Summary
Background
We previously found plasma levels of CD40 ligand (CD40L), chemokine (C-X-C motif) ligand 5 (CXCL5), chemokine (C-C motif) ligand 5 (CCL5), and epidermal growth factor (EGF) to be low in aplastic anemia (AA) patients and to be correlated with the platelet count.
Objectives
To study the association of CD40L, CXCL5, CCL5, and EGF with platelets.
Patients/Methods
We measured cytokines in the plasma of immune thrombocytopenic purpura (ITP) and AA patients using the Luminex assay and confirmed the results in a mouse model and in vitro experiments.
Results
Both ITP and AA showed similarly low levels of CD40L, CXCL5, CCL5, and EGF, compared with healthy controls. In ITP, levels of these proteins were significantly greater in patients with higher platelet counts than in those with lower platelet counts. In a murine thrombocytopenia model, levels of CD40L, CXCL5, CCL5, and EGF decreased with platelet count after immune-mediated destruction, while the cytokine levels increased when the platelet count recovered. In vitro, concentrations of these cytokines in the supernatants of platelet suspensions were proportional to platelet numbers, and levels in sera prepared by simple blood coagulation were equivalent to those in platelet-rich plasma-converted sera. mRNA expression for CXCL5, CCL5, and EGF was higher in platelets than in megakaryocytes, peripheral blood mononuclear cells, granulocytes, and non-megakaryocytic bone marrow cells.
Conclusions
Plasma CD40L, CXCL5, CCL5, and EGF are mainly platelet-derived, suggesting a role of platelets in immune responses and inflammation. Measurement of CD40L, CXCL5, CCL5, and EGF in human blood allowed testable inferences concerning physiology and pathophysiology in quantitative platelet disorders.
Keywords: cytokine, immune thrombocytopenic purpura, aplastic anemia
Introduction
Thrombocytopenia is a defining laboratory feature of both aplastic anemia (AA) and immune thrombocytopenic purpura (ITP). In AA, thrombocytopenia results from decreased or absent megakaryocyte numbers, while in ITP thrombocytopenia is caused by peripheral destruction of platelets and concomitant decrease in platelet production, due to anti-platelet antibodies cross reactive with megakaryocytes. In contrast to AA, in which the bone marrow is hypocellular, in ITP the marrow is normocellular and the number of megakaryocytes is usually normal or increased.
We recently reported a comprehensive analysis of circulating cytokines in AA and myelodysplasia (MDS) patients. We found that high levels of thrombopoietin (Tpo) and granulocyte colony-stimulating factor (G-CSF), and low levels of CD40 ligand (CD40L), chemokine (C-X-C motif) ligand 5 (CXCL5), epidermal growth factor (EGF), chemokine (C-C motif) ligand 5 (CCL5), vascular endothelial growth factor (VEGF), and CCL11, were a cytokine signature profile of AA. In these patients, levels of CD40L, CXCL5, EGF, and CCL5 in the plasma correlated with the patients’ platelet counts 1. CD40L (also known as CD154) is a 39 kDa transmembrane glycoprotein and a member of the tumor necrosis factor α (TNF-α) family, and it is mainly expressed on the surface of activated CD4+ T cells. Plasma CD40L is a truncated form (18 kDa) of the CD40L protein, named soluble CD40L (sCD40L).2 sCD40L is a platelet agonist and can promote coagulation.3, 4 CXCL5 was reported to be secreted by peripheral blood monocytes and involved in neutrophil activation. EGF stimulates the growth of various epidermal and epithelial tissues in vivo and in vitro and of some fibroblasts in cell culture. CCL5, a chemoattractant for blood monocytes, memory T-helper cells, and eosinophils, causes the release of histamine from basophils and activates eosinophils. Interestingly, EGF, CCL5, and CXCL5 also share similar functions in platelet activation and coagulation.5, 6 Previous studies have reported the existence of CCL5, CXCL5, EGF, and CD40L in platelets or megakaryocytes,6–10 but their correlation to the platelet count and origin were uncertain. As platelet alpha-granules contain a variety of growth factors, cytokines, and chemokines, in addition to hemostatic proteins, platelets might be a source of CD40L, CXCL5, EGF, and CCL5. We hypothesized that these cytokines were related to the megakaryocyte-platelet lineage, and we investigated this possibility in another heamatologic disorder characterized by severe thrombocytopenia, ITP. Identification of the cellular sources of these cytokines would potentially be a first step into additional insights into the roles of platelets in immune responses and inflammation.
Design and Methods
A total of 42 ITP, 33 severe AA (SAA), and 52 healthy controls were included in this study. ITP and SAA were defined as previously described. 11, 12 Written informed consent from all subjects was obtained in accordance with protocols approved by the institutional review boards of the National Heart, Lung, and Blood Institute (Bethesda, MD) and of Weill Medical College of Cornell University (New York, NY). Of the 42 ITP patients, three were receiving corticosteroids; ten were receiving immunomodulatory therapies (e.g. IVIg, Anti-D, Rituximab); 16 were on treatment with Tpo-receptor agonists (Romiplostim or Eltrombopag); and 13 had no active therapy at the time of sampling. Twenty-one of the ITP patients had platelet counts < 50 × 109/L, and 21 platelet counts > 50 × 109/L at sampling. The distribution of various treatments was similar between these two groups. Healthy controls and patients’ characteristics are shown in Table 1.
Table 1.
