Abstract
Objective
To characterize the natural history of intestinal failure (IF) among 14 pediatric centers during the intestinal transplantation (ITx) era.
Study design
The Pediatric Intestinal Failure Consortium performed a retrospective analysis of clinical and outcome data for a multi-center cohort of infants with IF. Entry criteria included infants <12 mo receiving parenteral nutrition (PN) for >60 continuous days. Enteral autonomy was defined as discontinuation of PN for >3 consecutive months. Values are presented as median (25th, 75th percentiles) or as (n, %).
Results
272 infants with a gestational age of 34 wks (30, 36) and birth weight of 2.1 kg (1.2, 2.7) were followed for 25.7 mo (11.2, 40.9). Residual small bowel length in 144 patients was 41 cm (25.0, 65.5). Diagnoses were necrotizing enterocolitis (71, 26%), gastroschisis (44, 16%), atresia (27, 10%), volvulus (24, 9%), combinations of these diagnoses (46, 17%), aganglionosis (11, 4%), and other single or multiple diagnoses (48, 18%). Prescribed medications included oral antibiotics (207, 76%), H2 blockers (187, 69%), and PPIs (156, 57%). Enteral feeding approaches varied among centers; 19% of the cohort received human milk. The cohort experienced 8.9 new catheter-related blood stream infections per 1,000 catheter days. The cumulative incidences for enteral autonomy, death, and ITx were 47%, 27%, and 26%, respectively. Enteral autonomy continued into the 5th year after study entry.
Conclusions
Children with IF endure significant mortality and morbidity. Enteral autonomy may require years to achieve. Improved medical, nutritional, and surgical management may reduce time on PN, mortality and need for transplantation.
Keywords: Intestinal failure, short bowel syndrome, parenteral nutrition, intestinal transplantation, PIFCon
Intestinal failure (IF) in infants and children is a devastating condition that can be broadly defined as the inability of the gastrointestinal tract to sustain life without supplemental parenteral nutrition (PN).(1–2) The most common type of IF is short bowel syndrome (SBS) with an estimated incidence between 3 to 5 per 100,000 births per year.(3–4) Advances in neonatal intensive care, anesthesia, nutrition support and surgical techniques have improved survival of children such that the prevalence of short bowel syndrome has likely increased in recent years.(5)
Human and societal costs for this rare condition are substantial. Long-term use of PN can be complicated by life-threatening conditions that include sepsis and chronic liver disease. Mortality and outcomes in patients with IF/SBS are historically linked to residual bowel length, absence of the ileocecal valve, inability to achieve enteral autonomy, incidence of sepsis, presence of cholestasis, and timing of ostomy closure.(6–7) Mortality has decreased from 30% of patients over 5 years(5) to 10–15% over 4 years(8–11) and likely reflects the impact of multidisciplinary intestinal rehabilitation programs on patient outcomes. However, children with IF are at risk for multiple morbidities, including metabolic derangement from gastrointestinal electrolyte losses, mechanical and infectious complications of central venous catheters, structural and functional bowel disorders, missed school and a lower quality of life. With approximately 16,000 children on home PN (HPN) in the United States, the economic burden of this population is substantial, with estimated individual medical charges exceeding $500,000 in the first year of life.(12) Data regarding the prevalence of children with SBS weaned from PN are scarce, but some remain at risk for nutritional and growth abnormalities as a consequence of their altered intestinal anatomy.(13–14)
We created a consortium of academic medical centers to perform a national retrospective study characterizing the largest and most geographically diverse cohort of infants with IF/SBS ever studied in order to describe the natural history of this condition and its outcomes.
Methods
The Pediatric Intestinal Failure Consortium (PIFCon) was initiated in June, 2006 and consists of 14 sites with established multi-disciplinary pediatric intestinal rehabilitation programs comprised of medical, surgical, nutritional, nursing and other specialists. Nine sites are intestinal transplant centers.
This was a multicenter retrospective cohort study. Inclusion criteria consisted of all infants with IF who were no more than 12 months of age and had received prolonged parenteral nutrition (PN) as a consequence of IF/SBS. PN was defined as an intravenous solution containing protein, carbohydrate, electrolytes, as well as vitamins and trace elements. Prolonged PN was defined as receiving PN in 60 out of 74 consecutive days at any time in the first year of life. This allowed for brief interruptions in PN for loss of intravenous access or surgery. Participants at 13 sites met the age and PN criteria between January 1, 2000 and December 31, 2004, whereas at one site the end date for study entry was extended to December 31, 2005. Data were collected through December 31, 2006 for 13 sites and December 31, 2007 at the one site to allow at least a two year follow-up for all children.
Following IRB approval at each site, data were collected retrospectively by chart review of patients with IF/SBS who were followed by one of the site investigators. Data were entered into de-identified case report forms and electronically transmitted to a central location at the University of Pittsburgh Graduate School of Public Health. Time intervals for collecting data after entry criteria were met were 1, 3, 6, 9, and 12 months and annually thereafter. Windows to record data were contiguous to ensure maximum data collection. If data elements were available on more than one occasion within a time interval, those closest to the designated month were selected.
