Abstract
This study investigated the effects of proline-serine (PS) and valine-serine (VS) dipeptides on melanogenesis in Mel-Ab cells. Proline-serine and VS significantly inhibited melanin synthesis in a concentration-dependent manner, though neither dipeptide directly inhibited tyrosinase activity in a cell-free system. Both PS and VS down-regulated the expression of microphthalmia-associated transcription factor (MITF) and tyrosinase. In a follow-up study also described here, the effects of these dipeptides on melanogenesis-related signal transduction were quantified. Specifically, PS and VS induced ERK phosphorylation, though they had no effect on phosphorylation of the cAMP response element binding protein (CREB). These data suggest that PS and VS inhibit melanogenesis through ERK phosphorylation and subsequent down-regulation of MITF and tyrosinase. Properties of these dipeptides are compatible with application as skin-whitening agents.
Keywords: Dipeptide, ERK, Melanogenesis, MITF, Tyrosinase
INTRODUCTION
Melanin is the most important determinant of skin, hair, and eye color in mammals. In melanocytes, melanin is produced within specialized organelles called melanosomes. Tyrosinase, the rate-limiting enzyme in melanin synthesis, catalyses the hydroxylation of tyrosine to 3,4-dihydroxyphenylalanine (DOPA) and the oxidation of DOPA to dopaquinone [1]. Melanosomes may move into melanocytic dendrites and later be transported into neighboring keratinocytes [2]. Non-uniform patterns of melanin synthesis result in cosmetically unappealing conditions such as melasma, age spots and freckles, and generate a need for safe and effective skin-whitening agents.
Specific signaling cascades control melanogenesis. Alphamelanocyte stimulating hormone (α-MSH) up-regulates cyclic AMP (cAMP) through binding to melanocortin-1 receptor (MC1R) [3]. Protein kinase A (PKA), a downstream effector of cAMP, induces cAMP responsive element-binding protein (CREB) phosphorylation, thereby promoting the expression of microphthalmia-associated transcription factor (MITF), the major transcription factor for tyrosinase expression [4,5]. Conversely, when extracellular signal-regulated kinase (ERK) is phosphorylated, it down-regulates MITF expression by phosphorylating MITF at serine 73, thereby also down-regulating tyrosinase [6,7].
Synthetic peptides show wide-ranging activities, some with significant effects on inflammation, immunity, antioxidant response, resistance to infection and melanogenesis [8-10]. While peptide synthesis is relatively simple, synthetic peptides may be expensive, depending on the number and type of amino acids required [11]. Our interest focuses on several peptides that have the capacity to modulate skin pigmentation [12,13]. Several tripeptides have recently been added to this list [8] and oligopeptides with specific amino acid sequences may reduce pigmentation through inhibition of tyrosinase activity [14-16]. However, the effects of dipeptides on melanogenesis have not been thoroughly evaluated. Dipeptides may be significant therapeutically because molecular size may influence skin penetration.
In this study, we screened dipeptides from an internal dipeptide library for the capacity to inhibit melanogenesis. Of the dipeptides screened, proline-serine (PS) and valine-serine (VS) showed hypopigmentary effects suggesting they might be used to develop whitening agents. To investigate the mechanism of action of PS and VS in melanin synthesis, we measured the effects of these dipeptides on MITF and tyrosinase expression, and on the activation of melanogenesis-related signaling pathways in Mel-Ab cells.
METHODS
Materials
All dipeptides were supplied by Beadtech Inc. (Seoul, Korea). PS and VS were dissolved in dimethyl sulfoxide (DMSO) and stored at 4℃ as stock solutions (10 mg/ml). Fetal bovine serum (FBS) was purchased from Hyclone (Logan, UT, USA), while 12-O-tetradecanoylphorbol-13-acetate (TPA), cholera toxin (CT), 3,4-dihydroxy-L-phenylalanine (L-DOPA), and mushroom tyrosinase were obtained from Sigma-Aldrich Co. (St. Louis, MO, USA). Dulbecco's modified Eagle's medium (DMEM), antibiotic-antimycotic mix (penicillin, streptomycin), and trypsin-EDTA were purchased from WelGENE (Dalseogu, Daegu, South Korea). Antibodies specific for phospho-ERK1/2 (Thr202/Tyr204, #9101S) and phospho-CREB (Ser133, #9198) were obtained from Cell Signaling Technology (Beverly, MA, USA). Antibodies specific for tyrosinase (C-19) and actin (I-19), and for microphthalmia Ab-1 (C5, MS-771-P0) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA and NeoMarkers (Fremont, CA, USA), respectively. Secondary antibodies (anti-goat IgG (PI-9500), anti-mouse IgG (PI-2000) and anti-rabbit IgG (PI-1000)) were purchased from Vector Laboratories (Burlingame, CA, USA).
