Abstract
Objective
To report the results of cochlear implant-elicited cortical auditory evoked potentials (eCAEP) in children with cochlear nerve deficiency (CND).
Study Design
Case control series.
Setting
Tertiary academic referral center.
Patients
Seven children with CND that have a cochlear implant in their affected ear. Four children without CND served as controls.
Intervention(s)
eCAEPs were elicited by activation of individual cochlear implant electrodes.
Main Outcome Measure(s)
Onset responses (P1-N1-P2 complex).
Results
Three of 7 CND children demonstrated eCAEP responses across a broad range of electrodes despite having limited or no open set speech perception abilities using their implants. Two of these children had eCAEPs that were characterized by substantial variability in latency, amplitude, and number of electrodes with identifiable responses. The remaining 4 ears with CND and poor speech perception had multiphasic responses that are inconsistent with eCAEPs. Non-CND ears with excellent speech perception abilities demonstrated robust responses on all electrodes stimulated.
Conclusions
Abent eCAEP responses were indicative of poor open-set speech perception skills in all cases. However, eCAEP onset responses were measurable in some children with imaging evidence of CND, indicating probable cochlear nerve hypoplasia rather than aplasia. That some children with CND and poor speech perception had robust eCAEPs in some instances makes this particular measure of limited utility for predicting good speech perception outcomes following cochlear implantation in these children. The origin of multiphasic responses remains to be determined but may be of somatosensory origin in some instances.
Keywords: cochlear implant, cochlear nerve deficiency, hypoplasia, aplasia, cortical potential
INTRODUCTION
Cochlear implants provide sound awareness and speech understanding for hearing-impaired individuals. Improved access to previously inaudible sound enhances spoken language acquisition for pre-linguistic children and restores communication for post-linguistic children and adults (1,2). The benefits of this intervention are far-reaching. Cochlear implants enhance educational and employment opportunities and achievement, socialization with alleviation of isolation and depression, and overall quality of life (3,4).
The only absolute contraindication to cochlear implantation is an absent cochlea or cochlear nerve. In such instances, the multiple electrode array lacks the necessary neural interface for discrete auditory stimulation. While the diagnosis of cochlear aplasia (i.e. Michel) is readily apparent on imaging studies, cochlear nerve aplasia or hypoplasia is decidedly more challenging to diagnose, owing to the limits of spatial resolution in currently available modalities. Because of this uncertainty in diagnosis, the term cochlear nerve deficiency (CND) was coined to refer to the situation where the cochlear nerve or its osseous conduit is not visible on imaging (5-7). The clinical diagnosis of CND includes these imaging findings in the setting of profound hearing loss. Auditory brainstem response testing that shows a present cochlear microphonic but absent neural waves can support the diagnosis (8).
An absent internal auditory canal (IAC) on either high-resolution computed tomography (CT) or magnetic resonance imaging (MRI) is clear evidence for cochlear nerve aplasia. When the IAC is narrow (<3 mm), an absent cochlear nerve aperture or bony cochlear nerve canal (BCNC) also provides good evidence for CND. In the setting of a normal dimension IAC, MRI can usually resolve a normal cochlear nerve; however, a very small or absent nerve might have a similar appearance (5-7).
Because of this diagnostic uncertainty in cochlear nerve imaging, cochlear implantation has been undertaken in a number of children with CND (9-12). Results of these interventions suggest that most children with CND gain some sound awareness from their device using higher levels of electrical stimulation. However, achievement of open set speech perception for the purposes of speech and language development has been largely unsuccessful, probably as a result of a poor electrode-neural interface and other associated conditions. In one study, only 19% of children with CND developed any open set speech perception using their cochlear implant, and none of these children were able to communicate using auditory-only information (i.e. all used some supplemental sign language) (9).
Bilateral CND in children is often times only one manifestation of a larger syndromic etiology such as CHARGE, VATER, or congenital hydrocephalus, among others. In such instances, the lack of developmental progress with a cochlear implant might as easily be attributable to associated learning disabilities rather than the anatomic cochlear nerve issue. In an effort to distinguish auditory from non-auditory activation in implanted children with CND, previous studies have used electrically evoked compound action potential (ECAP) and brainstem response (EABR) testing (9,10,13,14). The results of these studies demonstrate limited or no ECAPs in CND children. In rare instances, EABR testing demonstrates normal wave Vs but more commonly it reveals abnormal peaks with atypical latencies and morphologies or no reliable waves are evident on such testing (10, 13, 14).
The cortical auditory evoked potential (CAEP) is a cortically generated potential that provides information about the integrity of the auditory system and the neural processing of sound beyond the auditory brainstem. It can be recorded using a passive listening paradigm using an acoustic or electric stimulus. In the latter case it is referred to as the electrically evoked CAEP (eCAEP). In normal-hearing adults, the CAEP consists of a series of peaks (P1, N1, P2) occurring within a time window of 50-250 ms after stimulus onset. Responses from normal-hearing children less than 5 years of age show only a large, broad P1 (around 100 ms) followed by the N2 component (15,16). The emergence of the P1-N1-P2 complex occurs by 9-12 years of age (17). In cochlear implant children, the eCAEP can be reliably recorded in response to sound field stimuli transduced by the implant's speech processor or by electrical pulse trains delivered directly to specific implant electrodes (18-21). In early-implanted children who have extensive listening experience with their devices, eCAEPs are of a similar morphology to those of age-matched peers with normal hearing. Children implanted at older ages may not show age-dependent changes in the eCAEP (18).