Characteristics of patients and healthy controls
| Controls | SAA | ITP | ||
|---|---|---|---|---|
| PLT < 50 ×109/L | PLT > 50 ×109/L | |||
| Total number | 52 | 33 | 21 | 21 |
| Mean age ± SD |
46±14 | 33 ± 21** | 45 ± 24 | 40 ± 26 |
| Age range | 22 ~ 73 | 5 ~ 80 | 6 ~ 81 | 2 ~ 83 |
| Male/Female (%) |
52/48 | 58/42 | 45/55 | 14/86 |
| Median blood counts × 109/L (25–75 IQ) | ||||
| ANC | 5.2 (4.1 ~ 6.2) |
0.5*** (0.2 ~ 0.7) |
4.8 (2.8 ~ 9.0) |
3.6 (1.9 ~ 5.9) |
| ARC | - | 25.2 (11.2 ~ 44.7) |
90.3 (73.4 ~ 109.4) |
68.7 (49.6 ~ 101.0) |
| Platelet | 239 (202 ~ 289) |
15*** (10 ~ 25) |
25*** (18 ~ 29) |
170 (124 ~ 315) |
SAA, severe aplastic anemia, ITP, immune thrombocytopenic purpura; PLT, platelet; ANC, absolute neutrophil count; ARC, absolute reticulocyte count; 25–75 IQ, 25–75 interquartile range;
p < .01;
p < .001 (compared with healthy controls).
Mice and Induction of ITP
Six to 8-week-old C57BL6 (B6) mice were purchased from Jackson Laboratories (Bar Harbor, ME) and were acclimated for at least one week prior to use. All mice were housed in the National Institutes of Health animal facility with standard care and nutrition. All animal study protocols were approved by the Animal Care and Use Committee of the National Heart, Lung and Blood Institute.
Experimental ITP was induced in B6 mice following procedures described by Wei et al.13 Rabbit anti-mouse thrombocyte antiserum was obtained from Inter-Cell Technologies (Jupiter, FL), and its optimal dose for mouse platelet destruction was experimentally determined to be 0.2 mL at 1:5 dilution, and delivered through intraperitoneal injection. Control mice were injected with 0.2 mL sterile saline. Peripheral blood was collected from the retro-orbital sinus six and 24 hours after antiserum injection in tubes containing tripotassium ethylenediaminetetraacetic acid. Complete blood counts (CBC) were performed using a Hemavet 950 analyzer (Drew Scientific Inc., Oxford, CT), and plasma samples were prepared by centrifugation for cytokine measurement.
Platelet preparation and plasma conversion to serum
Peripheral blood from healthy controls was collected in tubes containing 10% final volume of acid-citrate-dextrose (ACD, 38 mmol/L citric acid, 75 mmol/L trisodium citrate, and 100 mmol/L dextrose) and centrifuged at 200 × g for 15 minutes, and platelet-rich plasma (PRP) was carefully aspirated and re-centrifuged at 200 × g for 5 minutes to remove residual red and white blood cells. PRP was centrifuged again at 1500 × g for 10 minutes to separate the platelets into pellets, and the plasma without platelets was denoted platelet-poor plasma (PPP). The pellet was then washed with phosphate-buffered saline and assessed for purity in a Cell-Dyn Coulter counter (Abbott Diagnostics, Abbott Park, Ill). Leucocyte contamination in the purified platelet preparation was assessed by transcript levels of CD45 by real-time quantitative polymerase chain reaction (Q-PCR), Ct values were above 35, indicating efficient leucocyte depletion (data not shown). To determine if cytokines in the blood were derived from platelets or other cell types after activation, we used Ca2+ as an agonist to maximize cytokine release. Samples were prepared by adding 50 µL of 2.5 M CaCl2 to 1 mL ACD anti-coagulated whole blood, PRP, and PPP to induce coagulation. Among these preparations, almost no cells were present in PPP and cytokines detected were baseline, while the majority of the cell components were platelets in PRP, which excluded other cell types, and cytokines measured were only derived from platelets after activation. In the whole blood-converted serum, all cell types were included, and cytokines might be derived from various cell types upon activation. Converted sera from these preparations were collected for cytokine measurement. To confirm that whole blood-converted serum was similar to conventionally prepared serum, sera from the same individuals were made by withdrawal of blood into tubes without anticoagulants, followed by centrifugation.
In vitro incubation of peripheral platelets
Isolated peripheral platelets from healthy controls were suspended in 10% fetal calf serum (FCS)/RPMI1640 medium at 5 × 108, 1 × 108, 2 × 107, and 4 × 106/mL, respectively, and incubated at 37°C over night. Neither aggregation nor obvious apoptosis (annexin V+ cells) of platelets was observed by microscopy and flow cytometry, respectively. Supernatants were collected by centrifugation at 1500 × g for 10 minutes and stored at −80°C until thawed for cytokine measurement.
Cytokine Analysis
The following cytokines were measured in human plasma: Tpo, G-CSF, CD40L, CXCL5, EGF, CCL5, VEGF, CXCL10, and CCL11. Measurement of all cytokines was performed by the Luminex assay according to the instructions of the manufacturer. All materials for the Luminex assay were purchased from R&D Systems (Minneapolis, MN). Mouse CXCL5, CCL5 (R&D Systems), CD40L (eBiosciences, San Diego, CA), and EGF (Signosis, Sunnyvale, CA) levels in the plasma were measured using an enzyme-linked immunosorbent assay (ELISA). To confirm if platelets were the sources of EGF, CCL5, CXCL5, and CD40L, the levels of these cytokines in regular sera, whole blood-derived sera, PRP-derived sera, PPP-derived sera, and PPP from 4 healthy volunteers were measured using ELISA kits (R&D Systems, Minneapolis, MN).
Megakaryocyte differentiation
Bone marrow CD34+ cells were isolated by magnetic bead separation from four healthy volunteers. To induce megakaryocytic differentiation, CD34+ cells were cultured for 14 days in StemSpan serum-free medium supplemented with recombinant human interleukin-6 (IL-6), IL-9, stem cell factor (SCF), and Tpo (all from Stem Cell Technologies, Vancouver, Canada). Isolation of CD61+ (megakaryocytes) and CD61− (non-megakaryocytic bone marrow cells) populations was performed by CD61+ magnetic bead separation (Miltenyi Biotech, Auburn, CA), according to the manufacturer's instructions, to purity 97%.