Cell culture
The Mel-Ab cell line is a spontaneously immortalized murine melanocyte line that produces large quantities of melanin [17]. Mel-Ab cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS), 100 nM TPA, 1 nM CT, 50 µg/ml streptomycin, and 50 µg/ml of penicillin in 5% CO2 at 37℃.
Cell viability assay
Cell viability was determined by crystal violet assay [17]. After incubating cells with PS or VS at 1~50 µg/ml for 24 hours, the medium was removed and the cells were stained with 0.1% crystal violet in 10% ethanol for 5 minutes at room temperature. The cells were then rinsed four times with distilled water, and any crystal violet retained by adherent cells was extracted using 95% ethanol. Absorbance was measured at 590 nm using an ELISA plate reader (VersaMax; Molecular Devices, Sunnyvale, CA, USA).
Assessment of melanin content and microscopy
After incubation with PS or VS at 1~20 µg/ml for 4 days, Mel-Ab cells were visualized by phase contrast microscopy (Olympus Optical Co., Tokyo, Japan) and photographed using a DCM300 digital camera (Scopetek, Inc., Hangzhou, China). The melanin content of the cells was quantified as previously described [18] with some modifications. Briefly, the cell pellets were dissolved in 1 ml of 1 N NaOH at 100℃ for 30 minutes, then centrifuged for 20 minutes at 16,000× g. The relative optical densities (OD) of the resulting supernatants were then measured at 400 nm using an ELISA reader. Triplicate samples of synthetic melanin (0~300 µg/ml) were used to prepare standard curves for each experiment.
Tyrosinase activity
Tyrosinase activity was assayed to determine DOPA oxidase activity. Mel-Ab cells were incubated for 4 days in six-well plates (2×105 cells/well) containing PS in DMEM. The cells were then washed with PBS, lysed with lysis buffer (0.1 M phosphate buffer [pH 6.8] containing 1% Triton X-100), and disrupted by freeze-thawing. The lysates were clarified by centrifugation at 15,000 rpm for 30 min. After protein quantification using a protein assay kit (Bio-Rad, Hercules, CA, USA), the cell lysates were adjusted with lysis buffer so that all contained equal amounts of protein. Next, 90 µl of each lysate and 10 µl of 10 mM L-DOPA were pipetted into the wells of a 96-well plate. Control wells containing 90 µl of lysis buffer and 10 µl of 10 mM L-DOPA were also prepared. After incubation at 37℃ for 20 minutes, dopachrome formation was quantified from the absorbance at 475 nm using an ELISA reader.
The direct effect of PS on tyrosinase activity was tested in a cell-free assay system. Briefly, 70 µl of phosphate buffer containing PS was mixed with 20 µl of mushroom tyrosinase (stock solution 53.7 units/ml) and 10 µl of 10 mM L-DOPA. Following incubation at 37℃ for 20 min, the absorbance was measured at 475 nm.
Western blot analysis
Mel-Ab cells were lysed in a cell lysis buffer (62.5 mM Tris-HCl [pH 6.8], 2% SDS, 5% β-mercaptoethanol, 2 mM phenylmethylsulfonyl fluoride, and protease inhibitors [Complete™; Roche, Mannheim, Germany], 1 mM Na3VO4, 50 mM NaF and 10 mM EDTA). Proteins in the total cell lysates were separated by SDS-polyacrylamide gel electrophoresis using 20 µg of protein per lane, blotted onto polyvinylidene fluoride (PVDF) membranes, and blocked with 5% dried milk in Tris-buffered saline containing 0.5% Tween 20. Blots were then incubated with the appropriate primary antibodies at a dilution of 1:1,000, and then re-incubated with horseradish peroxidase-conjugated secondary antibody. Bound antibody was identified using an enhanced chemiluminescence testing kit (Thermo Scientific Inc., Bremen, Germany). All images of the immunoblots were obtained using an LAS-1000 lumino-image analyzer (Fuji Film, Tokyo, Japan).