Data elements sought at baseline included patient demographics, diagnosis leading to IF, intestinal anatomy (including residual small bowel length as determined by operative reports), composition of parenteral and enteral nutrition, and clinical laboratory tests. Diagnosis consisted of the principal etiology of IF/SBS in each patient. When more than one diagnosis contributed to this process, patients fell into the multiple single diagnoses category. Data sought at follow-up included anthropometric z-scores to enable calculation of underweight (weight for age z-score <−2), stunting (height for age z-score <−2), and wasting (weight-for-height z-score <−2), clinical laboratory tests that included micronutrient levels, details related to the type, volume and percent energy provided by enteral and parenteral nutrition, medications, sentinel events, and whether an outcome occurred during the interval of observation. Sentinel events included frequency of bacteremia, cholestasis (defined as serum total bilirubin ≥ 5 mg/dL or conjugated or direct bilirubin ≥ 2 mg/dL), intestinal bleeding, admission to hospital, medical and surgical interventions, and referral for intestinal transplant evaluation. Outcome variables included death, intestinal transplant (ITx), and enteral autonomy defined as the discontinuation of PN for more than 3 consecutive months.
Statistical Analyses
Values are presented as median (25th, 75th percentiles) or as (n, %). World Health Organization (WHO) growth charts were used to obtain z-scores when gestational age was greater than 40 weeks.(15) When gestational age was 40 weeks or less, an alternative reference standard was used to calculate z-scores for weight and length.(16) Competing risks analysis(17) was used to obtain cumulative incidence rates for enteral autonomy, death, and transplantation where death was a competing risk for transplantation and both death and transplantation were competing risks for enteral autonomy. The log rank test was used to compare survival of children with and children without cholestasis at baseline.
Results
There were 272 infants who met entry criteria (Table I). Follow-up data were collected for a median 25.7 mo (11.2, 40.9). The majority were male and the majority were Caucasian. One-half of the cohort initiated PN within 3 days after birth, 77% were premature, and 30% of the cohort met criteria for very low birth weight (≤1500 grams). Residual small bowel length was recorded for slightly more than half of the cohort and the median small bowel length remaining was 41 cm (range 1 to 166 cm). At study entry, 31 (21%; 98 missing) were underweight, 38 (34%, 161 missing) had stunted growth, and 10 (11%; 183 missing) met criteria for wasting. Cholestasis was present at study entry in nearly 75 percent of infants. Only 65 patients had an INR measured at or near the time of study entry, and of those that were measured, fewer than 10 percent had an INR greater than 1.5.
TABLE 1.
Characteristics at birth and at study entry of 272 infants with intestinal failure
| FEATURE (N=data available) | Number (%) | Median (25th-75th) |
|---|---|---|
| AT BIRTH | ||
| Male | 156 (57.4) | |
| Caucasian (250) | 204 (81.6) | |
| Hispanic | 42 (15.4) | |
| Gestation (wks) (264) | 34 (30–36) | |
| < 37 wk (premature) | 202 (76.5) | |
| Birth weight (kg) (221) | 2.1 (1.2–2.7) | |
| ≤ 1.5 kg (very low birth weight) | 66 (29.9) | |
| AT STUDY ENTRY | ||
| Age when entry criteria met (days) | 63 (61, 74) | |
| Small bowel length (cm) (144) | 41 (25 – 65.5) | |
| Weight age z-score < −2 (174) | 37 (21.3) | |
| Height age z-score <−2 (111) | 38 (34.2) | |
| Weight-for-height z-score <−2 (89) | 10 (11.2) | |
| ALT ≥ 80 IU/ml (163) | 77 (47.2) | |
| AST ≥ 80 IU/ml (138) | 92 (66.7) | |
| Cholestasis present* (168) | 125 (74.4) | |
| Albumin < 2.8 g/dl (167) | 82 (49.1) | |
| INR > 1.5 (65) | 6 (9.2) |
Cholestasis defined as either a total bilirubin ≥ 5 mg/dl or direct/conjugated bilirubin ≥ 2 mg/dl
Diagnoses underlying IF/SBS are listed in Table II. Necrotizing enterocolitis, gastroschisis, intestinal atresia, and volvulus were the most common single diagnoses. Infants with multiple single diagnoses, such as gastroschisis and atresia, necrotizing enterocolitis and atresia, and other combinations occurred in 28% of the cohort.
TABLE 2.