There are sparse data on eCAEPs in children with well-defined CND. Such data are important for at least two reasons. First, information concerning auditory evoked cortical activity might be of significance to decisions concerning recommended communication strategies for these children and their families. Second, there is a growing interest in the possibility of auditory brainstem implantation as an intervention option in CND children who do not benefit from cochlear implantation. Knowledge of cochlear-implant evoked cortical activity may contribute to an informative index of benefit. The purpose of this study therefore is to measure eCAEPs in a group of children with well-defined CND and who have significant device experience such that their outcome performance is known and stable.
MATERIALS AND METHODS
Subjects
The biomedical institutional review board (IRB) approved this study. The procedures followed were in accordance with the ethical standards of the IRB and with the Helsinki Declaration. All subjects signed an approved informed consent and were paid for their participation.
Seven implanted CND children (3 to 15 yrs) and 3 implanted non-CND children (5 to 12 yrs) participated in the study. Demographic information, inner ear malformation type, age at implantation, duration of use, electrode array and presence/absence of ECAPs for these patients are listed in Table 1. The diagnosis of CND was made preoperatively in all but one child (CND6) according to previously reported diagnostic imaging protocols (5-8). The details regarding cochlear implant outcomes and the utility of ECAPs in these children were reported as a part of a larger study previously (9). CND6 had unilateral CND (right) and bilateral cochlear implants. Sensorineural hearing loss (SNHL) children with normal cochlear nerves who were roughly age-matched to the CND children with speech perception abilities served as controls. All patients were using Cochlear Corporation devices (Englewood, CO) in their test ear. Data from both ears (left non-CND, right CND) of CND6 are reported in this study.
Table 1.
General Characteristics.
| Subject | Gender | Test Ear | Inner Ear Anatomy | Age at test (yr) | Age at implant (yr) | Device | ECAP response |
|---|---|---|---|---|---|---|---|
| CND1 | F | L | Hypoplastic type 1 | 6.6 | 2.3 | 24RE (Straight) | Present |
| CND2 | M | L | Hypoplastic type 3 | 11.8 | 3.1 | 24R (CS) | Absent |
| CND3 | M | L | Hypoplastic type 2 | 7.9 | 4.7 | 24RE (Straight) | Present |
| CND4* | M | R | Hypoplastic type 3 | 14.9 | 6.1 | 24R (CS) | Absent |
| CND5 | F | R | Common Cavity | 15.1 | 9.9 | 24RE (Straight) | Absent |
| CND6 | M | L** | Normal | 12.1 | 5.8 | 24RE (CA) | Present |
| R | Normal | 12.1 | 4.0 | 24R (CS) | Absent | ||
| CND7 | M | L | Hypoplastic type 1 | 3.0 | 1.7 | 24RE (Straight) | Absent |
| SNHL1 | F | R | Normal | 5.8 | 2.0 | 24RE (CA) | Present |
| SNHL2 | M | R | Normal | 11.9 | 6.3 | 24RE (CA) | Present |
| SNHL3 | F | R | Normal | 8.5 | 1.5 | 24R (CA) | Present |
CHARGE syndrome
normal auditory nerve. ECAP: electrically evoked compound action potential
General Procedure
The experimental protocol included aided (i.e. cochlear implant) pure-tone thresholds and aided speech perception measures (Table 2). All of these tests were carried out on the same day.
Table 2.
Audiological Information.
| Aided Testing# | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Test Ear | Pure tone thresholds (dB HL) |
SAT | Speech perception scores |
|||||||
| 250 Hz | 500 Hz | 1000 Hz | 2000 Hz | 3000 Hz | 4000 Hz | 6000 Hz | PBK word | PBK phoneme | |||
| CND1 | L | 40 | 35 | 40 | 40 | 40 | 40 | 25 | 36% | 72% | |
| CND2 | L | 30 | 30 | 25 | 30 | 30 | 35 | 30 | 10 | 30% | 70% |
| CND3 | L | 30 | 30 | 30 | 35 | 35 | 35 | 35 | 10 | 16% | 45% |
| CND4 | R | 25 | 25 | 35 | 35 | 35 | 35 | 30 | 25 | 0% | 0% |
| CND5 | R | 25 | 25 | 30 | 20 | 30 | 30 | 30 | 10 | 0% | 0% |
| CND6 | L* | 20 | 20 | 35 | 20 | 30 | 25 | 20 | 10 | 96% | 98% |
| R | 40 | 45 | 35 | 30 | 30 | 30 | 30 | 25 | 0% | 0% | |
| CND7 | L | 50 | 55 | 45 | 45 | 45 | 45 | 0% | 0% | ||
| SNHL1 | R | 30 | 30 | 15 | 20 | 25 | 20 | 15 | 10 | 88% | 95% |
| SNHL2 | R | 25 | 25 | 15 | 30 | 25 | 20 | 20 | 10 | 92% | 96% |
| SNHL3 | R | 20 | 20 | 20 | 15 | 20 | 10 | 15 | 10 | 90% | 96% |
Using cochlear implant
normal auditory nerve
PBK: Phonetically Balanced Kindergarten
Behavioral Audiometry and Speech Perception Testing
During programming sessions, observation and conditioned behavioral audiometry techniques were used to determine electrical thresholds and comfortable listening levels. When stimulation levels resulted in visible or painful facial contractions, higher levels were generally not attempted. After device fitting, speech perception assessment was attempted with children at 6-month intervals when possible. Standard tests included a hierarchical battery of measures as previously described (9). For this study, the results of the open-set, Phonetically Balanced Kindergarten word lists (PBK words) are shown. Tests were administered in a sound controlled environment using live-voice or recorded stimuli presented at 60 dB SPL. Due to age and attention restrictions, it was generally necessary to use live voice presentations for younger children to complete the tests.
