Abstract
We studied the effects of acetylcholinesterase inhibitors, donepezil and galantamine, and an N-methyl-D-aspartate (NMDA) receptor blocker, memantine, on sleep-wake architecture in rats. Screw electrodes were chronically implanted into the frontal and parietal cortex for the electroencephalography (EEG). EEG was recorded with a bio-potential amplifier for 8 h from 09:30 to 17:30. Vibration was recorded to monitor animal activity with a vibration measuring device. Sleep-wake states such as wake (W), slow-wave sleep (S) and paradoxical or rapid eye movement sleep (P), were scored every 10 sec by an experimenter. We measured mean episode duration and number of episode to determine which factor sleep disturbance was attributed to. Donepezil and memantine showed a significant increase in total W duration and decreases in total S and P duration and delta activity. Memantine showed increases in sleep latency and motor activity. Changes of S and P duration in memantine were attributed from changes of mean episode duration. Galantamine had little effect on sleep architecture. From these results, it is showed that galantamine may be an anti-dementia drug that does not cause sleep disturbances and memantine may be a drug that causes severe sleep disturbance.
Keywords: Donepezil, Electroencephalography, Galantamine, Memantine, Sleep-wake state
INTRODUCTION
Alzheimer's disease (AD) is a neurodegenerative disorder that is characterized by progressive loss of memory and deterioration of the cognitive functions. In addition, patients with AD often exhibit sleep disturbances, which are characterized by an irregular sleep-wake rhythm such as increases of daytime sleep and nighttime sleep loss correlated to disease severity [1]. It seems to be related to not only disease progression but also environmental factors which are reduced daily activity, increased daytime sleep and light exposure during night for patient care [2]. Sleep disturbance and nighttime activity in the patients are a major factor of physical stress, psychological burden for the caregiver and a major determinant of institutionalization [3].
Anti-dementia drugs may themselves disturb sleep-wake cycle in addition to cognitive improvement in patients. Although the effects of anti-dementia drugs have been widely studied, limited data are available comparing the adverse effects of these drugs on sleep architecture and daytime wakefulness using EEG. Acetylcholinesterase inhibitors, such as donepezil and galantamine, are currently approved for the treatment of mild-to-moderate AD [4,5]. Though sleep disturbances are one of the most common adverse effects in treating with acetylcholinesterase inhibitors, they are not evident in chronic treatment of acetylcholinesterase inhibitors in AD [6-9]. Memantine is used for the treatment of moderate or severe AD. It is well known that memantine acts as a blocker on N-methyl-D-aspartate (NMDA) receptors. It produces wake promotion and insomnia in AD patients [10]. However, a few studies showed that memantine increases total awake time and reduces total sleep time [11,12].
There is no report comparing the effect of three anti-dementia drugs (galantamine, donepezil, and memantine) on sleep with EEG after administration of minimal doses. And there is as yet little information as to whether these drug-related sleep disturbances attributed from mean episode duration or number of episodes.
Therefore, we compared the effect of two acetylcholinesterase inhibitors, galantamine and donepezil, and one NMDA channel blocker, memantine, on parameters reflecting sleep-wake patterns in rats. The parameters tested in this study were duration and frequency of each sleep-wake state, mean duration of each state, delta activity, and delta density during slow-wave sleep, and latencies to slow-wave sleep and REM sleep. We used smaller doses than previous study, considering rat's body weight and human usual dosage. After drug administration, parameters were analyzed for every 30 min, 2 h periods. Next, we measured mean episode duration and number of episode to determine which factor sleep disturbance was attributed to. Furthermore we measured power of vibration, reflecting motor activity.