Cytokine gene expression by quantitative real-time polymerase chain reaction
Total cellular RNA was isolated from platelets, differentiated megakaryocytes, peripheral blood mononuclear cells (PBMCs), granulocytes, and non-megakaryocytic bone marrow cells using the RNeasy kit (Qiagen, Valencia, CA), and treated with DNase I (Qiagen). First strand synthesis was performed using Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA) with random hexamers following the manufacturer’s instructions. Synthesized first strand cDNA was subjected to SYBR green (Bio-Rad Life Science Research, Hercules, CA) based Q-PCR using PTC-200 Peltier Thermal Cycler (MJ Research, Waltham, MA). All PCRs were performed in duplicate. The levels of CXCL5, CCL5, CD40L, and EGF mRNAs, relative to β-actin mRNA, were calculated using the formula: relative RNA expression=2−(Ct of cytokine - Ct of β-actin), where Ct is the threshold cycle value. The results were expressed as fold-expression compared with non-megakaryocytic bone marrow cells. Primer sequences were shown below.
CXCL5: 5’-TTTACAGACCACGCAAGGAG-3’ and 5’-TCTTCAGGGAGGCTACCACT-3’;
CD40L: 5’-CTCAGAGCTGCAAATACCCA-3’ and 5’-CTCCTCCCAAGTGAATGGAT-3’;
CCL5: 5’- AAGGAAGTCAGCATGCCTCT-3’ and 5’-TTTGCCAGTAAGCTCCTGTG-3’;
EGF: 5’-ACCAAGACCTCAAGAATGGG-3’ and 5’-TCCATGAAGTTGGTTGCATT-3’;
actin: 5’-CATGGGTCAGAAGGATTCCT-3’ and 5’-AGCTCGTAGCTCTTCTCCAG-3’.
Statistical analysis
The rank-based Kruskal-Wallis one-way analysis of variance (ANOVA) method was used to evaluate the differences in cytokine levels among AA, ITP patients and the healthy controls, as well as the differences in cytokine levels among different sera preparations. Differences in CBC or cytokines between ITP mice and control mice were evaluated by student’s t-test and Mann-Whitney U test, respectively. Cytokine mRNA expression in various haematological cells versus platelets was compared using student’s t-test. Statistical significance was set at 0.05 level for all the tests.
Results
Cytokines in the plasma of ITP and AA patients
In all ITP patients, either with high or low platelet counts, Tpo and G-CSF levels were similar to those observed in healthy controls, but there were significantly higher levels of CXCL10 in ITP compared to controls (Figure 1). Levels of CXCL5, CCL5, CD40L, and EGF were similarly low in ITP patients who had low platelet counts (< 50 × 109/L) and in thrombocytopenic AA patients, as compared to controls. Levels of CXCL5, CCL5, CD40L, and EGF were higher in ITP patients who had platelet counts > 50 × 109/L, as compared to those patients who had low platelet counts, suggesting that they were associated with circulating platelet number. VEGF and CCL11 levels were lower in AA but not in ITP, compared with healthy controls (Figure.1). A moderate to strong correlation was found between platelet counts and the levels of the following cytokines in ITP patients: CD40L (r = 0.7638), CCL5 (r = 0.8868), CXCL5 (r = 0.6101), EGF (r = 0.7702), and VEGF (r = 0.5583) (p < 0.0001 for all of them); no correlation was found between platelet counts and Tpo, G-CSF, or CCL11 levels.
Figure 1. Plasma cytokine levels in patients with immune thrombocytopenic purpura, aplastic anemia, and healthy controls.
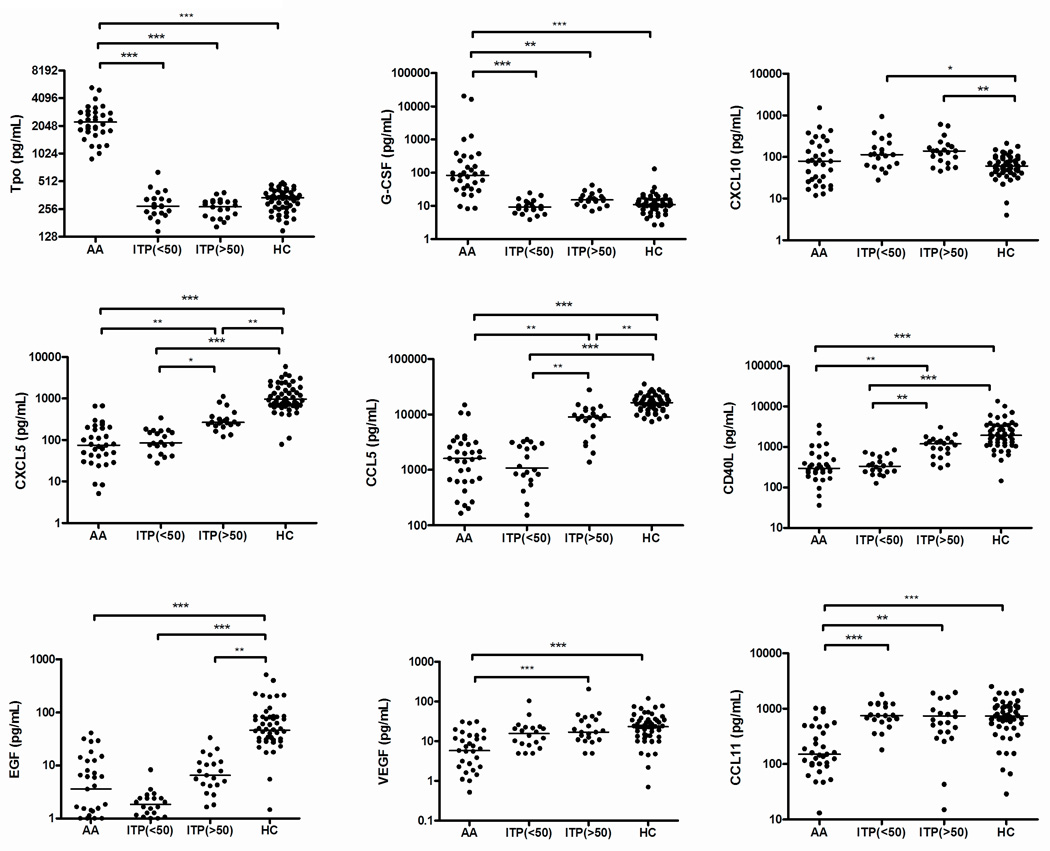
Cytokine levels in the plasma samples from 21 immune thrombocytopenic purpura (ITP) patients with platelet counts < 50 × 109/L -ITP (<50), 21 ITP with platelet counts > 50 × 109/L -ITP (>50), 33 untreated aplastic anemia (AA) patients, and 52 healthy controls (HC) were measured using a Luminex assay. The bars represent median values. ***, p < .001; **, p < .01; *, p < .05 (Kruskall-Wallis). Tpo, thrombopoietin; G-CSF, granulocyte colony-stimulating factor; CXCL5, chemokine (C-X-C motif) ligand 5; CXCL10, chemokine (C-X-C motif) ligand 10; CCL5, chemokine (C-C motif) ligand 5; CCL11, chemokine (C-C motif) ligand 11; CD40L, CD40 ligand; EGF, epidermal growth factor; VEGF, vascular endothelial growth factor.