Diagnoses Associated with Intestinal Failure and Short Bowel Syndrome in Infants (N=272)
| DIAGNOSIS | N (%) |
|---|---|
| Necrotizing enterocolitis | 71 (26) |
| Gastroschisis | 44 (16) |
| Intestinal atresia (large/small) | 27 (10) |
| Volvulus | 24 (9) |
| Long segment Hirschsprung disease | 11 (4) |
| Tufting or Microvillus inclusion | 3 (1) |
| Other single diagnoses | 14 (5) |
| Unknown | 1 |
| Multiple single diagnoses | 77 (28) |
Medical and surgical Interventions
Children with IF/SBS were exposed to multiple classifications of medications (Table III; available at www.jpeds.com). The list represents any exposure to medications within that classification anytime during any interval of observation for patients in the cohort. Oral antibiotics for bacterial overgrowth, histamine-2 blockers and proton pump inhibitors were each given to over 50% of patients. Infants with IF underwent a considerable number of abdominal procedures (Table IV; available at www.jpeds.com). Numbers represent all abdominal procedures performed from birth. Multiple abdominal procedures could be performed during a single surgical operation. There were 26 children who underwent 28 bowel lengthening procedures; 11 patients received a longitudinal intestinal lengthening and tailoring procedure, 13 received a serial transverse enteroplasty (STEP) procedure, and 2 children had both a STEP and a longitudinal lengthening procedure.
TABLE 3.
Medication Classifications used in the Management of Infants with IF/SBS (N=272)
| CLASSIFICATION | N (%) |
|---|---|
| Oral antibiotics for bacterial overgrowth | 207 (76) |
| Histamine-2 Blockers | 187 (69) |
| Proton pump inhibitors | 156 (57) |
| Pro-motility agents | 118 (43) |
| Anti-motility agents | 111 (41) |
| Bile salt binding medication | 55 (20) |
| Anti-secretory agent | 47 (17) |
| Antibiotic lock therapy | 41 (15) |
TABLE 4.
Abdominal Surgical Procedures Performed at any Time from Birth until the Last Period of Observation
| SURGICAL PROCEDURE | N |
|---|---|
| Small bowel resection | 336 |
| Exploratory laparotomy | 202 |
| Ostomy creation, revision, closure | 209 |
| Gastrostomy creation/revision/closure | 218 |
| Lengthening procedure | 28 |
| Intestinal lengthening and tailoring = 13 | |
| Serial transverse enteroplasty = 15 | |
| Tapering procedure only | 14 |
| Fundoplication | 19 |
| Other procedures | 200 |
| TOTAL PROCEDURES | 1226 |
Septic Complications
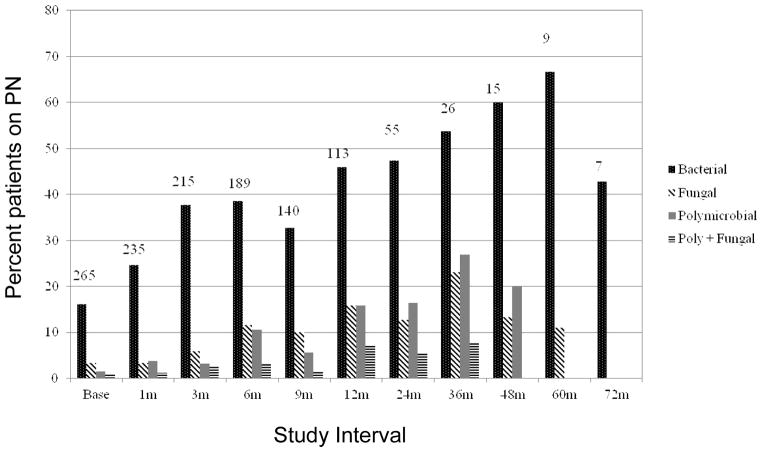
The cohort experienced 8.9 new catheter-related blood stream infections per 1,000 catheter days. The percent of patients on PN with positive blood cultures during the intervals following enrollment into the study is depicted in Figure 1. Single bacterial infections predominated in each of the respective intervals. In the first 548 days of observation, i.e. across the first six intervals of observation, 185 (68%) of children had at least one septic event for a total of 711 septic events while on PN. Furthermore, 177, 61, 43 and 18 children experienced at least one bacterial, fungal, polymicrobial, and polymicrobial and fungal event, respectively. Of the children not experiencing a septic event, 59 (22%) received PN for 3 or more intervals of observation.
Figure 1.
Percent of Patients on PN with Septic Events by Study Interval. Time intervals were contiguous, with the designated time coinciding with the mid-point of the interval. The numbers on top of the bars represent the number of children on PN for that study interval.
Nutrition
Breast milk was reported to be given to 52 (19%) of children, and 20 different infant formulas were used as the initial enteral diet, and 40 different formulas were used overall.
Outcomes
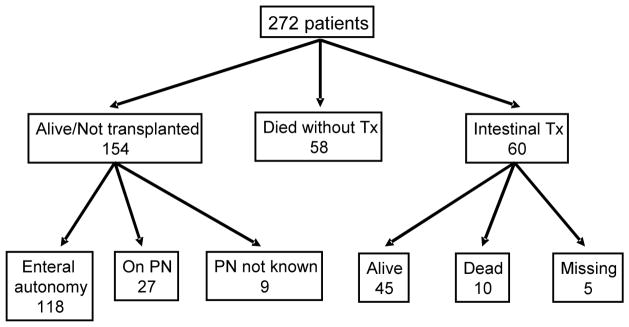
Death was reported for 68 children and 60 children received ITx. Of the 68 who died, 10 had received an ITx (Figure 2). Causes of death are listed in Table V (available at www.jpeds.com). Median time to ITx following meeting study entry criteria was 14.7 months. There were 154 children alive without ITx when the last data were abstracted from the medical record. 118 (77%) had achieved enteral autonomy at least once; 6/118 lost enteral autonomy at least once and 5/6 achieved enteral autonomy a second time. There were 27 (18%) who had not achieved enteral autonomy and continued on PN through the last period of observation, and in 9 the PN status was unknown.