METHODS
Animals
Eight male Sprague-Dawley rats (Samtaco, Osan, Korea) weighing from 280 to 310 g (295.60±3.65) at surgery were used. All animals were housed individually in Plexiglas cages (28×42×18 cm high), were kept in a controlled environment with 23±2℃ ambient temperature, 12:12 h light-dark cycle (lights on from 07:00 to 19:00), and were fed with a commercial food (Hyochang Science, Daegu, Korea) and tap water available ad libitum, except on the days of the behavioral recordings. All procedures were conducted in accordance with the National Institutes of Health Guidelines for Care and Use of Laboratory Animal (1996).
Surgery
The animal was anesthetized with an intraperitoneal injection of a 2 ml/kg cocktail of 10 ml of ketamine hydrochloride (50 mg/ml, Ketamine, Yuhan Co., Seoul, Korea), 1.1 ml of xylazine hydrochloride (23.32 mg/ml, Rumpun, Bayer Co., Seoul, Korea), and 2.67 ml of saline. After fixed to a stereotaxic apparatus (David-Kopf Instrument Co., CA, USA), the scalp was locally anesthetized with a subcutaneous injection of 2% lidocaine with 1:100,000 epinephrine (Lidocaine hydrochloride Epinephrine, Huons Co., Seoul, Korea) and then, the midline of the scalp was incised and the skull surface was cleaned. For electroencephalogram (EEG), four stainless steel screws (1.1 mm in diameter), served as recording electrodes, were inserted into the bilateral frontal (+2.5 mm anterior and ±2.5 mm lateral to the bregma) and parietal bones (5.0 mm posterior and ±2.5 mm lateral to the bregma), without perforation the dura mater, and two stainless steel screws, served as reference and ground electrodes, were inserted into the inter-parietal bone (10.0 mm posterior and ±2.5 mm lateral to the bregma). The electrodes were soldered to connecting pins which were arranged in a 3×2 matrix, and the whole assembly was fixed over the skull with dental cement (Ortho-jet, Lang Dental Manufacturing Co., PA, USA).
Recordings of EEG and vibration
At least 7 days were allowed to recover from the surgery. The animal was acclimated to the recording setup from 09:30 to 17:30 (8 h) on the day before the recording. Recording chamber was a sound-attenuated and electrically shielded box (80 cm wide×60 cm deep×80 cm high). The vibration measurement plate, constructed in our lab [13] was placed on the floor of the chamber to measure animal activity. On the day of the recording, a Plexiglas cage was put on the vibration measurement plate and the rat was placed into the cage at 09:30. Its head pins were connected to the amplifier via a flexible cable and swivel system. EEG signals from the frontal and parietal cortices and vibration signal were fed to amplifiers (Model 3500, A-M systems, Inc., WA, USA and CyberAmp 380, Axon instruments Inc., CA, USA) from 09:30 to 17:30 (8 h). The EEG signals and the vibration signal were amplified with 10,000× and 10× gain, respectively, and were filtered with 1~100 Hz. The signals were digitized at a rate of 1 KHz (DAQ PAD6015, National Instrument Inc., CA, USA), averaged with 5 consecutive samples, and stored at a rate of 200 Hz with custom-made LabView program (National Instrument INC., CA, USA).
Sleep-wake state scoring
Sleep-wake states were scored by an experienced experimenter with EEG and vibration signals. Each 10-second epoch was assigned to one of the following sleep-wake states: active wake (aW), quiet wake (qW), transition to slow wave sleep (tSWS), slow wave sleep (SWS), transition to paradoxical sleep (tPS) and paradoxical sleep (PS). As described previously [14], aW was scored according to the presence of high and phasic vibration together with high-frequency and theta activity on the parietal EEG; qW by the presence of moderate vibration with low-voltage fast EEG activity; tSWS by the presence of low vibration with delta activity less than 50% of the epoch and/or a sleep spindle; SWS by the presence of minimal vibration with delta activity more than 50% of the epoch; tPS by absent vibration with almost continuous spindle activity and interlaced theta activity; and PS by absent vibration with prominent theta activity on the parietal cortex.