Cytokines in ITP mouse model
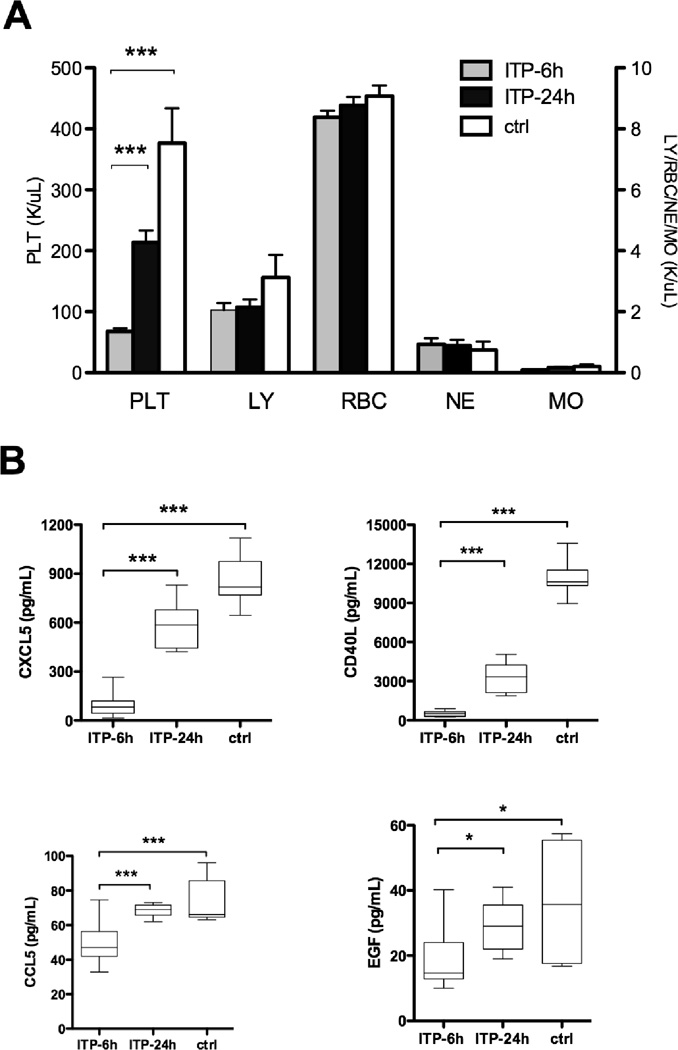
To confirm a correlation between platelet count and circulating levels of CCL5, CD40L, CXCL5 and EGF, we created an ITP animal model by injecting rabbit anti-mouse thrombocyte antiserum into B6 mice. Although thrombocytopenia induced by passive immunization may not be entirely identical to the chronic long-lasting course of ITP in humans, mice with ITP showed a significant decrease in platelet counts while maintaining normal levels of other blood cell types six hours after antiserum injection, and platelet counts recovered 24 hours later (Figure 2A). Bone marrow cellularity and megakaryocyte number were similar in ITP mice and controls (data not shown). As in human ITP, thrombocytopenic mice at six hours had significantly lower levels of plasma CD40L, CXCL5, CCL5, and EGF, compared to control mice. These cytokines increased with the platelet counts recovery at 24 hours (Figure 2B).
Figure 2. CXCL5, CD40L, CCL5, and EGF levels in ITP mouse model.
A) Mouse model developed with anti-thrombocyte antiserum showed significantly decreased numbers of platelet (PLT) but not other cells such as lymphocytes (LY), red blood cells (RBC), neutrophils (NE), and monocytes (MO) at 6 hours, and platelet counts recovered at 24 hours. B) CXCL5, CD40L, CCL5, and EGF in the plasma of ITP mice at 6 hours (n = 8) and at 24 hours (n = 6), and control mice (n = 7). Box-and-whiskers plot, whiskers show the range of data.