Figure 2.
Outcome for the 272 children on the last date for which data are recorded at the clinical site.
TABLE 5.
CAUSE OF DEATH IN INFANTS WITH IF/SBS BEFORE AND AFTER INTESTINAL TRANSPLANT
| CAUSE OF DEATH | N (%) |
|---|---|
| PRIOR TO INTESTINAL TRANSPLANT | 58 |
| Multi-organ system failure | 28 (62) |
| Sepsis | 9 (20) |
| Hemorrhage | 4 (9) |
| Central nervous system | 2 (4) |
| Meningitis-Palliation | 1 (2) |
| Nephroblastoma-Palliation | 1 (2) |
| Unknown | 13 |
| FOLLOWING INTESTINAL TRANSPLANT | 10 |
| Respiratory failure | 4 (40) |
| Multi-organ system failure | 3 (30) |
| Sepsis | 2 (20) |
| Hemorrhage | 1 (10) |
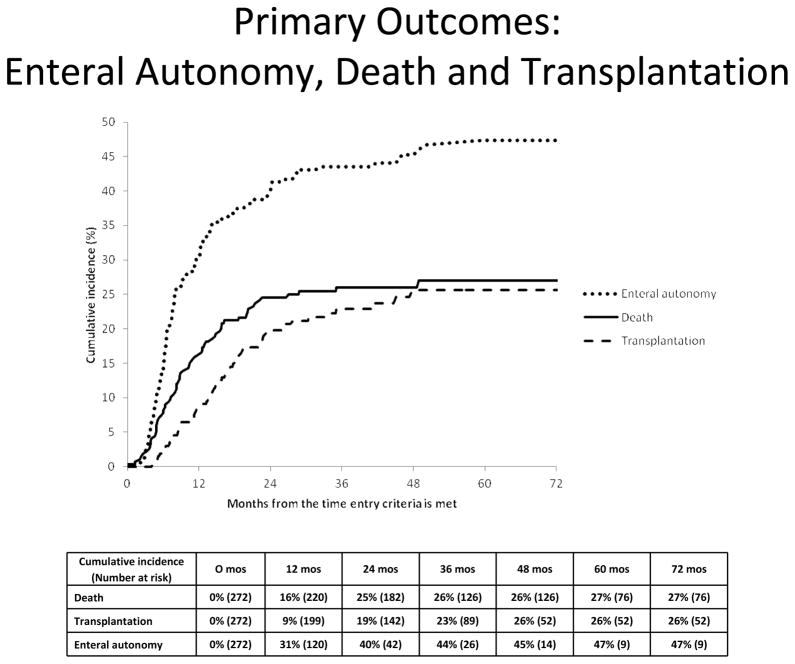
By 12 months following study enrollment, a cumulative percentage of 31% of the cohort had achieved enteral autonomy, 16% had died, and 9% had received an intestinal transplant (Figure 3). By 36 months, the cumulative percentages of events were 44% for enteral autonomy, 26% for death, and 23% for ITx. Beyond 48 months after study entry, no one received an ITx, 1 child died at 48.8 months, and 3 children achieved enteral autonomy at 48.8, 50.3, and 59.6 months respectively.
Figure 3.
Primary outcomes: enteral autonomy, death, and intestinal transplantation The data below the graph show the cumulative incidence of and the number of children who remain at risk for developing the outcome.
Among the 168 infants with sufficient data to assess for the presence of cholestasis at baseline, 125 children had cholestasis and their cumulative percentage of survival was significantly lower than in the 43 without cholestasis (79% vs. 95% at 1 year, and 73% vs. 88% at 3 years; p = 0.03). For 26 children who ever received a bowel lengthening procedure, by the end of their follow-up 9 underwent ITx, 8 continued on PN, 6 had achieved enteral autonomy and 3 died without ITx.
Discussion
Infants and children with IF are at risk for myriad complications as well as death. Large societal and financial costs of this relatively small sub-group of patients underlie the importance of understanding the natural history and outcomes of IF. The rarity of the condition combined with changing care practices make single center prospective studies that are adequately powered difficult to perform.
For 272 infants with IF, the cumulative percentage who achieved enteral autonomy (with death and intestinal transplantation as competing risks) was almost 50% at 3 years and achievement of enteral autonomy continued through 60 months of study. Our findings along with others(18) suggest infants with IF can hope to achieve enteral autonomy even after many years on PN, which is in sharp contrast to adults who rarely achieve enteral autonomy beyond 2 years on PN.(19) However, the path to enteral autonomy is not linear as a few children fell back to PN dependency before achieving enteral autonomy a second time. The cumulative percentage of children who died was 27%, and the cumulative percentage receiving ITx was 26%. Mortality and morbidity remain high in this population, and is similar to other reports.(20) Nearly all of the deaths occurred within 2 years after study entry. Therefore, improvements in the early management of infants with IF may improve outcomes.