Power spectrum analysis
It was adapted from Lee et al. [15]. The raw EEG signals were visually inspected prior to analysis and 10-sec EEG epochs with artifacts were excluded from analysis. The one-day recording was divided into sixteen 30-min segments from 09:30 and was analyzed. Ten power spectra were calculated by using a fast Fourier transform from 10 Hanning-windowed 2-sec FFT epochs (1-sec overlap) of one 10-sec EEG epoch and then averaged to a power spectrum. The spectrum was divided to 5 frequency bands (delta, 0.5~4.0; theta, 4.5~8.0; alpha 8.5~12.0; beta, 12.5~24.5; and gamma, 25~49.5 Hz). All the calculations were performed by custom-made programs using MatLab (MathWorks Inc., Natick, MA, USA).
Measurements
Sleep-wake states were estimated as W, S and P: W consists of aW and qW. S consists of tSWS and SWS. P consists of tPS and PS. Seven sleep-related parameters were evaluated as follows: (1) total duration, total time spent in each sleep-wake stage; (2) number of episode, total number of occurrence of each sleep-wake stage; and (3) mean episode duration, mean duration of each sleep-wake stage when it was once occurred for 2-h period (from 30 to 150 min after drug injection); (4) latency to 5-min S and (5) latency to 1-min P, the latency time in min from drug injection to the accumulation of total 5-min S and of total 1-min P, respectively.
The power parameters were calculated as follows: (1) delta activities, as power density of delta band (0.5~4.5 Hz) in the average spectrum of 2- and 8-h recording; (2) band power density during S and P, calculation respective delta (0.5~4.5 Hz), theta (4.5~6.5 Hz), alpha (8.5~12.5 Hz), beta (12.5~25 Hz) and gamma (25~50 Hz) of average spectrum in S and P during 2-h sleep after injection.
Drugs
Galantamine hydrobromide (Reminyl, Janssen Korea Co., Seoul, Korea), donepezil hydrochloride (Aricept, Eisai Korea Co., Seoul, Korea), and memantine hydrochloride (Ebixa, Lundbeck Korea Co., Seoul, Korea) were gifted from each company. Animals were received all of the following drugs once: vehicle (1 ml/kg, Con), donepezil (0.15 mg/kg, Don), galantamine (0.25 mg/kg, Gal) and memantine (0.25 mg/kg, Mem). Each rat had 7-day washout period between drugs. The dosage of each drug was chosen from clinical dosing recommendations in a previous report [16]. All drugs were dissolved in distilled water before the administration. Animals received an intraperitoneal injection at a volume of 1 ml/kg at 09:00.
Statistics
All data were represented as mean±S.E.M. One-way analysis of variance (ANOVA) with Tukey HSD test was used for statistical analysis to estimate the effects of the drugs (SPSS v. 17.0 for Window, Data solution, Seoul, Korea). A p-value below 0.05 was considered significantly different.