Electrophysiological Tests
Stimuli
The stimuli to elicit the eCAEP were created using custom-designed software incorporating Nucleus Implant Communicator (NIC) programming routines. Electrical stimulation was delivered to individual electrodes by way of the NIC interface. The software also generated a trigger pulse coincident with the onset of the stimulus pulse train that was used to synchronize the stimulating and recording systems.
The stimulus was a 100-ms train of biphasic pulses with an inter-stimulation interval of 900 ms delivered in the monopolar mode (MP1). The stimulating electrodes, pulse widths, inter pulse gaps and rates were selected based on programming MAPs of individual patients. These stimulating parameters used for each patient are listed in Table 3. Stimulation levels were set at the maximum comfortable level measured for individual electrodes for each patient, and held constant throughout the entire stimulation period.
Table 3.
Stimulation parameters.
| Subject | Test Ear | Stimulating electrode | Processing rate (Hz) | Pulse width (μV) | Inter pulse gap (μV) |
|---|---|---|---|---|---|
| CND1 | L | 10,11, 12,13,16,19,22 | 500 | 62 | 8 |
| CND2 | L | 5, 9, 13, 16, 19, 22 | 900 | 75 | 8 |
| CND3 | L | 11,13,16,19,22 | 900 | 37 | 8 |
| CND4 | R | 3,8,12,16,20 | 250 | 150 | 8 |
| CND5 | R | 9,12,16,19,22 | 1200 | 50 | 8 |
| CND6 | L* | 11,17,22 | 2400 | 12 | 6 |
| R | 6,11 | 900 | 50 | 8 | |
| CND7 | L | 15,19 | 250 | 150 | 8 |
| SNHL1 | R | 4,8,12,16,20 | 900 | 25 | 8 |
| SNHL2 | R | 4,8,12,16,20 | 900 | 25 | 8 |
| SNHL3 | R | 4,8,12,16,20 | 900 | 25 | 8 |
normal auditory nerve
Recording Procedure
Patients were tested in a single-walled sound booth. CND7 was tested while seated on his parent's lap due to his young age. All others were seated in a reclining chair and watched a silent movie with captions or played computer games on an iPad 2® (Apple Corp, Cupertino, CA). They were instructed to ignore the stimulus and relax but not fall asleep. Breaks were provided as needed. Each recording session took approximately 2 hours.
Electroencephalographic (EEG) activity was recorded using an Intelligent Hearing System SmartEP (Miami, FL) for CND6 and a Compumedics Neuroscan® (Charlotte, NC) for all other patients. Disposable, sterile Ag-AgCl surface electrodes were used to record the EEG. Responses were differentially recorded between electrodes positioned at Fz (positive) and contralateral mastoid (reference). A ground electrode was placed on the low forehead (Fpz). Eye-blink activity was monitored using two recording electrodes placed superior and inferior to the eye contralateral to the implant ear. Ocular artifacts exceeding ±100 μV were rejected from averaging. Electrode impedances were maintained below 5000 Ohms with an inter-electrode impedance difference of less than 2000 Ohms. The recording window consisted of a 100-ms pre-stimulus baseline and a 412-ms peri/post-stimulus time for CND6. For all other patients, the recording window included a 100-ms pre-stimulus baseline and a 600-ms peri/post-stimulus time. For CND6, responses were amplified with a gain of 10k, analog filtered online between (1-100 Hz), and sampled at a rate of 1000 Hz. For all other patients, responses were amplified (10× gain), analog filtered online between 0.1 and 100 Hz (24 dB/octave roll-off) prior to averaging, and sampled at a rate of 1000 Hz. After eye-blink rejection, the remaining (at least 200) sweeps per stimulating electrode were averaged and digitally filtered between 1-30 Hz (24 dB/octave) offline using custom-designed MATLAB software. Averaged responses were smoothed using a 40-msec wide boxcar filter before response identification and amplitude measurements.
Data Analysis
Electrophysiological responses were independently assessed by two experienced observers. Visual identification of the eCAEPs was based on peak latency and waveform morphology. The presence/absence of each response was determined on the basis of mutual agreement between these two researchers. For each patient, peak amplitude and latency were measured for each identifiable component of the eCAEP that was recorded from different electrodes. Peak latency was measured at the most positive or negative point and peak amplitude was measured from baseline to peak.