We found greater heterogeneity among the diagnostic categories than previously described as 26% had complex intestinal anomalies or insults, which can be associated with unique medical and surgical challenges.(21) Recording of residual bowel length varied between sites. Reasons to account for these differences include the potential the infant was too sick for detailed measurements, inability to measure due to multiple adhesions, or clinical judgment that knowledge of the bowel length would not impact management. The relative importance of the residual bowel length is also dependent upon other factors that include gestational age, presence of an ileal segment and/or ileo-cecal valve, and colon length. Therefore, a standard approach to measuring technique as well as documentation of important co-variables should be incorporated into future prospective studies.
For those patients in whom the presence or absence of cholestasis could be assessed, we demonstrated that cholestatic disease is associated with a significant decrease in the probability of survival. The preponderance of our data were collected prior to initiation of lipid management strategies to prevent or treat cholestasis. An omega-3 fish oil-based parenteral emulsion was first introduced at one PIFCon site in 2004 and their findings were reported after completion of our data collection.(22) No other sites were able to access the omega-3 intravenous lipid preparation at any time during the study. A lipid reduction protocol to treat or prevent cholestasis was not initiated until 2007 at another PIFCon site.(23) Therefore, the impact on our data set by the evolving changes in clinical practice related to lipid management is likely small. Whether these strategies prevent PN-related liver disease or just improve cholestasis remains in question.(24) Newer lipid formulations containing medium-chain triglycerides, olive oil in addition to soy and fish oil may be available in the future.(25) The impact of alternative lipid components or reduced lipid infusions upon neurocognitive development, neuronal maturation, or cellular membrane composition in seriously ill neonates and infants is not known. Future studies will need to address these concerns.
Catheter-related blood stream infections (CRBSIs) were common, consistent with prior reports.(26) Sepsis was the second leading cause of death among those who died without an ITx, making infectious complications associated with IF a leading cause of morbidity and mortality in this population. Ethanol lock therapy to prevent CRBSIs in selected patients has recently been advocated, but adequately powered trials to support its use are lacking in pediatric populations.(27–28) New technologies related to impregnating central catheters with molecules to prevent adherence of biofilm or bacteria to the catheter may provide future options to prevent or reduce CRBSIs.(29) Antibiotic lock therapy has been used to salvage the central catheter following some CRBSIs and was used in 41 (15%) patients in our study; this likely represents an evolving change in clinical practice. Lock therapy with antibiotics is now recommended for catheter salvage (30–31), thus the frequency of antibiotic lock therapy has likely increased since 2006.
Infants with IF are exposed to multiple medications, none of which have been subjected to an adequately powered, prospective, randomized study in this population.(32) Although the prevalence and clinical relevance of bacterial overgrowth in SBS is unknown(33), 76% of children in our study were given oral antibiotics for presumed bacterial overgrowth with regimens that differed widely among the centers. Random manipulation of the gut microbiota with oral antibiotics may negatively impact energy absorption and intestinal secretion.(34) Ranitidine may reduce gastric acid secretion in patients with SBS (35), but the impact of prolonged use of more potent proton pump inhibitors alone or in combination with other histamine 2 antagonists is unknown.(36–37) Likewise, the use of pro- or anti-motility agents was noted in over 40% of our cohort with little evidence of safety or efficacy in this population. Alterations in enteral drug absorption and metabolism in the setting of SBS is an additional confounding variable that impacts a rational use of oral medications.(38)
Enteral feeding is essential for intestinal adaptation. Breast milk has been associated with decreased duration of PN in IF patients.(7) The beneficial effects of breast milk are attributable to its immunoprotective properties, effect on postnatal development of intestinal flora, and its nutrient composition that includes long chain triglycerides, free amino acids, nucleotides, and growth factors as well as complex protein and fat.(39) Only 52/272 (19%) of infants were reported to have received breast milk. Variations in practice regarding the choice of enteral formulas were considerable between and within the 14 sites. Opportunities to improve oral and enteral feeding strategies aimed to enhance intestinal adaptation should be addressed in future studies.
One limitation of this study is the gaps in data collected that can occur with retrospective studies. Reasons for missing data include absence of an electronic medical record, miss-filed or un-retrievable paper records of these frequently hospitalized children and incomplete records from the patients’ home institution. A second limitation is the variation in clinical practice between and within PIFCon sites. Although not unexpected, identification of practice variations provide opportunities to pursue comparative effectiveness strategies that will establish a “best practice” within the consortium. Development of a prospective, multi-center study would enhance the completeness, consistency and accuracy of the data and its analysis.