RESULTS
Total duration, number of episode and mean episode duration
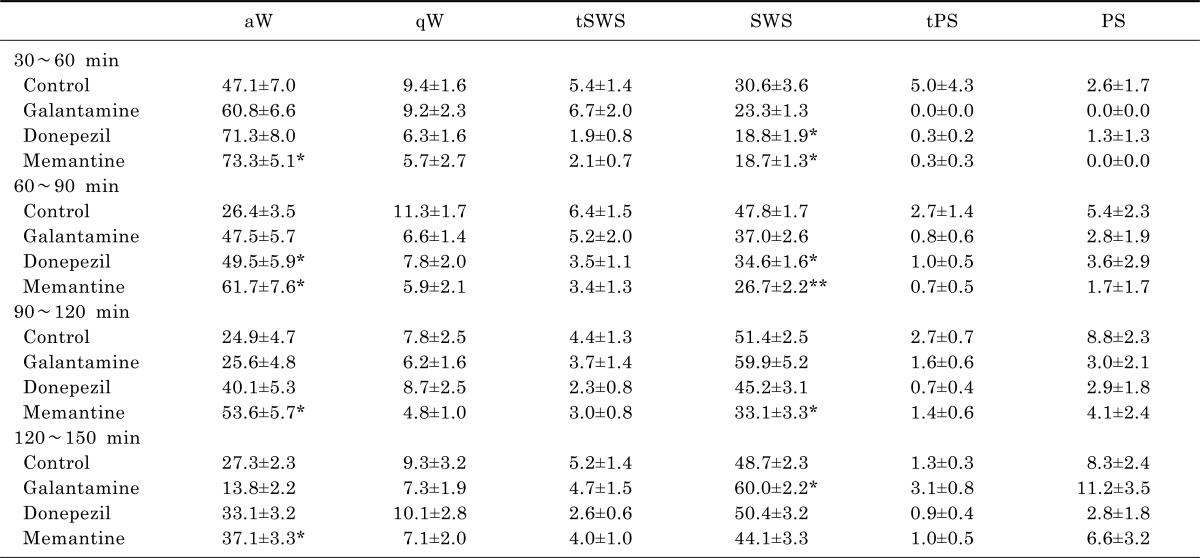
Three anti-dementia drugs, galantamine, donepezil and memantine, had differential effect on the sleep-wake architecture in rats. Donepezil and memantine increased active wake and decreased slow-wave sleep significantly. Memantine had stronger and longer effect. Its effect on active wake was continued to 150 min and its effect on slow-wave sleep was continued to 120 min after the injection. Donepezil has effect on active wake and on slow-wave sleep to 90 min after the injection. On the other hand, galantamine did not produce significant changes on duration of any sleep-wake stages, but rather increased slow-wave sleep during 120~150 min after the injection (Table 1).
Table 1.
Total duration (%) of sleep-wake stages for 2-hour period (from 30 min to 150 min) after drug injection
Each value represents the mean±S.E.M. (n=8). aW, active wake; qW, quiet wake; tSWS, transition to SWS; SWS, slow-wave sleep; tPS, transition to PS; and PS, paradoxical sleep or REM sleep. *p<0.05, **p<0.001 significantly different from the control group.
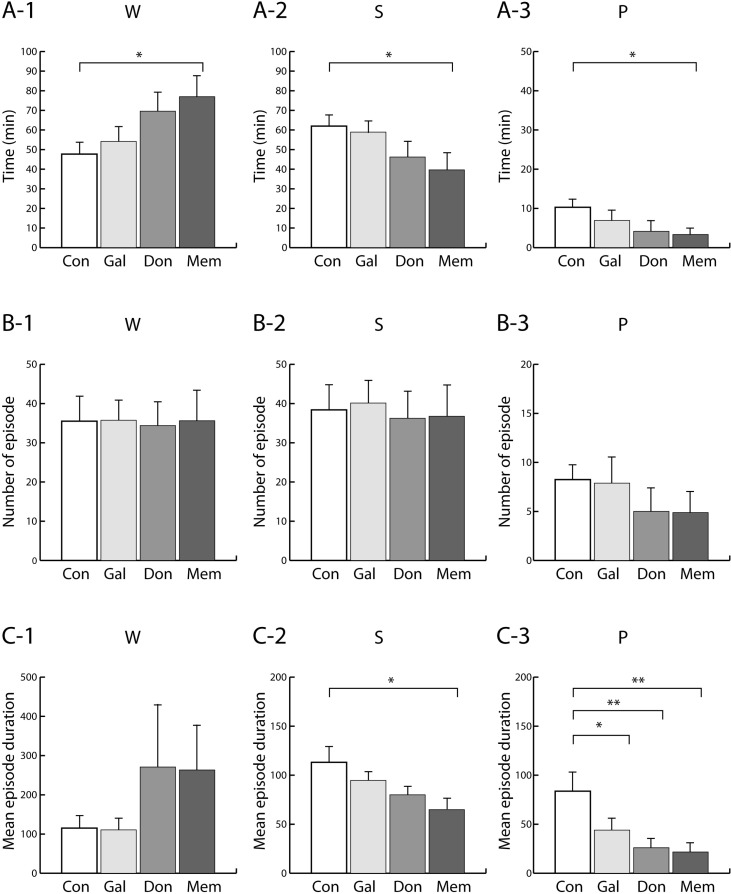
Only memantine significantly increased W (aW+qW) and decreased S (tSWS+SWS) and P (tPS+PS) as compared to control group (Fig. 1A), in which the total durations were pooled for 2-h period. Three anti-dementia drugs caused no significant changes in number of episode. (Fig. 1B). Mean episode duration of S was significantly decreased by memantine while that of P was significantly decreased by all three anti-dementia drugs (Fig. 1C).