Statistics
The statistical significance of the intergroup differences was assessed by analysis of variance (ANOVA) followed by Student's t-test. All p-values <0.01 were considered significant.
RESULTS
Effects of PS and VS on cell viability
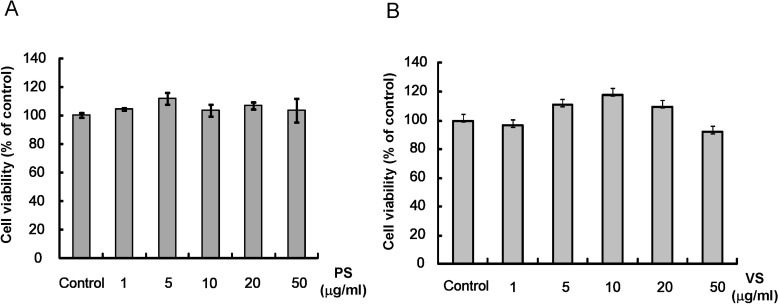
At concentrations between 1 and 50 µg/ml in 24-hr incubations, PS and VS showed no cytotoxicity (Fig. 1). Therefore, dipeptides at concentrations from 1 µg/ml to 20 µg/ml were used for the subsequent experiments.
Fig. 1.
Effects of PS and VS on Mel-Ab cell viability. Mel-Ab cells were treated with PS (A) or VS (B) at 1~50 µg/ml for 24 h. Cell viability was determined using a crystal violet assay as described in Methods. All data were expressed as the mean±S.D. of triplet viability assays.
Effects of PS and VS on melanin synthesis and tyrosinase activity
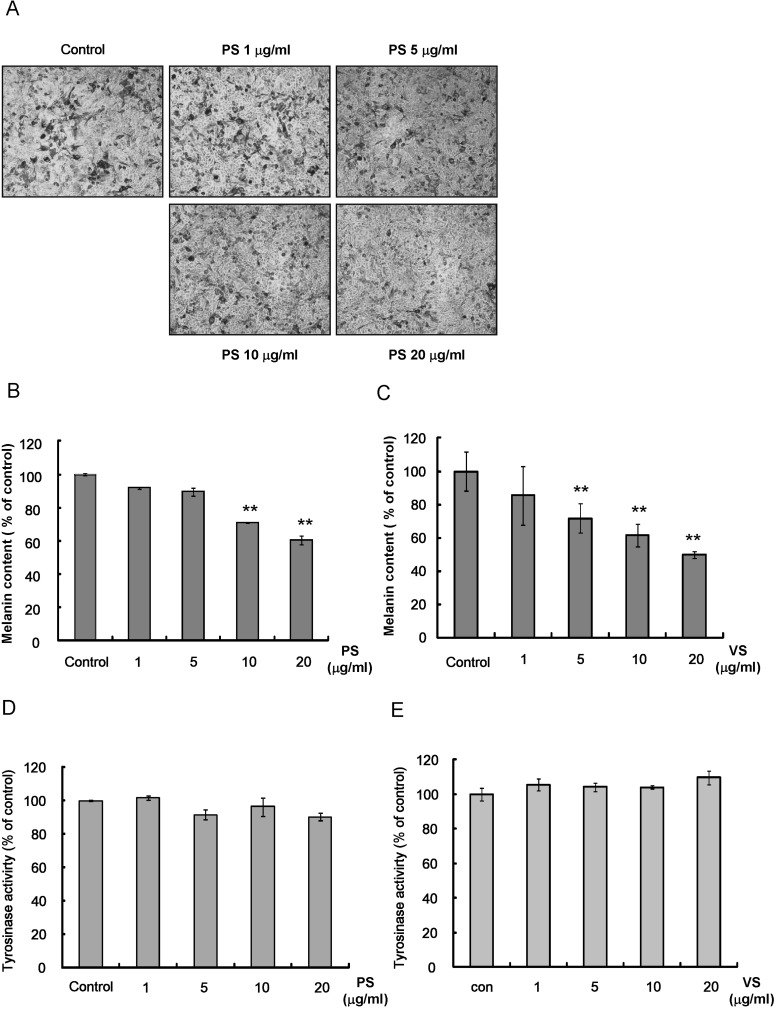
Mel-Ab cells were treated with PS or VS at 1~20 µg/ml for 4 days. Before measuring the melanin content, the cells were observed under a phase contrast microscope. Melanin pigmentation decreased dose-dependently in PS-treated cells (Fig. 2A). Similarly, cell melanin content was reduced in a dose-dependent manner after treatment with either PS or VS (Fig. 2B, C). Accordingly, the capacity of PS and VS to inhibit tyrosinase directly in a cell-free system was examined, and neither dipeptide showed such an effect (Fig. 2D, E).