RESULTS
Table 1 details patient demographics, inner ear morphology, electrode array, and ECAP status for the implanted ear. Five CND children had inner ear malformations characterized as hypoplastic spectrum, one had a common cavity, and one had normal morphology. One child (CND6) had a CND ear (implanted first) and a non-CND ear (implanted second) and thus is represented in both CND and non-CND data sets. Mean (SD) age at implantation, duration of device use, and age at testing was 4.5 (2.8), 5.7 (2.9), and 10.2 (4.5) yrs. in the CND ears and 3.9 (2.5), 5.7 (1.4), and 9.6 (3.0) yrs. in the non-CND ears. Only 2 (28.6%) of 7 ears with CND had present ECAPs while all 4 ears without CND had present responses.
Pure tone thresholds, speech awareness thresholds (SAT), PBK word and phoneme scores from all subjects are listed in Table 2. All patients had sound detection across the various frequencies. CND patients tended to have higher pure tone thresholds than non-CND patients. However, mean (SD) SAT was 21.4 dB (12.8) for the CND ears and 12.5 dB (5.0) for the non-CND ears. Three CND ears (CND1, CND2, CND3) had limited open set speech perception (mean PBK word 27%, range 16-36%; mean PBK phoneme 62%, range 45-72%) while the remaining 4 CND ears had 0% PBK word and phoneme scores. By contrast, the mean PBK word and phoneme scores for the 4 non-CND ears were 91.5% (3.4) and 96.3% (1.3), respectively.
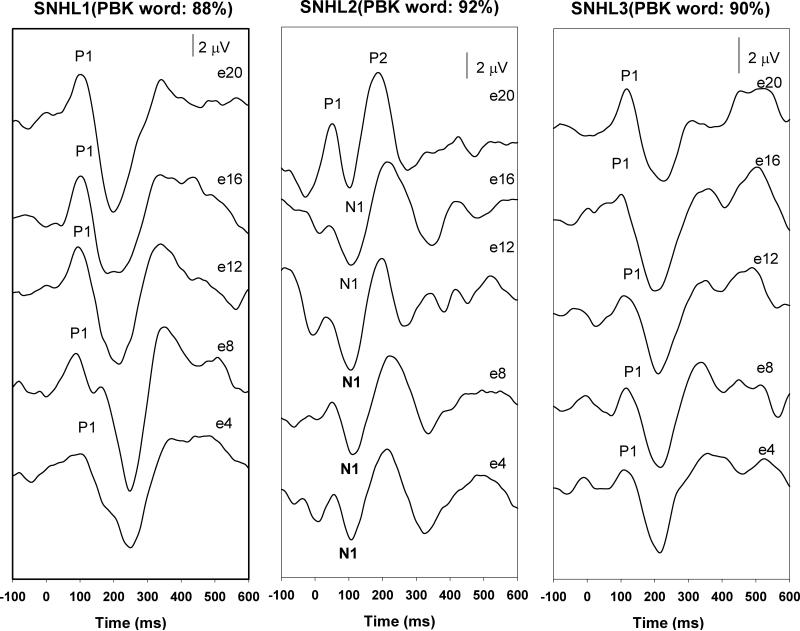
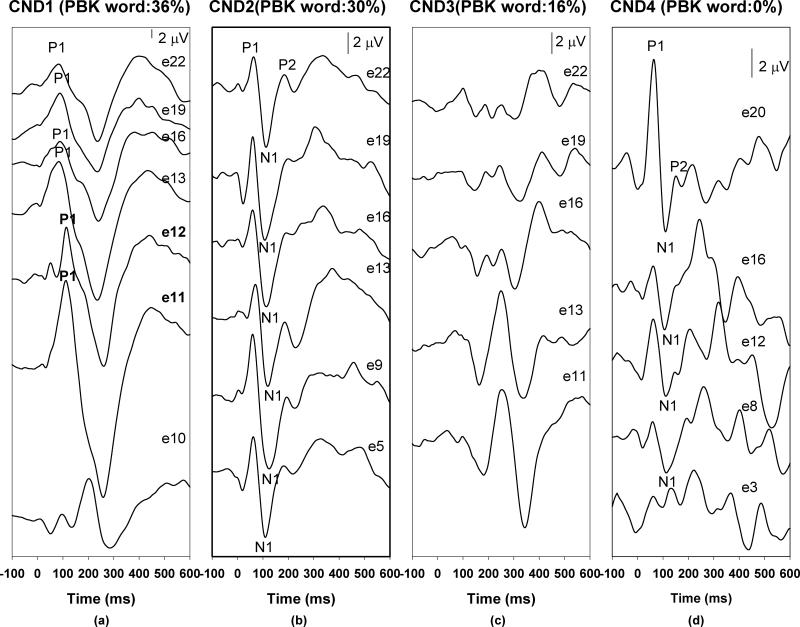
Figure 1 shows the eCAEPs recorded from non-CND ears for reference with the respective speech perception measures. In general, all electrodes tested had robust eCAEP responses within the 100-250 ms epoch, with age-appropriate waveform morphologies. Within this group, the oldest child (SNHL2) demonstrated a very well formed P1-N1-P2 complex. Responses recorded from two other children are dominated by P1 components. Figures 2 and 3 show the responses and PBK word results for the CND ears with and without identifiable responses, respectively. While the responses of CND2 look very similar to those recorded from the non-CND ears, the onset responses for CND1 and CND4 are characterized by substantial variability in latency and amplitude across the electrodes tested. For instance, responses recorded for e11, e12, e13, e16, e19 and e22 from CND1 are dominated by a P1 component followed by an N2 peak similar to those recorded from SNHL1, a child with a comparable age of implantation and duration of use. However, the most basal electrode tested (e10) in CND1 shows a multi-peaked morphology with none of the peaks matching expected eCAEP latencies. CND4 also showed robust onset responses on many electrodes with a similar multi-peaked response on the basal electrode e3. Peak amplitudes and latencies of the eCAEP measured for these children are listed in Table 4.