Fig. 1.
(A) Total duration (min), (B) number of episode (per 2-h period) and (C) mean episode duration of each sleep-wake stages after drug treatment. The rats were given saline (Con), galantamine (Gal), donepezil (Don) and memantine (Mem). Two-h period following each drug treatment was scored. Data were expressed as mean±S.E.M. (n=8 per group). *p<0.05 and **p<0.01; significantly different from the Con group. W, active and quiet wake; S, transition to slow-wave sleep and slow-wave sleep; and P, transition to paradoxical sleep and paradoxical sleep.
Sleep latency and motor activity
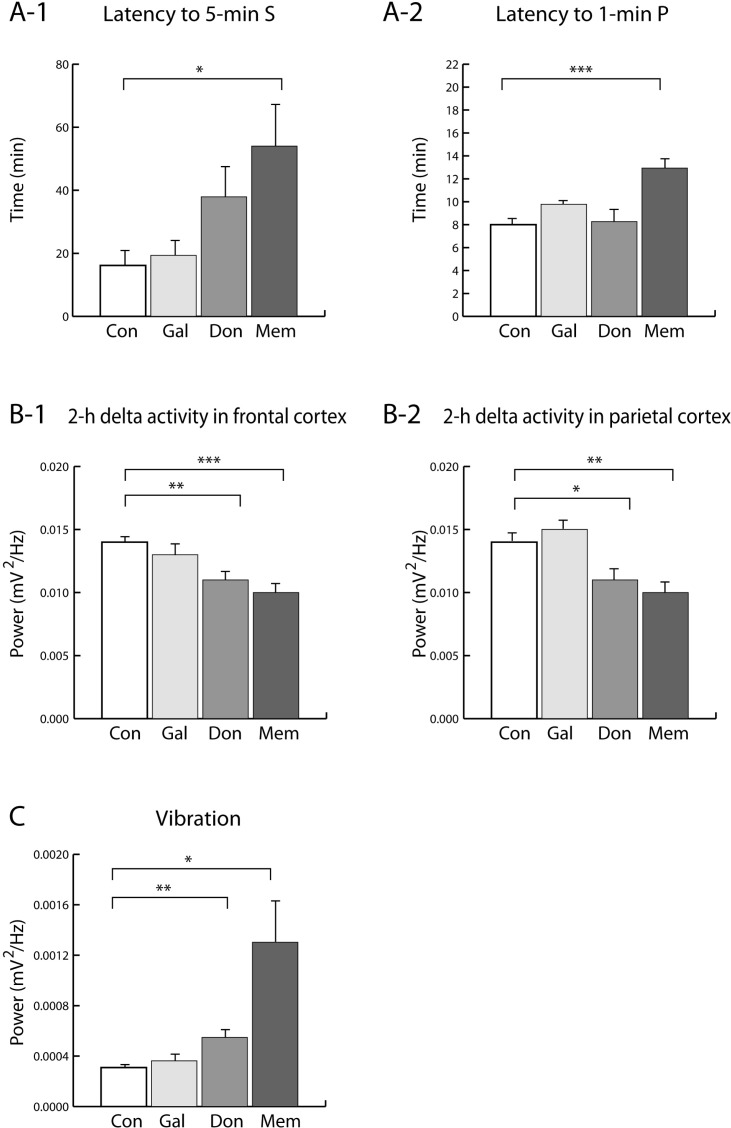
Memantine caused significant increases both in latency to S and latency to P, while donepezil and galantamine did not (Fig. 2A). Activity was estimated by power of vibration in this study. Memantine and donepezil significantly increased movement while galantamine did not (Fig. 2C).