Fig. 2.
Effects of PS and VS on melanogenesis in Mel-Ab cells. Mel-Ab cells were treated with PS or VS at 1~20 µg/ml for 4 days. (A) Phase contrast photographs were taken using a digital video camera. Melanin content (B and C) and tyrosinase activity (D and E) were measured as described in Methods. Data are expressed as the mean±S.D. of triplicate assays. **p<0.01 compared to the untreated control.
PS and VS down-regulated MITF and tyrosinase expression
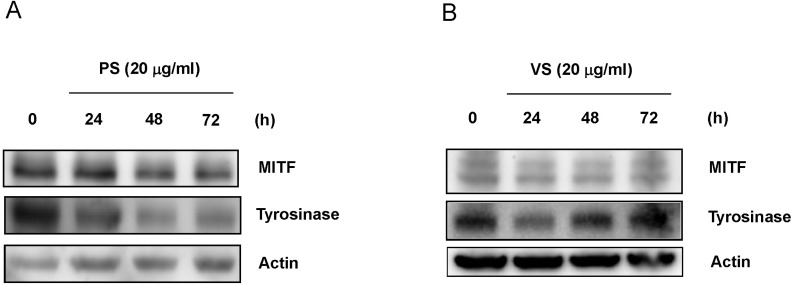
We determined the levels of MITF and tyrosinase proteins by western blot analysis during culture of cells with PS or VS (20 µg/ml) for 4 days. MITF protein levels were reduced after 48 to 72 hours of treatment with PS, and tyrosinase levels declined in parallel with the reduction in MITF (Fig. 3A). Treatment with VS also decreased levels of MITF and tyrosinase proteins at 24 hours. Levels of both proteins recovered gradually and returned to the normal range by 72 hours (Fig. 3B). These results provide evidence that PS and VS may decrease melanogenesis by down-regulating MITF and tyrosinase.
Fig. 3.
The dipeptides PS and VS downregulate MITF and tyrosinase expression in Mel-Ab cells. Mel-Ab cells were treated with PS (A) or VS (B) (20 µg/ml) for the times indicated. Western blot analyses were performed with MITF- and tyrosinase-specific antibodies. Equal protein loading was confirmed by probing for actin expression.
PS and VS induced the phosphorylation of ERK but not CREB
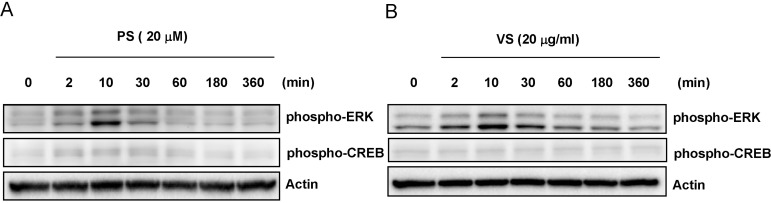
Emerging data suggest that ERK phosphorylation leads to MITF degradation by phosphorylation of MITF at serine 73 [6,19]. Therefore, we investigated whether PS- or VS-induced hypopigmentation was related to ERK phosphorylation. Cells were treated with PS or VS (20 µg/ml) for 0~360 min and the whole cell lysates were subjected to western blot analysis. Cellular levels of phospho-ERK increased for at least 30 min after treatment with either PS or VS (Fig. 4). We also measured the phosphorylation of cyclic AMP response element-binding protein (CREB), a transcription factor that promotes melanogenesis through MITF regulation [20,21]. However, neither PS nor VS affected CREB phosphorylation. These results suggest that PS and VS modulate melanogenesis by promoting ERK phosphorylation.