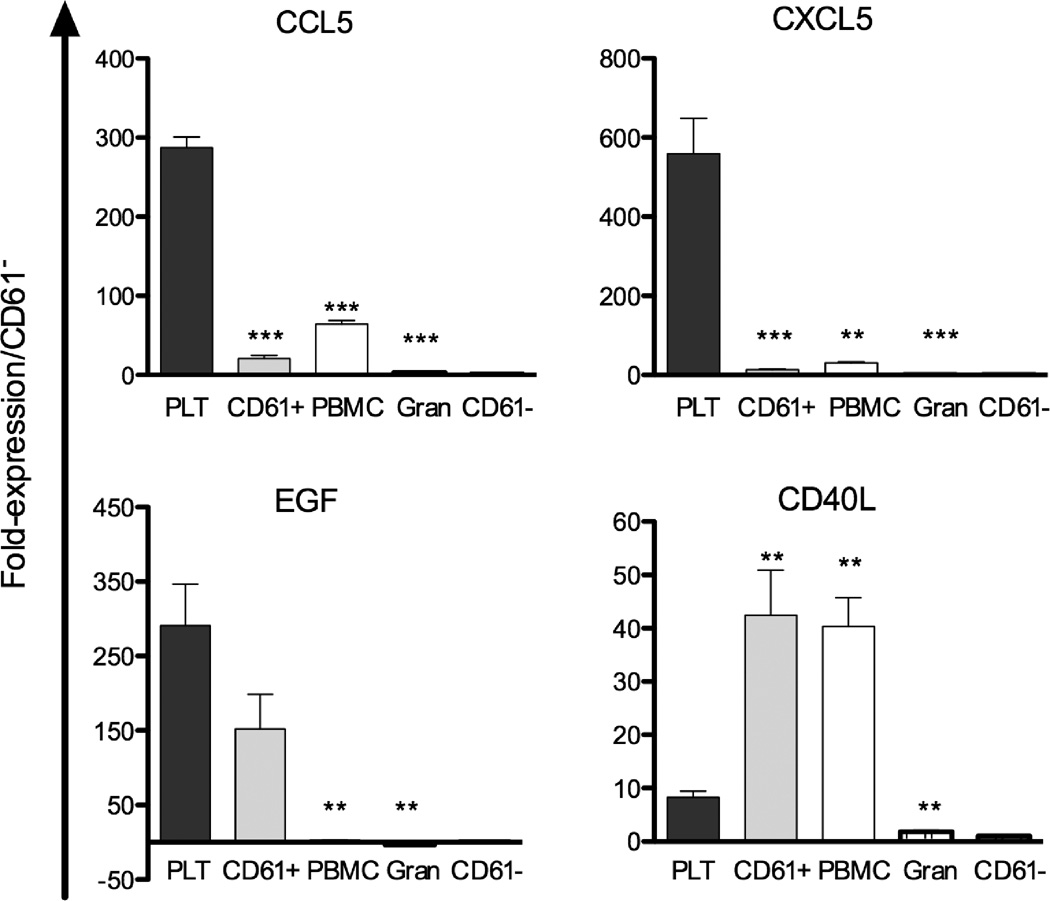
Cytokine gene expression in platelets and various haematological cells
To further investigate the association of these cytokines with platelets or their precursors, we examined mRNA expression of the relevant genes in platelets, megakaryocytes (CD61+), PBMCs, granulocytes, and non-megakaryocytic bone marrow cells (CD61−) (Figure 3). mRNA expression in platelets was much higher for CCL5, CXCL5, and EGF than was expression of these genes in megakaryocytes (CD61+), PBMCs, granulocytes, and CD61− cells. However, expression of CD40L (including surface and soluble forms) in megakaryocytes and PBMCs was higher than expression in platelets. CXCL10 levels were not affected by platelet count, and expression of CXCL10 in platelets, megakaryocytes and non-megakaryocytic bone marrow cells was similar (data not shown).
Figure 3. mRNA expression of CCL5, CXCL5, EGF, and CD40L in human platelets, megakaryocytes, peripheral blood mononuclear cells, granulocytes, and non-megakaryocytic bone marrow cells.
Data are expressed as fold-expression of CCL5, CXCL5, EGF, and CD40L mRNA in platelets (PLT), megakaryocytes (CD61+), peripheral blood mononuclear cells (PBMCs), and granulocytes (gran) compared with non-megakaryocytic bone marrow cells (CD61−). Bars indicate standard errors from four independent experiments. ***, p < .001; **, p < .01; *, p < .05, compared with platelets.
Platelets secrete CD40L, CXCL5, CCL5, and EGF, and are the main contributor of these cytokines in the serum
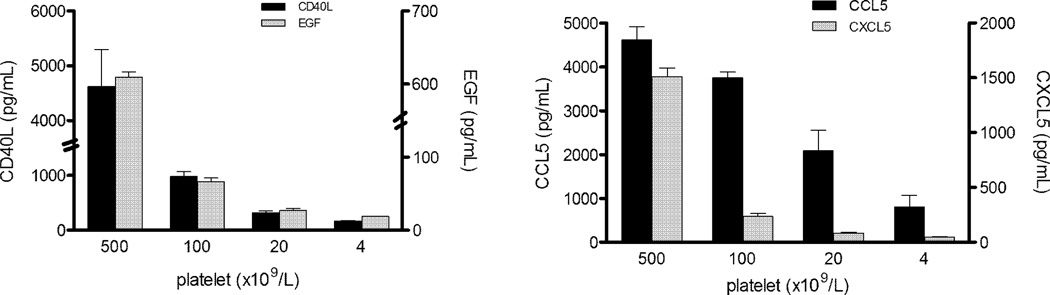
To investigate if platelets secreted CD40L, CXCL5, CCL5, and EGF, we incubated unstimulated platelets purified from the blood of healthy donors in tissue culture medium. Further corroborating our hypothesis, cytokine levels in culture supernatants indeed were proportional to the platelet counts (Figure 4). Finally, we measured the levels of these cytokines in standard preparations of sera and after the generation of sera under activating conditions with the addition of exogenous Ca2+ to whole blood, PRP, and PPP. As shown in Figure 5, for the first three forms of sera there was comparable level of cytokines, while cytokine levels were much lower in the PPP-derived sera or PPP, suggesting that the platelets (a main component of PRP) are a principal source of these cytokines.
Figure 4. Cytokine levels in the supernatants of platelet cultures.
Different concentrations of platelet were incubated at 37°C over night, levels of CD40L, EGF, CCL5, and CXCL5 in the supernatants were measured by Luminex. The bars indicate standard errors from four independent experiments.
Figure 5. Cytokine levels in sera of different preparations.