Fig. 2.
(A) Latency to 5-min S and to 1-min P. (B) Delta activity in the frontal and parietal cortex for 2-h period after drug treatment. (C) Vibration. The rats were given saline (Con), galantamine (Gal), donepezil (Don) and memantine (Mem). Data were expressed as mean±S.E.M. (n=8 per group). *p<0.05, **p <0.01 and ***p<0.001: significantly different from the Con group. S, transition to slow-wave sleep and slow-wave sleep, and P, transition to paradoxical sleep and paradoxical sleep.
Delta activity
Donepezil and memantine decreased delta activity of the frontal and parietal cortex during 2-h period (Fig. 2B). Memantine and donepezil cause significantly decrease of delta activity in frontal and parietal cortex. There were no significant differences of delta activity in frontal and parietal cortex by galantamine.
DISCUSSION
We tested whether anti-dementia drugs produce sleep disturbing effect by measuring several sleep parameters, such as sleep-wake duration, latency to the sleep, number of episode and mean episode duration, motor activity and delta activity. Previous studies showed that donepezil and memantine caused significant increases in sleep latency and total waking time, while galantamine caused no sleep disturbance [11,12]. Similarly, it was found that memantine showed significant decrease of slow-wave sleep time and paradoxical sleep time and donepezil showed the similar tendency although not significant statistically.
We have also found memantine had longer and earlier effects on sleep architecture and donepezil had effects on only early sleep periods. On the other hand, galantamine had little effects. It is well known memantine has a longer half life than donepezil. It seems longer effects of memantine may be due to longer half life. These results suggest that sleep disturbing effect of memantine and donepezil occurs very acute period of total sleep cycle and effect gradually decreased during 2 h. Interestingly, galantamine produced rebound increase in slow-wave sleep during last 30 min period even though it did not produce sleep disturbance in a few periods after drug administration.
Decrease of slow-wave sleep time and paradoxical sleep time resulted from decrease of mean episode duration, not resulted from number of episode. It is supported that slow-wave sleep and paradoxical sleep were fragmented by memantine and donepezil. Furthermore, we have also found that memantine and donepezil showed significant increase of motor activity. About sleep latency, memantine showed significant increase of sleep latency and donepezil showed the similar tendency although not significant statistically. These results can also support the sleep disturbing effects of memantine and donepezil.
Delta activity, particularly in the frontal cortex, during slow-wave sleep means the depth of sleep and quality of sleep. Different from Ishida and Kamei [11,12], Delta activity during sleep were significantly decreased with donepezil and memantine. The results indicate that donepezil and memantine caused not only a decrease in non-REM sleep, but also deterioration of sleep quality in rats. And this discordance may result from difference of recording time and dosage of drugs used. In this study, EEG recording analyzed was done for 2-h period, which are forepart of sleep cycle. We used the minimum dosage for memory enhancing.