Figure 1.
The eCAEPs recorded from non-CND ears
Figure 2.
Neural responses recorded from four CND ears.
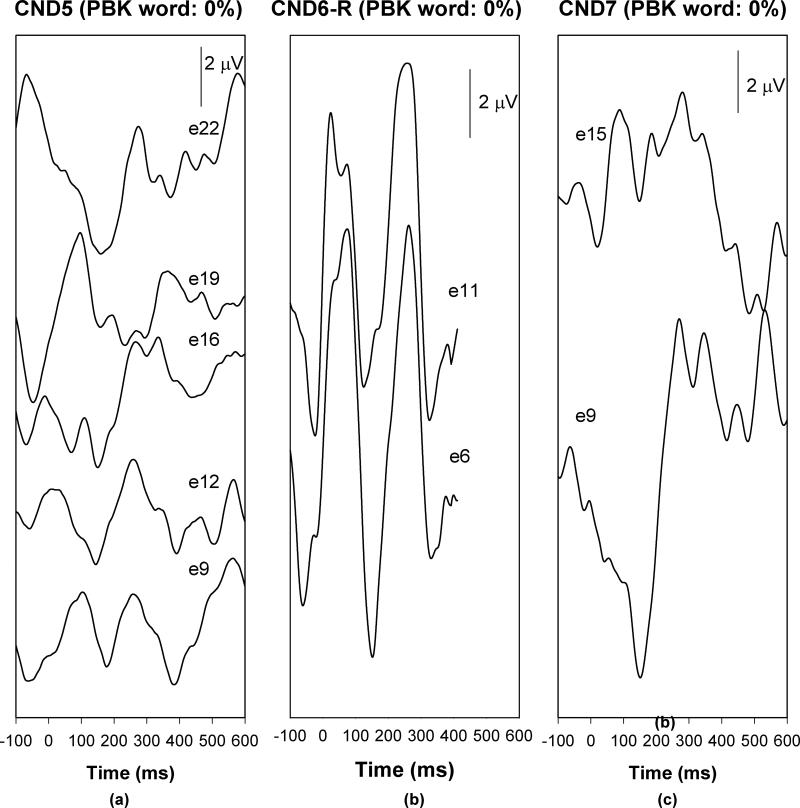
Figure 3.
Traces without any identifiable neural responses recorded from three CND ears.
Table 4.
Mean amplitudes and latencies (standard deviation) of the eCAEP.
| Subject | Amplitude (μV ± SD) | Latency (ms ± SD) | ||||
|---|---|---|---|---|---|---|
| P1 | N1 | P2 | P1 | N1 | P2 | |
| CND1 | 7.80 (2.34) | 91 (8.58) | ||||
| CND2 | 3.56 (1.20) | -6.21 (0.78) | 0.54 (0.90) | 63.67 (5.85) | 115.83 (6.91) | 189.33 (6.19) |
| CND4 | 3.70 (2.77) | -2.53 (1.15) | 63.25 (2.22) | 110 (1.41) | ||
| SNHL1 | 3.87 (1.12) | 99.6 (6.15) | ||||
| SNHL2 | 0.04 (1.20) | -4.00 (1.07) | 4.36 (1.05) | 49.4 (7.89) | 113.8 (4.38) | 209.4 (14.53) |
| SNHL3 | 1.40 (0.75) | 99.6 (6.15) | ||||
SD: Standard deviation
eCAEP: electrically evoked cortical auditory evoked potential
For CND3, responses recorded from all electrodes exhibited multiphasic morphologies, which are inconsistent with a typical eCAEP response. Specifically, responses recorded from stimulation of e11 and e13 consisted of two positive peaks and two negative peaks. The first positive peak occurred around 100 ms and the second positive peak had a latency of 250 ms. The latency for the first and the second negative peak was 200 and 350 ms, respectively. Consistent morphology was observed for responses recorded for e16, e19 and e22. However, these responses did not exhibit any waveform peak latencies or morphology that would be expected for a typical eCAEP.
Figure 3 shows the recorded responses from 3 CND ears (CND5, CND6, CND7) without any measurable speech perception. These responses do not show morphological characteristics of typical eCAEPs. No replicable peaks can be identified in any of these recording traces.