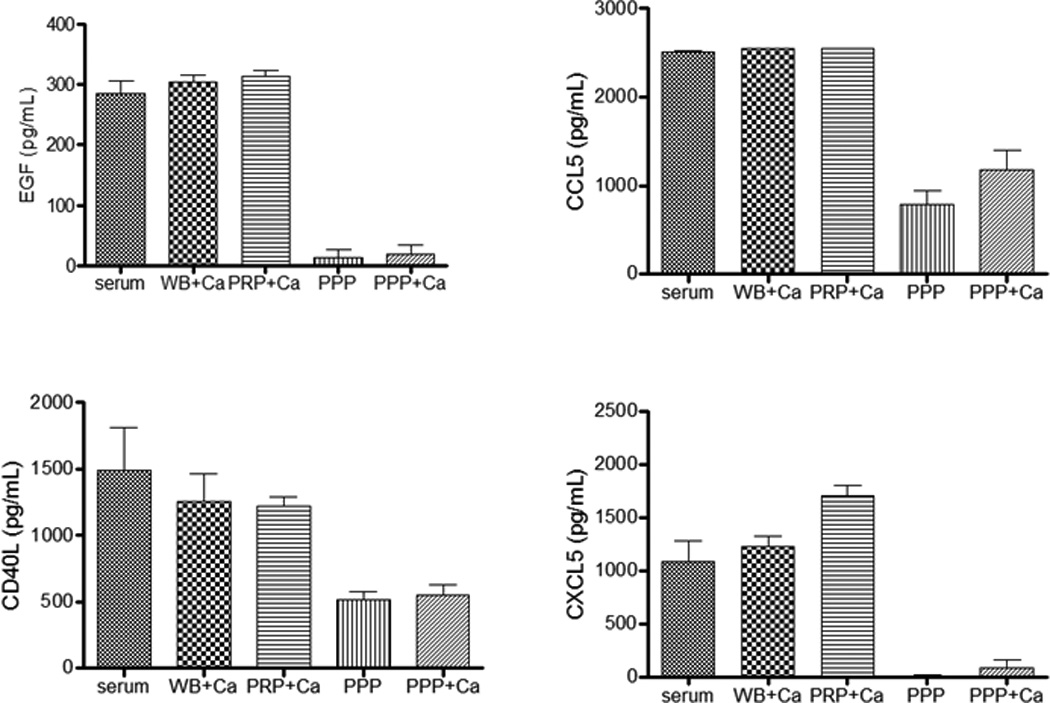
CD40L, EGF, CCL5, and CXCL5 in regular sera, whole blood-derived sera (WB+Ca), PRP-derived sera (PRP+Ca), PPP-derived sera (PPP+Ca), and PPP from four healthy volunteers were detected by ELISA. For WB, PRP and PPP, exogenous Ca2+ was added as a stimulant.
Discussion
Based on our previous study, we hypothesized that circulating CD40L, CXCL5, CCL5, and EGF were derived from platelets. To test this hypothesis, we measured these cytokines in the plasma of ITP and AA patients, since thrombocytopenia is the common feature of both diseases but the pathophysiology is entirely different. Our results showed similarly low levels of CD40L, CXCL5, CCL5, and EGF in both diseases; furthermore, the cytokine levels strongly correlated with the platelet counts, suggesting that plasma levels of these cytokines are very likely platelet derived.
Other cytokines tested had different relationships. Higher CXCL10 levels in ITP patients were consistent with a role of interferon-γ (IFNγ) in ITP pathogenesis, as CXCL10 is secreted by monocytes, endothelial cells, lymphocytes, and keratinocytes in response to IFNγ.14 Tpo levels were much higher in AA as compared to ITP patients and to controls, reflecting the large difference in megakaryocyte mass between these conditions.15–17
The strong correlation between cytokine levels of CD40L, CXCL5, CCL5, and EGF and platelet count in ITP patients is consistent with that found in AA patients.1 Conversely, VEGF levels were also correlated with platelet count, but overall the plasma levels were not low in ITP, suggesting other sources of VEGF besides platelets.
Low levels of CD40L and CCL5 in AA and ITP are in contrast to the reports in diabetes, 18, 19 in which high levels of these cytokines were identified and considered as evidence of immune system activation. 18, 19 Low levels of these cytokines in AA and ITP may not relate to inflammation or immune system activation but rather to the common characteristic of decreased platelets. Contradictory results regarding circulating CCL5 in systemic lupus erythematosus (SLE) have been reported. 20, 21 Based on our findings, it is possible that the low levels of these cytokines in SLE relate to the extent of thrombocytopenia in the tested patients.
Viallard et al. demonstrated increased soluble and platelet-associated CD40L in essential thrombocythemia and reactive thrombocytosis, suggesting that platelets were a source of CD40L,22 - a finding consistent with ours. Another study from the same group demonstrated that CD40L in platelet lysate is increased in ITP, a finding that was attributed to the activated state of platelets in ITP patients. 23 Despite possible platelet activation, very low absolute number of platelets still results in low CD40L levels in ITP. Activated CD4+ T cells mainly express membrane CD40L, not soluble CD40L, because in T-cell receptor (TCR)-activated T cells, calcium is sufficient to induce membrane CD40L expression but not the production of soluble CD40L.24 It remains to be determined whether soluble CD40L in the plasma and on the cell surface of T cells have the same functions. Although we cannot exclude the effects of corticosteroids or other drugs on the release of these cytokines by platelets, as the majority of ITP patients in this study either were presently receiving or had had previous treatments, our results do demonstrate that CD40L, CCL5, CXCL5, and EGF levels in the plasma were proportional to platelet counts in AA and ITP patients, suggesting that platelets (or their precursors) are probably the common source of these cytokines.
The transcriptome analysis supported the hypothesis that platelets and megakaryocytes were the cellular origin of these four cytokines in AA and ITP. Q-PCR data (Figure 3) confirmed that CXCL5, CCL5 and EGF were expressed at much higher levels in platelets than in megakaryocytes, PBMCs and granulocytes. However, expression of CD40L was higher in megakaryocytes and PBMCs than in platelets, and we could not distinguish between soluble and surface CD40L. Although megakaryocytes also expressed CD40L, CCL5, CXCL5, and EGF, since their absolute number is much smaller and they reside solely in the marrow compartment, it seems likely that platelets are the main contributors to plasma levels of these four cytokines. This inference would be consistent with the similarly low levels of these cytokines in ITP and AA despite a substantially greater megakaryocyte mass in ITP.