There are some reports that acetylcholinesterase inhibiting activity is responsible for sleep disturbance in both humans and animals. Prolonged sleep latency and increased wake time may result from acetylcholinesterase-inhibiting action. It is supposed that donepezil induces sleep disturbances result from this action. However, galantamine having another acetylcholinesterase-inhibiting action did not show sleep disturbing effect. It was also been reported that galantamine had no remarkable effect on the sleep-wake pattern in humans [17] and rats [11] and it was explained that the acetylcholinesterase-inhibiting action of galantamine is weaker than those of donepezil, rivastigmine and physostigmine [18]. Therefore, it is suggested that differences of sleep disturbing effects in two acetylcholinesterase-inhibiting agents may be due to the potency of acetylcholinesterase-inhibiting activity.
Memantine effect on sleep was the most strongest in this study. Memantine is known to have noncompetitive NMDA receptor blocking effect and its wake promoting effect is due to enhancement of dopaminergic neurotransmission [12]. It is well known that elevated dopamine level makes large contribution to the arousal effect. Hanania and Zahniser [19] reported that NMDA receptor antagonist increased locomotor activity by enhanced dopamine neurotransmission.
In contrast to sleep disturbing effect of acute treatment of acetylcholinesterase inhibiting agents, chronic administration of galantamine and donepezil does not show significant effect on sleep [7]. Sleep disorder in Alzheimer's dementia patients is most important for the caregiver and drug treatment has compounding effect on sleep. Acute treatment induces sleep fragmentation but repeated treatment is tolerated with patient. So, titrating antidementia drug slowly can be helpful method for avoiding adverse effect on sleep architecture. Moreover, considering the effect of drug occur very acute stage and gradually decreased over 2 h, drug administration at the beginning of wake could be better than at the beginning of sleep as current usual method.
From the results, it is suggested that galantamine may be an anti-dementia drug that does not cause sleep disturbances and memantine and donepezil may be a drug that causes significant sleep disturbance in acute treatment by shortening sleep episode duration rather than decreased episode number. In addition taking small doses at first, slowly elevating the dose and taking a medicine at morning can be better to avoid sleep disturbance in demented patients.
ACKNOWLEDGEMENTS
This work was supported by Biomedical Research Institute Grant (2010), Kyungpook National University Hospital.
ABBREVIATIONS
- AD
Alzheimer's disease
- aW
active wake
- EEG
electroencephalography
- NMDA
N-methyl-D-aspartate
- P
paradoxical sleep
- PS
paradoxical sleep
- qW
quiet wake
- REM
rapid eye movement
- S
slow-wave sleep
- SWS
slow-wave sleep
- tPS
transition to paradoxical sleep
- tSWS
transition to slow-wave sleep
- W
wake
References
- 1.Dauvilliers Y. Insomnia in patients with neurodegenerative conditions. Sleep Med. 2007;8(Suppl 4):S27–S34. doi: 10.1016/S1389-9457(08)70006-6. [DOI] [PubMed] [Google Scholar]
- 2.Yesavage JA, Friedman L, Ancoli-israel S, Bliwise D, Singer C, Vitiello MV, Monjan AA, Lebowitz B. Development of diagnostic criteria for defining sleep disturbance in Alzheimer's disease. J Geriatr Psychiatry Neurol. 2003;16:131–139. doi: 10.1177/0891988703255684. [DOI] [PubMed] [Google Scholar]
- 3.Hope T, Keene J, Gedling K, Fairburn CG, Jacoby R. Predictors of institutionalization for people with dementia living at home with a carer. Int J Geriatr Psychiatry. 1998;13:682–690. doi: 10.1002/(sici)1099-1166(1998100)13:10<682::aid-gps847>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 4.Thompson S, Lanctôt KL, Herrmann N. The benefits and risks associated with cholinesterase inhibitor therapy in Alzheimer's disease. Expert Opin Drug Saf. 2004;3:425–440. doi: 10.1517/14740338.3.5.425. [DOI] [PubMed] [Google Scholar]