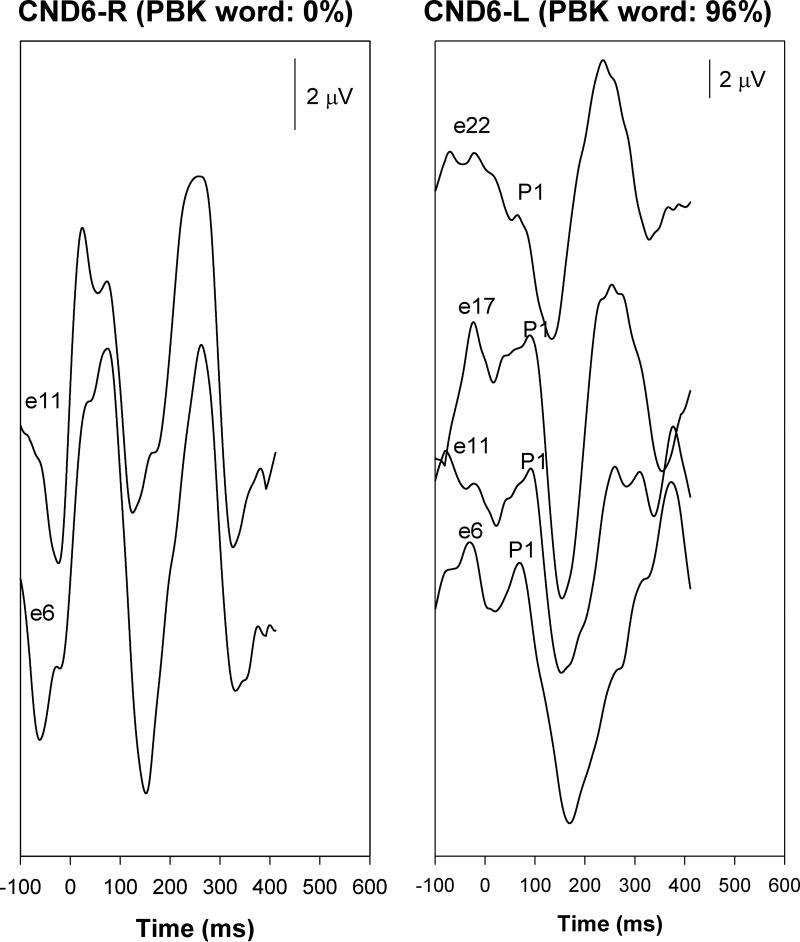
Figure 4 shows responses recorded from CND6, a child with unilateral CND (right ear) and bilateral implants. Recordings from the left ear demonstrate the expected P1-N1 morphology similar to that seen in other non-CND, good performing ears. By contrast, responses elicited from stimulation of the poor performing CND ear (right) in panel (b) appear multiphasic with symmetric, positive-negative characteristics. These responses also start concurrently with the stimulus onset. Importantly, this child claimed to “feel” stimulation in the CND (right) ear and “hear” the stimulation in the left ear. The child experienced no dizziness or facial twitching during stimulation.
Figure 4.
Responses recorded from CND6.
DISCUSSION
Synchronous and discrete activation of the auditory system through electrical stimulation is, in part, a necessary precondition for effective cochlear implant use. Such activation requires well-positioned intracochlear electrodes and sufficient neural substrate. In children with congenital hearing loss, neural integrity is determined prior to implantation through assessments of residual hearing (auditory brainstem response and behavioral testing) and imaging (MRI and/or CT). Following implantation, neural integrity is assumed when behavioral responses to electrode activation occur at low charge levels, robust implant-evoked electrophysiological responses are evident on ECAP testing and speech perception and language develop at expected rates. When performance is less than expected, there are limited tools available for truly objective assessment.
CND on high-resolution imaging is a poor prognostic sign for the development of open set speech perception using a cochlear implant (9-11). Most children with CND can however, show some degree of sound awareness with their implants using higher than normal charge levels for stimulation. These responses, in theory, can be related to auditory stimulation of residual cochlear nerve fibers that are not resolvable on imaging or to non-auditory stimulation (vestibular, somatosensory, or motor). The present study is a preliminary work to compare eCAEPs in implanted children with and without evidence of CND in an effort to better understand auditory system activation in CND children.
Implanted children without CND exhibited robust eCAEP onset responses (P1-N1-P2) across a wide range of stimulated electrodes (basal→apical). CND children demonstrated either good (CND2), absent (CND5-7) or mixed (CND1, CND4) onset responses to varying degrees and locations of electrode stimulation. Three children (CND1 CND2, CND4) that demonstrated good morphology onset response complexes across a broad range of electrodes had limited or no open set speech perception using their implants. These results imply that cochlear nerve aplasia is highly unlikely in this group of children. That CND1 had robust response ECAPs and some open set perception suggests that synchronizable neural responses were inducible from the distal cochlear nerve and the auditory cortex. For this child, the diagnosis of CND might be questionable except that the child's performance is far less than expected despite having good therapy and cognitive abilities. For CND1, CND2, CND4, varying degrees of cochlear nerve hypoplasia might explain their relative poor performance even with present eCAEPs. Considering the fact that CND1 and CND4 had variable onset responses on some electrodes but not on others might support the premise that regional differences in neural populations exist. This finding might be consistent with a hypoplastic nerve.
