry and morphometry. Virchows Arch A Pathol Anat Histopathol. 1988;412:553–62. doi: 10.1007/BF00844291. [DOI] [PubMed] [Google Scholar]
- 15.Thiry A, Delvenne P, Fontaine MA, et al. Comparison of bone marrow sections, smears and immunohistological staining for immunoglobulin light chains in the diagnosis of benign and malignant plasma cell proliferations. Histopathology. 1993;22:423–8. doi: 10.1111/j.1365-2559.1993.tb00155.x. [DOI] [PubMed] [Google Scholar]
- 16.Turley H, Jones M, Erber W, et al. VS38: a new monoclonal antibody for detecting plasma cell differentiation in routine sections. J Clin Pathol. 1994;47:418–22. doi: 10.1136/jcp.47.5.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costes V, Magen V, Legouffe E, et al. The Mi15 monoclonal antibody (anti–syndecan-1) is a reliable marker for quantifying plasma cells in paraffin-embedded bone marrow biopsy specimens. Hum Pathol. 1999;30:1405–11. doi: 10.1016/s0046-8177(99)90160-0. [DOI] [PubMed] [Google Scholar]
- 18.Chilosi M, Adami F, Lestani M, et al. CD138/syndecan-1: a useful immunohistochemical marker of normal and neoplastic plasma cells on routine trephine bone marrow biopsies. Mod Pathol. 1999;12:1101–6. [PubMed] [Google Scholar]
- 19.Dhodapkar MV, Abe E, Theus A, et al. Syndecan-1 is a multifunctional regulator of myeloma pathobiology: control of tumor cell survival, growth, and bone cell differentiation. Blood. 1998;91:2679–88. [PubMed] [Google Scholar]
- 20.Dhodapkar MV, Sanderson RD. Syndecan-1 (CD 138) in myeloma and lymphoid malignancies: a multifunctional regulator of cell behavior within the tumor microenvironment. Leuk Lymphoma. 1999;34:35–43. doi: 10.3109/10428199909083378. [DOI] [PubMed] [Google Scholar]
- 21.Stanley MJ, Liebersbach BF, Liu W, et al. Heparan sulfate–mediated cell aggregation. Syndecans-1 and -4 mediate intercellular adhesion following their transfection into human B lymphoid cells. J Biol Chem. 1995;270:5077–83. doi: 10.1074/jbc.270.10.5077. [DOI] [PubMed] [Google Scholar]
- 22.Ridley RC, Xiao H, Hata H, et al. Expression of syndecan regulates human myeloma plasma cell adhesion to type I collagen. Blood. 1993;81:767–74. [PubMed] [Google Scholar]
- 23.Liebersbach BF, Sanderson RD. Expression of syndecan-1 inhibits cell invasion into type I collagen. J Biol Chem. 1994;269:20013–9. [PubMed] [Google Scholar]
- 24.Bayer-Garner IB, Sanderson RD, Dhodapkar MV, et al. Syndecan-1 (CD138) immunoreactivity in bone marrow biopsies of multiple myeloma: shed syndecan-1 accumulates in fibrotic regions. Mod Pathol. 2001;14:1052–8. doi: 10.1038/modpathol.3880435. [DOI] [PubMed] [Google Scholar]
- 25.Jego G, Robillard N, Puthier D, et al. Reactive plasmacytoses are expansions of plasmablasts retaining the capacity to differentiate into plasma cells. Blood. 1999;94:701–12. [PubMed] [Google Scholar]
- 26.O’Connell FP, Pinkus JL, Pinkus GS. CD138 (syndecan-1), a plasma cell marker immunohistochemical profile in hematopoietic and nonhematopoietic neoplasms. Am J Clin Pathol. 2004;121:254–63. doi: 10.1309/617D-WB5G-NFWX-HW4L. [DOI] [PubMed] [Google Scholar]
- 27.Nanki N, Fujita J, Yang Y, et al. Expression of oncofetal fibronectin and syndecan-1 mRNA in 18 human lung cancer cell lines. Tumour Biol. 2001;22:390–6. doi: 10.1159/000050642. [DOI] [PubMed] [Google Scholar]
- 28.Ng AP, Wei A, Bhurani D, et al. The sensitivity of CD138 immunostaining of bone marrow trephine specimens for quantifying marrow involvement in MGUS and myeloma, including samples with a low percentage of plasma cells. Haematologica. 2006;91:972–5. [PubMed] [Google Scholar]
- 29.Shrout P, Fleiss J. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–8. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 30.Rajkumar SV, Greipp PR. Prognostic factors in multiple myeloma. Hematol Oncol Clin North Am. 1999;13:1295–1314. xi. doi: 10.1016/s0889-8588(05)70128-3. [DOI] [PubMed] [Google Scholar]
- 31.Rajkumar S, Fonseca R, Lacy M, et al. Abnormal cytogenetics predict poor survival after high-dose therapy and autologous blood cell transplantation in multiple myeloma. Bone Marrow Transplant. 1999;24:497–503. doi: 10.1038/sj.bmt.1701943. [DOI] [PubMed] [Google Scholar]
- 32.Rawstron AC, Davies FE, DasGupta R, et al. Flow cytometric disease monitoring in multiple myeloma: the relationship between normal and neoplastic plasma cells predicts outcome after transplantation. Blood. 2002;100:3095–100. doi: 10.1182/blood-2001-12-0297. [DOI] [PubMed] [Google Scholar]
- 33.San Miguel JF, Garcia-Sanz R, Gonzalez M, et al. DNA cell content studies in multiple myeloma. Leuk Lymphoma. 1996;23:33–41. doi: 10.3109/10428199609054799. [DOI] [PubMed] [Google Scholar]
- 34.Ocqueteau M, Orfao A, Almeida J, et al. Immunophenotypic characterization of plasma cells from monoclonal gammopathy of undetermined significance patients. Implications for the differential diagnosis between MGUS and multiple myeloma. Am J Pathol. 1998;152:1655–65. [PMC free article] [PubMed] [Google Scholar]
- 35.Smock KJ, Perkins SL, Bahler DW. Quantification of plasma cells in bone marrow aspirates by flow cytometric analysis compared with morphologic assessments. Arch Pathol Lab Med. 2007;131:951–5. doi: 10.5858/2007-131-951-QOPCIB. [DOI] [PubMed] [Google Scholar]