- 5.Wilkinson DG, Francis PT, Schwam E, Payne-Parrish J. Cholinesterase inhibitors used in the treatment of Alzheimer's disease: the relationship between pharmacological effects and clinical efficacy. Drugs Aging. 2004;21:453–478. doi: 10.2165/00002512-200421070-00004. [DOI] [PubMed] [Google Scholar]
- 6.Rogers SL, Doody RS, Mohs RC, Friedhoff LT Donepezil Study Group. Donepezil improves cognition and global function in Alzheimer disease: a 15-week, double-blind, placebo-controlled study. Arch Intern Med. 1998;158:1021–1031. doi: 10.1001/archinte.158.9.1021. [DOI] [PubMed] [Google Scholar]
- 7.Stahl SM, Markowitz JS, Papadopoulos G, Sadik K. Examination of nighttime sleep-related problems during double-blind, placebo-controlled trials of galantamine in patients with Alzheimer's disease. Curr Med Res Opin. 2004;20:517–524. doi: 10.1185/030079904125003214. [DOI] [PubMed] [Google Scholar]
- 8.Ancoli-Israel S, Amatniek J, Ascher S, Sadik K, Ramaswamy K. Effects of galantamine versus donepezil on sleep in patients with mild to moderate Alzheimer disease and their caregivers: a double-blind, head-to-head, randomized pilot study. Alzheimer Dis Assoc Disord. 2005;19:240–245. doi: 10.1097/01.wad.0000189052.48688.36. [DOI] [PubMed] [Google Scholar]
- 9.Schredl M, Weber B, Leins ML, Heuser I. Donepezil-induced REM sleep augmentation enhances memory performance in elderly, healthy persons. Exp Gerontol. 2001;36:353–361. doi: 10.1016/s0531-5565(00)00206-0. [DOI] [PubMed] [Google Scholar]
- 10.Guay DR. Drug forecast: memantine, prototype of a new approach to treatment of dementia. Consult Pharm. 2003;18:625–634. [PubMed] [Google Scholar]
- 11.Ishida T, Kamei C. Characteristic effects of anti-dementia drugs on rat sleep patterns. J Pharmacol Sci. 2009;109:449–455. doi: 10.1254/jphs.08229fp. [DOI] [PubMed] [Google Scholar]
- 12.Ishida T, Obara Y, Kamei C. Studies on wakefulness-promoting effect of memantine in rats. Behav Brain Res. 2010;206:274–278. doi: 10.1016/j.bbr.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 13.Jung JY. Characterization of vibration signal patterns for rat behaviors using specially designed vibration sensors. Kyungpook National University Graduate School; 2010. MS Thesis. [Google Scholar]
- 14.Kwon DH, Won SH, Kim KM, Chang SM, Kim SH, Lee MG. Effect of caffeine on sleep and EEG spectra in rats. J Korean Soc Biol Ther Psychiatry. 2006;12:252–267. [Google Scholar]
- 15.Lee MG, Kim M, Roh M, Jang IS, Won SH. Differences between physostigmine- and yohimbine-induced states are visualized in canonical space constructed from EEG during natural sleep-wake cycle in rats. Exp Neurobiol. 2011;20:54–65. doi: 10.5607/en.2011.20.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farlow MR, Cummings JL. Effective pharmacologic management of Alzheimer's disease. Am J Med. 2007;120:388–397. doi: 10.1016/j.amjmed.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 17.Blesa R. Galantamine: therapeutic effects beyond cognition. Dement Geriatr Cogn Disord. 2000;11(Suppl 1):28–34. doi: 10.1159/000051229. [DOI] [PubMed] [Google Scholar]
- 18.Geerts H. Indicators of neuroprotection with galantamine. Brain Res Bull. 2005;64:519–524. doi: 10.1016/j.brainresbull.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Hanania T, Zahniser NR. Locomotor activity induced by noncompetitive NMDA receptor antagonists versus dopamine transporter inhibitors: opposite strain differences in inbred long-sleep and short-sleep mice. Alcohol Clin Exp Res. 2002;26:431–440. [PubMed] [Google Scholar]