Enteral autonomy can be achieved in this high risk population, but physicians and families must be patient and vigilant as it may require many years. Current management strategies are imperfect as many are not evidence-based. Emerging strategies to prevent or treat cholestasis and CRBSIs will likely improve outcomes for children with IF. Comprehensive, multi-center, prospective studies will be required to design adequately powered clinical trials to assess medical, nutritional, and surgical interventions in children with IF that will reduce time on PN, morbidity, mortality and need for transplantation.
Acknowledgments
Supported by the National Institutes of Health NIDDK (grant 1 R21 DK081059-01). C.D. received funding from NICHD (K24 HD058795).
Appendix
Key individuals who actively participated in the establishment of PIFCon, as well as portions of this study include (by site): Children’s Hospital of Pittsburgh of UPMC: Cartland Burns, MD, George Mazariegos,, MD, Anita Nucci, PhD, RD, Jane Anne Yawarski, RN, Danielle Sebbens, DNP, CRNP, Rhonda Cunningham. Children’s Hospital, Boston: Daniel Kamin, MD, Tom Jaksic, MD, PhD, Hueng Bae Kim, MD, Sharon Collier, RD, LD, Melanie Connolly, RD, LD. C.S. Mott Children’s Hospital: Pamela Brown, M.D., Michele Johnson, LD, Robert Drongowski, MA, Research Coordinator. Nationwide Children’s Hospital: Christina. Valentine, MD, Steven Teich, MD, Beth Skaggs, Clinical Research Coordinator. Mattel Children’s Hospital UCLA: Martin G. Martin, MD, M.P.P., Patty Beckwith, RD, CDE, James Dunn, MD, Ph.D, Douglas G. Farmer, MD, F.A.C.S.. Laurie Reyen, RN, MN. UCSF Benioff Children’s Hospital: Diana Farmer, MD, Sang-Mo Kang, MD, Lane Bower, Dietician. Children’s Hospital Omaha: Dean L. Antonson, MD, Steve C. Raynor, MD, Brandy Sunderman, RD, Kris Seipel, Clinical Studies Program Coordinator. Children’s Hospital at Vanderbilt: Brent Polk, MD, Martha Ballew, MEd, RD. Texas Children’s Hospital: Mary Brandt, MD, Saul Karpen, MD, PhD, Sara Philips, RD, Kristin Brown, RD, Alejandro De La Torre. Children’s Hospital Colorado: Sara Fidanza, NP, Kristin Brown, RD. Seattle Children’s Hospital: Frances Malone, ARNP, Ph.D, Patrick Healey, MD, Jorge Reyes, MD, Cheryl Davis, RD. Cincinnati Children’s Hospital: Greg Tiao, MD, Jacqueline Wessel, Nurse Coordinator. Children’s Memorial Hospital: Valeria Cohran, MD, Kimberley Kazmerski, RD, Lisa Keys, BSN, RN, CCTNMargaret “Peggy” Richard, RN. University of Calgary: David Sigalet, MD, PhD. Emory-Children’s Center: Conrad Cole, MD
Footnotes
The authors declare no conflicts of interest.
Abstract presented Digestive Diseases Week May 6–10, 2011.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- 1.Goulet O, Ruemmele F, Lacaille F, Colomb V. Irreversible intestinal failure. J Pediatr Gastroenterol Nutr. 2004 Mar;38:250–69. doi: 10.1097/00005176-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Kocoshis SA, Beath SV, Booth IW, Garcia Oliva CA, Goulet O, Kaufman SS, et al. Intestinal failure and small bowel transplantation, including clinical nutrition: Working Group report of the second World Congress of Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2004 Jun;39( Suppl 2):S655–61. doi: 10.1097/00005176-200406002-00012. [DOI] [PubMed] [Google Scholar]
- 3.Wallander J, Ewald U, Lackgren G, Tufveson G, Wahlberg J, Meurling S. Extreme short bowel syndrome in neonates: an indication for small bowel transplantation? Transplant Proc. 1992 Jun;24:1230–5. [PubMed] [Google Scholar]
- 4.DeLegge M, Alsolaiman MM, Barbour E, Bassas S, Siddiqi MF, Moore NM. Short bowel syndrome: parenteral nutrition versus intestinal transplantation. Where are we today? Dig Dis Sci. 2007 Apr;52:876–92. doi: 10.1007/s10620-006-9416-6. [DOI] [PubMed] [Google Scholar]
- 5.Schalamon J, Mayr JM, Hollwarth ME. Mortality and economics in short bowel syndrome. Best Pract Res Clin Gastroenterol. 2003 Dec;17:931–42. doi: 10.1016/s1521-6918(03)00079-9. [DOI] [PubMed] [Google Scholar]
- 6.Quiros-Tejeira RE, Ament ME, Reyen L, Herzog F, Merjanian M, Olivares-Serrano N, et al. Long-term parenteral nutritional support and intestinal adaptation in children with short bowel syndrome: a 25-year experience. J Pediatr. 2004 Aug;145:157–63. doi: 10.1016/j.jpeds.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 7.Andorsky DJ, Lund DP, Lillehei CW, Jaksic T, Dicanzio J, Richardson DS, et al. Nutritional and other postoperative management of neonates with short bowel syndrome correlates with clinical outcomes. J Pediatr. 2001 Jul;139:27–33. doi: 10.1067/mpd.2001.114481. [DOI] [PubMed] [Google Scholar]