Fig. 4.
Effects of PS and VS on melanogenesis-related signaling. Mel-Ab cells were treated with PS (A) or VS (B) (20 µg/ml) for 0~360 min, after which the whole cell lysates were analyzed by western blotting using antibodies specific for phospho-ERK and phospho-CREB. Equal protein loading was confirmed by probing for actin expression.
DISCUSSION
Dipeptides, the simplest product of peptide bond formation between amino acids, may intervene in a variety of physiologic functions depending on experimental context. Thus anti-inflammatory, neuroprotective and antitumor activities of dipeptides are reported [22-24]. The peptides that inhibit tyrosinase activity [14,15] may potentially be used to develop new skin care products. Although the hypopigmentary properties of tripeptides have been investigated, the effects of dipeptides on melanogenesis have not been comprehensively assessed [8]. We screened dipeptides from an internal library for activities that influence melanogenesis. We found that while PS and VS inhibited melanin synthesis in a dose-dependent manner, single amino acids, including proline, valine, and serine, had significantly lower inhibitory effects (data not shown).
Various peptides may reduce melanogenesis by directly inhibiting tyrosinase activity [12]. In contrast, PS and VS did not directly inhibit the enzyme, but did reduce MITF protein expression, thereby decreasing levels of tyrosinase protein (Fig. 3). Mechanisms for the activities of dipeptides in melanogenesis, including potential target molecules or protein receptors for dipeptides, await further investigation. It seems unlikely that PS or VS can permeate cell membranes in view of their hydrophilic structures. In addition, phosphorylation of ERK suggests that the dipeptide acts primarily at the cell surface. To probe the mechanisms of dipeptides, we tested the effects of PS and VS on regulatory signaling in melanin synthesis. We tested the effects of PS and VS on CREB phosphorylation because CREB is believed to up-regulate MITF expression [25]. At the dosages tested, neither dipeptide affected CREB phosphorylation in Mel-Ab cells (Fig. 4).
In contrast, PS and VS did activate ERK, a classical mitogen-activated protein kinase (MAPK) that may regulate melanogenesis by phosphorylating MITF at serine 73 and thus marking MITF for degradation [26]. In the present study, extended treatment with either PS or VS decreased levels of MITF and tyrosinase proteins. Concerning the mechanism of dipeptides, it is significant that MITF and tyrosinase protein levels were decreased as early as 24 h after treatment with PS or VS, while the decrease in melanin synthesis was measured after treatment with either dipeptide for 4 days. This temporal relationship is consistent with a mechanism that targets an early signal in the melanin biosynthetic pathway. We hypothesize that PS and VS inhibit melanin synthesis by activating ERK and thereby down-regulating MITF and tyrosinase expression.
While kojic acid is one of the most widely accepted tyrosinase inhibitors used as a skin-whitening agent, it is unstable in cosmetic formulations and its safety is not confirmed. Noting that kojic acid-tripeptides and kojic acid-tripeptide amides exert greater tyrosinase-inhibition than free kojic acid [12,27], we suggest that attachment of kojic acid to certain dipeptides may also increase tyrosinase inhibition as compared to either agent alone. Released from these compounds, the dipeptides may further downregulate tyrosinase and thereby enhance hypopigmentary effects.
In conclusion, PS and VS suppressed melanogenesis in Mel-Ab cells. The action of PS and VS occurred through inhibition of MITF and tyrosinase expression subsequent to ERK phosphorylation. These results suggest that dipeptides may prove useful in development of new skin-whitening agents.
ACKNOWLEDGEMENTS
This study was supported by a grant (A100179) from the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea.