Results of this study demonstrate that children with CND and absent eCAEPs fail to achieve open-set speech perception skills with their implant. However, children with robust eCAEP onset responses are also not good performers. Thus, while an absent eCAEP is a clear sign for poor performance, present or even robust responses are less predictive. These finding are noteworthy and in contrast to previous investigations that used sound field activation of the speech processor to evoke cortical responses. These differences might be related to differences in the stimulation paradigm. One advantage of the set up used in the present study is that individual electrodes can be stimulated, allowing regional differences in intracochlear neural populations to be explored. Future studies, using a greater number of stimulation sites might be needed to better decipher the relevance of these regional stimulation differences.
Results of this study also suggest that electrically evoked compound action potentials (ECAPs) do not provide predictive information about electrically evoked neural activation in these CND children. For example, the ECAP could not be recorded from CND 2 and CND4. However, robust eCAEP responses were recorded from some, if not all, electrodes in these two patients. In contrast, for CND3, robust ECAP responses with normal morphologies, amplitudes, and latencies were recorded from the same set of electrodes that were used for the eCAEP recordings. Amplitude growth functions of the ECAP recorded from electrode 19 and 22 showed characteristics that were comparable to those described for SNHL children (22). However, cortical responses recorded from this patient failed to show characteristics of a typical eCAEP response. These findings in CND3 could be caused by concurrent lesions in the central auditory system. Nevertheless, the possibility that the neural generators of the “ECAP” recorded from this patient are located in vestibular system instead of auditory system could not be excluded (23).
The source of multiphasic responses observed in 4 CND ears remains to be determined. Options might include vestibular, somatosensory, or facial myogenic responses. That subject CND6 described a non-auditory “feeling” during electrode activation that did not induce dizziness, rotation, visual disturbance, facial contraction or twitching suggests a somatosensory origin.
Overall, our results clearly indicate that the eCAEP onset response cannot be used as the sole criteria for predicting good cochlear implant performance in CND children. The ability to understand speech depends on how well the auditory system can detect incoming signals (auditory detection) and discriminate differences among varying signals (auditory discrimination). The CAEP evoked by a brief acoustic/electrical stimulus only provides information about the neural encoding of the physical characteristics of sound that allows for auditory detection. It does not appear to provide information about the necessary neural processing that underlies auditory discrimination (24). Future work should focus on identifying electrophysiologic predictors of auditory discrimination as these measures will help families of children with CND (and other poor prognostic factors) make communication choices such as the need for supplementary sign language or whether to consider auditory brainstem implantation in lieu of their cochlear implant.
ACKNOWLEDGMENT
Supported, in part, by grants (to C.A.B.) from the Deafness Research Foundation (DRF) and the National Institutes of Health (DC011383). The authors would like to thank Holly Teagle, Pat Roush, Corrine Macpherson, Sarah Martinho, Nicelle Franco, Deb Hatch, Lisa Park, and Jennifer Woodard for the expert care of these children and their logistical assistance in carrying out these studies. We also wish to thank Carolyn Brown and Paul Abbas for generously providing the stimulus software and Harold C. Pillsbury for his unwavering support of this work.
Supported, in part, by grants (to C.A.B.) from the Deafness Research Foundation (DRF) and the National Institutes of Health (R21DC011383-01A1).
Footnotes
Presented at the Annual Meeting of the American Neurotology Society, Manchester Grand Hyatt, San Diego, CA, April 21-22, 2012.
All procedures were in accordance with the ethical standards of the University of North Carolina at Chapel Hill IRB and with the Helsinki Declaration. Informed consent was obtained for all subjects enrolled in the study. Protocol number 09-1202.
Financial disclosures: Dr. Buchman serves as a surgical advisory board member for Advanced Bionics, Anspach, Cochlear, and MedEL corporations.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bassim MK, Buss E, Clark MS, et al. MED-EL Combi40+ cochlear implantation in adults. Laryngoscope. 2005;115:1568–73. doi: 10.1097/01.mlg.0000171023.72680.95. [DOI] [PubMed] [Google Scholar]
- 2.Niparko JK, Tobey EA, Thal DJ, et al. Spoken language development in children following cochlear implantation. JAMA. 2010;21303:1498–506. doi: 10.1001/jama.2010.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barton GR, Stacey PC, Fortnum HM, et al. Hearing-impaired children in the United Kingdom, IV: cost-effectiveness of pediatric cochlear implantation. Ear Hear. 2006;27:575–88. doi: 10.1097/01.aud.0000233967.11072.24. [DOI] [PubMed] [Google Scholar]