- 8.Sudan D, DiBaise J, Torres C, Thompson J, Raynor S, Gilroy R, et al. A multidisciplinary approach to the treatment of intestinal failure. J Gastrointest Surg. 2005 Feb;9:165–76. doi: 10.1016/j.gassur.2004.10.014. discussion 76–7. [DOI] [PubMed] [Google Scholar]
- 9.Torres C, Sudan D, Vanderhoof J, Grant W, Botha J, Raynor S, et al. Role of an intestinal rehabilitation program in the treatment of advanced intestinal failure. J Pediatr Gastroenterol Nutr. 2007 Aug;45:204–12. doi: 10.1097/MPG.0b013e31805905f9. [DOI] [PubMed] [Google Scholar]
- 10.Hess RA, Welch KB, Brown PI, Teitelbaum DH. Survival Outcomes of Pediatric Intestinal Failure Patients: Analysis of Factors Contributing to Improved Survival over the Past Two Decades. J Surg Res. 2011 Apr 15; doi: 10.1016/j.jss.2011.03.037. [DOI] [PubMed] [Google Scholar]
- 11.Modi BP, Langer M, Ching YA, Valim C, Waterford SD, Iglesias J, et al. Improved survival in a multidisciplinary short bowel syndrome program. J Pediatr Surg. 2008 Jan;43:20–4. doi: 10.1016/j.jpedsurg.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spencer AU, Kovacevich D, McKinney-Barnett M, Hair D, Canham J, Maksym C, et al. Pediatric short-bowel syndrome: the cost of comprehensive care. Am J Clin Nutr. 2008 Dec;88:1552–9. doi: 10.3945/ajcn.2008.26007. [DOI] [PubMed] [Google Scholar]
- 13.Miyasaka EA, Brown PI, Kadoura S, Harris MB, Teitelbaum DH. The adolescent child with short bowel syndrome: new onset of failure to thrive and need for increased nutritional supplementation. J Pediatr Surg. 2010 Jun;45:1280–6. doi: 10.1016/j.jpedsurg.2010.02.100. [DOI] [PubMed] [Google Scholar]
- 14.Jeffrey Yang CF, Duro D, Zurakowski D, Lee M, Jaksic T, Duggan C. High prevalence of multiple micronutrient deficiencies in children with intestinal failure: a longitudinal study. J Pediatr. 2011 Jul;159:39–44. e1. doi: 10.1016/j.jpeds.2010.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grummer-Strawn LM, Reinold C, Krebs NF. Use of World Health Organization and CDC growth charts for children aged 0–59 months in the United States. MMWR Recomm Rep. 2010 Sep 10;59:1–15. [PubMed] [Google Scholar]
- 16.Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics. 2010 Feb;125:e214–24. doi: 10.1542/peds.2009-0913. [DOI] [PubMed] [Google Scholar]
- 17.Pepe MS, Mori M. Kaplan-Meier, marginal or conditional probability curves in summarizing competing risks failure time data? Stat Med. 1993 Apr 30;12:737–51. doi: 10.1002/sim.4780120803. [DOI] [PubMed] [Google Scholar]
- 18.Spencer AU, Neaga A, West B, Safran J, Brown P, Btaiche I, et al. Pediatric short bowel syndrome: redefining predictors of success. Ann Surg. 2005 Sep;242:403–9. doi: 10.1097/01.sla.0000179647.24046.03. discussion 9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Messing B, Crenn P, Beau P, Boutron-Ruault MC, Rambaud JC, Matuchansky C. Long-term survival and parenteral nutrition dependence in adult patients with the short bowel syndrome. Gastroenterology. 1999 Nov;117:1043–50. doi: 10.1016/s0016-5085(99)70388-4. [DOI] [PubMed] [Google Scholar]
- 20.Cole CR, Hansen NI, Higgins RD, Ziegler TR, Stoll BJ. Very low birth weight preterm infants with surgical short bowel syndrome: incidence, morbidity and mortality, and growth outcomes at 18 to 22 months. Pediatrics. 2008 Sep;122:e573–82. doi: 10.1542/peds.2007-3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kronfli R, Bradnock TJ, Sabharwal A. Intestinal atresia in association with gastroschisis: a 26-year review. Pediatr Surg Int. 2010 Sep;26:891–4. doi: 10.1007/s00383-010-2676-4. [DOI] [PubMed] [Google Scholar]
- 22.Gura KM, Lee S, Valim C, Zhou J, Kim S, Modi BP, et al. Safety and efficacy of a fish-oil-based fat emulsion in the treatment of parenteral nutrition-associated liver disease. Pediatrics. 2008 Mar;121:e678–86. doi: 10.1542/peds.2007-2248. [DOI] [PubMed] [Google Scholar]
- 23.Cober MP, Teitelbaum DH. Prevention of parenteral nutrition-associated liver disease: lipid minimization. Curr Opin Organ Transplant. 2010 Jun;15:330–3. doi: 10.1097/MOT.0b013e328338c2da. [DOI] [PubMed] [Google Scholar]