ABBREVIATIONS
- CREB
cAMP response element binding protein
- DOPA
3,4-dihydroxyphenylalanine
- ERK
extracellular signal-regulated kinase
- MAPKs
mitogen-activated protein kinases
- MC1R
melanocortin 1 receptor
- MITF
microphthalmia-associated transcription factor
- α-MSH
α-melanocyte stimulating hormone
- PS
proline-serine
- UV
ultraviolet
- VS
valine-serine
References
- 1.del Marmol V, Beermann F. Tyrosinase and related proteins in mammalian pigmentation. FEBS Lett. 1996;381:165–168. doi: 10.1016/0014-5793(96)00109-3. [DOI] [PubMed] [Google Scholar]
- 2.Ando H, Niki Y, Yoshida M, Ito M, Akiyama K, Kim JH, Yoon TJ, Matsui MS, Yarosh DB, Ichihashi M. Involvement of pigment globules containing multiple melanosomes in the transfer of melanosomes from melanocytes to keratinocytes. Cell Logist. 2011;1:12–20. doi: 10.4161/cl.1.1.13638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwahn DJ, Xu W, Herrin AB, Bales ES, Medrano EE. Tyrosine levels regulate the melanogenic response to alpha-melanocyte-stimulating hormone in human melanocytes: implications for pigmentation and proliferation. Pigment Cell Res. 2001;14:32–39. doi: 10.1034/j.1600-0749.2001.140106.x. [DOI] [PubMed] [Google Scholar]
- 4.Goding CR. Mitf from neural crest to melanoma: signal transduction and transcription in the melanocyte lineage. Genes Dev. 2000;14:1712–1728. [PubMed] [Google Scholar]
- 5.Ohguchi K, Akao Y, Nozawa Y. Involvement of calpain in melanogenesis of mouse B16 melanoma cells. Mol Cell Biochem. 2005;275:103–107. doi: 10.1007/s11010-005-1081-0. [DOI] [PubMed] [Google Scholar]
- 6.Hemesath TJ, Price ER, Takemoto C, Badalian T, Fisher DE. MAP kinase links the transcription factor Microphthalmia to c-Kit signalling in melanocytes. Nature. 1998;391:298–301. doi: 10.1038/34681. [DOI] [PubMed] [Google Scholar]
- 7.Buscà R, Ballotti R. Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigment Cell Res. 2000;13:60–69. doi: 10.1034/j.1600-0749.2000.130203.x. [DOI] [PubMed] [Google Scholar]
- 8.Kim SY, Na JI, Park SJ, Choi HR, Kim DS, Park KC. Novel tri-peptides with hypopigmenting activity. J Dermatol Sci. 2012;65:68–69. doi: 10.1016/j.jdermsci.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Bardan A, Nizet V, Gallo RL. Antimicrobial peptides and the skin. Expert Opin Biol Ther. 2004;4:543–549. doi: 10.1517/14712598.4.4.543. [DOI] [PubMed] [Google Scholar]
- 10.Kim H, Lee BJ, Lee MH, Hong SG, Ryu PD. Mechanisms of selective antimicrobial activity of gaegurin 4. Korean J Physiol Pharmacol. 2009;13:39–47. doi: 10.4196/kjpp.2009.13.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang L, Falla TJ. Cosmeceuticals and peptides. Clin Dermatol. 2009;27:485–494. doi: 10.1016/j.clindermatol.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Noh JM, Kwak SY, Kim DH, Lee YS. Kojic acid-tripeptide amide as a new tyrosinase inhibitor. Biopolymers. 2007;88:300–307. doi: 10.1002/bip.20670. [DOI] [PubMed] [Google Scholar]
- 13.Abdel-Malek ZA, Kadekaro AL, Kavanagh RJ, Todorovic A, Koikov LN, McNulty JC, Jackson PJ, Millhauser GL, Schwemberger S, Babcock G, Haskell-Luevano C, Knittel JJ. Melanoma prevention strategy based on using tetrapeptide alpha-MSH analogs that protect human melanocytes from UV-induced DNA damage and cytotoxicity. FASEB J. 2006;20:1561–1563. doi: 10.1096/fj.05-5655fje. [DOI] [PubMed] [Google Scholar]
- 14.Abu Ubeid A, Zhao L, Wang Y, Hantash BM. Short-sequence oligopeptides with inhibitory activity against mushroom and human tyrosinase. J Invest Dermatol. 2009;129:2242–2249. doi: 10.1038/jid.2009.124. [DOI] [PubMed] [Google Scholar]