As CD40/CD40L interaction plays important roles in Th2 response,25 decreased CD40L might result in an imbalance in CD40-CD40L “cross-talk” between T cells and B cells or dendritic cells, and provide a cytokine environment that promotes Th1 polarization. Platelets might suppress this process by secreting different cytokines. Tacke et al. recently reported lower plasma CXCL5 levels in patients with liver cirrhosis;26 CXCL5 exerts mitogenic properties on hepatocytes. They demonstrated an association of decreased CXCL5 plasma levels with fibrosis in liver histology and with the clinical signs of decompensation in patients with liver cirrhosis including bleeding disorders. Their results also showed a correlation between CXCL5 levels and platelet count. The low levels of certain cytokines (CD40L, EGF) may also have a direct or indirect negative impact on marrow function, as suggested by reports correlating these cytokines with heamatopoiesis27, 28. Future systematic studies should explore platelets and platelet-associated cytokines such as CD40L, CXCL5, EGF, and CCL5 in the setting of thrombocytopenia and the effects of heamatopoiesis and Th1/Th2 balance.
In summary, this study confirmed our hypothesis that platelets secrete and account for circulating CD40L, CXCL5, CCL5, and EGF by detecting mRNA expression in platelets and megakaryocytes, by measuring cytokine levels in platelet culture supernatants and PRP-derived sera, and in a murine ITP model. Association of platelets with these cytokines expands our understanding of the effects of platelets on other target cells via secreting cytokines, as well as the functions of platelets in inflammation in other diseases, and in haematopoiesis. Measurement of platelet-specific cytokines will allow inferences concerning physiology and pathophysiology in quantitative platelet disorders.
Acknowledgement
This work was supported by the Intramural Research Program of the National Heart, Lung and Blood Institute, National Institutes of Health, JBB grants and CCBF
Footnotes
Addendum:
X. Feng designed the research, performed experiments, analyzed data and wrote the manuscript. P. Scheinberg designed the research, analyzed data and wrote the manuscript. L. Samsel and J.P. McCoy performed the Luminex experiments and analyzed data. O. Rios was involved in the handling and collection of samples and provided clinical data. J. Chen performed the mice experiment. W. Ghanima and J.B. Bussel provided patient samples and wrote the manuscript. N.S. Young was involved in primary conception, interim discussions, data analysis, and manuscript preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
References
- 1.Feng X, Scheinberg P, Wu CO, Samsel L, Nunez O, Prince C, Ganetzky RD, McCoy JP, Jr, Maciejewski JP, Young NS. Cytokine signature profiles in acquired aplastic anemia and myelodysplastic syndromes. Haematologica. 2011;96:602–606. doi: 10.3324/haematol.2010.030536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ludewig B, Henn V, Schroder JM, Graf D, Kroczek RA. Induction, regulation, and function of soluble TRAP (CD40 ligand) during interaction of primary CD4+ CD45RA+ T cells with dendritic cells. Eur J Immunol. 1996;26:3137–3143. doi: 10.1002/eji.1830261246. [DOI] [PubMed] [Google Scholar]
- 3.Andre P, Prasad KS, Denis CV, He M, Papalia JM, Hynes RO, Phillips DR, Wagner DD. CD40L stabilizes arterial thrombi by a beta3 integrin--dependent mechanism. Nat Med. 2002;8:247–252. doi: 10.1038/nm0302-247. [DOI] [PubMed] [Google Scholar]
- 4.Prasad KS, Andre P, He M, Bao M, Manganello J, Phillips DR. Soluble CD40 ligand induces beta3 integrin tyrosine phosphorylation and triggers platelet activation by outside-in signaling. Proc Natl Acad Sci U S A. 2003;100:12367–12371. doi: 10.1073/pnas.2032886100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kato S, Pinto M, Carvajal A, Espinoza N, Monso C, Bravo L, Villalon M, Cuello M, Quest AF, Suenaga A, Brosens JJ, Owen GI. Tissue factor is regulated by epidermal growth factor in normal and malignant human endometrial epithelial cells. Thromb Haemost. 2005;94:444–453. doi: 10.1160/TH05-01-0066. [DOI] [PubMed] [Google Scholar]
- 6.Gear AR, Camerini D. Platelet chemokines and chemokine receptors: linking hemostasis, inflammation, and host defense. Microcirculation. 2003;10:335–350. doi: 10.1038/sj.mn.7800198. [DOI] [PubMed] [Google Scholar]
- 7.Solanilla A, Dechanet J, El Andaloussi A, Dupouy M, Godard F, Chabrol J, Charbord P, Reiffers J, Nurden AT, Weksler B, Moreau JF, Ripoche J. CD40-ligand stimulates myelopoiesis by regulating flt3-ligand and thrombopoietin production in bone marrow stromal cells. Blood. 2000;95:3758–3764. [PubMed] [Google Scholar]
- 8.Crist SA, Sprague DL, Ratliff TL. Nuclear factor of activated T cells (NFAT) mediates CD154 expression in megakaryocytes. Blood. 2008;111:3553–3561. doi: 10.1182/blood-2007-05-088161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Power CA, Clemetson JM, Clemetson KJ, Wells TN. Chemokine and chemokine receptor mRNA expression in human platelets. Cytokine. 1995;7:479–482. doi: 10.1006/cyto.1995.0065. [DOI] [PubMed] [Google Scholar]