- 4.Francis HW, Chee N, Yeagle J, et al. Impact of cochlear implants on the functional health status of older adults. Laryngoscope. 2002;112:1482–8. doi: 10.1097/00005537-200208000-00028. [DOI] [PubMed] [Google Scholar]
- 5.Glastonbury CM, Davidson HC, Harnsberger HR, et al. Imaging findings of cochlear nerve deficiency. AJNR Am J Neuroradiol. 2002;23:635–43. [PMC free article] [PubMed] [Google Scholar]
- 6.Adunka OF, Roush PA, Teagle HF, et al. Internal auditory canal morphology in children with cochlear nerve deficiency. Otol Neurotol. 2006;27:793–801. doi: 10.1097/01.mao.0000227895.34915.94. [DOI] [PubMed] [Google Scholar]
- 7.Adunka OF, Jewells V, Buchman CA. Value of computed tomography in the evaluation of children with cochlear nerve deficiency. Otol Neurotol. 2007;28:597–604. doi: 10.1097/01.mao.0000281804.36574.72. [DOI] [PubMed] [Google Scholar]
- 8.Buchman CA, Roush PA, Teagle HF, et al. Auditory neuropathy characteristics in children with cochlear nerve deficiency. Ear Hear. 2006;27:399–408. doi: 10.1097/01.aud.0000224100.30525.ab. [DOI] [PubMed] [Google Scholar]
- 9.Buchman CA, Teagle HF, Roush PA, et al. Cochlear implantation in children with labyrinthine anomalies and cochlear nerve deficiency: implications for auditory brainstem implantation. Laryngoscope. 2011;121:1979–88. doi: 10.1002/lary.22032. [DOI] [PubMed] [Google Scholar]
- 10.Valero J, Blaser S, Papsin BC, et al. Electrophysiologic and behavioral outcomes of cochlear implantation in children with auditory nerve hypoplasia. Ear Hear. 2012;33:3–18. doi: 10.1097/AUD.0b013e3182263460. [DOI] [PubMed] [Google Scholar]
- 11.Kutz JW, Jr, Lee KH, Isaacson B, et al. Cochlear implantation in children with cochlear nerve absence or deficiency. Otol Neurotol. 2011;32:956–61. doi: 10.1097/MAO.0b013e31821f473b. [DOI] [PubMed] [Google Scholar]
- 12.Warren FM, 3rd, Wiggins RH, 3rd, Pitt C, et al. Apparent cochlear nerve aplasia: to implant or not to implant? Otol Neurotol. 2010;31:1088–94. doi: 10.1097/MAO.0b013e3181eb3272. [DOI] [PubMed] [Google Scholar]
- 13.Walton J, Gibson WP, Sanli H, et al. Predicting cochlear implant outcomes in children with auditory neuropathy. Otol Neurotol. 2008;29:302–9. doi: 10.1097/MAO.0b013e318164d0f6. [DOI] [PubMed] [Google Scholar]
- 14.Song MH, Bae MR, Kim HN, et al. Value to intracochlear electrically evoked auditory brainstem response after cochlear implantation in patients with narrow internal auditory canal. Laryngoscope. 2010;120:1625–31. doi: 10.1002/lary.21008. [DOI] [PubMed] [Google Scholar]
- 15.Ponton CW, Eggermont JJ, Kwong B, et al. Maturation of human central auditory system activity: evidence from multi-channel evoked potentials. Clin Neurophysiol. 2000;111:220–36. doi: 10.1016/s1388-2457(99)00236-9. [DOI] [PubMed] [Google Scholar]
- 16.Wunderlich JL, Cone-Wesson BK, Shepherd R. Maturation of the cortical auditory evoked potential in infants and young children. Hear Res. 2006;212:185–202. doi: 10.1016/j.heares.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Ponton CW, Eggermont JJ, Khosla D, et al. Maturation of human central auditory system activity: separating auditory evoked potentials by dipole modeling. Clin Neurophysiol. 2002;113:407–20. doi: 10.1016/s1388-2457(01)00733-7. [DOI] [PubMed] [Google Scholar]
- 18.Sharma A, Dorman MF, Spahr AJ. A sensitive period for the development of the central auditory system in children with cochlear implant: implications for age of implantation. Ear Hear. 2002;23:532–9. doi: 10.1097/00003446-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Ponton CW, Eggermont JJ. Of kittens and kids: altered cortical maturation following profound deafness and cochlear implant use. Audiol Neurootol. 2001;6:363–80. doi: 10.1159/000046846. [DOI] [PubMed] [Google Scholar]
- 20.Gordon KA, Tanaka S, Wong DDE, Papsin BC. Characterizing responses from auditory cortex in young people with several years of cochlear implant experience. Clin Neurophysiol. 2008;119:2347–62. doi: 10.1016/j.clinph.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 21.Gordon KA, Tanaka S, Wong DDE, Stockley T, Ramsden JD, Brown T, Jewell S, Papsin BC. Multiple effects of childhood deafness on cortical activity in children receiving bilateral cochlear implants simultaneously. Clin Neurophysiol. 2011;122:823–33. doi: 10.1016/j.clinph.2010.10.037. [DOI] [PubMed] [Google Scholar]
- 22.Brown CJ, Abbas PJ, Etler CP, O'Brient S, Oleson JJ. Effects of long-term use of a cochlear implant on the electrically evoked compound action potential. J Am Acad Audiol. 2010;21:5–15. doi: 10.3766/jaaa.21.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nie K, Bierer SM, Ling L, Oxford T, Rubinstein JT, Phillips JO. Characterization of the electrically evoked compound action potential of the vestibular nerve. Otol Neurotol. 2011;32:88–97. doi: 10.1097/mao.0b013e3181f6ca45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin BA, Tremblay KL, Korczak P. Speech evoked potentials: from the laboratory to the clinic. Ear Hear. 2008;29:285–313. doi: 10.1097/AUD.0b013e3181662c0e. [DOI] [PubMed] [Google Scholar]