- 15.Schurink M, Van BW, Wichers HJ, Boeriu CG. Novel peptides with tyrosinase inhibitory activity. Peptides. 2007;28:485–495. doi: 10.1016/j.peptides.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 16.Ishikawa M, Kawase I, Ishii F. Combination of amino acids reduces pigmentation in B16F0 melanoma cells. Biol Pharm Bull. 2007;30:677–681. doi: 10.1248/bpb.30.677. [DOI] [PubMed] [Google Scholar]
- 17.Dooley TP, Gadwood RC, Kilgore K, Thomasco LM. Development of an in vitro primary screen for skin depigmentation and antimelanoma agents. Skin Pharmacol. 1994;7:188–200. doi: 10.1159/000211294. [DOI] [PubMed] [Google Scholar]
- 18.Tsuboi T, Kondoh H, Hiratsuka J, Mishima Y. Enhanced melanogenesis induced by tyrosinase gene-transfer increases boron-uptake and killing effect of boron neutron capture therapy for amelanotic melanoma. Pigment Cell Res. 1998;11:275–282. doi: 10.1111/j.1600-0749.1998.tb00736.x. [DOI] [PubMed] [Google Scholar]
- 19.Kim DS, Park SH, Jeong YM, Kwon SB, Miller AJ, Fisher DE, Park KC. Sphingosine-1-phosphate decreases melanin synthesis via microphthalmia-associated transcription factor phosphorylation through the S1P3 receptor subtype. J Pharm Pharmacol. 2011;63:409–416. doi: 10.1111/j.2042-7158.2010.01223.x. [DOI] [PubMed] [Google Scholar]
- 20.Buscà R, Bertolotto C, Ortonne JP, Ballotti R. Inhibition of the phosphatidylinositol 3-kinase/p70(S6)-kinase pathway induces B16 melanoma cell differentiation. J Biol Chem. 1996;271:31824–31830. doi: 10.1074/jbc.271.50.31824. [DOI] [PubMed] [Google Scholar]
- 21.Kondo T, Hearing VJ. Update on the regulation of mammalian melanocyte function and skin pigmentation. Expert Rev Dermatol. 2011;6:97–108. doi: 10.1586/edm.10.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang YA, Na JI, Choi HR, Choi JW, Kang HY, Park KC. Novel anti-inflammatory peptides as cosmeceutical peptides. Peptides. 2011;32:2134–2136. doi: 10.1016/j.peptides.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 23.Nitta A, Nishioka H, Fukumitsu H, Furukawa Y, Sugiura H, Shen L, Furukawa S. Hydrophobic dipeptide Leu-Ile protects against neuronal death by inducing brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor synthesis. J Neurosci Res. 2004;78:250–258. doi: 10.1002/jnr.20258. [DOI] [PubMed] [Google Scholar]
- 24.Anisimov VN, Khavinson VK, Morozov VG. Immunomodulatory synthetic dipeptide L-Glu-L-Trp slows down aging and inhibits spontaneous carcinogenesis in rats. Biogerontology. 2000;1:55–59. doi: 10.1023/a:1010042008969. [DOI] [PubMed] [Google Scholar]
- 25.Shibahara S, Takeda K, Yasumoto K, Udono T, Watanabe K, Saito H, Takahashi K. Microphthalmia-associated transcription factor (MITF): multiplicity in structure, function, and regulation. J Investig Dermatol Symp Proc. 2001;6:99–104. doi: 10.1046/j.0022-202x.2001.00010.x. [DOI] [PubMed] [Google Scholar]
- 26.Kim DS, Hwang ES, Lee JE, Kim SY, Kwon SB, Park KC. Sphingosine-1-phosphate decreases melanin synthesis via sustained ERK activation and subsequent MITF degradation. J Cell Sci. 2003;116:1699–1706. doi: 10.1242/jcs.00366. [DOI] [PubMed] [Google Scholar]
- 27.Kim H, Choi J, Cho JK, Kim SY, Lee YS. Solid-phase synthesis of kojic acid-tripeptides and their tyrosinase inhibitory activity, storage stability, and toxicity. Bioorg Med Chem Lett. 2004;14:2843–2846. doi: 10.1016/j.bmcl.2004.03.046. [DOI] [PubMed] [Google Scholar]