- 10.Ben-Ezra J, Sheibani K, Hwang DL, Lev-Ran A. Megakaryocyte synthesis is the source of epidermal growth factor in human platelets. Am J Pathol. 1990;137:755–759. [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenfeld SJ, Kimball J, Vining D, Young NS. Intensive immunosuppression with antithymocyte globulin and cyclosporine as treatment for severe acquired aplastic anemia. Blood. 1995;85:3058–3065. [PubMed] [Google Scholar]
- 12.Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, Bussel JB, Cines DB, Chong BH, Cooper N, Godeau B, Lechner K, Mazzucconi MG, McMillan R, Sanz MA, Imbach P, Blanchette V, Kuhne T, Ruggeri M, George JN. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113:2386–2393. doi: 10.1182/blood-2008-07-162503. [DOI] [PubMed] [Google Scholar]
- 13.Wei H, Ding X, Ren J, Liu K, Tan P, Li D, Ma RZ. A murine model for human immune thrombocytopenic purpura and comparative analysis of multiple gene expression in bone marrow and spleen. J Genet Genomics. 2008;35:665–671. doi: 10.1016/S1673-8527(08)60088-0. [DOI] [PubMed] [Google Scholar]
- 14.Luster AD, Ravetch JV. Biochemical characterization of a gamma interferon-inducible cytokine (IP-10) J Exp Med. 1987;166:1084–1097. doi: 10.1084/jem.166.4.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aledort LM, Hayward CP, Chen MG, Nichol JL, Bussel J. Prospective screening of 205 patients with ITP, including diagnosis, serological markers, and the relationship between platelet counts, endogenous thrombopoietin, and circulating antithrombopoietin antibodies. Am J Hematol. 2004;76:205–213. doi: 10.1002/ajh.20104. [DOI] [PubMed] [Google Scholar]
- 16.Koike Y, Yoneyama A, Shirai J, Ishida T, Shoda E, Miyazaki K, Sunaga S, Horie R, Aoki K, Koike K, Ogata I, Tahara T, Kato T, Nakahara K, Kariya T, Higashihara M. Evaluation of thrombopoiesis in thrombocytopenic disorders by simultaneous measurement of reticulated platelets of whole blood and serum thrombopoietin concentrations. Thromb Haemost. 1998;79:1106–1110. [PubMed] [Google Scholar]
- 17.Emmons RV, Reid DM, Cohen RL, Meng G, Young NS, Dunbar CE, Shulman NR. Human thrombopoietin levels are high when thrombocytopenia is due to megakaryocyte deficiency and low when due to increased platelet destruction. Blood. 1996;87:4068–4071. [PubMed] [Google Scholar]
- 18.Nomura S, Shouzu A, Omoto S, Nishikawa M, Fukuhara S. Significance of chemokines and activated platelets in patients with diabetes. Clin Exp Immunol. 2000;121:437–443. doi: 10.1046/j.1365-2249.2000.01324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varo N, Libby P, Nuzzo R, Italiano J, Doria A, Schonbeck U. Elevated release of sCD40L from platelets of diabetic patients by thrombin, glucose and advanced glycation end products. Diab Vasc Dis Res. 2005;2:81–87. doi: 10.3132/dvdr.2005.014. [DOI] [PubMed] [Google Scholar]
- 20.Kaneko H, Ogasawara H, Naito T, Akimoto H, Lee S, Hishikawa T, Sekigawa I, Tokano Y, Takasaki Y, Hirose SI, Hashimoto H. Circulating levels of beta-chemokines in systemic lupus erythematosus. J Rheumatol. 1999;26:568–573. [PubMed] [Google Scholar]
- 21.Lit LC, Wong CK, Tam LS, Li EK, Lam CW. Raised plasma concentration and ex vivo production of inflammatory chemokines in patients with systemic lupus erythematosus. Ann Rheum Dis. 2006;65:209–215. doi: 10.1136/ard.2005.038315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viallard JF, Solanilla A, Gauthier B, Contin C, Dechanet J, Grosset C, Moreau JF, Praloran V, Nurden P, Pellegrin JL, Nurden AT, Ripoche J. Increased soluble and platelet-associated CD40 ligand in essential thrombocythemia and reactive thrombocytosis. Blood. 2002;99:2612–2614. doi: 10.1182/blood.v99.7.2612. [DOI] [PubMed] [Google Scholar]
- 23.Solanilla A, Pasquet JM, Viallard JF, Contin C, Grosset C, Dechanet-Merville J, Dupouy M, Landry M, Belloc F, Nurden P, Blanco P, Moreau JF, Pellegrin JL, Nurden AT, Ripoche J. Platelet-associated CD154 in immune thrombocytopenic purpura. Blood. 2005;105:215–218. doi: 10.1182/blood-2003-07-2367. [DOI] [PubMed] [Google Scholar]
- 24.Matthies KM, Newman JL, Hodzic A, Wingett DG. Differential regulation of soluble and membrane CD40L proteins in T cells. Cell Immunol. 2006;241:47–58. doi: 10.1016/j.cellimm.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Khan WI, Motomura Y, Blennerhassett PA, Kanbayashi H, Varghese AK, El-Sharkawy RT, Gauldie J, Collins SM. Disruption of CD40-CD40 ligand pathway inhibits the development of intestinal muscle hypercontractility and protective immunity in nematode infection. Am J Physiol Gastrointest Liver Physiol. 2005;288:G15–G22. doi: 10.1152/ajpgi.00159.2004. [DOI] [PubMed] [Google Scholar]
- 26.Tacke F, Zimmermann HW, Trautwein C, Schnabl B. CXCL5 plasma levels decrease in patients with chronic liver disease. J Gastroenterol Hepatol. 2011;26:523–529. doi: 10.1111/j.1440-1746.2010.06436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seijkens T, Engel D, Tjwa M, Lutgens E. The role of CD154 in haematopoietic development. Thromb Haemost. 2010;104:693–701. doi: 10.1160/TH10-03-0174. [DOI] [PubMed] [Google Scholar]
- 28.Kilroy GE, Foster SJ, Wu X, Ruiz J, Sherwood S, Heifetz A, Ludlow JW, Stricker DM, Potiny S, Green P, Halvorsen YD, Cheatham B, Storms RW, Gimble JM. Cytokine profile of human adipose-derived stem cells: expression of angiogenic, hematopoietic, and pro-inflammatory factors. J Cell Physiol. 2007;212:702–709. doi: 10.1002/jcp.21068. [DOI] [PubMed] [Google Scholar]