- 24.Soden JS, Lovell MA, Brown K, Partrick DA, Sokol RJ. Failure of resolution of portal fibrosis during omega-3 fatty acid lipid emulsion therapy in two patients with irreversible intestinal failure. J Pediatr. 2010 Feb;156:327–31. doi: 10.1016/j.jpeds.2009.08.033. [DOI] [PubMed] [Google Scholar]
- 25.Tomsits E, Pataki M, Tolgyesi A, Fekete G, Rischak K, Szollar L. Safety and efficacy of a lipid emulsion containing a mixture of soybean oil, medium-chain triglycerides, olive oil, and fish oil: a randomised, double-blind clinical trial in premature infants requiring parenteral nutrition. J Pediatr Gastroenterol Nutr. 2010 Oct;51:514–21. doi: 10.1097/MPG.0b013e3181de210c. [DOI] [PubMed] [Google Scholar]
- 26.Cole CR, Frem JC, Schmotzer B, Gewirtz AT, Meddings JB, Gold BD, et al. The rate of bloodstream infection is high in infants with short bowel syndrome: relationship with small bowel bacterial overgrowth, enteral feeding, and inflammatory and immune responses. J Pediatr. 2010 Jun;156:941–7. 7, e1. doi: 10.1016/j.jpeds.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones BA, Hull MA, Richardson DS, Zurakowski D, Gura K, Fitzgibbons SC, et al. Efficacy of ethanol locks in reducing central venous catheter infections in pediatric patients with intestinal failure. J Pediatr Surg. Jun;45:1287–93. doi: 10.1016/j.jpedsurg.2010.02.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slobbe L, Doorduijn JK, Lugtenburg PJ, El Barzouhi A, Boersma E, van Leeuwen WB, et al. Prevention of catheter-related bacteremia with a daily ethanol lock in patients with tunnelled catheters: a randomized, placebo-controlled trial. PLoS One. 2010;5:e10840. doi: 10.1371/journal.pone.0010840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Danese PN. Antibiofilm approaches: prevention of catheter colonization. Chem Biol. 2002 Aug;9:873–80. doi: 10.1016/s1074-5521(02)00192-8. [DOI] [PubMed] [Google Scholar]
- 30.Kim EY, Saunders P, Yousefzadeh N. Usefulness of anti-infective lock solutions for catheter-related bloodstream infections. Mt Sinai J Med. 2010 Sep-Oct;77:549–58. doi: 10.1002/msj.20213. [DOI] [PubMed] [Google Scholar]
- 31.Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O’Grady NP, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009 Jul 1;49:1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barclay AR, Beattie LM, Weaver LT, Wilson DC. Systematic review: medical and nutritional interventions for the management of intestinal failure and its resultant complications in children. Aliment Pharmacol Ther. 2010 Jan;33:175–84. doi: 10.1111/j.1365-2036.2010.04514.x. [DOI] [PubMed] [Google Scholar]
- 33.Dibaise JK, Young RJ, Vanderhoof JA. Enteric microbial flora, bacterial overgrowth, and short-bowel syndrome. Clin Gastroenterol Hepatol. 2006 Jan;4:11–20. doi: 10.1016/j.cgh.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 34.Musso G, Gambino R, Cassader M. Gut microbiota as a regulator of energy homeostasis and ectopic fat deposition: mechanisms and implications for metabolic disorders. Curr Opin Lipidol. 2009 Feb;21:76–83. doi: 10.1097/MOL.0b013e3283347ebb. [DOI] [PubMed] [Google Scholar]
- 35.Hyman PE, Everett SL, Harada T. Gastric acid hypersecretion in short bowel syndrome in infants: association with extent of resection and enteral feeding. J Pediatr Gastroenterol Nutr. 1986 Mar-Apr;5:191–7. [PubMed] [Google Scholar]
- 36.Williams C, McColl KE. Review article: proton pump inhibitors and bacterial overgrowth. Aliment Pharmacol Ther. 2006 Jan 1;23:3–10. doi: 10.1111/j.1365-2036.2006.02707.x. [DOI] [PubMed] [Google Scholar]
- 37.Williams NS, Evans P, King RF. Gastric acid secretion and gastrin production in the short bowel syndrome. Gut. 1985 Sep;26:914–9. doi: 10.1136/gut.26.9.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Severijnen R, Bayat N, Bakker H, Tolboom J, Bongaerts G. Enteral drug absorption in patients with short small bowel : a review. Clin Pharmacokinet. 2004;43:951–62. doi: 10.2165/00003088-200443140-00001. [DOI] [PubMed] [Google Scholar]
- 39.Levy J. Immunonutrition: the pediatric experience. Nutrition. 1998 Jul-Aug;14:641–7. doi: 10.1016/s0899-9007(98)00007-0. [DOI] [PubMed] [Google Scholar]